Abstract
Purpose:
There is little data on the safety and efficacy of endovascular treatment (EVT) in comparison with intravenous thrombolysis (IVT) in acute ischemic stroke due to isolated posterior cerebral artery occlusion (IPCAO). We aimed to investigate the functional and safety outcomes of stroke patients with acute IPCAO treated with EVT (with or without prior bridging IVT) compared to IVT alone.
Methods:
We did a multicenter retrospective analysis of data from the Swiss Stroke Registry. The primary endpoint was overall functional outcome at 3 months in patients undergoing EVT alone or as part of bridging, compared with IVT alone (shift analysis). Safety endpoints were mortality and symptomatic intracranial hemorrhage. EVT and IVT patients were matched 1:1 using propensity scores. Differences in outcomes were examined using ordinal and logistic regression models.
Findings:
Out of 17,968 patients, 268 met the inclusion criteria and 136 were matched by propensity scores. The overall functional outcome at 3 months was comparable between the two groups (EVT vs IVT as reference category: OR = 1.42 for higher mRS, 95% CI = 0.78–2.57, p = 0.254). The proportion of patients independent at 3 months was 63.2% in EVT and 72.1% in IVT (OR = 0.67, 95% CI = 0.32–1.37, p = 0.272). Symptomatic intracranial hemorrhages were overall rare and present only in the IVT group (IVT = 5.9% vs EVT = 0%). Mortality at 3 months was also similar between the two groups (IVT = 0% vs EVT = 1.5%).
Conclusion:
In this multicenter nested analysis, EVT and IVT in patients with acute ischemic stroke due to IPCAO were associated with similar overall good functional outcome and safety. Randomized studies are warranted.
Keywords: Acute ischemic stroke, thrombectomy, posterior cerebral artery
Introduction
Acute ischemic strokes (AISs) due to isolated posterior cerebral artery occlusion (IPCAO) are less common than anterior circulation strokes, 1 but can lead to severe clinical deficits2–4 and unfavorable outcomes. 5 Reperfusion therapies appear safe and effective in posterior cerebral artery strokes, but available data are driven by non-randomized small-sample observational studies, susceptible to selection bias and often limited to intravenous thrombolysis (IVT) 6 or intra-arterial thrombolysis. 7 Recent studies have also investigated the outcome of endovascular treatment (EVT) in patients with IPCAO,8–13 but without a direct comparison between EVT, bridging therapy (i.e. IVT followed by EVT) and IVT alone. Moreover, patients with IPCAO were excluded from anterior-circulation large-vessel thrombectomy trials. 14 As a result, current guidelines concerning EVT of IPCAO are based on class IIb (weak) strength of recommendation and on “expert opinion” level of evidence. 15
In this study, we aimed to investigate the functional and safety outcomes at 90 days in patients with AIS due to documented IPCAO, and treated with EVT or bridging therapy, compared to IVT alone, using a propensity score (PS) matching approach.
Methods
Study population
We performed a multicenter retrospective analysis of routinely collected data in the Swiss Stroke Registry in the period 2014–2020.16,17 We extracted the following variables: age, sex, cardiovascular risk factors (hypertension, diabetes, hyperlipidemia, smoking), anticoagulation prior to stroke, time of symptoms onset and arrival to hospital, clinical and disability scales on admission and at 90 days (National Institutes of Health stroke scale [NIHSS], modified Rankin scale [mRS]), type and timing of reperfusion therapy (IVT, EVT, or bridging), occlusion site (P1 or P2), recanalization rate (modified thrombolysis in cerebral infarction [mTICI] score in EVT patients), and in-hospital complications (symptomatic hemorrhage, angioedema, seizures, cranial decompression, death). Implausible values were set as missing.
