Graphical abstract
Keywords: Trichoderma species, Terpene, Structural diversity, Pharmacological activity, Biosynthesis
Highlights
-
•
Trichoderma species from soil and plants are rich source of bioactive secondary metabolites.
-
•
A total of 253 terpenoids with diverse skeletons and bioactivities were isolated from Trichoderma species.
-
•
The promising biosynthetic potential of Trichoderma species for terpenoid diversity were generalized and discussed.
-
•
Trichoderma species are of great significance for exploring drug lead compounds and potential application in agriculture.
Abstract
Background
Trichoderma species are rich source of bioactive secondary metabolites. In the past decades, a series of secondary metabolites were reported from different Trichoderma fungi, among which terpenoids possessing versatile structural diversities and extensive pharmacological activities are one of the particularly important categories.
Aim of Review
The review aims to summarize the terpenoids isolated from Trichoderma species regarding their structural diversities, biological activities, and promising biosynthetic potentials.
Key Scientific Concepts of Review
So far, a total of 253 terpenoids, including 202 sesquiterpenes, 48 diterpenes, 2 monoterpenes and 1 meroterpenoid, were isolated and identified from Trichoderma species between 1948 and 2022. Pharmacological investigations of Trichoderma terpenoids mainly focused on their antibacterial activities, antifungal activities, inhibitory activities on marine plankton species and cytotoxic activities, indicating that Trichoderma species are important microbial agents for drug discovery and environmentally friendly agrochemicals development. Intriguing chemistry and enzymology involved in the biosynthesis of Trichoderma terpenoids were also presented to facilitate further precise genome mining-guided novel structure discovery. Taken together, the abundance of novel skeletons, bioactivities and biosynthetic potentials presents new opportunities for drug and agrochemicals discovery, genome mining and enzymology exploration from Trichoderma species. The work will provide references for the profound study of terpenoids derived from Trichoderma, and facilitate further studies on Trichoderma species in the areas of chemistry, medicine, agriculture and microbiology.
Introduction
Trichoderma is a genus of fungi which has a long history of at least 100 million years. By the late 1970 s, it began to enter the scientific spotlight and gradually attracted people’s attention. Trichoderma fungi have a wide variety of different ecological habitats including natural soils [1], [2], decaying wood [3], agricultural habitats [4], living plants [5], mushroom-related substrata [6], [7], human body [8], water-related environments [9], air and settled dust [10]. Trichoderma genus are classified into approximately 10,000 species according to morphological features [11]. Up to now, Trichoderma species have been broadly applied in agriculture as biocontrol agent for various plant diseases [12], and as sustainable bio-organisms for plant growth promotion [13], [14], natural decomposition [15], [16] and bioremediation [17], [18].
Previous literatures have demonstrated that the secondary metabolites derived from Trichoderma possess diverse and promising bioactivities, which have the potential to be considered as lead compounds for agrochemicals and drug development. In the past decades, many types of secondary metabolites from Trichoderma genus have been isolated and identified, such as peptaibols [19], [20], polyketides [21], pyrones [22], and terpenoids [23] that may be associated with the pivotal antimicrobial potential of Trichoderma [24], [25], [26], [27]. Increasing evidences have confirmed the extremely important roles of those secondary metabolites in the favorable effects of Trichoderma on crops and human health [28]. Due to the diversity, complexity and abundance of Trichoderma species and their secondary metabolites, more and more attentions have been attracted and casted on the exploration of this genus.
Terpenoids, an important class of plant and microbial secondary metabolites, not only possess versatile pharmacological activities, but also have great potential in agricultural, food, cosmetics industries and even as substitute of certain fossil fuel [29], [30]. Currently, most of the terpenoids are obtained either from plant extraction or artificially synthesis with apparent drawbacks of low yields, resource unsustainability, and environmental unfriendliness, thus preventing the large scale of discovery and utilization of terpenoids. Consequently, exploring new and sustainable resources for terpenoids biosynthesis is vital for discovering and industrializing the biotechnological production of valuable terpenoids. Nowadays, advanced metabolic engineering and synthetic biology make microorganisms efficient platforms for the green industrial production of natural compounds with high value [31], [32], [33]. Trichoderma species, known as one group of fungi mainly residing in plants and soil, is an excellent resource and effective platform served as hosts for terpenoids production owing to their characteristic of fast and easily growing. Additionally, terpenoids are the most abundant secondary metabolites in Trichoderma fungi and have been attracting extensive interest from chemists and agronomist aiming at searching for novel bioactive molecules for new drug and agrochemicals development.
In the present paper, a total of 253 terpenoids isolated from Trichoderma species since 1948 were included and classified into sesquiterpenes, diterpenes, monoterpenes and meroterpenoid. The review aims to summarize the structural diversity, biological activities and plausible biosynthetic genes and routes of Trichoderma terpenoids in hope of providing reference for further studies on Trichoderma species in the areas of chemistry, medicine, agriculture and microbiology.
Structural diversity of Trichoderma terpenoids
Terpenoids are the most common secondary metabolites of Trichoderma, assembled by multiple activated forms of the isoprene unit and endowed with great structural variety. Up to now, there have been 253 terpenoids in all discovered and identified from Trichoderma fungi, including 202 sesquiterpenes, 48 diterpenes, 2 monoterpenes and 1 meroterpenoid (Table 1).
Table 1.
Terpenoids from Trichoderma species.
| Type | No. | Names | Sources | Reference |
|---|---|---|---|---|
| Cyclonerane-type sesquiterpenes | 1 | cyclonerodiol | Trichoderma polysporum | [34] |
| 2 | cyclonerodiol oxide | Trichoderma polysporum | [34] | |
| 3 | epicyclonerodiol oxide | Trichoderma polysporum | [34] | |
| 4 | cyclonerotriol | Trichoderma virens | [35] | |
| 5 | 10,11-dihydrocyclonerotriol | Trichoderma longibrachiatum YM311505 | [36] | |
| 6 | cyclonerodiol B | Trichoderma sp. Xy24 | [37] | |
| 7 | (10E)-12-acetoxy-10-cycloneren-3,7-diol | Trichoderma harzianum P1-4 | [38] | |
| 8 | 12-acetoxycycloneran-3,7-diol | Trichoderma harzianum P1-4 | [38] | |
| 9 | 9-cycloneren-3,7,11-triol | Trichoderma asperellum cf44-2 | [39] | |
| 10 | 11-cycloneren-3,7,10-triol | Trichoderma asperellum cf44-2 | [39] | |
| 11 | 7,10-epoxycycloneran-3,11,12-triol | Trichoderma asperellum cf44-2 | [39] | |
| 12 | 11-methoxy-9-cycloneren-3,7-diol | Trichoderma harzianum X-5 | [40] | |
| 13 | 10-cycloneren-3,5,7-triol | Trichoderma harzianum X-5 | [40] | |
| 14 | methyl 3,7-dihydroxy-15-cycloneranate | Trichoderma harzianum X-5 | [40] | |
| 15 | 3,7,11-trihydroxycycloneran-10-one | Trichoderma asperellum A-YMD-9–2 | [41] | |
| 16 | cycloneran-3,7,10,11-tetraol | Trichoderma asperellum A-YMD-9–2 | [41] | |
| 17 | cycloneran-3,7,11-triol | Trichoderma asperellum A-YMD-9–2 | [41] | |
| 18 | 11,12,15-trinorcycloneran-3,7,10-triol | Trichoderma asperellum A-YMD-9–2 | [41] | |
| 19 | 7,10S-epoxycycloneran-3,15-diol | Trichoderma asperellum A-YMD-9–2 | [41] | |
| 20 | 7,10R-epoxycycloneran-3,15-diol | Trichoderma asperellum A-YMD-9–2 | [41] | |
| 21 | (10Z)-15-acetoxy-10-cycloneren-3,7-diol | Trichoderma asperellum A-YMD-9–2 | [41] | |
| 22 | (10E)-isocyclonerotriol | Trichoderma citrinoviride A-WH-20–3 | [42] | |
| 23 | (10Z)-isocyclonerotriol | Trichoderma citrinoviride A-WH-20–3 | [42] | |
| 24 | 3,7,11-trihydroxy-cycloneran | Trichoderma harzianum (XS-20090075) | [43] | |
| 25 | 5-hydroxyepicyclonerodiol oxide | Trichoderma hamatum Z36 − 7 | [44] | |
| 26 | 4-hydroxyepicyclonerodiol oxide | Trichoderma hamatum Z36 − 7 | [44] | |
| 27 | cycloner-3-en-7,11-diol | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 28 | isoepicyclonerodiol oxide | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 29 | norepicyclonerodiol oxide | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| Cadinane-type sesquiterpenes | 30 | trichocadinin A | Trichoderma virens Y13-3 | [46] |
| 31 | 4-cadinen-11,12-diol | Trichoderma asperellum A-YMD-9–2 | [47] | |
| 32 | 4-cadinen-11,13-diol | Trichoderma asperellum A-YMD-9–2 | [47] | |
| 33 | trichocadinin B | Trichoderma virens QA-8 | [48] | |
| 34 | trichocadinin C | Trichoderma virens QA-8 | [48] | |
| 35 | trichocadinin D | Trichoderma virens QA-8 | [48] | |
| 36 | trichocadinin E | Trichoderma virens QA-8 | [48] | |
| 37 | trichocadinin F | Trichoderma virens QA-8 | [48] | |
| 38 | trichocadinin G | Trichoderma virens QA-8 | [48] | |
| 39 | trichodermapene A | Trichoderma reesei PSU-SPSF013 | [49] | |
| 40 | trichodermapene B | Trichoderma reesei PSU-SPSF013 | [49] | |
| 41 | trichodermapene C | Trichoderma reesei PSU-SPSF013 | [49] | |
| 42 | 11-hydroxy-15-drimeneoic acid | Trichoderma koningiopsis A729 | [50] | |
| 43 | trichodermaloid A | Trichoderma sp. SM16 | [51] | |
| 44 | trichodermaloid B | Trichoderma sp. SM16 | [51] | |
| 45 | trichodermaloid C | Trichoderma sp. SM16 | [51] | |
| 46 | aspergilloid G | Trichoderma sp. SM16 | [51] | |
| 47 | rhinomilisin E | Trichoderma sp. SM16 | [51] | |
| 48 | rhinomilisin G | Trichoderma sp. SM16 | [51] | |
| 49 | trichocadinin H | Trichoderma virens RR-dl-6–8 | [52] | |
| 50 | trichocadinin I | Trichoderma virens RR-dl-6–8 | [52] | |
| 51 | trichocadinin J | Trichoderma virens RR-dl-6–8 | [52] | |
| 52 | trichocadinin K | Trichoderma virens RR-dl-6–8 | [52] | |
| 53 | trichocadinin L | Trichoderma virens RR-dl-6–8 | [52] | |
| 54 | trichocadinin M | Trichoderma virens RR-dl-6–8 | [52] | |
| 55 | cadin-4-en-11-ol | Trichoderma asperelloides | [45] | |
| Carotane-type sesquiterpenes | 56 | 8-daucene-3,4-diol(CAF-603) | Trichoderma virens | [53] |
| 57 | 7-β-hydroxy CAF-603 | Trichoderma virens | [46] | |
| 58 | 14-hydroxy CAF-603 oleate | Trichoderma virens | [54] | |
| 59 | 8α,9α-epoxy-3β,4β-dihydroxycarotane | Trichoderma virens | [35] | |
| 60 | 3β,4β,14-trihydroxycarota-8-ene | Trichoderma virens | [35] | |
| 61 | 3β,4β,14-trihydroxycarotane | Trichoderma virens | [35] | |
| 62 | 3β,4β,11,14-tetrahydroxycarota-8-ene | Trichoderma virens | [35] | |
| 63 | trichocarotin A | Trichoderma virens Y13-3 | [46] | |
| 64 | trichocarotin B | Trichoderma virens Y13-3 | [46] | |
| 65 | trichocarotin C | Trichoderma virens Y13-3 | [46] | |
| 66 | trichocarotin D | Trichoderma virens Y13-3 | [46] | |
| 67 | trichocarotin E | Trichoderma virens Y13-3 | [46] | |
| 68 | trichocarotin F | Trichoderma virens Y13-3 | [46] | |
| 69 | trichocarotin G | Trichoderma virens Y13-3 | [46] | |
| 70 | trichocarotin H | Trichoderma virens Y13-3 | [46] | |
| 71 | 14-O-methyltrichocarotin G | Trichoderma virens RR-dl-6–8 | [52] | |
| 72 | 14-O-methyl CAF-603 | Trichoderma virens RR-dl-6–8 | [52] | |
| 73 | trichocarotin I | Trichoderma virens QA-8 | [55] | |
| 74 | trichocarotin J | Trichoderma virens QA-8 | [55] | |
| 75 | trichocarotin K | Trichoderma virens QA-8 | [55] | |
| 76 | trichocarotin L | Trichoderma virens QA-8 | [55] | |
| 77 | trichocarotin M | Trichoderma virens QA-8 | [55] | |
| Acorane-type sesquiterpenes | 78 | trichoacorenol | Trichoderina koningii | [56] |
| 79 | 15-hydroxyacorenone | Trichoderma harzianum | [57] | |
| 80 | 2β-hydroxy trichoacorenol | Trichoderma sp. PR-35 | [58] | |
| 81 | 1α-isopropyl-4α,8-dimethylspiro[4.5]dec-8-ene-2β,7α-diol | Trichoderma sp. YMF1.02647 | [59] | |
| 82 | 1α-isopropyl-4α,8-dimethylspiro[4.5]dec-8-ene-3β,7α-diol | Trichoderma sp. YMF1.02647 | [59] | |
| 83 | 2β-hydroxy-1α-isopropyl-4α,8-dimethylspiro[4.5]dec-8-en-7-one | Trichoderma sp. YMF1.02647 | [59] | |
| 84 | 15-hydroxy-trichoacorenol | Trichoderma sp. Xy24 | [37] | |
| 85 | 8β-(hydroxymethyl)-1β-isopropyl-4β-methylspiro[4.5]dec-9-en-7α,8α-diol | Trichoderma sp. Xy24 | [37] | |
| 86 | 8-acoren-3,11-diol | Trichoderma harzianum X-5 | [40] | |
| 87 | trichoacorin A | Trichoderma brevicompactum A-DL-9–2 | [60] | |
| 88 | trichoacorside A | Trichoderma longibrachiatum EN-586 | [61] | |
| Trichothecene-type sesquiterpenes | 89 | trichothecin | Trichothecium roseum | [62] |
| 90 | trichodermin | Trichoderma viride | [63] | |
| 91 | trichodermol | Trichoderma polysporum,Trichoderma sporulosum | [64] | |
| 92 | trichoderminol | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 93 | 2,4,12-trihydroxyapotrichothecene | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 94 | harzianum A | Trichoderma harzianum | [67] | |
| 95 | harzianum B | Trichoderma harzianum | [67] | |
| 96 | trichobreol A | Trichoderma cf.brevicompactum | [68] | |
| 97 | trichobreol B | Trichoderma cf.brevicompactum | [68] | |
| 98 | trichobreol C | Trichoderma cf.brevicompactum | [68] | |
| 99 | trichobreol D | Trichoderma sp. TPU199 (cf. Trichoderma brevicompactum) | [68] | |
| 100 | trichobreol E | Trichoderma sp. TPU199 (cf. Trichoderma brevicompactum) | [68] | |
| 101 | trichodermol chlorohydrin | Trichoderma hamatum Z36 − 7 | [44] | |
| 102 | 8-deoxy-trichothecin | Trichoderma brevicompactum A-DL-9–2 | [66] | |
| 103 | trichothecinol A | Trichoderma brevicompactum A-DL-9–2 | [66] | |
| 104 | trichothecinol B | Trichoderma brevicompactum A-DL-9–2 | [66] | |
| 105 | trichodermarin A | Trichoderma brevicompactum A-DL-9–2 | [66] | |
| 106 | trichodermarin B | Trichoderma brevicompactum A-DL-9–2 | [66] | |
| 107 | trichodermarin G | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 108 | trichodermarin H | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 109 | trichodermarin I | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 110 | trichodermarin J | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 111 | trichodermarin K | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 112 | trichodermarin L | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 113 | trichodermarin M | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 114 | trichodermarin N | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| Bisabolane-type sesquiterpenes | 115 | trichoderic acid | Trichoderma sp. PR-35 | [58] |
