Abstract
Introduction:
Intravenous thrombolysis (IVT) and mechanical thrombectomy (MT) in women with ischemic stroke (IS) during pregnancy/post-partum is challenging, and recent guidelines claimed for additional information to better argue its effectiveness and safety. This observational national study aimed to describe characteristics, rates and outcomes of pregnant/post-partum women receiving acute revascularization therapy for IS compared to their non-pregnant counterparts, and pregnant women with IS not receiving such therapy.
Patients and methods:
In this cross-sectional study, all women aged 15–49 years hospitalized in France for IS between 2012 and 2018 were retrieved from the French hospital discharge databases. Pregnant or post-partum (⩽6 weeks after delivery) women were identified. Data about patients’ characteristics, risk factors, revascularization therapy, delivery, post-stroke survival, and recurrent vascular events during follow-up were recorded.
Results:
Over the study period, 382 women with pregnancy-related IS were registered. Among them, 7.3% (n = 28) received a revascularization therapy, including nine cases during pregnancy, one the same day as delivery, and 18 during the post-partum period, compared with 8.5% (n = 1285) in women with non-pregnancy-related IS (n = 15,084). Treated pregnant/post-partum women had more severe IS than not-treated pregnant/post-partum. Compared with treated not-pregnant women, they were younger, but did not differ regarding other characteristics including stroke severity. There were no differences in systemic or intracranial hemorrhages or in the length of hospital stay between pregnant/post-partum women compared with treated not-pregnant women. All women receiving revascularization during pregnancy had a live baby. After a mean follow-up of 4.3 years, all pregnant/post-partum women were alive, one had recurrent IS and none had other vascular events.
Discussion and conclusion:
Only a few women with pregnancy-related IS were treated with acute revascularization therapy, but this was proportionately similar to their non-pregnant counterparts, from whom they did not differed regarding characteristics, survival, and risk of recurrent events. These findings suggest that stroke physicians applied treatment strategies of IS in a similar way regardless of pregnancy in France, and this attitude was an anticipation but consistent with the recently published guidelines on the topic.
Keywords: Stroke, ischemic stroke, pregnancy, thrombolysis, mechanical thrombectomy, epidemiology
Introduction
Although available data are scarce, the incidence of ischemic stroke (IS) during pregnancy and post-partum has been estimated to range between 5 and 35 per 100,000 pregnancies per year.1–3 Many questions remain about the best acute treatment of pregnancy-related IS. In 2019, the American Heart Association/American Stroke Association issued a class IIb recommendation with a level of evidence C due to limited data that intravenous thrombolysis (IVT) administration may be considered in pregnancy when the anticipated benefits of treating moderate or severe stroke outweigh the anticipated increased risks of uterine bleeding. 4 The recent European Stroke Organisation (ESO) guidelines on stroke in women failed to make evidence-based recommendations on the use of IVT or mechanical thrombectomy (MT) in the absence of randomized clinical trials. 5 Nevertheless, the expert consensus suggested that these treatments could be used during pregnancy after appropriate assessment of the benefit/risk profile on an individual basis. This statement was made based on data from the US Get With The Guidelines (GWTG) Stroke Registry 6 and a few number of single case reports, 5 but pointed out the fact that additional information is required to better argue the effectiveness and safety of such therapeutic strategies.
Therefore, the aim of this study was to provide a nationwide perspective of acute therapy for IS during pregnancy and post-partum, and to compare characteristics and outcomes of treated women to those of their non-pregnant counterparts, and to women with pregnancy-related IS not receiving acute revascularization therapy.
