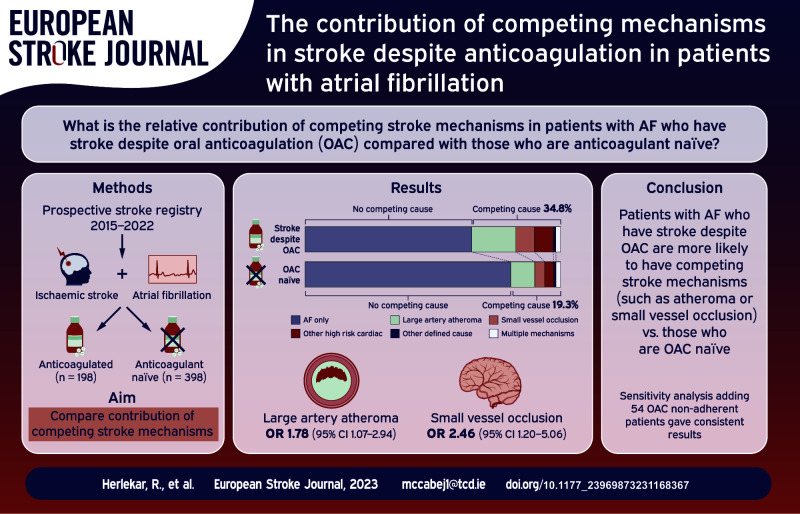
Abstract
Background:
For reasons poorly understood, strokes frequently occur in patients with atrial fibrillation (AF) despite oral anticoagulation. Better data are needed to inform randomised trials (RCTs) of new strategies to prevent recurrence in these patients. We investigate the relative contribution of competing stroke mechanisms in patients with AF who have stroke despite anticoagulation (OAC+) compared with those who are anticoagulant naïve (OAC−) at the time of their event.
Patients and methods:
We performed a cross-sectional study leveraging data from a prospective stroke registry (2015–2022). Eligible patients had ischemic stroke and AF. Stroke classification was performed by a single stroke-specialist blinded to OAC status using TOAST criteria. The presence of atherosclerotic plaque was determined using duplex ultrasonography, computerised tomography (CT) or magnetic resonance (MR) angiography. Imaging was reviewed by a single reader. Logistic regression was used to identify independent predictors of stroke despite anticoagulation.
Results:
Of 596 patients included, 198 (33.2%) were in the OAC+ group. A competing cause for stroke was more frequent in patients with OAC+ versus OAC− (69/198 (34.8%)) versus 77/398 (19.3%), p < 0.001). After adjustment, both small vessel occlusion (odds ratio (OR): 2.46, 95% CI: 1.20–5.06) and arterial atheroma (⩾50% stenosis) (OR: 1.78, 95% CI: 1.07–2.94) were independently associated with stroke despite anticoagulation.
Discussion and conclusion:
Patients with AF-associated stroke despite OAC are much more likely than patients who are OAC-naïve to have competing stroke mechanisms. Rigorous investigation for alternative stroke causes in stroke despite OAC has a high diagnostic yield. These data should be used to guide patient selection for future RCTs in this population.
Keywords: Ischaemic stroke, atrial fibrillation, recurrence, prognosis, atrial size, anticoagulation
Graphical abstract.
Introduction
Atrial fibrillation (AF) is a leading cause of ischemic stroke. Despite the availability of proven oral anticoagulant (OAC) therapy, the incidence of AF-associated stroke is expected to increase substantially in the coming decades due to population ageing. 1 Oral anticoagulation with vitamin K antagonists (VKA) 2 or direct oral anticoagulants (DOACs) 3 reduce the risk of stroke in patients with AF by approximately 60%. However, strokes that occur despite OAC therapy represent up to one-third of all AF-associated stroke.4,5 These patients have a high mortality and a high burden of disability. 6 Importantly, patients who have stroke despite anticoagulation have an annualised risk of stroke recurrence of approximately 9%, 5 which is substantially greater than that observed in randomised control trials (RCTs), which reported stroke rates of 1.1%–2.4% per year on DOACs.7,8 There are limited strategies to reduce this residual stroke risk and guidelines have not made any recommendations on best practice in such cases. 9
Better data are clearly needed to inform clinical practice and future RCTs of new therapeutic strategies in AF. However, the causes of stroke despite OAC in AF are not well understood. Previous studies have reported a myriad of potential reasons for ‘OAC failure’ including therapeutic non-adherence or under-dosing of OAC.6,10 It is also possible that patients with stroke despite anticoagulation are particularly prone to thrombo-embolic events in the setting of a more advanced atrial cardiopathy. On the other hand, competing mechanisms such as cerebral small vessel disease (CSVD) and atherosclerosis may play a prominent role. The purpose of this study is to better understand the aetiology of stroke on anticoagulation in patients with AF. By comparing the background prevalence of competing mechanisms in patients who are not taking anticoagulation at the time of stroke (OAC−) versus those who are taking anticoagulation (OAC+), a more accurate determination of the prevalence of ‘true anticoagulant failure’ can be made. Therefore, in the present work we investigate the relative contribution of competing stroke mechanisms in patients with and without OAC in AF-associated stroke.
