Abstract
Background/Aims
Extended wireless pH monitoring (WPM) is used to investigate gastroesophageal reflux disease (GERD) as subsequent or alternative investigation to 24-hour catheter-based studies. However, false negative catheter studies may occur in patients with intermittent reflux or due to catheter-induced discomfort or altered behavior. We aim to investigate the diagnostic yield of WPM after a negative 24-hour multichannel intraluminal impedance pH (MII-pH) monitoring study and to determine predictors of GERD on WPM given a negative MII-pH.
Methods
Consecutive adult patients (> 18 years) who underwent WPM for further investigation of suspected GERD following a negative 24-hour MII-pH and upper endoscopy between January 2010 and December 2019 were retrospectively included. Clinical data, endoscopy, MII-pH, and WPM results were retrieved. Fisher’s exact test, Wilcoxon rank sum test, or Student’s t test were used to compare data. Logistic regression analysis was used to investigate predictors of positive WMP.
Results
One hundred and eighty-one consecutive patients underwent WPM following a negative MII-pH study. On average and worst day analysis, 33.7% (61/181) and 34.2% (62/181) of the patients negative for GERD on MII-pH were given a diagnosis of GERD following WPM, respectively. On a stepwise multiple logistic regression analysis, the basal respiratory minimum pressure of the lower esophageal sphincter was a significant predictor of GERD with OR = 0.95 (0.90-1.00, P = 0.041).
Conclusions
WPM increases GERD diagnostic yield in patients with a negative MII-pH selected for further testing based on clinical suspicion. Further studies are needed to assess the role of WPM as a first line investigation in patients with GERD symptoms.
Keywords: Diagnosis, Esophageal pH Monitoring, Gastroesophageal reflux
Introduction
Wireless pH monitoring (WPM) is a useful method for the prolonged analysis of esophageal pH,1 particularly for subsequent investigation or as an alternative to catheter based studies.2 A peculiar advantage of WPM is the accommodation of day to day variation and patients’ normal activities of daily living.3 It has been shown that false negative catheter studies may occur if reflux is intermittent so that a 24-hour window of assessment is insufficient.4 In this regard, it has been estimated that a significant proportion of patients would have a positive finding of GERD if assessed with extended pH studies rather than a 24-hour pH assessment.3,5
Ambulatory pH impedance monitoring has been demonstrated to increase the diagnostic yield for gastroesophageal reflux disease (GERD) when compared with standard pH catheter studies due to non-acid reflux detection.6-8 However, the yield of WPM over standard catheter-based pH impedance has not been yet determined. Calculating the yield has been difficult to undertake in the past due to the lack of validated threshold for a diagnosis of GERD based on the acid exposure time (AET) at WPM. Only recently, these thresholds were validated and are now available.9
When deciding on the need for further pH testing given a negative 24-hour study, a clinician may take into account a number of factors including the nature and frequency of the symptoms, or other tests such as the endoscopy or manometry which may provide circumstantial evidence of GERD.1 There is no guidance however, as to who should undergo prolonged acid monitoring in the presence of a negative pH impedance study. Furthermore, novel metrics of GERD, such as the post-reflux swallow induced peristaltic wave (PSPW) index6,10 and the mean nocturnal baseline impedance (MNBI)6,11-13 have not been assessed in this cohort.
The primary aim of the current study is to establish the diagnostic yield of WPM after a negative 24-hour multichannel intraluminal impedance pH (MII-pH) monitoring study, by average and worst day analysis. The secondary aim is to determine potential predictors of GERD on WPM given a negative MII-pH.
Materials and Methods
Patient Selection
Consecutive adult patients (> 18 years) who underwent WPM for further investigation of suspected GERD following a negative 24-hour MII-pH between January 2010 and December 2019 were retrospectively included. A negative pH impedance test was defined as having less than 40 reflux episodes and an AET for ≤ 4.2% of a 24-hour period.1 Patients with a normal AET but with > 40 reflux episodes were also excluded on the basis that they have non-acid reflux. A WPM study was defined as positive according to the validated threshold of ≥ 4.3% on average day analysis or ≥ 7.1% on any 24-hour period for the worst day analysis.9 All included studies were performed off acid suppressing medication for the previous 2 weeks. Symptoms were categorized as follows: typical (heartburn, regurgitation, and chest pain), atypical (belching, cough, throat symptoms, abdominal pain, vomiting, and nausea), and mixed (presence of both typical and atypical symptoms).
