Highlights
-
•
Magnetic resonance-guided stereotactic body radiation therapy (MRgSBRT)
-
•
MRgSBRT with optional adaptation can deliver ablative doses to primary liver cancer.
-
•
Adaptation was associated with having radiation targets ≤ 1 cm of organs at risk.
-
•
Duodenum, small bowel, or stomach were key organs at risk for adaptive radiation.
Abstract
Purpose
Magnetic resonance-guided stereotactic body radiation therapy (MRgSBRT) with optional online adaptation has shown promise in delivering ablative doses to unresectable primary liver cancer. However, there remain limited data on the indications for online adaptation as well as dosimetric and longer-term clinical outcomes following MRgSBRT
Methods and Materials
Patients with unresectable hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and combined biphenotypic hepatocellular-cholangiocarcinoma (cHCC-CCA) who completed MRgSBRT to 50 Gy in 5 fractions between June of 2015 and December of 2021 were analyzed. The necessity of adaptive techniques was evaluated. The cumulative incidence of local progression was evaluated and survival and competing risk analyses were performed.
Results
Ninety-nine analyzable patients completed MRgSBRT during the study period and 54 % had planning target volumes (PTVs) within 1 cm of the duodenum, small bowel, or stomach at the time of simulation. Online adaptive RT was used in 53 % of patients to correct organ-at-risk constraint violation and/or to improve target coverage. In patients who underwent adaptive RT planning, online replanning resulted in superior target coverage when compared to projected, non-adaptive plans (median coverage ≥ 95 % at 47.5 Gy: 91 % [IQR: 82–96] before adaptation vs 95 % [IQR: 87–99] after adaptation, p < 0.01). The median follow-up for surviving patients was 34.2 months for patients with HCC and 10.1 months for patients with CCA/cHCC-CCA. For all patients, the 2-year cumulative incidence of local progression was 9.8 % (95 % CI: 1.5–18 %) for patients with HCC and 9.0 % (95 % CI: 0.1–18) for patients with CCA/cHCC-CCA. Grade 3 through 5 acute and late clinical gastrointestinal toxicities were observed in < 10 % of the patients.
Conclusions
MRgSBRT, with the option for online adaptive planning when merited, allows delivery of ablative doses to primary liver tumors with excellent local control with acceptable toxicities. Additional studies evaluating the efficacy and safety of MRgSBRT in the treatment of primary liver cancer are warranted.
Introduction
Liver and intrahepatic bile duct cancers are the fifth leading cause of cancer death in the United States and are estimated to account for 42,230 new cases and 30,230 deaths in 2021 [1]. In patients with early-stage hepatocellular carcinoma (HCC) without significant background hepatic comorbidity, surgical resection and orthotopic liver transplant are considered potentially curative treatments [2], [3]. Similarly, for patients with early-stage hilar cholangiocarcinoma (CCA), transplant regimens can offer a definitive cure [4], [5], [6]. Patients with locally advanced primary liver cancer who are not candidates for surgery or transplant have been shown to benefit from locoregional therapies, which can slow disease progression and potentially downstage patients to orthotopic liver transplant [7], [8].
With advancement in radiation planning and delivery, stereotactic body radiation therapy (SBRT) has emerged as a promising locoregional modality for primary liver cancers [9], [10]. SBRT delivers highly conformal radiation to the target volume while minimizing radiation dose to the non-target organs-at-risk (OAR). Multiple retrospective studies [11], [12], [13] and phase 1–2 prospective trials [14], [15], [16], [17], [18] have reported on the use of SBRT primarily in patients who were ineligible for or developed a recurrence following standard locoregional therapies. Studies also have shown favorable comparisons between SBRT and standard locoregional liver-directed therapies, such as trans-arterial chemoembolization (TACE) [19], [20] and radiofrequency ablation (RFA) [21], [22]. Together, these studies report excellent local control and acceptable toxicities, supporting the safety and efficacy of SBRT in the management of primary liver cancers [9], [10].
Combining SBRT with real-time magnetic resonance (MR) guidance has been demonstrated to be an accurate and reproducible treatment modality, allowing for superior soft tissue visualization, tumor tracking, and, in otherwise-challenging anatomic locations, enabling the delivery of higher radiation doses [23], [24]. MR-guided SBRT (MRgSBRT) has been utilized to treat malignancies in the thorax [25], [26] and abdomen and pelvis [25], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. While some recent studies have reported the early clinical experience and feasibility of MRgSBRT for primary liver cancer [27], [28], [31], [32], [34], [38], there remain limited data on the indications for online adaptation and long-term clinical outcomes following MRgSBRT. Here we report our six-year institutional experience with MRgSBRT for primary liver cancers.
Methods and Materials
Patient and tumor characteristics
Patients with unresectable primary liver tumors, including hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and combined biphenotypic hepatocellular-cholangiocarcinoma (cHCC-CCA), who received MRgSBRT between June of 2015 and December of 2021 (N = 125) were identified from an Institutional Review Board-approved institutional database at a single academic institution. Patients were excluded for receiving regimens other than 50 Gy in 5 fractions (N = 22), treatment to regional lymph nodes (N = 3), or incomplete radiation therapy (RT) (N = 1). Ninety-nine analyzable patients completed MRgSBRT to 50 Gy in 5 fractions to a biologic equivalent dose (BED10) of 100 Gy (Table 1). Fifty-one (52 %) patients were treated on an MR tri-cobalt-60 device (Viewray ©), 47 (47 %) were treated on an MR-linear accelerator (MR-Linac, ViewRay MRIdian ©), and 1 (1 %) patient was treated on both devices. Selection criteria for MRgSBRT included patients with unresectable primary liver tumors with disease progression or disease not amenable to alternative locoregional therapies, Child-Pugh Class A-B (for patients with cirrhosis), and the ability to undergo magnetic resonance imaging (MRI). Patients were generally considered inoperable due to medical comorbidities, underlying hepatic dysfunction, or tumor-related factors. The primary selection criteria for patients who underwent optional daily adaptive radiotherapy (ART) planning as part of their MRgSBRT was a tumor location within 2 cm of a mobile, luminal GI structure (stomach, duodenum, small bowel, or large bowel).
Table 1.