Patients and methods
Hypothesis and aims
We hypothesised that competing stroke mechanisms would occur more frequently in AF patients in the OAC+ group than in the comparator group of patients not on OAC. The pre-specified primary aims of this study were to evaluate the association between: (1) intra-cranial/extra-cranial atherosclerosis; and (2) small vessel occlusion (SVO) with stroke despite anticoagulation. The secondary aims were to investigate the association between: (1) competing high-risk cardiac source of embolism; (2) echocardiographic biomarkers; and (3) cardiovascular risk factors with stroke despite anticoagulation.
Patient selection and ethical approval
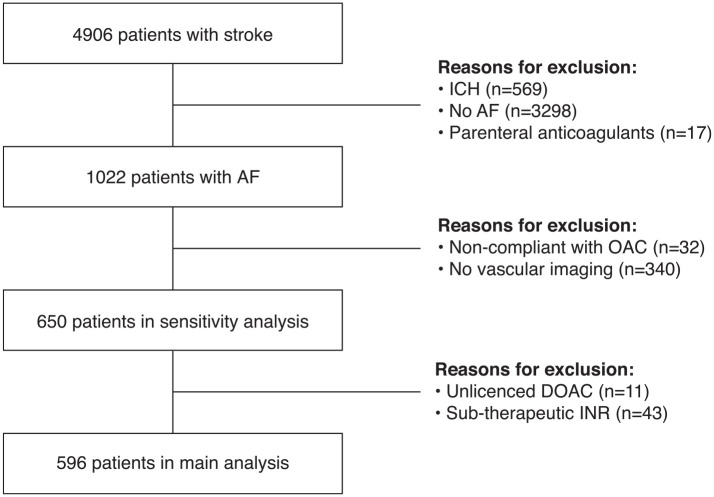
The Cambridge AF Failure of Anticoagulation Study (CAFFAS) is a cross-sectional study which leveraged data collected in a prospective stroke registry (UK Sentinel Stroke National Audit Programme (SSNAP)) from a single comprehensive stroke centre (Addenbrooke’s Hospital, Cambridge). Details of consecutive patients with a diagnosis of stroke are recorded in SSNAP. Participants included had: (1) imaging-confirmed acute ischemic stroke; (2) a new/pre-existing diagnosis of permanent, persistent, or paroxysmal AF; and (3) presented between January 2015 and January 2022. The following patients were excluded: (1) transient ischemic attack (TIA) without radiological evidence of infarction; (2) intra-cerebral haemorrhage; (3) no extracranial vascular imaging with either computerised tomography angiography (CTA), duplex ultrasonography, or magnetic resonance angiography; (4) participants non-compliant with prescribed OAC therapy; or (5) participants receiving parenteral therapeutic anticoagulation for AF. This study was registered with Cambridge University Hospitals’ Quality Surveillance Team (ID 14396). Formal confirmation was received that ethical approval from the Institutional Review Board was not required and the need to obtain written informed patient consent was therefore waived.
