Abstract
Background:
Observational studies have demonstrated improved outcomes with the adjunctive use of balloon guide catheters (BGC) during endovascular thrombectomy (EVT) for anterior circulation acute ischaemic stroke (AIS). However, the lack of high-level evidence and global practice heterogeneity justifies a randomised controlled trial (RCT) to investigate the effect of transient proximal blood flow arrest on the procedural and clinical outcomes of patients with AIS following EVT.
Hypothesis:
Proximal blood flow arrest in the cervical internal carotid artery during EVT for proximal large vessel occlusion is superior to no flow arrest in achieving complete vessel recanalisation.
Methods:
ProFATE is an investigator-initiated, pragmatic, multicentre RCT with blinding of participants and outcome assessment. An estimated 124 participants with an anterior circulation AIS due to large vessel occlusion, an NIHSS of ⩾2, ASPECTS ⩾ 5 and eligible for EVT using a first-line combined technique (contact aspiration and stent retriever) or contact aspiration only will be randomised (1:1) to receive BGC balloon inflation or no inflation during EVT.
Outcomes:
The primary outcome is the proportion of patients achieving near-complete/complete vessel recanalisation (eTICI 2c-3) at the end of the EVT procedure. Secondary outcomes include the functional outcome (modified Rankin Scale at 90 days), new or distal vascular territory clot embolisation rate, near-complete/complete recanalisation after the first pass, symptomatic intracranial haemorrhage, procedure-related complications and death at 90 days.
Discussion:
This is the first RCT to investigate the effect of proximal blood flow arrest during EVT using a BGC on the procedural and clinical outcomes of patients with AIS due to large vessel occlusion.
Keywords: Stroke, thrombectomy, endovascular, balloon guide catheter, emboli
Introduction
Endovascular thrombectomy (EVT) is the standard of care for large vessel occlusion in acute ischaemic stroke.1–3 Using balloon guide catheters (BGC) as an adjunctive device during EVT in the anterior circulation allows transient proximal blood flow arrest by inflating the balloon at its tip, and even flow reversal when concomitant aspiration is applied. This is thought to limit distal clot migration or embolisation to new vascular territories during EVT.4–6
Recent meta-analyses of non-randomised studies have suggested that the use of BGC during EVT leads to improved rates of successful reperfusion, first-pass effect (successful reperfusion after the first pass), procedural time and functional outcomes, particularly in patients treated using the stent-retriever (SR) technique only.7,8 However, due to the paucity of data, meaningful conclusions could not be drawn for patients treated using BGC with the contact aspiration (CA)-only technique or the combined first-line technique of SR and CA. 8 This is relevant as both techniques have demonstrated high rates of successful reperfusion and good functional outcomes following EVT and are now increasingly used whilst the use of the SR-only technique of EVT is diminishing.9–11
Despite recommendations in the current guidelines,2,3 there is a state of clinical equipoise globally, with approximately only one in four interventionists reporting routine use of BGCs during EVT. 12 Reasons cited for not using BGCs include: potential device related complications (arterial dissection), handling properties such as lack of support, restricted compatibility with larger bore aspiration catheters and higher costs. 12
Due to practice heterogeneity, lack of high-level evidence data and potential for either improved or worsened outcomes with BGC use during EVT, there is an urgent need for a dedicated randomised controlled trial (RCT) to investigate the effect of transient proximal blood flow arrest during EVT using a BGC on the procedural and clinical outcomes of patients with AIS.
Methods
Study aim
The ProFATE trial aims to test the hypothesis that proximal blood flow arrest in the cervical internal carotid artery (ICA) using a BGC during EVT for proximal large vessel occlusion in the anterior circulation is superior to no flow arrest in achieving complete vessel recanalisation.