Methods
Study population
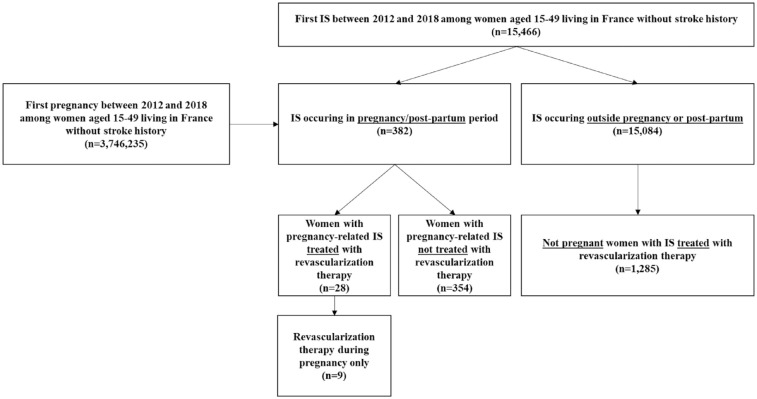
For this cross-sectional study, data about pregnancy-related IS were obtained from the CONCEPTION study, the methodology of which has been previously described extensively. 7 Briefly, all French women aged between 15 and 49 years old who gave birth in a hospital after 22 weeks of amenorrhea between 1 January, 2012 and 31 December, 2018 were identified through the French National Health Insurance Information System database (Système National des Données de Santé, SNDS). This database contains data from the national hospital discharge database (PMSI) which records information about all public and private hospital stays diagnosis and data from the national health-insurance database, containing information regarding reimbursements of healthcare expenditures, such as outpatient medical care. Pregnancy was defined as lasting from the first day of pregnancy until the beginning of hospitalization for childbirth. Pregnancy start date was computed as the date of delivery minus the gestational age at delivery. Postpartum was defined as starting at the end of hospitalization for childbirth until 6 weeks after birth. For the present analysis, only women with a hospital diagnosis of IS (International Classification of Diseases-Tenth Revision codes (ICD-10) codes: I63-I64; excluding I63.6), were considered. Non-pregnant women aged between 15 and 49 years old with acute IS were also identified from the SNDS database over the same period, using a similar procedure. Women with a history of stroke between 2006 and the inclusion date (1st of January 2012) were excluded. A flow chart of the study is shown in Figure 1.
Figure 1.
Flow-chart of population study.
Data collected
Sociodemographic data, medical history, information related to antepartum, peripartum, and postpartum, and information regarding healthcare management of the women were recorded in the French National Health Insurance Information System. Quintile of social deprivation of the town of living was recorded for each women. The level of social deprivation was estimated from the French deprivation index (FDep) developed by Rey et al., 8 which included the following dimension for a town: the median household income, the percentage of high school graduates in the population aged 15 years and older, the percentage of blue-collar workers in the active population, and the unemployment rate. A deprivation index was then attributed to each French town. Then all individuals were grouped together into quintile of the social deprivation of the town where they lived. Tobacco use was identified by specific coding at the hospital (ICD-10 codes F17, Z716, T652, and Z720) or by the reimbursement of tobacco replacement therapy before or during pregnancy. Obesity was identified in the hospital discharge summaries of the childbirth stay. Pre-existing diabetes mellitus was determined using an algorithm based on delivery of three antidiabetic drugs on three different dates in the year preceding pregnancy (or on two dates if at least one large package of antidiabetic drugs was dispensed). Gestational diabetes mellitus was defined using an algorithm combining delivery of insulin or glucose strips, or a diagnosis of diabetes mellitus during pregnancy, and no pre-existing diabetes mellitus. Chronic hypertension was identified by at least three deliveries of antihypertensive drugs on different dates over a 12-month period, or on two dates if at least one large pack (90 pills) of antihypertensive drugs was dispensed or a hospitalization with a diagnosis of chronic hypertension during pregnancy. Hypertensive disorders of pregnancy (HDP) were considered using an algorithm based on hospitalizations for preeclampsia (O14), HELLP syndrome (O14.2), or eclampsia (O15). In women without preexisting chronic hypertension, gestational hypertension was identified in our database by a hospitalization with a primary diagnosis of gestational hypertension during pregnancy (O13), or at least one delivery of antihypertensive medication between 20 weeks of gestation and 6 weeks of postpartum without differential diagnosis of preterm labor. Personal history of cardiovascular diseases (CVD) of each women, including acute coronary syndrome or heart failure, personal history of venous thromboembolism, inflammatory disease, cardiac malformation were all recorded using all hospital discharge summaries between 2006 and their pregnancy. Finally, comorbidities reported during hospitalization as defined by the Charlson index score were recorded. 9
Follow-up
All women were followed from the start of the first pregnancy occurring between 2012 and 2018 until 31 December 2020 with the record of hospitalization for stroke recurrence, cardiovascular diseases, venous thrombo-embolism diseases, and the registration of potential date of death.