Co-variates
Clinical, laboratory and imaging information for each acute stroke episode is prospectively recorded in the SSNAP database and was acquired for the purposes of this study (Supplemental Methods). AF was confirmed by medical record documentation or if newly diagnosed during hospital admission for the index event. Patients prescribed and recently adherent to warfarin, apixaban, dabigatran, rivaroxaban, or edoxaban at the time of the qualifying stroke were defined as the OAC+ group. Patients who experienced a stroke and were not taking OAC were defined as OAC naïve (OAC−). Participants who had not ingested prescribed OAC therapy in the previous 24 h prior to stroke onset were considered non-adherent and excluded from the analysis. Non-adherence was determined by patient enquiry by the stroke physician or from a reliable collateral historian. Patients who were taking unlicensed low-dose DOAC (Supplemental Table 1) or had a sub-therapeutic (<2.0) International Normalised Ratio (INR) on VKAs were considered as under-treated on OAC therapy. Our pre-specified primary analysis plan excluded these patients. However, we also performed a sensitivity analysis with under-treated patients included. The rationale of excluding patients under-dosed on OAC in the main analysis was to avoid biasing any association between competing mechanisms and stroke despite OAC toward the null. We considered both clinical events and radiological evidence of chronic brain infarcts as evidence of previous stroke.
Stroke classification
Stroke classification was performed by an experienced stroke specialist (JMC) blinded to OAC status (Web-Supplement). A high-risk competing mechanism was defined as per TOAST criteria. 11 A small vessel occlusion was considered as a potential competing cause to AF only if there was imaging evidence of a single and clinically relevant acute infarct <15 mm in diameter within the territory of basal or brainstem penetrating arteries in the absence of any focal pathology in the parent artery. Large artery atherosclerosis was considered relevant if there was ⩾50% stenosis or occlusion (⩽50% if evidence of acute thrombus or plaque rupture) of the extra/intra-cranial vasculature proximal to the acute infarct. As the primary aim of the study was to determine the prevalence of competing stroke aetiologies other than AF in both groups, we also captured information regarding the presence of other high-risk cardio-embolic sources. Any of the following were considered as competing high-risk cardioembolic causes: infective endocarditis, left atrial (LA) myxoma, myocardial infarction <4 weeks prior, dilated cardiomyopathy, akinetic left ventricular (LV) segment, sick sinus syndrome, patent foramen ovale with concurrent systemic embolism, LV/LA thrombus, mechanical heart valve, or moderate-severe mitral stenosis.
Cranial and vascular imaging
The size/location of acute infarcts were recorded after review of neuroimaging. Vascular imaging was acquired at the discretion of the treating physicians. The presence and degree of atherosclerotic plaque was recorded on duplex ultrasonography, CT angiography (CTA), or MRA. All neuroimaging was reviewed by a single reader (JMC) blinded to OAC status.
Echocardiography
Transthoracic echocardiograms (TTE) were performed by British Society of Echocardiography (BSE)-accredited sonographers. Ejection fraction (EF) was calculated by the Simpson’s Biplane or the Teichholtz method where available. Severe LV impairment was defined as an ejection fraction of <35%, as per the BSE. 12 Resting wall motion abnormalities (RWMA) were defined as evidence of focal or global hypokinesia, dyskinesia or akinesia and were recorded as per the 17-segment model of myocardial segmentation (Supplemental Methods). LA size was calculated using the ellipsoid area-length method of two-dimensional volumetric assessment based on the blood-tissue interface on apical four- and two-chamber views. LA volume was calculated using the area-length method and indexed to body surface area. 13
Statistical analyses
Clinical characteristics were compared using t tests, Mann-Whitney, or χ2 tests. Simple and stepwise backward multivariate logistic regression was used to identify independent predictors of stroke despite OAC. Variables with a p value <0.10 were retained in the final model. Model 1 separated out the individual components of the CHA2DS2-VASc score in the regression model. Model 2 included the CHA2DS2-VASc score rather than its individual constituents. STATA 15.0 (StataCorp, College Station, TX) software was used for statistical analyses. A p value <0.05 was considered statistically significant.
