Abstract
Background
Brain-derived neurotrophic factor (BDNF) is a modulator of neuroplasticity in the brain. It plays an important role in the pathophysiology of depression through the stress pathway. The information about correlation of BDNF levels with depression severity and treatment response in Indian population is scarce.
Methods
Consecutive 60 never treated cases with depression reporting to a large tertiary care psychiatry unit and 60 healthy matched controls from 01 January 2016 to 31 December 2016 were enrolled for study. Sociodemographic data were collected. Diagnosis of depression was carried out as per International Classification of Diseases-10th revision (ICD-10) diagnostic criteria for research. The Hamilton Rating Scale for Depression (HRSD) was administered and accordingly scored. Venous blood for BDNF levels was collected from all cases and controls. Cases were reassessed after 04 weeks of treatment with HRSD and BDNF levels.
Results
The mean level of serum BDNF among cases (18.56 ng/ml) was found to be reduced significantly as compared with healthy controls (32.41 ng/ml). The mean serum BDNF level (18.56 ng/ml) in never treated cases was significantly negatively correlated with the median clinical HRSD score (18.5). There was a significant increase in the mean level of serum BDNF after antidepressant treatment.
Conclusion
The study has revealed statistically significant low levels of serum BDNF in cases not exposed to treatment with depression compared with healthy controls. There was significant negative correlation of levels of serum BDNF with depression severity. The levels of serum BDNF significantly increased after four weeks of treatment.
Keywords: Brain-derived neurotrophic factor, Depression, Antidepressants
Introduction
Major depression is a serious neuropsychiatric illness and has been predicted to be second to ischaemic heart disease in terms of premature death or disability. The lifetime prevalence of major depressive episodes as per Diagnostic and Statistical Manual of Mental disorders, fourth edition criteria was found to be ranging from 11.1% to 14.6%.1 Brain-derived neurotrophic factor (BDNF) is a 14 kDa protein and is secreted primarily by neurons in the brain. It is present in most parts of the brain. The hippocampus has been found to have highest levels of BDNF in the brain. It serves an important function of promoting neurogenesis and modulation of neuroplasticity in the brain.2
The theory of stress mechanism dysfunction has been hypothesized in the neurobiology of depression. As per this theory, stress induces hypothalamic-pituitary-adrenal axis hyperactivity, which results in sustained high levels of glucocorticoids. The aforementioned process results in decreased BDNF levels in the brain, especially in the hippocampus. This deficiency of BDNF induces cell damage, reduction in neurogenesis and decreased dendritic arborisation, which leads to hippocampal damage and structural changes.3,4,5,6 The ‘neurotrophin’ hypothesis of depression states that BDNF reduction induced by stress leads to damage to the hippocampus which may result in depression in predisposed individuals. The hippocampus damage improves with increase in BDNF after treatment of depression. This also helps in preventing further damage.7,8,9,10 Thus, poor ability of the nervous system to develop proper neuroplasticity, due to deficiency in BDNF, would contribute to the pathogenesis of depression.4 Researchers have also found that, higher neuroticism scores are associated with low BDNF levels, suggesting its utility in measurement in mood disorders.11 Hence, there is a role of assessment of BDNF levels in patients with depression.