| 116 | bisabolan-1,10,11-triol | Trichoderma asperellum cf44-2 | [69] | |
| 117 | 12-nor-11-acetoxybisabolen-3,6,7-triol | Trichoderma asperellum cf44-2 | [69] | |
| 118 | trichaspin | Trichoderma asperellum cf44-2 | [39] | |
| 119 | trichaspside A | Trichoderma asperellum cf44-2 | [39] | |
| 120 | trichaspside B | Trichoderma asperellum cf44-2 | [39] | |
| 121 | trichobisabolin Q | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 122 | trichobisabolin R | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 123 | trichobisabolin S | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 124 | trichobisabolin T | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 125 | trichobisabolin U | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 126 | trichobisabolin V | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 127 | trichobisabolin W | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 128 | trichobisabolin X | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 129 | trichobisabolin Y | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 130 | trichobisabolin Z | Trichoderma asperelloides RR-dl-6–11 | [45] | |
| 131 | trichobisabolin O1 | Trichoderma brevicompactum A-DL-9–2 | [60] | |
| 132 | trichobisabolin O2 | Trichoderma brevicompactum A-DL-9–2 | [60] | |
| 133 | trichobisabolin P | Trichoderma brevicompactum A-DL-9–2 | [60] | |
| 134 | trichobisabolin M | Trichoderma atroviride RR-dl-3–9 | [70] | |
| 135 | trichobisabolin N | Trichoderma atroviride RR-dl-3–9 | [70] | |
| 136 | trichodone A | Trichoderma asperellum | [71] | |
| 137 | trichodone B | Trichoderma asperellum | [71] | |
| 138 | trichodone C | Trichoderma asperellum | [71] | |
| Botryane-type sesquiterpenes | 139 | 4,10,15-trihydroxybotry-1(9)-en | Trichoderma oligosporum | [72] |
| 140 | 15-O-acetylbotry-1(10),5(9)-dien-4-ol | Trichoderma oligosporum | [72] | |
| 141 | 15-O-acetyldehydrobotrydienol | Trichoderma oligosporum | [72] | |
| 142 | 10,15-di-O-acetyldehydrobotrydienol | Trichoderma oligosporum | [72] | |
| 143 | 10-demethyldehydrobotrydien-1,15-diol | Trichoderma oligosporum | [72] | |
| 144 | norpupukeanane A | Trichoderma longibrachiatum | [66] | |
| Drimane sesquiterpenes | 145 | neomacrophorin I | Trichoderma sp. 1212–03 | [73] |
| 146 | neomacrophorin II | Trichoderma sp. 1212–03 | [73] | |
| 147 | neomacrophorin III | Trichoderma sp. 1212–03 | [73] | |
| 148 | 3-deoxyneomacrophorin IV | Trichoderma sp. 1212–03 | [74] | |
| 149 | 3-oxoneomacrophorin I | Trichoderma sp. 1212–03 | [74] | |
| 150 | 3-oxoneomacrophorin II | Trichoderma sp. 1212–03 | [74] | |
| 151 | neomacrophorin VII | Trichoderma sp. 1212–03 | [74] | |
| 152 | 5′-epimacrophorin B | Trichoderma sp. 1212–03 | [74] | |
| 153 | 5′-deoxyneomacrophorin IV | Trichoderma sp. 1212–03 | [74] | |
| 154 | premacrophorin III | Trichoderma sp. 1212–03 | [74] | |
| 155 | premacrophorindiol | Trichoderma sp. 1212–03 | [74] | |
| 156 | premacrophorintriol I | Trichoderma sp. 1212–03 | [74] | |
| 157 | premacrophorintriol II | Trichoderma sp. 1212–03 | [74] | |
| Other-type sesquiterpenes | 158 | gliocladic acid | Trichoderma viride | [75] |
| 159 | trichodermanene | Trichoderma reesei PSU-SPSF013 | [49] | |
| 160 | koningic acid | Trichoderina koningii | [76] | |
| 161 | lignoren | Trichoderma lignorum | [77] | |
| 162 | trichodermoside | Trichoderma sp. PT2 | [78] | |
| 163 | trichoderiol A | Trichoderma atroviride | [79] | |
| 164 | trichoderiol B | Trichoderma atroviride | [79] | |
| 165 | microsphaeropsisin B | Trichoderma sp. (Strain 307) | [80] | |
| 166 | microsphaeropsisin C | Trichoderma sp. (Strain 307) | [80] | |
| 167 | harzianoic acid A | Trichoderma harzianum | [81] | |
| 168 | harzianoic acid B | Trichoderma harzianum | [81] | |
| 169 | atrichodermone C | Trichoderma atroviride | [82] | |
| 170 | trichodermadione B | Trichoderma atroviride S361 | [83] | |
| 171 | 3-acetylgliocladic acid | Trichoderma virens CMB-TN16 | [84] | |
| 172 | 14-acetylgliocladic acid | Trichoderma virens CMB-TN16 | [84] | |
| 173 | diacetylgliocladic acid | Trichoderma virens | [85] | |
| 174 | koninginol D | Trichoderma koningiopsis A729 | [50] | |
| 175 | tricinoloniol acid A | Trichoderma hypoxylon | [86] | |
| 176 | tricinoloniol acid B | Trichoderma hypoxylon | [86] | |
| 177 | tricinoloniol acid C | Trichoderma hypoxylon | [86] | |
| 178 | trichocuparin A | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 179 | trichocuparin B | Trichoderma brevicompactum A-DL-9–2 | [65] | |
| 180 | trichonerolin A | Trichoderma species | [60] | |
| 181 | trichonerolin B | Trichoderma species | [60] | |
| 182 | ethyl hydroheptelidate | Trichoderma species | [87] | |
| 183 | rhinomilisin B | Trichoderma virens | [85] | |
| 184 | xylaric acid B | Trichoderma virens | [85] | |
| 185 | hydroheptelidic acid | Trichoderma virens | [85] | |
| 186 | chlorine heptelidic acid | Trichoderma virens | [85] | |
| 187 | rhinomilisin A | Trichoderma virens | [85] | |
| 188 | divirensol A | Trichoderma virens CMB-TN16 | [84] | |
| 189 | divirensol B | Trichoderma virens CMB-TN16 | [84] | |
| 190 | divirensol C | Trichoderma virens CMB-TN16 | [84] | |
| 191 | divirensol D | Trichoderma virens CMB-TN16 | [84] | |
| 192 | divirensol E | Trichoderma virens CMB-TN16 | [84] | |
| 193 | divirensol F | Trichoderma virens CMB-TN16 | [84] | |
| 194 | divirensol G | Trichoderma virens CMB-TN16 | [84] | |
| 195 | divirensol H | Trichoderma virens CMB-TN16 | [85] | |
| 196 | trivirensol A | Trichoderma virens FY06, Trichoderma virens CMB-TN16 | [88] | |
| 197 | trivirensol B | Trichoderma virens FY06, Trichoderma virens CMB-TN16 | [88] | |
| 198 | trivirensol C | Trichoderma virens CMB-TN16 | [88] | |
| 199 | trivirensol D | Trichoderma virens CMB-TN16 | [88] | |
| 200 | trivirensol E | Trichoderma virens CMB-TN16 | [88] | |
| 201 | trivirensol F | Trichoderma virens CMB-TN16 | [88] | |
| 202 | trivirensol G | Trichoderma virens CMB-TN16 | [88] | |
| Harziane-type diterpenes | 203 | harziandione | Trichoderma harzianum | [89], [91] |
| 204 | isoharziandione | Trichoderma viride. | [90], [91] | |
| 205 | harzianone | Trichoderma longibrachiatum. | [91] | |
| 206 | trichodermaerin | Trichoderma erinaceum; Trichoderma asperellum | [92], [93] | |
| 207 | (9R,10R)-dihydro-harzianone | Trichoderma sp. Xy24 | [94] | |
| 208 | harzianelactone | Trichoderma sp. Xy24 | [94] | |
| 209 | 11-hydroxy-9-harzien-3-one | Trichoderma asperellum cf44-2 | [39] | |
| 210 | 3R-hydroxy-9R,10R-dihydroharzianone | Trichoderma harzianum X-5 | [40] | |
| 211 | 3S-hydroxyharzianone | Trichoderma asperellum A-YMD-9–2 | [47] | |
| 212 | harzianelactone A | Trichoderma harzianum XS-20090075 | [95] | |
| 213 | harzianelactone B | Trichoderma harzianum XS-20090075 | [95] | |
| 214 | harzianone A | Trichoderma harzianum XS-20090075 | [95] | |
| 215 | harzianone B | Trichoderma harzianum XS-20090075 | [95] | |
| 216 | harzianone C | Trichoderma harzianum XS-20090075 | [95] | |
| 217 | harzianone D | Trichoderma harzianum XS-20090075 | [95] | |
| 218 | harziane | Trichoderma harzianum XS-20090075 | [95] | |
| 219 | koninginol C | Trichoderma koningiopsis A729 | [50] | |
| 220 | harzianone E | Trichoderma harzianum (XS-20090075) | [43] | |
| 221 | deoxytrichodermaerin | Trichoderma longibrachiatum A-WH-20–2 | [96] | |
| 222 | harzianol A | Trichoderma atroviride B7 | [97] | |
| 223 | harzianol F | Trichoderma atroviride B7 | [98] | |
| 224 | harzianol G | Trichoderma atroviride B7 | [98] | |
| 225 | harzianol H | Trichoderma atroviride B7 | [98] | |
| 226 | harzianol I | Trichoderma atroviride B7 | [98] | |
| 227 | harzianol J | Trichoderma atroviride B7 | [98] | |
| 228 | 3S-hydroxy-9R,10R-dihydroharzianone | Trichoderma sp.SCSIOW21 | [100] | |
| 229 | 3S-hydroxytrichodermaerin | Trichoderma sp.SCSIOW21 | [100] | |
| 230 | methyl 3S-hydroxy-10,11-seco-harzianate | Trichoderma sp.SCSIOW21 | [100] | |
| 231 | harzianol K | Trichoderma sp.SCSIOW21 | [99] | |
| 232 | harzianol L | Trichoderma sp.SCSIOW21 | [99] | |
| 233 | harzianol M | Trichoderma sp.SCSIOW21 | [99] | |
| 234 | harzianol N | Trichoderma sp.SCSIOW21 | [99] | |
| 235 | harzianol O | Trichoderma sp.SCSIOW21 | [99] | |
| Diterpenes with a tetracyclic 6–5-6–6 ring system | 236 | trichodermanin A | Trichoderma atroviride (strain no. S361) | [101] |
| 237 | wickerol A | Trichoderma atroviride FKI-3849 | [102] | |
| 238 | wickerol B | Trichoderma atroviride FKI-3849 | [102] | |
| 239 | trichodermanin C | Trichoderma harzianum OUPS-111D-4 | [103] | |
| 240 | trichodermanin D | Trichoderma harzianum OUPS-111D-4 | [103] | |
| 241 | trichodermanin E | Trichoderma harzianum OUPS-111D-4 | [103] | |
| 242 | trichodermanin F | Trichoderma harzianum OUPS-111D-4 | [104] | |
| 243 | trichodermanin G | Trichoderma harzianum OUPS-111D-4 | [104] | |
| 244 | trichodermanin H | Trichoderma harzianum OUPS-111D-4 | [104] | |
| Other-type diterpenes | 245 | trichocitrin | Trichoderma citrinoviride cf-27 | [105] |
| 246 | 11R-methoxy-5,9,13-proharzitrien-3-ol | Trichoderma harzianum X-5 | [40] | |
| 247 | koninginol A | Trichoderma koningiopsis A729 | [50] | |
| 248 | koninginol B | Trichoderma koningiopsis A729 | [50] | |
| 249 | citrinovirin | Trichoderma citrinoviride cf-27 | [106] | |
| 250 | harzianolic acid A | Trichoderma harzianum (XS-20090075) | [43] | |
| Monoterpenes | 251 | (7S)- and (7R)-1-hydroxy-3-p-menthen-9-oic acids | Trichoderma asperellum cf44-2 | [69] |
| 252 | [69] | |||
| Meroterpenes | 253 | neomacrophorin X | Trichoderma sp. 1212–03 | [107] |
Sesquiterpenes
Sesquiterpenes account for the largest proportion of Trichoderma terpenes. According to the type of skeleton, 202 sesquiterpenes can be divided into nine categories: 29 cyclonerane-type, 26 cadinane-type, 22 carotane-type, 11 acorane-type, 26 trichothecene-type, 24 bisabolane-type, 6 botryane-type, 13 drimane-type and 45 other type sesquiterpenes.
Cyclonerane-type sesquiterpenes
Cyclonerane-type sesquiterpenes comprise a very large group of Trichoderma sesquiterpenes. These structures of cyclonerane-type sesquiterpenes are characterized by a tri-substituted cyclopentane moiety. The first report of cyclonerane-type sesquiterpenes from Trichoderma fungi can be traced back to 1980 s, when cyclonerodiol (1), cyclonerodiol oxide (2) and epicyclonerodiol oxide (3) were isolated from T. polysporum [34]. Later, cyclonerotriol (4) was isolated from a strain of T. virens in 1995 [35]. 10,11-Dihydrocyclonerotriol (5) was obtained from T. longibrachiatum YM311505, an endophytic fungus of Azadirachta indica [36], while cyclonerodiol B (6), from the endophytic fungus Trichoderma sp. Xy24 in mangrove plant, was a monocyclic sesquiterpene and established to be 10,11-dihydroxycyclonerodiol in 2014 [37]. From then on, another 23 analogues (7–29) were reported in succession from the cultures of Trichoderma species residing in different origins, including marine sediment, marine alga and soft coral [38], [39], [40], [41], [42], [43], [44], [45]. It is worth mentioning that compounds 22 and 23 represented the first occurrence of ring-isomerized cycloneranes [42]. The chemical structures of all these compounds mentioned above (1–29, Table 1) were presented in Figure S1 and some representative structures with reported bioactivities were displayed in Fig. 1.
Fig. 1.
Representative cyclonerane-type, cadinane-type and carotane-type sesquiterpenes produced by Trichoderma species.
Cadinane-type sesquiterpenes
The basic skeleton of cadinane-type sesquiterpenes is decahydronaphthalene with each a methyl at C-5 and C-9, and one isopropyl at C-2. In recent years, an increasing number of cadinene-type sesquiterpenes (30–55, Table 1) have been isolated from Trichoderma fungi. The first cadinene sesquiterpene, trichocadinin A (30), was isolated from the culture of T. virens Y13-3 obtained from the surface of a marine red alga in 2018 [46]. Subsequently, 4-cadinen-11,12-diol (31) and 4-cadinen-11,13-diol (32) were obtained as the second and third new members from the culture of T. asperellum A-YMD-9–2 also from a marine red alga in 2019 [47]. More recently, a serial of chemical investigations has been conducted on different Trichoderma strains, including T. virens QA-8 [48], T. reesei [49], T. koningiopsis A729 [50], Trichoderma sp. SM16 [51], T. virens RR-dl-6–8 [52] and T. asperelloides [45], yielding trichocadinins B − G (33–38) [48], trichodermapenes A–C (39–41) [49], 11-hydroxy-15-drimeneoic acid (42) [50], trichodermaloids A-C (43–45), aspergilloid G (46), rhinomilisin E (47), rhinomilisin G (48) [51], trichocadinins H − M (49–54) [52] and cadin-4-en-11-ol (55) [45], respectively. Among these compounds, trichocadinin H (49) was a halogenated 2,3-seco-cadinane sesquiterpene, while the epimeric 50 and 51 featured a 2-nor-cadinane skeleton [52]. All these structures were described in Figure S2 and some representative structures with reported bioactivities were displayed in Fig. 1.
Carotane-type sesquiterpenes
Carotane-type sesquiterpenes possess a bicycle-decane system with three substitutes, including two methyl groups at C-4, C-7 and one isopropyl group at C-10. So far, a total of 22 carotane sesquiterpenes have been reported from Trichoderma metabolites. In 1990, Gliocladium virens (also known as T. virens) IFO 9166 was found to produce a new carotene sesquiterpene, CAF-603 (56), which was elucidated as 8-daucene-3,4-diol [53]. 7β-hydroxy CAF-603 (57) [46] and14-hydroxy CAF-603 oleate (58) [54] were then discovered also from T. virens as two new analogues of compound 56. Subsequently, a serial of new carotane sesquiterpenes, including trichocaranes A-D (59–62) [35], trichocarotins A − H (63–70) [46], 14-O-methyltrichocarotin G (71), 14-O-methyl CAF-603 (72) [52] and trichocarotins I–M (73–77) [55], were isolated and identified from T. virens. Chemical structures of compounds 56–77 were presented in Figure S3 and some representative structures with reported bioactivities were displayed in Fig. 1. Interestingly, all these compounds were reported from different strains of T. virens, which indicated that carotane sesquiterpenes may be used as chemotaxonomic markers for T. virens.
