Graphical abstract
Keywords: Bone loss, Sympathetic nervous system, YAP/TAZ, Piezo 1, Immobilization, Muscle-bone crosstalk
Highlights
-
•
The development of scientific research in acute bone loss after fracture was elaborated in a complete and clear perspective.
-
•
The possible mechanisms, including the new achievements in microgravity, muscle-bone crosstalk and the sympathetic nervous system, of acute bone loss after fracture were reviewed.
-
•
Several key molecules and current treatments as well as the potential targets and promising therapies were also briefly reviewed and compared.
-
•
The challenges we faced and the future directions are also summarized and elaborated.
Abstract
Background
Acute bone loss after fracture is associated with various effects on the complete recovery process and a risk of secondary fractures among patients. Studies have reported similarities in pathophysiological mechanisms involved in acute bone loss after fractures and osteoporosis. However, given the silence nature of bone loss and bone metabolism complexities, the actual underlying pathophysiological mechanisms have yet to be fully elucidated.
Aim of review
To elaborate the latest findings in basic research with a focus on acute bone loss after fracture. To briefly highlight potential therapeutic targets and current representative drugs. To arouse researchers' attention and discussion on acute bone loss after fracture.
Key scientific concepts of review
Bone loss after fracture is associated with immobilization, mechanical unloading, blood supply damage, sympathetic nerve regulation, and crosstalk between musculoskeletals among other factors. Current treatment strategies rely on regulation of osteoblasts and osteoclasts, therefore, there is a need to elucidate on the underlying mechanisms of acute bone loss after fractures to inform the development of efficacious and safe drugs. In addition, attention should be paid towards ensuring long-term skeletal health.
Introduction
Acute bone loss usually occurs between 1 and 2 years after fractures, especially the first 6 to 8 weeks after acute immobilization. At this stage, the patient’s bone mass sharply drops to the lowest level [1], [2]. Acute bone loss after fracture is a common occurrence, especially in osteoporotic patients [3], and this clinical challenge is closed related to osteoporosis with regards to pathogenesis and clinical treatment. Osteoporosis is an age-related metabolic disease [4] that is associated with loss of bone mass, destruction of bone microstructure and increased bone fragility [5]. Therefore, studies should aim at elucidating on the pathomechanisms of osteoporosis and bone loss after fractures.
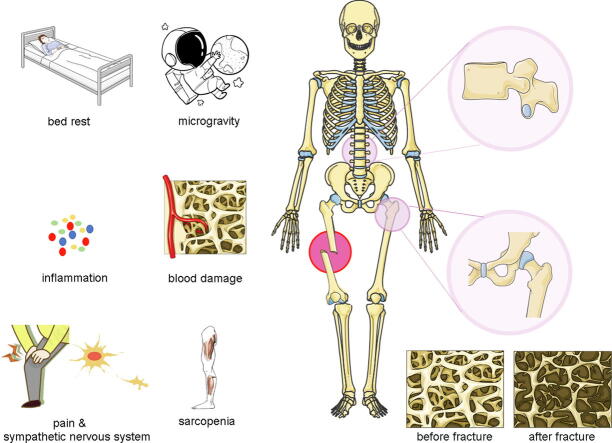
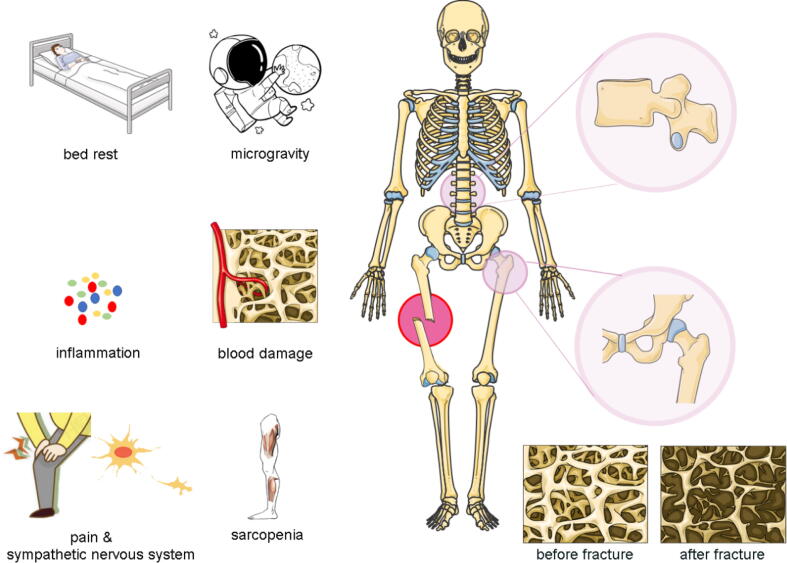
The most severe consequences of osteoporosis are fractures [6] while the most severe outcomes of bone loss after fractures are secondary fractures [7], [8], [9], [10]. Multiple fractures in elderly osteoporotic patients are a clinical challenge. Subsequent fractures in osteoporotic patients mostly occur in different parts [11], [12] and patients with fractures are also predisposed to varying degrees of osteoporosis [13], [14], [15]. Bone loss after fractures is an important reason for occurrence of re-fractures, however, the exact pathomechanisms have yet to be fully established. After fracture, bone remodeling increases in both skeletal and systemic regions adjacent to the fracture site. The increase in bone turnover near the fracture is referred to as the regional acceleratory phenomenon (RAP), while the increase in bone turnover in other regions is referred to as the systemic acceleratory phenomenon (SAP). Both RAP and SAP enhance bone formation by osteoblasts and bone resorption by osteoclasts, however, the rate of bone resorption usually exceeds the rate of bone formation, leading to bone loss in the fractured area and systemic regions. RAP and SAP have different performances in different regions and different fractures have different characteristics, even if it is the same form of bone loss phenomenon. For instance, bone loss of the cortex, subcortex, and trabecular compartment exhibit different features [16]. Accelerated bone remodeling after fracture supports the hypothesis that fractures cause long-term systemic osteopenia, however, etiologies of bone loss after fractures are complex. Generally, immediate immobilization after injury, reduction of activity, excitement of pain-related sympathetic nerves, inflammation, destruction of blood supply and sarcopenia are potential causes of bone loss [17]. These factors are shown in Fig. 1.
Fig. 1.
Immediate immobilization after injury, reduction of activity, excitement of pain-related sympathetic nerves, and destruction of blood supply are hypothesized as the causes of bone loss in an injured area. Reduced body exercise caused by bed rest or self-cultivation, and the related changes in the crosstalk of the musculoskeletal system, represent the main reasons for loss of bone mass in other parts of the body.
Studies on pathogenesis are useful for guiding the development of approaches for early prevention, precise identification and effective treatment of acute bone loss after fracture. In this review, we comprehensively discuss the pathophysiological mechanisms of acute bone loss after fractures to provide new ideas for clinical treatment and further research.
The article will mainly review the following:
1). Elucidating on the physiological and pathological mechanisms underlying acute bone loss after fractures.
2). Summarizing the differences in bone loss after fractures, osteoporosis and bone remodeling.
3). Highlighting the drugs and systemic treatment strategies used in management of bone fractures.
Pathophysiological mechanisms underlying acute bone loss after fractures
Immediate immobilization
It is recommended that patients should stop working and rest for a period of time. Sometimes, fractures in central and important parts require absolute bed rest, otherwise the healing and functional recovery times of the fractures may be prolonged, especially if these fractures occur in the spine, limbs or other load-bearing parts [18], [19]. Necessary limb immobilization is beneficial, although unscientific immobilization can lead to disuse muscle atrophy and acute loss of bone mass.
Cast or brace immobilization
Plaster fixation is one of the most common and primitive external fixation methods [20]. Technological advances in orthopaedics and industry have resulted in development of new technologies and materials, including polymer-made plaster and 3D-printed personality customized braces to replace plasters. Plaster fixation has several advantages, key among them being the use of a strong structure to support, fix, and maintain the correct bone shape as well as mechanical curve, thereby stabilizing the injured environment, and enhancing fracture healing [21].
Advances in medical technology coupled with improvement of perioperative management have enhanced the attitudes of patients and doctors towards surgery. Although the time taken for plaster fixation has significantly shortened, it is undeniable that plaster still have a place today for its low costs and wide applicability. Nevertheless, immediate immobilization of limbs may be a potential cause for acute bone loss after fractures.
The key molecular of unloading-induce bone loss
Wolff's law states that bone growth is affected by mechanical stimulation to optimize their structure [22], [23], [24]. The growth direction of bones, especially trabecular ones, has strong adaptability to the mechanical environment with the smallest mass and the largest mechanical efficiency. Generally, mechanical stress enhances bone strength by affecting collagen arrangement. Moreover, it upregulates the expressions of bone-derived cytokines, thereby improving the activity of osteoblasts and promoting cell proliferation as well as differentiation. Under normal circumstances, the human body is either in an upright state or in constant motion. Under gravity, human bones and muscles share the body’s weight. The musculoskeletal system bears most of the physiological gravity load, which continuously stimulates the body to strengthen the bones and muscles while promoting osteoblast mobilization and osteoclast activation. This complex and mechanically mediated bone turnover is essential for maintaining healthy bone metabolism and density [25], [26]. When a patient suffers from acute immobilization or plaster fixation due to fractures, trauma, hip replacement, knee replacement, spinal cord injury, and conservative treatment of intervertebral disc disease or orthopaedic traction, weightlessness (also known as microgravity or unloading) will occur, and this effect is magnified with increasing immobilization time [27].
Apart from the primary osteoporosis that is caused by aging and decreased gonadal functions, the microgravity environment has been implicated in occurrence of disuse and secondary osteoporosis. Moreover, the microgravity environment is associated with a high rate of bone loss, relative to that on the ground. It takes 6 months to 2 years to recover a patient’s bone mass after fractures, which is a long period [28], [29], [30].
Mechanoreceptors
Osteocytes comprise more than 90 % of all bone tissue cells in adult bones. Almost all bone matrix surfaces are covered by osteocytic bodies and processes. The complex pore system, comprising bone lacunas and tubules, is extensively distributed in the cortical bone. Interstitial fluid in bone lacuna and tubules provides nourishment, facilitates excretion of metabolites as well as other material exchange functions and plays an important role in force perception [31]. Osteocytes are the most important mechanical receptors. Application of an external force on bone cortex generates a slight deformation on bone matrix, causing pressure differences in tissue fluids, which triggers its flow into bone tubules [32]. These changes in fluid mechanics are detected by the sensor in osteocytes, which enhances its effects. This form of oscillating fluid flow (OFF) of tissue fluids plays a role in conversion of mechanical signals into biological signals and their amplification [33].
Based on the above theory, the dynamic process of solute convection and fluid flow in the lacunar-canalicular system (LCS) of the bone can be understood [34] by using a new experimental method. This method is based on fluorescence recovery after photobleaching (FRAP) as well as simultaneous mechanical loading and imaging, and successful quantification transport of a fluorescent tracer in bone LCS. Under the influence of microgravity, flow direction of the tissue fluid in bone tubules changes from the original longitudinal to lateral or disordered direction, which is attributed to the inability to be affected by gravity [35]. At this point, mechanical signals cannot be effectively converted into biochemical ones. Moreover, under microgravity conditions, mass transfer cannot reach the deep voids under microgravity conditions, a phenomenon that results in apoptosis of bone cells and bone loss. Notably, under microgravity, there is drastic drop in mechanical signals, and this cannot be compared with the situation under normal gravity.
During the transformation of fluid flow into cellular responses in bone cells, the primary cilia of bone cells gets connected and are regulated by downstream cell responses through a Ca2+ entry and polycystin-2 independent signaling mechanism [36]. This allows mechanical signals to alter cell activities via tissue-specific pathways, thereby regulating homeostasis across different tissues. Upon exposures to appropriate mechanical stimulation conditions, osteogenic-related genes are upregulated in human mesenchymal stem cells (hMSC), and this process requires primary cilia [26], [37]. Then, RNA-based inhibition of primary cilia downregulates bone formation-associated genes in cells, even in the presence of mechanical stimulation [27], [37], [38]. Under microgravity conditions, primary cilia on surfaces of osteoblasts gradually atrophy, become shorter and eventually disappear, whereas cell microtubules are depolymerized, and the cytoskeleton undergoes significant changes [38]. Additional evidences have further shown that primary cilia are closely associated with the cytoskeleton [39], [40]. These findings imply that primary cilia are potential interstitial sensors of gravity (under normal or microgravity conditions) and their disappearance results in microgravity-induced osteo-inhibition.
Hippo-YAP/TAZ
The Hippo signaling pathway regulates cell proliferation, apoptosis, and controls normal volume of tissues and organs as well as homeostasis of the internal environment [41]. Moreover, it is associated with other processes, including embryonic development, growth regulation, tumorigenesis as well as metastasis [42] and also plays a regulatory role in bone transduction related to mechanical load [43], [44]. The Yes-associated protein (YAP) and its analogue, transcriptional co-activator with PDZ binding motif (TAZ) in its kinase chain act as response molecules during mechanical signaling [45], [46]. They are subjected to extracellular mechanical stimulation and cytoskeleton activation under the influence of tension, where they are transferred from the cytoplasm to the nucleus to co-activate RUNX2 through transcription to regulate osteogenic gene expressions [47]. In addition, YAP/TAZ regulates the differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) [48]. Downregulated YAP/TAZ activities were found to promote adipogenesis while their upregulation resulted in induction of MSCs to enter osteogenesis [49], [50]. Furthermore, YAP/TAZ activity decreases under microgravity conditions, and this is accompanied by suppressed osteogenic differentiation of MSCs [38], [47].
During the translation of mechanical signals into biochemical ones, some cytokines are involved in growth regulation and initiation of cascade reactions. This process leads to activation of nuclear transcription factors that control gene transcription of downstream molecules. The complex crosstalk between mechanical transduction and growth factors should be investigated further. Bone morphogenetic protein 2 (BMP-2), which belongs to the transforming growth factor β (TGF-β) superfamily, is involved in promoting osteogenesis [51], [52]. The efficiency of BMP-2-induced osteogenic differentiation is highly dependent on cell shape, cytoskeletal tension, cell-ligand interactions and matrix stiffness [53], [54]. Notably, BMP-2 activates and induces the phosphorylation of the Smad family signal transduction protein (Smad1/5/8), thereby forming a heteromeric complex [55], [56]. These complexes are transferred to the nucleus where they bind target genes. However, initiation of BMP-2-induced Smad signaling is not associated with cytoskeletal tension.