Blood and plasma levels of BDNF were found to be similar to hippocampus BDNF levels in the published literature. This information can be used for peripheral measures of BDNF as a marker for brain BDNF levels.12The literature search on BDNF in depression across the world revealed common finding of lower BDNF levels in patients with depression not exposed to treatment as compared with controls. The average BDNF levels were around 20 ng/ml in patients with depression not exposed to treatment in a meta-analysis involving 15 studies. The BDNF levels of controls were in the range of 26-32 ng/ml in above meta-analysis.13 Serum BDNF was found to be lower in first episode depression and recurrent depressive disorder in an Indian study of 85 subjects compared with controls.14 In a meta-analysis of six studies, it was found that BDNF levels increase with most of the antidepressant treatment.15 Yoga therapy, alone or in combination with medications, is associated with improved neuropsychological functions and neuroplastic effects in patients with depression.16
Studies have also shown that the levels of BDNF correlate with the depression severity.17,18 However, a study involving 962 drug-free depressed patients did not reveal any correlation with severity of depression or with the number of episodes.19
Very few Indian studies have been conducted on BDNF and depression. There have been few studies on correlation of BDNF levels with severity of depression and follow-up treatment response in Indian population. The study was, therefore, undertaken to assess serum BDNF levels in patients with depression who are not exposed to treatment and to correlate in terms of its severity, followed by reassessment after four weeks of treatment.
Material and methods
The study was conducted in a large tertiary care hospital psychiatry unit of India, and the BDNF estimation was carried out at its laboratory. The proposal of the study was approved by the hospital ethics committee.
Cases
Drug-naïve Indian patients with depression in the age group of 18–60 years were included as cases in the study. The exclusion criteria were subjects having mental subnormality, alcohol and drug abuse, central nervous system disorders, psychiatric symptomatology of psychosis, bipolar disorder, catatonia and lastly treated depression cases.
Controls
Controls included healthy consenting Indian volunteers who were adequately matched for age and sex. The same exclusion criteria were used as for the cases.
Methods
The study design was a case-control study. The sample size of 60 was considered depending on the availability of fresh cases of depression meeting the inclusion and exclusion criteria. Sixty never treated consecutive cases with depression reporting to a large tertiary care psychiatry unit from 01 January 2016 to 31 December 2016 were enrolled for the study. Sociodemographic data were collected. Diagnosis of depression was carried out as per ICD-10 diagnostic criteria for research by one psychiatrist. The Hamilton Rating Scale for Depression (HRSD) was assessed by the coworker psychiatrist, who was blind to diagnosis of cases. Healthy controls were also enrolled for the study. Informed consent was obtained from all subjects. Venous blood for BDNF levels was collected from all cases (before starting medication) and controls. All cases were given antidepressants (Tab Escitalopram 10–20 mg per day) depending on the severity of depression and response to treatment and were also given psychoeducation by the psychiatrist managing the case. None of the patients were exposed to brain stimulation therapy. The cases were reassessed after 04 weeks of treatment. The HRSD was reassessed by the coworker psychiatrist, who not only was blind to the diagnosis of cases but also was not involved in the management of cases. The BDNF levels were reassessed.
Assessment tools
HRSD-17 item version:HRSD-17 is a clinician administered scale, used in adults to assess severity of depression and subsequent response to treatment. The assessment is based on clinical evaluation, caregiver information and nurse report. The rating scale assesses multiple facets of depression. These includes self-harm ideations, biodrives, affective, cognitive and somatic components. The total score of rating scale is 52. The score of <7 is considered as normal. The score of 8–13 denotes mild depression. The score of 14–18 is suggestive of moderate severity of depression. The score of 19–22 suggests severe depression and ≥23 is suggestive of very severe depression.20
Serum BDNF estimation
Serum BDNF estimation was carried out by human BDNF enzyme-linked immunosorbent assay kits (Boster Immuno leader, Boster Biological Technology Co. Ltd., CA, USA, Code: EK0307). The sensitivity of the kit is < 2 pg/ml, and the measuring interval is from 31.2 pg/ml to 2000 pg/ml. The intraassay precision of the test is 3.5–4.9, and interassay precision of the test is 7.2–7.9. The venous sample was collected from cases before administration of any treatment and also from controls in anticoagulant-free containers between 0900 h and 1100 h. The serum was separated by centrifugation after 30 min. The collected samples were given coding. The laboratory staff was blinded to the study subjects. The samples were stored at -20° C. The test is based on sandwich enzyme-linked immunosorbent assay technology and was performed as per the manufacturer's instructions.12
Statistical analysis
Data were entered and statistically analysed using Statistical Package for Social Sciences program, version 21. The results were reported as mean and median with 95 percent confidence intervals. The Wilcoxon signed-rank test was performed to compare scores of the HRSD and BDNF levels of cases before and after treatment. The Mann-Whitney U test was performed to compare cases (after treatment) and healthy controls in terms of HRSD scores and serum BDNF levels. Spearman's correlation coefficient was computed between pretreatment and posttreatment BDNF and HRSD scores. In this study, the level of significance was kept at P < 0.05 for all analysis.