Baseline patient and clinical characteristics for all patients and for patients with HCC and CCA/cHCC-CCA.
| Characteristic | N |
All (N = 991) |
HCC (N = 521) |
CCA/cHCC-CCA (N = 471) |
p-value2 |
|---|---|---|---|---|---|
| Age, IQR | 99 | 69 (63 – 76) | 70 (64 – 75) | 69 (63 – 77) | 0.85 |
| Sex (Male) | 99 | 70 (71) | 40 (77) | 30 (64) | 0.15 |
| Ethnicity | 99 | 0.84 | |||
| White | 89 (90) | 46 (88) | 43 (92) | ||
| Black | 8 (8) | 5 (10) | 3 (6) | ||
| Asian | 2 (2) | 1 (2) | 1 (2) | ||
| ECOG | 99 | 0.8 | |||
| 0 | 38 (38) | 19 (37) | 19 (40) | ||
| 1 | 41 (42) | 22 (42) | 19 (40) | ||
| 2 | 19 (19) | 10 (19) | 9 (20) | ||
| 3 | 1 (1) | 1 (2) | 0 (0) | ||
| AFP (ng/mL), IQR3 | 74 | 5.6 (2.8 – 18.5) | 7.7 (3.0 – 48.8) | 3.5 (2.0 – 6.2) | 0.004 |
| Bilirubin, Total (mg/dL), IQR3 | 97 | 0.5 (0.4 – 0.9) | 0.7 (0.4 – 1.0) | 0.5 (0.4 – 0.6) | 0.02 |
| Albumin (g/dL), IQR3 | 97 | 3.7 (3.3 – 4.0) | 3.7 (3.3 – 4.0) | 3.7 (3.3 – 4.0) | 0.82 |
| BCLC | 524 | ||||
| A | See HCC | 3 (6) | NA | ||
| B | See HCC | 8 (15) | NA | ||
| C | See HCC | 40 (77) | NA | ||
| D | See HCC | 1 (2) | NA | ||
| Child-Pugh Class | 505 | 0.56 | |||
| A | 45 (90) | 41 (91) | 4 (80) | ||
| B | 4 (8) | 3 (7) | 1 (20) | ||
| C | 1 (2) | 1 (2) | 0 (0) | ||
| ALBI Grade | 99 | 0.74 | |||
| 1 | 43 (44) | 24 (46) | 19 (42) | ||
| 2 | 51 (53) | 27 (52) | 24 (54) | ||
| 3 | 3 (3) | 1 (2) | 2 (4) | ||
| T Stage6 | 99 | <0.001 | |||
| 1 | 25 (25) | 3 (6) | 22 (47) | ||
| 2 | 40 (41) | 24 (46) | 16 (34) | ||
| 3 | 12 (12) | 10 (19) | 2 (4) | ||
| 4 | 21 (21) | 15 (29) | 6 (13) | ||
| X | 1 (1) | 0 (0) | 1 (2) | ||
| N Stage6 | 99 | 0.053 | |||
| 0 | 91 (92) | 51 (98) | 40 (86) | ||
| 1 | 5 (5) | 1 (2) | 4 (8) | ||
| X | 3 (3) | 0 (0) | 3 (6) | ||
| M Stage6 | 99 | 0.26 | |||
| 0 | 95 (96) | 51 (98) | 44 (94) | ||
| 1 | 4 (4) | 1 (2) | 3 (6) | ||
| Group Stage6 | 99 | <0.001 | |||
| I | 22 (22) | 3 (6) | 19 (40) | ||
| II | 38 (39) | 23 (44) | 15 (32) | ||
| III | 19 (19) | 10 (19) | 9 (19) | ||
| IV | 20 (20) | 16 (31) | 4 (9) | ||
| Liver Lesion Number | 99 | 0.052 | |||
| 1 | 69 (70) | 31 (60) | 38 (81) | ||
| 2 | 13 (13) | 7 (13) | 6 (13) | ||
| 3 | 10 (10) | 8 (15) | 2 (4) | ||
| >3 | 7 (7) | 6 (12) | 1 (2) | ||
| Tumor Size (cm) | 89 | 2.9 (2.0 – 4.7) | 3.2 (2.3 – 4.9) | 2.8 (1.7 – 4.1) | 0.12 |
| Portal Vein Involvement | 99 | 18 (18) | 11 (21) | 7 (15) | 0.42 |
| Systematic Therapy | 99 | 32 (32) | 3 (6) | 29 (62) | <0.001 |
5-FU, 5-Fluorouracil; AFP, Alpha-Fetoprotein; ALBI, Albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; cHCC-CCA, combined biphenotypic hepatocellular-cholangiocarcinoma; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma.
Statistics presented: Median (IQR); n (%).
Statistical tests performed: Wilcoxon rank-sum test; chi-square test of independence.
Pre-treatment laboratory values.
BCLC is only available for patients with HCC.
Child-Pugh Class is only available for patients with cirrhosis.
UNOS Staging for patients with HCC and AJCC 8th ed. Staging for patients with cholangiocarcinoma and biphenotypic tumor.
Simulation and planning
Details of the MRgSBRT workflow involving simulation and initial radiation planning have been previously described [25], [27], [39]. Prescribed dose for all plans was 50 Gy in 5 fractions to the planning target volume (PTV), which was defined as a 0.5 cm volumetric expansion of the gross tumor volume (GTV). The prescription goal was to cover 95 % of the PTV with 95 % of prescription dose (47.5 Gy) while meeting strict constraints for OARs (Table 2). The ranges of the hotpots for the targets are described in Table S2. If the goal PTV coverage could not be met without violation of strict OAR constraints, PTV coverage was sacrificed following an isotoxicity approach [27]. Additionally, OARs near the PTV on the simulation scan were assessed using symmetric volumetric expansion of the PTV in 0.5 cm to 2.0 cm in 0.5 cm increments.
Table 2.