In contrast, YAP/TAZ is regulated by cytoskeletal tension [57]. YAP/TAZ is mainly localized in the nucleus of cells cultured on a stiff surface and in the cytoplasm of those cultured on a soft surface [46], [58]. This indicates that YAP/TAZ is an important molecular sensor for cytoskeletal tension. Moreover, this shuttle to and from the nucleus does not depend on the BMP-2 signaling pathway. Complete osteogenic differentiation requires cytoskeletal tension-induced accumulation of YAP/TAZ in the nucleus, while translocation of YAP/TAZ in the cell enhances the activation of BMP-2 induced osteogenic genes. These two signaling pathways synergistically interact to enhance the expressions of osteogenic-related genes.
Microgravity interferes with f-actin and inhibits nuclear translocation of TAZ, thereby suppressing the osteogenic differentiation ability of BMSCs [59]. However, the addition of lysophosphatidic acid (LPA) restores the positioning of TAZ in the nucleus and promotes the secretion of osteogenic indicators. RNA-based inhibition of TAZ expression does not restore osteogenic differentiation of bone marrow mesenchymal stem cells under microgravity, even after LPA addition. This indicates that LPA can restore and retain the osteogenic differentiation ability of bone marrow mesenchymal stem cells in microgravity via the f-actin-TAZ signaling pathway. LPA has been shown to effectively reverse microgravity-induced decrease in osteogenic differentiation of MSCs via the Rock-TAZ signaling pathway [48]. Moreover, daily applications of low-intensity vibrations (LIV) can rescue the microgravity-induced decrease in nuclear YAP levels [60]. In addition, LPA treatment has also been shown to increase nuclear YAP levels, while daily applications of LIV alleviated microgravity-induced LPA-induced YAP nuclear entry.
Piezo1
Piezo1/2 protein is a novel type of mechanically sensitive ion channel protein that is closely associated with mechanical stress stimulation signals [61], [62]. Piezo1 is widely expressed in various tissues and regulates the development of vital organs and important physiological processes. Piezo2 is highly expressed in neurons and plays an important role in regulating the mechanical transduction of central nervous system neurons and dorsal root ganglion neurons [63], [64]. This ion channel can be directly activated by mechanical stress stimulation. The channel can also non-selectively pass through divalent ions, namely Ca2+, Mg2+, Mn2+ and Ba2+, as well as monovalent alkaline ions, including K+, Na+, Cs+ and Li+, among others, thereby generating local transmembrane ion flux currents [65], [66]. Studies on orthopaedic diseases revealed that the Piezo1 protein is not only expressed in chondrocytes, but is also associated with osteoarthritis and bone loss [67]. Conditional absence of piezo1 in osteoblasts and osteocytes was associated with reduced cortical thickness and cancellous bone volume. Conversely, applications of piezo1 agonists significantly increased bone mass. In addition, piezo1 is associated with subchondral osteogenesis [68]. For instance, piezo1 knockout mice were found to exhibit skeletal hypoplasia and trabecular insufficiency, with adults manifesting symptoms such as osteoporosis and multiple fractures.
Microgravity changes have been implicated in induction of dynamic changes of calcium signal transduction in cells. The intracellular calcium signaling pathway, a secondary messenger that activates various cellular functions, is one of the earliest events in mechanical transduction. Piezo1 transduces mechanical signals induced by low-intensity pulsed ultrasounds into intracellular calcium ions, while an influx of Ca2+ acts as a second messenger to activate ERK1/2 phosphorylation and perinuclear f-actin filament polymerization, thereby regulating MC3T3-E1 cell proliferations [69]. Moreover, the Ca2+ signal plays an important role in regulation of RUNX2 protein stability [56]. RUNX2 plays an essential role in differentiation of mesenchymal cells into osteoblasts as well as in blocking osteoblast differentiation into adipocytes and chondrocytes. RUNX2 stability is regulated by precision and complexity, of which the TMCO1–CaMKII–HDAC4 axis is regulated in a local Ca2+ signal-dependent manner [70]. Bone samples from osteoporotic patients and mice exhibited significantly low levels of TMCO1, relative to healthy individuals. In addition, a lack of TMCO1 has been associated with impaired endoplasmic reticulum Ca2+ homeostasis, resulting in endoplasmic reticulum calcium overload, thereby affecting bone formation.
Piezo1 regulates the expressions of Wnt1 and other osteogenic-related genes in bone cells via YAP1 and TAZ [69]. Piezo1 activation can mimic the effects of mechanical stimulation in bone cells to increase bone mass. In response to mechanical stimulation, Piezo1 in osteoblasts can also regulate the expressions of different proteins in the bone matrix, including several collagens, by regulating the YAP signaling pathway. In turn, these collagen subtypes regulate osteoclast differentiation and coordinate the osteoblast-osteoclast crosstalk in the bones for controlled bone homeostasis. Once a limb breaks, the level of BMSCs that could be used for both muscle and bone regeneration decreases, while their activities are further suppressed [71], [72]. These outcomes limit the sources of osteoblasts, resulting in reduced development of new bones and exacerbating the extent of bone loss.
Considering that the current in vivo animal experiment designs involve unilateral or bilateral long bone fractures, the fractures faced in clinical work are not only simple transverse fractures, but more complex comminuted fractures or multiple fractures. The fractures involve injuries that require the patient to stay in bed for a longer time or to be completely non-weight-bearing. Therefore, the current experimental results are not sufficient to deduce acute bone loss after fractures. More radical views seem to be biased towards the effects of microgravity.
Muscle injury
The musculoskeletal system is an important part of the locomotion system and a crucial factor in many exercise-related physiological and pathological processes, including assisting in breathing, protecting important internal organs and endocrine regulation [73], [74]. Apart from being involved in the body's normal daily physiological activities, as a whole system, bones and muscles have a sophisticated and complex crosstalk [25], [75]. This not only refers to mechanical conduction [76], but also through effective growth factors. Interactions between bones and muscles regulate various processes, including fluid circulation, cellular and molecular processes, as well as mechanical transmission [25], [75]. This crosstalk is both bilateral and direct, that is, from muscles to bones and vice versa. However, due to the involvement of different physiological activities, participation of various cells and small biologically active molecules as well as soft tissues, ligaments, nerves, blood vessels and other tissues outside the musculoskeletal system, this crosstalk is more complicated and sophisticated.
Biomechanical factors
Under physiological conditions, the skeletal muscle is always in a state of dynamic equilibrium, thus, maintenance of its homeostasis requires synergistic actions involving many aspects, key among them being mechanical homeostasis. The bundle structure formed by fast-contracting muscle fibers (type II fibers), slow muscle fibers (type I fibers), collagen and motor neurons, enhances its sensitivity to mechanical loads, including fluid shear force, pulling force, and gravity among others. In addition, normal gravity physical exercises can effectively increase the mechanical load of skeletal muscles, thereby strengthening them. This phenomenon is specifically manifested in improved quality of skeletal muscles and construction of its fiber bundles [77]. In contrast, a decreased mechanical load causes a reduction in mass and volume of muscle fibers. In addition, muscle atrophy occurs before bone changes [78], [79], leading to a tilt in bone balance towards enhanced bone resorption. This occurrence is evident when analyzing bone health of astronaut groups and long-term bedridden patients.
Long-term bed rest or lack of activity is associated with the loss of body proteins, which is mainly attributed to reduced synthesis in skeletal muscles and the whole body [80], [81]. The muscle is attached to the muscle insert in the bone. Mechanical stimuli, including compression, stretching, bending, torsion and shear generated by muscle movements directly transmit the mechanical load onto the bones, thereby affecting bone tissue microstructure and mass. When acting on the strain generated by the bone, these forces have important stimulating effects on osteoblasts, thereby promoting the continuous formation of osteoblasts in situ to increase bone mass. When skeletal muscle contraction forces decreases, the mechanical force or the load exerted on the bones also decreases, and once this stimulation is weakened, it can reduce bone formation, accelerate bone resorption and lead to bone loss or osteoporosis. The muscle contraction strength is affected by many factors, including myogenic, neurogenic, and hormonal effects. An increase in age or occurrence of disuse muscle atrophy due to limb fixation markedly reduces the diameter and number of muscle fibers. These muscle fibers are replaced by fat and collagen, a phenomenon that results in reduced muscle elasticity and volume [82], [83]. In addition, a decrease in activities of enzymes that regulate muscle contraction, coupled with a reduction in the number of motor neurons, eventually reduces muscle strength and activity speed [84], [85]. These factors are shown in Fig. 2.
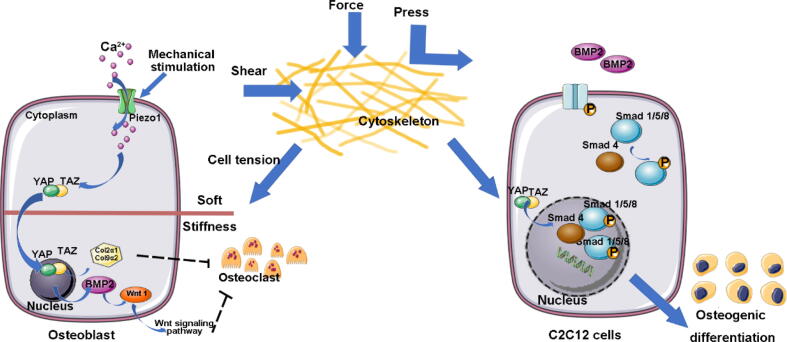
Fig. 2.
YAP/TAZ is an important molecular sensor for cytoskeletal tension. Mechanoreception protein piezo1 may control the expression of Wnt1 and other osteogenic-related genes through YAP1 and TAZ. And the translocation of YAP/TAZ in the cell enhances activation of BMP-2 induced osteogenic genes. These two signaling pathways synergistically interact to enhance expression of osteogenic-related genes. Besides, BMP-2 was found to activate and induce phosphorylation of the Smad family signal transduction protein Smad1/5/8, thereby forming a heteromeric complex, which will be transferred to the nucleus where they bind to target genes.
Biochemical factors
Various bone-derived factors and myogenic factors are involved in the crosstalk between muscles and the bone, including interleukin-6 (IL-6), irisin, pro-prostaglandin E2 (PGE2), insulin-like growth factor-1 (IGF-1), mechanical growth factors (MGF), vascular endothelial growth factors (VEGF), hepatocyte growth factors (HGF), and transformation growth factors (TGF-β). There are many comparable effects associated with these cytokines, as well as their own specific effects, which are mainly reflected in muscle cell proliferation and differentiation, muscle cell repair, bone balance, and fracture repairs. The functions of specific cytokines are summarized in Table 1.
Table 1.
The role of myogenic cytokines in bone-muscle crosstalk.