Study design
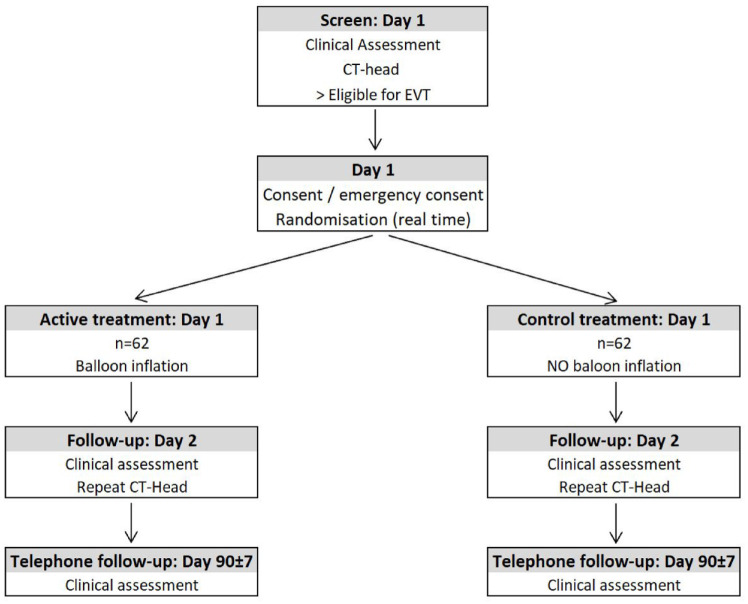
ProFATE is an investigator-initiated, pragmatic, multicentre, randomised controlled (1:1), parallel-group and participant and outcome-blinded trial of temporary proximal blood flow arrest (balloon inflation using a BGC) versus no proximal flow arrest (no balloon inflation using a BGC) when first-line combined clot-retrieval technique (SR and CA) or CA only is used during EVT treatment (Figure 1). Patients admitted with AIS due to anterior circulation large vessel occlusion and eligible for EVT treatment will be recruited at four high-volume (⩾100 EVTs/year) EVT centres in the United Kingdom. No upper limit of the stroke onset-to-EVT initiation time is prescribed due to the similar treatment effect demonstrated in patients treated beyond 24 h compared to those treated within 24 h from stroke onset, provided that the trial imaging and clinical eligibility criteria are met.13,14 Each interventionist involved in the trial should be familiar with and regularly perform EVT in the anterior circulation using a BGC (⩾20/year). The ProFATE trial received a favourable ethical opinion from the Health Research Authority, the Health and Care Research Wales, and the Wales Research Ethics Committee (Ref: 21/WA/0199) on 03/09/2021. Nottingham University Hospitals NHS Trust is the study sponsor (Ref: 21DI004). The trial is funded by the Royal College of Radiologists Kodak Fund Scholarship and is registered with ClinicalTrials.gov (NCT05020795). The trial will be conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice.
Figure 1.
Study flow-chart.
Intervention
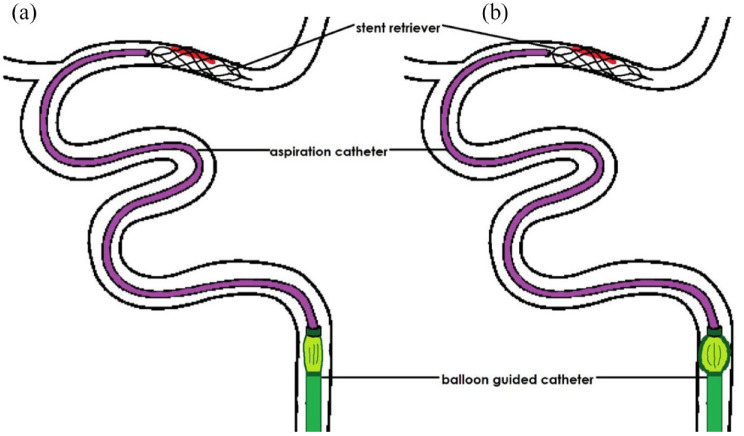
Participants will be randomised to (i) temporary proximal blood flow arrest (balloon inflation) in the cervical ICA during clot retrieval or (ii) no temporary proximal blood flow arrest (no balloon inflation) during clot-retrieval (Figure 2).
Figure 2.
(a) Control trial arm: Combined technique of contact aspiration and stent-retriever (or contact aspiration only – not shown) used without balloon inflation of the balloon guide catheter (BGC) during endovascular thrombectomy (EVT) and (b) treatment trial arm: EVT using the combined technique with balloon inflation of the BGC within the internal carotid artery.
In either trial arm, CA only (a direct aspiration first pass technique (ADAPT) technique) 11 or any variation of the combined SR and CA techniques are permitted as the intended first-line technique during EVT. However, it is recommended that the ‘balloon guide with large bore distal access catheter with dual aspiration with stent-retriever as standard approach’ (BADDASS) technique is preferentially used if a combined technique is selected first line. 10 For each procedure, the BGC will be used as a delivery system, and the balloon will or will not be inflated during clot retrieval according to the randomisation outcome. It is strongly recommended that the balloon inflation is performed under direct fluoroscopy, and the degree of inflation should sufficiently oppose the vessel wall (following the instructions for use). Balloon inflation is only permitted in the extracranial ICA to ensure adequate flow arrest (as opposed to inflation within the common carotid artery). 15
The choice of any endovascular device (catheter, stent-retriever or aspiration device) will be left to the discretion of the interventionist provided the devices are FDA-approved and CE-marked and are routinely used by the interventionist. At present, the only CE-marked BGCs available for commercial use in the United Kingdom are the 8 Fr Flowgate 2 BGC (Stryker, USA) and the 8 Fr Cello BGC (Medtronic, USA). Preparation of the balloon prior to its use should be according to the product’s instructions for use by active aspiration of any residual air and its inflation using at least a 50:50 iodinated contrast-saline mixture. It is recommended that dual aspiration using a continuous vacuum pump and/or a 60 ml syringe for manual aspiration are applied to both the distal access catheter and the BGC at the point of clot retrieval. At least three thrombectomy attempts/passes with or without balloon inflation must be made (if required) according to the randomisation outcome, beyond which the use of balloon inflation or a change in guide catheter for any further attempts will be at the interventionist’s discretion. The decision for the mode of anaesthesia (general anaesthesia or conscious sedation), and any rescue therapy, including intracranial angioplasty with or without stenting and intra-arterial pharmacological therapy, is at the discretion of the interventionist.