Details of patients who underwent online adaptation for OAR constraint violation or to improve target coverage.
| OAR Constraint Violation | Hard Constraint | Violation Frequency | Violation % | |||
|---|---|---|---|---|---|---|
| Duodenum or small bowel | < 0.5 cc at 36 Gy | 94 / 495 | 19.0 | |||
| Stomach | < 0.5 cc at 36 Gy | 51 / 495 | 10.3 | |||
| Large bowel | < 0.5 cc at 36 Gy | 25 / 495 | 5.1 | |||
| Heart | < 15 cc at 32 Gy | 9 / 495 | 1.8 | |||
| Uninvolved liver | < 700 cc at 20 Gy < 33 % at 25 Gy |
2 / 495 | 0.4 | |||
| Esophagus | < 0.5 cc at 36 Gy | 1 / 495 | 0.2 | |||
| Kidneys | Mean < 18 Gy | 1 / 495 | 0.2 | |||
| Spinal cord | < 0.5 cc at 25 Gy | 0 / 495 | 0.0 | |||
| Target Coverage | N | Statistic | Pre-adaptation | Post-adaptation | Change in PTV Coverage (Δ) | P-value |
| PTV ≥ 95 % at 50 Gy | 39 | Median (IQR) | 87 (78 – 93) | 92 (81 – 97) | 3.8 (1.0 – 9.1) | <0.01 |
| Range | 41 – 98 | 36 – 100 | −4.6 – 20.8 | |||
| PTV ≥ 95 % at 47.5 Gy | 34 | Median (IQR) | 91 (82 – 96) | 95 (87 – 99) | 3.2 (1.3 – 5.2) | <0.01 |
| Range | 56 – 100 | 68 – 100 | −1.6 – 20.3 | |||
| PTV Optimized ≥ 95 % | 34 | Median (IQR) | 98 (95 – 100) | 98 (97 – 100) | 0.11 (-0.10 – 0.70) | 0.04 |
| at 47.5 Gy | Range | 56 – 100 | 53 – 100 | −5.1 – 15.9 | ||
1Statistical test performed: Wilcoxon signed rank test.
PTV, Planning Target Volume.
Daily treatment delivery and optional adaptive planning
For each SBRT fraction, patients underwent MRI for daily setup and localization. Patients were aligned to the centroid of the GTV. An established deformable registration algorithm was utilized for automatic cine MRI gating directly upon the GTV during treatment delivery, without a need for fiducial markers or other surrogates [27].
For patients with tumors located within 2 cm of a luminal GI structures and therefore considered for online adaptive planning as part of their MRgSBRT course, details of the daily online plan adaptation, quality assurance, and treatment delivery processes have been previously published [25], [27], [39], [40]. Following MRI imaging for daily setup as per above, the treating physician edited target and OAR contours to match the daily anatomy. The initial or prior adaptive fraction’s plan was then projected onto the daily anatomy and evaluated. If the initial or prior plan violated a strict OAR constraint (Table 2) or if the PTV coverage could be improved by at least 5 %, an online adaptive plan was generated. Adaptive plans were compared to the prior plans based on dose to OARs and target coverage, without dose accumulation, using a fraction-by fraction, strict isotoxicity approach, and the superior plan was then delivered. The delivered adaptive plan became the default plan for the subsequent fraction.
Clinical Follow-up
After completing MRgSRBT, patients were generally followed with clinical, laboratory, and imaging (MRI abdomen liver protocol preferred, triphasic CT liver protocol accepted if MRI cannot be performed) every 3 months for the first year. Local progression was defined as a radiographic increase in tumor size or enhancement and/or development or expansion of vascular invasion on the post-treatment imaging from the completion of MRgSRBT. Equivocal post-treatment changes were reviewed at multidisciplinary tumor board. Further locoregional treatments, systemic therapy, and/or liver transplant were performed at the discretion of the treating physicians after multidisciplinary consensus. Clinical and biochemical toxicities were evaluated at clinical follow-up visits using the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 [41].
Statistical analysis
Baseline characteristics were compared using Chi-square and Wilcoxon rank-sum tests. PTV coverage pre- and post-RT adaptation was compared using the Wilcoxon signed-rank test for non-parametric paired samples. Univariate and multivariate logistic regression was performed to evaluate predictors of MRgSBRT online adaptation use based on OAR proximity to PTV. Sensitivity and specificity of OAR proximity to PTV for MRgSBRT online adaptation use was also calculated.
A competing risk analysis using the Fine and Gray method [42] was used to assess the cumulative incidence of local progression, with death from any cause and/or liver transplant treated as competing events. Competing risk analysis was also used to analyze the cumulative incidence of regional, elsewhere liver, and distant progression, with death as a competing event. Regional progression was defined as progression of existing metastatic regional lymph nodes or development of new metastatic regional lymph nodes. Elsewhere liver progression was defined as the earlier of disease progression of existing lesions in the untreated liver or development of new LI-RADS 5 or LI-RADS M hepatic lesions, excluding progression of the lesion(s) treated by MRgSRBT. Distant progression was defined as progression of existing distant extrahepatic metastatic disease or development of new distant extrahepatic metastatic disease.
Overall survival (OS), defined as time to death from any cause, was estimated using the Kaplan-Meier method and compared using the log-rank test. Time to recurrence or death was calculated from the date of MRgSBRT completion to the date of the event of interest and patients who did not develop events during the study period were censored at last follow-up. A two-sided p-value of < 0.05 was considered statistically significant. All analyses were performed in R, version 4.0.2 (R Foundation for Statistical Computing).
Results
Patient, Tumor, and treatment characteristics
Baseline patient and tumor characteristics are detailed in Table 1. Fifty-two (53 %) patients had HCC, 45 (45 %) patients had CCA, and 2 (2 %) patients had cHCC-CCA. All patients completed MRgSBRT to 50 Gy in 5 fractions. For patients with HCC, the median follow-up was 14.2 months (IQR: 5.0–33.5) for all patients and 34.2 months (IQR: 11.8–42.0) for surviving patients. For patients with CCA/cHCC-CCA, the median follow-up was 9.6 months (IQR: 4.6–16.2) for all patients and 10.1 months (IQR: 5.6–20.8) for surviving patients.