Statistical analyses
Comparisons of categorical variables between pregnant women with IS receiving revascularization, non-pregnant counterparts, and women with pregnancy-related IS not receiving acute revascularization therapy, were performed using Mantel–Haenszel Chi-squared, or Fisher’s exact test when necessary, and using Wilcoxon-Mann-Whitney test for quantitative variables.
Statistical analysis was performed with SAS Guide software SAS (version 7.11, SAS Institute Inc., Cary, NC, USA).
Results
From 2012 to 2018, 3,746,235 women without previous stroke gave birth in France, corresponding to a total of 4,831,172 pregnancies. Among these women, 382 had acute IS (incidence rate: 10.3 per 100,000 person-years). Twenty-eight patients (7.3%) received revascularization therapy (Table 1) including 19 patients with IVT alone, eight patients with MT alone, and one patient with bridging therapy. In detail, nine women were treated during pregnancy (seven patients with IVT alone, one patient with MT alone, and one patient with bridging therapy), one woman on the same day as delivery (IVT alone), and 18 women during the post-partum period (11 patients with IVT alone and 7 patients with MT alone) (Supplemental Table). Among women with revascularization therapy during pregnancy, treatment was administered between 4 and 24 weeks of amenorrhea (median: 12 weeks). During the post-partum period, time between delivery and revascularization therapy ranged between 1 and 42 days (median: 11 days). One patient had IS 17 days after a medical termination of pregnancy. Among the remaining 27 patients, all had a live baby. Median gestational age at delivery was 39 weeks of amenorrhea (range: 34–39) for women treated during pregnancy, 40 weeks of amenorrhea in the woman treated on the same day as delivery, and 38 weeks of amenorrhea (range: 27–40) in women treated during post-partum period.
Table 1.
Characteristics of women with pregnancy-related IS treated or not with revascularization therapy and not pregnant women with IS, in France between 2012 and 2018.
| Women with pregnancy-related IS treated with revascularization therapy (n = 28) | Women with pregnancy-related IS not treated with revascularization therapy (n = 354) | Not pregnant women with IS treated with revascularization therapy (n = 1285) | p * | p ** | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Age, mean ± SD | 32.4 ± 6.8 | 32.0 ± 5.5 | 39.9 ± 7.8 | |||||
| Age, median ± IQR | 33.5 ± 11 | 32 ± 8 | 42 ± 11 | 0.78 | <0.001 | |||
| Age group | 0.63 | <0.001 | ||||||
| 15–24 | 3 | 10.7 | 39 | 11.0 | 74 | 5.8 | ||
| 25–29 | 8 | 28.6 | 77 | 21.8 | 91 | 7.1 | ||
| 30–34 | 5 | 17.9 | 114 | 32.2 | 134 | 10.4 | ||
| 35–39 | 7 | 25.0 | 94 | 26.6 | 195 | 15.2 | ||