Prior to a thrombectomy attempt, cervico-cranial digital subtraction angiography (DSA) is performed to confirm adequate antegrade flow (particularly in cases of moderate proximal ICA stenosis), the occlusion site and any presence of emboli in a different vascular territory. Blinded adjudication of the vessel recanalisation (eTICI) score, the presence of any distal or new vascular territory emboli and the presence of carotid dissection or vasospasm, is performed for each thrombectomy pass.
All AIS patients are admitted to a dedicated hyperacute stroke unit and are treated according the National Institute for Health and Care Excellence (NICE) guidelines 16 which includes 300 mg Aspirin on admission if they are ineligible for intravenous thrombolysis/alteplase (recombinant tissue type-plasminogen activator at 0.9 mg/kg), adequate blood pressure and blood glucose control.
Eligibility criteria
Inclusion
■ Age ⩾18 years.
■ Acute ischaemic stroke presenting with a neurological deficit (National Institutes of Health Stroke Scale (NIHSS) ⩾2).
■ Intracranial arterial occlusion of the distal ICA or middle cerebral artery (MCA; M1/proximal M2 segments) demonstrated on clinical neuroimaging such as computed tomography angiogram (CTA), magnetic resonance imaging angiogram (MRA) or DSA.
■ Alberta Stroke Program Early CT Score (ASPECTS) of ⩾5.
■ Pre-morbid disability (modified Rankin Scale, mRS ⩽ 2).
■ Intention to treat with first-line CA only, or combined technique of SR + CA during EVT.
Exclusion
■ Severe stenosis (>90%) or tandem occlusion of the ipsilateral extracranial ICA.
■ Previously deployed stents in the ipsilateral ICA.
■ Dissection of the ipsilateral ICA.
■ Unlikely to be available for 90 days follow-up (e.g. no fixed home address, a visitor from overseas).
■ Subject participating in a study involving an investigational drug or device that would impact this study.
Randomisation and blinding
All participants eligible for inclusion and for whom consent has been obtained will be randomised centrally using a secure internet site in real time, directly after the CTA. Randomisation will be 1:1 (balloon inflation vs no balloon inflation), stratified by use of thrombolysis, and minimised based on key prognostic factors: age (⩽70 vs >70 years), time since stroke onset (⩽6 vs >6 h), ASPECTS (amount of brain tissue infarcted on the non-contrast CT Head scan on admission; ⩽7 vs >7), blood clot location (ICA vs MCA) and stroke severity (NIHSS; ⩽12 vs >12). The computer-based randomisation will allocate a number corresponding to the choice of treatment, and the participant will receive treatment from the allocated number. In the event of computer failure (e.g. server failure), investigators will follow the working practice document for computer system disaster recovery, which will allow the participant to be randomised following a standardised operating procedure.
Multiple efforts will be taken to minimise bias through concealment of allocation, blinded central telephone follow-up (eliminating bias from local measurement), and core laboratory adjudication of the baseline and follow-up imaging outcome measures, as well as analysis by intention-to-treat with adjustment for key prognostic variables. Minimisation of key prognostic variables will minimise differences in baseline prognostic variables and improve statistical power to help improve precision. 17 As all patients will undergo this procedure under conscious sedation/general anaesthesia, the patient will be blinded to the allocation. Though it will not be possible to blind the interventionist performing the intervention, the baseline imaging and outcome measures by central core laboratory adjudicators, and trained specialist nurses who perform the telephone follow-up interview at 90 days, will be blinded to the treatment allocation.
Primary outcome
The primary outcome is the proportion of patients achieving near-complete/complete vessel recanalisation (eTICI 2c-3) at the end of the EVT procedure, as assessed by the central core laboratory blinded adjudication. Near-complete/complete vessel recanalisation (procedural efficacy) was chosen as the primary outcome measure as it is a key surrogate marker strongly associated with increased functional independence and has been reliably used in previous EVT trials.9,18,19
Secondary outcomes
Secondary procedural outcome measures include (i) near-complete/complete recanalisation (eTICI 2c-3) and (ii) complete recanalisation (eTICI 3) after the first pass, (iii) number of passes/attempts of clot retrieval to achieve near-complete/complete vessel recanalisation (eTICI2c-3), (iv) procedural time (arterial puncture to final angiography), (v) new or distal vascular territory clot embolisation and (vi) change in the automated core infarct volume based on the unenhanced CT imaging between baseline and at 24 h.
The clinical efficacy outcomes include the (i) distribution of the mRS score at 90 days (ordinal shift) and (ii) the rate of functional independence (mRS 0–2) at 90 days.