Results
Baseline characteristics
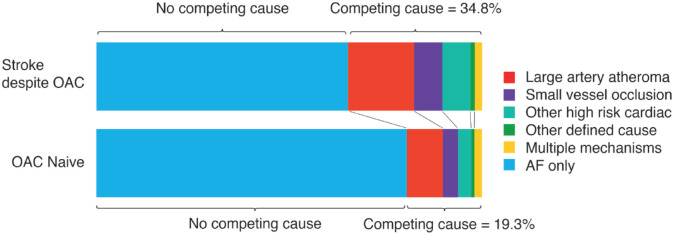
The derivation of the final cohort is shown in Figure 1. The proportion of patients in each group (OAC+ vs OAC−) who had vascular imaging was similar (p = 0.20). After exclusion of patients without vascular imaging and those non-adherent to OAC, there were 596 patients in the main analysis and 650 participants in the sensitivity analysis. Of the 596 patients, 198 (33.2%) taking OAC at the time of their stroke (n = 57 (28.8%) warfarin, n = 141 (71.2%) DOAC, Supplemental Table 2). The baseline characteristics of the final cohort are given in Table 1. Patients in the OAC+ group were slightly older, had a higher CHA2DS2-VASc score, had a higher prevalence of known AF prior to stroke, and more vascular risk factors. Patients with stroke despite anticoagulation more frequently presented with a clinical lacunar syndrome. In the primary analysis, a competing explanation for stroke other than AF was identified in 69/198 (34.8%) of patients in the OAC+ versus 77/398 (19.3%) of the OAC− group (p < 0.001). The mechanistic classification of stroke despite OAC is illustrated in Figure 2. Additional details regarding the mechanistic subtypes are provided in Supplemental Table 3.
Figure 1.
Derivation of final study cohort.
Table 1.
Baseline characteristics.
| Variable | OAC− (n = 398) | OAC+ (n = 198) | Overall | p |
|---|---|---|---|---|
| Age | 80.6 (74.1–86.4) | 82.5 (76.5–86.9) | 81.4 (74.7–86.7) | 0.05 |
| Male | 201 (50.5) | 103 (52.0) | 304 (51.0) | 0.73 |
| Hypertension | 286 (71.9) | 168 (84.9) | 454 (76.2) | <0.001 |
| History of vascular disease | 68 (17.1) | 52 (26.3) | 120 (20.1) | 0.008 |
| Diabetes mellitus | 89 (22.4) | 54 (27.3) | 143 (24.0) | 0.19 |
| CCF | 69 (17.3) | 48 (24.2) | 117 (19.6) | 0.05 |
| Hyperlipidaemia | 230 (57.8) | 135 (68.2) | 365 (61.2) | 0.01 |
| Smoking | 140 (35.2) | 76 (38.4) | 216 (36.2) | 0.44 |
| Systolic BP (n = 474) | 131 (120–143) | 135 (120–147) | 133 (120–144) | 0.10 |
| Diastolic BP (n = 474) | 72 (65–78) | 71 (66–78) | 71 (65–78) | 0.84 |
| Previous stroke/TIA or systemic embolism | 99 (24.9) | 96 (48.5) | 195 (32.7) | <0.001 |
| CHA2DS2-VASC | 4 (3–5) | 5 (4–6) | 4 (3–5) | <0.001 |
| Known AF | 180 (45.2) | 196 (99.0) | 376 (63.1) | <0.001 |
| NIHSS (n = 524) | 6 (3–12) | 6 (2.5–11) | 6 (3–12) | 0.46 |
| Lacunar syndrome | 66 (16.6) | 51 (25.8) | 117 (19.6) | 0.008 |
| MRI | 117 (29.4) | 57 (28.8) | 174 (29.2) | 0.88 |
| Any acute infarct | 346 (86.9) | 161 (81.3) | 507 (85.1) | 0.07 |
| Any proximal large artery atheroma (n = 589) | 284 (72.3) | 158 (80.6) | 442 (75.0) | 0.03 |
| Any contralateral atheroma (n = 521) | 244 (70.3) | 137 (78.7) | 381 (73.1) | 0.04 |
| Contralateral large artery atheroma ⩾50% (n = 521) | 30 (8.7) | 19 (10.9) | 49 (9.4) | 0.40 |
| Proximal large artery atheroma ⩾50% (n = 589) | 45 (11.5) | 36 (18.4) | 81 (13.8) | 0.02 |
| Other high risk cardiac mechanism | 19 (4.8) | 17 (8.6) | 36 (6.0) | 0.07 |
| Small vessel occlusion | 18 (4.5) | 17 (8.6) | 35 (5.9) | 0.05 |
| Plasma CRP (mg/l) (n = 505) | 5 (2–16) | 7 (2–22) | 6 (2–18) | 0.17 |
| Serum creatinine (mmol/l) (n = 589) | 82 (68–99) | 85 (67–104) | 82.6 (67.6–102) | 0.43 |
| LA area (n = 251) | 24.1 (20.4–27.7) | 26.4 (21.1–31.1) | 24.9 (20.4–29.9) | 0.006 |
| LA volume (ml/m2) (n = 179) | 43.2 (34.7–52.7) | 47.0 (38.2–62.0) | 45.2 (36.5–57.5) | 0.07 |
| Any RWMA (n = 252) | 30 (19.6) | 18 (18.2) | 48 (19.1) | 0.78 |
| Severe LV impairment (n = 258) | 21 (13.5) | 12 (11.8) | 33 (12.8) | 0.69 |
Categorical data is presented as number of observations (%). Skewed data is presented as median (IQR). There were 596 observations for each variable unless otherwise stated.