Patients were excluded for the following reasons: previous esophageal surgery or intervention such as endoscopic mucosal resection or radiofrequency ablation; less than 24 hours of recording on MII-pH; less than 48 hours of recording on WPM study. This study protocol was conducted in full compliance with the Declaration of Helsinki and was approved by the local Ethics Committee (IRAS 18/NW/0120).
High-resolution Manometry Protocol
High-resolution manometry (HRM) was performed following local analgesia of the nares. The catheter was introduced trans-nasally and patients were instructed to drink water through a straw whilst the HRM catheter was advanced to the stomach. The HRM catheter depth was adjusted to ensure manometric visual of the upper esophageal sphincter, the gastroesophageal junction, the lower esophageal sphincter (LES), and gastric pressures. Ten single swallows of 5 mL were performed with each being 20 seconds apart. HRM tracings were assessed in accordance to Chicago classification version 3.0 using Manoview software version 3.0 (Sierra Scientific Instruments, Los Angeles, CA, USA).14
pH Impedance Protocol
After HRM, patients underwent a 24-hour MII-pH reflux monitoring after a 6-hour fasting period using Sandhill Scientific multichannel impedance pH catheters (ZAN-BG-44) while off anti-reflux medications for 2 weeks prior to the procedure. The dual pH sensors of the catheter were positioned 5 cm below and above the manometric LES with impedance sensors positioned above the LES by 3, 5, 9, 15, and 19 cm. Each reflux event was categorized as an acid non-acid reflux event based on the distal esophageal pH capture (pH < 4 = acid reflux event; pH 4-8 = non-acid). PSPW was defined as an antegrade impedance drop by 50% within 30 seconds of the reflux event. The PSPW index was manually calculated and defined as the number of reflux events followed within 30 seconds by a swallow-induced peristaltic wave as a proportion of the total number of reflux events (normal value > 61%).10 The MNBI was assessed using a recent protocol of assessment of the overall average baseline impedance readings throughout the complete nocturnal period (normal value > 2292 ohms).12 The data was captured by a ZepHrTM recording device (Sandhill Scietific, Whitney, UK) and data was analyzed using the BioVIEW Analysis software (5.7.1.0).
Wireless pH Monitoring Protocol
The 96-hour WPM procedure was performed after a 6-hour fasting period while off anti-reflux medications for 2 weeks prior to the procedure. Under conscious sedation, the calibrated WPM capsule was inserted endoscopically to the wall of the esophagus 6 cm proximal to the Z line. The following parameters were obtained from the analysis and compared against normal reference values1: number of reflux episodes per day; total and postprandial percent of time spent in reflux; percent of time spent in reflux in the upright or supine position; the DeMeester score. All patients completed a concurrent diary of symptoms and meal/drink times and registered symptoms on the recording device as it occurred.
Endoscopy Assessment
The degree of esophagitis recorded by the endoscopist at the time of WPM insertion was recorded for each patient. All endoscopists inserting WPM catheters used the Los Angeles (LA) esophagitis grading system. LA grade A to D was included as classifying the patient as having esophagitis.15
Statistical Methods
WPM, HRM, and pH impedance results were ordered according to the patient identifier and date of the study. To ensure that there was no duplicated data for patients who had undergone more than 2 of any test, only tests chronologically closest to the test to be merged were chosen. To ensure relevance, tests were merged only if there was less than 1 year’s difference between the two. Variables were selected on the basis of lack of multicollinearity and an adequate number of data points to remove skew from missing data. All quantitative data were presented as mean ± SD. Fisher’s exact test or Wilcoxon rank sum test was used to compare non-parametric numerical data. Student’s t test was used to compare parametric numerical data. Categorical data was compared using a chi-squared test of independence. A one-way repeated measures ANOVA was used to compare day to day variation between patients grouped according to the day of their highest AET. Analysis was performed on symptoms grouped as typical, atypical, and mixed. Results for the univariate analysis were performed using both average and worst day analysis. Statistical comparison was carried out on all recorded parameters and those with P < 0.05 were selected for evaluation in a multivariate model with a stepwise method (forward selection/backward elimination). A P < 0.05 was taken as the threshold of significance for the multivariate model and the strength of association was expressed as odds ratio (OR) with 95% confidence interval.