The secondary safety outcome measures are (i) early neurological deterioration defined as a change in the stroke severity (NIHSS) of ⩾4 between baseline and at 24 h post-EVT, (ii) symptomatic intracranial haemorrhage, defined using the European Cooperative Acute Stroke Study II (ECASS II) classification as any intracranial haemorrhage with an increase in the NIHSS ⩾ 4 within 24 h or death, 20 (iii) procedure-related complications (including vessel dissection/vasospasm/perforation, vascular access site haematoma or pseudoaneurysm) and (iv) all-cause death at 90 days.
Consent
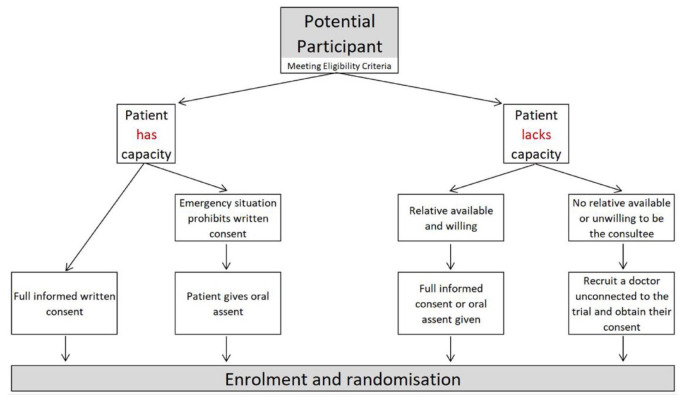
Due to the time-critical nature of performing EVT for patients with an acute ischaemic stroke due to a large vessel occlusion, deferred (two-stage) informed consent was approved by the ethics committee for this trial due to ethical reasons in the potential delay of treatment initiation. 21 Specifically, an initial brief informed verbal consent is sought from participants prior to enrolment into the trial (Figure 3). Later, full written informed consent is confirmed with the participant, ideally within 24–48 h after EVT. If a participant lacks the mental capacity to provide the initial verbal consent due to lack of consciousness or aphasia, then a personal consultee (relative/next of kin or independent medical practitioner in case no relative is available) is approached to consent for the trial enrolment. Full informed consent is later sought from the recruited participant within 24–48 h post-EVT if full mental capacity is regained. It will be explained to the potential participant or their legal representative that entry into the trial is entirely voluntary and that they can withdraw at any time without giving a reason. In the event of their withdrawal, their participation in the trial will be terminated. However, it will be explained that their data collected up to the given point cannot be erased, and we will seek consent to use the data in the final analyses where appropriate.
Figure 3.
Deferred informed consent flow-chart.
Data collection and management
By integrating the pragmatic trial into routine patient pathways, the imaging, clinical data and outcome measures are collected as part of the routine standard of clinical care in the participating institutions thus reducing the chance of missing data and reducing participant burden (Table 1). An electronic Case Report Form (eCRF) will be completed for each study participant summarising all clinical screening and study data. The eCRF used for this trial is hosted on Research Electronic Data Capture (REDCap®), a secure, web-based application designed to support data collection and management for research studies. Each participant will be assigned a trial identity code number, allocated at randomisation, for use on the eCRF and other trial documents. Participants will only be referred to in the eCRF by their participant number in order to retain participant confidentiality. The completed original eCRF data is to be submitted via REDCap® online to the Sponsor as soon as practical after completion. A trial recruitment log will be kept to ascertain the consecutive recruitment of eligible participants into the trial. Regular scheduled data monitoring audits (at least every 6 months) are performed by members of the research department from the Sponsor’s institution to ensure the accuracy and completeness of the data entry.
Table 1.
Summary of data collection at specified time intervals.
| Randomisation | During procedure | 24 h | 90 days | |
|---|---|---|---|---|
| Baseline information | ||||
| Demographics | Yes | |||
| NIHSS | Yes | |||
| mRS | Yes | |||
| Consent* | Yes | Yes | ||
| Comorbidities | Yes | |||
| Medications | Yes | |||
| Neuroimaging (NCCT/CTA) | Yes | |||
| Thrombolysis | Yes | |||
| Time metrics (patient pathway) | Yes | |||
| Procedural outcomes | ||||
| EVT imaging (eTICI) | Yes | |||
| Time metrics | Yes | |||
| Number of passes | Yes | |||
| Complications | Yes | |||
| Emboli in new/distal vascular territory | Yes | |||
| Functional and safety outcomes | ||||
| NIHSS | Yes | |||
| mRS | Yes | |||
| Mortality | Yes | Yes | ||
| Follow-up neuroimaging (NCCT) | Yes | |||
EVT: endovascular thrombectomy; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; eTICI: extended thrombolysis in cerebral infarction; NCCT: non-contrast computed tomography; CTA: computed tomography angiography.
Two-stage consent process.
