Highlights
-
•
This prospective study used Cicaderma® to prevent grade ≥ 2 radiation dermatitis.
-
•
The study did not show a superiority of Cicaderma® over routine practice skin care.
-
•
Less patients treated with Cicaderma® reported grade ≥ 2 pruritus.
Keywords: Breast cancer, Radiotherapy, Radiation Oncology, Radiation dermatitis, Radiation induced skin toxicity, Skin care, Prevention, Topical agents
Abstract
Background and purpose
To prevent the occurrence of grade ≥ 2 radiodermatitis after post-operative breast irradiation in patients with non metastatic breast cancer.
Methods
This prospective randomised open-label multicenter study allocated patients from 3 French institutions, ≥18 years, requiring postoperative radiotherapy for histologically proven, early-stage (non-metastatic) unilateral breast adenocarcinoma or in situ breast cancer, with R0 or R1 post-operative status, to receive hygiene rules, associated with either Cicaderma® (Arm A), or preventive treatment according to the investigator preference (mainly hyaluronic acid (ialuset®), essential oils, or water spray, or no medication (Arm B). The primary outcome was to compare the efficacy of Cicaderma® versus local standard management in preventing the occurrence of grade ≥ 2 radiodermatitis. Main secondary objectives include Cicaderma® impact on radiotherapy discontinuation and on skin toxicity (pruritus), pain, quality of life, satisfaction.
Results
The CICA-RT study enrolled from June 2020 to April 2021, 258 women with a median age of 61 (22–91) years in 3 institutions. Patients received either Cicaderma® (A: N = 130) or standard practice (B: N = 128). In the 123 patients who initiated radiotherapy in each arm, 95 (77%, 95%CI 68.8%–84.3%) patients did not develop grade ≥ 2 dermatitis. Sensitivity and per-protocol analyses confirmed the absence of differences between arms.
Conclusion
This prospective study did not meet its primary endpoint of superiority of Cicaderma® over routine practice skin care in terms of prevention of acute radioinduced dermatitis of grade 2 or higher. However, Cicaderma® showed a significant decrease in the occurrence of pruritus with less patients reporting at least once grade ≥ 2 pruritus (A: N = 38, 31%; B: N = 58, 47%; p = 0.009).
ClinicalTrials.gov identifier NCT04300829.
Introduction
Breast cancer (BC) is the most common cancer in women, accounting for nearly 12% of all cancer diagnoses and remains the leading cause of cancer deaths in women [1]. BC multimodal treatment approaches substantially improved the outcome of BC patients during the last decades, with emphasis on biologically-directed therapies and treatment de-escalation to reduce related adverse effects. BC management usually includes lumpectomy or mastectomy with or without neoadjuvant chemotherapy, and adjuvant treatments including radiation therapy (RT), +/- chemotherapy, and/or targeted therapy [2], [3], [4]. Histological and molecular characteristics largely trigger treatment decision [2]. Technology developments in diagnostic imaging and RT showed a significant efficacy of RT on disease control and treatment-related mortality [5]. Efforts are focused on leveraging treatments using novel techniques to maximize the benefits of RT in BC patients, while limiting adverse events in critical organs. Ionizing radiation (photons, electrons, etc.) targeting cancer cells should preserve as much as possible healthy tissues and nearby organs. The areas considered are breast gland or tumor bed (according to surgery conservative or not), chest wall, nodes of the internal mammary chain and supra-clavicular nodes regardless of the type of surgery, and axillary lymph node area. Advanced radiation techniques aimed to increase the therapeutic ratio by improving target coverage and/or reducing the exposure of critical organs, and adequate dose escalation, intensification, or hypofractionation (HF) showed improved outcomes [6], [7]. Despite significant improvement in RT techniques, dermatitis related to ionizing radiation frequently occur in the next coming days or weeks, and dry or exudative radiodermatitis, or acute radionecrosis may be observed. Up to 90% of the patients develop acute radiation dermatitis, and up to 25% pruritus [8]. Besides negative impacts on patient quality of life (pain, sensitivity to UV radiation, feelings of discomfort, etc.), these adverse events may also limit the therapeutic dose delivered and/or lead to temporary or permanent discontinuation of RT with associated risk of decreasing tumor control [9]. Indeed, further research to improve management of radiation dermatitis and skin toxicities should be conducted.
Recommendations to prevent and manage acute radiation dermatitis have been recently provided by the multinational association of supportive care in cancer (MASCC) consensus, based on the current state of evidence, after several attempts from different cancer care agencies to provide guidelines [10]. At the time of study initiation, clinical practice mainly followed guidelines provided a decade ago by Wong et al. [11], however disparities between practices and guidelines still exist, and heterogeneity in clinical practice remain due to the scarcity of high quality data or conflicting findings across studies [11], [12], [13], [14]. Several topical products were used such as aqueous cream, aloe vera, trolamine (Biafine®) [15], [16], [17], [18]. Recent results with trolamine and hyaluronic acid showed no significant reduction of the radiodermatitis incidence, however Biafine® emulsion can prevent grade 3 radiodermatitis [19]. The European medical device Class IIa Xonrid® did not reduced the incidence of grade 2 radiodermatitis at 5 weeks post-RT [20]. If Calendula officinalis showed reduced radiodermatitis in patients with early breast cancer with post-operative RT, controversies regarding its efficacy still exist, and texture-related constraints preclude its development [21], [22]. Consequently, even if the use of a topical after each session may be locally recommended with decision left at the discretion of each investigator, compelling evidence regarding their role in the prevention of radiation dermatitis were still lacking at the time of study conception. Cicaderma® ointment (soft paraffin extract from Calendula officinalis L.Hyperycum. perforatum L., Achilea millefolium L., and mother tincture of Ledum palustre) (Laboratoire Boiron, Lyon, France) appeared as a promising treatment for burns and radiation-induced dermatitis [23]. Cicaderma® is indicated in the treatment of wounds, small superficial burns, and insect bites, and recommended to improve healing through inhibition of the inflammatory process and activity of matrix metalloproteinases [24], [25], [26]. In addition, re-epithelialization, and accelerate healing with Cicaderma® was reported in a murine skin ulcer model [27].
This randomised study aimed to compare the efficacy of Cicaderma® versus the local investigator practice in each investigation center to prevent the occurrence of grade ≥ 2 radiation dermatitis after post-operative breast irradiation in patients with non metastatic breast cancer.
Patient and methods
Study design and patients
This prospective, multicenter, comparative, open-label study randomised patients older than 18 years, requiring postoperative RT (after mastectomy or breast conserving surgery) for both histologically proven, early-stage (non-metastatic) unilateral breast adenocarcinoma or in situ breast cancer; with no tumor residue (R0 or R1) in a 1:1 ratio to receive either hygiene rules (cotton clothing, neutral soap, etc.) and preventive treatment with Cicaderma® ointment (Arm A) or preventive treatment according to the investigator's practice (hygiene rules, possibly associated with a single topical treatment in the comparator arm, defined according to clinical practice at each investigation site, in the current absence of standard recommendations, namely essential oils, or water spray, or no medication, Arm B). The RT was initiated from 4 to 8 weeks, and no>12 weeks after surgery, and from 3 to 8 weeks after neoadjuvant chemotherapy discontinuation. Conformational RT or modulated intensity (tomotherapy or VMAT) may be used.