| Growth factors | Introduction | Origin | Function | Ref. |
|---|---|---|---|---|
| BDNF | A protein with neurotrophic effects, and it has the highest content in the central nervous system. | muscle | 1. It plays an important role in the growth and development, survival, differentiation, and differentiation of neurons. 2. As a paracrine factor, BDNF could regulate the differentiation of satellite cells into slow vs fast myofibers. 3. Affect the synaptic connections between neurons and muscles. |
[209] |
| CXCL-1 | A small molecular weight cytokine that belongs to the CXC chemokine family. CXCL-1 is expressed by macrophages, neutrophils and epithelial cells, and has neutrophil chemotactic activity. | muscle | CXCL-1 overexpression increases muscular fatty acid oxidation with concomitant attenuation of diet-induced fat accumulation in the adipose tissue | [210], [211] |
| FAM5C |
It was originally identified in mouse brain as a gene that is induced by BMP and retinoic acid signaling | muscle | FAM5C enhances osteoblast differentiation | [212] |
| FGF-2 | a secreted protein and belongs to the heparin-binding growth factors family. | muscle | FGF-2 not only stimulates muscle growth but also promotes intramuscular adipogenesis. | [213] |
| IGF-1 | A peptide material necessary for the physiological effects of growth hormone, and is also called somatomedins. | muscle | 1. Improve the ability of muscle cell proliferation and increase muscle abundance 2. Improve protein synthesis efficiency 3. Improve the regeneration of nerve |
[214], [215] |
| IL-5 | inflammatory cytokines | muscle | IL-5 induces local accumulation of eosinophils and their release of major basic protein. The secreted proteins adhere to the muscle fiber membrane, resulting in muscle damage. | [216] |
| IL-6 | inflammatory cytokines | muscle | IL-6 must signal in osteoblasts to favor osteoclast differentiation and the release of bioactive osteocalcin in the general circulation to increase exercise capacity. | [217] |
| IL-7 | inflammatory cytokines | muscle | IL-7 is a novel myokine regulated both in vitro and in vivo, and it may play a role in the regulation of muscle cell development. | [218] |
| IL-15 | inflammatory cytokines | muscle | IL-15Rα has a role in defining the phenotype of fast skeletal muscles in vivo | [219] |
| Irisin | A protein produced by muscles after exercise. | muscle | 1.Irisin induces the expression of pro-myogenic and exercise response genes in myotubes. 2. Irisin increases myogenic differentiation and myoblast fusion by activating IL6 signaling. 3. Irisin rescues the loss of skeletal muscle mass after denervation through the activation of satellite cells and the enhancement of protein synthesis and reduction of protein degradation. |
[220] |
| LIF | A cytokine with multiple functions, but its most important application is to maintain the undifferentiated state of mouse embryonic stem cells | muscle | LIF exerts its effect locally, stimulating the proliferation of muscle satellite cells and participating in muscle hypertrophy and regeneration to promote muscle adaptation to exercise. | [221] |
| Myostatin | TGF-β family | muscle | A key negative regulator of muscle repair and growth. | [222] |
| NT-3 | Neurotrophin-3 (NT-3) is a member of the NGF family of neurotrophic factors. | muscle | NT-3 enhances axon regeneration and has potential clinical effects in preventing muscle atrophy after nerve injury. | [223] |
| PGE2 | inflammatory cytokine | muscle | 1. PGE2 directly targets muscle-specific stem cells (MuSC) through the EP4 receptor, leading to the expansion of MuSC. 2. PGE2 can enhance muscle regeneration and accelerate the repair of damaged muscles. |
[224] |
| TGF-β1 | TGFβ belongs to the TGF-β superfamily and works by binding to the type 2 TGF-β receptor (TGFBR2). |
muscle | 1. Participate in embryogenesis and cell differentiation and apoptosis. 2. TGFβ1 is recognized as critical negative regulator of skeletal muscle repair. |
[225] |
| BMP-2 | BMP2 belongs to the transforming growth factor-β family. | bone | BMP-2 plays an important role in the progress of bone development, bone diseases, and the repair of bone and joint injury. | [52] |
| DMP-1 | A non-collagenous matrix protein found in dentine and bone. | bone | An acidic non-collagen protein necessary for the biomineralization of bone, cartilage, enamel, cementum and dentin. | [226] |
| DKK-1 | DKK is a group of secreted glycoproteins, which are inhibitory molecules of Wnt pathway. | Chondrocytes, bone | Dkk-1 blocks osteoblast differentiation, induces sclerostin expression and leads to osteocyte death. | [227] |
| FGF-23 | A kind of fibroblast growth factor. | bone | FGF-23 participate in the homeostasis of vitamin D and phosphate. | [228] |
| HGF | hepatocyte growth factor | bone | HGF promotes the differentiation of MSCs into osteoblasts, and plays a role in bone development, health and repair. | [229] |
| IGF-1 | An active protein peptide substance | bone | IGF-1 promotes bone anabolism and maintains its normal structure and function. | [230] |
| MEPE | A non-collagenous phosphorylated glycoprotein of extracellular matrix, mainly expressed in bone tissue, dental tissue and renal proximal tubules | bone | Regulate phosphate metabolism and bone mineralization. | [231] |
| MGF | a splicing variant of insulin-like growth factor 1 | bone | mechanical stimuli influence the physiological responses of osteoblasts by increasing the expression of MGF, which is regulated by splicing factors. | [232] |
| NO | A colorless, odorless gas that is hardly soluble in water | bone | Bone cells can promote the production of NO after being stimulated by inflammatory cytokines and mechanical stress. NO directly facilitates osteoblastic differentiation. | [233] |
| OCN | It belongs to non-collagen acid glycoprotein and is a vitamin K-dependent calcium binding protein. | Bone, osteoblast | 1. Osteocalcin (OCN) secreted by osteoblasts regulates systemic glucose and energy metabolism, reproduction, and cognition. 2. Promote bone formation and regulate bone metabolism. |
[234] |
| OPG | A member of the tumor necrosis factor receptor family | Bone, osteoblast | 1. Markers of bone turnover. 2. Promote bone formation. |
[235] |
| PGE2 | inflammatory cytokine | Bone, osteoblast | PGE2 mediates sensory nerve to control bone homeostasis and promote regeneration. | [236] |
| RANKL | RANKL belongs to the tumor necrosis factor family. | Bone, osteoblast | The functions of RANKL-RANK forward and reverse signaling in the regulation of osteoblast differentiation and bone formation. | [237] |
| SOST | A secreted protein | Bone, osteoblast | A negative regulator of bone formation. | [238] |
| TGF-β | A number of TGF-β superfamily that regulate cell growth and differentiation | bone | Promote cartilage and bone repair | [239] |
| VEGF | A highly specific vascular endothelial cell growth factor | Bone, osteoblast | VEGF is important for bone development, postnatal bone homeostasis and bone repair and regeneration. | [240] |
| Wnt3a | wnt3a is the main ligand of the canonical wnt signaling pathway | Bone, osteoblast | 1. Canonical Wnt signaling is central to central to normal bone homeostasis. 2. Wnt3a can stimulate bone regeneration and inhibit the growth of multiple myeloma. |
[241] |
| Abbreviation | ||||
| Brain-derived neurotrophic factor,BDNF Bone morphogenetic protein 2, BMP-2 Bone gammacarboxyglutamate protein, BGLAP Dentin Matrix Acidic Phosphoprotein 1, DMP-1 Dickkopf-1, DKK-1 Family with sequence similarity 5, member C, FAM5C Fibroblast Growth Factor-2, FGF2 Fibroblast growth factor-23, FGF23 Hepatocyte growth factor, HGF Interleukin-6, IL-6 Interleukin-7, IL-7 Interleukin-15, IL-15 Insulin-like growth factor-1, IGF-1 Leukemia Inhibitory Factor, LIF Mechano growth factor, MGF Matrix extracellular phosphoglycoprotein, MEPE Osteocalcin, OCN Osteoprotegerin,OPG Prostaglandin E2, PGE2 Receptor activator of nuclear factor-κ B ligand, RANKL Sclerostin, SOST Transforming growth factor β, TGF-β Vascular endothelial growth factor, VEGF | ||||
Blood supply
The bone is a highly vascularized tissue [86], [87], [88]. Blood circulation and regeneration of blood vessels in the bone are essential for bone development, growth, remodeling, and repair. Blood circulation provides a steady stream of oxygen and nutrients to the bones as well as calcium and phosphate for bone mineralization [89]. Moreover, blood is the medium in paracrine circulation between bones and muscles. The damage to the perforator vessel is inevitable during the injury. However, due to network distribution of capillaries, which has a sufficient compensatory capacity, the impact of vascular damage on fracture healing is limited. Besides, when severe open injury occurs, especially with large blood vessel damage or large segmental bone defects [90], bone regeneration will be affected [91], [92]. The negative consequence of poor local blood circulation is that it cannot meet the material needs for repair and healing of injured parts. Therefore, non-union of fractures or tissue necrosis may occur in severe cases.
In summary, blood vessel formation and bone formation affect each other, be it in maintenance of bone homeostasis or in the process of fracture healing. Angiogenesis indicates the possibility of bone regeneration, and blood vessel formation disorders lead to bone regeneration disorders.
VEGF is the most important growth factor in promotion of blood vessel growth [93]. It is also a bone metabolism regulator that is mainly involved in bone formation by promoting endothelial cell proliferation and blood vessel formation. In the skeletal microenvironment, VEGF is regulated by ATF4 and it affects bone angiogenesis via endothelial sprouting from embryonic metatarsals [94]. When the bone is unloaded, VEGF secretion in osteoblasts significantly increases to promote angiogenesis and resist the negative effects of bone unloading [95]. The crosstalk between osteoblasts and endothelial cells is an important pathophysiological mechanism during osteoporosis. However, excess VEGF enhances the recruitment of TRAP and cathepsin K-positive osteoclasts [96], which may involve VEGF-C, a lymphatic growth factor, as an autocrine factor that regulates osteoclast activities [97], [98]. A lack of VEGF reduces bone mass [99], [100]. Osteoblast-secreted VEGF levels among the elderly are low, and are associated with aging-related osteoporosis [101].
During fracture healing, VEGF is at the center of vascularization at the fracture site. VEGF-A from early osteoblasts (Osx+) is essential for rapid formation of periosteal blood vessels and formation of woven bones after bone injury [102]. VEGF-A from other cells is necessary for intramedullary angiogenesis in largest cortical defects at the time of injury. In chondrocytes, FOXO1 directly binds the VEGF-A promoter and stimulates the transcriptional activities of VEGF-A [103]. This suggests that it is involved in regulation of angiogenesis in the cartilage during bone fracture healing. The SDF1/CXCR4 related pathways regulate the recruitment of endothelial progenitor cells (EPC) in tissues, which is an important prerequisite for angiogenesis at fracture sites [104].
During fracture healing, there is enhanced crosstalk between important signaling pathways, including those involving VEGF and BMP2 [105]. For instance, biglycan is significantly involved in regulation of bone homeostasis. Biglycan regulates bone cell functions by directly binding BMP2 and regulating its ability to stimulate downstream signal transduction, thereby promoting bone cell differentiation [106]. Besides, it can bind VEGF and stimulate its expressions via the TLR pathway [107]. However, specific mechanisms through which it regulates downstream of the VEGF pathway have not yet been elucidated. VEGF was found to be markedly suppressed in the callus when compared between biglycan conditionally knocked out mice with the control group seven days after the fracture [108]. At 14 days after fracture, the newly formed cartilage and braided bone in the biglycan-KO group were compared. There was less collagen formation while the degree of vascularization was low, which means the injured bone repair capacity. Expressions of NTs and their receptors were upregulated during the bone repair process. Additional treatment with recombinant NT-3 increased the mRNA levels of BMP-2, VEGF, and expressions of the endothelial cell marker (CD31) at the injury site, which promoted injury repair [109]. Tissue vascularization promotes bone repair. Therefore, vascularization is vital in fracture healing, and is affected by many signaling pathways as well as biomolecules.
Special mechanical changes
In physics, stress is defined as the internal force that interacts with various parts of an object when an object deforms due to external factors. This internal force is aimed at restoring the object from its deformed position to its pre-deformed position [110], [111]. During fracture treatment, stress is involved in repair of fracture trauma and a reasonable use of stress can promote fracture healing. Inappropriate mechanical forces can delay fracture healing.
Stress shielding effects
The stress shielding effect suggests that the load and redistribution of stress as well as strain will occur when two or more components with elastic modulus form a loading system [112], [113]. The component with higher elastic modulus will bear more load, whereas the material with a low elastic modulus bears less load, thus, there is less deformation. In fracture healing, this phenomenon is reflected in stress shunt of the fixing material to the bone. A very strong internal plate fixation will compress the blood supply under the plate, compress the periosteum, and reduce the force pressed on the bone, resulting in thinning of the cortical bone, decreased bone density, bone structure disorders, and local osteoporosis on the fixed side [114], [115]. Comparable challenges have received attention in strong external fixation, artificial prosthesis implantation, and spinal pedicle screw internal fixation. Although the stress shielding effect directly hinders callus formation [116], [117], [118], its biological effects and its effects on fracture healing have yet to be fully established.
Improvement of the internal fixation material [119], [120], geometric appearance of the steel plate [121], distribution and angle of the nail hole [122], and steel plate elasticity [123] are used to reduce stress shielding effects during steel plate fixation. Moreover, finite element simulation analysis and simulation experiments of in vitro specimens on multi-dimensional biomechanical machines significantly increased the application potential of virtual technologies in assessing changes in biomechanics [88], [124].
Special vertebral multiple fractures
Osteoporotic vertebral fractures have different characteristics. Incidences of re-fractures after surgery are extremely high in patients with osteoporotic vertebral fractures (OVF) [125]. The most important factor is the progress of osteoporosis [126], and the direct factor is the poor rigidity and strength of the vertebral body and adjacent vertebrae. Spinal fractures are often accompanied by varying degrees of kyphosis and changes in physiological curvatures of the spine, which makes the body's center of gravity to move forward. In addition, when stress of the anterior and middle columns of the vertebral body increases, vertebral fractures are more likely to occur [127].
The thoracolumbar spine is a segment that migrates from the thoracic vertebra with less mobility to the lumbar vertebra with greater mobility [128], thus, biomechanics stress is relatively concentrated in thoracolumbar spine. Spinal fractures often occur at the thoracolumbar vertebra, especially at T11 to L2 segments. The injured vertebrae became extremely hard after surgery, leading to changes in spine biomechanics. This change is also considered to involve natural progression of degenerative lumbar spine disease, thereby accelerating intervertebral disc degeneration, intervertebral space stenosis, and vertebral body instability. This leads to changes in physiological curvatures and biomechanics of the spine, and ultimately affects the spine and fracture [129].
Neuromodulation
The central and peripheral nervous systems are jointly involved in regulation of bone development, bone metabolism, bone repair, and bone regeneration via peripheral nerve synapses and secretion of neurotransmitters. The relationship between the sympathetic nervous system and the skeleton has been studied. The sensory nervous system is associated with pain perception. A limited number of studies have reported on the association between the parasympathetic nervous system and bone metabolism. Nerve fibers in the bone tissue contain a large number of neuropeptides, including calcitonin gene-related peptide (CGRP), neuropeptide Y (NPY), substance P (SP), vasoactive and intestinal peptides (VIP). Among them, SP and CGRP exist in the sensory nervous system, whereas VIP and NPY exist in the sympathetic nervous system. Various neuropeptide receptors are distributed on human osteoblasts and osteoclasts [130]. The peripheral nervous system binds specific receptors on osteocytes and acts on specific signaling pathways to regulate osteoblasts and osteoclasts, including their proliferation, differentiation, calcification, and reabsorption pathways to achieve a balance between bone resorption and reconstruction [131], [132], [133].
Nervous system distribution
Regulation of bone mass may depend on nerve fibers that are distributed in the periosteum and bone tissue. Periosteum structures differ depending on anatomy and age. Traditionally, the periosteum is often divided into a superficial fibrous layer and a deep cambium layer, with no clear boundaries between the layers. In addition to thick collagen fiber bundles that fix the periosteum and ligaments, the periosteum is rich in blood vessels and cells, including osteoprogenitor cells, osteoblasts, osteoclasts, and vascular endothelial cells. The periosteum is also rich in innervation, including myelinated and unmyelinated sensory nerve fibers, mainly unmyelinated nerve fibers. Sympathetic nerves in the bone, especially noradrenergic nerves, are mainly distributed at the location near blood vessels, and the most abundant are in areas with high osteogenic activities, such as near the epiphyseal growth plate. Mature osteoblasts and osteoclasts can form synapses with appropriate axons. The extension of sympathetic nerve axons to osteoblasts and osteoclasts is subject to dynamic neuroregulation of local bone metabolism [134].
The effects of sympathetic nervous system
Under physiological and pathological conditions, the sympathetic nervous system can directly or indirectly affect bone remodeling, and its functional disorders can also affect bone metabolism to result in various bone diseases. When the sympathetic nervous system is excited, the adrenal medulla secretes epinephrine (EP) and norepinephrine (NE). NE is the most common transmitter released by the sympathetic postganglionic fibers. There are various adrenergic receptors on membranes of human osteoblasts and osteoclasts. These receptors are divided into two: α-adrenergic receptors (α-ARs) and β-adrenergic receptors (β-ARs). The β-ARs are closely associated with calcaneal metabolism. When the sympathetic nerve is excited, plasma norepinephrine levels increase, osteogenesis is inhibited, and bone resorption is promoted through β-adrenergic receptors in the bone to reduce bone mass. The effect of sympathetic nervous system on bone metabolism are briefly outlined in Fig. 3.