All participants will have baseline non-contrast CT imaging on admission to the primary stroke centre or the EVT-capable centre (prior to enrolment) to exclude intracranial haemorrhage or other stroke mimics, and to assess the baseline infarct size scored using the ASPECTS. CTA will be performed as part of the centre’s local practice to identify the proximal large vessel occlusion site (ICA, M1 or M2 MCA segments) and the presence of collateral supply. Cranial DSA will be performed to confirm the occlusion site, the presence of the anterior or posterior communicating artery, the presence of any emboli in a different vascular territory and the recanalisation score. Repeat non-contrast CT imaging will be performed 24 h after admission to evaluate the presence of any intracranial haemorrhage. Neurological severity will be assessed by trained clinicians or specialist nurses using the NIHSS at baseline and at 24 h after admission. The functional outcome will be assessed with the mRS at baseline and at 90 days by trained specialist nurses using a standardised telephone follow-up interview proforma, blinded to group allocation.
Sample size
No previous RCT assessing the procedural and clinical outcomes of temporary proximal blood flow arrest during EVT has been completed. Hence, the sample size calculation has been informed by our meta-analysis of observational studies and recent published retrospective studies.7,8,22–26 The mean proportion of patients expected to achieve the binary primary outcome measure of near-complete/complete vessel recanalisation (eTICI 2c-3) in the balloon inflation treatment group is 70% versus 45% in the non-balloon inflation group, giving rise to an absolute difference of 25%. Assuming an overall significance (alpha) = 0.05; power (1−beta) = 0.80; the calculation (Chi-squared test) aiming to prove treatment superiority revealed a sample size of 120 participants. To account for any (rare) eventuality of the BGC not being utilised during EVT due to tortuous vessel anatomy, a 4% increase adjustment was made, amounting to 124 patients. If a patient is enrolled into the trial but, due to spontaneous recanalisation no thrombectomy has taken place or commenced, no further data will be collected from the patient and their trial enrolment will be terminated. Instead, another eligible patient will be enrolled to ensure sufficient numbers of randomised patients have the procedure undertaken. The primary outcome is based on the vessel recanalisation success rate identified at the end of the EVT procedure. Hence, any potential patient lost to follow-up will not affect the primary outcome measure. In summary, a trial of 124 participants will have 80% power to detect a 25% proportional difference of successful vessel reperfusion.
Data and safety monitoring board
A Data and Safety Monitoring Board (DSMB) independent from the Trial Management Group, will be convened to monitor the safety of participants and will include a stroke physician, neuroradiologist and an independent statistician. The DSMB will receive safety reports every 3 months, or more frequently if requested and perform unblinded reviews of safety data. The DSMB will perform a formal interim analysis at 50% recruitment with 90-day follow-up.
Statistical analysis
Statistical analyses will be carried out independently by the trial statistician. Study characteristics will be summarised using descriptive statistics for patient demographics, clinical characteristics, co-morbidities and time metrics.
The primary outcome analysis of the near-complete/complete vessel recanalisation scores (eTICI 2c-3) between treatment groups will be analysed using a mixed-effects logistic regression model with adjustment for IV thrombolysis and age, time from stroke onset-to-randomisation, ASPECTS, blood clot location and NIHSS, with the recruiting site as the random centre and centre × treatment interaction.
Secondary outcome measure analyses will also use the mixed-effects logistic (binary for dichotomised outcomes) or linear regression models as appropriate using the same adjustment variables as the primary outcome, including the mixed-effects ordinal logistic regression for the full-scale mRS distribution. Analyses of adjusted and unadjusted outcome estimates will be expressed as an odds ratio (OR) (for logistic and ordinal regression) or a coefficient estimate (for linear regression), both with 95% confidence intervals (CI). A two-tailed p-value of <0.05 will be considered statistically significant. All analyses will be performed on the intention-to-treat population, and the robustness of the primary and key secondary analyses will be assessed in the per-protocol population.
Pre-specified subgroup analyses in all minimisation variables, including IV thrombolysis, age (⩽70 vs >70 years), time since stroke to randomisation (⩽6 vs >6 h), ASPECTS (⩽7 vs >7), blood clot location (ICA vs MCA), stroke severity (NIHSS; ⩽12 vs >12) and exploratory analyses of the collateral status and presence of the communicating arteries are planned. Analysis of the primary outcome in these pre-specified sub-groups does not comprise the primary analysis and has not informed the sample size calculation. The interpretation of any subgroup effects will be based on interaction tests and are hypothesis-generating.
Missing data
No missing data are anticipated for the primary outcome measure based on the vessel recanalisation success rate as identified at the end of the EVT procedure. For any missing secondary outcome mRS data, multiple regression imputation will be performed.
Trial status
Recruitment for ProFATE commenced on 17th October 2021, and is ongoing in January 2023 with 92 patients randomised, following the favourable recommendation of the DSMB to continue enrolment after the interim safety analysis in September 2022. Recruitment is expected to be completed by May 2023, and the final follow-up by August 2023.
Discussion
ProFATE is a pragmatic multicentre RCT with a participant and outcome-blinded design of temporary proximal blood flow arrest (balloon inflation using a BGC) versus no proximal flow arrest (no balloon inflation using a BGC) when first line combined clot-retrieval technique (SR and CA) or contact aspiration only is used during EVT treatment for patients presenting with an AIS due to an anterior circulation large vessel occlusion. To the best of our knowledge, ProFATE is the first RCT to investigate the effect of proximal blood flow arrest during EVT by primarily evaluating the procedural efficacy of near-complete/complete vessel recanalisation at the end of the EVT treatment.