| 40–44 | 5 | 17.9 | 29 | 8.2 | 320 | 24.9 | ||
| 45–49 | 0 | 0 | 1 | 0.3 | 471 | 36.7 | ||
| Quintile of social deprivation | 0.63 | 0.76 | ||||||
| Quintile 1 (the least deprived) | 6 | 21.4 | 58 | 16.4 | 186 | 14.5 | ||
| Quintile 2 | 4 | 14.3 | 69 | 19.5 | 233 | 18.1 | ||
| Quintile 3 | 4 | 14.3 | 72 | 20.3 | 244 | 19.0 | ||
| Quintile 4 | 5 | 17.9 | 65 | 18.4 | 220 | 17.1 | ||
| Quintile 5 (the most deprived) | 7 | 25.0 | 57 | 16.1 | 313 | 24.4 | ||
| Missing | 2 | 7.1 | 33 | 9.3 | 89 | 6.9 | ||
| Weeks of amenorrhea at delivery, median (IQR) | 38.5 ± 1.5 | 39 ± 3 | – | 0.51 | – | |||
| Diabetes mellitus | 0 | 0 | 8 | 2.3 | 31 | 2.6 | 0.43 | 0.40 |
| Obesity | 6 | 21.4 | 26 | 7.3 | 180 | 14.0 | 0.001 | 0.27 |
| Chronic hypertension | 2 | 7.4 | 17 | 4.9 | 192 | 16.2 | 0.57 | 0.22 |
| Tobacco use | 8 | 28.6 | 54 | 15.3 | 346 | 27.0 | 0.07 | 0.84 |
| Atrial fibrillation | 2 | 7.1 | 6 | 1.7 | 61 | 4.7 | 0.053 | 0.56 |
| Chronic heart failure | 1 | 3.6 | 3 | 0.9 | 27 | 2.1 | 0.17 | 0.59 |
| History of venous thromboembolism | 3 | 10.7 | 13 | 3.7 | 54 | 4.2 | 0.07 | 0.09 |
| History of coronary artery diseases | 0 | 0 | 4 | 1.1 | 54 | 4.2 | 0.57 | 0.27 |
| Gestational Diabetes mellitus | 4 | 14.3 | 31 | 8.8 | – | – | 0.33 | – |
| Hypertensive disorders of pregnancy | 3 | 10.7 | 51 | 14.4 | – | – | 0.59 | – |
| Outcomes linked to delivery | ||||||||
| Post-partum hemorrhage | 5 | 17.9 | 44 | 12.4 | – | – | 0.41 | – |
| Cesarean section | 6 | 21.4 | 111 | 31.4 | – | – | 0.27 | – |
| Numbers of Charlson comorbidities, % | <0.001 | 0.96 | ||||||
| 0 | 7 | 25.0 | 228 | 64.4 | 314 | 24.4 | ||
| 1 | 0 | 0.0 | 2 | 0.6 | 14 | 1.1 | ||
| 2 | 19 | 67.9 | 109 | 30.8 | 852 | 66.3 | ||
| 3 | 0 | 0.0 | 6 | 1.7 | 40 | 3.1 | ||
| ⩾4 | 2 | 7.1 | 9 | 2.5 | 65 | 5.1 | ||
| Stroke severity | ||||||||
| Hemiplegia | 21 | 75.0 | 107 | 30.2 | 878 | 68.33 | <0.001 | 0.45 |
| Aphasia | 17 | 60.7 | 97 | 27.4 | 754 | 58.68 | <0.001 | 0.83 |
| Decreased consciousness | 2 | 7.1 | 20 | 5.7 | 135 | 10.51 | 0.74 | 0.56 |
| Cerebral hemorrhagic complications | 0 | 0 | 9 | 2.5 | 18 | 1.4 | 0.39 | 0.53 |
| ICH | 0 | 0 | 4 | 1.1 | 14 | 1.4 | 0.57 | 0.58 |
| Subarachnoid hemorrhage | 0 | 0 | 4 | 1.1 | 4 | 0.3 | 0.57 | 0.77 |
| Other cerebral hemorrhage | 0 | 0 | 1 | 0.3 | 0 | 0 | 0.78 | – |
| Systemic hemorrhagic complications | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
| Patient with stay in a stroke unit | 27 | 96.4 | 263 | 74.3 | 1224 | 95.3 | 0.009 | 0.56 |
| Patient with stay in a resuscitation unit | 7 | 52 | 30 | 8.5 | 200 | 15.6 | 0.005 | 0.17 |
| Home discharge | 18 | 64.3 | 281 | 79.4 | 863 | 67.2 | 0.06 | 0.18 |
| Length of hospital stay in days, median (IQR) | 9 (13.5) | 6 (7) | 8 (8) | <0.001 | 0.41 | |||
Bold: p-value < 0.05. IQR = Inter-quartile range.