On the breast, the dose delivery was 50 Gy in 25 fractions, complemented by a sequential boost of 16 Gy in 8 fractions according to the age. Delineation of organs at risk was performed according to ESTRO recommendations [28]. The objective was to avoid 110% and limit to 107%. For intensity modulation, an integrated boost using 25 fractions of 2 Gy on the breast, and 2.35 Gy on the tumoral bed, equivalent to 66 Gy delivered using normo-fractionation dosing (NF-RT), considering biologically equivalent dosing and repopulation time (0.6 Gy per day and alpha/beta of 10 Gy). Hypofractionation protocole (HF-RT) using 40.05 Gy in 15 fractions of 2.67 Gy was allowed. In case of boost requirement, NF-RT with 16 Gy delivered in 8 fractions of 2 Gy was preferred in patients ≥ 60 years.
In the case of mastectomy, dose delivery was 50 Gy in 25 fractions. In patients ≥ 60 years, 40.05 Gy in 15 fractions of 2.67 Gy can be used. Supraclavicular and internal breast node area were irradiated according to local thesaurus, with dosing between 46 and 50 Gy, in 23 to 25 fractions. Owing to the COVID-19 pandemia, dosing regimen, fractionating in breast irradiation required specific adaptations, in compliance with international guidelines [29], [30], and breast and parietal area doses were changed to 40.05 Gy in 15 fractions of 2.67 Gy, 5 times a week. If a boost was required, a dose of 10 Gy in 4 fractions of 2.5 Gy, 4 times a week may be administrated. Node area (3–4 level or interpectoral) are irradiated at the same dosing (40.05 Gy in 15 fractions of 2.67 Gy, 5 times a week). For intensity modulation, irradiation of the breast was 42.3 Gy in 18 fractions of 2.35 Gy, with a boost of 52.2 Gy in 18 fractions of 2.9 Gy each. Node area (3–4 level or interpectoral or internal mammary or axillary chain) are irradiated with a dose of 42.3 Gy in 18 fractions of 2.35 Gy. The study was stratified by investigation center, RT fractionation (NF-RT vs HF-RT, and body mass index (BMI) (≤25 vs > 25), and used a minimization technique.
Main exclusion criteria included unresolved skin toxicities from any previous treatment; hormone therapy initiated prior to radiation therapy; concomitant use of topical treatment in arm A; concomitant use of more than one topical treatment in the area to be irradiated in arm B; dermocorticoids as preventive treatment; concomitant chemotherapy and/or targeted therapies; known hypersensitivity to Cicaderma® or one of the components. The phototype was evaluated according to Fitzpatrick classification [31].
This randomised phase 3 study was set up in 3 authorized institutions (Centre Léon Bérard (CLB), Institut Sainte Catherine (ISC), Institut Curie (Curie), performed according to the declaration of Helsinki and the International conference on Harmonization on Good Medical Practices after local approval (Ethic committee of Lyon Sud-Est IV). All patients provided written informed consent before enrolment. The trial was registered with clinical trials NCT04300829.
Treatment
Cicaderma® should be applied by spreading 2 dots of ointment over the entire area treated with radiation. Cicaderma® should be applied after the radiation session and in the evening, from the first radiation session, and then continuously twice a day for 30 days after RT discontinuation.
Weekly follow-up of the patients was performed during RT, then 1 month after the end of the RT.
Endpoints
The primary endpoint was the rate of patients with no grade ≥ 2 radiation dermatitis within 30 days after completion of radiation therapy. Radiation dermatitis was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE-version 5) [32].
Secondary endpoints included the evaluation of patient satisfaction in preventive management of the radiation dermatitis; quality of life (based on the score from the dermatology life quality index questionnaire (DLQI, French dermatology society) [33]; self-reported pain assessment in the irradiated area using a 0–100 mm visual analogue scale (VAS) with 0 indicating no pain, and 100 untolerable pain; rates of temporary and permanent RT discontinuations related to the occurrence of grade 3 radiation dermatitis; Rate of occurrence of pruritus of any grade; RT doses received; time to first grade ≥ 2 cutaneous event (radiation dermatitis or pruritus) according to NCI-CTCAE-version 5 [32]; In arm A, compliance of Cicaderma® application; identify pre-treatment factors associated with grade ≥ 2 radiation dermatitis.
Statistics
The study was designed to detect an improvement in the success rate in the experimental arm from 50% (in the standard arm) to 70% (in the experimental arm). A patient with no grade ≥ 2 radiation dermatitis within 30 days post-RT was considered as successful. Based on a Chi-2 test, with 1-sided a = 0.25 and 90% power, 248 patients had to be enrolled (i.e., 124 per treatment arm). The two futility interim analyses initially planned after 33% and 50% of patients included and follow-up, were not performed owing to enrollment faster than expected.
Efficacy analyses were performed in the intention-to-treat population (all randomly assigned patients, N = 258 pts), and in the per-protocol population (N = 210) excluding patients with non-authorized concomitant topical treatment, multiple concurrent topical medications before grade 2 event, no RT initiated, non-compliant with allocation arm, and too short follow-up post-RT (Fig. 1). A sensitivity analysis was performed considering as successful all patients with no radiation dermatitis within 30 days after completion of RT.
Fig. 1.
Trial profile. *number of patients with no grade ≥ 2 radiodermatitis. ‡Non evaluable patients (A: N = 7 [consent withdrawal (N = 5); limited follow-up (N = 2)]; B: consent withdrawal (N = 5)).
Qualitative criteria were compared using Chi-2 tests and quantitative criteria were compared using Student's t test. The time to first grade ≥ 2 skin event (radiation dermatitis or pruritus) was estimated using Kaplan Meier method and described in terms of median survivals, along with the associated 2-sided 95% confidence interval (CI).
Logistic regression models were performed to determine the factors associated with the development of grade ≥ 2 radiation dermatitis. Odds Ratios (ORs) were presented with 95% CI.
SAS software version 9.4 was used for all statistical analyses.
Role of funding
The Centre Léon Bérard as sponsor was responsible for trial conception and coordination, data analysis and publication writing. All authors were involved in writing, in reviewing the report and in the decision for publication. Principal coordinators, project leader and statisticians had full access to all study data and full responsibility to submit the manuscript for publication. Laboratoire Boiron provided the study drug Cicaderma® and funding to the sponsor to conduct this study. Laboratoire Boiron had no role in the design of the study, analysis or writing.
Results
Patient characteristics
From June 2020 to April 2021, the CICA-RT study enrolled 258 breast cancer patients from 3 investigation sites and allocated them to receive either Cicaderma® (A: N = 130) or standard practice (B: N = 128) (Fig. 1).