Inclusion and exclusion criteria
Patients fulfilling the following criteria were included: (1) age ⩾18 years; (2) diagnosis of AIS; (3) presence of IPCAO in either P1 or P2 on acute cerebral angiographic-imaging (CT-angiography or MR-angiography); (4) Treatment with EVT and/or IVT; (5) pre-stroke mRS ⩽ 2. All patients included were treated in Stroke Units or Comprehensive Stroke Centers, where reperfusion treatments are routinely performed. We excluded patients with additional intracranial occlusion other than PCA, as well as (1) ipsilateral carotid occlusion (in the case of fetal posterior cerebral artery variant), (2) concomitant vertebral or basilar artery occlusion.
Outcomes
The primary outcome of the study was the overall functional outcome at 3 months in patients undergoing EVT alone, or as part of bridging therapy, compared to IVT alone (shift analysis, see below). Secondary outcomes were: (1) the proportion of functionally independent patients at 3 months (mRS = 0–2); (2) differences between EVT and IVT in overall functional and independence outcomes after stratifying for occlusion site, that is, P1 segment versus P2 segment; (3) differences in overall functional and independence outcomes in IVT versus EVT alone, and in IVT versus bridging. Safety endpoints were mortality at 3 months, and occurrence of symptomatic intracranial hemorrhage (defined as ⩾4-points NIHSS worsening associated with brain hemorrhage).
Statistical analysis
Categorical variables were described by counts and percentages, continuous and ordinal variables by median and interquartile range (IQR). After selection of patients based on inclusion criteria, EVT (alone or as part of bridging) and IVT alone patients were matched 1:1 using PS matching to limit imbalances in relevant baseline variables (age, sex, baseline NIHSS score, pre-stroke mRS, pre-stroke anticoagulation, time of stroke onset, time between stroke and treatment initiation and PCA occlusion site [P1 vs P2 segment], with caliper width of 0.1 standard deviations of the PS logit). We compared overall functional outcome at 3 months between groups using ordinal regression models (shift analysis) and binary outcomes using logistic models, with type of treatment as independent variable (EVT vs IVT as reference category). Analyses were performed using R (https://www.r-project.org/) and the R package “match-it.”
Ethics
The registry and the present study were both approved by the responsible ethics committee (CE Req-2020-01042). This study complies with the Declaration of Helsinki.
Results
Patient selection and baseline characteristics
Out of 17,968 AIS patients admitted between January 1, 2014 and February 29, 2020, 268 met the inclusion criteria. Of these, 119 were treated with EVT (n = 50 with EVT alone, n = 69 with bridging) and 149 with IVT only (Table 1). The most prominent differences between EVT and IVT were the occlusion site (IVT: P1 = 26.2%, P2 = 73.8%; EVT: P1 = 57.1%; P2 = 42.9%; p < 0.001) and onset-to-treatment time (IVT: 145 [100–200] min; EVT: 182 [120–291] min; p < 0.001). There were also significant differences between the two groups in terms of age, pre-anticoagulation treatment, and NIHSS at admission. There were no differences between the two groups regarding sex or pre-stroke functional status (mRS). Pre-stroke antiplatelet therapy and vascular risk factors were also comparable between the two groups (not shown). Among EVT patients, 70.6% (84/119) reached successful reperfusion (defined as Modified treatment in cerebral ischemia [mTICI] score of 2b or 3).
Table 1.
Demographic and clinical characteristics of stroke patients included in the study before and after propensity score (PS) matching (based on age, sex, pre-stroke mRS, type of stroke onset, time to treatment, admission NIHSS, occlusion site, and pre-stroke anticoagulation). Categorical variable are reported as counts (percentage), continuous and ordinal variables as median (IQR). p values are calculated using chi-square (for categorical variables) and Mann-Whitney (for continuous and ordinal variables).