Acorane-type sesquiterpenes
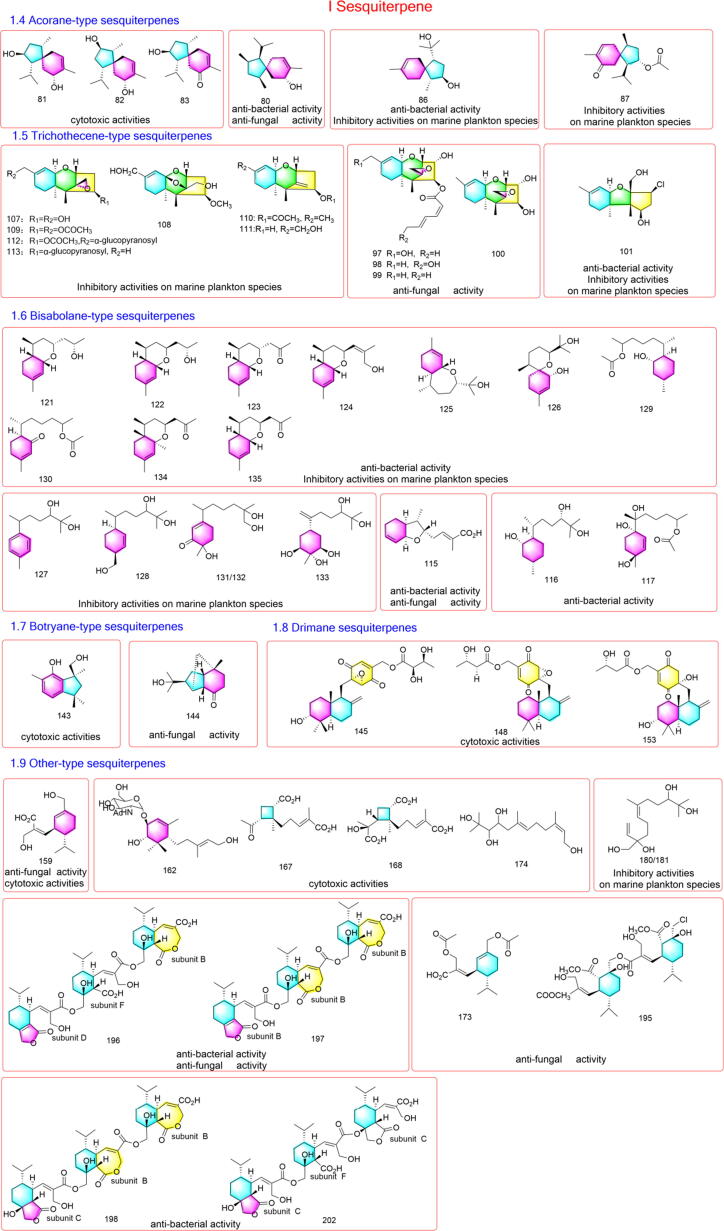
Spiro[4.5]decane system features the basic skeleton of acorane-type sesquiterpenes, with each a methyl at C-1 and C-8, and an isopropyl at C-4. There have been 11 acorane sesquiterpenes reported from the fermentation broth of Trichoderma species up to now. The first acorane-type sesquiterpene, named trichoacorenol (78), was obtained from the culture broth of T. koningiioudemans in 1995, with the chemical structure established as (1S,4S,5S,7R)-1-isopropyl-4,8-dimethyl-spiro[4.5]dec-8-en-7-ol [56]. Two years later, the second new member of this class, 15-hydroxyacorenone (79), was isolated from the T. harzianum. and identified as {(1S,4S,SS)-8-hydroxyrnethyl-1-isopropyl-4-methylspiro-[4.5]dec-8-en-7-one} [57]. Since 2011, the other 9 acorane sesquiterpenes, including 2β-hydroxytrichoacorenol (80) [58], 1α-isopropyl-4α,8-dimethylspiro[4.5]dec-8-ene-2β,7α-diol (81), 1α-isopropyl-4α,8-dimethylspiro[4.5]dec-8-ene-3β,7α-diol (82), 2β-hydroxy-1α-isopropyl-4α,8-dimethylspiro[4.5]dec-8-en-7-one (83) [59], trichoacorenols B and C (84, 85) [37], 8-acoren-3,11-diol (86), trichoacorin A (87) [60] and trichoacorside A (88) [61], have been isolated from different Trichoderma strains. The chemical structures of these compounds mentioned above were described in Figure S4 and some representative structures with reported bioactivities were displayed in Fig. 2.
Fig. 2.
Representative acorane-type, trichothecene-type, bisabolane-type, botryane-type, drimane-type and other-type sesquiterpenes produced by Trichoderma species.
Trichothecene-type sesquiterpenes
Trichothecene-type sesquiterpenes are characterized by the presence of a 6–6-5 ring system. The earliest member discovered in this genus can be dated back to 1940 s that trichothecin (89) was isolated from T. roseum [62]. More than twenty years later, trichodermin (90) was obtained from T. viride in 1965 [63], while trichodermol (91) was isolated from T. polysporum and T. sporulosum in 1972 [64]. In the following decades, trichoderminol (92), 2,4,12-trihydroxyapotrichothecene (93), 8-deoxy-trichothecin (1 0 2), trichothecinols A and B (103, 104), trichodermarins A and B (105, 106) [65], [66], trichodermol chlorohydrin (1 0 1) [44] and harzianums A and B (94, 95) [67], were isolated from soil-borne or plant derived Trichoderma fungi. More recently, another five trichothecene sesquiterpenes, trichobreols A-E (96–100), were isolated from the culture broth of marine-derived Trichoderma sp. TPU199 in 2020 [68], while trichodermarins G-N (107–114) were reported from T. brevicompactum A-DL-9–2 from a marine red alga in 2021 [65]. So far, a total of 26 trichothecene analogues (89–114) have been reported from Trichoderma species and their chemical structures were presented in Figure S5. Some representative structures with bioactivities reported were displayed in Fig. 2.
Bisabolane-type sesquiterpenes
The basic skeleton of bisabolane-type sesquiterpenes composes of a six-membered ring with diverse substitution and cyclization. Twenty-four bisabolene sesquiterpenes (115–138) have been discovered from Trichoderma so far. Trichoderic acid (1 1 5) represented the first example of bisabolane-type sesquiterpenes isolated from Trichoderma sp. PR-35 in 2011 [58]. Later, bisabolan-1,10,11-triol (1 1 6) and a new norbisabolane derivative, 12-nor-11-acetoxybisabolen-3,6,7-triol (1 1 7) [69], together with trichaspin (1 1 8), trichaspsides A and B (119, 120) [39], were isolated from a marine-alga-endophytic fungus T. asperellum cf44-2. Among those, trichaspin was characterized by an ethylated bisabolane skeleton, while trichaspsides A and B represented the first aminoglycosides of bisabolane and norbisabolane sesquiterpenes, respectively. Subsequently, trichobisabolins Q–Z (121–130) [45], trichobisabolins O1, O2 and P (131–133) [60], trichobisabolins M and N (134, 135) [70] and trichodones A–C (136–138) [71] were also discovered from T. asperelloides RR-dl-6–11, T. brevicompactum A-DL-9–2, T. atroviride RR-dl-3–9 and T. asperellum, respectively. The chemical structures of these compounds were all displayed in Figure S6 and selected bioactive compounds were presented in Fig. 2.
Botryane-type sesquiterpenes
Bicyclo[4.3.0]nonane serves as the basic skeleton of botryane-type sesquiterpenes. Up to present, only six members of this category have been reported from Trichoderma fungi. In 2018, the first five new members identified as 4,10,15-trihydroxybotry-1(9)-en (1 3 9), 15-O-acetylbotry-1(10),5(9)-dien-4-ol (1 4 0), 15-O-acetyldehydrobotrydienol (1 4 1), 10,15-di-O-acetyldehydrobotrydienol (1 4 2), 10-demethyldehydrobotrydien-1,15-diol (1 4 3), were isolated from T. oligosporum [72], while the sixth one, norpupukeanane A (1 4 4), was obtained from T. longibrachiatum in 2020, characterized by a novel tricyclic-6/5/5- [4.3.1.01,6]-decane skeleton [66]. The chemical structures of six compounds (139–144) were described in Figure S7 and bioactive analogues were selected to be displayed in Fig. 2.
Drimane sesquiterpenes
Drimane sesquiterpenes in Trichoderma species were firstly discovered in 2014 that neomacrophorins I-III (145–147), characterized as three novel drimenyl cyclohexanes, was reported from Trichoderma sp. 1212–03 [73]. The authors also described these structures as daucanes [73]. Later in 2019, a continuous study conducted by the same group and on the same strain led to the discovery of another 10 analogues, including 3-deoxyneomacrophorin IV (1 4 8), 3-oxoneomacrophorins I and II (149, 150), neomacrophorin VII (1 5 1), 5′-epimacrophorin B (1 5 2), and 5′-deoxyneomacrophorin IV (1 5 3), premacrophorin III (1 5 4), premacrophorindiol (1 5 5), and premacrophorintriols I and II (156, 157) [74]. These thirteen structures (145–157) were described in Figure S8 and bioactive ones were shown in Fig. 2.
Other-type sesquiterpenes
Besides above-mentioned diverse sesquiterpene categories, there are still a large portion of varied structures (158–202) that cannot be classified properly and thus go to the other-type. Interestingly, gliocladic acid (1 5 8) and its varied monomer analogues (159, 160, 171–173, 182–186) as well as their novel dimeric (187–195) and trimeric (196–202) derivatives occupy the majority of this type (Figure S9), which were proposed to be biosynthetically originated from the “cadinene scaffold” and branched from a key epoxy-lactone-acid intermediate, koningic acid (1 6 0). Gliocladic acid (1 5 8) was identified from T. viride in 1982 [75], while the key oxidative intermediate, koningic acid (1 6 0), was firstly reported from a strain of T. koningii in 1985 [76]. Compound 160 subsequently undergoes hydrolysis, ring cleavage, re-lactonization and decarboxylation to possibly yield diverse monomeric units, including acetylated analogues 3-acetylgliocladic acid (1 7 1), 14-acetylgliocladic acid (1 7 2) [84] and diacetylgliocladic acid (1 7 3) [85], re-lactonized products ethyl hydroheptelidate (1 8 2) [87] and hydroheptelidic acid (1 8 5) [85], and chlorinated analogues xylaric acid B (1 8 4) and chlorine heptelidic acid (1 8 6) [85]. Those monomeric units further experience multimerization to give rarely reported dimeric congeners, rhinomilisin A (1 8 7) [85] and divirensols A − H (188–195) [84], [85], and trimeric congeners trivirensols A − G (196–202) [85], [88]. Trivirensols A and B (196 and 197) were coincidentally isolated from T. virens FY06 residing in Litchi chinensis [85] and a termite nest-derived fungus T. virens CMB-TN16 [88]. However, an alternative opinion indicated this class of compounds may be classified to monoterpene derivatives. Trichodermanene (1 5 9), established as a Z-isomer of gliocladic acid (1 5 8), was reported from the soil-derived fungus T. reesei PSU-SPSF013, was regarded as a new limonene derivative [49]. Though much more evidence favored the sesquiterpene side, further in-depth biosynthetic studies should be conducted to make it clear.
In addition, lignoren (1 6 1) from T. lignorum HKI 0257 [77] and trichoderiols A and B (163, 164) from T. atroviride S361 [79] bearing a 2-oxabicyclo[2], [2], [1]heptane ring system, may also be considered as cyclonerane-type sesquiterpenes undergoing 3,7-epoxidation. Trichodermoside (1 6 2) [78], atrichodermone C (1 6 9) [82] and trichodermadione B (1 7 0) [83] were cyclohexenone derivatives. Microsphaeropsisins B and C (165, 166) were furan-type isoeremophilane sesquiterpenes [80], while harzianoic acids A (1 6 7) and B (1 6 8) were structurally characterized by a cyclobutane nucleus [81]. Trichocuparins A and B (178, 179) were two cuparene derivatives [65], whereas koninginol D (1 7 4) [50], tricinoloniol acids (TRAs) A–C (175–177) [86] and trichonerolins A and B (180, 181) [60] were linear sesquiterpenes discovered in Trichoderma species. Chemical structures of some bioactive molecules were selected to be presented in Fig. 2.
Diterpenes
Diterpene is the characteristic type secondary metabolites of Trichoderma species and consisted of four isoprene units typically with 20 carbon atoms. Up to date, a total of 48 Trichoderma diterpenes have been isolated and identified, which can be divided into three classes, including 33 harziane-type, 9 with a tetracyclic 6–5-6–6 ring system and 6 other-type diterpenes.
Harziane-type diterpenes
Harziane diterpenes are novel secondary metabolites exclusively discovered in Trichoderma fungi, typically containing a 4/7/5/6-fused tetracyclic scaffold. Harziandione (2 0 3) represents the first diterpene isolated from Trichoderma species in 1992 [89]. Later, isoharziandione (2 0 4) was obtained as 3,7,11,15,15-pentamethyl tetracyclo [3–2–1–04–12–5–2–08–11]tetradec-7-en-1–10 dione in 1997 [90], but was subsequently revised to harziandione (2 0 3) on the basis of 13C NMR data comparison and calculation in 2012 by Miao et al. and they also got another novel congener harzianone (2 0 5), from T. longibrachiatum [91]. Afterwards, trichodermaerin (2 0 6), a novel diterpenoid lactone with a tetracyclic 5/7/5/6 ring system, was isolated both from T. erinaceum in 2013 [92] and from T. asperellum in 2014 [93], which represents a sub-type with the typical 4-membered cyclobutanone ring varied to a 5-membered lactone ring, also including harzianelactone (2 0 8) [94], harzianelactones A and B (212, 213) [95] and 3S-hydroxytrichodermaerin (2 2 9) [100]. And methyl 3S-hydroxy-10,11-seco-harzianate (2 3 0) was the first example with the cyclobutanone ring cleaved [100], while the other analogues differ mainly in the configuration and substitution on the basic skeleton, including (9R,10R)-dihydro-harzianone (2 0 7) [94], 11-hydroxy-9-harzien-3-one (2 0 9) [39], 3R-hydroxy-9R,10R-dihydroharzianone (2 1 0) [40], 3S-hydroxyharzianone (2 1 1) [47], harzianones A–D (214–217), harziane (2 1 8) [95], koninginol C (2 1 9) [50], harzianone E (2 2 0) [43], deoxytrichodermaerin (2 2 1) [96], harzianol A (2 2 2) [97], [99], harzianols F-O (223–227, 231–235) [98], [99], and 3S-hydroxy-9R,10R-dihydroharzianone (2 2 8) [100]. All these chemical structures were described in Figure S10 and selected bioactive ones were shown in Fig. 3.
Fig. 3.
Representative diterpenes, monoterpenes and meroterpenoid produced by Trichoderma species.
Diterpenes with a tetracyclic 6–5-6–6 ring system
So far, only nine diterpenes possessing a fused 6–5-6–6 ring have been identified from Trichoderma species (Figure S11). In 2011, our group firstly identified a structurally unique diterpenoid with skeletal carbons arranged compactly in a novel fused 6–5-6–6 ring system, named trichodermanin A (2 3 6), from T. atroviride S361 [101]. Later in 2012, wickerols A and B (237, 238) were also found to have a fused 6–5-6–6 ring skeleton from T. atroviride FKI-3849 [102]. Detailed structural and NMR data comparison revealed that the structure of wickerol B (2 3 8) was totally the same as that of trichodermanin A (2 3 6). Subsequently, six analogues of this type, trichodermanins C–E (239–241) and trichodermanins F-H (242–244), have been reported from T. harzianum OUPS-111D-4 [103], [104]. The chemical structures of some bioactive trichodermanins were displayed in Fig. 3.
Other-type diterpenes
In addition to the above-mentioned types of diterpenes, there were still other types reported from Trichoderma species. Trichocitrin (2 4 5) represented the first Trichoderma-derived and furan-bearing fusicoccane diterpene [105], while 11R-methoxy-5,9,13-proharzitrien-3-ol (2 4 6) was a proharziane diterpene discovered from T. harzianum X-5 [40]. Koninginols A and B (247, 248) were two intriguing diterpene alkaloids from T. koningiopsis A729 [50], while citrinovirin (2 4 9) was a little bit similar but rare norditerpene produced by T. citrinoviride cf-27 [106]. In addition, harzianolic acid A (2 5 0) was a chlorinated cleistanthane-type diterpene obtained from T. harzianum (XS-20090075) [43]. Chemical structures of these six compounds (245–250) were described in Figure S12 and selected bioactive ones were shown in Fig. 3.