Figure 2.
Stroke aetiology stratified by OAC status. Patients non-adherent or under-dosed on OAC are excluded (primary analysis) (n = 596). Multiple mechanisms refer to patients with ⩾2 mechanisms in addition to AF.
AF, atrial fibrillation; OAC, oral anticoagulant.
The clinical characteristics of patients with stroke despite anticoagulation stratified by the presence or absence of competing stroke mechanisms were similar (Supplemental Table 4). However, patients with competing mechanisms had a higher background prevalence of vascular disease in other arterial beds (26/69 (37.7%) vs 26/129 (20.2%), p = 0.01) than patients without competing mechanisms.
Competing stroke mechanisms associated with stroke despite anticoagulation
The results of the logistic regression analysis are shown in Table 2/Supplemental Table 5. SVO was identified as a competing mechanism in 17/198 (8.6%) of patients in the OAC+ group versus 18/398 (4.5%) in the OAC− group. On univariate analysis, SVO was associated with stroke despite anticoagulation (crude OR: 1.98, 95% confidence interval (CI): 1.00–3.94). In the final model, after adjustment for other clinical factors associated with stroke despite OAC (model 1), SVO remained associated with stroke events on anticoagulation (adjusted OR (aOR): 2.46, 95% CI: 1.20–5.06). The presence of proximal arterial atheroma (⩾50% stenosis) or occlusive atheromatous disease was identified in 36/196 (18.4%) versus 45/393 (11.5%) of patients with and without OAC (crude OR: 1.74, 95% CI: 1.08–2.80). However, contralateral asymptomatic arterial stenosis (⩾50%) was not more prevalent in patients with stroke despite OAC (crude OR: 1.30, 95% CI: 0.71–2.37), suggesting that there is a causal role for proximal steno-occlusive disease in stroke on OAC. After adjustment, proximal arterial stenosis remained associated with stroke despite anticoagulation (aOR: 1.78, 95% CI: 1.07–2.94, model 1). When CHA2DS2-VASc was included in the final model (model 2), there was so significant change to these findings. The other factors associated with stroke on OAC after adjustment were hypertension (aOR: 1.93, 95% CI: 1.22–3.07) and previous stroke/TIA or systemic embolism (aOR: 2.90, 95% CI: 2.00–4.20). High-risk cardiac sources of embolism (other than AF) were not associated with stroke despite anticoagulation after adjustment (Supplemental Table 5).
Table 2.
Clinical associations with stroke despite anticoagulation.
| Variable | Crude OR (95% CI) | p Value | Model 1 adjusted OR (95% CI) | p Value | Model 2 adjusted OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Hypertension | 2.19 (1.40–3.42) | 0.001 | 1.93 (1.22–3.07) | 0.005 | ||
| CCF | 1.53 (1.01–2.31) | 0.05 | 1.50 (0.96–2.33) | 0.07 | ||
| CHA2DS2-VASC (per point) | 1.41 (1.26–1.57) | <0.001 | 1.41 (1.26–1.58) | <0.001 | ||
| Previous stroke/TIA or systemic embolism | 2.84 (1.98–4.07) | <0.001 | 2.90 (2.00–4.20) | <0.001 | ||
| Proximal large artery atheroma ⩾50% | 1.74 (1.08–2.80) | 0.02 | 1.78 (1.07–2.94) | 0.03 | 1.67 (1.02–2.74) | 0.04 |
| Small vessel occlusion | 1.98 (1.00–3.94) | 0.05 | 2.46 (1.20–5.06) | 0.01 | 2.41 (1.18–4.92) | 0.02 |
Odds ratio (OR) and 95% CIs were calculated from logistic regression. The adjusted models were derived from a stepwise backward logistic regression analysis.