Patients were female, with a median age of 61 (range, 22–91) years, with good Eastern Cooperative Oncology Group Performance Status (ECOG-PS 0: 218, 85%; ECOG-PS 1: 34, 13%), cup sizes A-B (40%), C-D (52%), and E and over (8%), and predominantly classified in phototype II (53%) and III (35%) according to the Fitzpatrick classification. Histology results reported 99 (38.4%) patients had an invasive carcinoma of no special type and 89 (34.5%) had an invasive ductal carcinoma. Patient, tumor, and treatment characteristics are detailed in Table 1.
Table 1.
Patients, tumors and treatment characteristics. ECOG-PS: Eastern Cooperative Oncology Group Performance Status. UICC: Union for international cancer group. RT: radiation therapy; NF: Normofractionated. HF: Hypofractionated. CT: Chemotherapy. *3 patients were marginally treated with ultra HF-RT (5x5.2 Gy (N = 2); 6x6 Gy (N = 1) (A:3;B:0). **Consent withdrawal.
| Arm A Cicaderma® ointment |
Arm B Standard practice |
All |
||||
|---|---|---|---|---|---|---|
| N = 130 | N = 128 | N = 258 | ||||
| Median age (years)(min–max) | 60.0 (31–91) | 62.0 (22–86) | 61.0 (22–91) | |||
| Median weight (kg)(min–max) | 67.0 (38–118) | 66.5 (42–110) | 67.0 (38–118) | |||
| Not specified | 1 | 0 | 1 | |||
| ECOG-PS | ||||||
| 0 | 111 | (85.4%) | 107 | (84.3%) | 218 | (84.8%) |
| 1 | 17 | (13.1%) | 17 | (13.4%) | 34 | (13.2%) |
| 2 | 2 | (1.5%) | 2 | (1.6%) | 4 | (1.6%) |
| 3 | 0 | (0.0%) | 1 | (0.8%) | 1 | (0.4%) |
| Not specified | 0 | 1 | 1 | |||
| Median BMI (min–max) | 24.8 (16–48) | 24.5 (16–40) | 24.7 (16–48) | |||
| Not specified | 1 | 0 | 1 | |||
| Cup size | ||||||
| A-B | 53 | (41.1%) | 49 | (39.8%) | 102 | (40.4%) |
| C-D | 69 | (35.7%) | 64 | (51.8%) | 133 | (52.8%) |
| E-F-G | 7 | (5.4%) | 10 | (8.1%) | 17 | (6.8%) |
| Not specified | 1 | 5 | 6 | |||
| Phototype according to Fitzpatrick classification | ||||||
| Phototype I | 8 | (6.2%) | 6 | (4.7%) | 14 | (5.4%) |
| Phototype II | 74 | (56.9%) | 62 | (48.4%) | 136 | (52.7%) |
| Phototype III | 39 | (30.0%) | 51 | (39.8%) | 90 | (34.9%) |
| Phototype IV | 6 | (4.6%) | 7 | (5.5%) | 13 | (5.0%) |
| Phototype V | 3 | (2.3%) | 2 | (1.6%) | 5 | (1.9%) |
| History of the tumor | ||||||
| Tumour localisation | ||||||
| Left breast | 71 | (54.6%) | 67 | (52.3%) | 138 | (53.5%) |
| Right breast | 59 | (45.4%) | 61 | (47.7%) | 120 | (46.5%) |
| pT | ||||||
| (P)T0 | 12 | (9.2%) | 7 | (5.5%) | 19 | (7.4%) |
| (P)T1 | 67 | (51.5%) | 75 | (58.6%) | 142 | (55.0%) |
| (P)T2 | 34 | (26.2%) | 32 | (25.0%) | 66 | (25.6%) |
| (P)T3 | 12 | (9.2%) | 10 | (7.8%) | 22 | (8.5%) |
| (P)T4 | 0 | (0.0%) | 1 | (0.8%) | 1 | (0.4%) |
| (P)TX | 5 | (3.8%) | 3 | (2.3%) | 8 | (3.1%) |
| pN | ||||||
| (P)N0 | 80 | (61.5%) | 87 | (68.0%) | 167 | (64.7%) |
| (P)N1 | 30 | (23.1%) | 29 | (22.7%) | 59 | (22.9%) |
| (P)N2 | 5 | (3.8%) | 4 | (3.1%) | 9 | (3.5%) |
| (P)N3 | 4 | (3.1%) | 0 | (0.0%) | 4 | (1.6%) |
| (P)NX | 11 | (8.5%) | 8 | (6.3%) | 19 | (7.4%) |
| Histology | ||||||
| Invasive ductal carcinoma | 46 | (35.4%) | 43 | (33.6%) | 89 | (34.5%) |
| Invasive lobular carcinoma | 17 | (13.1%) | 14 | (10.9%) | 31 | (12.0%) |
| In situ ductal carcinoma | 15 | (11.5%) | 15 | (11.7%) | 30 | (11.6%) |
| In situ lobular carcinoma | 3 | (2.3%) | 0 | (0.0%) | 3 | (1.2%) |
| Mixed invasive | 1 | (0.8%) | 1 | (0.8%) | 2 | (0.8%) |
| Invasive carcinoma of no special type (nst) | 46 | (35.4%) | 53 | (41.4%) | 99 | (38.4%) |
| Invasive papillary carcinoma | 1 | (0.8%) | 1 | (0.8%) | 2 | (0.8%) |
| Intraductal papilloma | 1 | (0.8%) | 0 | (0.0%) | 1 | (0.4%) |
| Invasive cribriform carcinoma | 0 | (0.0%) | 1 | (0.8%) | 1 | (0.4%) |
| UICC stade | ||||||
| 0 | 14 | (10.9%) | 10 | (8.0%) | 24 | (9.4%) |
| I | 55 | (42.6%) | 59 | (47.2%) | 114 | (44.9%) |
| II | 47 | (36.4%) | 45 | (36.0%) | 92 | (36.2%) |
| III | 13 | (10.1%) | 11 | (8.8%) | 24 | (9.4%) |
| Not specified | 1 | 3 | 4 | |||
| SBR grade | ||||||
| I | 35 | (26.9%) | 28 | (21.9%) | 63 | (24.4%) |
| II | 52 | (40.0%) | 58 | (45.3%) | 110 | (42.6%) |
| III | 36 | (27.7%) | 31 | (24.2%) | 67 | (26.0%) |
| Non evaluable | 7 | (5.4%) | 11 | (8.6%) | 18 | (7.0%) |
| Mitotic index | ||||||
| Low | 63 | (48.8%) | 62 | (48.8%) | 125 | (48.8%) |
| Moderate | 34 | (26.4%) | 25 | (19.7%) | 59 | (23.0%) |
| High | 24 | (18.6%) | 29 | (22.8%) | 53 | (20.7%) |
| Unknown | 8 | (6.2%) | 11 | (8.7%) | 19 | (7.4%) |
| Not specified | 1 | 1 | 2 | |||
| HER2 status | ||||||
| Positive | 15 | (11.5%) | 13 | (10.2%) | 28 | (10.9%) |
| Negative | 107 | (82.3%) | 102 | (79.7%) | 209 | (81.0%) |
| Non evaluable | 8 | (6.2%) | 13 | (10.2%) | 21 | (8.1%) |
| Oestrogen receptor status (RE) | ||||||