Monoterpenes and meroterpenoid
In the process of secondary metabolites exploration from Trichoderma species, there have been merely-two monoterpenes and one meroterpenoid discovered until now. (7S)- and (7R)-1-hydroxy-3-p-menthen-9-oic acids (251, 252) were two new naturally occurring monoterpenes found in a marine brown-alga-endophytic strain T. asperellum cf44-2 [69]. Additionally, chemical investigation on Trichoderma sp. 1212–03 led to the isolation of the only meroterpenoid, neomacrophorin X (2 5 3), bearing a unique [4.4.3]propellane moiety [107] (Fig. 3).
Pharmacological activities
To the best of our knowledge, there have been more than 1000 compounds produced and reported from Trichoderma species, among which terpenes account for a large portion. As presented in this review, a total of 252 terpenes have been recorded since 1948 and their pharmacological activities have been extensively investigated. Generally, pharmacological investigations on Trichoderma terpenes mainly focused on their antibacterial activities, antifungal activities, inhibitory activities on marine plankton species, cytotoxic activities and anti-virus activities (Fig. 4).
Fig. 4.
Bioactivities of representative terpenoids from Trichoderma species.
Antibacterial activities
It is well known that Trichoderma is a fungal genus possessing the ability to antagonize various plant pathogens [108] and a growing number of researches reported the antibacterial activities of Trichoderma terpenoids, which were summarized in Table 2.
Table 2.
Antibacterial activities of Trichoderma terpenoids.
| NUMBER | NAME | Antibacterial activity | Reference | |
|---|---|---|---|---|
| 12 | 11-methoxy-9-cycloneren-3,7-diol | Vibrio anguillarum | 6.1 mm (20 μg/disk) | [40] |
| 13 | 10-cycloneren-3,5,7-triol | Vibrio anguillarum | 6.1 mm (20 μg/disk) | [40] |
| 14 | methyl 3,7-dihydroxy-15-cycloneranate | Vibrio anguillarum | 6.2 mm (20 μg/disk) | [40] |
| 86 | 8-acoren-3,11-diol | Vibrio anguillarum | 7.2 mm (20 μg/disk) | [40] |
| Vibrio splendidus | 6.1 mm (20 μg/disk) | |||
| 210 | 3R-hydroxy-9R,10R-dihydroharzianone | Vibrio anguillarum | 6 mm (20 μg/disk) | [40] |
| Vibrio splendidus | 6.2 mm (20 μg/disk) | |||
| 246 | 11R-methoxy-5,9,13-proharzitrien-3-ol | Vibrio harveyi | 6.8 mm (20 μg/disk) | [40] |
| 116 | bisabolan-1,10,11-triol | Vibrio parahaemolyticus | 6.7 mm (20 μg/disk) | [69] |
| Vibrio anguillarum | 6.5 mm (20 μg/disk) | |||
| Vibrio harveyi | 6.5 mm (20 μg/disk) | |||
| Vibrio Splendidus | 6.3 mm (20 μg/disk) | |||
| 117 | 12-nor-11-acetoxybisabolen-3,6,7-triol | Vibrio parahaemolyticus | 6.4 mm (20 μg/disk) | [69] |
| Vibrio anguillarum | 7.5 mm (20 μg/disk) | |||
| Vibrio harveyi | 7 mm (20 μg/disk) | |||
| Vibrio Splendidus | 6.3 mm (20 μg/disk) | |||
| 245 | trichocitrin | Escherichia coli | 8 mm (20 μg/disk) | [105] |
| 205 | harzianone | Escherichia coli | 8.3 mm (30 μg/disk) | [91] |
| Staphylococcus aureus | 7 mm (30 μg/disk) | |||
| 25 | 5-hydroxyepicyclonerodiol oxide | Vibrio anguillarum | 10 mm (40 μg/disk) | [44] |
| Vibrio harveyi | 8.5 mm (40 μg/disk) | |||
| Vibrio parahaemolyticus | 7.7 mm (40 μg/disk) | |||
| Vibrio splendidus | 7.7 mm (40 μg/disk) | |||
| 26 | 4-hydroxyepicyclonerodiol oxide | Vibrio anguillarum | 9 mm (40 μg/disk) | [44] |
| Vibrio harveyi | 7.7 mm (40 μg/disk) | |||
| Vibrio parahaemolyticus | 8 mm (40 μg/disk) | |||
| Vibrio splendidus | 6.7 mm (40 μg/disk) | |||
| 101 | trichodermol chlorohydrin | Vibrio parahaemolyticus | 7 mm (40 μg/disk) | [44] |
| 31 | 4-cadinen-11,12-diol | Vibrio anguillarum | 7.5 mm (40 μg/disk) | [47] |
| Vibrio harveyi | 7 mm (40 μg/disk) | |||
| Vibrio parahaemolyticus | 7 mm (40 μg/disk) | |||
| Vibrio splendidus | 7.1 mm (40 μg/disk) | |||
| Pseudoalteromonascitrea | 7.8 mm (40 μg/disk) | |||
| 32 | 4-cadinen-11,13-diol | Vibrio anguillarum | 7 mm (40 μg/disk) | [47] |
| Vibrio harveyi | 7 mm (40 μg/disk) | |||
| Vibrio parahaemolyticus | 6.2 mm (40 μg/disk) | |||
| Vibrio splendidus | 6.2 mm (40 μg/disk) | |||
| Pseudoalteromonascitrea | 8 mm (40 μg/disk) | |||
| 211 | 3S-hydroxyharzianone | Vibrio anguillarum | 6.2 mm (40 μg/disk) | [47] |
| Vibrio harveyi | 6.5 mm (40 μg/disk) | |||
| Vibrio parahaemolyticus | 6.5 mm (40 μg/disk) | |||
| Vibrio splendidus | 6 mm (40 μg/disk) | |||
| Pseudoalteromonascitrea | 7.5 mm (40 μg/disk) | |||
| 22 | (10E)-isocyclonerotriol | Vibrio harveyi | 7 mm (50 μg/disk) | [42] |
| Vibrio parahaemolyticus | 6.5 mm (50 μg/disk) | |||
| Vibrio splendidus | 6.5 mm (50 μg/disk) | |||
| 23 | (10Z)-isocyclonerotriol | Vibrio anguillarum | 7 mm (50 μg/disk) | [42] |
| Vibrio harveyi | 6.5 mm (50 μg/disk) | |||
| Vibrio parahaemolyticus | 6.5 mm (50 μg/disk) | |||
| Vibrio splendidus | 6.5 mm (50 μg/disk) | |||
| 134 | trichobisabolin M | Pseudoalteromonas citrea | 7 mm(50 μg/disk) | [70] |
| Vibrio splendidus | 7.3 mm(50 μg/disk) | |||
| 135 | trichobisabolin N | Vibrio splendidus | 5.7 mm(50 μg/disk) | [70] |
| 209 | 11-hydroxy-9-harzien-3-one | Vibrio parahaemolyticus | 6.2 mm | [39] |
| 28 | isoepicyclonerodiol oxide | Vibrio splendidus | 7 mm(100 μg/disk) | [45] |
| Pseudoalteromonas citrea | 9 mm(100 μg/disk) | |||
| 29 | norepicyclonerodiol oxide | Vibrio splendidus | 7 mm(100 μg/disk) | [45] |
| Pseudoalteromonas citrea | 9 mm(100 μg/disk) | |||
| 55 | cadin-4-en-11-ol | Pseudoalteromonas citrea | 8 mm(100 μg/disk) | [45] |
| 121 | trichobisabolin Q | Vibrio splendidus | 7.5 mm(100 μg/disk) | [45] |
| Pseudoalteromonas citrea | 7 mm(100 μg/disk) | |||
| 122 | trichobisabolin R | Vibrio splendidus | 8 mm(100 μg/disk) | [45] |
| Pseudoalteromonas citrea | 7 mm(100 μg/disk) | |||
| 123 | trichobisabolin S | Vibrio harveyi | 7.5 mm(100 μg/disk) | [45] |
| Vibrio parahaemolyticus | 7 mm(100 μg/disk) | |||
| Pseudoalteromonas citrea | 7 mm(100 μg/disk) | |||
| 124 | trichobisabolin T | Pseudoalteromonas citrea | 7 mm(100 μg/disk) | [45] |
| 125 | trichobisabolin U | Pseudoalteromonas citrea | 7 mm(100 μg/disk) | [45] |
| 126 | trichobisabolin V | Pseudoalteromonas citrea | 8 mm(100 μg/disk) | [45] |
| Vibrio anguillarum | 7 mm(100 μg/disk) | |||
| Vibrio harveyi | 7.5 mm(100 μg/disk) | |||
| Vibrio parahaemolyticus | 7.5 mm(100 μg/disk) | |||
| Vibrio splendidus | 8 mm(100 μg/disk) | |||
| 129 | trichobisabolin Y | Pseudoalteromonas citrea | 9 mm(100 μg/disk) | [45] |
| 130 | trichobisabolin Z | Pseudoalteromonas citrea | 6 mm(100 μg/disk) | [45] |
| Vibrio harveyi | 8 mm(100 μg/disk) | |||
| Vibrio splendidus | 9 mm(100 μg/disk) | |||
| 33 | trichocadinin B | Escherichia coli EMBLC-1 | MIC = 8 μg/mL | [48] |
| Aeromonas hydrophilia QDIO-1 | MIC = 8 μg/mL | |||
| Micrococcus luteus QDIO-3 | MIC = 32 μg/mL | |||
| Pseudomonas aeruginosa QDIO-4 | MIC = 8 μg/mL | |||
| Vibrio harveyi QDIO-7 | MIC = 8 μg/mL | |||
| Vibrio parahemolyticus QDIO-8 | MIC = 4 μg/mL | |||
| Vibrio vulnificus QDIO-9 | MIC = 64 μg/mL | |||
| 34 | trichocadinin C | Escherichia coli EMBLC-1 | MIC = 16 μg/mL | [48] |
| Aeromonas hydrophilia QDIO-1 | MIC = 64 μg/mL | |||
| Pseudomonas aeruginosa QDIO-4 | MIC = 8 μg/mL | |||
| Vibrio harveyi QDIO-7 | MIC = 16 μg/mL | |||
| Vibrio parahemolyticus QDIO-8 | MIC = 32 μg/mL | |||
| 35 | trichocadinin D | Escherichia coli EMBLC-1 | MIC = 8 μg/mL | [48] |
| Aeromonas hydrophilia QDIO-1 | MIC = 8 μg/mL | |||
| Micrococcus luteus QDIO-3 | MIC = 32 μg/mL | |||
| Pseudomonas aeruginosa QDIO-4 | MIC = 4 μg/mL | |||
| Vibrio harveyi QDIO-7 | MIC = 2 μg/mL | |||
| Vibrio parahemolyticus QDIO-8 | MIC = 8 μg/mL | |||
| Vibrio vulnificus QDIO-9 | MIC = 64 μg/mL | |||
| 38 | trichocadinin G | Edwardsiellatarda QDIO-2 | MIC = 1 μg/mL | [48] |
| Vibrio anguillarum QDIO-6 | MIC = 2 μg/mL | |||
| 73 | trichocarotin I | Escherichia coli EMBLC-1 | MIC = 16 µg/mL | [55] |
| 74 | trichocarotin J | Escherichia coli EMBLC-1 | MIC = 32 µg/mL | [55] |
| Micrococcus luteus QDIO | MIC = 32 µg/mL | |||
| 75 | trichocarotin K | Escherichia coli EMBLC-1 | MIC = 0.5 µg/mL | [55] |
| 76 | trichocarotin L | Escherichia coli EMBLC-1 | MIC = 0.5 µg/mL | [55] |
| 77 | trichocarotin M | Escherichia coli EMBLC-1 | MIC = 0.5 µg/mL | [55] |
| Micrococcus luteus QDIO | MIC = 8 µg/mL | |||
| 247 | koninginol A | Staphylococcus aureus (CMCC 26003) | MIC>100 μg/mL | [50] |
| Bacillus subtilis (CMCC 63501) | MIC = 10 μg/mL | |||
| 248 | koninginol B | Staphylococcus aureus (CMCC 26003) | MIC = 40 μg/mL | [50] |
| Bacillus subtilis (CMCC 63501) | MIC = 2 μg/mL | |||
| 115 | trichoderic acid | Escherichia coli | MIA = 25 mg/disk | [58] |
| Staphylococcus albus | MIA = 25 mg/disk | |||
| Shigella sonnei | MIA = 100 mg/disk | |||
| 80 | 2β-hydroxytrichoacorenol | Escherichia coli | MIA = 50 mg/disk | [58] |
| Staphylococcus albus | MIA = 150 mg/disk | |||
| 196 | trivirensol A | Enterococcus faecalis (AUS-RBWH-VRE-01) | IC50 = 1 μM | [88] |
| 197 | trivirensol B | Enterococcus faecalis (AUS-RBWH-VRE-01) | IC50 = 1.6 μM | [88] |
| 198 | trivirensol C | Enterococcus faecalis (AUS-RBWH-VRE-01) | IC50 = 8 μM | [88] |
| 202 | trivirensol G | Enterococcus faecalis (AUS-RBWH-VRE-01) | IC50 = 10 μM | [88] |
| 226 | harzianol I | Staphylococcus aureus | EC50 = 7.7 ± 0.8 μg/mL | [98] |
| Bacillus subtilis | EC50 = 7.7 ± 1.0 μg/mL | |||
| Micrococcus luteus | EC50 = 9.9 ± 1.5 μg/mL | |||
Compounds 12–14, 86, 116, 117, 210 and 246 showed promising effects against the marine-derived bacteria Vibrio parahaemolyticus, V. anguillarum, V. harveyi, and V. splendidusat, with inhibitory zone diameters of 6.0–8.0 mm at 20 µg/disk [40], [69], while 11-hydroxy-9-harzien-3-one (2 0 9) also showed inhibition against V. parahaemolyticus with a 6.2 mm zone [39]. Harzianone (2 0 5) (30 μg/disk) could suppress the growth of Escherichia coli and Staphylococcus aureus with inhibitory diameters of 8.3 and 7.0 mm, respectively [91], while trichocitrin (2 4 5), the first fusicoccane diterpene of Trichoderma, exhibited an 8.0 mm inhibition zones against E. coli at 20 μg/disk [105]. Trichoderic acid (1 1 5) also exhibited potent antibacterial activities against E. coli and S. albus with MIA (minimal inhibitory amount) value of 25 mg/disk [58]. Trichocadinins B − D (33–35) exhibited a broad antibacterial spectrum against various bacteria, while trichocadinin G (38) showed good activity against Edwardsiella tarda and V. anguillarum with MIC values of 1 and 2 μg/mL, respectively [48]. Furthermore, compounds 247 and 248 showed obvious antibacterial effects on Bacillus subtilis with MIC values of 10 and 2 μg/mL, respectively [50]. Interestingly, trivirensols A − C, G (196–198, 202), four trimeric sesquiterpenes displayed strong inhibitory effects against a clinical vancomycin-resistant Enterococcus faecalis with IC50 values of 1.0, 1.6, 8.0 and 10.0 μM [88]. In addition, harzianol I (2 2 6) showed good antibacterial effects against S. aureus, Bacillus subtilis and Micrococcus luteus with IC50 values of 7.7, 7.7 and 9.9 μg/mL, respectively [98]. Those terpenoids possessing broad antibacterial spectrum and supreme efficacy against drug-resistant strains may serve as promising candidates for antibacterial drug development.
Antifungal activities
Trichoderma species and the abundant terpenoids they produced are also well known for their antifungal activities against both human and plant pathogenic fungi. CAF-603 (56) displayed a broad antifungal spectrum against various fungi (Candida albicans, C. krusei, Saccbaromyces cerevisiae, Torulopsis glabrata, Aspergillus fumigatus and A. niger), especially showing remarkable antifungal activity against different strains of Candida albicans with MIC values ranging from 0.4 to 12.5 μg/mL [53]. Trichodermapenes B and C (40, 41) and trichodermanene (1 5 9) showed interesting antifungal effects against Cryptococcus neoformans ATCC90113 with MIC values of 32, 8 and 8 μg/mL, respectively, while only trichodermapene C (41) could inhibit the growth of C. neoformans ATCC90112 (MIC = 32 μg/mL) [49]. Furthermore, trichobreol A (96) showed potent antifungal activity against both C. albicans and C. neoformans with MIC values of 3.1 and 1.6 μg/mL, respectively, while trichobreol D (99) gave equivalent MIC values of 6.3 μg/mL [68]. Trichocadinins B − G (33–38) could inhibit the growth of Fusarium oxysporum f. sp. cucumebrium with MIC values of 1–64 μg/mL [48] and trichoderic acid (1 1 5) was active against F. avenaceum and Hormodendrum dermatitidis with MIA values of 125 and 75 mg/disk, respectively, while 2β-hydroxytrichoacorenol (80) showed antifungal activities against Botrytis cinerea and Pyricularia oryzae with MIA values at 175 and 200 mg/disk, respectively [58]. Interestingly, compound 141, a novel norsesquiterpene, exhibited promising antifungal activities on two Colletotrichum species and two carbendazim-resistant Botrytis cinerea with MIC values ranging from 8 to 64 µg/mL [66]. In addition, divirensol H (1 9 5) was effective against Fusarium oxysporum, Colletotrictum gloeosporioides, C. musae, Penicillium italicum, F. graminearum with MIC values of 12.5, 6.25, 25, 6.25 and 6.25 µg/mL, respectively. The efficacy of Trichoderma terpenoids against both human and plant pathogenic fungi suggests and potentializes their utilization as potential antifungal drugs and agrochemicals.