We chose near-complete/complete vessel recanalisation (procedural efficacy) as the primary outcome measure as it is a key surrogate marker strongly associated with an increase in functional independence and has been reliably used in previous EVT trials comparing thrombectomy devices.9,18,19 Although an absolute difference of 25% in the procedural efficacy may be considered large, a smaller difference in the eTICI scores may not result in a clinically meaningful difference in the functional outcome (mRS at 90 days). For instance, in a recent non-randomised study, 23 an absolute difference of 26.5% in the eTICI2c-3 rates (76.8% vs 50.3% between the BGC and non-BGC groups respectively) only amounted to a 4% difference in the functional independence rate. Furthermore, the smaller reported differences in the functional independence between BGC and non-BGC groups in various non-randomised studies23,25 would necessitate an estimated large sample size of over 3000 patients to detect a statistically significant improvement in clinical outcome.
There has been rapid development of larger bore BGCs with an internal diameter of up to 0.087″ (e.g. BOBBY; Microvention, USA, Walrus; Q’Apel Medical, USA, EMBOGUARD; Cerenovus, USA), which are able to accommodate large bore distal aspiration catheters (e.g. Sofia 6 Plus), similar to those currently used alongside conventional (non-balloon) guide catheters.27,28 However, only the 8 Fr Flowgate 2 (Stryker, USA) BGC, which has a smaller internal diameter of 0.084″, and the 8 Fr Cello BGC (Medtronic, USA) are available for commercial use in the United Kingdom at present, thereby limiting the compatibility with large bore aspiration catheters, which have been associated with improved procedural and clinical outcomes. 29 Nevertheless, this trial sought to investigate the concept of proximal flow arrest during EVT as opposed to the efficacy of specific BGCs themselves. We sought to investigate the effect of proximal flow arrest by mandating the use of a BGC (Flowgate 2 ) in both the treatment and control arms, the latter performed without balloon inflation. This ensures as many variables are standardised as possible to minimise potential confounders by allowing a direct comparison of similarly sized guide and aspiration catheters across both groups. Hence, in the future, findings from this study may also inform potential differences between the similarly sized new generation large bore BGCs compared to the conventional (non-balloon) guide catheters. The results of this study could also potentially be applicable to other neurointerventional procedures where proximal flow arrest may be considered, such as in carotid artery stenting for symptomatic carotid stenosis. 30
Although there is an increasing trend of EVT treatment using the radial artery approach, there remains some reluctance amongst interventionists to use large bore BGCs for anterior circulation EVT, particularly in patients with a small radial arterial diameter due to the theoretical risk of arterial injury or occlusion. 31 However, recent studies have reported on the successful completion of EVT procedures performed using a sheathless BGC via the transradial approach. 28 Hence, it is probable that BGC use with the transradial approach may be equally feasible and safe as the transfemoral approach, and its uptake amongst interventionists should not solely be limited by the preferred vascular access site.
An important outcome measure investigated in this study is the frequency of emboli to distal and new vascular territories. Recent studies have demonstrated poorer functional outcomes amongst patients with distal emboli, and including those with microemboli formed during EVT which are thought to be play a role in microperfusion delay (no-reflow phenomenon) despite successful vessel recanalisation.4,6,32 There is no consensus on the optimal method of identifying emboli to new or distal vascular territories due to limitations in each imaging method. 33 We chose to identify emboli to new and distal vascular territories by comparing the pre- and post-EVT angiographic images as opposed to surrogate measures using the susceptibility or diffusion-weighted MR imaging sequence. This is due to the likelihood of selection bias by potentially super-selecting patients with early functional recovery who are more likely to be able to tolerate the MRI investigation post-EVT and excluding those who suffer from early deterioration or reduced consciousness. Furthermore, due to the high incidence of iatrogenic embolic shower during EVT (up to 82% depending on the MR sequence or imaging used 33 ), it is reasonable to assume that new distal emboli demonstrated on catheter angiography post-EVT are largely treatment-related. In addition, the same standardised method of outcome assessment is employed across both treatment groups, minimising any potential inherent biases.
Implementation of the deferred informed consent, which has been successfully employed in recent hyperacute stroke trials, as well as the pragmatic study design, will likely increase patient enrolment and reduce selection bias, thereby increasing the generalisability of the results. 21
Conclusion
This is the first RCT to investigate the effect of proximal blood flow arrest during EVT using a BGC on the procedural and clinical outcomes of patients with AIS due to large vessel occlusion.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231166194 for Effect of proximal blood flow arrest during endovascular thrombectomy (ProFATE): Study protocol for a multicentre randomised controlled trial by Permesh Singh Dhillon, Waleed Butt, Anna Podlasek, Pervinder Bhogal, Norman McConachie, Robert Lenthall, Sujit Nair, Luqman Malik, Jeremy Lynch, Tony Goddard, Emma Barrett, Kailash Krishnan, Robert A Dineen and Timothy J England in European Stroke Journal
Acknowledgments
We would like to thank the DSMB committee, and the research collaborators and investigators at the participating sites for their contributions to this trial. We also thank the Neurointerventionalists, Stroke physicians, Mechanical Thrombectomy specialist nurses, the wider Stroke teams at all participating institutions for the excellent care of all patients.