Patients in the OAC+ group had a higher burden (158/196 (80.6%) vs 284/393 (72.3%)) of any atheromatous plaque (defined as 1%–99% stenosis/occlusion) proximal to the acute infarct than patients in the OAC− group (crude OR: 1.60, 95% CI: 1.05–2.42). However, the presence of non-contributory contralateral atherosclerotic plaque was also more prevalent in the OAC+ group and to a similar degree (137/174 (78.7%) vs 197/347 (70.3%), crude OR: 1.56, 95% CI: 1.02–2.40, p = 0.04), thereby suggesting that not all ipsilateral atheromatous plaque is causally relevant in patients with AF patients and stroke despite anticoagulation (Supplemental Table 5).
Echocardiographic factors associated with stroke despite anticoagulation
Echocardiographic data was available in 260 patients. The LA area was significantly greater in the OAC+ group (26.4 cm2, IQR: 21.1–31.1) than the OAC− group (24.1 cm2, IQR: 20.4–27.7) (p = 0.006). LA volume was also non-significantly increased compared to patients not on OAC (47.0 ml/m2, IQR: 38.2–62.0 vs 43.2 ml/m2, IQR: 34.7–52.7; p = 0.07). However, neither severe LV impairment nor the presence of RWMA differed between either groups (Table 1). Both LA area and LA volume were strongly associated with stroke despite OAC after adjustment (Supplemental Table 6). Compared with patients in the bottom quarter (Q1) of LA area measurements (⩽20.8 cm2), those in the top quarter (Q4, >30.5 cm2) were threefold more likely to have suffered a stroke whilst on OAC (aOR: 3.26, 95% CI: 1.45–7.32, p = 0.004, model 1). Similarly, patients in Q4 of LA volume measurement (>62.0 ml/m2) were significantly more likely to be in the OAC+ group (aOR: 5.12, 95% CI: 1.81–14.46, p = 0.002, model 1) than those in Q1 (⩽37.44 ml/m2).
Sensitivity analyses
The analysis was repeated including all participants on unlicensed DOAC or with sub-therapeutic INR on warfarin. Proximal arterial atheroma (⩾50% stenosis) was associated with stroke despite OAC (aOR: 1.61, 95% CI: 1.01–2.58, p = 0.05, model 1). A non-significant, but directionally-similar, trend towards association was seen for SVO (aOR 1.88, 95% CI 0.94–3.78, p = 0.08, model 1) (Supplemental Table 7).
Discussion
Strokes that occur on anticoagulation are a common phenomenon and account for approximately one-third of all AF-associated stroke. As these patients have a very high risk of recurrent stroke,5,14 new strategies to reduce this risk are clearly needed. However, before embarking on RCTs of new therapies in this population, a better understanding of the aetiology of stroke despite anticoagulation is required. This study provides new and important information in several ways. First, in patients with stroke despite anticoagulation the attributable causes differ significantly from patients with AF who have events without anticoagulation. In patients who undergo rigorous investigation, over one-third with stroke on anticoagulation have an alternative explanation for their stroke other than oral anticoagulant ‘failure’. Second, CSVD and large artery atherosclerosis are particularly important factors in stroke despite anticoagulation even after accounting for a higher burden of risk factors in this patient group. Third, increased LA size is strongly associated with stroke on anticoagulation, even after adjusting for CHA2DS2-VASC score.
Compared with our findings, previous studies reported a similar high prevalence of competing mechanisms and insufficient anticoagulation in these patients.4,6,10,14 However, these studies have limitations. Imaging was not adjudicated by a single reader blinded to OAC status. Information was not given regarding CSVD and other high-risk cardiac mechanisms in two studies.4,14 Just one study reported the prevalence of competing causes relative to OAC-naïve comparator. 4 This makes it challenging to establish to what extent stroke despite anticoagulation represents a distinct pathological process in AF when compared with stroke events that occur off anticoagulation.