Anti-marine plankton species
Trichoderma terpenoids have been proven to possess inhibitory activities against diverse marine plankton species. The inhibitory effects of compounds 12–23, 30–32, 49–54, 65–67, 70–72, 86, 210, 211, 225, 226, 246 on six marine phytoplankton species (including Chattonella marina, Heterosigma akashiwo, Karlodinium veneficum, Prorocentrum donghaiense, Amphidinium carterae and Heterocapsa circularisquama) were recorded and summarized in Table 3, and their MIC values were ranging from 0.27 to 75 μg/mL [40], [41], [42], [46], [47], [52], [98]. These results suggested that Trichoderma terpenoids may be useful for the development of novel marine antifouling agent.
Table 3.
Inhibitory activities of Trichoderma terpenoids on six marine plankton species (MIC, μg/mL).
| No. | Name | C. marina | H. akashiwo | K. veneficum | P. donghaiense | A. carterae | H. circularisquama | References |
|---|---|---|---|---|---|---|---|---|
| 12 | 11-methoxy-9-cycloneren-3,7-diol | 0.66 | 23 | 2.2 | 37 | – | – | [40] |
| 13 | 10-cycloneren-3,5,7-triol | 9.9 | 75 | 14 | 66 | – | – | [40] |
| 14 | methyl 3,7-dihydroxy-15-cycloneranate | 12 | 68 | 41 | 55 | – | – | [40] |
| 86 | 8-acoren-3,11-diol | 2.8 | 56 | 54 | 54 | – | – | [40] |
| 210 | 3R-hydroxy-9R,10R-dihydroharzianone | 7 | 42 | 24 | 70 | – | – | [40] |
| 246 | 11R-methoxy-5,9,13-proharzitrien-3-ol | 1.2 | 1.3 | 3.2 | 4.3 | – | – | [40] |
| 15 | 3,7,11-trihydroxycycloneran-10-one | 5.2 | 8 | 10 | 9.9 | – | – | [41] |
| 16 | cycloneran-3,7,10,11-tetraol | 8.8 | 21 | 76 | 6.5 | – | – | [41] |
| 17 | cycloneran-3,7,11-triol | 61 | 73 | 71 | 40 | – | – | [41] |
| 18 | 11,12,15-trinorcycloneran-3,7,10-triol | 13 | 73 | 6.3 | 34 | – | – | [41] |
| 19 | 7,10S-epoxycycloneran-3,15-diol | 2.4 | 26 | 3.9 | 20 | – | – | [41] |
| 20 | 7,10R-epoxycycloneran-3,15-diol | 5.8 | 37 | 5.5 | 15 | – | – | [41] |
| 21 | (10Z)-15-acetoxy-10-cycloneren-3,7-diol | 59 | 14 | 35 | 7.3 | – | – | [41] |
| 22 | (10E)-isocyclonerotriol | – | 17 | 8.1 | 51 | – | – | [42] |
| 23 | (10Z)-isocyclonerotriol | – | 70 | 22 | 54 | – | – | [42] |
| 30 | trichocadinin A | – | – | – | – | – | – | [46] |
| 65 | trichocarotin C | 0.24 | 3.1 | 5.2 | 3.8 | – | – | [46] |
| 66 | trichocarotin D | 0.33 | 4.4 | 7.6 | 3.2 | – | – | [46] |
| 67 | trichocarotin E | 0.27 | 4.2 | 5 | 3.6 | – | – | [46] |
| 70 | trichocarotin H | 1.2 | 6.2 | 12 | 6.5 | – | – | [46] |
| 31 | 4-cadinen-11,12-diol | 1.8 | 1.1 | 2.1 | 8.9 | – | – | [47] |
| 32 | 4-cadinen-11,13-diol | 4.3 | 2.7 | 3.2 | 5.8 | – | – | [47] |
| 211 | 3S-hydroxyharzianone | 6.9 | 3.1 | 4.5 | 7.7 | – | – | [47] |
| 49 | trichocadinin H | – | 0.87 | – | 0.83 | 1.5 | 2.6 | [52] |
| 50 | trichocadinin I | – | 3.2 | – | 1.7 | 6.2 | 3.6 | [52] |
| 51 | trichocadinin J | – | 2.3 | – | 1.4 | 1.7 | 1.3 | [52] |
| 52 | trichocadinin K | – | 1.5 | – | 2.4 | 6.7 | 2.7 | [52] |
| 53 | trichocadinin L | – | 0.88 | – | 0.68 | 2.4 | 1.8 | [52] |
| 54 | trichocadinin M | – | 2.1 | – | 0.54 | 1.8 | 5.2 | [52] |
| 71 | 14-O-methyltrichocarotin G | – | 22 | – | 10 | 24 | 19 | [52] |
| 72 | 14-O-methyl CAF-603 | – | 9.2 | – | 8.3 | 4.7 | 9.8 | [52] |
| 225 | harzianol H | – | 27 | – | – | – | 30 | [98] |
| 226 | harzianol I | – | 10 | – | 14 | 15 | 8.4 | [98] |
| 25 | 5-hydroxyepicyclonerodiol oxide | – | – | – | 35 | – | – | [44] |
| 101 | trichodermol chlorohydrin | – | – | – | 35 | 97 | – | [44] |
| 27 | cycloner-3-en-7,11- diol | 5.3 | 3.1 | – | 5.2 | 4.5 | – | [45] |
| 28 | isoepicyclonerodiol oxide | 2.5 | 3.3 | – | 5.1 | 4.2 | – | [45] |
| 29 | norepicyclonerodiol oxide | 3.1 | 5.4 | – | 5.6 | 3.1 | – | [45] |
| 55 | cadin-4-en-11-ol | 0.54 | 5.4 | – | 4.3 | 1.4 | – | [45] |
| 87 | trichoacorin A | 13 | – | – | 26 | – | – | [60] |
| 107 | trichodermarin G | – | 16 | – | 19 | 21 | 13 | [65] |
| 108 | trichodermarin H | – | 63 | – | – | 53 | 66 | [65] |
| 109 | trichodermarin I | – | 25 | – | – | 14 | 31 | [65] |
| 110 | trichodermarin J | – | 30 | – | 23 | 28 | [65] | |
| 111 | trichodermarin K | – | 64 | – | 55 | 68 | – | [65] |
| 112 | trichodermarin L | – | 24 | – | 20 | 18 | [65] | |
| 113 | trichodermarin M | – | 34 | – | 25 | 22 | [65] | |
| 121 | trichobisabolin Q | 0.54 | 6.2 | – | 5.7 | 1.4 | – | [45] |
| 122 | trichobisabolin R | 3.4 | 1 | – | 4.5 | 12 | – | [45] |
| 123 | trichobisabolin S | 5.4 | 7.2 | – | 8.3 | 4.6 | – | [45] |
| 124 | trichobisabolin T | 7 | 4.2 | – | 3.4 | 1.6 | – | [45] |
| 125 | trichobisabolin U | 1.6 | 13 | – | 1.7 | 5.2 | – | [45] |
| 126 | trichobisabolin V | 8.4 | 11 | – | 5.7 | 6.6 | – | [45] |
| 127 | trichobisabolin W | 4.8 | 2.5 | – | 6.8 | 2 | – | [45] |
| 128 | trichobisabolin X | 6.4 | 7.1 | – | 4.7 | 4.6 | – | [45] |
| 129 | trichobisabolin Y | 2.2 | 5 | – | 3.9 | 4.6 | – | [45] |
| 130 | trichobisabolin Z | 3.3 | 6.3 | – | 8.4 | 3.5 | – | [45] |
| 131/132 | trichobisabolin O1, O2 | 8.5 | – | – | 19 | – | – | [60] |
| 133 | trichobisabolin P | 12 | – | – | 37 | 1.8 | – | [60] |
| 134 | trichobisabolin M | 15 | 25 | – | 24 | 23 | – | [70] |
| 135 | trichobisabolin N | 11 | 83 | – | 33 | 71 | – | [70] |
| 180/181 | trichonerolin A and B | 1.2 | – | – | – | 55 | – | [60] |
| 228 | 3S-hydroxy-9R,10R-dihydroharzianone | 31 | 35 | – | 18 | 34 | – | [100] |
| 229 | 3S-hydroxytrichodermaerin | 25 | 22 | – | 18 | 32 | – | [100] |
| 230 | methyl −3S-hydroxy-10,11-seco-harzianate | 22 | 31 | – | 20 | 47 | – | [100] |
Cytotoxic activities
The cytotoxic activities of Trichoderma terpenoids have also been broadly evaluated and reported. Trichodermaloids A-C (43–45) showed promising antiproliferative effects on NCIH-460, NCIC-H929 and SW620 cells with IC50 values ranging from 6.8 to 12.7 μM [51], while 10-demethyldehydrobotrydien-1,15-diol (1 4 3) showed good inhibitory rate of 45–60 % on K562 cells at 6.25 µM [71]. In the cytotoxic assay of trichodermanins C—H (239–244) using three cancer cell lines (P388, HL-60, L1210), trichodermanin C (2 3 9) showed potent cytotoxic effects on 3 tested cell-lines with IC50 values of 7.9, 6.8, 7.6 μM, respectively, whereas other trichodermanins only exhibited modest activities or inactive (IC50>40 μM) [103], [104]. In addition, 1α-isopropyl-4α,8-dimethylspiro[4.5]dec-8-ene-3β,7α-diol (82) showed moderate antiproliferative activities against HL-60, A-549 and MCF-7 cell lines with IC50 values of 22.8, 22.4 and 36.1 μM, respectively [59], while (9R,10R)-dihydro-harzianone (2 0 7) also showed antiproliferative effect against HeLa and MCF-7 cell lines (IC50 = 30.1 and 30.7 μM) [94] and koninginol B (2 4 8) exhibited modest cytotoxic effects on A549 cell (IC50 = 46.6 μM) [50]. Harzianol I (2 2 6) also showed weak cytotoxicity against NCI-H1975, HepG2, MCF-7 cell lines with IC50 values of 58.72, 60.88 and 53.92 μM, respectively [98]. Importantly, intraperitoneal administration of gliocladic acid (1 5 8) (3 mg/kg) exhibited significant antitumor activity against Sarcoma-37 tumor growth in ICR mice with an inhibition rate of 46 %. Preliminary safety evaluation revealed that the maximum tolerated dose (MTD) of gliocladic acid (1 5 8) may be more than 200 mg/kg in mice [75].
Anti-virus activities
In addition to the above-mentioned bioactivities, limited Trichoderma terpenoids also exhibited anti-virus properties. Harzianoic acids A and B (167, 168), two cyclobutene-containing sesquiterpenes, displayed promising anti-HCV activities and low toxicity by reducing the HCV RNA levels and possibly blocking the entry step in the HCV life cycle through targeting the viral E1/E2 and the host cell CD81 proteins [81]. In addition, wickerols A and B (237, 238) were two novel anti-virus diterpenes that displayed potent anti-influenza virus activity against H1N1 with IC50 values of 0.07 and 5.0 μg/mL [102], thus providing novel scaffold for anti-virus drug development.
Terpenoid gene clusters and biosynthesis in Trichoderma
Currently, significant progress has been achieved in the discovery of novel secondary metabolites from Trichoderma species under varied fermentation conditions (Fig. 5A) and in the bioactivity exploration of Trichoderma secondary metabolites. Recently, Trichoderma genome sequencing projects have targeted 86 Trichoderma strains and 22 have already been published (https://genome.jgi-psf.org/programs/fungi), which provides a great opportunity and a feasible way for high-efficiency production of structurally varied secondary metabolites based on biosynthesis and engineering.
Fig. 5.
Genomics, biosynthetic pathways and gene clusters of Trichoderma species terpenoid. (A) Representative figures of Trichoderma species (left: early growth stage; middle: middle: sporogenic stage; right: liquid medium culture). (B) Trichoderma species with published genomics and the biosynthetic pathways of terpenoids in organisms. (C-E) Terpenoid clusters or genes have been reported in Trichoderma.
According to the structure types of Trichoderma metabolites, the main enzymes involved in biosynthesis are terpenoid synthases/cyclases (TSs/TCs), polyketide synthases (PKSs), non-ribosomal peptide synthases (NRPSs) and PKS-NRPS hybrid. In 2008, the first genome from T. reesei was sequenced, which revealed the presence of 12 TSs, 8 NRPSs and 11 PKSs [109]. Later, T. virens was predicted to include 16 TSs, 22 NRPSs, 18PKSs, while T. atroviride contained 14 TSs, 14 NRPSs and 18 PKSs [110]. These diverse synthase-encoding genes in Trichoderma species indicated the presence of abundant biosynthetic gene clusters (BGC) for secondary metabolites production. Focusing on TSs for terpenoids biosynthesis, we herein summarized the mining of TPSs in Trichoderma strains with published genomics, and a large number of TSs have been annotated in 13 species (Fig. 5B) [110], [111], [112].
The structural diversity and biological versatility of Trichoderma terpenoids are fascinating us in the interpretation of their biosynthetic pathways. Exploration and elucidation of key genes and enzymes involved in terpenoid biosynthetic pathways of Trichoderma has been essential for taking full advantage of Trichoderma fungi in industry and agriculture. Generally, all terpenoids are biosynthesized from isoprenyl diphosphate formed by the consecutive condensation of the five-carbon monomer isopentenyl diphosphate (IPP) to its isomer dimethylallyl diphosphate (DMAPP) (Fig. 5B). The linear chain of prenyl diphosphate of varying lengths are produced by successive head-to-tail condensation of these C-5 units, which become the substrates for different kinds of key enzymes involved in terpene biosynthesis [113].
In microorganisms, genes encoding key enzymes responsible for consecutive steps in a biosynthetic pathway tend to be co-localized as biosynthetic gene clusters (BGCs) on microbial chromosome. As for Trichoderma terpenoids, the most well-explored BGC is the tri gene cluster for trichothecene biosynthesis characterized in T. arundinaceum, T. brevicompactum and T. roseum (Fig. 5C) [114]. Especially, the biosynthetic pathway for two trichothecene-type sesquiterpenes, trichodermin (90) and harzianum A (94), in T. arundinaceum has been elucidated and most of the involved genes have been functionally annotated. The tri BGC in T. arundinaceum IBT 40,837 includes three tri loci with eleven candidate genes (Fig. 5C). As is illustrated, farnesyl diphosphate (FPP) is initially catalyzed to trichodiene (TD) by trichodiene synthase TRI5. Subsequent oxygenation of TD at C2, C11 and C13 are catalyzed by TRI4 to get isotrichodiol, which is nonenzymatically cyclized to give trichothecene (EPT). TRI22 catalyzes the hydroxylation of EPT at C4 to yield trichodermol. Subsequently, trichodermol undergoes TRI3-catalyzed acetylation of the C4 hydroxyl to get trichodermin (90) and the acetyl group of trichodermin can be replaced by octa-2,4,6-trienedioyl to produce harzianum A (94) [115], [116], [117]. Up to date, tri 12 and tri 14 have not been characterized yet, which may play roles in unknown tailoring steps requiring further in-depth studies. In addition, a vir gene cluster was identified from T. virens (Fig. 5D), which includes candidate genes that encode putative terpene cyclases, cytochrome P450s and an oxidoreductase, predicted to be responsible for viridin and viridiol biosynthesis, though the viridin family is of steroidal antibiotic rather than a diterpene origin [118]. However, a later study demonstrated that vir4 of the vir BGC is responsible for the biosynthesis of volatile sesquiterpenes in T. virens and the vir4-containing gene cluster contributes to a unique spectrum of volatile terpenes in this species [119]. Further exploration is urgently needed to fully characterize the precise function and structure of this gene cluster.