Prior to treatment with MRgSBRT, liver-directed locoregional therapies were delivered to any lesions in the liver in 60 (61 %) patients. For the lesion(s) treated with MRgSRBT, 41 (41 %) patients had prior liver-directed locoregional therapies, for which MRgSBRT was delivered as consolidation or salvage therapy for persistent or progressive disease. Details on the types of locoregional therapies for the entire cohort and for the HCC and CCA/cHCC-CCA subgroups are summarized in Table S1. No systemic therapy was given concurrently with MRgSBRT. Systemic therapy within 3 months before or after the administration of MRgSBRT included gemcitabine-based chemotherapy in 21 (21 %), 5-fluorouracil (5-FU)-based chemotherapy in 4 (4 %), capecitabine in 2 (2 %), and targeted therapies in 5 (5 %) for all patients. For the subset of patients with HCC, 1 (2 %) received 5-FU-based chemotherapy and 2 (4 %) received targeted therapy. For the subset of patients with CCA/cHCC-CCA, 21 (46 %) received gemcitabine-based chemotherapy, 3 (6 %) received 5-FU-based chemotherapy, 2 (4 %) received capecitabine, and 3 (6 %) received targeted therapy.
Initial radiation therapy plan
Dosimetric data from simulation-based RT plans for targets and OARs are summarized in Table S2 and Table S3, respectively. The median target volumes were 49 cc (IQR: 28–93) for GTV and 114 cc (IQR: 68–192) for PTV (Table S2). OAR constraints are listed in Table 2. The baseline RT plans (i.e. original RT plan with the anatomy at the time of simulation) met all the constraints for the duodenum, small bowel, large bowel, stomach, esophagus, kidneys, and spinal cord.
Online adaptive radiotherapy (ART)
Of the 99 total patients, 52 (53 %) patients underwent any RT adaptation, corresponding with 181 (37 %) adapted fractions out of the 495 total delivered fractions. In total, 44 of the patients underwent adaptation for fraction 1, 36 for fraction 2, 34 for fraction 3, 37 for fraction 4, and 30 for fraction 5. Patients who underwent RT adaptation had a median of 4 (IQR: 3–5) adapted fractions. Adaptation was primarily performed to reverse OAR constraint violations when the prior plan was applied to anatomy-of-the-day on treatment days in 48 % (48/99) of patients over 29 % (141/495) of fractions. The frequencies of strict constraint violations for each OAR are summarized in Table 2. Additionally, adaptation was primarily performed to improve the target coverage of the PTV by the prescribed isodose line in 27 % (27/99) of patients over 7.9 % (39/495) of fractions. Adaptation was to reverse both OAR constraint violation and poor target coverage in 24 % (24/99) of patients. For patient undergoing MRgSBRT, adaptive plans resulted in superior PTV coverage when compared to projected, non-adaptive plans (Table 2).
Patients who underwent RT adaptation were more likely to have PTVs within 1 cm of the duodenum or small bowel (75 % vs 6 %, p < 0.001), stomach (62 % vs 9 %, p < 0.001), and large bowel (29 % vs 11 %, p = 0.02) at the time of simulation (Table S4). Compared to patients with HCC, patients with CCA/cHCC-CCA were more likely to have PTVs within 1 cm of the duodenum or small bowel (62 % vs 25 %, p < 0.001) and stomach (53 % vs 21 %, p < 0.001) and were more likely to undergo adaptation (67 % vs 33 %, p < 0.001, Table S4). Multivariate logistic regression demonstrated that having PTVs within 1 cm of the duodenum, small bowel, or stomach were significant predictors of online adaptation use (Table S5) with a sensitivity of 88 % (95 % CI: 77–96 %) and a specificity of 85 % (95 % CI: 72–94 %).
Compared to patients who did not require adaptive RT, patients who underwent RT adaptation had lower baseline PTV95 coverage (median: 93 % [IQR: 85–97] vs 100 % [IQR: 96–100], p < 0.001, Table S2) and higher maximum dose to the duodenum (median: 38 Gy [IQR: 35–40] vs 14 Gy [IQR: 4–26], p < 0.001), small bowel (median: 11 Gy [IQR: 5–20] vs 2 Gy [IQR: 1–9], p = 0.004), large bowel (median: 27 Gy [IQR: 16–37] vs 21 Gy [IQR: 3–27], p = 0.01), and stomach (median: 35 Gy [IQR: 26–39] vs 17 Gy [IQR: 10–23], p < 0.001) on the initial plan (Table S3).
Tumor control and survival
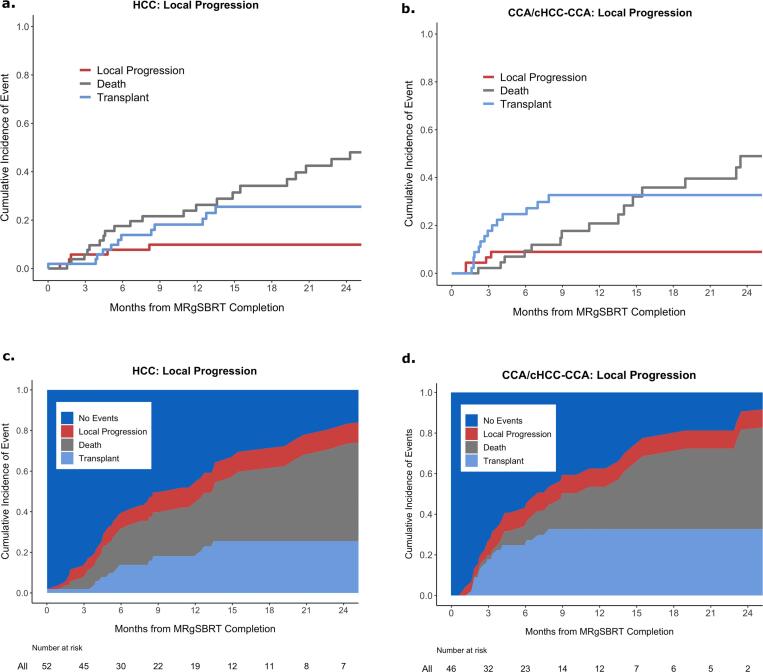
Local progression of the index tumor treated with MRgSBRT was observed in 12 % (6 / 52) of patients with HCC and in 8.5 % (4 / 47) of patients with CCA/cHCC-CCA during the study period. These local progressions occurred at a median time of 3.3 months (IQR: 1.7–8.1) and 2.0 months (IQR: 1.1–3.0) after MRgSBRT completion for patients with HCC and CCA/cHCC-CCA, respectively. The 1 and 2-year cumulative incidences of local progression for patients with HCC were both 9.8 % (95 % CI: 1.5–18) (Fig. 1a). The 1 and 2-year cumulative incidences of local progression for patients with CCA/cHCC-CCA were both 9.0 % (95 % CI: 0.1–18) (Fig. 1b).