| Positive | 101 | (77.7%) | 96 | (75.0%) | 197 | (76.4%) |
| Negative | 21 | (16.2%) | 19 | (14.8%) | 40 | (15.5%) |
| Non evaluable | 8 | (6.2%) | 13 | (10.2%) | 21 | (8.1%) |
| Progesterone receptor status (RP) | ||||||
| Positive | 86 | (66.2%) | 87 | (68.0%) | 173 | (67.1%) |
| Negative | 36 | (27.7%) | 28 | (21.9%) | 64 | (24.8%) |
| Non evaluable | 8 | (6.2%) | 13 | (10.2%) | 21 | (8.1%) |
| Surgery at inclusion | ||||||
| Type of surgery | ||||||
| Breast conserving surgery | 106 | (81.5%) | 109 | (85.2%) | 215 | (83.3%) |
| Mastectomy | 24 | (18.5%) | 19 | (14.8%) | 43 | (16.7%) |
| Number of removed lymph node | ||||||
| N (missing) | 129 (1) | 126 (2) | 255 (3) | |||
| Median (min–max) | 3.0 (0–45) | 3.0 (0–24) | 3.0 (0–45) | |||
| Appearance of a lymphocele | ||||||
| No | 117 | (90.0%) | 113 | (88.3%) | 230 | (89.1%) |
| Yes | 13 | (10.0%) | 15 | (11.7%) | 28 | (10.9%) |
| If lymphocele appearance, spcification | ||||||
| Chest wall | 6 | 4 | 10 | |||
| Tumour bed area | 7 | 11 | 18 | |||
| Residual disease | ||||||
| R0 (no residual disease) | 113 | (86.9%) | 114 | (89.1%) | 227 | (88.0%) |
| R1 (microscopic residual disease) | 17 | (13.1%) | 14 | (10.9%) | 31 | (12.0%) |
| Re-excision surgery (tumour bed area) | ||||||
| No | 116 | (89.2%) | 118 | (92.2%) | 234 | (90.7%) |
| Yes | 14 | (10.8%) | 10 | (7.8%) | 24 | (9.3%) |
| Axillary dissection | ||||||
| No | 63 | (48.5%) | 61 | (47.7%) | 124 | (48.1%) |
| Yes | 67 | (51.5%) | 67 | (52.3%) | 134 | (51.9%) |
| Chemotherapy (CT) | ||||||
| Neo-adjuvant CT | 22 | (16.9%) | 19 | (14.8%) | 41 | (15.9%) |
| Histological response to neo-adjuvant CT (%) | ||||||
| <10% viable tumour cells | 12 | 11 | 23 | |||
| ≥10% viable tumour cells | 7 | 3 | 10 | |||
| Not specified | 3 | 5 | 8 | |||
| Adjuvant CT | 37 | (28.5%) | 36 | (28.1%) | 73 | (28.3%) |
| Radiation therapy (RT) | ||||||
| RT technique | ||||||
| 3D | 78 (62.4%) | 67 (54.5%) | 145 (58.5%) | |||
| IMRT tomotherapy, IMRT, VMAT | 47 (37.6%) | 56 (48.9%) | 103 (41.5%) | |||
| Integrated or sequential boost (tumour bed) | 69 (55.2%) | 61 (49.6%) | 130 (52.4%) | |||
| Radiotherapy RT type | ||||||
| NF-RT | 65 (52%) | 63 (51.2%) | 128 (51.6%) | |||
| HF-RT* | 60 (48%) | 60 (48.7%) | 120 (48.4%) | |||
| No RT** | 5 | 5 | 10 | |||
Among the patients in the arm A (Cicaderma®), 65 (52%) received NF-RT, and 60 (48%) received HF-RT; in the arm B (local investigator practice), 63 (51.2%) patients received NF-RT and 60 (48.7%) were treated with HF-RT. To note, 12 patients were considered as non evaluable: 10 patients with consent withdrawal before RT initiation (A: N = 5; B: N = 5), and 2 patients with too short follow-up (A: N = 2; B: N = 0) (Fig. 1). The median total RT dose was 50 (26–66) Gy regardless the groups. To note, 3 patients were patients were marginally treated with ultra HF-RT (5 fractions of 5.2 Gy (N = 2); 6 fractions of 6 Gy (N = 1)(A:3; B:0). Most of the patients were treated with 3D technique (N = 145, 58.5%; A: N = 78, 62%; B: N = 67, 54.5%), or IMRT technique (N = 73, 29%; A: N = 28, 22%; B: N = 45, 37%). All evaluable patients received irradiation on breast or breast wall area (N = 236, 95.2%). RT was performed on tumour bed (N = 130, 52.4%), on lymph node area (N = 81, 32.7%), or on sub-clavicular (N = 74, 91.4%). No patient prematurely discontinued RT permanently.
In the experimental arm, Cicaderma® was applied in all but 3 patients. The average quantity of Cicaderma® used was 4.5 (0–13) ointment tubes. To note, more than half of the patients (60%) used between 3 and 5 ointment tubes, and 18 (16%) patients required more than the 5 tubes provided in the standard kit of treatment. Patients in the arm B (local investigator practice) mainly applied hyaluronan/ialuset® (N = 69, 56%) and/or betamethasone dipropionate/diprosone® (N = 24, 20%), silver sulfadiazine/flamazine (N = 11, 8.9%) and 31 (25%) patients did not used any topical products during the study.
In the follow-up period, the hygiene rules were respected in all but seven patients (compliance failures, once: A: 1, 0.8%; B: 5, 4.2%; three times: A: N = 0; B: N = 1, 0.8%). Complementary alternative methods (fire helmsman/magnetizer) were requested at least once by 63 (25.4%) patients (A: N = 36, 29%; B: N = 27, 22%).
Impact on radiation dermatitis
The primary endpoint was evaluated in 246 patients. Reasons for the 12 failures were withdrawals of consent (N = 10) and reduced (<20 days) post-RT follow-up (N = 2).
Among the 123 patients evaluable in each group, a total of 95 (77%, 95 %CI 68.8%-84.3%) patients did not develop grade ≥ 2 radiation dermatitis. No differences in the occurrence of grade ≥ 2 radiation dermatitis between groups were evidenced.