Results
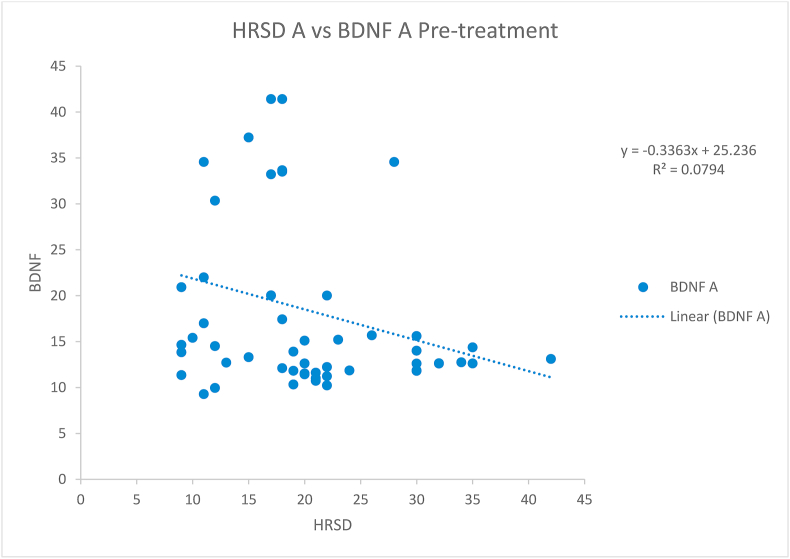
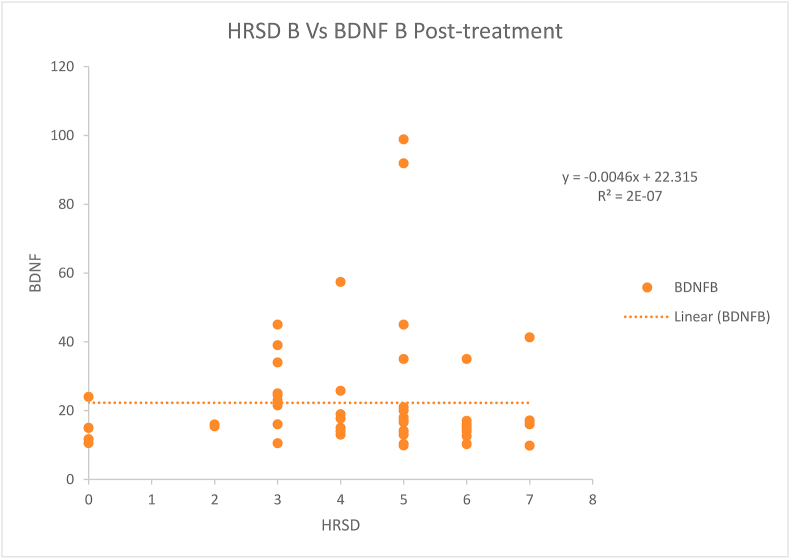
The difference between cases and controls regarding age, income, sex and education was not statistically significant (Table 1). The mean BDNF levels (18.56 ng/ml) before starting the treatment among the patients were negatively correlated (statistically significant rs = −0.317, p-value = 0.013) with the HRSD score (median = 18.5) taken before starting the treatment (Fig. 1). However, after treatment, the negative correlation (rs=−0.151, p-value = 0.246) of mean BDNF levels (22.29) and HRSD scores (median 5) was not found to be statistically significant (Fig. 2). The difference of HRSD scores and BDNF levels before and after treatment of cases was statistically significant (Table 2, Table 3). The difference of HRSD scores and BDNF levels of controls and of cases after treatment was statistically significant (Table 4, Table 5). The mean BDNF level was lowest in severe depression and has shown statistically significant improvement after treatment (Table 6).