| Baseline variables | Before matching |
After PS matching |
|||||
|---|---|---|---|---|---|---|---|
| EVT (n = 119) | IVT (n = 149) | p | EVT (n = 68) | IVT (n = 68) | p | ||
| Age | Years | 72.2 (62.1–80.0) | 75.7 (66.5–83.6) | 0.022 | 71.5 (61.9–79.7) | 75.9 (62.3–82.9) | 0.150 |
| Sex | Males | 77 (64.7) | 81 (54.4) | 0.113 | 41 (60.3) | 43 (63.2) | 0.859 |
| Pre-stroke mRS | 0 | 90 (75.6) | 109 (73.2) | 0.075 | 52 (76.5) | 51 (76.5) | 0.972 |
| 1 | 15 (12.6) | 31 (20.8) | 10 (14.7) | 11 (16.2) | |||
| 2 | 14 (11.8) | 9 (6.0) | 6 (8.8) | 6 (8.8) | |||
| Time stroke onset | Known | 85 (71.4) | 129 (86.6) | 0.007 | 53 (77.9) | 53 (77.9) | 1.000 |
| Wake-up | 16 (13.4) | 11 (7.4) | 8 (11.8) | 8 (11.8) | |||
| Unknown | 18 (15.1) | 9 (6.0) | 7 (10.3) | 7 (10.3) | |||
| Time to treatment | Minutes | 182 (120–291) | 145 (100–200) | <0.001 | 175 (120–262) | 148 (100–229) | 0.166 |
| Admission NIHSS | Score | 7 (5–13) | 5 (3–8) | <0.001 | 7 (4–9) | 7 (4–10) | 0.743 |
| Occlusion site | P1 | 68 (57.1) | 39 (26.2) | <0.001 | 26 (38.2) | 30 (44.1) | 0.601 |
| P2 | 51 (42.9) | 110 (73.8) | 42 (61.8) | 38 (55.9) | |||
| Anticoagulation | Yes | 15 (12.6) | 6 (4.0) | 0.018 | 7 (10.3) | 4 (5.9) | 0.529 |
After PS matching, 68 patients in each group were available for analysis. Baseline differences between the two groups were greatly reduced and not statistically significant. In this matched EVT-treated population, 66.2% of patients (45/68) reached successful reperfusion.
Primary and secondary outcomes
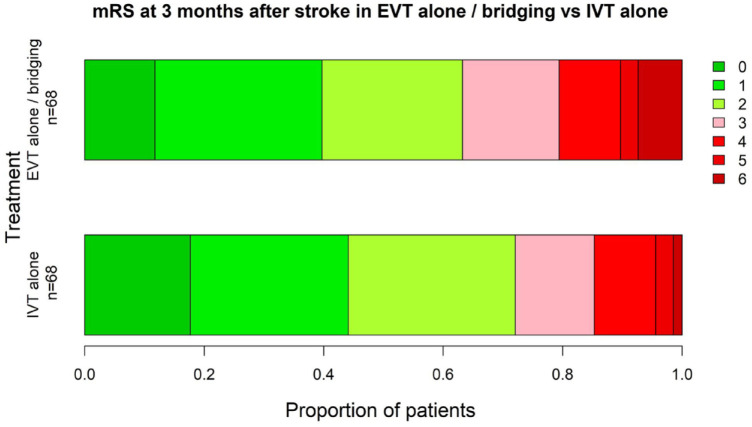
The overall functional outcome at 3 months (Figure 1) (insertfig1) was comparable between the two groups (EVT vs IVT as reference category: OR = 1.42 for higher mRS, 95% CI = 0.78–2.57, p = 0.254). The proportion of patients who were independent at 3 months was 63.2% in EVT and 72.1% in IVT (OR = 0.67, 95% CI = 0.32−1.37, p = 0.272).
Figure 1.
Distribution of Modified Rankin Scale Scores at 90 days. A modified Rankin scale score of 0 indicates no disability, 1, no clinically-significant disability, 2, slight disability, 3, moderate disability but able to walk unassisted, 4, moderately severe disability, 5, severe disability and 6, death.
To further investigate whether results were influenced by proximal versus distal occlusions, which may imply different clinical severity and technical requirements, we stratified patients by occlusion site (P1 vs P2). No significant differences emerged in terms of overall functional outcome and independence between EVT and IVT in either P1 or P2 occlusions (Table 2). Similarly, despite a greater proportion of patients with mRS ⩾ 3 at 3 months in EVT alone (41.7%) as compared to bridging (34.1%), differences in functional outcomes were not significant in either PS-matched IVT versus EVT alone or IVT versus bridging.