p Value for comparison between women with pregnancy-related IS treated with revascularization therapy and women with pregnancy-related IS not treated with revascularization therapy.
p Value for comparison between women with pregnancy-related IS treated with revascularization therapy and not pregnant women with IS treated with revascularization therapy.
Characteristics of women with pregnancy-related IS according to revascularization therapy are shown in Table 1. Compared with IS women who did not receive revascularization therapy (n = 354), treated women were more frequently admitted to a stroke unit (96% vs 74%, p = 0.009) and/or a resuscitation unit (25% vs 8%, p = 0.005), and had a longer length of stay (median: 9 vs 6 days, p < 0.0001). In addition, they had more severe IS with more frequent hemiplegia (75% vs 30%, p < 0.0001), and aphasia (61% vs 27%, p < 0.001), more frequent Charslon co-morbidities, and more frequent obesity. In contrast, they did not differ regarding other vascular risk factors or pregnancy-related co-morbidities. Of note, postpartum hemorrhage was observed in five patients (18%) with treated IS. Postpartum hemorrhage occurred in two women with IS during pregnancy (treated with IVT at 3 and 16 weeks of amenorrhea, respectively, with a delivery at 39 weeks of amenorrhea for both patients), and in three women with IS during post-partum period (one patients with IS treated with MT the same day as delivery, one patient treated with IVT 9 days after delivery, and one patient treated with IVT 34 days after delivery). In comparison, postpartum hemorrhage occurred in 44 women (12%) with IS not receiving acute revascularization therapy (p = 0.41). Also among women with pregnancy-related IS, those receiving revascularization therapy had similar rate of caesarian section compared to those who did not received revascularization therapy (n = 6 (21.4%) vs n = 111 (31.4%), p = 0.27) (Table 1). This observation was also true when restricting the comparison to women receiving revascularization during pregnancy only (Supplemental Table). Finally, during the hospital stay for stroke, we did not record any hemorrhagic complication (cerebral or systemic) in women with pregnancy-related IS who were treated with revascularization therapy (Table 1).
Over the same study period, 1285 not pregnant women aged 15–44 years old had acute IS and were treated with revascularization therapy including 1117 (86.9%) with IVT alone, 249 (19.4%) with MT alone, and 81 (6.3%) with bridging therapy, accounting for 8.5% of the total number of women with IS in this age group (1285 on the 15,084 non-pregnant women with IS aged 15–49 years). Compared to them, treated pregnant/post-partum women were younger (median age 33 vs 42 years old, p < 0.0001), but did not differ regarding vascular risk factors, Charlson co-morbidities, pregnancy-related co-morbidities, stroke severity, admission to a stroke unit and/or a resuscitation unit, length of stay, and home discharge (Table 1). Same results were observed when we restricted our comparison to women with treated IS during pregnancy (Supplemental Table).