Our results are important because they illustrate that stroke despite anticoagulation in AF is a heterogeneous condition and clinicians need to carefully evaluate the patient for drug non-adherence, under-dosing and competing stroke mechanisms. We have illustrated that a comprehensive evaluation for other stroke aetiologies has a high diagnostic yield and can facilitate an individualised approach to secondary prevention strategies. On the other hand, we found that despite extensive investigation, two-thirds of patients with stroke on anticoagulation do not have an alternative explanation for their stroke, and therefore such cases may represent ‘true’ treatment failure. It is possible that these patients may be a high-risk subgroup of patients with AF, for whom treatment with proven anticoagulation therapies may be insufficient to prevent stroke.
In the present work, we demonstrated strong associations between LA size and stroke despite anticoagulation even after adjusting for CHA2DS2-VASC. It is possible therefore that some patients who have stroke on anticoagulation are at a more advanced stage of atrial cardiopathy and carry a greater risk of thrombo-embolism. Previous work has demonstrated that increased LA size was strongly associated with recurrent stroke, even in patients with AF who were anticoagulated. The addition of LA diameter to CHA2DS2-VASC also improved the identification of recurrent stroke. 15 The CHA2DS2-VASC score performs best in determining which patient is at low risk of stroke but is less accurate in identifying high-risk groups such as patients with stroke. 16 Identifying patients who are at greatest risk of recurrence is important for patient selection for RCTs of new therapeutic strategies in AF-associated stroke. Therefore, the value of echocardiographic biomarkers of atrial cardiopathy for risk stratification purposes in such patients should be the focus of future work.
There is no randomised evidence to support a specific therapeutic strategy in patients with stroke despite anticoagulation. The addition of anti-platelet therapy to OAC may have a role in selected cases, but observational data suggests that this approach is associated with an excessive bleeding risk and no additional benefit for stroke prevention. 6 However, these findings may be confounded by indication and the addition of short-term anti-platelets may still be beneficial in select subgroups of patients with stroke despite anticoagulation, particularly in suspected atherosclerotic mechanisms. Future studies, using larger sample sizes, should investigate the effect of adding anti-platelet therapy according to the presence/absence of competing atherosclerotic mechanisms. Percutaneous LA appendage occlusion (LAAO) in patients with AF has been compared with warfarin in two trials, which demonstrated similar efficacy to OAC for stroke prevention.17,18 The majority of patients in these trials did not have a history of stroke and therefore it is difficult to extrapolate these findings to patients with stroke on anticoagulation. The absence of proven secondary prevention strategies in this high-risk group of patients with AF demonstrates the clear need for RCTs in this population.
Our study has several strengths. It is the first study to analyse the contribution of competing stroke mechanisms in stroke despite anticoagulation in a systematic and comprehensive manner. Competing mechanisms were determined using a standardised and validated tool 11 by a single stroke physician blinded to OAC status. Blinded vascular and brain imaging was also reviewed by a single reader. Patients underwent comprehensive investigation, thereby minimising the risk of under-detection of alternative mechanisms. Finally, the work provides important information regarding the aetiology of stroke in patients on OAC, which will inform the design of future RCTs in this population. We acknowledge some limitations. Some patients were excluded as they did not undergo vascular imaging and the rates of competing mechanisms may be different in these patients compared to those included in the study. However, we feel this is unlikely to alter our findings significantly as a similar proportion of patients with and without OAC had vascular imaging available. We used a strict definition of non-adherence and did not have DOAC plasma levels available, which might have contributed to an underestimation of therapeutic non-adherence. As expected, more patients in the OAC+ group had known AF prior to stroke than patients in the OAC− group. It is possible that some of the differences between the groups may reflect different stages in the disease process or that patients in the OAC+ group are a higher risk group by virtue of the fact they have a greater burden of risk factors. However, we controlled for CHA2DS2-VASC in the analysis, and this should reduce the risk of residual confounding. As our study was performed in a single centre in the UK, its findings may not be generalisable to other populations. We did not have comprehensive data on the prevalence of malignancy in both groups, but this may also be a relevant cause for stroke despite anticoagulation. However, previous work suggests malignancy plays a minor role in these cases.