Table 2.
mRS at 3 months after stroke (percentage) and results of ordinal and logistic regression models testing overall functional outcome and independence between treatment groups in all patients and after stratifying by site of occlusion (P1 vs P2).
| Occlusion site | Treatment | mRS at 3 months after stroke (%) |
Favorable shift analysis |
Independence |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | OR (95% CI) | p | OR (95% CI) | p | ||
| P1 and P2 | IVT (n = 68) | 17.6 | 26.5 | 27.9 | 13.2 | 10.3 | 2.9 | 1.5 | - | - | - | - |
| EVT alone/bridging (n = 68) | 11.8 | 27.9 | 23.5 | 16.2 | 10.3 | 2.9 | 7.4 | 0.71 (0.38–1.28) | 0.253 | 0.67 (0.32–1.38) | 0.272 | |
| P1 | IVT (n = 30) | 13.3 | 26.7 | 33.3 | 10.0 | 13.3 | 0.0 | 3.3 | - | - | - | - |
| EVT alone/bridging (n = 26) | 19.2 | 30.8 | 15.4 | 11.5 | 11.5 | 0.0 | 11.5 | 1.06 (0.42–2.74) | 0.891 | 0.69 (0.22–2.15) | 0.519 | |
| P2 | IVT (n = 38) | 21.1 | 26.3 | 23.7 | 15.8 | 7.9 | 5.3 | 0.0 | - | - | - | - |
| EVT alone/bridging (n = 42) | 7.1 | 26.2 | 28.6 | 19.0 | 9.5 | 4.8 | 4.8 | 0.54 (0.24–1.19) | 0.124 | 0.66 (0.26–1.69) | 0.389 | |
| P1 and P2 | IVT (n = 44) | 22.7 | 31.8 | 20.5 | 11.4 | 9.1 | 4.5 | 0.0 | - | - | - | - |
| Bridging (n = 44) | 11.4 | 38.6 | 15.9 | 15.9 | 11.4 | 2.3 | 4.5 | 0.66 (0.31–1.40) | 0.281 | 0.64 (0.25–1.62) | 0.351 | |
| P1 and P2 | IVT (n = 24) | 8.3 | 16.7 | 41.7 | 16.7 | 12.5 | 0.0 | 4.2 | - | - | - | - |
| EVT alone (n = 24) | 12.5 | 8.3 | 37.5 | 16.7 | 8.3 | 4.2 | 12.5 | 0.71 (0.25–1.97) | 0.509 | 0.70 (0.22–2.26) | 0.552 | |
Safety outcomes
Symptomatic intracranial hemorrhages were uncommon and present only in the IVT group (IVT = 4 [5.9%] vs EVT = 0 [0%]). Mortality at 3 months was similar between the two groups (IVT = 0 [0%] vs EVT = 1 [1.5%]). Regression analyses were not performed because of the small number of safety events.
Discussion
In this nested multicenter analysis, we found that EVT and IVT are similarly effective in terms of overall functional outcome and safety at 90 days after stroke. In particular, a substantial proportion (i.e. more than 60%) of IPCAO patients who underwent EVT were independent at 3 months, in line with two other studies of smaller sample size (in which 60% and 59% of EVT patients, respectively remained independent).10,18 Symptomatic hemorrhages were uncommon in both groups. In terms of angiographic outcomes, the successful recanalization rate in our matched EVT-treated population (66% of patients reached mTICI 2b or 3) was also consistent with the study of Strambo etal. 10 and Cunha etal. 13 (both 68%). Analyses were performed to address if the occlusion site might influence outcomes of reperfusion therapies, but no differences emerged when comparing EVT versus IVT in either P1 or P2 segment occlusions.