Some key enzymes in the biosynthetic pathway of Trichoderma terpenoids have also been excavated. It was reported that deletion of a NRPS encoding gene Tex7 in T. virens could led to the notable over-production of heptelidic acid (1 6 0), also named koningic acid [120]. The heterologous expression of TaTC6, a terpenoid cyclase identified from T. atroviride FKI-3849, in E. coli BL21 (DE3) could convert FPP into trichobrasilenol with some other sesquiterpenes as by-products (Fig. 5E). The first enzyme in the mevalonate pathway is hydroxy-methylglutaryl-CoA reductase (HMGR), which is encoded by the hmgR gene and associated with the isoprene building block. erg1 was a gene encoding a squalene epoxidase essential for triterpene biosynthesis characterized in T. harzianum. Partial silencing of the hmgR or erg1 gene in T. harzianum both led to lower production of ergosterol and a decrease of antifungal activity but an increase in the expression of erg7, a gene encoding an oxidosqualene lanosterol-cyclase [121], [122].
Besides those characterized BGCs and annotated key genes or enzymes, there have been quite a lot proposed biosynthetic pathways of Trichoderma terpenes speculated based on key precursors and biosynthetic principles. A total of 31 terpenes (24, 160, 171, 172, 175–177, 188–194, 196–202, 220, 221–227, 237, 238, 246, 249, 253) have been recorded in reported plausible biosynthetic pathways until now, mainly focused on the biosynthesis of sesquiterpenes (Fig. 6A) and diterpenes (Fig. 6B). Jiao et al. isolated 16 sesquiterpenes from T. virens CMB-TN16, including two acetylated analogues, 3-acetylgliocladic acid (1 7 1), 14-acetylgliocladic acid (1 7 2), seven dimeric sesquiterpenes, divirensols A − G (188–194) [84], and seven trimeric sesquiterpenes, trivirensols A − G (196–202) [88], which provided a great opportunity for interpreting and integrating the related pathway. It was biosynthetically speculated that these congeners are likely generated from a “cadinene scaffold”, which first undergoes oxidation to form the epoxy-lactone-acid A (heptelidic acid, 160) as a pivotal intermediate. Subsequent hydrolysis of A delivers the monomeric units B − F. And monomeric unit F can decarboxylate to yield G, whereas G can undergo an intramolecular addition to the ether H [84]. The monomeric unit B undergoes trans lactonization and dehydration to produce subunits C and D, respectively. Also, subunit B can be hydrated to give subunit F, which can undergo dehydration and decarboxylation to form subunit G, followed by intramolecular addition to yield subunit H [84]. Therefore, a plausible biosynthetic relationship of all these compounds (monomers, dimers and trimers) could be found in Fig. 6A. In addition, a putative biosynthetic pathway for tricinoloniol acids (TRAs) A–C (175–177) was also proposed. TraA functions as a terpene cyclase catalyzing a farnesyl pyrophosphate (FPP) to germacrene D. Subsequent oxidation and reduction steps led to the formation of TRA A (1 7 5) and TRA B (1 7 6), while rearrangement of TRA A (1 7 5) finally gave TRA C (1 7 7) [86] (Fig. 6A). And 3,7,11-trihydroxy-cycloneran (24) was proposed to start from NPP followed by cyclization and oxidation [43].
Fig. 6.
Proposed biosynthetic pathway of Trichoderma terpenoids from different precursors (A) Proposed biosynthetic pathway of sesquiterpenes from FPP (from left to right: yellow grey pathway, plausible biosynthetic relationship between sesquiterpene monomeric subunits (158–160, 171–173, 182–186), dimers (188–195) and trimers (196–202); gray pathway, proposed biosynthetic pathway of TRAs A-C (175–177) in T. hypoxylon; purple pathway, plausible biogenetic pathways proposed for 24). (B) Proposed biosynthetic pathway of diterpenes from GGPP (from left to right: minium pathway and lavender pathway, plausible biogenetic pathway for 250 and 245 from GGPP, respectively; pink pathway, proposed mechanism of cyclization from GGPP to wickerols 237 and 238; water blue pathway, plausible biosynthetic pathway for compound 220, 223–227). (C) Proposed biosynthetic pathway of meroterpenoid 253. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Varied cyclization of the common precursor GGPP catalyzed by different terpene cyclases, combined with a wide variety of tailoring steps, produces high structural diversity of Trichoderma diterpenes. Harzianols F-J (223–227) are 4/7/5/6-fused tetracyclic diterpenoids exclusively isolated from Trichoderma species. Though their cyclization mechanism from GGPP has not been clarified yet, some biosynthetic steps could be plausibly proposed based on related studies (Fig. 6B). A pivotal harzianyl cation was supposed to be initially formed via diphosphate ionization-mediated cyclization of GGPP, which would be quenched by a molecule of water to yield 226 or undergo deprotonation followed by Δ9,20 double-bond reduction and sequential oxidation to give 227. Selective hydroxylation of 226 at C-2 and C-3 generated 223 and 225, respectively, while hydroxylation of 227 at C-20 produces 220 [98]. Wickerols A and B (237, 238) are novel Trichoderma diterpenes possessing 6–5-6–6 ring skeleton, which have been proven to be biosynthesized from GGPP based on feeding experiment of [1-13C]-, [2-13C]- and [1,2-13C2]-acetates [102]. The cyclization mechanism of GGPP to wickerols can be predicted initially from the boat-like transition state of GGPP, from which pyrophosphate is released to form a verticillen-12-yl cation intermediate (Fig. 6B). Subsequent 1,2-rearrangements of β-methyl and α-hydride followed by ring inversion, cyclization and rearrangement-initiated ring expansion finally formed the fused 6–5-6–6 ring system. Cytochrome P450 catalyzed hydroxylation at C-8 of 237 forms 238 [102]. Trichocitrin (2 4 5) represents the first Trichoderma-derived and furan-bearing fusicoccane diterpene with unclear but interesting cyclization mechanisms [105] (Fig. 6B), while harzianolic acid A (2 5 0) was the first reported chlorinated cleistanthane diterpene, probably derived from CPP to cleistanthadiene through cyclization and rearrangement, followed by halogenation and sequential oxidation of cleistanthadiene [43]. Additionally, neomacrophorin X (2 5 3) was the only one meroterpenoid isolated from Trichoderma species [107] and premacrophorin is proposed to be a presumably biosynthetic precursor followed by cyclization and fusion with reduced form of anthraquinone to give 253, as outlined in Fig. 6C.
Taken together, Trichoderma genome mining and enabling technologies provide a great opportunity for comprehensively understanding and extending the chemical diversity of Trichoderma secondary metabolites. However, compared with the large number of Trichoderma terpenoids already reported, limited BGCs and key genes/enzymes have been fully illustrated. More direct evidence is therefore needed to characterize the BGC and validate the biosynthetic pathways of Trichoderma terpenoids regarding those intriguing enzymology and novel chemistry to be unveiled.
Conclusion and future directions
Trichoderma fungi produce a variety of novel terpenoids with clinically and agriculturally relevant biological activities, which have been playing crucial roles in the fields of agriculture and pharmaceutical industry. This review summarized a total of 253 terpenoids isolated from Trichoderma species between 1948 and 2022. These Trichoderma terpenoids display highly diverse structures, mainly containing varied types of sesquiterpenes and diterpenes, and exhibit versatile bioactivities, mainly including antibacterial activities, antifungal activities, inhibitory activities on marine plankton species and cytotoxic activities, which justifies Trichoderma species as important microbial agents for drug discovery and environmentally friendly agrochemicals development. Therefore, Trichoderma species represents an infinite natural treasure that provides a wealth of opportunities for the discovery and production of terpenoids with an expansive array of structures and functionalities. Furthermore, intriguing chemistry and enzymology involved in the biosynthesis of Trichoderma terpenoids were also presented to facilitate further precise genome mining-guided structural diversity expansion. This review may thus facilitate further studies on Trichoderma species in the areas of chemistry, medicine, agriculture and microbiology.
Though great progress has been made to date on Trichoderma species and their secondary metabolites, there are several future directions for taking full advantage of this invaluable microbial resource and the produced terpenoids. Firstly, previous studies on the structure–activity relationship (SAR) and mechanism of actions of Trichoderma terpenoids were far from sufficient either in depth or in extent. To comprehensively elucidate the SAR and drug targets of bioactive Trichoderma terpenoids may help facilitate the development of highly effective drugs and environmentally friendly agrochemicals. Secondly, the large number of enzymes and reactions as well as novel BGCs involved in Trichoderma terpenoids biosynthetic pathways are still unknown, uncharacterized and undiscovered. Thus, to identify potential BGCs and key enzymes and elucidate important biosynthetic steps based on genome mining and genetic engineering are needed for the full exploitation of Trichoderma as platform strains for terpenoids biosynthesis. Thirdly, on the basis of well explored biosynthetic pathways, synthetic biology approach is an intriguing strategy that enables Trichoderma strains as chemical factory not only for unknown terpenoids but also for specialized terpenoids production with high efficiency. So, it is promising to utilize synthetic biology to expand our search space for novel terpenoids synthesis and unlock the great potential for terpenoids production of Trichoderma.
From what have been discussed above, it is not difficult to draw the conclusion that Trichoderma species can be regarded as an excellent natural source for terpenoids biosynthesis. Searching for high-value terpenoids and understanding their biosynthetic pathways with complicated regulation are critical for efficient utilization of Trichoderma species. So, by integrating metabolic engineering, synthetic biology, genetic engineering and other enabling technologies, much more progress will be made to discover more bioactive terpenoids with structural and functional diversities, and uncover the precise mechanisms of Trichoderma terpenoids biosynthesis.
Compliance with ethics requirements
This review does not involve any studies with animal or human subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This review is financially supported by the National Natural Science Foundation of China (82174081) and Shanghai Pujiang Program (21PJD082).
Biographies

Dr. Bingke Bai received her bachelor's degree from Henan University of Traditional Chinese Medicine (HNUTCM) in July 2020 and continued her master and doctoral studies at Naval Medical University (NMU) in September of the same year. At present, Dr. Bingke Bai has participated in the development of 2 papers (including 1 as co-first author), and 1 patent. Her main research direction is to explore bioactive natural products and their potential molecular mechanisms from traditional Chinese herbs and their symbiotic microbes.

Dr. Chang Liu is studying for his Ph.D. Degree at Shanghai University of Traditional Chinese Medicine (SHUTCM) in July 2018. Currently, Dr. Liu is a lecturer of the Department of Chinese Medicine Authentication in the Faculty of Pharmacy, NMU. At present, he has participated in the publication of more than 10 research papers (among these, over 7 as first or co-first author). Now he is engaged in the research of plant natural product mining and screening of key enzyme genes for biosynthesis of secondary metabolites. Moreover, he focuses on new strategies with mining glycosyltransferases from secondary metabolites of medicinal plants.

Dr. Chengzhong Zhang is a lecturer of the Department of Chinese Medicine Authentication in the Faculty of Pharmacy, NMU. He has published over 20 research papers. His research interest focuses on the authentication and processing of traditional Chinese medicine and their bioactive constituents.

Xuhui He received his bachelor's degree at Naval Medical University (NMU) in June 2019. Currently, He is an assistant of the Department of Chinese Medicine Authentication in the Faculty of Pharmacy, NMU, and focuses on the biofunctions and biosynthesis of natural products from traditional Chinese herbs and their symbiotic microbes.

Hongrui Wang received his bachelor's degree at Naval Medical University (NMU) in June 2019. Currently, He is an assistant of the Department of Chinese Medicine Authentication in the Faculty of Pharmacy, NMU, and focuses on the bioactive natural products and their potential molecular mechanisms from traditional Chinese herbs.

Dr. Wei Peng, World’s Top 2% Scientists 2020 (Pharmacology & Pharmacy), obtained his Ph.D. Degree at Chengdu university of Traditional Chinese Medicine (CDUTCM) in July 2017. Currently, Dr. Peng is an Associate Professor in the School of Pharmacy, CDUTCM, and has published over 100 research papers (among these, over 50 as first or corresponding authors) with total citation over 2100. His current main interests are focused on the natural products with anti-oxidative stress, antitumor, anti-inflammatory & immunological activities and their potential molecular mechanisms. In addition, Dr. Peng also focuses on new strategies for treating tumor, inflammatory & immunological disorders and the new pathogenesis of these diseases.

Dr. Chengjian Zheng, World’s Top 2% Scientists 2020 (Pharmacology & Pharmacy), obtained his Ph.D. Degree at Naval Medical University (NMU) in July 2010. In 2019, he went to the United States and undertook post-doctoral research in natural products biosynthesis and drug discovery at the Department of Chemistry of The Scripps Research Institute, Jupiter, Florida. Currently, Dr. Zheng is a Full Professor and the director of the Department of Chinese Medicine Authentication in the Faculty of Pharmacy, NMU, and has published over 90 research papers (H-index 30). Zheng’s group is interested in the chemistry, biofunctions and biosynthesis of natural products from traditional Chinese herbs and their symbiotic microbes.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.09.010.
Contributor Information
Wei Peng, Email: pengwei@cdutcm.edu.cn.