After a mean follow-up of 4.3 years (range: 2.3–8.5), all patients with pregnancy-related IS treated with acute revascularization therapy were alive (Table 2). One patient had recurrent IS 38 months after post-partum treated IS. None had acute coronary syndrome and two patients suffered a venous thromboembolism event. Despite some numerical differences, women with pregnancy-related IS not receiving acute revascularization therapy and not pregnant women with IS treated with revascularization therapy did not statistically differ from pregnancy-related IS treated with acute revascularization therapy regarding major vascular outcomes. Of note, no hemorrhagic stroke nor systemic bleeding were reported during the follow-up among the 28 women who received revascularization therapy for pregnancy-related IS.
Table 2.
Outcomes of women with pregnancy-related IS treated or not with revascularization therapy and not pregnant women with IS, in France between 2012 and 2018.
| Women with pregnancy-related IS treated with revascularization therapy (n = 28) | Women with pregnancy-related IS not treated with revascularization therapy (n = 354) | Not pregnant women with IS treated with revascularization therapy (n = 1285) | p * | p ** | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Duration of follow-up in days, mean ± SD | 1,560.5 ± 699.8 | 1,834.1 ± 870.6 | 1,398.6 ± 862.9 | |||||
| Duration of follow-up in days, median ± IQR | 1294 ± 1015 | 1888 ± 1137 | 1287 ± 1172 | 0.78 | <0.001 | |||
| Stroke recurrence | ||||||||
| Ischemic stroke | 1 | 3.6 | 20 | 5.7 | 99 | 7.7 | 0.64 | 0.41 |
| Hemorrhagic stroke | 0 | 0 | 2 | 0.6 | 4 | 0.3 | 0.69 | 0.77 |
| ICH | 0 | 0 | 2 | 0.6 | 2 | 0.2 | 0.69 | 0.83 |
| Other systemic bleedings | 0 | 0 | 11 | 3.1 | 52 | 4.0 | 0.34 | 0.28 |
| Acute coronary syndrome | 0 | 0 | 3 | 0.9 | 7 | 0.5 | 0.63 | 0.70 |
| Venous thromboembolism | 0 | 0 | 2 | 0.6 | 19 | 1.5 | 0.69 | 0.51 |
| Death | 0 | 0 | 11 | 3.1 | 79 | 6.5 | 0.34 | 0.18 |
p Value for comparison between women with pregnancy-related IS treated with revascularization therapy and women with pregnancy-related IS not treated with revascularization therapy.
p Value for comparison between women with pregnancy-related IS treated with revascularization therapy and not pregnant women with IS treated with revascularization therapy.
Discussion
This study demonstrated that a few number of women with pregnancy-related IS were treated with acute revascularization therapy, but in a similar proportion than their non-pregnant counterparts, from whom they did not differed regarding characteristics, survival, and risk of recurrent events.
Women with pregnancy-related IS treated with revascularization therapy had a greater clinical severity compared with not treated pregnant women, as already reported in the GWTG study. 6 This reflects the fact that the indication for acute revascularization takes into account the severity of the stroke, patients with minor IS being generally excluded from it. Moreover, it cannot be totally ruled out that a treatment selection has occurred, with clinicians reluctant to administer acute revascularization therapy to pregnant IS women with mild symptoms, all the more so since that pregnancy was still listed as a contraindication of intravenous tPA in the French marketing authorization. However, the fact that women with pregnancy-related IS who received acute revascularization therapy did not differ from non-pregnant women with treated IS in terms of severity argues against this hypothesis and suggests instead that stroke neurologists tended to treat pregnant women in a similar way than their non-pregnant counterparts.