Table 1.
Comparison of cases and controls – matched for age, income, sex and education.
| Socio-demographic variables | Cases (N = 60) | Controls (N = 60) | t/x2 | p value |
|---|---|---|---|---|
| Age (in years) – mean (SD) | 32.38 (6.5) | 33 (9) | 0.43 (t) | 0.66a |
| Monthly income (Rs) – mean (SD) | 22450 (14546) | 25850 (14560) | 1.28 (t) | 0.20a |
| Sex (numbers) | M (42), F (18) | M (44), F (16) | 0.16 (x2) | 0.68b |
| Education (percentage) | P (6%), H (72%), G (22%) | P (17%), H (73%), G (10%) | 1.65 (x2) | 0.44b |
M, male; F, female; P, primary and middle school; H, high school; G, graduate.
Two-sample t test is not significant at 0.05 level.
Chi square (x2) test is not significant at 0.05 level.
Fig. 1.
Scatter plot showing Spearman correlation between the Hamilton Rating Scale for Depression (HRSD) and brain-derived neurotrophic factor (BDNF) levels before treatment of cases. HRSD A: HRSD score before starting treatment in cases. BDNF A: BDNF levels before starting treatment in patients (ng/ml). The scatter plot showed a significant linear negative correlation (rs = −0.317, p-value = 0.013) between HRSD scores and BDNF levels before treatment.
Fig. 2.
Scatter plot showing Spearman correlation between the Hamilton Rating Scale for Depression (HRSD) and brain-derived neurotrophic factor (BDNF) levels after treatment of cases. HRSD B: HRSD score after 4 weeks of treatment in cases. BDNF B: BDNF levels after 4 weeks of treatment in cases (ng/ml). The scatter plot showed no significant negative correlation (rs=−0.151, p-value = 0.246) between HRSD scores and BDNF levels after treatment.
Table 2.
HRSD scores before and after treatment of cases (N = 60).
| HRSD score | HRSD (before treatment) | HRSD (after treatment) | p-value |
|---|---|---|---|
| Min | 9 | 0 | <0.001 |
| Max | 42 | 7 | |
| Median | 18.5 | 5 | |
| Q1 | 14.5 | 3 | |
| Q3 | 22.25 | 6 |
p-value <0.05 (significant). Wilcoxon signed-rank test is used. There is significant reduction in HRSD score with treatment (p = <0.001). HRSD, Hamilton Rating Scale for Depression
Table 3.
BDNF scores before and after treatment of cases (N = 60).
| BDNF score | BDNF (before treatment) (ng/ml) | BDNF (after treatment (ng/ml) | p-value |
|---|---|---|---|
| Min | 9.27 | 9.80 | 0.003 |
| Max | 41.40 | 98.84 | |
| Mean | 18.56 | 22.29 | |
| SD | 9.18 | 17.06 | |
| Median | 14.44 | 16.00 | |
| Q1 | 12.04 | 14.00 | |
| Q3 | 20.25 | 23.25 |
p-value < 0.05 (significant). The Wilcoxon signed-rank test is used. The difference in BDNF levels before and after treatment is significant (p value = 0.003). BDNF, brain-derived neurotrophic factor.
Table 4.