Fig. 1.
Cumulative incidence of local progression with death and transplant as competing risks in patients with a) HCC and b) CCA/cHCC-CCA; combined cumulative incidence of the events for patients with c) HCC and d) CCA/cHCC-CCA.
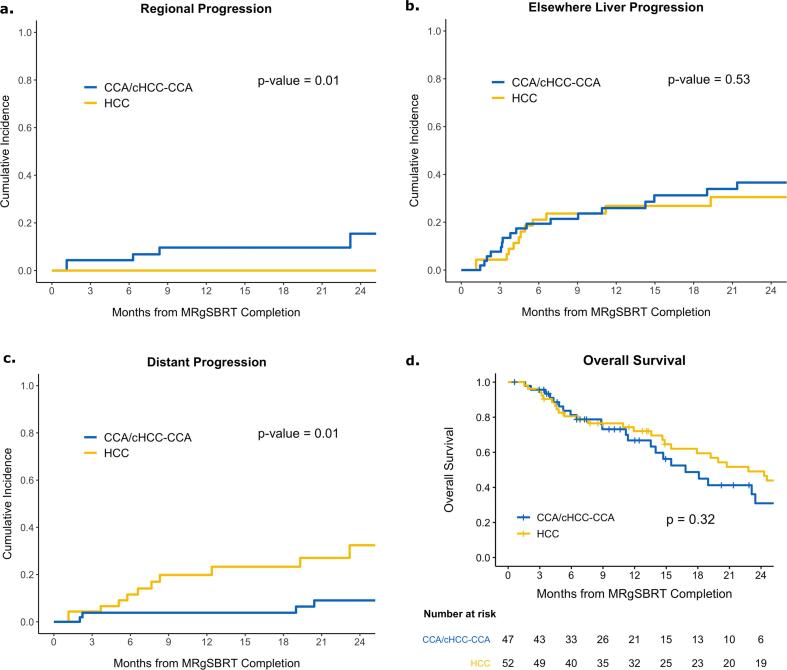
The 1- and 2-year cumulative incidences of regional progression (defined as progressive regional lymphadenopathy) for patients with HCC were both 0 % (95 % CI: 0–0), compared to 9.6 % (95 % CI: 0.1–19) and 16 % (95 % CI: 0.1–30) for patients with CCA/cHCC-CCA (p = 0.01, Fig. 2a). The 1- and 2-year cumulative incidences of elsewhere liver progression for patients with HCC were 26 % (95 % CI: 14–38) and 37 % (95 % CI: 22–51), respectively, compared to 27 % (95 % CI: 13–41) and 31 % (95 % CI: 15–46) for patients with CCA/cHCC-CCA (p = 0.53, Fig. 2b). The 1- and 2-year cumulative incidence of distant progression for patients with HCC were 3.8 % (95 % CI: 0–9.1) and 9.1 % (95 % CI: 0.1–17.8), respectively, compared to 20 % (95 % CI: 7.2–33) and 32 % (95 % CI: 15–50) for patients with CCA/cHCC-CCA (p = 0.01, Fig. 2c).
Fig. 2.
Cumulative incidence of a) regional, b) elsewhere liver, c) distant progression, and d) overall survival for patients with HCC and CCA/cHCC-CCA.
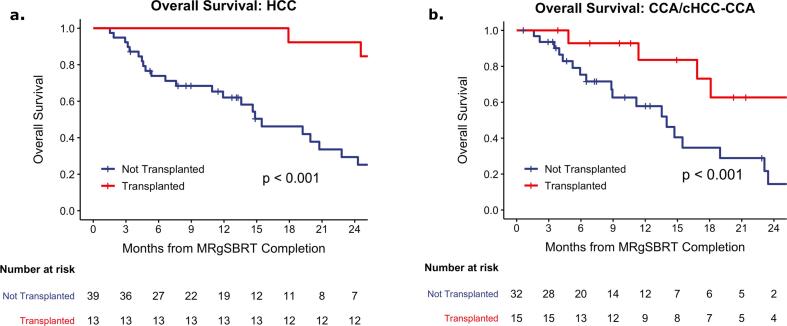
The 1- and 2-year OS for patients with HCC were 72 % (95 % CI: 61–86) and 49 % (95 % CI: 36–67), respectively, compared to 67 % (95 % CI: 53–84) and 31 % (95 % CI: 17–56) (p = 0.32, Fig. 2d) for patients with CCA/cHCC-CCA. Following MRgSBRT completion, 25 % (13 / 52) of the HCC subgroup and 32 % (15 / 47) of the CCA/cHCC-CCA subgroup underwent liver transplant at a median of 5.9 months (IQR: 4.2–13) and 2.9 months after SBRT (IQR: 1.8–5.1), respectively. Patients who underwent liver transplant were younger (median age: 63 [IQR: 59–70] vs 71 [IQR: 66–79], p < 0.001), more likely to be male (86 % vs 65 %, p = 0.04), and trended towards having an improved ECOG status (p = 0.08) (Table S6). OS was significantly improved in the HCC subgroup that underwent liver transplant following MRgSBRT (2-year: 92 % [95 % CI: 79–100] vs 29 % [95 % CI: 16–53], p < 0.001, Fig. 3a). Similarly, the CCA/cHCC-CCA subgroup that underwent liver transplant following MRgSBRT had significantly improved OS (2-year: 63 % [95 % CI: 39–100] vs 14 % [95 % CI: 4.3–48], p < 0.001, Fig. 3b).
Fig. 3.
Overall survival for patients who underwent liver transplant vs not after completion of MRgSBRT in the a) HCC and b) CCA/cHCC-CCA subset.