In addition, no difference in the primary endpoint according to strata subgroups was reported. Success rates between arms were similar regardless the centers (CLB: N = 75/106, 70.8%; ISC: N = 90/115, 78.3%; Curie: N = 25/25, 100%), RT type (NF-RT: N = 85/127, 66.9%; HF-RT: N = 105/119, 88.2%), and comparable when RT duration was < 50 days (N = 164/206, 79.6%). A slight imbalance was observed in patients with RT ≥ 50 days (N = 26/40, 65%, 48.3%-79.4%; A: 55.6%, 30.8%-78.5%; B: 72.7%, 49.8%-89.3%). A slight imbalance was observed according to BMI, with more women with BMI ≤ 25 reporting no grade ≥ 2 radiation dermatitis (BMI ≤ 25 N = 103/131, 78.6%; A: 83.1%, 72%-91%; B:74.2%, 62%-84%) and less women with BMI > 25 reporting no grade ≥ 2 radiation dermatitis after having applied Cicaderma® (BMI > 25 N = 87/115, 75.7%; A: 70.7%, 57%-82%; B: 80.7%, 68%-90%).
Success rates were comparable regardless of the radiation therapy technique (3D RT: N = 112/143, 78.3%; A: 78.9%; B: 77.6%; intensity modulated radiation therapy (IMRT)/Tomotherapy IMRT, Volumetric Modulated Arc Therapy (VMAT) technics (N = 78/103, 75.7%; A: 74.5%; B: 76.8%). Sensitivity analysis and per-protocol analysis confirmed the absence of difference between arms.
Impact on radiation therapy discontinuation
No permanent discontinuation due to skin toxicity was reported. No temporary discontinuations due to dermatitis of grade 3 occurred. Temporary discontinuations -more precisely postponed RT sessions- occurred in 61 (24.6%) patients (A: N = 31, 24.8%; B: N = 30, 24.4%) mainly due to technical or maintenance reasons (A: N = 13; B: N = 11), or patient decision (A: N = 2; B: N = 3). To note, 3 patients had temporary discontinuations for grade 1 radiation dermatitis (A: N = 1, 0.8%; B: N = 2, 1.6%), with 2 to 7 postponed sessions; Seven patients reported temporary discontinuations for other toxicities (exsudative wound: A: N = 4; B: N = 1), bone pain: (A: N = 0; B: N = 1; breast pain (A: N = 1; B: N = 0)). These temporary discontinuations had no significant impact on the median RT duration (A: 35 (6–69) days; B: 34 (17–71) days), and RT duration < 50 days were reported in most patients (A: N = 107,82.3%; B: N = 101,78.9%).
Impact on skin toxicity
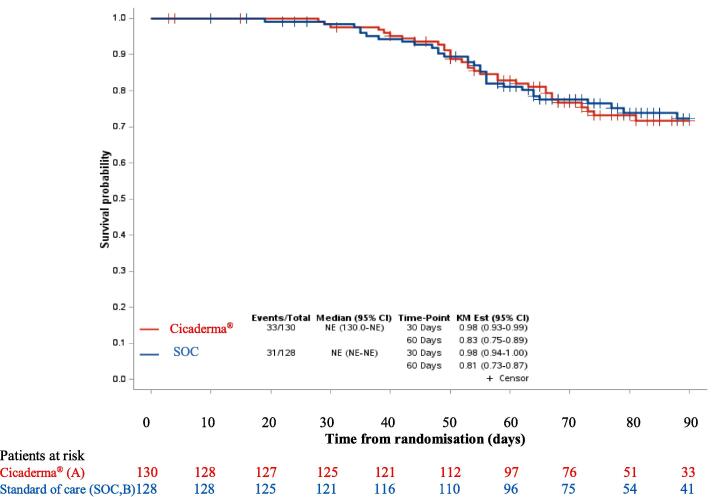
A total of 64 patients experienced grade ≥ 2 cutaneous event (radiation dermatitis or pruritus) (A: N = 33; B: N = 31) with similar time to occurrence of skin toxicity (radiation dermatitis or pruritus) in the two arms (median time not reached), from randomisation (Fig. 2) Similar results are reported from RT initiation. At 30 day- and 60 day-post-RT initiation, 92% and 73% of the patients did not experienced grade ≥ 2 skin toxicity in arm A, and 89% and 75% had no grade ≥ 2 skin toxicity in the arm B, respectively.
Fig. 2.
Skin toxicity-free survival. Standard of care (SOC).
A total of 96 (39%) patients reported at least one occurrence of pruritus of any grade. Cicaderma® showed a significant impact on the occurrence of pruritus with less patients reporting pruritus occurrence of any grade (A: N = 38, 30.9%; B: N = 58, 47.2%; p = 0.009). To note, the same trend was reported regardless the RT technique (HF-RT: A: N = 13, 22%; B:N = 25, 41.7%; p = 0.022); NF-RT: A: N = 25, 39.1%; B: N = 33, 52.4%; p = 0.132).
Satisfaction
Patients were satisfied regarding the preventive management for radiation dermatitis, with 185 (83%) patients being satisfied or very satisfied (satisfied: N = 82, 36.9%; very satisfied: N = 103, 46.4%). To note, the rate of patients being very satisfied was slightly higher in the patients treated with Cicaderma® compared with the standard arm (A: N = 60, 53.1%; B: N = 43, 39.4%).
Pain
Overall, a large proportion of patients reported no pain in the irradiated area (A: N = 52, 43%; B: N = 49, 41%) or mild pain scores from 1 to 3 (A: N = 51, 42%; B: N = 43, 36%). The reported average pain slightly increased during RT (at 7 days: 0.6; at 35 days post-RT: 1.6). A slight increase in mean levels between arms was observed at 35 day-post-radiation (A: 1.1; B: 2, respectively). However, these results must be interpreted with caution considering that pain was scored in only 90/258 patients at this timepoint. After RT, pain score decreased back to inclusion level, and was similar in both arms (Fig. 3A).
Fig. 3.
A) Pain (measured on a 0–100 mm visual analogue scale), and B) self-reported quality of life assessment according to the final score of Dermatology Life Quality index (DLQI)), at different timepoints after RT discontinuation, in Cicaderma® (A) and Standard of care (SOC, B).
Quality of life (QoL)
Overall QoL was not impaired, with a maximum DLQI score of 4.8, translating a marginal effect on the QoL. To note, QoL after RT was slightly reduced (baseline: 1.4; 35 days post-RT: 4.0). If deteriorated QoL was reported during treatment, at 30 days post- RT, patient QoL was less impaired, with a mean score of 2.4.
Fig. 3B is summarizing the evaluation of QoL in both arms.
Factors associated with grade ≥ 2 radiation dermatitis
We performed a logistic regression analysis to determine factors associated with grade ≥ 2 radiation dermatitis. The multivariate model identified that patients with a total dose ≥ 50 Gy are more likely to develop grade ≥ 2 radiation dermatitis than patients with a total dose < 50 Gy (greater risk of 4.5, 95 %CI 1.87–10.66). The RT total dose was correlated to the age of the patient.
To note, Cicaderma® related hypersensitivity has been reported (at 21 days, N = 1; at 30 days, N = 1) in 2 patients who respectively applied Cicaderma® during 16 days and 34 days.