HRSD scores of controls (N = 60) and cases (after treatment, N = 60).
| HRSD score | HRSD |
p-value | |
|---|---|---|---|
| HRSD of controls | HRSD of cases (after treatment) | ||
| Min | 0 | 0 | <0.001 |
| Max | 6 | 7 | |
| Median | 2 | 5 | |
| Q1 | 2 | 3 | |
| Q3 | 3 | 6 | |
p value < 0.05 (significant). The Mann-Whitney U test is used. The difference between HRSD scores of cases (after treatment) and controls is significant (p value=<0.001). HRSD, Hamilton Rating Scale for Depression.
Table 5.
BDNF levels of controls (N = 60) and cases (after treatment, N = 60).
| BDNF score | BDNF of controls (ng/ml) | BDNF of cases (after treatment) (ng/ml) | p-value |
|---|---|---|---|
| Min | 8.20 | 9.80 | 0.001 |
| Max | 106.52 | 98.84 | |
| Mean | 32.41 | 22.29 | |
| SD | 28.01 | 17.06 | |
| Median | 20.84 | 16.00 | |
| Q1 | 17.37 | 14.00 | |
| Q3 | 31.06 | 23.25 |
p-value < 0.05 (significant). The Mann-Whitney U test is used. The difference between BDNF levels of controls and cases (after treatment) is significant (p value = 0.001). BDNF, brain-derived neurotrophic factor.
Table 6.
BDNF level improvement of cases (N = 60) after treatment with respect to severity of depression.
| Depression Severity | BDNF (before treatment) (ng/ml) |
BDNF (after treatment) (ng/ml) |
p-value within the group | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Mild (n = 15) | 19.42 | 8.9 | 20.64 | 9.86 | 0.172 |
| Moderate (n = 15) | 27.16 | 10.22 | 34.7 | 26.75 | 0.172 |
| Severe (n = 15) | 12.91 | 3.15 | 19.63 | 12.54 | 0.006∗# |
| Very severe (n = 15) | 14.74 | 6.64 | 14.19 | 3.15 | 0.733 |
| p-value | 0.001∗ | 0.003∗ | |||
∗Significant (p-value < 0.05). The Kruskal-Wallis test is used.
∗#Significant (p-value < 0.05). The Wilcoxon signed-rank test is used.
BDNF, brain-derived neurotrophic factor.
Discussion
In this study, 70% (42) of cases were men and 30% (18) were women, as the hospital caters largely for male population. The mean age of the never treated cases with depression was 32.38 years (Standard Deviation (SD) 6.5), which was in the similar range as in other studies.14,21 Majority (96.7%) of the cases were married and were educated (71%) up to high school. The mean BDNF levels of both cases and controls obtained in our study were in the similar range as of the other studies.16,21 The aforementioned findings suggested that BDNF levels become low in never treated cases with depression and have a role in pathophysiology of depression. With this, we can infer that low serum BDNF level suggests ‘state’ marker for depression. The mean serum BDNF level significantly increased from 18.56 ng/ml to 22.29 ng/ml with antidepressant treatment, which was in consonance with other studies and meta-analyses.15,19 This rise in BDNF levels after treatment supports the ‘neurotrophin’ hypothesis of depression. The same can be used as a marker for assessing the antidepressant efficacy in future.
The findings of negative correlation of serum BDNF levels with severity of depression are consistent with those of other studies.17,21 The possible explanation for no statistically significant correlation after treatment is that serum BDNF levels might continue to be low, despite clinical improvement. This could indicate that low levels of BDNF are a consequence of depressive symptoms that persist into early remission.