Toxicity
Table 3 summarizes the clinical and biochemical toxicities following MRgSBRT. The most frequently noted clinical acute grade 3 or higher toxicities were ascites and bile duct stenosis. The most common gastrointestinal luminal toxicities were 2 (2 %) patients with grade 3 obstruction and 2 (2 %) patients with grade 3 gastrointestinal bleeds. Grades 3 through 4 ascites was seen in 5 (5 %) patients, all of whom had underlying cirrhosis. Grades 3–4 bile duct stenosis occurred in 7 (7 %) patients, 6 of whom had CCA/cHCC-CCA (6 of 47, 13 %). There was one acute grade 5 toxicity with peritoneal infection that occurred two months after MRgSBRT. There was also a late grade 5 toxicity with cholangitis that occurred 1.2 years after MRgSBRT. The most common acute or late grade 3 to 4 biochemical toxicity observed was hyperbilirubinemia, which was seen in 8 (8 %) patients with CCA/cHCC-CCA and 7 (7 %) patients with HCC.
Table 3.
Acute (≤90 days of MRgSBRT start) and late toxicity (>90 days of MRgSBRT start) at least possibly attributable to MRgSBRT for all patients (N = 99).
| Toxicity1 | Acute Toxicity2 | Late Toxicity2 | ||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | |
| Clinical | ||||||
| Hepatic | 5 (5) | 0 (0) | 0 (0) | 5 (5) | 1 (1) | 0 (0) |
| Biliary | 5 (5) | 1 (1) | 0 (0) | 5 (5) | 0 (0) | 1 (1) |
| Gastrointestinal, Other | 5 (5) | 0 (0) | 1 (1) | 3 (3) | 1 (1) | 0 (0) |
| Biochemical | ||||||
| AST/ALT | 1 (1) | 1 (1) | N/A | 5 (5) | 5 (5) | N/A |
| ALP | 4 (4) | 1 (1) | N/A | 8 (8) | 0 (0) | N/A |
| Total bilirubin | 6 (6) | 0 (0) | N/A | 11 (11) | 0 (0) | N/A |
AST, Aspartate Transaminase; ALT, Alanine Transaminase; ALP, Alkaline Phosphatase.
Toxicity ≥ grade 3 (CTCAE V 5.0) in all patients. Toxicity reported includes acute (≤90 days of MRgSBRT start) and late toxicity (>90 days of MRgSBRT start) at least possibly attributable to MRgSBRT. 2Statistics presented: n (%).
Discussion
SBRT has emerged as an alternative to ablation and/or embolization techniques when these therapies have failed or are contraindicated in patients with unresectable primary liver cancer [10], [43], [44], and MR-guidance has enabled delivery of higher ablative doses in select patients with tumors in otherwise-challenging anatomic locations [23], [24]. In this study of 99 patients with unresectable primary liver cancer, MRgSBRT to a BED10 of 100 Gy, with the option for online adaptation (performed in 53 % of patients), resulted in a 2-year cumulative incidence of local progression of 9.8 % and 9.0 % for patients with HCC and CCA/cHCC-CCA, respectively. This translated to a 2-year local control of 90 % and 91 % for the HCC and CAA/cHCC-CC subgroups, respectively. Forty-one (41 %) patients had prior liver-directed locoregional therapies to the lesion(s) treated with MRgSRBT, for which MRgSBRT was delivered as consolidation or salvage therapy for persistent or progressive disease.
The excellent local control following MRgSBRT in this study compares favorably with the results from prospective studies on SBRT for primary liver cancer [9], [10]. A meta-analysis of 1950 patients with HCC from 32 studies treated with SBRT resulted in a pooled 2-year local control rate of 83.6 % (95 % CI: 77.4–88.3) [12]. In a phase II clinical trial of patients with liver tumors, Feng et al. demonstrated a 2-year local control rate of 95 % (95 % CI: 91–99) after SBRT to a median delivered dose of 49 Gy (range: 23–60) in 3 or 5 fractions, and total dose trended towards improved local control [18]. The 1-year local control was 82 % in another prospective trial, in which patients with liver tumors received a median dose of 45 Gy (range: 27.5–45) in 3 or 5 fractions [45]. Dose of 45 Gy versus < 45 Gy was found to be the only factor associated with local control [45]. Radiation dose is also important in maintaining local control in CCA. In a series of 79 patients with inoperable intrahepatic CCA, Tao et al. demonstrated higher doses were correlated with an improved local control and OS, with a 3-year local control rate of 78 % after a BED>80.5 Gy and 45 % after lower doses [11].
While radiation dose is crucial to achieving local control, delivery of a high dose to the primary liver tumor can be challenging in some patients due to proximity of OARs, inter- and intrafraction motion, and need for tumor and OAR localization [24], [46]. To overcome these challenges, MRgSBRT with real-time visualization of the tumors and OARs has shown promise of ensuring treatment accuracy. Rosenberg et al. described the earliest multi-institutional clinical experience with MRgSBRT for primary liver cancer and liver metastases [29]. In the study of 26 patients comprised of 6 patients with HCC and 2 patients with CCA, MRgSBRT to a median of 50 Gy (range: 30–60) to a median of 10 Gy (range: 6–12) per fraction, reporting no local failures in the HCC subgroup at a median follow-up of 21.2 months [29]. Several other small clinical series also supported the feasibility of MRgSBRT in treating primary liver cancer, though the studies have a small sample size, contain heterogeneous patients with a mix of primary and secondary liver tumors, and have limited data on tumor control and clinical outcomes [27], [28], [31], [32], [34], [38]. This study is the largest series to date of MRgSBRT for primary liver tumors, and it demonstrates that MRgSBRT, with use of online adaptation when deemed anatomically necessary, enabled the delivery of 50 Gy in 5 fractions to 99 patients with primary liver tumors and resulted in a local control of 90 % and 91 % for the HCC and CCA/cHCC-CCA subgroups, respectively.
Regarding the safety of SBRT for patients with primary liver cancer, the reported rates of hepatic toxicity have been variable, with generally <20 % grade 3 or higher toxicities in patients with well-compensated liver function [18], [45], [47], [13], [48], [11]. The toxicities from our study compare similarly to those in published literature. Our study reports <10 % acute or late clinical gastrointestinal and biochemical grade 3 or higher toxicities except for 11 % late grade 3 hyperbilirubinemia. Notably, grade 5 toxicity occurred in 2 % of patients (N = 2). These results are especially notable considering that 61 % of all patients received liver-directed therapies prior to MRgSBRT and 16 % received prior radiation with either radioembolization (N = 14) or SBRT (N = 2). However, given the absence of a standard comparator arm and the significant heterogeneity of the patients, tumors, and prior liver-directed therapies, it is difficult to assess the absolute reduction of radiation-related adverse events with MRgSBRT compared to conventional SBRT or other modalities. As a whole, the collective published data support that SBRT is safe with acceptable toxicity in carefully selected patients with primary liver cancer [44]. Further studies are necessary to optimize the patient selection criteria, determine timing and sequencing of therapies, assess cumulative radiation dose (e.g. with radioembolization and SBRT), and refine radiation-planning constraints when combining MRgSBRT with other liver-directed therapies to minimize toxicities.