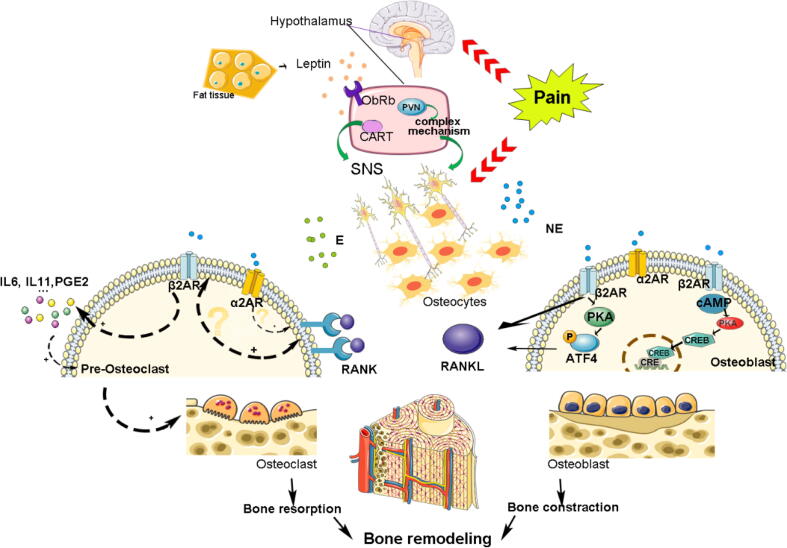
Fig. 3.
As the target organ of the nervous system, the bone is innervated by the sympathetic nervous system. Specifically, the brainstem and hypothalamus integrate internal and external signals, and noradrenergic fibers are present in the periosteum, bone, and bone marrow. The extension of sympathetic nerve axons to osteoblasts and osteoclasts is the anatomical basis for the dynamic neuroregulation of bone metabolism. After fracture, the enhancement of sympathetic tone, caused by fracture pain, increases the level of norepinephrine, which will bind to specific receptors (such as β2R) on osteoblasts or pre-osteoclast. The osteoblasts differentiation and osteoclasts maturation were regulated though specific signaling pathways to achieve the balance between bone resorption and bone reconstruction.
A previous study found that sympathetic excitability of an astronauts’ muscles was higher after a flight test than before the flight, and serum NE levels were also higher after the flight test than before [135]. In addition, the peripheral sympathetic nervous system in bed nucleus of stria terminalis -ventromedial hypothalamus (BNST-VMH) neural circuitry is involved in regulation of stress-induced bone loss [136]. The bone marrow adipose tissue is a fat depot and an endocrine tissue, and is also regulated by sympathetic nerves for orderly energy metabolism and endocrine regulation [137], [138]. Bone and bone marrow adipose tissues may also modulate bone metabolism under precise regulation of the sympathetic nervous system. The crosstalk between the two has been elucidated [139]. The central nervous system, especially the sympathetic nervous system (SNS), regulates bone mass [140], [141], however, the specific signaling pathways or key molecules have not yet been clearly determined.
A crosstalk between the sympathetic nervous system and leptin has also been found in bone metabolism [142]. Leptin acts on neurons in the ventral midline of the hypothalamus and stimulates NE sympathetic nerve fibers to release NE [136]. The released NE specifically interacts with β2-AR on the osteoblasts [143], thereby inhibiting the activities of osteoblasts and bone formation. Therefore, leptin regulates bone remodeling via at least two different antagonistic pathways. On one hand, the sympathetic nerve signal of Adrb2 promotes osteoclast differentiation [144] while on the other hand, realization of this sympathetic nerve function is controlled by phosphorylation of ATF4 [141].
Administration of low doses of leptin in ovariectomized osteoporotic female rats effectively reduced estrogen deficiency-associated trabecular bone loss and structural changes [140]. Thus, leptin may play an important protective role in bone metabolism by inhibiting bone resorption. This could be because leptin is a potential inhibitor of bone formation via the central nervous system and also enhances bone metabolism via peripheral mechanisms.
Given that the sympathetic nerve system is plastic [145], pain will result in sympathetic nerve excitement, whose hyperactivity may amplify pain signals [146]. In ovariectomized mice, destruction of sympathetic nerve fibers was associated with a reduction in their movements after the fracture, which affected bone tissue remodeling in the latter stages of fracture repair. However, the destruction had no effect on pain-related processes [147]. Our current research shows that fracture pain may contribute to systemic bone loss by activating sympathetic nerves through the central nervous system (Unpublished data).
Pain and sensory nervous system
Pain is a central feature in tissue damage. The pain after fracture is very severe [148], and orthopaedic surgery is considered to be the most painful operation [149]. Fracture-associated pain may be caused by a combination of local inflammation, cytokine release, neurotrophic factor release, sympathetic axon sprouting, abnormal activation of DRGs, and glial cell activation [150].
NGF-TrkA
The combination of NGF and its receptor (TrkA) is one of the main molecular signals that produce bone pain. Most of the sensory nerve fibers innervating the bone express TrkA+ [151], [152]. TrkA can guide sensory nerve axons to the initial ossification site. In addition, NGF produced by osteochondral progenitor cells activate TrkA to act as a skeletal neurotrophic factor [153]. Therefore, NGF-related signaling pathways play an important role in coordinating sensory innervation, vascularization, and ossification of long bone development, which is essential for normal primary and secondary ossification.
Under physiological conditions, mechanical signals upregulate NGF expressions in osteoblasts, which in turn activate TrkA sensory nerves, leading to regulation of Wnt/β-catenin signal transduction and enhanced bone formation [154]. Piezo2, a mechanically gated ion channel, aids the sensitivity and responsiveness of bone afferent neurons to harmful/normal mechanical stimuli [52], [63]. Piezo2 knockout animals exhibited reduced firing frequencies and inhibited NGF-induced sensitization of bone afferent neurons [155]. The combination of NGF and its receptor (TrkA) forms the main molecular signal that produces bone pain. Under pathological conditions, soft tissue trauma leads to high expressions of NGF and NGF-responsive axon invasion occurs before osteoclast differentiation. Cartilage and bone formation were significantly delayed when the sciatic nerve was surgical removed [156]. Single-cell sequencing showed that the signal of prechondral cells shifted from TGF-β to FGF activation after denervation. Therefore, activation of the FGF signal may indicate inhibition of bone and cartilage differentiation of MSCs.
The use of opioids or NSAID drugs during fracture healing can effectively relieve pain, however, there is the potential harm of impeded fracture healing. Autophosphorylation of TrkA receptors can be prevented by using specific neutralizing antibodies to block NGF/TrkA signaling, thus, the subsequent signaling cascade is not activated, which eliminates pain transmission without delaying fracture healing [157]. This was also confirmed by Lilian et al. [158], who found that blocking NGF activities by inhibiting TrkA reduced pain in an osteoarthritis rat model. The possible reasons are the asymmetry of weight-bearing caused by the irregular wear of articular cartilage in patients with osteoarthritis, and the standing pain caused by the combination of pain and allergies. In this process, NGF/TrkA plays a crucial role in establishment and amplification of pain sensations. However, there are different points of view with regards to the fact that the use of the TrkA agonist (gambogic amide (GA)) can increase NGF-TrkA signaling in the bone, thereby enhancing bone adaptation to mechanical forces [159]. This results in increased load-induced bone formation and anabolic signaling without inducing significant thermal pain or mechanical hyperalgesia. Skeletal sensory nerves play a role in pain and fracture healing, therefore, any pharmacological method that changes sensory nerve functions may affect bone anabolism.
Pge2-EP4
Prostaglandin E2 (PGE2) is an inflammatory factor [160], [161] and a neuromodulator that alters neuronal excitability [162], [163]. Excess PGE2 activates sensory neurons and mediates pain hypersensitivity by binding PGE2/PGE2 receptor 4 (EP4) receptors. Moreover, PGE2 is a multifunctional regulator of bone metabolism and the intra-skeletal sensory regulates bone homeostasis via the concentration of PGE2 in the bone [164].
The processes by which PGE2 regulates bone homeostasis include regulation of bone resorption and bone formation, that is, by up-regulating the expressions of NFκB ligand receptor activator (RANKL) to promote osteoclast differentiation and bone resorption [165]. When bone density decreases, the PGE2 secreted by osteoblasts increases, thereby inhibiting sympathetic nerve activities via the central nervous system and promoting bone formation [161], [166], [167]. The use of bone-targeted prostaglandin E2 receptor 4 agonists also increased the therapeutic effects of bisphosphonates in a mouse model of severe osteogenesis imperfect [168].
PGE2 is also involved in pain processes in diseases such as osteoarthritis and rheumatoid arthritis [166]. For patients with low back pain, the increase in PGE2 in the porous endplate stimulates sensory nerves, leading to spinal cord pain [167], [169]. The use of low-dose celecoxib can inhibit endplate porosity, sensory innervation, as well as spinal pain and allergies [170].
Inflammation
Fractures, like any other trauma or injury, are accompanied by inflammatory responses that play important roles in repair processes. An association between chronic/systemic inflammation and bone loss has been reported. However, the impact of the inflammatory state on bone fracture has not been fully elucidated.
At 1 day after injury, the secretion of inflammatory factors significantly increases, remain high for 2 weeks, and begin to decline at 3 to 4 weeks after injury. Mice with multiple fractures [171], older mice [14], and male mice [172] tend to exhibit high levels of inflammation and corresponding bone loss. TNF-α, IL-1, and IL-6 are common pro-inflammatory cytokines that directly activate osteoclasts or increase osteoclast differentiation by inducing osteoblasts and osteoblasts to produce RANKL. In addition, pro-inflammatory cytokines suppress the production of bone matrix by enhancing the secretion of sclerostin (SOST) [173] or Dickkopf-related protein 1 (DKK1) [174]. Among them, IL-6 affects bone metabolism by regulating osteoclast and osteoblast development as well as function. IL-6 stimulates the differentiation of osteoclast precursor cells into active and mature osteoclasts with concomitant bone loss following OVX [175] or unloading [176] or upon aging [177]. The use of IL-6-neutralizing antibodies [176] or IL-6 gene knockout [178] reduced the generation of osteoclasts and alleviated bone loss.
The current research focuses on the detection of the concentration of inflammatory factors. There is no definite evidence to identify the relationship between inflammation and bone loss after fractures, but existing research results suggest that increased concentrations of pro-inflammatory cytokines may trigger increase the bone loss. There is a need to investigate the dynamic changes in inflammatory factor concentrations to identify the inflammatory factors that have the greatest impact on bone remodeling, and to inform the development of appropriate therapeutic approaches.
Therapy
Active drug interventions
The pathological mechanism of acute bone loss after fracture is mainly associated with osteoporosis. Therefore, standard anti-osteoporosis drugs should be actively used after fractures. These drugs alleviate pain, inhibit acute bone loss, reduce progressive bone loss, and restore the bone mass to the level before the injury, thereby reducing re-fracture incidences. Drug interventions are mainly divided into basic drug therapy and anti-osteoporosis drug therapy.
Calcium and vitamin D
The bone is a calcium-rich tissue [179], accounting for 99 % of the body's calcium. Calcium is necessary for fracture callus mineralization, thus, during the fracture healing process, the demand for calcium is more than usual. When dietary calcium supply does not meet the calcium requirements for callus mineralization, more calcium is mobilized from distal bones to ensure adequate bone repair [180]. Therefore, it is important to timely replenish calcium to enhance its absorption.
Calcium and vitamin D [181], [182], as the basic treatment drugs for osteoporosis, are routinely used in the treatment of osteoporosis patients, with the purpose of promote bone formation, mineralization and reduce the risk of fractures. The drugs can also be used in combination with other anti-osteoporosis drugs during fracture treatment. The recommended calcium supplement dose should not be less than 1000 mg/d for adults [183], and it should be taken continuously for at least half a year, with vitamin D (800–1200 IU/d) supplementation [183], [184], [185]. Although other types of vitamins have also been studied [186], [187], [188], we will not discuss them here because of inconclusive findings regarding them.
Anti-osteoporosis drugs
Anti-osteoporosis drugs are divided into anti-bone resorption drugs (bisphosphonate drugs, estrogen, selective estrogen receptor modulators, and RANKL inhibitors) and bone formation drugs (thyroid parathyroid hormone analogs).
Estrogen and SERMs
Estrogen has a wide range of physiological effects, including skeletal system maturation and bone mass maintenance [189], [190]. Postmenopausal osteoporosis is significantly associated with declining estrogen levels. Estrogen replacement therapy (ERT) can inhibit bone turnover, prevent bone loss, and increase bone density of the vertebral body and hip [191]. Notably, ERT or estrogen-progesterone hormone replacement therapy (HRT) are usually prescribed for postmenopausal osteoporotic fracture patients. Selective estrogen receptor modulators (SERMs) can selectively act on target organs of estrogen, combine with different forms of estrogen receptors, reduce bone resorption, and prevent bone loss. However, SERMs and estrogen drugs are not recommended for acute phases of fractures.
Calcitonin
Calcitonins inhibit the biological activities of osteoclasts, reduce the number of osteoclasts, and have good therapeutic effects on acute bone loss and pain after osteoporotic fractures [192], [193]. Some commonly used drugs include salmon calcitonin and eel calcitonin.
Bisphosphonates
Bisphosphonates can specifically bind hydroxyphosphatidite in the bone and inhibit bone resorption by suppressing osteoclast activities [194], [195]. This increases bone density of the lumbar spine and hip while reducing re-fracture incidences. Currently, bisphosphonates are the most successful and commonly used drugs for osteoporosis treatment, especially osteoporosis that is characterized by bone loss and bone structure destruction. Some commonly used drugs include alendronate sodium, risedronate sodium, zoledronic acid, and ibandronate sodium.
RANKL inhibitors
RANKL inhibitors, such as denosumab, inhibit the activation of the osteoclast RANK signaling pathway by combining with RANKL, thereby suppressing ostoclasst activation and maturation, and reducing bone loss [196], [197].
Parathyroid hormone analogue
Parathyroid hormone analogues (PTHa), mainly teriparatide [198] and abaloparatide [199], stimulate osteoblast activities, increase osteoblast secretion of collagen, enhance bone matrix formation as well as mineralization, promote bone formation, and improve bone remodeling [200].
There are differences between the healing process of fractures and the pathophysiological mechanisms of bone reconstruction. The factors influencing bone reconstruction do not necessarily affect fracture healing. It has been reported that bisphosphonates have no effects on intrachondral osteogenesis but have an effect on intramembranous osteogenesis [201]. Therefore, when deciding on a treatment plan, various considerations are required.
Furthermore, drugs that affect bone regeneration, including hormones, diuretics, psychiatric drugs, and chemotherapeutic drugs should be adjusted according to the condition. Importantly, applications of anti-osteoporosis drugs should strictly follow the indications and contraindications because excess use will cause overcorrection, leading to gastrointestinal reactions, allergies, headaches, urinary tract stones, and other side effects.
Combined therapy
The degrees of bone loss among patients after fractures differ, and personalized treatment is needed. Combined treatment may achieve better results with the most minimal side effects [201], [202], [203]. However, not every fracture patient requires anti-osteoporosis medications. Therefore, clinicians should formulate an individualized treatment plan based on the degree of osteoporosis, the severity of injury, and the overall state of the patient, combined with indications, contraindications, and drug safety.