Conclusion
Patients with AF who experience stroke on OAC represent a distinct high-risk cohort, with an increased prevalence of competing mechanisms than the background prevalence in patients not on OAC at the time of stroke. Rigorous interrogation for competing mechanisms and therapeutic non-adherence has a high diagnostic yield. These data should be used to guide patient selection for future RCTs in this population.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231168367 for The contribution of competing mechanisms in stroke despite anticoagulation in patients with atrial fibrillation by Rahul Herlekar, Akangsha Sur Roy, Saur Hajiev, Isuru Induruwa, Smriti Agarwal, Nicholas R Evans, Kayvan Khadjooi, Hugh Markus, Eoin O’Brien, Elizabeth Warburton, George Zachariah and John J McCabe in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231168367 for The contribution of competing mechanisms in stroke despite anticoagulation in patients with atrial fibrillation by Rahul Herlekar, Akangsha Sur Roy, Saur Hajiev, Isuru Induruwa, Smriti Agarwal, Nicholas R Evans, Kayvan Khadjooi, Hugh Markus, Eoin O’Brien, Elizabeth Warburton, George Zachariah and John J McCabe in European Stroke Journal
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: II is funded by a NIHR Academic Clinical Lectureship (RG85316).
Informed consent and ethical approval: This study was registered with Cambridge University Hospitals’ Quality Surveillance Team. Formal confirmation was received that ethical approval from the Institutional Review Board was not required and the need to obtain written informed patient consent was waived.
Guarantor: John J McCabe.
Contributorship: All authors reviewed and edited the manuscript and approved the final version of the manuscript. JM conceived the study hypothesis, interpreted the imaging, drafted the manuscript and performed the statistical analysis. RH, AS and SH were involved in data acquisition and data management. II and KK contributed to the study hypothesis.
ORCID iDs: Nicholas R Evans  https://orcid.org/0000-0002-7640-4701
https://orcid.org/0000-0002-7640-4701
John J McCabe  https://orcid.org/0000-0003-2029-1303
https://orcid.org/0000-0003-2029-1303
Data availability: Data are available on reasonable request.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Yiin GS, Howard DP, Paul NL, et al. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: a population-based study. Circulation 2014; 130: 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 3.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 4.Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: the IAC study. J Neurol Neurosurg Psychiatry 2021; 92: 1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol 2020; 87(5): 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2022; 93: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. New Engl J Med 2013; 369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 8.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 9.Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. Eur Stroke J 2019; 4: 198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purrucker JC, Holscher K, Kollmer J, et al. Etiology of ischemic strokes of patients with atrial fibrillation and therapy with anticoagulants. J Clin Med 2020; 9: 20200911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 12.Harkness A, Ring L, Augustine DX, et al. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: a guideline from the British Society of Echocardiography. Echo Res Pract 2020; 7: G1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 14.Paciaroni M, Caso V, Agnelli G, et al. Recurrent Ischemic stroke and bleeding in patients with atrial fibrillation who suffered an acute stroke while on treatment with nonvitamin K antagonist oral anticoagulants: the RENO-EXTEND Study. Stroke 2022; 53: 2620–2627. [DOI] [PubMed] [Google Scholar]
- 15.Ogata T, Matsuo R, Kiyuna F, et al. Left atrial size and long-term risk of recurrent stroke after acute ischemic stroke in patients with nonvalvular atrial fibrillation. J Am Heart Assoc 2017; 6: e006402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lip GY. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J 2013; 34: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 17.Holmes DR, Jr., Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014; 64: 1–12. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014; 312: 1988–1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231168367 for The contribution of competing mechanisms in stroke despite anticoagulation in patients with atrial fibrillation by Rahul Herlekar, Akangsha Sur Roy, Saur Hajiev, Isuru Induruwa, Smriti Agarwal, Nicholas R Evans, Kayvan Khadjooi, Hugh Markus, Eoin O’Brien, Elizabeth Warburton, George Zachariah and John J McCabe in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231168367 for The contribution of competing mechanisms in stroke despite anticoagulation in patients with atrial fibrillation by Rahul Herlekar, Akangsha Sur Roy, Saur Hajiev, Isuru Induruwa, Smriti Agarwal, Nicholas R Evans, Kayvan Khadjooi, Hugh Markus, Eoin O’Brien, Elizabeth Warburton, George Zachariah and John J McCabe in European Stroke Journal