Discussion
The randomised trial CICA-RT demonstrated no significant difference between Cicaderma® and local standard practice for the prevention of grade ≥ 2 radiation dermatitis. The trial reported that 77% of the patients with breast cancer did not develop grade ≥ 2 radiation dermatitis, regardless the allocation group. Similarly, skin care satisfaction, pain, and quality of life were not statistically different between the two arms. Despite a very low toxicity was reported in both arms, the trial showed a clinically significant reduced rate of patients developing at least one pruritus after RT after Cicaderma® application (A: N = 38, 31%; B: N = 58, 47%; p = 0.009), regardless the RT technique. The same trend was observed in the patients treated with HF-RT (A:13; B:25; p = 0.022), or NF-RT (A: 25; B:33; p = 0.132).
Initial hypotheses were based on a success rate of 50% in the control arm compared to 70% in Cicaderma® arm (reflecting an Odds Ratio of 0.429). However, the trial reported a basal rate of success much higher than anticipated in the control arm (77%). Indeed, the proportion of patients treated with HF-RT was higher than anticipated, and emphasized by the ongoing amendment owing to COVID-19 pandemia. The HF /NF-RT strata considered for the primary endpoint showed that almost half of the patients received HF-RT (HF-RT: 119, 46%; NF-RT: 127, 49%; not specified 5%) and patients treated with HF-RT globally reported less grade ≥ 2 skin toxicities (dermatitis: 12%; pruritus: 32%) than patients with NF-RT (dermatitis: 33%; pruritus: 46%). Previous studies led us to define assumptions which appeared not to adequately estimate the occurrence of skin toxicity [21]. These assumptions were too pessimistic considering current RT techniques in CICA-RT study; An unexpected elevated rate of patients treated with HF-RT was also reported in the Mepitel study during the COVID-19 pandemia [10]. The study would have required a higher power level to evidence reduction in RT-related toxicities, especially in a population with high rates of patients treated with HF-RT. The rate of grade ≥ 2 radiation dermatitis limited to 23% in the CICA-RT study was consistent with results showing reduced skin toxicity using HF-RT, but contrasted with the recently reported rate of 45% in the control arm in the Mepitel population [10]. However, differences in treatments (aqueous creams) in the standard arms and in patient characteristics (more mastectomies (40%), more patients treated with HF-RT (92–94%), and less patients with boost (29%) in Mepitel study) prevent direct comparisons between studies. Interpretation of our results may be limited by the lack of a standard recommendations as a comparator; a placebo would allow easier interpretation, but was not easy to propose and not ethically acceptable in the current context. Even if the results of CICA-RT study did not evidence an improvement in the onset of grade ≥ 2 acute dermatitis, an improvement in the occurrence of grade ≥ 2 pruritus was reported. Cicaderma® was well accepted by both patients and nurses. The multicenter randomised phase III trial HYPO-G01 (NCT03127995) comparing HF-RT versus NF-RT in breast cancer with an indication for regional lymph node irradiation reported CTCAE-based acute toxicity assessments, and showed frequent skin toxicities, mostly grade 1 toxicities (dermatitis: up to 90%; pruritus: up to 28%), and a limited number of patients reported grade 2–3 dermatitis and pruritus occurrence. In practice, patients with HF-RT showed less RT-associated grade 2 skin toxicities (dermatitis: 15%; pruritus: 2%), than patients treated with NF-RT (dermatitis: 33%; pruritus: 3%). No grade 4 or 5 were observed [35]. Similar results showing less toxicities and improved QOL have been recently reported and supported the benefits of HF-RT [36].
Whereas the 10-year follow-up showed that appropriately dosed HF-RT is safe and effective in patients with breast cancer,[2] guidelines still differ worldwide, and normo-fractionated (NF-RT) regimens are still recommended in some European countries including France, until robust results from prospective meta-analysis are reported. In Europe, the Danish (NCT00909818) randomised trial investigating NF-RT and moderate HF-RT regimens recently showed no more grade 2–3 breast induration with low risk for locoregional recurrence at 9-year [3]. The final long-term follow-up results from the French randomised trial HYPOG-01 (NCT03127995) are eagerly expected to determine whether moderate HF-RT should become locally the standard for regional treatment. Preliminary results showed that women receiving 3-week moderately HF locoregional RT showed reduced acute toxicities and no important acute safety concerns [34]. Despite differences in grade ≥ 2 acute skin toxicity in favor to accelerated partial breast RT with external beam RT or brachytherapy have been reported [4], most guidelines in France still recommend NF-RT when lymph node areas need to be irradiated.
While acute radiation dermatitis, and pruritus have been frequently reported [8], most patients experienced grade 1 dermatitis and pruritus, and up to 32% of grade 2–3 skin toxicities, and HF-RT showed reduced skin toxicity occurrence compared to NF-RT (dermatitis, HF-RT: N = 13 (2%); NF-RT: N = 29 (3%); pruritus, HF-RT: N = 2; NF-RT: N = 3)[35]. Besides negative impacts on patient quality of life (pain, sensitivity to UV radiation, feelings of discomfort, etc.), these adverse events may adversely limit the therapeutic dose delivered and/or lead to temporary or permanent discontinuation of RT with associated risk of decreasing tumor control [7].
In addition, other factors can support the current favourable tolerance profile. Notably, patients included in CICA-RT study received systematic risk prevention based on detailed oral and written information underlining the importance of hygiene rules. Such particular vigilance may have contributed to limit and/or delay the occurrence of radiation dermatitis. Careful monitoring of patient diary may have contributed to closely consider comfort and increase satisfaction. In addition, the enhancement in modern irradiation techniques including intensity modulation and better conformation related to patient anatomy, contribute to reduce acute toxicity [37], [38], [39]. Other factors, such as elevated BMI, regional node radiation, and chemotherapy were reported to be associated with increased acute skin toxicity [40]. Regular clinical follow-up with evaluation by the attending physician once a week may contribute to limit the emergence of adverse events and the development of complications, with topical medication, and to adjust RT schedule with temporary discontinuation/ postponed sessions, when necessary.
Among several skin care products such as calendula, trolamine, aloe vera, or hydrosorb investigated, no efficacy for the curative treatment of radiation dermatitis was demonstrated [41], [42], [43], [44], [45], [46].
Beyond RT-irritated skin local sequelae, associated discomfort may contribute to induce depressive psychological status and deteriorate patient quality of life [47]. Reinforcing care to better manage adverse consequences related to irradiated skin, including potential psychological impact after receiving radiation therapy is required. Composite endpoints using clinician rating and patient reported outcomes would help to better appreciate the discomfort related to skin toxicities, and better anticipate potential consequences. Further studies involving quality of life and cost effectiveness analysis would provide complementary perspectives. Up to recently, most series were limited by reduced sample sizes and faced with inter- and intra-observer variability despite implementation of CTCAE criteria, preventing robust interpretation and conclusions. The MASCC oncodermatology study group recently published clinical practice guidelines for the prevention and management of acute radiation dermatitis, and reported reduction in radiation dermatitis in patients with breast cancer with the use of Mepitel film [10]. Further studies are required to better prevent and manage radiation induced skin reactions and their consequences.