Footnotes
Abbreviations: EVT = endovascular thrombectomy, AIS = acute ischaemic stroke, mRS = modified Rankin Scale, NIHSS = National Institutes of Health Stroke Scale, ASPECTS = Alberta Stroke Program Early CT Score, eTICI = extended thrombolysis in cerebral infarction, sICH = symptomatic intracranial haemorrhage, NCCT = non-contrast computed tomography, CTA = computed tomography angiography.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PB reported travel support from Perflow; compensation from Cerenovus, Balt USA, LLC, Vesalio, Phenox Inc. and Brainomix for consultant services. No other disclosures or competing interests were declared by the remaining authors.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This investigator-initiated study is independently funded by the Royal College of Radiologists United Kingdom Kodak Scholarship Fund. PSD receives funded research time to undertake this trial from Nottingham University Hospitals NHS Trust. The funder had no role in the study design, data collection, analysis, interpretation and writing of the manuscript.
Ethical approval: The ProFATE trial received a favourable ethical opinion from the Health Research Authority, the Health and Care Research Wales, and the Wales Research Ethics Committee (Ref: 21/WA/0199).
Informed consent: Informed consent was obtained from all subjects before the study recruitment.
Guarantor: PSD.
Contributorship: Conception and design: PSD, WB, RAD, TJE. Writing of the manuscript: PSD, WB, AP. Critical revision of the manuscript: PSD, WB, AP, PB, NM, RL, SN, LM, JL, EB, KK, RAD, TJE. All authors approved the final version of the manuscript.
Trial registration: ClinicalTrials.gov (NCT05020795).
ORCID iDs: Permesh Singh Dhillon  https://orcid.org/0000-0003-4353-4515
https://orcid.org/0000-0003-4353-4515
Anna Podlasek  https://orcid.org/0000-0001-7297-7169
https://orcid.org/0000-0001-7297-7169
Robert Lenthall  https://orcid.org/0000-0002-2386-1335
https://orcid.org/0000-0002-2386-1335
Kailash Krishnan  https://orcid.org/0000-0002-6486-3783
https://orcid.org/0000-0002-6486-3783
Supplemental material: Supplemental material for this article is available online.
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 3.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg 2019; 11: 535–538. [DOI] [PubMed] [Google Scholar]
- 4.Chueh JY, Puri AS, Wakhloo AK, et al. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J Neurointerv Surg 2016; 8: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schönfeld MH, Kabiri R, Kniep HC, et al. Effect of balloon guide catheter utilization on the incidence of sub-angiographic peripheral emboli on high-resolution DWI after thrombectomy: a prospective observational study. Front Neurol 2020; 11: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bala F, Kappelhof M, Ospel JM, et al. Distal embolization in relation to radiological thrombus characteristics, treatment details, and functional outcome. Stroke 2023; 54: 448–456. [DOI] [PubMed] [Google Scholar]
- 7.Brinjikji W, Starke RM, Murad MH, et al. Impact of balloon guide catheter on technical and clinical outcomes: a systematic review and meta-analysis. J Neurointerv Surg 2018; 10: 335–339. [DOI] [PubMed] [Google Scholar]
- 8.Podlasek A, Dhillon PS, Jewett G, et al. Clinical and procedural outcomes with or without balloon guide catheters during endovascular thrombectomy in acute ischemic stroke: a systematic review and meta-analysis with first-line technique subgroup analysis. AJNR Am J Neuroradiol 2021; 42: 1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapergue B, Blanc R, Costalat V, et al. Effect of thrombectomy with combined contact aspiration and stent retriever vs stent retriever alone on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER2 randomized clinical trial. JAMA 2021; 326: 1158–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ospel JM, Volny O, Jayaraman M, et al. Optimizing fast first pass complete reperfusion in acute ischemic stroke - the BADDASS approach (BAlloon guiDe with large bore Distal Access catheter with dual aspiration with Stent-retriever as Standard approach). Expert Rev Med Devices 2019; 16: 955–963. [DOI] [PubMed] [Google Scholar]
- 11.Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014; 6: 260–264. [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Kappelhof M, Ospel JM, et al. Balloon guide catheters: use, reject, or randomize? Neuroradiology 2021; 63: 1179–1183. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon PS, Butt W, Jovin TG, et al. Endovascular thrombectomy vs best medical management beyond 24 hours from last known well in acute ischaemic stroke due to large vessel occlusion stroke: vascular and interventional neurology. Stroke 2023. DOI: 10.1161/SVIN.122.000790. [Google Scholar]