Results
Baseline Characteristics of Included Patients
During the study period, a total of 181 consecutive patients underwent WPM following a negative MII-pH study and were included in the analysis. Among included patients, 126 were females, 55 were males, and the mean age was 47.5 ± 15 years. Overall, 95 patients reported both typical and atypical symptoms, 49 patients reported atypical symptoms only, 21 typical only, and 16 patients recorded symptoms without providing a specific label. On endoscopy, 7 patients had LA grade A, 3 grade B, 1 grade C, and 1 grade D esophagitis (Table 1). The diagnoses at pH impedance included functional heartburn (n = 50, 27.6%) and hypersensitive oesophagus (n = 32, 17.7%). At HRM the diagnoses included distal esophageal spasm (n = 11, 6.1%), esophagogastric outlet obstruction (n = 1, 0.6%), frequent failed peristalsis (n = 4, 2.2%), ineffective esophageal motility (n = 33, 18.2%), and normal motility (n = 101, 55.6%).
Table 1.
Patient-registered Symptoms and Endoscopy Findings at the Time of Performance of Wireless pH Monitoring
| Clinical and endoscopy findings | Average day analysis | Worst day analysis | |||||
|---|---|---|---|---|---|---|---|
| Negative (n = 120) |
Positive (n = 61) |
P-value | Negative (n = 119) |
Positive (n = 62) |
P-value | ||
| Atypical symptoms | 32 (27) | 17 (28) | 0.490 | 34 (29) | 15 (24) | 0.641 | |
| Mixed symptoms | 63 (52) | 32 (52) | 61 (51) | 34 (55) | |||
| Typical symptoms | 12 (10) | 9 (15) | 12 (10) | 9 (15) | |||
| Unlabelled symptoms | 13 (11) | 3 (5) | 12 (10) | 4 (6) | |||
| Presence of any endoscopic finding | 2 (2) | 10 (16) | < 0.001 | 2 (2) | 10 (16) | < 0.001 | |
| Esophagitis LA grade A | 1/2 (50) | 6/10 (60) | 0.793 | 1/2 (50) | 6/10 (60) | 0.790 | |
| Esophagitis LA grade B | 1/2 (50) | 2/10 (20) | 1/2 (50) | 2/10 (20) | |||
| Esophagitis LA grade C | 0/2 (0) | 1/10 (10) | 0/2 (0) | 1/10 (10) | |||
| Esophagitis LA grade D | 0/2 (0) | 1/10 (10) | 0/2 (0) | 1/10 (10) | |||
LA, Los Angeles classification.
Data are presented as n (%) or n/N (%).
Wireless pH Monitoring Results
Using average day analysis 61 studies were positive and 120 were negative for GERD. Overall, 33.7% (61/181) of the patients initially diagnosed as not having GERD were given a diagnosis of GERD following WPM.
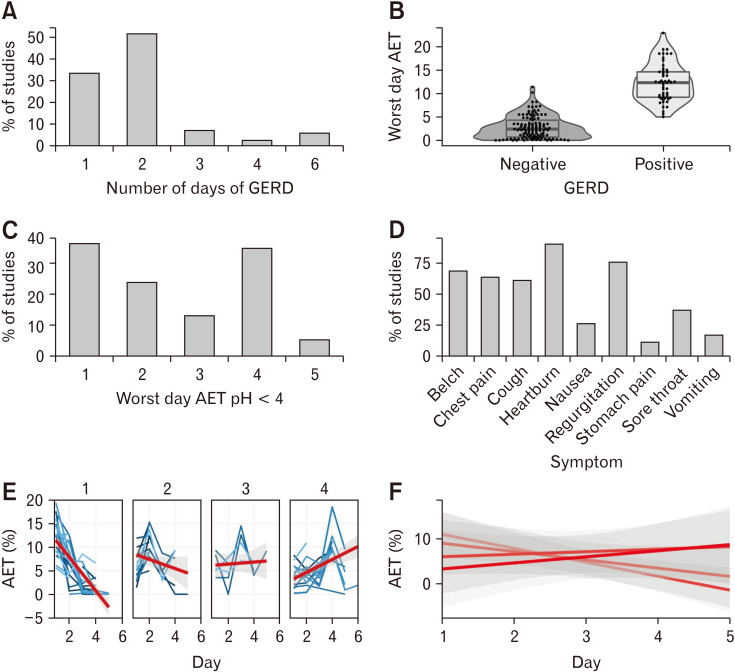
Using a worst day analysis, of the patients who were negative for GERD at MII-pH, 62 studies were positive at WPM (34.2%). Overall, 92.0% of the patients diagnosed with GERD on worst day analysis were also positive on average day analysis. Twenty (33.3%) of patients classified as GERD on WPM were positive for only 1 day. The number of studies that were negative for GERD in the first 24 hours and 48 hours of the WPM study was 19 (31.1 %) and 11 (18.0%), respectively. The worst day symptom association probability (SAP) was positive in 66.1%. The majority of the worst SAP positive days (38.3%) occurred in the first 24 hours for both positive and negative WPM studies. For all WPM studies which demonstrated a positive result for GERD, a one way ANOVA demonstrated no significant variation between each day of the measured acid exposure time (P = 0.068).