Integrated management
Effective immobilization is necessary, whether surgical treatment or conservative treatment. Scientific management and education during the immobilization period are needed [21], [204], [205]. Identifying high-risk patients and commencing active intervention as early as possible can significantly eliminate the influence of unfavorable factors. High-risk factors include aging, women, diabetes, high blood pressure, severe fractures, multiple fractures, long-term use of corticosteroids or other drugs, and a history of previous fractures [11], [12], [206]. In the perioperative period of fracture patients, it is suitable to apply an effective combination of a personalized treatment plan and modular multidisciplinary cooperation, as well as active early rehabilitation training [207], [208].
Conclusion and future directions
Rapid bone loss after fracture is increasingly being studied. Its pathophysiological mechanisms are complicated and involves multiple tissues, organs and systems. In addition, the complexity of bone loss after fracture is not only due to the complex crosstalk between key molecules or signaling pathways, but is also closely associated with pathological changes caused by the fracture itself and osteoporosis progression. Fracture patients with acute bone loss require special attention because of increased risk of secondary fractures. The advantages and disadvantages of the existing drug therapy regimens suggest that there is a need to proceed with caution during treatment, implying the need for further research on bone loss.
CRediT authorship contribution statement
Xuan-Qi Zheng: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Jie Huang: Methodology, Formal analysis, Investigation, Writing – original draft. Jia-liang Lin: Methodology, Formal analysis, Investigation, Writing – original draft. Chun-Li Song: Conceptualization, Methodology, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Funds
This work was supported by the National Key Research and Development Program (2020YFC2009004, 2021YFC2501700), the National Natural Science Foundation of China (81874010, 82272554) and PKU-Baidu Fund (2020BD014).
Biographies

Xuan-Qi Zheng, MD My name is Xuan-Qi Zheng, I am studying in Peking University Third Hospital, my mentor is Chun-Li Song. In June 2021, I completed my postgraduate degree from Wenzhou Medical University, and in September 2021, I was admitted as a PhD student of Peking University Health Science Center, majoring in orthopaedics in Beijing Key Laboratory of Spinal Disease. Currently, I am mainly engaged in osteoporosis-related research, especially in the fields of pain and bone loss. In August 2021, I published an article titled Neurophysiological mechanisms of cancer-induced bone pain on Journal of Advanced Research, demonstrating the latest advances in pain-related basic research. In the same year, we started to study the problems of bone loss after fractures.

Jie Huang, MD My name is Jie Huang, I am studying in Peking University Health Science Center as a doctoral candidate majoring in orthopedics. My mentor is Chun-Li Song. Currently, I am mainly engaged in osteoporosis-related research, especially in the fields of metabolic bone disease.

Jia-Liang Lin, MD My name is Jia-Liang Lin, I am now studying at Peking University Third Hospital, Peking University. I completed my master's degree and graduated from Wenzhou Medical University in July 2020. In September of the same year, I enrolled in Peking University to start my PhD career, majoring in orthopedics. Up to now, I have mainly focused on research related to heterotopic ossification and osteogenic differentiation.

Chun-Li Song, MD PhD Member of the Chinese Society of Osteoporosis and Bone Mineral Research (CSOBMR), Vice Chairman of the Chinese Geriatrics Society Medical Equipment and Aging Support Deputy Director of the Osteoporosis and Bone Mineral Research of the Beijing Medical Association, Deputy Director of the Osteoporosis Academic Working Committee of the Bone and Joint Branch of the Chinese Geriatric Society Member of the Innovation and Conversion of the Orthopedic Branch of the Chinese Medical Association Leader of the Basic Science Group of the Geriatric Orthopedic Branch of the Chinese Society of Gerontology and Geriatrics Commission Editor of “Chinese Journal of Medical Research Management”.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Epstein S., Inzerillo A.M., Caminis J., Zaidi M. Disorders Associated with Acute Rapid and Severe Bone Loss. J Bone Miner Res. 2003 doi: 10.1359/jbmr.2003.18.12.2083. [DOI] [PubMed] [Google Scholar]
- 2.Osipov B., Emami A.J., Christiansen B.A. Systemic Bone Loss After Fracture. Clin Rev Bone Miner Metab. 2018 doi: 10.1007/s12018-018-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lems W.F., Dreinhöfer K.E., Bischoff-Ferrari H., Blauth M., Czerwinski E., Da Silva J., et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2016-210289. [DOI] [PubMed] [Google Scholar]
- 4.Compston J.E., McClung M.R., Leslie W.D. Osteoporosis Lancet. 2019 doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S., Hofbauer L.C. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017 doi: 10.1016/S2213-8587(17)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qaseem A., Forciea M.A., McLean R.M., Denberg T.D. Treatment of low bone density or osteoporosis to prevent fractures in men and women: A clinical practice guideline update from the American college of physicians. Ann Intern Med. 2017 doi: 10.7326/M15-1361. [DOI] [PubMed] [Google Scholar]
- 7.Black D.M., Arden N.K., Palermo L., Pearson J., Cummings S.R. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. J Bone Miner Res. 1999;14:821–828. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- 8.Klotzbuecher C.M., Ross P.D., Landsman P.B., Abbott T.A., Berger M. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 9.Haentjens P., Autier P., Collins J., Velkeniers B., Vanderschueren D., Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women a meta-analysis. J Bone Jt Surg - Ser A. 2003;85:1936–1943. doi: 10.2106/00004623-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Clinton J., Franta A., Polissar N.L., Neradilek B., Mounce D., Fink H.A., et al. Proximal humeral fracture as a risk factor for subsequent hip fractures. J Bone Jt Surg - Ser A. 2009;91:503–511. doi: 10.2106/JBJS.G.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson C.M., Royds M., Abraham A., McQueen M.M., Court-Brown C.M., Christie J. Refractures in patients at least forty-five years old: A prospective analysis of twenty-two thousand and sixty patients. J Bone Jt Surg - Ser A. 2002;84:1528–1533. doi: 10.2106/00004623-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Wu F., Mason B., Horne A., Ames R., Clearwater J., Liu M., et al. Fractures between the ages of 20 and 50 years increase women’s risk of subsequent fractures. Arch Intern Med. 2002;162:33–36. doi: 10.1001/archinte.162.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen B.A., Harrison S.L., Fink H.A., Lane N.E. Incident fracture is associated with a period of accelerated loss of hip BMD: the Study of Osteoporotic Fractures. Osteoporos Int. 2018;29:2201–2209. doi: 10.1007/s00198-018-4606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emami A.J., Toupadakis C.A., Telek S.M., Fyhrie D.P., Yellowley C.E., Christiansen B.A. Age Dependence of Systemic Bone Loss and Recovery Following Femur Fracture in Mice. J Bone Miner Res. 2019;34:157–170. doi: 10.1002/jbmr.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapurlat R., Bui M., Sornay-Rendu E., Zebaze R., Delmas P.D., Liew D., et al. Deterioration of Cortical and Trabecular Microstructure Identifies Women With Osteopenia or Normal Bone Mineral Density at Imminent and Long-Term Risk for Fragility Fracture: A Prospective Study. J Bone Miner Res. 2020;35:833–844. doi: 10.1002/jbmr.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervinka T., Sievänen H., Hyttinen J., Rittweger J. Bone loss patterns in cortical, subcortical, and trabecular compartments during simulated microgravity. J Appl Physiol. 2014;117:80–88. doi: 10.1152/japplphysiol.00021.2014. [DOI] [PubMed] [Google Scholar]
- 17.Krolner B., Toft B. Vertebral bone loss: An unheeded side effect of therapeutic bed rest. Clin Sci. 1983;64:537–540. doi: 10.1042/cs0640537. [DOI] [PubMed] [Google Scholar]
- 18.Denaro V., Longo U.G., Denaro L. Vertebroplasty versus conservative treatment for vertebral fractures. Lancet. 2010 doi: 10.1016/S0140-6736(10)62289-1. [DOI] [PubMed] [Google Scholar]
- 19.Handoll H.H.G., Parker M.J. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD000337.pub2. [DOI] [PubMed] [Google Scholar]
- 20.DeMaio M., McHale K., Lenhart M., Garland J., McIlvaine C., Rhode M. Plaster: Our orthopaedic heritage - AAOS exhibit selection. J Bone Jt Surg - Ser A. 2012 doi: 10.2106/JBJS.L.00183. [DOI] [PubMed] [Google Scholar]
- 21.Bakody E. Orthopaedic plaster casting: nurse and patient education. Nurs Stand. 2009 doi: 10.7748/ns2009.08.23.51.49.c7224. [DOI] [PubMed] [Google Scholar]
- 22.Chen J.H., Liu C., You L., Simmons C.A. Boning up on Wolff’s Law: Mechanical regulation of the cells that make and maintain bone. J Biomech. 2010 doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Pearson O.M., Lieberman D.E. The aging of Wolff’s “law”: Ontogeny and responses to mechanical loading in cortical bone. Yearb Phys Anthropol. 2004 doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 24.Frost H.M.A. update of bone physiology and Wolff s law for clinicians. Angle Orthod. 2003;2004 doi: 10.1043/0003-3219(2004)074<0003:AUOBPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann M., Engelke K., Ebert R., Müller-Deubert S., Rudert M., Ziouti F., et al. Interactions between muscle and bone—where physics meets biology. Biomolecules. 2020;10:1–29. doi: 10.3390/biom10030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao Z., Quarles L.D. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev Endocr Metab Disord. 2015;16:115–129. doi: 10.1007/s11154-015-9313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolvien T., Amling M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif Tissue Int. 2021 doi: 10.1007/s00223-021-00836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittweger J., Felsenberg D. Recovery of muscle atrophy and bone loss from 90 days bed rest: Results from a one-year follow-up. Bone. 2009 doi: 10.1016/j.bone.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 29.Cooke N.J., Rodgers L., Rawlings D., McCaskie A.W., Holland J.P. Bone density of the femoral neck following Birmingham hip resurfacing. Acta Orthop. 2009 doi: 10.3109/17453670903486992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingle B.M., Hay S.M., Bottjer H.M., Eastell R. Changes in bone mass and bone turnover following distal forearm fracture. Osteoporos Int. 1999 doi: 10.1007/s001980050246. [DOI] [PubMed] [Google Scholar]
- 31.Way F., Ralston A., Wilf H.S. Mathematical Methods for Digital Computers. Am Math Mon. 1961 doi: 10.2307/2312521. [DOI] [Google Scholar]
- 32.Scheuren A.C., Vallaster P., Kuhn G.A., Paul G.R., Malhotra A., Kameo Y., et al. Mechano-Regulation of Trabecular Bone Adaptation Is Controlled by the Local in vivo Environment and Logarithmically Dependent on Loading Frequency. Front Bioeng Biotechnol. 2020 doi: 10.3389/fbioe.2020.566346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu X., Wang N., Wang Z., Yu W., Wang Y., Guo Y., et al. Mathematically modeling fluid flow and fluid shear stress in the canaliculi of a loaded osteon. Biomed Eng Online. 2016 doi: 10.1186/s12938-016-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price C., Zhou X., Li W., Wang L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: Direct evidence for load-induced fluid flow. J Bone Miner Res. 2011;26:277–285. doi: 10.1002/jbmr.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H.Y., Zhao S., Zhang H., Huang S.Y., Peng W.T., Zhang C.Q., et al. Research on solute transport behaviors in the lacunar-canalicular system using numerical simulation in microgravity. Comput Biol Med. 2020;119 doi: 10.1016/j.compbiomed.2020.103700. [DOI] [PubMed] [Google Scholar]
- 36.Malone A.M.D., Anderson C.T., Tummala P., Kwon R.Y., Johnston T.R., Stearns T., et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoey D.A., Tormey S., Ramcharan S., O’Brien F.J., Jacobs C.R. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells. 2012;30:2561–2570. doi: 10.1002/stem.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W., Zhang Y., Chen K., He J., Feng X., Wei W., et al. Primary cilia act as microgravity sensors by depolymerizing microtubules to inhibit osteoblastic differentiation and mineralization. Bone. 2020;136 doi: 10.1016/j.bone.2020.115346. [DOI] [PubMed] [Google Scholar]
- 39.Smith C.E.L., Lake A.V.R., Johnson C.A. Primary Cilia, Ciliogenesis and the Actin Cytoskeleton: A Little Less Resorption, A Little More Actin Please. Front Cell Dev Biol. 2020 doi: 10.3389/fcell.2020.622822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiesel P., Alvarez Viar G., Tsoy N., Maraspini R., Gorilak P., Varga V., et al. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat Struct Mol Biol. 2020 doi: 10.1038/s41594-020-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moya I.M., Halder G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019 doi: 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- 42.Dey A., Varelas X., Guan K.L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov. 2020 doi: 10.1038/s41573-020-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rausch V., Hansen C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020 doi: 10.1016/j.tcb.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Codelia V.A., Sun G., Irvine K.D. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol. 2014 doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013 doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 46.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011 doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z., Luo Q., Lin C., Kuang D., Song G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci Rep. 2016;6:1–11. doi: 10.1038/srep30322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z., Luo Q., Lin C., Song G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem Biophys Res Commun. 2015;468:21–26. doi: 10.1016/j.bbrc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Zemke N.R., Gou D., Berk A.J. Dedifferentiation by adenovirus E1A due to inactivation of hippo pathway effectors YAP and TAZ. Genes Dev. 2019 doi: 10.1101/gad.324814.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J., Yan J.F., Wan Q.Q., Shen M.J., Ma Y.X., Gu J.T., et al. Matrix stiffening by self-mineralizable guided bone regeneration. Acta Biomater. 2021 doi: 10.1016/j.actbio.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji K., Bandyopadhyay A., Harfe B.D., Cox K., Kakar S., Gerstenfeld L., et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006 doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 52.Salazar V.S., Gamer L.W., Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016 doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 53.Sefkow-Werner J., Machillot P., Sales A., Castro-Ramirez E., Degardin M., Boturyn D., et al. Heparan sulfate co-immobilized with cRGD ligands and BMP2 on biomimetic platforms promotes BMP2-mediated osteogenic differentiation. Acta Biomater. 2020 doi: 10.1016/j.actbio.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Zhao C., Wang X., Gao L., Jing L., Zhou Q., Chang J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018 doi: 10.1016/j.actbio.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 55.Wei Q., Holle A., Li J., Posa F., Biagioni F., Croci O., et al. BMP-2 Signaling and Mechanotransduction Synergize to Drive Osteogenic Differentiation via YAP/TAZ. Adv Sci. 2020;7:1–15. doi: 10.1002/advs.201902931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S., Hu J., Zhang G., Qi W., Zhang P., Li P., et al. Extracellular Ca2+ Promotes Odontoblastic Differentiation of Dental Pulp Stem Cells via BMP2-Mediated Smad1/5/8 and Erk1/2 Pathways. J Cell Physiol. 2015 doi: 10.1002/jcp.24945. [DOI] [PubMed] [Google Scholar]