Women with pregnancy-related IS were approximately 9 years younger than non-pregnant IS women in our study (median age 33 vs 42 years). This age-gap was consistent with the results of GWTG study in which median age of IS pregnant/post-partum women was 31 versus 39 years in their non-pregnant counterparts, 6 and may be explained by the fact that pregnancy is associated with an increased risk of IS, especially during peripartum and post-partum, 2 in line with various physiological changes including hormonal modifications, hypercoagulability, and hypertensive disorders of pregnancy such as preeclampsia. 6
The use of MT was as common among pregnant IS women as among non-pregnant women. Hence, MT either alone or as bridging therapy was performed in nine pregnant women (32% of treated pregnant women, corresponding to 2.4% of overall pregnancy-related IS), and in 330 non-pregnant women (26% of treated non-pregnant women, corresponding to 2.6% of overall IS outside pregnancy). These proportions appear to be low in comparison with data from the population-based Dijon Stroke Registry that showed that in this age group, about 15% of women with IS have a large vessel occlusion, that is, potentially eligible for MT. 10 Nevertheless, it should be kept in mind that at the beginning of our study period, this treatment was not routinely applied, the recommendations on the use of MT having been elaborated since 2015 after the demonstration of its effectiveness by randomized clinical trials. 11
There was no difference in outcomes of women with pregnancy-related IS treated or not with revascularization therapy and non-pregnant women with IS regarding subsequent vascular events and death. The GWTG study reported similar rates of in-hospital death, home discharge, and independent ambulation at discharge in pregnant/postpartum and non-pregnant women who received reperfusion therapy, but no information on long-term outcome was available. 6 Consistently with this study, we observed a longer duration of hospital stay in pregnant women treated with revascularization therapy which may suggest a medical attitude toward a longer surveillance of these patients. Finally, all but one woman with IS treated with acute revascularization therapy had a live baby, and the remaining patient suffered IS 17 days after a medical termination of pregnancy. Although this result should be interpreted with caution in the absence of clinical data about newborns, it is consistent with a recent review of case reports in which a healthy baby was born to 28 out of 32 patients treated with IVT, and to 18 out of 19 patients treated with MT or intraarterial thrombolysis (the remaining pregnant women had an abortion or a medically terminated pregnancy). 5
Our study has several strengths and limitations. The use of a nationwide database allowed us to record all cases of pregnant women who gave birth after 22 weeks of amenorrhea with IS receiving revascularization therapy in France over a long period. We were able to follow women included in the study during a quite long time-period. Although some clinical data are missing, only this type of database can be used to obtain enough patients to draw conclusions because of the rarity of stroke in pregnant/post-partum women. The lack of information on stroke severity based on dedicated scales such the NIHSS score limited comparisons between patients, but the use of clinical proxies of severity recorded in the database made it possible to partially overcome this limitation. In addition, we had no information on functional outcome that limited the interpretation of the results. Only pregnancy of at least 22 weeks of amenorrhea could be exhaustively identified in the SNDS, therefore our study potentially missed in the pregnant women group with IS in the early pregnancy who aborted, and the treatment related to IS they might have received. This probably concerned very few cases but could have caused a classification bias by considering these women in the group of non-pregnant women. The SNDS database did not precisely provide the time at which revascularization treatment was administrated. Therefore, for women who received acute IS treatment the same day as delivery, we were not able to confirm which came first between the delivery and the revascularization therapy. Finally, subgroups analyses were limited by the small number of cases.
To conclude, a small part of women with pregnancy-related IS were treated with acute revascularization therapy, although in a similar proportion than their non-pregnant counterparts. No difference in baseline characteristics were observed between pregnant/postpartum and not pregnant women, suggesting that stroke physicians applied treatment strategies of IS in a similar way regardless of pregnancy. This attitude was an anticipation but consistent with the recently published guidelines on the topic. Although there were not signal for safety concern for babies, and similar long-term survival and risk of recurrent events than in non-pregnant women, additional observational studies focusing on maternal and newborns functional outcomes would be needed.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231156208 for Acute revascularization therapy for ischemic stroke during pregnancy and post-partum in France by Yannick Béjot, Valérie Olié, Grégory Lailler, Clémence Grave, Nolwenn Regnault, Jacques Blacher, Gauthier Duloquin and Amélie Gabet in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231156208 for Acute revascularization therapy for ischemic stroke during pregnancy and post-partum in France by Yannick Béjot, Valérie Olié, Grégory Lailler, Clémence Grave, Nolwenn Regnault, Jacques Blacher, Gauthier Duloquin and Amélie Gabet in European Stroke Journal
Acknowledgments
None.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yannick Béjot received honoraria for lectures or consulting fees from BMS, Pfizer, Medtronic, Amgen, Servier, Boehringer-Ingelheim, and Novo-Nordisk, outside the submitted work. JB reports, outside the submitted work, personal fees from Abbott, Bayer, Bottu, Egis, Ferring, Steripharma, Kantar, Sanofi, Servier and Teriak, personal fees and non-financial support from Pfizer and Quantum Genomics.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval and informed consent: According to the French governmental regulations and the National Ethics Committee, no patient consent was required. The databases used in the study contained pseudonymized patient information. Furthermore, full access to the SNDS which includes the French National Hospital Databases is granted to the National Agency for Public Health (Santé Publique France) by decree (regulatory decision DE-2011-078).