Chengjian Zheng, Email: cjzheng1984@126.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Danielson R.M., Davey C.B. The abundance of Trichoderma propagules and the distribution of species in forest soils. Soil Biol Biochem. 1973;5:485–494. doi: 10.1016/0038-0717(73)90038-2. [DOI] [Google Scholar]
- 2.Sun R.-Y., Liu Z.-C., Fu K., Fan L., Chen J. Trichoderma biodiversity in China. J Appl Genet. 2012;53:343–354. doi: 10.1007/s13353-012-0093-1. [DOI] [PubMed] [Google Scholar]
- 3.Błaszczyk L., Popiel D., Chełkowski J., Koczyk G., Samuels G.J., Sobieralski K., et al. Species diversity of Trichoderma in Poland. J Appl Genet. 2011;52(2):233–243. doi: 10.1007/s13353-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Y., Wang J.-L., Chen J., Mao L.-J., Feng X.-X., Zhang C.-L., et al. Trichoderma biodiversity of agricultural fields in east China reveals a gradient distribution of species. PLoS ONE. 2016;11(8):e0160613. doi: 10.1371/journal.pone.0160613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuels G.J., Suarez C., Solis K., Holmes K.A., Thomas S.E., Ismaiel A., et al. Trichoderma theobromicola and T. paucisporum: two new species isolated from cacao in South America. Mycol Res. 2006;110(4):381–392. doi: 10.1016/j.mycres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Hatvani L., Antal Z., Manczinger L., Szekeres A., Druzhinina I.S., Kubicek C.P., et al. Green mold diseases of agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology. 2007;97(4):532–537. doi: 10.1094/PHYTO-97-4-0532. [DOI] [PubMed] [Google Scholar]
- 7.Hatvani L., Sabolić P., Kocsubé S., Kredics L., Czifra D., Vágvölgyi C., et al. The first report on mushroom green mould disease in Croatia. Arh Hig Rada Toksikol. 2012;63:481–487. doi: 10.2478/10004-1254-63-2012-2220. [DOI] [PubMed] [Google Scholar]
- 8.Kredics L., Antal Z., Dóczi I., Manczinger L., Kevei F., Nagy E. Clinical importance of the genus Trichoderma: A review. Acta Microbiol Immunol Hung. 2003;50:105–117. doi: 10.1556/AMicr.50.2003.2-3.1. [DOI] [PubMed] [Google Scholar]
- 9.Rocha L.C., Luiz R.F., Rosset I.G., Raminelli C., Seleghim M.H.R., Sette L.D., et al. Bioconversion of iodoacetophenones by marine fungi. Mar Biotechnol. 2012;14(4):396–401. doi: 10.1007/s10126-012-9463-2. [DOI] [PubMed] [Google Scholar]
- 10.Madsen A.M., Hansen V.M., Meyling N.V., Eilenberg J. Human exposure to airborne fungi from genera used as biocontrol agents in plant production. Ann Agric Environ Med. 2007;14:5–24. doi: 10.1002/adic.200690081. [DOI] [PubMed] [Google Scholar]
- 11.Rajesh R.W., Rahul M.S., Ambalal N.S. A significant fungus for agriculture and environment. Afr J Agr Res. 2016;11(22):1952–1965. [Google Scholar]
- 12.Woo S.L., Ruocco M., Vinale F., Nigro M., Marra R., Lombardi N., et al. Trichoderma-based products and their widespread use in agriculture. The Open Mycology Journal. 2014;8(1):71–126. [Google Scholar]
- 13.Cai F., Yu G., Wang P., Wei Z., Fu L., Shen Q., et al. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol Biochem. 2013;73:106–113. doi: 10.1016/j.plaphy.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Contreras-Cornejo H.A., Macías-Rodríguez L., Alfaro-Cuevas R., López-Bucio J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol Plant Microbe Interact. 2014;27:503–514. doi: 10.1094/mpmi-09-13-0265-r. [DOI] [PubMed] [Google Scholar]
- 15.Dayana-Amira R., Roshanida A.R., Rosli M.I., Siti-Fatimah-Zahrah M.F., Mohd-Anuar J., Nazrul-Adha C.M. Bioconversion of empty fruit bunches (EFB) and palm oil mill effluent (POME) into compost using Trichoderma virens. Afr J Biotechnol. 2011;10:18775–18780. doi: 10.5897/AJB11.2751. [DOI] [Google Scholar]
- 16.Sharma B.L., Singh S.P., Sharma M.L. Bio-degradation of crop residues by Trichoderma species vis-à vis nutrient quality of the prepared compost. Sugar Tech. 2012;14:174–180. doi: 10.1007/s12355-011-0125-x. [DOI] [Google Scholar]
- 17.Vázquez M.B., Barrera V., Bianchinotti V. Molecular identification of three isolates of Trichoderma harzianum isolated from agricultural soils in Argentina, and their abilities to detoxify in vitro metsulfuron methyl. Botany-botanique. 2015;93(11):793–800. [Google Scholar]
- 18.Yadav U., Choudhury P.P. Biodegradation of sulfosulphuron in agricultural soil by Trichoderma sp. Lett Appl Microbiol. 2014;59:479–486. doi: 10.1111/lam.12306. [DOI] [PubMed] [Google Scholar]
- 19.Brewer D., Mason F.G., Taylor A. The production of alamethicins by Trichoderma spp. Can J Microbiol. 1987;33:619–625. doi: 10.1139/m87-108. [DOI] [PubMed] [Google Scholar]
- 20.Neuhof T., Dieckmann R., Druzhinina I.S., Kubicek C.P., Döhren H. Intact-cell MALDI-TOF mass spectrometry analysis of peptaibol formation by the genus Trichoderma/Hypocrea: can molecular phylogeny of species predict peptaibol structures? Microbiology+ 2007;153:3417–3437. doi: 10.1099/mic.0.2007/006692-0. [DOI] [PubMed] [Google Scholar]
- 21.Baker S.E., Perrone G., Richardson N.M., Gallo A., Kubicek C.P. Phylogenomic analysis of polyketide synthase-encoding genes in Trichoderma. Microbiology+ 2012;158:147–154. doi: 10.1099/mic.0.053462-0. [DOI] [PubMed] [Google Scholar]
- 22.Collins R.P., Halim A.F. Characterization of the major aroma constituent of the fungus Trichoderma viride. J Agr Food Chem. 1972 doi: 10.1021/jf60180a010. [DOI] [Google Scholar]
- 23.Jiang M., Wu Z., Guo H., Liu L., Chen S. A review of terpenes from marine-derived fungi: 2015–2019. Mar Drugs. 2020;18(6):321. doi: 10.3390/md18060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vizcaino J.A., Sanz L., Basilio A., Vicente F., Gutierrez S., Hermosa M.R., et al. Screening of antimicrobial activities in Trichoderma isolates representing three Trichoderma sections. Mycol Res. 2005;109(12):1397–1406. doi: 10.1017/s0953756205003898. [DOI] [PubMed] [Google Scholar]
- 25.Vinale F., Marra R., Scala F., Ghisalberti E.L., Lorito M., Sivasithamparam K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett Appl Microbiol. 2006;43:143–148. doi: 10.1111/j.1472-765X.2006.01939.x. [DOI] [PubMed] [Google Scholar]
- 26.Song X.-Y., Shen Q.-T., Xie S.-T., Chen X.-L., Sun C.-Y., Zhang Y.-Z. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. Fems Microbiol Lett. 2006;260:119–125. doi: 10.1111/j.1574-6968.2006.00316.x. [DOI] [PubMed] [Google Scholar]
- 27.Vinale F., Flematti G., Sivasithamparam K., Lorito M., Marra R., Skelton B.W., et al. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J Nat Prod. 2009;72(11):2032–2035. doi: 10.1021/np900548p. [DOI] [PubMed] [Google Scholar]
- 28.Zeilinger S., Gruber S., Bansal R., Mukherjee P.K. Secondary metabolism in Trichoderma – chemistry meets genomics. Fungal Biol Rev. 2016;30:74–90. doi: 10.1016/j.fbr.2016.05.001. [DOI] [Google Scholar]
- 29.Li Z.-J., Wang Y.-Z., Wang L.-R., Shi T.-Q., Sun X.-M., Huang H. Advanced strategies for the synthesis of terpenoids in Yarrowia lipolytica. J Agr Food Chem. 2021;69(8):2367–2381. doi: 10.1021/acs.jafc.1c00350. [DOI] [PubMed] [Google Scholar]
- 30.Abbas F., Ke Y., Yu R., Yue Y., Amanullah S., Jahangir M.M., et al. Volatile terpenoids: multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta. 2017;246(5):803–816. doi: 10.1007/s00425-017-2749-x. [DOI] [PubMed] [Google Scholar]
- 31.Chemler J.A., Koffas M.A.G. Metabolic engineering for plant natural product biosynthesis in microbes. Curr Opin Biotech. 2008;19:597–605. doi: 10.1016/j.copbio.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y., Li B.-Z., Liu D., Zhang L., Chen Y., Jia B., et al. Engineered biosynthesis of natural products in heterologous hosts. Chem Soc Rev. 2015 doi: 10.1002/chin.201537290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marienhagen J., Bott M. Metabolic engineering of microorganisms for the synthesis of plant natural products. J Biotechnol. 2013;163:166–178. doi: 10.1016/j.jbiotec.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Fujita T., Takaishi Y., Takeda Y., Fujiyama T., Nishi T.J.C., Bulletin P. Fungal metabolites. II. structural elucidation of minor metabolites, valinotricin, cyclonerodiol oxide, and epicyclonerodiol oxide, from Trichoderma polysporum. Chem Pharm Bull. 1984;32:4419–4425. doi: 10.1248/cpb.32.4419. [DOI] [Google Scholar]
- 35.Macías F.A., Varela R.M., Simonet A.M., Cutler H.G., Cutler S.J., Eden M.A., et al. Bioactive carotanes from Trichoderma virens. J Nat Prod. 2000;63(9):1197–1200. doi: 10.1021/np000121c. [DOI] [PubMed] [Google Scholar]
- 36.Xuan Q.-C., Huang R., Chen Y.-W., Miao C.-P., Ma K.-X., Wang T., et al. Cyclonerol derivatives from Trichoderma longibrachiatum YM311505. Nat Prod Commun. 2014;9:313–314. doi: 10.1177/1934578X1400900307. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Zhao J.-L., Liu J.-M., Chen R.-D., Xie K.-B., Chen D.-W., et al. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J Asian Nat Prod Res. 2017;19(7):651–658. doi: 10.1080/10286020.2016.1251908. [DOI] [PubMed] [Google Scholar]
- 38.Fang S.-T., Wang Y.-J., Ma X.-Y., Yin X.-L., Ji N.-Y. Two new sesquiterpenoids from the marine-sediment-derived fungus Trichoderma harzianum P1–4. Nat Prod Res. 2019;33:3127–3133. doi: 10.1080/14786419.2018.1522314. [DOI] [PubMed] [Google Scholar]
- 39.Song Y.-P., Liu X.-H., Shi Z.-Z., Miao F.-P., Fang S.-T., Ji N.-Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry. 2018;152:45–52. doi: 10.1016/j.phytochem.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Song Y.-P., Fang S.-T., Miao F.-P., Yin X.-L., Ji N.-Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J Nat Prod. 2018;81:2553–2559. doi: 10.1021/acs.jnatprod.8b00714. [DOI] [PubMed] [Google Scholar]
- 41.Song Y.-P., Miao F.-P., Liu X.-H., Yin X.-L., Ji N.-Y. Cyclonerane derivatives from the algicolous endophytic fungus Trichoderma asperellum A-YMD-9-2. Mar Drugs. 2019;17(5):252. doi: 10.3390/md17050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X.-H., Hou X.-L., Song Y.-P., Wang B.-G., Ji N.-Y. Cyclonerane sesquiterpenes and an isocoumarin derivative from the marine-alga-endophytic fungus Trichoderma citrinoviride A-WH-20-3. Fitoterapia. 2020;141 doi: 10.1016/j.fitote.2020.104469. [DOI] [PubMed] [Google Scholar]
- 43.Shi T., Shao C.-L., Liu Y., Zhao D.-L., Cao F., Fu X.-M., et al. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) Induced by chemical epigenetic manipulation. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma X.-Y., Song Y.-P., Shi Z.-Z., Ji N.-Y. Three sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma hamatum Z36–7. Phytochem Lett. 2021;43:98–102. doi: 10.1016/j.phytol.2021.03.020. [DOI] [Google Scholar]
- 45.Zou J.-X., Song Y.-P., Liu X.-H., Li X.-N., Ji N.-Y. Bisabolane, cadinane, and cyclonerane sesquiterpenes from an algicolous strain of Trichoderma asperelloides. Bioorg Chem. 2021;115 doi: 10.1016/j.bioorg.2021.105223. [DOI] [PubMed] [Google Scholar]
- 46.Shi Z.-Z., Fang S.-T., Miao F.-P., Yin X.-L., Ji N.-Y. Trichocarotins A-H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg Chem. 2018;81:319–325. doi: 10.1016/j.bioorg.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Song Y.-P., Miao F.-P., Liang X.-R., Yin X.-L., Ji N.-Y. Harziane and cadinane terpenoids from the alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Phytochem Lett. 2019;32:38–41. doi: 10.1016/j.phytol.2019.05.001. [DOI] [Google Scholar]
- 48.Shi X.-S., Meng L.-H., Li X.-M., Li X., Wang D.-J., Li H.-L., et al. Trichocadinins B-G: antimicrobial cadinane sesquiterpenes from Trichoderma virens QA-8, an endophytic fungus obtained from the medicinal plant artemisia argyi. J Nat Prod. 2019;82(9):2470–2476. doi: 10.1021/acs.jnatprod.9b00139. [DOI] [PubMed] [Google Scholar]
- 49.Rukachaisirikul V., Chinpha S., Phongpaichit S., Saikhwan N., Sakayaroj J., Preedanon S.J.P.L. Sesquiterpene and monoterpene derivatives from the soil-derived fungus Trichoderma reesei PSU-SPSF013. Phytochem Lett. 2019;30:124–129. doi: 10.1016/j.phytol.2019.01.023. [DOI] [Google Scholar]
- 50.Chen S., Li H., Chen Y., Li S., Xu J., Guo H., et al. Three new diterpenes and two new sesquiterpenoids from the endophytic fungus Trichoderma koningiopsis A729. Bioorg Chem. 2019;86:368–374. doi: 10.1016/j.bioorg.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Cui J., Shang R.-Y., Sun M., Li Y.-X., Liu H.-Y., Lin H.-W., et al. Trichodermaloids A-C, cadinane sesquiterpenes from a marine sponge symbiotic Trichoderma sp. SM16 Fungus Chem Biodivers. 2020;17(4) doi: 10.1002/cbdv.202000036. [DOI] [PubMed] [Google Scholar]
- 52.Song Y.-P., Shi X.-S., Wang B.-G., Ji N.-Y. Cadinane and carotane derivatives from the marine algicolous fungus Trichoderma virens RR-dl-6-8. Fitoterapia. 2020;146 doi: 10.1016/j.fitote.2020.104715. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe N., Yamagishi M., Mizutani T., Kondoh H., Omura S., Hanada K., et al. CAF-603: a new antifungal carotane sesquiterpene. Isolation and structure elucidation. J Nat Prod. 1990;53(5):1176–1181. doi: 10.1021/np50071a006. [DOI] [PubMed] [Google Scholar]
- 54.Lee S.H., Hensens O.D., Helms G.L., Liesch J.M., Zink D.L., Giacobbe R.A., et al. L-735,334, a novel sesquiterpenoid potassium channel-agonist from Trichoderma virens. J Nat Prod. 1995;58(12):1822–1828. doi: 10.1021/np50126a004. [DOI] [PubMed] [Google Scholar]
- 55.Shi X.-S., Song Y.-P., Meng L.-H., Yang S.-Q., Wang D.-J., Zhou X.-W., et al. Isolation and characterization of antibacterial carotane sesquiterpenes from artemisia argyi associated endophytic Trichoderma virens QA-8. Antibiotics. 2021;10(2):213. doi: 10.3390/antibiotics10020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Q., Tezuka Y., Hatanaka Y., Kikuchi T., Nishi A., Tubaki K.J.C., et al. Studies on metabolites of mycoparasitic fungi. III. new sesquiterpene alcohol from Trichoderma koningii. Chem Pharm Bull. 1995;43:1035–1038. doi: 10.1002/chin.199548201. [DOI] [PubMed] [Google Scholar]
- 57.Tezuka Y., Tasaki M., Huang Q., Hatanaka Y., Kikuchi T. ChemInform Abstract: Studies on Metabolites of Mycoparasitic Fungi. Part 6. 15-Hydroxyacorenone: New Acorane-Type Sesquiterpene from the Culture Broth of the Mycoparasitic Fungus Trichoderma harzianum. ChemInform. 1998;29(11):no–no. [Google Scholar]
- 58.Wu S.-H., Zhao L.-X., Chen Y.-W., Huang R., Miao C.-P., Wang J. Sesquiterpenoids from the endophytic fungus Trichoderma sp. PR-35 of paeonia delavayi. Chem Biodivers. 2011;8:1717–1723. doi: 10.1002/cbdv.201000236. [DOI] [PubMed] [Google Scholar]
- 59.Li G.-H., Yang Z.-S., Zhao P.-J., Zheng X., Luo S.-L., Sun R., et al. Three new acorane sesquiterpenes from Trichoderma sp. YMF102647 Phytochem Lett. 2011;4(2):86–88. [Google Scholar]
- 60.Shi Z.-Z., Liu X.-H., Song Y.-P., Yin X.-L., Ji N.-Y. Sesquiterpenoids and a steroid from the algicolous Trichoderma brevicompactum. Fitoterapia. 2021;153 doi: 10.1016/j.fitote.2021.104983. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Li X.-M., Yang S.-Q., Zhang F.-Z., Wang B.-G., Li H.-L., et al. Sesquiterpene and sorbicillinoid glycosides from the endophytic fungus Trichoderma longibrachiatum EN-586 derived from the marine red alga laurencia obtusa. Mar Drugs. 2022;20(3):177. doi: 10.3390/md20030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freeman G.G., Morrison R.I. Trichothecin; an antifungal metabolic product of Trichothecium roseum Link. Nature. 1948;162(4105):30. doi: 10.1038/162030a0. [DOI] [PubMed] [Google Scholar]
- 63.Godtfredsen W.O., Vangedal S., Undheim K., Sjöberg B., Pederson C.T., Larsen E. Trichodermin, a new sesquiterpene antibiotic. Acta Chem Scand. 1965;19:1088–1102. doi: 10.3891/acta.chem.scand.19-1088. [DOI] [PubMed] [Google Scholar]
- 64.Adams P.M., Hanson J.R. Sesquiterpenoid metabolites of Trichoderma polysporum and T. sporulosum. Phytochemistry. 1972;11(1):423. [Google Scholar]
- 65.Shi Z.-Z., Liu X.-H., Li X.-N., Ji N.-Y. Antifungal and antimicroalgal Trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2. J Agr Food Chem. 2020;68(52):15440–15448. doi: 10.1021/acs.jafc.0c05586. [DOI] [PubMed] [Google Scholar]
- 66.Du F.-Y., Ju G.-L., Xiao L., Zhou Y.-M., Wu X. Sesquiterpenes and cyclodepsipeptides from marine-derived fungus Trichoderma longibrachiatum and their antagonistic activities against soil-borne pathogens. Mar Drugs. 2020;18(3):165. doi: 10.3390/md18030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corley D.G., Miller-Wideman M., Durley R.C. Isolation and structure of harzianum A: a new trichothecene from Trichoderma harzianum. J Nat Prod. 1994;57:422–425. doi: 10.1021/np50105a019. [DOI] [PubMed] [Google Scholar]
- 68.Yamazaki H., Yagi A., Takahashi O., Yamaguchi Y., Saito A., Namikoshi M., et al. Antifungal trichothecene sesquiterpenes obtained from the culture broth of marine-derived Trichoderma cf. brevicompactum and their structure-activity relationship. Bioorg Med Chem Lett. 2020;30(17):127375. doi: 10.1016/j.bmcl.2020.127375. [DOI] [PubMed] [Google Scholar]
- 69.Song Y.-P., Miao F.-P., Fang S.-T., Yin X.-L., Ji N.-Y. Halogenated and nonhalogenated metabolites from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Mar Drugs. 2018;16(8):266. doi: 10.3390/md16080266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X.-H., Song Y.-P., Wang B.-G., Ji N.-Y. Sesquiterpenes and lipids from the algicolous fungus Trichoderma atroviride RR-dl-3-9. Phytochem Lett. 2021;45:6–12. doi: 10.1016/j.phytol.2021.07.003. [DOI] [Google Scholar]
- 71.Ding G., Chen A.-J., Lan J., Zhang H., Chen X., Liu X., et al. Sesquiterpenes and cyclopeptides from the endophytic fungus Trichoderma asperellum SAMUELS. LIECKF & NIRENBERG Chem Biodivers. 2012;9:1205–1212. doi: 10.1002/cbdv.201100185. [DOI] [PubMed] [Google Scholar]
- 72.Chen B., Li E., Liu L., Liao M., Zhu Z., Zhuang W., et al. Botryane sesquiterpenoids, cyclopentadepsipeptides, xanthones, and Trichothecenes from Trichoderma oligosporum. Planta Med. 2018;84(14):1055–1063. doi: 10.1055/a-0593-6030. [DOI] [PubMed] [Google Scholar]
- 73.Hirose A., Maeda H., Tonouchi A., Nehira T., Hashimoto M. Neomacrophorin I, II, and III, novel drimenyl cyclohexanes with hydroxylated butanoates from Trichoderma sp. 1212–03. Tetrahedron. 2014;45:1458–63. doi: 10.1002/chin.201430203.