- 57.Dasgupta I., McCollum D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J Biol Chem. 2019 doi: 10.1074/jbc.REV119.007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panciera T., Azzolin L., Cordenonsi M., Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017 doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li L., Li H., He Y., Tang H., Dong J., Chen X., et al. Cyclic pulsation stress promotes bone formation of tissue engineered laminae through the F-actin/YAP-1/β-Catenin signaling axis. Npj Regen Med. 2021;6:1–13. doi: 10.1038/s41536-021-00164-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson M., Woods K., Newberg J., Oxford J.T., Uzer G. Low-intensity vibration restores nuclear YAP levels and acute YAP nuclear shuttling in mesenchymal stem cells subjected to simulated microgravity. npj Microgravity. 2020;6:1–11. doi: 10.1038/s41526-020-00125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin Y.C., Guo Y.R., Miyagi A., Levring J., MacKinnon R., Scheuring S. Force-induced conformational changes in PIEZO1. Nature. 2019 doi: 10.1038/s41586-019-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He L., Si G., Huang J., Samuel A.D.T., Perrimon N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature. 2018 doi: 10.1038/nature25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin L., He T., Chen S., Yang D., Yi W., Cao H., et al. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021;9:44. doi: 10.1038/s41413-021-00168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Y., Yang X., Jiang J., Xiao B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem Sci. 2021 doi: 10.1016/j.tibs.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Douguet D., Honoré E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated Ion Channels. Cell. 2019 doi: 10.1016/j.cell.2019.08.049. [DOI] [PubMed] [Google Scholar]
- 66.Coste B., Mathur J., Schmidt M., Earley T.J., Ranade S., Petrus M.J., et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science (80-) 2010 doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hendrickx G., Fischer V., Liedert A., von Kroge S., Haffner-Luntzer M., Brylka L., et al. Piezo1 Inactivation in Chondrocytes Impairs Trabecular Bone Formation. J Bone Miner Res. 2021;36:369–384. doi: 10.1002/jbmr.4198. [DOI] [PubMed] [Google Scholar]
- 68.Li X., Han L., Nookaew I., Mannen E., Silva M.J., Almeida M., et al. Stimulation of piezo1 by mechanical signals promotes bone anabolism. Elife. 2019;8:1–22. doi: 10.7554/eLife.49631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang G., Li X., Wu L., Qin Y.X. Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells. Bone Res. 2021;9:1–10. doi: 10.1038/s41413-020-00124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J., Liu C., Li Y., Zheng Q., Xu Y., Liu B., et al. TMCO1-mediated Ca 2+ leak underlies osteoblast functions via CaMKII signaling. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Čamernik K., Mihelič A., Mihalič R., Haring G., Herman S., Marolt Presen D., et al. Comprehensive analysis of skeletal muscle- A nd bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res Ther. 2020 doi: 10.1186/s13287-020-01657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao H., Wang L., Zhang T., Chen C., Chen H., Li S., et al. Periosteum progenitors could stimulate bone regeneration in aged murine bone defect model. J Cell Mol Med. 2020 doi: 10.1111/jcmm.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012 doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 74.Pedersen B.K. Physical activity and muscle–brain crosstalk. Nat Rev Endocrinol. 2019 doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 75.Digirolamo D.J., Kiel D.P., Esser K.A. Bone and skeletal muscle: Neighbors with close ties. J Bone Miner Res. 2013;28:1509–1518. doi: 10.1002/jbmr.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brotto M., Bonewald L. Bone and muscle: Interactions beyond mechanical. Bone. 2015 doi: 10.1016/j.bone.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alkner B.A., Bring D.K.I. Muscle activation during gravity-independent resistance exercise compared to common exercises. Aerosp Med Hum Perform. 2019 doi: 10.3357/AMHP.5097.2019. [DOI] [PubMed] [Google Scholar]
- 78.Lee S.J., Lehar A., Meir J.U., Koch C., Morgan A., Warren L.E., et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2014716117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zullo A., Fleckenstein J., Schleip R., Hoppe K., Wearing S., Klingler W. Structural and Functional Changes in the Coupling of Fascial Tissue, Skeletal Muscle, and Nerves During Aging. Front Physiol. 2020 doi: 10.3389/fphys.2020.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirzoev T.M., Shenkman B.S. Regulation of Protein Synthesis in Inactivated Skeletal Muscle: Signal Inputs, Protein Kinase Cascades, and Ribosome Biogenesis. Biochem. 2018 doi: 10.1134/S0006297918110020. [DOI] [PubMed] [Google Scholar]
- 81.Haus J.M., Carrithers J.A., Carroll C.C., Tesch P.A., Trappe T.A. Contractile and connective tissue protein content of human skeletal muscle: Effects of 35 and 90 days of simulated microgravity and exercise countermeasures. Am J Physiol - Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00292.2007. [DOI] [PubMed] [Google Scholar]
- 82.Mercuri E., Bönnemann C.G., Muntoni F. Muscular dystrophies. Lancet. 2019 doi: 10.1016/S0140-6736(19)32910-1. [DOI] [PubMed] [Google Scholar]
- 83.Miller B.F., Baehr L.M., Musci R.V., Reid J.J., Peelor F.F., Hamilton K.L., et al. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle. 2019 doi: 10.1002/jcsm.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller S.G., Hafen P.S., Brault J.J. Increased adenine nucleotide degradation in skeletal muscle atrophy. Int J Mol Sci. 2020 doi: 10.3390/ijms21010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohnishi Y.I., Iwatsuki K., Shinzawa K., Nakai Y., Ishihara M., Yoshimine T. Disuse muscle atrophy exacerbates motor neuronal degeneration caudal to the site of spinal cord injury. NeuroReport. 2012 doi: 10.1097/WNR.0b013e32834f4048. [DOI] [PubMed] [Google Scholar]
- 86.Ramasamy S.K., Kusumbe A.P., Schiller M., Zeuschner D., Bixel M.G., Milia C., et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun. 2016 doi: 10.1038/ncomms13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simunovic F., Finkenzeller G. Vascularization strategies in bone tissue engineering. Cells. 2021 doi: 10.3390/cells10071749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng X.Q., Huang J.F., Lin J.L., Yang D.J., Xu T.Z., Chen D., et al. 3D bioprinting in orthopedics translational research. J Biomater Sci Polym Ed. 2019 doi: 10.1080/09205063.2019.1623989. [DOI] [PubMed] [Google Scholar]
- 89.Tian T., Zhang T., Lin Y., Cai X. Vascularization in Craniofacial Bone Tissue Engineering. J Dent Res. 2018 doi: 10.1177/0022034518767120. [DOI] [PubMed] [Google Scholar]
- 90.Li L., Shi J., Ma K., Jin J., Wang P., Liang H., et al. Robotic in situ 3D bio-printing technology for repairing large segmental bone defects. J Adv Res. 2021 doi: 10.1016/j.jare.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Epple C., Haumer A., Ismail T., Lunger A., Scherberich A., Schaefer D.J., et al. Prefabrication of a large pedicled bone graft by engineering the germ for de novo vascularization and osteoinduction. Biomaterials. 2019 doi: 10.1016/j.biomaterials.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 92.Liu F., Wang X., Chen T., Zhang N., Wei Q., Tian J., et al. Hydroxyapatite/silver electrospun fibers for anti-infection and osteoinduction. J Adv Res. 2020 doi: 10.1016/j.jare.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Apte R.S., Chen D.S., Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019 doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu K., Jiao H., Li S., Cao H., Galson D.L., Zhao Z., et al. ATF4 promotes bone angiogenesis by increasing vegf expression and release in the bone environment. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Veeriah V., Paone R., Chatterjee S., Teti A., Capulli M. Osteoblasts Regulate Angiogenesis in Response to Mechanical Unloading. Calcif Tissue Int. 2019 doi: 10.1007/s00223-018-0496-z. [DOI] [PubMed] [Google Scholar]
- 96.Helmrich U., Di Maggio N., Güven S., Groppa E., Melly L., Largo R.D., et al. Osteogenic graft vascularization and bone resorption by VEGF-expressing human mesenchymal progenitors. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 97.Hominick D., Silva A., Khurana N., Liu Y., Dechow P.C., Feng J.Q., et al. VEGF-C promotes the development of lymphatics in bone and bone loss. Elife. 2018 doi: 10.7554/eLife.34323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Q., Guo R., Lu Y., Zhao L., Zhou Q., Schwarz E.M., et al. VEGF-C, a lymphatic growth factor, is a RANKL target gene in osteoclasts that enhances osteoclastic bone resorption through an autocrine mechanism. J Biol Chem. 2008 doi: 10.1074/jbc.M708055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duan X., Bradbury S.R., Olsen B.R., Berendsen A.D. VEGF stimulates intramembranous bone formation during craniofacial skeletal development. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tong L., Zhu G., Wang J., Sun R., He F., Zhai J. Suppressing angiogenesis regulates the irradiation-induced stimulation on osteoclastogenesis in vitro. J Cell Physiol. 2018 doi: 10.1002/jcp.26196. [DOI] [PubMed] [Google Scholar]
- 101.Grunewald M., Kumar S., Sharife H., Volinsky E., Gileles-Hillel A., Licht T., et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021 doi: 10.1126/science.abc8479. [DOI] [PubMed] [Google Scholar]
- 102.Buettmann E.G., McKenzie J.A., Migotsky N., Sykes D.A.W., Hu P., Yoneda S., et al. VEGFA From Early Osteoblast Lineage Cells (Osterix+) Is Required in Mice for Fracture Healing. J Bone Miner Res. 2019;34:1690–1706. doi: 10.1002/jbmr.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang C., Feinberg D., Alharbi M., Ding Z., Lu C., O’Connor J.P., et al. Chondrocytes Promote Vascularization in Fracture Healing Through a FOXO1-Dependent Mechanism. J Bone Miner Res. 2019;34:547–556. doi: 10.1002/jbmr.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawakami Y., Ii M., Matsumoto T., Kuroda R., Kuroda T., Kwon S.M., et al. SDF-1/CXCR4 axis in tie2-Lineage cells including endothelial progenitor cells contributes to bone fracture healing. J Bone Miner Res. 2015;30:95–105. doi: 10.1002/jbmr.2318. [DOI] [PubMed] [Google Scholar]
- 105.Li P., Bai Y., Yin G., Pu X., Huang Z., Liao X., et al. Synergistic and sequential effects of BMP-2, bFGF and VEGF on osteogenic differentiation of rat osteoblasts. J Bone Miner Metab. 2014 doi: 10.1007/s00774-013-0538-6. [DOI] [PubMed] [Google Scholar]
- 106.Miguez P.A. Evidence of biglycan structure-function in bone homeostasis and aging. Connect Tissue Res. 2020;61:19–33. doi: 10.1080/03008207.2019.1669577. [DOI] [PubMed] [Google Scholar]
- 107.Hu L, Zang M de, Wang H xiao, Li J fang, Su L ping, Yan M, et al. Biglycan stimulates VEGF expression in endothelial cells by activating the TLR signaling pathway. Mol Oncol 2016;10:1473–84. https://doi.org/10.1016/j.molonc.2016.08.002. [DOI] [PMC free article] [PubMed]
- 108.Berendsen A.D., Pinnow E.L., Maeda A., Brown A.C., McCartney-Francis N., Kram V., et al. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix Biol. 2014;35:223–231. doi: 10.1016/j.matbio.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su Y.W., Chung R., Ruan C.S., Chim S.M., Kuek V., Dwivedi P.P., et al. Neurotrophin-3 Induces BMP-2 and VEGF Activities and Promotes the Bony Repair of Injured Growth Plate Cartilage and Bone in Rats. J Bone Miner Res. 2016;31:1258–1274. doi: 10.1002/jbmr.2786. [DOI] [PubMed] [Google Scholar]
- 110.Discher D., Dong C., Fredberg J.J., Guilak F., Ingber D., Janmey P., et al. Biomechanics: Cell research and applications for the next decade. Ann Biomed Eng. 2009 doi: 10.1007/s10439-009-9661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kültz D. Defining biological stress and stress responses based on principles of physics. J Exp Zool Part A Ecol Integr Physiol. 2020 doi: 10.1002/jez.2340. [DOI] [PubMed] [Google Scholar]
- 112.Srinivasan P., Miller M.A., Verdonschot N., Mann K.A., Janssen D. Strain shielding in trabecular bone at the tibial cement-bone interface. J Mech Behav Biomed Mater. 2017 doi: 10.1016/j.jmbbm.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hnat W.P., Conway J.S., Malkani A.L., Yakkanti M.R., Voor M.J. The Effect of Modular Tapered Fluted Stems on Proximal Stress Shielding in The Human Femur. J Arthroplasty. 2009 doi: 10.1016/j.arth.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 114.Feng X., Lin G., Fang C.X., Lu W.W., Chen B., Leung F.K.L. Bone resorption triggered by high radial stress: The mechanism of screw loosening in plate fixation of long bone fractures. J Orthop Res. 2019 doi: 10.1002/jor.24286. [DOI] [PubMed] [Google Scholar]
- 115.Woo S.L., Simon B.R., Akeson W.H., Gomez M.A., Seguchi Y. A new approach to the design of internal fixation plates. J Biomed Mater Res. 1983 doi: 10.1002/jbm.820170304. [DOI] [PubMed] [Google Scholar]