Strengths and Limitations.
Limitations of this study include its retrospective study design, modest sample size and duration of follow-up, and heterogeneity of patients with varying tumor types, stages, and underlying hepatic function. Additionally, there was heterogeneity of the prior locoregional liver-directed therapies and systemic therapies received. Due to the patient, tumor, and treatment variations and the few incidences of local failures in this cohort, this study was unable to assess the predictors of local control. Most patients in the study had Child-Pugh A cirrhosis, so additional studies are necessary to evaluate the safety of MRgSBRT in patients with Child-Pugh B cirrhosis. While online adaptation was used to reverse OAR violations in half of the patients presented here, precise cumulative doses to luminal GI OARs remain challenging to estimate, given the limitations of current technology. This study did not include patients with primary liver tumors without challenging anatomy who were treated with standard SBRT without MR-guidance. Future studies may further delineate the optimal patient selection for and the extent of clinical benefit of MRgSBRT for OAR sparing.
Despite these limitations, this study represents the largest cohort of patients with primary liver tumors uniformly treated with MRgSBRT to 50 Gy over 5 fractions. Fifty-three patients (54 %) had planning target volumes (PTVs) within 1 cm of the duodenum, small bowel, or stomach at the time of simulation with online adaptation was successfully performed as needed to reverse OAR constraint violation and/or to improve target coverage. In patients selected for online ART due to tumor proximity to luminal OARs, comprehensive dosimetric data illustrated a statistically significant improvement in target coverage with daily plan adaptation. In addition, this study demonstrates not only a high local control in the treated lesions, but also successful tumor downstaging and bridging to liver transplant in a select subset of patients [6], [49]. Unsurprisingly, in eligible patients, liver transplant resulted in significantly improved overall survival, though larger sample size and further follow-up is needed to assess the long-term outcomes following treatment with MRgSBRT and liver transplant. Recently completed and ongoing phase 1–2 clinical trials on MRgSBRT for primary liver cancer are summarized in Table S7. Future randomized controlled trials comparing MRgSBRT to current standards of care with other liver-directed locoregional therapies are also necessary to determine the utility of MRgSBRT in the management of primary liver cancer.
Conclusion
MRgSBRT with online adaptive planning as indicated allows delivery of ablative doses to primary liver cancers with excellent local control and acceptable toxicity. Additional studies evaluating the role of MRgSBRT in the treatment of primary liver tumors are warranted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100627.
Contributor Information
Re-I Chin, Email: rchin@wustl.edu.
Shahed N. Badiyan, Email: sbadiyan@wustl.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 202CA: A Cancer Journal for Clinicians. 2021;71:7–33. [DOI] [PubMed]
- 2.Iwatsuki S., Gordon R.D., Shaw B.W., et al. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–407. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortner J.G., Maclean B.J., Kim D.K., et al. The seventies evolution in liver surgery for cancer. Cancer. 1981;47:2162–2166. doi: 10.1002/1097-0142(19810501)47:9<2162::aid-cncr2820470909>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Robles R., Figueras J., Turrión V.S., et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker N.S., Rodriguez J.A., Barshes N.R., et al. Outcomes analysis for 280 patients with cholangiocarcinoma treated with liver transplantation over an 18-year period. J Gastrointest Surg. 2008;12:117–122. doi: 10.1007/s11605-007-0335-4. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed O, Vachharajani N, Chang S-H, et al. Single-center experience of liver transplantation for perihilar cholangiocarcinoma. HPB (Oxford). 2021:S1365-182X(21)01584–7. [DOI] [PubMed]
- 7.Heckman J.T., Devera M.B., Marsh J.W., et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169–3177. doi: 10.1245/s10434-008-0071-3. [DOI] [PubMed] [Google Scholar]
- 8.Sapisochin G., Barry A., Doherty M., et al. Stereotactic body radiotherapy versus TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.02.022. xxx:1–8 [DOI] [PubMed] [Google Scholar]
- 9.Roberts H.J., Wo J.Y. Stereotactic body radiation therapy for primary liver tumors: An effective liver-directed therapy in the toolbox. Cancer. 2021 doi: 10.1002/cncr.34033. [DOI] [PubMed] [Google Scholar]
- 10.Mathew A.S., Dawson L.A. Current Understanding of Ablative Radiation Therapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:575–586. doi: 10.2147/JHC.S284403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao R., Krishnan S., Bhosale P.R., et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: A retrospective dose response analysis. J Clin Oncol. 2016;34:219–226. doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rim C.H., Kim H.J., Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144. doi: 10.1016/j.radonc.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Mathew A.S., Atenafu E.G., Owen D., et al. Long term outcomes of stereotactic body radiation therapy for hepatocellular carcinoma without macrovascular invasion. Eur J Cancer. 2020;134:41–51. doi: 10.1016/j.ejca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bujold A., Massey C.A., Kim J.J., et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 15.Takeda A., Sanuki N., Tsurugai Y., et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122:2041–2049. doi: 10.1002/cncr.30008. [DOI] [PubMed] [Google Scholar]
- 16.Hong T.S., Wo J.Y., Yeap B.Y., et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner A.A., Olsen J., Ma D., et al. Stereotactic body radiotherapy for primary hepatic malignancies – report of a phase I/II institutional study. Radiother Oncol. 2016;121:79–85. doi: 10.1016/j.radonc.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng M., Suresh K., Schipper M.J., et al. Individualized Adaptive Stereotactic Body Radiotherapy for Liver Tumors in Patients at High Risk for Liver Damage: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:40–47. doi: 10.1001/jamaoncol.2017.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen P.-C., Chang W.-C., Lo C.-H., et al. Comparison of Stereotactic Body Radiation Therapy and Transarterial Chemoembolization for Unresectable Medium-Sized Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2019;105:307–318. doi: 10.1016/j.ijrobp.2019.05.066. [DOI] [PubMed] [Google Scholar]