Two studies suggested that posterior strokes might particularly benefit from EVT alone as compared to IVT or best medical treatment.10,12 We did not confirm an apparent superiority of EVT in our cohort, probably because of the different inclusion criteria and matching procedures. We found that patients undergoing EVT alone had a greater mRS at 3 months after stroke than those undergoing bridging, without differences when compared to PS-matched IVT patients. This suggests additional non-measurable factors influencing treatment choices (e.g. unknown time of onset, contraindication to IVT); themselves, intrinsically associated with worse outcomes.
Our study has methodological strengths. We identified a large number of individuals from a prospectively generated national registry (SSR), which independently enrolls a homogenous cohort of consecutive patients from tertiary academic, non-academic centers and regional stroke units. More than 90% of patients were treated between 2015 and 2020, when all participating centers were using the latest EVT devices and techniques. We used PS matching to rectify for imbalances in several baseline factors that might influence outcomes. We also excluded patients with PCA occlusion from vertebral or carotid artery occlusion and from basilar artery occlusions.
There are, however, limitations to this study, the most important being the retrospective, observational, non-randomized design of the study, which is susceptible to selection bias and confounding (despite PS matching). Clinical outcomes and angiographic results were measured independently in each center, leading to potential data heterogeneity. Another limitation is that the scales traditionally used to quantify neurological and functional consequences of strokes (i.e. NIHSS and mRS), do not adequately describe the extent of clinical deficit and functional impact in PCA strokes. Furthermore, the relatively small sample size limits the statistical power to detect significant differences, especially in subgroup analyses in which a non-significant trend favoring IVT was present. Finally, we had no data regarding vessel recanalization in IVT patients since follow-up vessel imaging was not routinely performed after IVT.
To conclude, 3-month functional outcomes from IPCAO were comparable after EVT alone or with bridging versus IVT alone, but confidence limits were wide. While providing important information regarding the safety of EVT in PCA strokes, these results do not allow general treatment recommendations. This highlights the need of randomized clinical trials comparing EVT versus IVT in IPCAO, including visual fields and neuropsychological tests among the outcomes.
Acknowledgments
Data used in this article were obtained from the Swiss Stroke Registry. We are grateful to the patients, families and clinical staff for their cooperation during this study. We acknowledge the researchers who contributed to the study design and/or provided data but did not participate in the analysis or writing of the report. We thank Antonia Lenstra for her precious support.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CWC reports grants from the Swiss Heart Foundation during the conduct of the study and other grants from iSchemaView and Bayer, both outside of the submitted work. PMi reports grants from the Swiss National Science Foundation and from the Swiss Heart Foundation during the conduct of the study and grants from the ERISTA program (BMS/Pfizer) and personal fees from Medtronic, all outside of the submitted work. PMo reports research support from Siemens, Cerenovus, iSchemaview, Medtronic, Stryker, the Swiss Heart Foundation and the Swiss National Foundation, and consultant fees paid to the institution by Medtronic, Cerenovus, Phenox and Microvention during the conduct of the study, but unrelated to the submitted work. The other authors report no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Swiss Heart Foundation funds the Swiss Stroke Registry (www.swissheart.ch).
Informed consent: Not applicable
Ethical approval: The study complied with the Declaration of Helsinki. We obtained Ethics approval (Swissethics ID 2019-01781) from institutional review boards of all participating centers. In accordance with national law, patients were informed about the use of their routinely collected data for research purposes.
Guarantor: CWC
Contributorship: The study was designed by CWC (Principal Investigator). FM drafted the manuscript. GD provided statistical analysis. LB is the Coordinator of the Swiss Stroke Registry. All authors listed provided acquisition, analysis and interpretation of the data and made critical revisions to the manuscript.