- 116.Zhao X., Jing W., Yun Z., Tong X., Li Z., Yu J., et al. An experimental study on stress-shielding effects of locked compression plates in fixing intact dog femur. J Orthop Surg Res. 2021 doi: 10.1186/s13018-021-02238-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Koh Y.G., Son J., Kim H.J., Kwon S.K., Kwon O.R., Kim H.J., et al. Multi-objective design optimization of high tibial osteotomy for improvement of biomechanical effect by using finite element analysis. J Orthop Res. 2018 doi: 10.1002/jor.24072. [DOI] [PubMed] [Google Scholar]
- 118.Bottlang M., Feist F. Biomechanics of far cortical locking. J Orthop Trauma. 2011 doi: 10.1097/BOT.0b013e318207885b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ziegler P., Maier S., Stöckle U., Gühring M., Stuby F.M. The Treatment of Proximal Humerus Fracture Using Internal Fixation With Fixed-angle Plates. Dtsch Aerzteblatt Online. 2019 doi: 10.3238/arztebl.2019.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hak D.J., Banegas R., Ipaktchi K., Mauffrey C. Evolution of plate design and material composition. Injury. 2018 doi: 10.1016/S0020-1383(18)30295-X. [DOI] [PubMed] [Google Scholar]
- 121.Agarwal T., Chiesa I., Presutti D., Irawan V., Vajanthri K.Y., Costantini M., et al. Recent advances in bioprinting technologies for engineering different cartilage-based tissues. Mater Sci Eng C. 2021;123 doi: 10.1016/j.msec.2021.112005. [DOI] [PubMed] [Google Scholar]
- 122.Henley M.B., Meier M., Tencer A.F. Influences of some design parameters on the biomechanics of the unreamed tibial intramedullary nail. J Orthop Trauma. 1993 doi: 10.1097/00005131-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 123.Jain R., Podworny N., Hearn T., Anderson G.I., Schemitsch E.H. Effect of stainless steel and titanium low-Contact dynamic compression plate application on the vascularity and mechanical properties of cortical bone after fracture. J Orthop Trauma. 1997 doi: 10.1097/00005131-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 124.Lv Q.B., Gao X., Pan X.X., Jin H.M., Lou X.T., Li S.M., et al. Biomechanical properties of novel transpedicular transdiscal screw fixation with interbody arthrodesis technique in lumbar spine: A finite element study. J Orthop Transl. 2018 doi: 10.1016/j.jot.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma X., Xia H., Wang J., Zhu X., Huang F., Lu L., et al. Re-fracture and correlated risk factors in patients with osteoporotic vertebral fractures. J Bone Miner Metab. 2019 doi: 10.1007/s00774-018-0974-4. [DOI] [PubMed] [Google Scholar]
- 126.Wu F., Mason B., Horne A., Ames R., Clearwater J., Liu M., et al. Fractures between the ages of 20 and 50 years increase women’s risk of subsequent fractures. Arch Intern Med. 2002 doi: 10.1001/archinte.162.1.33. [DOI] [PubMed] [Google Scholar]
- 127.Kim W.J., Ma S.B., Shin H.M., Song D.G., Lee J.W., Chang S.H., et al. Correlation of Sagittal Imbalance and Recollapse after Percutaneous Vertebroplasty for Thoracolumbar Osteoporotic Vertebral Compression Fracture: A Multivariate Study of Risk Factors. Asian. Spine J. 2021 doi: 10.31616/asj.2021.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu J., Jiang G., Lu B., Shi C., Luo K., Yue B. The positive correlation between upper adjacent vertebral fracture and the kyphosis angle of injured vertebral body after percutaneous kyphoplasty: An in vitro study. Clin Neurol Neurosurg. 2015 doi: 10.1016/j.clineuro.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 129.Rahmani M.S., Takahashi S., Hoshino M., Takayama K., Sasaoka R., Tsujio T., et al. The degeneration of adjacent intervertebral discs negatively influence union rate of osteoporotic vertebral fracture: A multicenter cohort study. J Orthop Sci. 2018 doi: 10.1016/j.jos.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 130.Grässel S. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther. 2014 doi: 10.1186/s13075-014-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Niedermair T., Kuhn V., Doranehgard F., Stange R., Wieskötter B., Beckmann J., et al. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol. 2014 doi: 10.1016/j.matbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 132.Lee N.J., Doyle K.L., Sainsbury A., Enriquez R.F., Hort Y.J., Riepler S.J., et al. Critical role for Y1 receptors in mesenchymal progenitor cell differentiation and osteoblast activity. J Bone Miner Res. 2010 doi: 10.1002/jbmr.61. [DOI] [PubMed] [Google Scholar]
- 133.Park M.H., Lee J.K., Kim N., Min W.K., Lee J.E., Kim K.T., et al. Neuropeptide Y Induces Hematopoietic Stem/Progenitor Cell Mobilization by Regulating Matrix Metalloproteinase-9 Activity Through Y1 Receptor in Osteoblasts. Stem Cells. 2016 doi: 10.1002/stem.2383. [DOI] [PubMed] [Google Scholar]
- 134.Togari A. Adrenergic regulation of bone metabolism: Possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech. 2002;58:77–84. doi: 10.1002/jemt.10121. [DOI] [PubMed] [Google Scholar]
- 135.Levine B.D., Pawelczyk J.A., Ertl A.C., Cox J.F., Zuckerman J.H., Diedrich A., et al. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang F., Liu Y., Chen S., Dai Z., Yang D., Gao D., et al. A GABAergic neural circuit in the ventromedial hypothalamus mediates chronic stress–induced bone loss. J Clin Invest. 2020;130:6539–6554. doi: 10.1172/JCI136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robles H., Park S.J., Joens M.S., Fitzpatrick J.A.J., Craft C.S., Scheller E.L. Characterization of the bone marrow adipocyte niche with three-dimensional electron microscopy. Bone. 2019 doi: 10.1016/j.bone.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wee N.K.Y., Lorenz M.R., Bekirov Y., Jacquin M.F., Scheller E.L. Shared autonomic pathways connect bone marrow and peripheral adipose tissues across the central neuraxis. Front Endocrinol (Lausanne) 2019 doi: 10.3389/fendo.2019.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X., Hassan M.G., Scheller E.L. Neural regulation of bone marrow adipose tissue. Best Pract Res Clin Endocrinol Metab. 2021;35 doi: 10.1016/j.beem.2021.101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Burguera B. Leptin Reduces Ovariectomy-Induced Bone Loss in Rats. Endocrinology. 2001 doi: 10.1210/en.142.8.3546. [DOI] [PubMed] [Google Scholar]
- 141.Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L., et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/S0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 142.Kajimura D., Hinoi E., Ferron M., Kode A., Riley K.J., Zhou B., et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011 doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang H.H., Brennan T.C., Muir M.M., Mason R.S. Functional α1- and β2-adrenergic receptors in human osteoblasts. J Cell Physiol. 2009 doi: 10.1002/jcp.21761. [DOI] [PubMed] [Google Scholar]
- 144.Aitken S., Landao-Bassonga E., Ralston S.H., Idris A.I. Beta-2 adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Bone. 2008 doi: 10.1016/j.bone.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 145.Li A.L., Zhang J.D., Xie W., Strong J.A., Zhang J.M. Inflammatory Changes in Paravertebral Sympathetic Ganglia in Two Rat Pain Models. Neurosci Bull. 2018 doi: 10.1007/s12264-017-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li H., Shang M., Liu L., Lin X., Hu J., Han Q., et al. Protein kinase G signaling pathway is involved in sympathetically maintained pain by modulating ATP-sensitive potassium channels. Reg Anesth Pain Med. 2021 doi: 10.1136/rapm-2021-102539. [DOI] [PubMed] [Google Scholar]
- 147.Niedermair T., Straub R.H., Brochhausen C., Grässel S. Impact of the sensory and sympathetic nervous system on fracture healing in ovariectomized mice. Int J Mol Sci. 2020;21:1–18. doi: 10.3390/ijms21020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alves C.J., Neto E., Sousa D.M., Leitão L., Vasconcelos D.M., Ribeiro-Silva M., et al. Fracture pain-Traveling unknown pathways. Bone. 2016 doi: 10.1016/j.bone.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 149.Oostinga D., Steverink J.G., van Wijck A.J.M., Verlaan J.J. An understanding of bone pain: A narrative review. Bone. 2020 doi: 10.1016/j.bone.2020.115272. [DOI] [PubMed] [Google Scholar]
- 150.Wang Q., Yang J., Wang H., Shan B., Yin C., Yu H., et al. Fibroblast growth factor 13 stabilizes microtubules to promote Na+ channel function in nociceptive DRG neurons and modulates inflammatory pain. J Adv Res. 2021 doi: 10.1016/j.jare.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chapurlat R.D., Gensburger D., Jimenez-Andrade J.M., Ghilardi J.R., Kelly M., Mantyh P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis. 2012;7:1–9. doi: 10.1186/1750-1172-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castañeda-corral G, Jimenez-andrade JM, Bloom AP, Reid N, Mantyh WG, Kaczmarska MJ, et al. the Majority of Myelinated and Unmyelinated 2012:196–207. https://doi.org/10.1016/j.neuroscience.2011.01.039.THE. [DOI] [PMC free article] [PubMed]
- 153.Tomlinson R.E., Li Z., Zhang Q., Goh B.C., Li Z., Thorek D.L.J., et al. NGF-TrkA Signaling by Sensory Nerves Coordinates the Vascularization and Ossification of Developing Endochondral Bone. Cell Rep. 2016;16:2723–2735. doi: 10.1016/j.celrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tomlinson R.E., Li Z., Li Z., Minichiello L., Riddle R.C., Venkatesan A., et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A. 2017;114:E3632–E3641. doi: 10.1073/pnas.1701054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nencini S., Morgan M., Thai J., Jobling A.I., Mazzone S.B., Ivanusic J.J. Piezo2 Knockdown Inhibits Noxious Mechanical Stimulation and NGF-Induced Sensitization in A-Delta Bone Afferent Neurons. Front Physiol. 2021;12:1–13. doi: 10.3389/fphys.2021.644929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee S., Hwang C., Marini S., Tower R.J., Qin Q., Negri S., et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat Commun. 2021;12:1–20. doi: 10.1038/s41467-021-25143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rapp A.E., Kroner J., Baur S., Schmid F., Walmsley A., Mottl H., et al. Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. J Orthop Res. 2015;33:1235–1241. doi: 10.1002/jor.22892. [DOI] [PubMed] [Google Scholar]
- 158.Nwosu L.N., Mapp P.I., Chapman V., Walsh D.A. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis. 2016;75:554–561. doi: 10.1136/annrheumdis-2014-207203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fioravanti G., Hua P.Q., Tomlinson R.E. The TrkA agonist gambogic amide augments skeletal adaptation to mechanical loading. Bone. 2021;147 doi: 10.1016/j.bone.2021.115908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ulmann L., Hirbec H., Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010 doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park J.Y., Pillinger M.H., Abramson S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 162.Pinto LG, Souza GR, Kusuda R, Lopes AH, Sant’Anna MB, Cunha FQ, et al. Non-Peptidergic Nociceptive Neurons Are Essential for Mechanical Inflammatory Hypersensitivity in Mice. Mol Neurobiol 2019. https://doi.org/10.1007/s12035-019-1494-5. [DOI] [PubMed]
- 163.Zhou Y.M., Wu L., Wei S., Jin Y., Liu T.T., Qiu C.Y., et al. Enhancement of acid-sensing ion channel activity by prostaglandin E2 in rat dorsal root ganglion neurons. Brain Res. 2019 doi: 10.1016/j.brainres.2019.146442. [DOI] [PubMed] [Google Scholar]
- 164.Chen H., Hu B., Lv X., Zhu S., Zhen G., Wan M., et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat Commun. 2019;10 doi: 10.1038/s41467-018-08097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tsutsumi R., Xie C., Wei X., Zhang M., Zhang X., Flick L.M., et al. PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J Bone Miner Res. 2009;24:1753–1762. doi: 10.1359/jbmr.090412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blackwell K.A., Raisz L.G., Pilbeam C.C. Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol Metab. 2010 doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ni S., Ling Z., Wang X., Cao Y., Wu T., Deng R., et al. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat Commun. 2019;10 doi: 10.1038/s41467-019-13476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boraschi-Diaz I., Chen G., Polak-Nachumow J., Young R.N., Rauch F. Effects of treatment with a bone-targeted prostaglandin E2 receptor 4 agonist C3 (Mes-1007) in a mouse model of severe osteogenesis imperfecta. Bone. 2021 doi: 10.1016/j.bone.2021.115867. [DOI] [PubMed] [Google Scholar]
- 169.Liu S., Wang Q., Li Z., Ma L., Li T., Li Y., et al. TRPV1 Channel Activated by the PGE2/EP4 Pathway Mediates Spinal Hypersensitivity in a Mouse Model of Vertebral Endplate Degeneration. Oxid Med Cell Longev. 2021 doi: 10.1155/2021/9965737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xue P., Wang S., Lyu X., Wan M., Li X., Ma L., et al. PGE2/EP4 skeleton interoception activity reduces vertebral endplate porosity and spinal pain with low-dose celecoxib. Bone Res. 2021;9 doi: 10.1038/s41413-021-00155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang C., Zhu J., Jia J., Guan Z., Sun T., Zhang W., et al. Effect of Single Versus Multiple Fractures on Systemic Bone Loss in Mice. J Bone Miner Res. 2021 doi: 10.1002/jbmr.4211. [DOI] [PubMed] [Google Scholar]
- 172.Osipov B., Paralkar M.P., Emami A.J., Cunningham H.C., Tjandra P.M., Pathak S., et al. Sex differences in systemic bone and muscle loss following femur fracture in mice. J Orthop Res. 2022 doi: 10.1002/jor.25116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sarahrudi K., Thomas A., Albrecht C., Aharinejad S. Strongly enhanced levels of sclerostin during human fracture healing. J Orthop Res. 2012 doi: 10.1002/jor.22129. [DOI] [PubMed] [Google Scholar]
- 174.Ke H.Z., Richards W.G., Li X., Ominsky M.S. Sclerostin and dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012 doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 175.Kim M.H., Jung K., Nam K.H., Jang H.J., Lee S.W., Kim Y., et al. inhibits signal transduction of IL-6 and prevents ovariectomy-induced bone loss by suppressing osteoclastogenesis. Arch Pharm Res. 2016 doi: 10.1007/s12272-016-0810-0. [DOI] [PubMed] [Google Scholar]
- 176.He B., Yin X., Hao D., Zhang X., Zhang Z., Zhang K., et al. Blockade of IL-6 alleviates bone loss induced by modelled microgravity in mice. Can J Physiol Pharmacol. 2020 doi: 10.1139/cjpp-2019-0632. [DOI] [PubMed] [Google Scholar]
- 177.Wang J., Chen J., Zhang B., Jia X. IL-6 regulates the bone metabolism and inflammatory microenvironment in aging mice by inhibiting Setd7. Acta Histochem. 2021 doi: 10.1016/j.acthis.2021.151718. [DOI] [PubMed] [Google Scholar]