Given that the worst day was on the first day in 23 patients (38.3%) we investigated whether the increased yield of GERD in this population was attributable to a false positive GERD measurement on the first day by comparing the distribution of mean AET between each day in each of the worst day groups. A one-way repeated measure ANOVA demonstrated no significant difference between any of the groups indicating that GERD on the first day of a WPM study is unlikely to be a false positive (F [1,3] = 0.530, P = 0.520). Figures 1 and 2 report WPM characteristics of patients with negative MII-pH.
Figure 1.
The characteristics of Gastroesophageal reflux disease (GERD) positive wireless pH monitoring (WPM) recordings for patients with negative impedance studies. (A) The number of days with GERD for GERD positive WPM studies. (B) The percentage acid exposure time (AET) for WPM-GERD positive and WPM-GERD negative studies. (C) Histogram of the worst day for WPM-GERD positive patients. (D) The symptoms registered by patients during WPM. (E) AET for patients grouped by the day on which the AET was worst. (F) Summarized regression lines across all worst day groups.
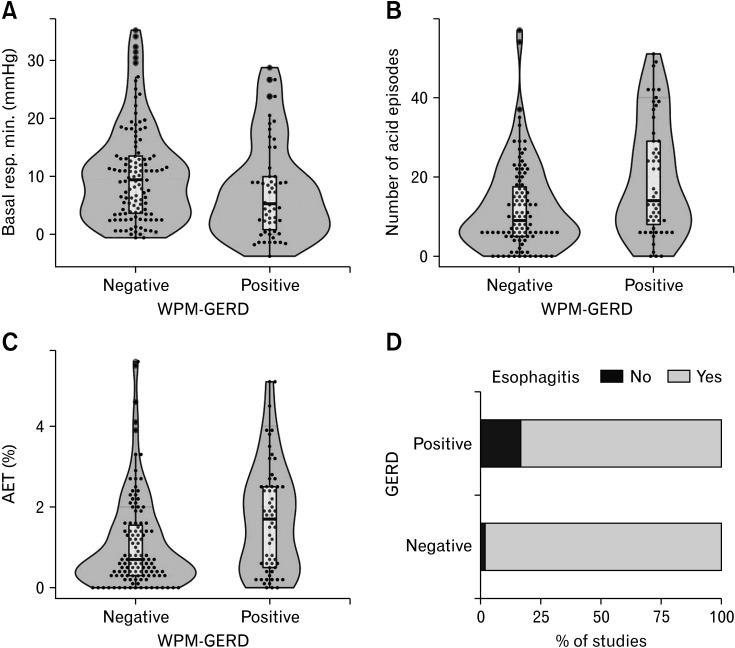
Figure 2.
Acid exposure time (AET) at pH impedance for wireless pH monitoring (WPM)-gastroesophageal reflux disease (GERD) positive versus WPM-GERD negative studies. (A) Basal respiratory minimum (resp. min.) (B) Number of acid episodes. (C) Acid exposure time. (D) Proportion of patients with esophagitis.
Predictors of Gastroesophageal Reflux Disease on Wireless pH Monitoring
To ascertain whether a variable at HRM could predict WPM-GERD-positive in pH impedance negative patients, the HRM for WPM GERD-positive and WPM GERD-negative patients were compared.
On average day analysis there was a significant difference in the basal respiratory minimum LES pressure between the 2 groups (WPM GERD-positive = 8 mmHg vs WPM GERD-negative = 10 mmHg; P = 0.010), but this was not seen in the worst day analysis (P = 0.051). No significant difference was noted for the other evaluated manometric findings on average day analysis (Table 2).
Table 2.