- 74.Nishiyama M., Maeda H., Tonouchi A., Hashimoto M. Neomacrophorin and premacrophorin congeners from Trichoderma sp. Tetrahedron. 2019;75(22):2993–3000. [Google Scholar]
- 75.Itoh Y., Takahashi S., Arai M. Structure of gliocladic acid. J Antibiot. 1982;35:541–542. doi: 10.7164/antibiotics.35.541. [DOI] [PubMed] [Google Scholar]
- 76.Endo A., Hasum K., Hasumi K., Sakai K., Kanbe T. Specific inhibition of glyceraldehyde-3-phosphate dehydrogenase by koningic acid (heptelidic acid) J Antibiot. 1985;38(7):920–925. doi: 10.7164/antibiotics.38.920. [DOI] [PubMed] [Google Scholar]
- 77.Berg A., Wangun H.V., Nkengfack A.E., Schlegel B. Lignoren, a new sesquiterpenoid metabolite from Trichoderma lignorum HKI 0257. J Basic Microb. 2004;44:317–319. doi: 10.1002/jobm.200410383. [DOI] [PubMed] [Google Scholar]
- 78.Du X.-P., Li Y.-Y., Lu C.-H., Zheng Z.-H., Shen Y.-M. A novel sesquiterpene glucoside from Trichoderma sp.PT2. Nat Prod Res Dev. 2010;22:544–547. [Google Scholar]
- 79.Zheng C.-J., Sun P.-X., Jin G.-L., Qin L.-P. Sesquiterpenoids from Trichoderma atroviride, an endophytic fungus in cephalotaxus fortunei. Fitoterapia. 2011;82:1035–1038. doi: 10.1016/j.fitote.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L., Niaz S., Khan D., Wang Z., Zhu Y., Zhou H., et al. Induction of diverse bioactive secondary metabolites from the mangrove endophytic fungus Trichoderma sp. (Strain 307) by co-cultivation with Acinetobacter johnsonii (Strain B2) Mar Drugs. 2017;15(2):35. doi: 10.3390/md15020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li B., Li L., Peng Z., Liu D., Si L., Wang J., et al. Harzianoic acids A and B, new natural scaffolds with inhibitory effects against hepatitis C virus. Bioorgan Med Chem. 2019;27(3):560–567. doi: 10.1016/j.bmc.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 82.Zhou P., Wu Z., Tan D., Yang J., Zhou Q., Zeng F., et al. Atrichodermones A-C, three new secondary metabolites from the solid culture of an endophytic fungal strain. Trichoderma atroviride Fitoterapia. 2017;123:18–22. doi: 10.1016/j.fitote.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Kong Z., Jing R., Wu Y., Guo Y., Geng Y., Ji J., et al. Trichodermadiones A and B from the solid culture of Trichoderma atroviride S361, an endophytic fungus in Cephalotaxus fortunei. Fitoterapia. 2018;127:362–366. doi: 10.1016/j.fitote.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Jiao W.-H., Dewapriya P., Mohamed O., Khalil Z.G., Salim A.A., Lin H.-W., et al. Divirensols: sesquiterpene dimers from the Australian termite nest-derived fungus Trichoderma virens CMB-TN16. J Nat Prod. 2019;82(1):87–95. doi: 10.1021/acs.jnatprod.8b00746. [DOI] [PubMed] [Google Scholar]
- 85.Hu Z., Tao Y., Tao X., Su Q., Cai J., Qin C., et al. Sesquiterpenes with phytopathogenic fungi inhibitory activities from fungus Trichoderma virens from Litchi chinensis Sonn. J Agric Food Chem. 2019;67(38):10646–10652. doi: 10.1021/acs.jafc.9b04053. [DOI] [PubMed] [Google Scholar]
- 86.Liu H., Pu Y.-H., Ren J.-W., Li E.-W., Guo L.-X., Yin W.-B. Genetic dereplication driven discovery of a tricinoloniol acid biosynthetic pathway in Trichoderma hypoxylon. Org Biomol Chem. 2020;18:5344–5348. doi: 10.1039/d0ob01202e. [DOI] [PubMed] [Google Scholar]
- 87.Qin C., Hu Z., Xiong Y., Chen M., Li C., Ding W. A new sesquiterpene derivative from the mangrove endophytic fungus Trichoderma harzianum (Strain No. R1) Chem Nat Compd+ 2021;57(2):312–314. [Google Scholar]
- 88.Jiao W.-H., Salim A.A., Khalil Z.G., Dewapriya P., Lin H.-W., Butler M.S., et al. Trivirensols: selectively bacteriostatic sesquiterpene trimers from the Australian termite nest-derived fungus Trichoderma virens CMB-TN16. J Nat Prod. 2019;82(11):3165–3175. doi: 10.1021/acs.jnatprod.9b00760. [DOI] [PubMed] [Google Scholar]
- 89.Ghisalberti E.L., Hockless D.C.R., Rowland C., White A.H. Harziandione, a new class of diterpene from Trichoderma harzianum. J Nat Prod. 1992;55:1690–1694. doi: 10.1021/np50089a023. [DOI] [Google Scholar]
- 90.Mannina L., Segre A.L., Ritieni A., Fogliano V., Vinale F., Randazzo G., et al. A new fungal growth inhibitor from Trichoderma viride. Tetrahedron. 1997;53(9):3135–3144. [Google Scholar]
- 91.Miao F.-P., Liang X.-R., Yin X.-L., Wang G., Ji N.-Y. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org Lett. 2012;14:3815–3817. doi: 10.1021/ol3014717. [DOI] [PubMed] [Google Scholar]
- 92.Xie Z.-L., Li H.-J., Wang L.-Y., Liang W.-L., Liu W., Lan W.-J. Trichodermaerin, a new diterpenoid lactone from the marine fungus Trichoderma erinaceum associated with the sea star Acanthaster planci. Nat Prod Commun. 2013;8:67–68. doi: 10.1007/s13594-012-0094-1. [DOI] [PubMed] [Google Scholar]
- 93.Chantrapromma S., Jeerapong C., Phupong W., Quah C.K., Fun H.K. Trichodermaerin: a diterpene lactone from Trichoderma asperellum. Acta Crystallogr E. 2014;70:o408–o409. doi: 10.1107/s1600536814004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang M., Liu J.-M., Zhao J.-L., Li N., Chen R.-D., Xie K.-B., et al. Two new diterpenoids from the endophytic fungus Trichoderma sp. Xy24 isolated from mangrove plant Xylocarpus granatum. Chinese Chem Lett. 2016;27(6):957–960. [Google Scholar]
- 95.Zhao D.-L., Yang L.-J., Shi T., Wang C.-Y., Shao C.-L., Wang C.-Y. Potent phytotoxic harziane diterpenes from a soft coral-derived strain of the fungus Trichoderma harzianum XS-20090075. Sci Rep-uk. 2019;9:13345. doi: 10.1038/s41598-019-49778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou J.-X., Song Y.-P., Ji N.-Y. Deoxytrichodermaerin, a harziane lactone from the marine algicolous fungus Trichoderma longibrachiatum A-WH-20-2. Nat Prod Res. 2021;35:216–221. doi: 10.1080/14786419.2019.1622110. [DOI] [PubMed] [Google Scholar]
- 97.Zhang M., Liu J., Chen R., Zhao J., Xie K., Chen D., et al. Microbial oxidation of harzianone by Bacillus sp. IMM-006. Tetrahedron. 2017;73(51):7195–7199. [Google Scholar]
- 98.Li W.-Y., Liu Y., Lin Y.-T., Liu Y.-C., Guo K., Li X.-N., et al. Antibacterial harziane diterpenoids from a fungal symbiont Trichoderma atroviride isolated from Colquhounia coccinea var. mollis. Phytochemistry. 2020;170:112198. doi: 10.1016/j.phytochem.2019.112198. [DOI] [PubMed] [Google Scholar]
- 99.Li H., Liu X., Li X., Hu Z., Wang L. Novel harziane diterpenes from deep-sea sediment fungus Trichoderma sp. SCSIOW21 and their potential anti-inflammatory effects. Mar Drugs. 2021;19(12):689. doi: 10.3390/md19120689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zou J.-X., Song Y.-P., Zeng Z.-Q., Ji N.-Y. Proharziane and harziane derivatives from the marine algicolous fungus Trichoderma asperelloides RR-dl-6-11. J Nat Prod. 2021;84(4):1414–1419. doi: 10.1021/acs.jnatprod.1c00188. [DOI] [PubMed] [Google Scholar]
- 101.Sun P.-X., Zheng C.-J., Li W.-C., Jin G.-L., Huang F., Qin L.-P. Trichodermanin A, a novel diterpenoid from endophytic fungus culture. J Nat Med-tokyo. 2011;65:381–384. doi: 10.1007/s11418-010-0499-1. [DOI] [PubMed] [Google Scholar]
- 102.Yamamoto T., Izumi N., Ui H., Sueki A., Masuma R., Nonaka K., et al. Wickerols A and B: novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron. 2012;68(45):9267–9271. [Google Scholar]
- 103.Yamada T., Suzue M., Arai T., Kikuchi T., Tanaka R. Trichodermanins C-E, new diterpenes with a fused 6–5-6-6 ring system produced by a marine sponge-derived fungus. Mar Drugs. 2017;15(6):169. doi: 10.3390/md15060169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamada T., Fujii A., Kikuchi T. New diterpenes with a fused 6–5-6-6 ring system isolated from the marine sponge-derived fungus Trichoderma harzianum. Mar Drugs. 2019;17(8):480. doi: 10.3390/md17080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liang X.-R., Miao F.-P., Song Y.-P., Guo Z.-Y., Ji N.-Y. Trichocitrin, a new fusicoccane diterpene from the marine brown alga-endophytic fungus Trichoderma citrinoviride cf-27. Nat Prod Res. 2016;30:1605–1610. doi: 10.1080/14786419.2015.1126264. [DOI] [PubMed] [Google Scholar]
- 106.Liang X.-R., Miao F.-P., Song Y.-P., Liu X.-H., Ji N.-Y. Citrinovirin with a new norditerpene skeleton from the marine algicolous fungus Trichoderma citrinoviride. Bioorg Med Chem Lett. 2016;26:5029–5031. doi: 10.1016/j.bmcl.2016.08.093. [DOI] [PubMed] [Google Scholar]
- 107.Kusakabe K., Honmura Y., Uesugi S., Tonouchi A., Maeda H., Kimura K., et al. Neomacrophorin X, a [4.4.3]propellane-type meroterpenoid from Trichoderma sp. 1212–03. J Nat Prod. 2017;80(5):1484–1492. doi: 10.1021/acs.jnatprod.6b01177. [DOI] [PubMed] [Google Scholar]
- 108.Harman G.E., Howell C.R., Viterbo A., Chet I., Lorito M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 109.Martinez D., Berka R.M., Henrissat B., Saloheimo M., Arvas M., Baker S.E., et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina) Nat Biotechnol. 2008;26(5):553–560. doi: 10.1038/nbt1403. [DOI] [PubMed] [Google Scholar]
- 110.Kubicek C.P., Herrera-Estrella A., Seidl-Seiboth V., Martinez D.A., Druzhinina I.S., Thon M., et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011;12(4) doi: 10.1186/gb-2011-12-4-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kubicek C.P., Steindorff A.S., Chenthamara K., Manganiello G., Henrissat B., Zhang J., et al. Evolution and comparative genomics of the most common Trichoderma species. BMC Genomics. 2019;20(1) doi: 10.1186/s12864-019-5680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li W.-C., Lee C.-Y., Lan W.-H., Woo T.-T., Liu H.-C., Yeh H.-Y., et al. Trichoderma reesei rad51 tolerates mismatches in hybrid meiosis with diverse genome sequences. P Natl Acad Sci Usa. 2021;118(8) doi: 10.1073/pnas.2007192118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumari N., Chapter S.S. In: Sharma V., Salwan R., Al-Ani L.K.T., editors. Vol. 24. Academic Press; 2020. Secondary metabolites and lytic tool box of trichoderma and their role in plant health; pp. 305–320. (Molecular Aspects of Plant Beneficial Microbes in Agriculture). [Google Scholar]
- 114.Cardoza R.E., Malmierca M.G., Hermosa M.R., Alexander N.J., McCormick S.P., Proctor R.H., et al. Identification of loci and functional characterization of Trichothecene biosynthesis genes in filamentous fungi of the genus Trichoderma. Appl Environ Microb. 2011;77(14):4867–4877. doi: 10.1128/AEM.00595-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gutiérrez S., McCormick S.P., Cardoza R.E., Lindo L., Alexander N.J., Proctor R.H. New and Future Developments in Microbial Biotechnology and Bioengineering: Elsevier. 2020. Chapter 13 - Trichoderma trichothecenes: beyond their toxic effect; pp. 281–301. [Google Scholar]
- 116.Chen H., Mao L., Zhao N., Xia C., Liu J., Kubicek C., et al. TRI3 verification of acetylation of Trichodermol to Trichodermin in the plant endophyte. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.731425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lindo L., McCormick S.P., Cardoza R.E., Busman M., Alexander N.J., Proctor R.H., et al. Requirement of two acyltransferases for 4-O-acylation during biosynthesis of Harzianum A, an antifungal Trichothecene produced by Trichoderma arundinaceum. J Agr Food Chem. 2019;67(2):723–734. doi: 10.1021/acs.jafc.8b05564. [DOI] [PubMed] [Google Scholar]
- 118.Mukherjee M., Horwitz B.A., Sherkhane P.D., Hadar R., Mukherjee P.K. A secondary metabolite biosynthesis cluster in Trichoderma virens: evidence from analysis of genes underexpressed in a mutant defective in morphogenesis and antibiotic production. Curr Genet. 2006;50:193–202. doi: 10.1007/s00294-006-0075-0. [DOI] [PubMed] [Google Scholar]
- 119.Crutcher F.K., Parich A., Schuhmacher R., Mukherjee P.K., Zeilinger S., Kenerley C.M., et al. A putative terpene cyclase, vir4, is responsible for the biosynthesis of volatile terpene compounds in the biocontrol fungus Trichoderma virens. Fungal Genet Biol. 2013;56:67–77. doi: 10.1016/j.fgb.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 120.Taylor J.T., Mukherjee P.K., Puckhaber L.S., Dixit K., Igumenova T.I., Suh C., et al. Deletion of the Trichoderma virens NRPS, Tex7, induces accumulation of the anti-cancer compound heptelidic acid. Biochem Bioph Res Comm. 2020;529(3):672–677. doi: 10.1016/j.bbrc.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cardoza R.E., Hermosa M.R., Vizcaíno J.A., González F., Llobell A., Monte E., et al. Partial silencing of a hydroxy-methylglutaryl-CoA reductase-encoding gene in Trichoderma harzianum CECT 2413 results in a lower level of resistance to lovastatin and lower antifungal activity. Fungal Genet Biol. 2007;44(4):269–283. doi: 10.1016/j.fgb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 122.Cardoza R.E., Vizcaíno J.A., Hermosa M.R., Sousa S., González F.J., Llobell A., et al. Cloning and characterization of the erg1 gene of Trichoderma harzianum: effect of the erg1 silencing on ergosterol biosynthesis and resistance to terbinafine. Fungal Genet Biol. 2006;43(3):164–178. doi: 10.1016/j.fgb.2005.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.