- 178.Li Y., Lu L., Xie Y., Chen X., Tian L., Liang Y., et al. Interleukin-6 Knockout Inhibits Senescence of Bone Mesenchymal Stem Cells in High-Fat Diet-Induced Bone Loss. Front Endocrinol (Lausanne) 2021 doi: 10.3389/fendo.2020.622950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vannucci L., Fossi C., Quattrini S., Guasti L., Pampaloni B., Gronchi G., et al. Calcium Intake in bone health: A focus on calcium-rich mineral waters. Nutrients. 2018 doi: 10.3390/nu10121930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fischer V., Haffner-Luntzer M., Amling M., Ignatius A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur. Cells Mater. 2018 doi: 10.22203/eCM.v035a25. [DOI] [PubMed] [Google Scholar]
- 181.Hu Z.C., Tang Q., Sang C.M., Tang L., Li X., Zheng G., et al. Comparison of fracture risk using different supplemental doses of Vitamin D, calcium or their combination: A network meta-analysis of randomised controlled trials. BMJ Open. 2019 doi: 10.1136/bmjopen-2018-024595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McAlindon T., LaValley M., Schneider E., Nuite M., Lee J.Y., Price L.L., et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA - J Am Med Assoc. 2013 doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rizzoli R., Stevenson J.C., Bauer J.M., Van Loon L.J.C., Walrand S., Kanis J.A., et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Maturitas. 2014 doi: 10.1016/j.maturitas.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 184.Gaffney-Stomberg E., Lutz L.J., Rood J.C., Cable S.J., Pasiakos S.M., Young A.J., et al. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: A randomized, double-blind, placebo controlled trial. Bone. 2014 doi: 10.1016/j.bone.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 185.Paschalis E.P., Gamsjaeger S., Hassler N., Fahrleitner-Pammer A., Dobnig H., Stepan J.J., et al. Vitamin D and calcium supplementation for three years in postmenopausal osteoporosis significantly alters bone mineral and organic matrix quality. Bone. 2017 doi: 10.1016/j.bone.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 186.Wu A.M., Huang C.Q., Lin Z.K., Tian N.F., Ni W.F., Wang X.Y., et al. The relationship between vitamin a and risk of fracture: Meta-analysis of prospective studies. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2237. [DOI] [PubMed] [Google Scholar]
- 187.Cheung A.M., Tile L., Lee Y., Tomlinson G., Hawker G., Scher J., et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): A randomized controlled trial. PLoS Med. 2008 doi: 10.1371/journal.pmed.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zheng L.Z., Wang J.L., Xu J.K., Zhang X.T., Liu B.Y., Huang L., et al. Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model. Biomaterials. 2020 doi: 10.1016/j.biomaterials.2020.119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hayashi M., Nakashima T., Yoshimura N., Okamoto K., Tanaka S., Takayanagi H. Autoregulation of Osteocyte Sema3A Orchestrates Estrogen Action and Counteracts Bone Aging. Cell Metab. 2019 doi: 10.1016/j.cmet.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 190.Chow J., Tobias J.H., Colston K.W., Chambers T.J. Estrogen maintains trabecular bone volume in rats not only by suppression of bone resorption but also by stimulation of bone formation. J Clin Invest. 1992 doi: 10.1172/JCI115588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bolognese M.A. SERMs and SERMs with estrogen for postmenopausal osteoporosis. Rev Endocr Metab Disord. 2010 doi: 10.1007/s11154-010-9137-1. [DOI] [PubMed] [Google Scholar]
- 192.Naot D., Musson D.S., Cornish J. The activity of peptides of the calcitonin family in bone. Physiol Rev. 2019 doi: 10.1152/physrev.00066.2017. [DOI] [PubMed] [Google Scholar]
- 193.Russell F.A., King R., Smillie S.J., Kodji X., Brain S.D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014 doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Surface L.E., Burrow D.T., Li J., Park J., Kumar S., Lyu C., et al. ATRAID regulates the action of nitrogen-containing bisphosphonates on bone. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aav9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Weinstein R.S., Roberson P.K., Manolagas S.C. Giant Osteoclast Formation and Long-Term Oral Bisphosphonate Therapy. N Engl J Med. 2009 doi: 10.1056/nejmoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bonnet N., Bourgoin L., Biver E., Douni E., Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019 doi: 10.1172/JCI125915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McDonald M.M., Khoo W.H., Ng P.Y., Xiao Y., Zamerli J., Thatcher P., et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021 doi: 10.1016/j.cell.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Collison J. Biosimilar for teriparatide safe and effective. Nat Rev Rheumatol. 2019 doi: 10.1038/s41584-019-0263-1. [DOI] [PubMed] [Google Scholar]
- 199.Erratum: Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: A randomized clinical trial (JAMA - Journal of the American Medical Association 316:7 (722–733)) JAMA - J Am Med Assoc. 2016;2017 doi: 10.1001/jama.2016.20139. [DOI] [PubMed] [Google Scholar]
- 200.Hodsman A.B., Bauer D.C., Dempster D.W., Dian L., Hanley D.A., Harris S.T., et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: A review of the evidence and suggested guidelines for its use. Endocr Rev. 2005 doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 201.Zhang C., Zhu J., Jia J., Guan Z., Sun T., Zhang W., et al. Long-term pretreatment with alendronate inhibits calvarial defect healing in an osteoporotic rat model. J Bone Miner Metab. 2021 doi: 10.1007/s00774-021-01235-0. [DOI] [PubMed] [Google Scholar]
- 202.Zhang C., Song C. Combination Therapy of PTH and Antiresorptive Drugs on Osteoporosis: A Review of Treatment Alternatives. Front Pharmacol. 2021 doi: 10.3389/fphar.2020.607017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang C., Zhu J., Jia J., Guan Z., Sun T., Zhang W., et al. Once-weekly parathyroid hormone combined with ongoing long-term alendronate treatment promotes osteoporotic fracture healing in ovariectomized rats. J Orthop Res. 2021 doi: 10.1002/jor.24953. [DOI] [PubMed] [Google Scholar]
- 204.Halanski M., Noonan K.J. Cast and splint immobilization: Complications. J Am Acad Orthop Surg. 2008 doi: 10.5435/00124635-200801000-00005. [DOI] [PubMed] [Google Scholar]
- 205.Boyd A.S., Benjamin H.J., Asplund C. Splints and casts: Indications and methods. Am Fam Physician. 2009 [PubMed] [Google Scholar]
- 206.Leslie W.D., Schousboe J.T., Morin S.N., Martineau P., Lix L.M., Johansson H., et al. Fracture risk following high-trauma versus low-trauma fracture: a registry-based cohort study. Osteoporos Int. 2020;31:1059–1067. doi: 10.1007/s00198-019-05274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hu Z.C., He L.J., Chen D., Bin L.X., Feng Z.H., Fu C.W., et al. An enhanced recovery after surgery program in orthopedic surgery: A systematic review and meta-analysis. J Orthop Surg Res. 2019 doi: 10.1186/s13018-019-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ljungqvist O., De Boer H.D., Balfour A., Fawcett W.J., Lobo D.N., Nelson G., et al. Opportunities and Challenges for the Next Phase of Enhanced Recovery after Surgery: A Review. JAMA Surg. 2021 doi: 10.1001/jamasurg.2021.0586. [DOI] [PubMed] [Google Scholar]
- 209.Delezie J., Weihrauch M., Maier G., Tejero R., Ham D.J., Gill J.F., et al. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc Natl Acad Sci U S A. 2019 doi: 10.1073/pnas.1900544116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pedersen L., Pilegaard H., Hansen J., Brandt C., Adser H., Hidalgo J., et al. Exercise-induced liver chemokine CXCL-1 expression is linked to muscle-derived interleukin-6 expression. J Physiol. 2011 doi: 10.1113/jphysiol.2010.200733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pedersen L., Hojman P. Muscle-to-organ cross talk mediated by myokines. Adipocyte. 2012 doi: 10.4161/adip.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tanaka K ichiro, Matsumoto E, Higashimaki Y, Sugimoto T, Seino S, Kaji H. FAM5C is a soluble osteoblast differentiation factor linking muscle to bone. Biochem Biophys Res Commun 2012. https://doi.org/10.1016/j.bbrc.2011.12.147. [DOI] [PubMed]
- 213.Mathes S., Fahrner A., Ghoshdastider U., Rüdiger H.A., Leunig M., Wolfrum C., et al. FGF-2–dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc Natl Acad Sci U S A. 2021 doi: 10.1073/pnas.2021013118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cong X.X., Gao X.K., Rao X.S., Wen J., Liu X.C., Shi Y.P., et al. Rab5a activates IRS1 to coordinate IGF-AKT-mTOR signaling and myoblast differentiation during muscle regeneration. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vassilakos G., Barton E.R. Insulin-like growth factor i regulation and its actions in skeletal muscle. Compr Physiol. 2019 doi: 10.1002/cphy.c180010. [DOI] [PubMed] [Google Scholar]
- 216.Murata K., Sugie K., Takamure M., Fujimoto T., Ueno S. Eosinophilic major basic protein and interleukin-5 in eosinophilic myositis. Eur J Neurol. 2003 doi: 10.1046/j.1468-1331.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 217.Chowdhury S., Schulz L., Palmisano B., Singh P., Berger J.M., Yadav V.K., et al. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J Clin Invest. 2020 doi: 10.1172/JCI133572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Haugen F., Norheim F., Lian H., Wensaas A.J., Dueland S., Berg O., et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am J Physiol - Cell Physiol. 2010 doi: 10.1152/ajpcell.00094.2009. [DOI] [PubMed] [Google Scholar]
- 219.Pistilli E.E., Bogdanovich S., Garton F., Yang N., Gulbin J.P., Conner J.D., et al. Loss of IL-15 receptor α alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest. 2011 doi: 10.1172/JCI44945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Reza M.M., Subramaniyam N., Sim C.M., Ge X., Sathiakumar D., McFarlane C., et al. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun. 2017 doi: 10.1038/s41467-017-01131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Broholm C., Pedersen B.K. Leukaemia inhibitory factor - An exercise-induced myokine. Exerc Immunol Rev. 2010 [PubMed] [Google Scholar]
- 222.He S., Fu T., Yu Y., Liang Q., Li L., Liu J., et al. IRE1α regulates skeletal muscle regeneration through myostatin mRNA decay. J Clin Invest. 2021 doi: 10.1172/JCI143737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sterne G.D., Coulton G.R., Brown R.A., Green C.J., Terenghi G. Neurotrophin-3-enhanced nerve regeneration selectively improves recovery of muscle fibers expressing myosin heavy chains 2b. J Cell Biol. 1997 doi: 10.1083/jcb.139.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ho A.T.V., Palla A.R., Blake M.R., Yucel N.D., Wang Y.X., Magnusson K.E.G., et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration & strength. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1705420114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cencetti F., Bernacchioni C., Tonelli F., Roberts E., Donati C., Bruni P. TGFβ1 evokes myoblast apoptotic response via a novel signaling pathway involving S1P4 transactivation upstream of Rho-kinase-2 activation. FASEB J. 2013 doi: 10.1096/fj.13-228528. [DOI] [PubMed] [Google Scholar]
- 226.Lu Y., Yuan B., Qin C., Cao Z., Xie Y., Dallas S.L., et al. The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa c-terminal fragment. J Bone Miner Res. 2011 doi: 10.1002/jbmr.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Heiland G.R., Zwerina K., Baum W., Kireva T., Distler J.H., Grisanti M., et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis. 2010 doi: 10.1136/ard.2010.132852. [DOI] [PubMed] [Google Scholar]
- 228.Wöhrle S., Bonny O., Beluch N., Gaulis S., Stamm C., Scheibler M., et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J Bone Miner Res. 2011 doi: 10.1002/jbmr.478. [DOI] [PubMed] [Google Scholar]
- 229.Frisch R.N., Curtis K.M., Aenlle K.K., Howard G.A. Hepatocyte growth factor and alternative splice variants - expression, regulation and implications in osteogenesis and bone health and repair. Expert Opin Ther Targets. 2016 doi: 10.1517/14728222.2016.1162293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yan J., Herzog J.W., Tsang K., Brennan C.A., Bower M.A., Garrett W.S., et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Atkins G.J., Rowe P.S., Lim H.P., Welldon K.J., Ormsby R., Wijenayaka A.R., et al. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res. 2011 doi: 10.1002/jbmr.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yi Q., Liu H., Feng J., Wu Y., Sun W., Ou M., et al. Splicing factor-modulated generation of mechano growth factor regulates physiological processes in osteoblasts under mechanical stimuli. Cell Adhes Migr. 2019 doi: 10.1080/19336918.2019.1686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jin U.H., Kim K.S., Park S.Y., Chung K.H., Kim D.S., Chang Y.C., et al. Effect of Buthus martensi Karsch on aromatase activity and cytokine-inducded NOS and NO production in osteoblasts and leukaemic cell line FLG 29.1. Immunopharmacol Immunotoxicol. 2006 doi: 10.1080/08923970600816723. [DOI] [PubMed] [Google Scholar]
- 234.Han Y., You X., Xing W., Zhang Z., Zou W. Paracrine and endocrine actions of bone - The functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018 doi: 10.1038/s41413-018-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yahiro Y., Maeda S., Morikawa M., Koinuma D., Jokoji G., Ijuin T., et al. BMP-induced Atoh8 attenuates osteoclastogenesis by suppressing Runx2 transcriptional activity and reducing the Rankl/Opg expression ratio in osteoblasts. Bone Res. 2020 doi: 10.1038/s41413-020-00106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen H., Hu B., Lv X., Zhu S., Zhen G., Wan M., et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat Commun. 2019 doi: 10.1038/s41467-018-08097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cao X. RANKL-RANK signaling regulates osteoblast differentiation and bone formation. Bone Res. 2018 doi: 10.1038/s41413-018-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Reppe S., Noer A., Grimholt R.M., Halldórsson B.V., Medina-Gomez C., Gautvik V.T., et al. Methylation of bone SOST, its mRNA, and serum sclerostin levels correlate strongly with fracture risk in postmenopausal women. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2342. [DOI] [PubMed] [Google Scholar]
- 239.Zhen G., Cao X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci. 2014 doi: 10.1016/j.tips.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hu K., Olsen B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016 doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Qiang Y.W., Shaughnessy J.D., Yaccoby S. Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood. 2008 doi: 10.1182/blood-2007-10-120253. [DOI] [PMC free article] [PubMed] [Google Scholar]