Conclusion
This prospective study did not meet its primary endpoint of superiority of Cicaderma® over routine practice skin care in terms of prevention of acute radiation-induced dermatitis of grade 2 or higher. However, Cicaderma® showed a significant decrease in the occurrence of grade ≥ 2 pruritus. Systematic prevention measures and modern breast cancer RT techniques led to acceptable tolerability, and the place of topical treatment to further optimize this tolerability has to be defined.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank all the patients who participated in the study, investigators, clinical and research staff from all the participating sites. The authors thank Sophie DARNIS PhD for helpful comments and valuable help for medical editorial assistance.
Data availability statements
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.
Funding statement
This study received the study drug Cicaderma® and partial support from Laboratoire Boiron. Laboratoire Boiron had no role in the design of the study, analysis or writing.
Author contributions
The Centre Léon Bérard as sponsor of the study was responsible for trial conception and coordination, data analysis, and writing of the report. The original idea and conception of the protocol: SR and YK. All authors were involved in patients’ inclusions, writing and reviewing the report. SR, SM, CS, YK had full access to study data and full responsibility to submit for publication.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M., et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overgaard J., Aznar M.C., Bacchus C., Coppes R.P., Deutsch E., Georg D., et al. Personalised radiation therapy taking both the tumour and patient into consideration. Radiother Oncol. 2022;166:A1–A5. doi: 10.1016/j.radonc.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Kerr A.J., Dodwell D., McGale P., Holt F., Duane F., Mannu G., et al. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat Rev. 2022;105 doi: 10.1016/j.ctrv.2022.102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelan, Timothy; Pignol, Jean-Philippe; Levine Mark; Julian Jim; MacKenzie, Robert; Parpia Sameer;Shelley, Wendy; Grimard, Laval; Bowen, Julie; Lukka, Himu; Perera, Francisko; Fyles, Anthony; Schneider, Ken; Gulavita, Sunil; and Freeman C. Long-term results of hypofractionated radiation therapy for breast cancer. Breast Dis 2010;21:267–8. https://doi.org/10.1016/S1043-321X(10)79594-1. [DOI] [PubMed]
- 7.Haviland J.S., Owen J.R., Dewar J.A., Agrawal R.K., Barrett J., Barrett-Lee P.J., et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 8.Pinnix C., Perkins G.H., Strom E.A., Tereffe W., Woodward W., Oh J.L., et al. Topical Hyaluronic Acid vs. Standard of Care for the Prevention of Radiation Dermatitis After Adjuvant Radiotherapy for Breast Cancer: Single-Blind Randomized Phase III Clinical Trial. Int. J Radiat Oncol. 2012;83(4):1089–1094. doi: 10.1016/j.ijrobp.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leventhal J., Young M.R. Radiation Dermatitis: Recognition, Prevention, and Management. Oncology (Williston Park) 2017;31(885–7):894–899. [PubMed] [Google Scholar]
- 10.Behroozian T., Bonomo P., Patel P., Kanee L., Finkelstein S., van den Hurk C., et al. Multinational Association of Supportive Care in Cancer (MASCC) clinical practice guidelines for the prevention and management of acute radiation dermatitis: international Delphi consensus-based recommendations. Lancet Oncol. 2023;24(4):e172–e185. doi: 10.1016/S1470-2045(23)00067-0. [DOI] [PubMed] [Google Scholar]
- 11.Wong R.K.S., Bensadoun R.-J., Boers-Doets C.B., Bryce J., Chan A., Epstein J.B., et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21(10):2933–2948. doi: 10.1007/s00520-013-1896-2. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein S., Kanee L., Behroozian T., Wolf J.R., van den Hurk C., Chow E., et al. Comparison of clinical practice guidelines on radiation dermatitis: a narrative review. Support Care Cancer. 2022;30(6):4663–4674. doi: 10.1007/s00520-022-06829-6. [DOI] [PubMed] [Google Scholar]
- 13.Behroozian T., Goldshtein D., Ryan Wolf J., van den Hurk C., Finkelstein S., Lam H., et al. MASCC clinical practice guidelines for the prevention and management of acute radiation dermatitis: part 1) systematic review. EClinicalMedicine. 2023;58 doi: 10.1016/j.eclinm.2023.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Society and College of Radiographers. Radiation dermatitis guidelines for radiotherapy healthcare professionals. April 2020. https://www.sor.org/learning-advice/professional-body-guidanceand-publications/documents-and-publications/policy-guidancedocument-library/radiation-dermatitis-guidelines-for-radiotherapy-h n.d.
- 15.Rosenthal A., Israilevich R., Moy R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558–567. doi: 10.1016/j.jaad.2019.02.047. [DOI] [PubMed] [Google Scholar]
- 16.Clark J.R., Rumcheva P., Veness M.J. Analysis and Comparison of the 7th Edition American Joint Committee on Cancer (AJCC) Nodal Staging System for Metastatic Cutaneous Squamous Cell Carcinoma of the Head and Neck. Ann Surg Oncol. 2012;19(13):4252–4258. doi: 10.1245/s10434-012-2504-2. [DOI] [PubMed] [Google Scholar]
- 17.Chan R.J., Webster J., Chung B., Marquart L., Ahmed M., Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:1–19. doi: 10.1186/1471-2407-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geara F.B., Eid T., Zouain N., Thebian R., Andraos T., Chehab C., et al. Randomized, Prospective, Open-label Phase III Trial Comparing Mebo Ointment With Biafine Cream for the Management of Acute Dermatitis During Radiotherapy for Breast Cancer. Am J Clin Oncol. 2018;41(12):1257–1262. doi: 10.1097/COC.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 19.Fatima S., Hirakawa S., Marta G.N., Caini S., Beveridge M., Bonomo P., et al. Topical non-steroidal agents for the prevention of radiation dermatitis: a systematic review and meta-analysis. Support Care Cancer. 2023;31(4) doi: 10.1007/s00520-023-07677-8. [DOI] [PubMed] [Google Scholar]
- 20.Ingargiola R., De Santis M.C., Iacovelli N.A., Facchinetti N., Cavallo A., Ivaldi E., et al. A monocentric, open-label randomized standard-of-care controlled study of XONRID®, a medical device for the prevention and treatment of radiation-induced dermatitis in breast and head and neck cancer patients. Radiat Oncol. 2020;15(1) doi: 10.1186/s13014-020-01633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pommier P., Gomez F., Sunyach M.P., D’Hombres A., Carrie C., Montbarbon X. Phase III randomized trial of Calendula Officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J Clin Oncol. 2004;22:1447–1453. doi: 10.1200/JCO.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 22.Siddiquee S., McGee M.A., Vincent A.D., Giles E., Clothier R., Carruthers S., et al. Efficacy of topical Calendula officinalis on prevalence of radiation-induced dermatitis: A randomised controlled trial. Australas J Dermatol. 2021;62:e35–e40. doi: 10.1111/ajd.13434. [DOI] [PubMed] [Google Scholar]