Guarantor: Amélie Gabet takes full responsibility for the data, the analyses and interpretation, the conduct of the research, and the accuracy and appropriateness of the reference list.
Contributorship: YB wrote the manuscript; AG, CG, GL, GD contributed to statistical analyses, discussion, and reviewed the manuscript; YB, VO, NR, and JB contributed to discussion and reviewed the manuscript; AG directed research, contributed to write the manuscript, contributed to statistical analyses, discussion, and reviewed the manuscript. All the authors have read and approved the manuscript and its publication in European Stroke Journal as a research article.
ORCID iD: Amélie Gabet  https://orcid.org/0000-0003-1273-8988
https://orcid.org/0000-0003-1273-8988
Supplemental material: Supplemental material for this article is available online.
References
- 1.Karjalainen L, Tikkanen M, Rantanen K, et al. Stroke in pregnancy and puerperium: validated incidence trends with risk factor analysis in Finland 1987-2016. Neurology 2021; 96: e2564–e2575. [DOI] [PubMed] [Google Scholar]
- 2.Martin A, Lailler G, Béjot Y, et al. Incidence and time trends of pregnancy-related stroke between 2010 and 2018: the nationwide CONCEPTION study. Neurology 2022; 99: e1598–e1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swartz RH, Cayley ML, Foley N, et al. The incidence of pregnancy-related stroke: a systematic review and meta-analysis. Int J Stroke 2017; 12: 687–697. [DOI] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 5.Kremer C, Gdovinova Z, Bejot Y, et al. European stroke organisation guidelines on stroke in women: management of menopause, pregnancy and postpartum. Eur Stroke J 2022; 7: I–XIX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leffert LR, Clancy CR, Bateman BT, et al. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol 2015; 125: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucheron P, Lailler G, Moutengou E, et al. Hypertensive disorders of pregnancy and onset of chronic hypertension in France: the nationwide CONCEPTION study. Eur Heart J 2022; 43: 3352–3361. [DOI] [PubMed] [Google Scholar]
- 8.Rey G, Jougla E, Fouillet A, et al. Ecological association between a deprivation index and mortality in France over the period 1997 - 2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health 2009; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 10.Duloquin G, Graber M, Garnier L, et al. Incidence of acute ischemic stroke with visible arterial occlusion: a population-based study (Dijon Stroke Registry). Stroke 2020; 51: 2122–2130. [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231156208 for Acute revascularization therapy for ischemic stroke during pregnancy and post-partum in France by Yannick Béjot, Valérie Olié, Grégory Lailler, Clémence Grave, Nolwenn Regnault, Jacques Blacher, Gauthier Duloquin and Amélie Gabet in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231156208 for Acute revascularization therapy for ischemic stroke during pregnancy and post-partum in France by Yannick Béjot, Valérie Olié, Grégory Lailler, Clémence Grave, Nolwenn Regnault, Jacques Blacher, Gauthier Duloquin and Amélie Gabet in European Stroke Journal