- 20.Wong T.C., Chiang C.-L., Lee A.-S., et al. Better survival after stereotactic body radiation therapy following transarterial chemoembolization in nonresectable hepatocellular carcinoma: A propensity score matched analysis. Surg Oncol. 2019;28:228–235. doi: 10.1016/j.suronc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Wahl D.R., Stenmark M.H., Tao Y., et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim N., Kim H.J., Won J.Y., et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol. 2019;131:81–87. doi: 10.1016/j.radonc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Hall W.A., Paulson E., Li X.A., et al. Magnetic resonance linear accelerator technology and adaptive radiation therapy: An overview for clinicians. CA Cancer J Clin. 2021:n/a. doi: 10.3322/caac.21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witt J.S., Rosenberg S.A., Bassetti M.F. MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol. 2020;21:e74–e82. doi: 10.1016/S1470-2045(20)30034-6. [DOI] [PubMed] [Google Scholar]
- 25.Fischer-Valuck B.W., Henke L., Green O., et al. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Advances in Radiation Oncology. 2017;2:485–493. doi: 10.1016/j.adro.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henke L.E., Kashani R., Hilliard J., et al. In Silico Trial of MR-Guided Midtreatment Adaptive Planning for Hypofractionated Stereotactic Radiation Therapy in Centrally Located Thoracic Tumors. Int J Radiat Oncol Biol Phys. 2018;102:987–995. doi: 10.1016/j.ijrobp.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Henke L., Kashani R., Robinson C., et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126:519–526. doi: 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Feldman A.M., Modh A., Glide-Hurst C., et al. Real-time Magnetic Resonance-guided Liver Stereotactic Body Radiation Therapy: An Institutional Report Using a Magnetic Resonance-Linac System. Cureus. 2019;11:e5774. doi: 10.7759/cureus.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg S.A., Henke L.E., Shaverdian N., et al. A Multi-Institutional Experience of MR-Guided Liver Stereotactic Body Radiation Therapy. Advances in Radiation Oncology. 2019;4:142–149. doi: 10.1016/j.adro.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudra S., Jiang N., Rosenberg S.A., et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019:2123–2132. doi: 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padgett K.R., Simpson G., Asher D., et al. Assessment of online adaptive MR-guided stereotactic body radiotherapy of liver cancers. Phys Med. 2020;77:54–63. doi: 10.1016/j.ejmp.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Luterstein E., Cao M., Lamb J.M., et al. Clinical Outcomes Using Magnetic Resonance-Guided Stereotactic Body Radiation Therapy in Patients With Locally Advanced Cholangiocarcinoma. Advances in Radiation Oncology. 2020;5:189–195. doi: 10.1016/j.adro.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hal W.A., Straza M.W., Chen X., et al. Initial clinical experience of Stereotactic Body Radiation Therapy (SBRT) for liver metastases, primary liver malignancy, and pancreatic cancer with 4D-MRI based online adaptation and real-time MRI monitoring using a 1.5 Tesla MR-Linac. PLoS One. 2020;15:e0236570. doi: 10.1371/journal.pone.0236570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogowski P., von Bestenbostel R., Walter F., et al. Feasibility and Early Clinical Experience of Online Adaptive MR-Guided Radiotherapy of Liver Tumors. Cancers. 2021;13:1523. doi: 10.3390/cancers13071523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ugurluer G., Mustafayev T.Z., Gungor G., et al. Stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of liver metastases in oligometastatic patients: initial clinical experience. Radiat Oncol J. 2021;39:33–40. doi: 10.3857/roj.2020.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayinger M., Ludwig R., Christ S.M., et al. Benefit of replanning in MR-guided online adaptive radiation therapy in the treatment of liver metastasis. Radiat Oncol. 2021;16:84. doi: 10.1186/s13014-021-01813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon S.M., Luterstein E., Chu F.-I., et al. Clinical outcomes of stereotactic magnetic resonance image-guided adaptive radiotherapy for primary and metastatic tumors in the abdomen and pelvis. Cancer Med. 2021;10:5897–5906. doi: 10.1002/cam4.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weykamp F., Hoegen P., Klüter S., et al. Magnetic Resonance-Guided Stereotactic Body Radiotherapy of Liver Tumors: Initial Clinical Experience and Patient-Reported Outcomes. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya S., Fischer-Valuck B.W., Kashani R., et al. Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications. Int J Radiat Oncol Biol Phys. 2016;94:394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Green O.L., Rankine L.J., Cai B., et al. First clinical implementation of real-time, real anatomy tracking and radiation beam control. Med Phys. 2018 doi: 10.1002/mp.13002. [DOI] [PubMed] [Google Scholar]
- 41.Anon. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
- 42.Fine J.P., Gray R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. null. 1999;94:496–509. [Google Scholar]
- 43.Anon. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(5) doi: 10.6004/jnccn.2021.0022. [DOI] [PubMed] [Google Scholar]
- 44.Apisarnthanarax S., Barry A., Cao M., et al. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2022;12:28–51. doi: 10.1016/j.prro.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Moon D.H., Wang A.Z., Tepper J.E. A prospective study of the safety and efficacy of liver stereotactic body radiotherapy in patients with and without prior liver-directed therapy. Radiother Oncol. 2018;126:527–533. doi: 10.1016/j.radonc.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Boldrini L., Corradini S., Gani C., et al. MR-Guided Radiotherapy for Liver Malignancies. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.616027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang W.I., Bae S.H., Kim M.-S., et al. A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: Safety and efficacy. Cancer. 2020;126:363–372. doi: 10.1002/cncr.32502. [DOI] [PubMed] [Google Scholar]
- 48.Durand-Labrunie J., Baumann A.-S., Ayav A., et al. Curative Irradiation Treatment of Hepatocellular Carcinoma: A Multicenter Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2020;107:116–125. doi: 10.1016/j.ijrobp.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Dageforde L.A., Vachharajani N., Tabrizian P., et al. Multi-Center Analysis of Liver Transplantation for Combined Hepatocellular Carcinoma-Cholangiocarcinoma Liver Tumors. J Am Coll Surg. 2021;232:361–371. doi: 10.1016/j.jamcollsurg.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.