- 23.Morin C., Roumegous A., Carpentier G., Barbier-Chassefière V., Garrigue-Antar L., Caredda S., et al. Modulation of inflammation by cicaderma ointment accelerates skin wound healing. J Pharmacol Exp Ther. 2012;343(1):115–124. doi: 10.1124/jpet.111.188599. [DOI] [PubMed] [Google Scholar]
- 24.Öztürk N., Korkmaz S., Öztürk Y. Wound-healing activity of St. John’s Wort (Hypericum perforatum L.) on chicken embryonic fibroblasts. J Ethnopharmacol. 2007;111:33–39. doi: 10.1016/j.jep.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Dell’Aica I, Caniato R, Biggin S, Garbisa S. Matrix proteases, green tea, and St. John’s wort: Biomedical research catches up with folk medicine. Clin Chim Acta 2007;381:69–77. https://doi.org/10.1016/j.cca.2007.02.022. [DOI] [PubMed]
- 26.Süntar I., Oyardi O., Akkol E.K., Ozçelik B. Antimicrobial effect of the extracts from Hypericum perforatum against oral bacteria and biofilm formation. Pharm Biol. 2016;54:1065–1070. doi: 10.3109/13880209.2015.1102948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuma C.H., Andrade T.A.M., Caetano G.F., Finci L.I., Maciel N.R., Topan J.F., et al. Development of lamellar gel phase emulsion containing marigold oil (Calendula officinalis) as a potential modern wound dressing. Eur J Pharm Sci. 2015;71:62–72. doi: 10.1016/j.ejps.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Offersen B.V., Boersma L.J., Kirkove C., Hol S., Aznar M.C., Biete Sola A., et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114(1):3–10. doi: 10.1016/j.radonc.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Loap P., Kirova Y., Takanen S., Créhange G., Fourquet A. Radiothérapie mammaire dans le contexte de la pandémie de COVID-19: astuces pratiques en période épidémique et conseils pour la reprise de l’activité en fin de crise. Cancer/Radiothérapie. 2020;24:196–198. doi: 10.1016/j.canrad.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coles C.E., Aristei C., Bliss J., Boersma L., Brunt A.M., Chatterjee S., et al. International Guidelines on Radiation Therapy for Breast Cancer During the COVID-19 Pandemic. Clin Oncol. 2020;32(5):279–281. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak M.A. In memory of Thomas Bernhard Fitzpatrick. J Invest Dermatol. 2004;122:xx–xxi. doi: 10.1046/j.1523-1747.2003.22248.x. [DOI] [PubMed] [Google Scholar]
- 32.NCI-CTCAE-version5 NCICTC for AE. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf n.d.
- 33.Finlay A.Y., Khan G.K. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 34.Loibl S., Sikov W., Huober J., Rugo H., Wolmark N., O’Shaughnessy J., et al. Event-free survival (EFS), overall survival (OS), and safety of adding veliparib (V) plus carboplatin (Cb) or carboplatin alone to neoadjuvant chemotherapy in triple-negative breast cancer (TNBC) after ≥4 years of follow-up: BrighTNess, a randomized phase. Ann Oncol. 2021:S407–S446. doi: 10.1016/annonc/annonc687. [DOI] [Google Scholar]
- 35.Rivera S., Brion T., Kirova Y., Racadot S., Benchalal M., et al. Acute toxicity associated with a 3-week versus a standard 5-week regimen for locoregional breast radiotherapy delivered in the UNICANCER HypoG-01 phase III trial. Ann Oncol. 2021;32:S407–S446. doi: 10.1016/annonc/annonc687. [DOI] [Google Scholar]
- 36.Arsenault J., Parpia S., Goldberg M., Rakovitch E., Reiter H., Doherty M., et al. Acute Toxicity and Quality of Life of Hypofractionated Radiation Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys. 2020;107(5):943–948. doi: 10.1016/j.ijrobp.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 37.Pignol J.-P., Olivotto I., Rakovitch E., Gardner S., Sixel K., Beckham W., et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 38.Donovan E., Bleakley N., Denholm E., Evans P., Gothard L., Hanson J., et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82(3):254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Allali S., Carton M., Sarrade T., Querel O., Jacquet A., Rivera S., et al. CANTO-RT: Skin toxicities evaluation of a multicentre large prospective cohort of irradiated patients for early-stage breast cancer. Int J Cancer. 2022;151:1098–1108. doi: 10.1002/ijc.34057. [DOI] [PubMed] [Google Scholar]
- 40.Parekh A., Dholakia A.D., Zabranksy D.J., Asrari F., Camp M., Habibi M., et al. Predictors of radiation-induced acute skin toxicity in breast cancer at a single institution: Role of fractionation and treatment volume. Adv Radiat Oncol. 2018;3(1):8–15. doi: 10.1016/j.adro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bazire L., Fromantin I., Diallo A., Lande B.d.l., Pernin V., Dendale R., et al. Hydrosorb® versus control (water based spray) in the management of radio-induced skin toxicity: Results of multicentre controlled randomized trial. Radiother Oncol. 2015;117(2):229–233. doi: 10.1016/j.radonc.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 42.Kirova Y.M., Fromantin I., De Rycke Y., Fourquet A., Morvan E., Padiglione S., et al. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy? Results of phase III randomised trial. Radiother Oncol. 2011;100(2):205–209. doi: 10.1016/j.radonc.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Chargari C., Fromantin I., Kirova Y.M. Intérêt des applications cutanées en cours de radiothérapie pour la prévention et le traitement des épithéliites radio-induites. Cancer/Radiotherapie. 2009;13:259–266. doi: 10.1016/j.canrad.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Heggie S., Bryant G.P., Tripcony L., Keller J., Rose P., Glendenning M., et al. A Phase III Study on the Efficacy of Topical Aloe Vera Gel on Irradiated Breast Tissue. Cancer Nurs. 2002;25(6):442–451. doi: 10.1097/00002820-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Elliott E.A., Wright J.R., Swann R.S., Nguyen-Tân F., Takita C., Bucci M.K., et al. Phase III Trial of an Emulsion Containing Trolamine for the Prevention of Radiation Dermatitis in Patients With Advanced Squamous Cell Carcinoma of the Head and Neck: Results of Radiation Therapy Oncology Group Trial 99–13. J Clin Oncol. 2006;24(13):2092–2097. doi: 10.1200/JCO.2005.04.9148. [DOI] [PubMed] [Google Scholar]
- 46.Williams M.S., Burk M., Loprinzi C.L., Hill M., Schomberg P.J., Nearhood K., et al. Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol Biol Phys. 1996;36(2):345–349. doi: 10.1016/s0360-3016(96)00320-3. [DOI] [PubMed] [Google Scholar]
- 47.Chu C.-N., Hu K.-C., Wu R.-C., Bau D.-T. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy. BMC Cancer. 2021;21(1) doi: 10.1186/s12885-021-08047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.