The observation of association between BDNF levels and severity of depression has not been consistent in various studies. The findings in our study of low levels of BDNF in severe depression and significant improvement after treatment were elucidated in some studies.22,23,24 The isolated finding of comparable high mean BDNF levels in moderate depression could be due to absence of adverse life events, increased resilience and the duration of illness, which have not been taken into account in the present study. Exposure to adverse life events has been elucidated by researchers as an alternative factor for low BDNF levels in patients with depression and bipolar disorders.25,26 Hence, it is recommended that multiple variables of childhood trauma, stressful life events, genetic vulnerability and environmental perspectives should be considered while studying BDNF and depression.27
The finding of significantly low serum BDNF levels after treatment with respect to controls was also observed by other researchers. They have observed that serum BDNF levels in early remission phase or even in euthymic phase of depression remained low compared to healthy controls.28 These low BDNF levels during the aforementioned period may be explained by the possibility of a scar of the depressive episode. This requires further research before coming to any conclusion. The importance of yoga therapy in depression especially in Indian settings needs to be considered while conducting research.16
The strengths of the study are translation research on finding the biomarker for depression with future clinical applicability. The limitations of the study are, firstly, the data were collected without considering causative factors such as childhood adversity, as the same may be an independent factor affecting BDNF levels. Secondly, serum BDNF was assumed to be the same as BDNF levels in the brain, although the same was validated in a previous study. Thirdly, confounding variables such as physical activity, smoking and body mass index were not factored in the study, as they may have association with the serum BDNF levels; fourthly, the sample size was modest, and finally, the study had comparable lesser female cases. The review process of the project after completion led to delay in the publication of the study.
To finally conclude, the study has revealed never treated cases of depression had statistically significant low serum BDNF levels in comparison with matched healthy controls. The low serum BDNF levels significantly increased after four weeks of treatment with improvement of depression among cases. Serum BDNF levels showed significant negative correlation with depression severity. However, further studies, with consideration of confounding factors and involvement of multiple centres, are required to ascertain evidence for the role of serum BDNF level assessment in diagnosis of depression, its severity and treatment response.
Declaration of publication/presentation of the article
The article was presented as part of “Col Kripal Singh Award” paper in 71st Annual National Conference of Indian Psychiatry Society (ANCIPS) held at Lucknow from 31 January to 03 Febuary 2019. The abstract of the article was published in the Indian Journal of Psychiatry (Supplementary Edition-Proceedings and Abstract of 71st ANCIPS-2019) with the reference as Col Kripal Sing Final Award Papers. Indian J Psychiatry 2019; 61, Suppl S3:362-3.http://www.indianjpsychiatry.org/text.asp?2019/61/9/362/250215. There is no DOI number.
Disclosure of competing interest
The authors have none to declare.
Acknowledgement
This article is based on Armed Forces Medical Research Committee No. 4617/2015 and granted by the office of Director General Armed Forces Medical Services and Defence Research Development Organisation, Government of India.
References
- 1.Bromet E., Andrade L.H., Hwang I., et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011 Dec;9(1):90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dokter M., von Bohlen O. Neurogenesis within the adult hippocampus under physiological conditions and in depression. Neural Regeneration research. 2012 Mar 5;7(7):552–559. doi: 10.3969/j.issn.1673-5374.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J., Lauren C., Siao C.J. Pro-BDNF negatively regulates neuronal remodeling, synaptic transmission and synaptic plasticity in hippocampus. Cell Rep. 2014 May 8;7(3):796–806. doi: 10.1016/j.celrep.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang T., Nie Z., Shu H., et al. The role of BDNF on neural plasticity in depression. Front Cell Neurosci. 2019;14:82. doi: 10.3389/fncel.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macewen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala and prefrontal cortex. Neuropsychopharmacology. 2015;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haroon E., Raison C.L., Miller A.H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee B.H., Kim Y.K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7:231–235. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade C., Rao N.S. How antidepressant drugs act: a primer on neuroplasticity as the eventual mediator of antidepressant efficacy. Indian J Psychiatr. 2010;52:378–386. doi: 10.4103/0019-5545.74318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy M.J., Boulle F., Steinbusch H.W., Vander Hove D.L.A., Kenis G., Laufumey L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology. 2018;235(8):2195–2220. doi: 10.1007/s00213-018-4950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou C., Zhong J., Zou B., et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terracciano A., Lobina M., Piras M.G., et al. Neuroticism, depressive symptoms, and serum BDNF. Psychosom Med. 2011 Oct;73(8):638–642. doi: 10.1097/PSY.0b013e3182306a4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein Anders B., Williamson Rebecca, Santini Martin A., et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 13.Bocchio-Chiavetto L., Bagnardi V., Zanardini R., et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatr. 2010;11:763–773. doi: 10.3109/15622971003611319. [DOI] [PubMed] [Google Scholar]
- 14.Jeenger J., Singroha V., Sharma M., Mathur D.M. C-reactive protein, brain-derived neurotrophic factor, interleukin-2, and stressful life events in drug-naive first-episode and recurrent depression: a cross-sectional study. Indian J Psychiatr. 2018;60(3):334–339. doi: 10.4103/psychiatry.IndianJPsychiatry_169_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugam V., John V.S., Augustine N., et al. The impact of antidepressant treatment on brain-derived neurotrophic factor level: an evidence-based approach through systematic review and meta-analysis. Indian J Pharmacol. 2017 May;49(3):236–242. doi: 10.4103/ijp.IJP_700_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halappa N.G., Thirthalli J., Varambally S., Rao M., Christopher R., Nanjundaiah G.B. Improvement in neurocognitive functions and serum brain-derived neurotrophic factor levels in patients with depression treated with antidepressants and yoga. Indian J Psychiatr. 2018;60(1):32–37. doi: 10.4103/psychiatry.IndianJPsychiatry_154_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurita M., Nishino S., Kato M., Numata Y., Sato T. Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PloS One. 2012;7(6) doi: 10.1371/journal.pone.0039212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He H.Y., Tian J.L., Deng Y.Q., et al. Association of brain-derived neurotrophic factor levels and depressive symptoms in young adults with acne vulgaris. BMC Psychiatr. 2019 Jun 24;19(1):193. doi: 10.1186/s12888-019-2182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molendijk M.L., Bus B.A., Spinhoven P., et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatr. 2011;16:1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varambally S., Naveen G.H., Rao M.G., et al. Low serum brain derived neurotrophic factor in non-suicidal out-patients with depression: relation to depression scores. Indian J Psychiatr. 2013;55(3 Suppl):397–399. doi: 10.4103/0019-5545.116311. S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jevtovic S., Karlovic D., Mihaljevic-Peles A., Seric V., Vrkic N., Jaksic N. Serum Brain-derived neurotrophic factor (BDNF): the severity and symptomatic dimensions of depression. Psychiatr Danub. 2011;23:363–369. [PubMed] [Google Scholar]
- 23.Emon M.P.Z., Das R., Nishuty N.L., et al. Reduced serum BDNF levels are associated with the increased risk for developing MDD: a case–control study with or without antidepressant therapy. BMC Res Notes. 2020;13(83) doi: 10.1186/s13104-020-04952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bus B.A., Tendolkar I., Franke B., et al. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatr. 2012;13:39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakofsky J., Ressler K., Dunlop B. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol Psychiatr. 2012;17:22–35. doi: 10.1038/mp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laura S., Velzen van, Lianne Schmaal, Rick Jansen, et al. Effect of childhood maltreatment and brain-derived neurotrophic factor on brain morphology. Soc Cognit Affect Neurosci. 2016;11(11):1841–1852. doi: 10.1093/scan/nsw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosang G.M., Shiles C., Tansey K.E., McGuffin P., Uher R. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med. 2014 Jan 16;7:12. doi: 10.1186/1741-7015-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee B.H., Park Y.M., Um T.H., Kim S. Lower serum brain-derived neurotrophic factor levels are associated with failure to achieve remission in patients with major depression after escitalopram treatment. Neuropsychiatric Dis Treat. 2014 Jul 23;10:1393–1398. doi: 10.2147/NDT.S64913. [DOI] [PMC free article] [PubMed] [Google Scholar]