- 14.Nguyen TN, Castonguay AC, Siegler JE, et al. Mechanical thrombectomy in the late presentation of anterior circulation large vessel occlusion stroke: a guideline from the Society of Vascular and Interventional Neurology Guidelines and Practice Standards Committee. Stroke 2023; 3(1): e000512. [Google Scholar]
- 15.Nikoubashman O, Wischer D, Hennemann HM, et al. Balloon-guide catheters are needed for effective flow reversal during mechanical thrombectomy. AJNR Am J Neuroradiol 2018; 39: 2077–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NICE. Stroke and transient ischaemic attack in over 16s: diagnosis and initial management, https://www.nice.org.uk/guidance/ng128/chapter/Recommendations (2022, accessed 20 January 2023). [PubMed]
- 17.Gray LJ, Bath PM, Collier T.Should stroke trials adjust functional outcome for baseline prognostic factors? Stroke 2009; 40: 888–894. [DOI] [PubMed] [Google Scholar]
- 18.Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 2019; 11: 433–438. [DOI] [PubMed] [Google Scholar]
- 19.Kaesmacher J, Dobrocky T, Heldner MR, et al. Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: success revisited. J Neurol Neurosurg Psychiatry 2018; 89: 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 21.Law ZK, Appleton JP, Scutt P, et al. Brief consent methods enable rapid enrollment in acute stroke trial: results from the TICH-2 randomized controlled trial. Stroke 2022; 53: 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berndt MT, Goyal M, Psychogios M, et al. Endovascular stroke treatment using balloon guide catheters may reduce penumbral tissue damage and improve long-term outcome. Eur Radiol 2021; 31: 2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blasco J, Puig J, Daunis-I-Estadella P, et al. Balloon guide catheter improvements in thrombectomy outcomes persist despite advances in intracranial aspiration technology. J Neurointerv Surg 2021; 13: 773–778. [DOI] [PubMed] [Google Scholar]
- 24.Blasco J, Puig J, López-Rueda A, et al. Addition of intracranial aspiration to balloon guide catheter does not improve outcomes in large vessel occlusion anterior circulation stent retriever based thrombectomy for acute stroke. J Neurointerv Surg 2022; 14: 863–867. [DOI] [PubMed] [Google Scholar]
- 25.Bourcier R, Marnat G, Labreuche J, et al. Balloon guide catheter is not superior to conventional guide catheter when stent retriever and contact aspiration are combined for stroke treatment. Neurosurgery 2020; 88: E83–E90. [DOI] [PubMed] [Google Scholar]
- 26.Kang DH, Kim BM, Heo JH, et al. Effect of balloon guide catheter utilization on contact aspiration thrombectomy. J Neurosurg. Epub ahead of print 1 November 2018. DOI: 10.3171/2018.6.JNS181045. [DOI] [PubMed] [Google Scholar]
- 27.Topiwala K, Quinn C, Mehta T, et al. BOBBY balloon guide catheter thrombectomy in large-vessel occlusion stroke: initial experience. Interv Neuroradiol. Epub ahead of print 30 May 2022. DOI: 10.1177/15910199221104920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waqas M, Monteiro A, Cappuzzo JM, et al. Mechanical thrombectomy with a balloon-guide catheter: sheathless transradial versus transfemoral approach. J Neurointerv Surg. Epub ahead of print 29 December 2022. DOI: 10.1136/jnis-2022-019607. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-García C, Maegerlein C, Rosati S, et al. Impact of aspiration catheter size on first-pass effect in the combined use of contact aspiration and stent retriever technique. Stroke Vasc Neurol 2021; 6: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cappuzzo JM, Monteiro A, Waqas M, et al. Carotid artery stenting using the walrus balloon guide catheter with flow reversal for proximal embolic protection: technical description and single-center case series. Oper Neurosurg 2023; 24: 11–16. [DOI] [PubMed] [Google Scholar]
- 31.Narsinh KH, Mirza MH, Caton MT, Jr, et al. Radial artery access for neuroendovascular procedures: safety review and complications. J Neurointerv Surg 2021; 13: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 32.Happi Ngankou E, Gory B, Marnat G, et al. Thrombectomy complications in large vessel occlusions: incidence, predictors, and clinical impact in the ETIS Registry. Stroke 2021; 52: e764–e768. [DOI] [PubMed] [Google Scholar]
- 33.Wong GJ, Yoo B, Liebeskind D, et al. Frequency, determinants, and outcomes of emboli to distal and new territories related to mechanical thrombectomy for acute ischemic stroke. Stroke 2021; 52: 2241–2249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231166194 for Effect of proximal blood flow arrest during endovascular thrombectomy (ProFATE): Study protocol for a multicentre randomised controlled trial by Permesh Singh Dhillon, Waleed Butt, Anna Podlasek, Pervinder Bhogal, Norman McConachie, Robert Lenthall, Sujit Nair, Luqman Malik, Jeremy Lynch, Tony Goddard, Emma Barrett, Kailash Krishnan, Robert A Dineen and Timothy J England in European Stroke Journal