ORCID iDs: F. Maulucci  https://orcid.org/0000-0003-0295-0571
https://orcid.org/0000-0003-0295-0571
U. Fischer  https://orcid.org/0000-0003-0521-4051
https://orcid.org/0000-0003-0521-4051
T. Kahles  https://orcid.org/0000-0002-1569-6376
https://orcid.org/0000-0002-1569-6376
L. Bonati  https://orcid.org/0000-0003-1163-8133
https://orcid.org/0000-0003-1163-8133
S. Wegener  https://orcid.org/0000-0003-4369-7023
https://orcid.org/0000-0003-4369-7023
References
- 1.Waqas M, Mokin M, Primiani CTet al. Large vessel occlusion in acute ischemic stroke patients: a dual-center estimate based on a broad definition of occlusion site. J Stroke Cerebrovasc Dis 2020; 29: 104504. [DOI] [PubMed] [Google Scholar]
- 2.Brandt T, Steinke W, Thie Aet al. Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature. Cerebrovasc Dis 2000; 10: 170–182. [DOI] [PubMed] [Google Scholar]
- 3.Cereda C, Carrera E.Posterior cerebral artery territory infarctions. Front Neurol Neurosci 2012; 30: 128–131. [DOI] [PubMed] [Google Scholar]
- 4.Ng YS, Stein J, Salles SSet al. Clinical characteristics and rehabilitation outcomes of patients with posterior cerebral artery stroke. Arch Phys Med Rehabil 2005; 86: 2138–2143. [DOI] [PubMed] [Google Scholar]
- 5.Sommer P, Posekany A, Serles Wet al. Is functional outcome different in posterior and anterior circulation stroke? Stroke 2018; 49: 2728–2732. [DOI] [PubMed] [Google Scholar]
- 6.Breuer L, Huttner HB, Jentsch Ket al. Intravenous thrombolysis in posterior cerebral artery infarctions. Cerebrovasc Dis 2011; 31: 448–454. [DOI] [PubMed] [Google Scholar]
- 7.Meier N, Fischer U, Schroth Get al. Outcome after thrombolysis for acute isolated posterior cerebral artery occlusion. Cerebrovasc Dis 2011; 32: 79–88. [DOI] [PubMed] [Google Scholar]
- 8.Weber R, Minnerup J, Nordmeyer Het al. Thrombectomy in posterior circulation stroke: differences in procedures and outcome compared to anterior circulation stroke in the prospective multicentre REVASK registry. Eur J Neurol 2019; 26: 299–305. [DOI] [PubMed] [Google Scholar]
- 9.Memon MZ, Kushnirsky M, Brunet MCet al. Mechanical thrombectomy in isolated large vessel posterior cerebral artery occlusions. Neuroradiol 2021; 63: 111–116. [DOI] [PubMed] [Google Scholar]
- 10.Strambo D, Bartolini B, Beaud Vet al. Thrombectomy and thrombolysis of isolated posterior cerebral artery occlusion: cognitive, visual, and disability outcomes. Stroke 2020; 51: 254–261. [DOI] [PubMed] [Google Scholar]
- 11.Meyer L, Papanagiotou P, Politi Met al. Feasibility and safety of thrombectomy for isolated occlusions of the posterior cerebral artery: a multicenter experience and systematic literature review. J Neurointerv Surg 2021; 13: 217–220. [DOI] [PubMed] [Google Scholar]
- 12.Meyer L, Stracke CP, Jungi Net al. Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol 2021; 78: 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha B, Baptista M, Pamplona Jet al. Acute treatment of isolated posterior cerebral artery occlusion: single center experience. J Stroke Cerebrovasc Dis 2022; 31: 106239. [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Menon BK, van Zwam WHet al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 15.Powers WJ, Rabinstein AA, Ackerson Tet al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 16.Manno C, Disanto G, Bianco Get al. Outcome of endovascular therapy in stroke with large vessel occlusion and mild symptoms. Neurology 2019; 93: e1618–e26. [DOI] [PubMed] [Google Scholar]
- 17.Meinel TR, Branca M, De Marchis GMet al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol 2021; 89: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarençon F, Baronnet F, Shotar Eet al. Should posterior cerebral artery occlusions be recanalized? Insights from the Trevo Registry. Eur J Neurol 2020; 27: 787–792. [DOI] [PubMed] [Google Scholar]