High-resolution Manometry Findings for Patients With a Positive Versus Negative Finding of Gastroesophageal Reflux Disease at Wireless pH Monitoring
| HRM parameters | Average day analysis | Worst day analysis | |||||
|---|---|---|---|---|---|---|---|
| Negative (n = 120) |
Positive (n = 61) |
P-value | Negative (n = 119) |
Positive (n = 62) |
P-value | ||
| LES midpoint from nares (cm) | 43.60 ± 3.34 | 44.10 ± 3.42 | 0.472 | 43.40 ± 3.31 | 44.40 ± 3.52 | 0.181 | |
| Basal LES pressure minimum (mmHg) | 10 (8) | 8 (8) | 0.010 | 10 (8) | 8 (9) | 0.054 | |
| Residual LES pressure mean (mmHg) | 5.70 ± 5.14 | 6.00 ± 6.82 | 0.725 | 5.70 ± 4.82 | 6.10 ± 7.23 | 0.510 | |
| DCI (mean mmHg/cm/sec) | 855 ± 741 | 947 ± 1421 | 0.401 | 842 ± 742 | 973 ± 1408 | 0.820 | |
| Contractile front velocity (m/sec) | 4.23 ± 5.27 | 4.12 ± 4.51 | 0.797 | 4.27 ± 5.29 | 4.04 ± 4.47 | 0.710 | |
| Distal latency (sec) | 7.06 ± 1.81 | 6.60 ± 1.33 | 0.144 | 7.04 ± 1.81 | 6.63 ± 1.32 | 0.222 | |
| % Failed peristalsis | 34 ± 32 | 40 ± 34 | 0.290 | 35 ± 32 | 38 ± 34 | 0.634 | |
| % Panoesophageal pressurization | 2.60 ± 9.52 | 1.70 ± 5.11 | 0.954 | 2.70 ± 9.51 | 1.50 ± 5.04 | 0.591 | |
| % Large breaks | 7 ± 14 | 5 ± 10 | 0.838 | 7 ± 14 | 5 ± 10 | 0.552 | |
| % Small breaks | 13 ± 15 | 15 ± 19 | 0.853 | 14 ± 16 | 14 ± 19 | 0.432 | |
HRM, high-resolution manometry; LES, lower esophageal sphincter; DCI, distal contractile integral.
Data are presented as mean ± SD or n (%).
Statistical tests performed: Wilcoxon rank-sum test; chi-square test of independence; Fisher's exact test. P < 0.05 was statistically significant.
With regards to MII-pH predictors of GERD on WPM, on univariate analysis WPM GERD-positive patients had a longer total AET at MII-pH when compared with WPM GERD-negative patients (P = 0.002). This was true for both average and worst day analysis. In addition, the upright AET and the number of refluxes were significantly increased in WPM GERD-positive patients when compared with the WPM GERD-negative group (P = 0.001 for both). There was no difference in the longest time spent in reflux, supine AET, MNBI, and PSPW index between WPM GERD-positive and negative patients (Table 3).
Table 3.
pH Impedance Findings for Patients With a Positive Versus Negative Finding of Gastroesophageal Reflux Disease at Wireless pH Monitoring
| Clinical and endoscopy findings | Average day analysis | Worst day analysis | |||||
|---|---|---|---|---|---|---|---|
| Negative (n = 120) |
Positive (n = 61) |
P-value | Negative (n = 119) |
Positive (n = 62) |
P-value | ||
| Number of non acid episodes | 18 ± 14 | 18 ± 14 | 0.921 | 18 ± 14 | 19 ± 15 | 0.594 | |
| Number of acid episodes | 12 ± 11 | 20 ± 14 | 0.001 | 12 ± 11 | 19 ± 15 | 0.010 | |
| AET | 1.07 ± 1.14 | 1.70 ± 1.35 | 0.002 | 1.04 ± 1.08 | 1.75 ± 1.39 | < 0.001 | |
| Longest acid episode | 8 ± 11 | 6 ± 8 | 0.810 | 8 ± 11 | 7 ± 9 | 0.711 | |
| MNBI | 2735 ± 1125 | 2379 ± 1071 | 0.061 | 2744 ± 1115 | 2367 ± 1085 | 0.047 | |
| PSPW index | 0.40 ± 0.26 | 0.41 ± 0.28 | 0.920 | 0.41 ± 0.26 | 0.39 ± 0.28 | 0.521 | |
AET, acid exposure time; MNBI, mean nocturnal baseline impedance; PSPW, post reflux swallowed-induced peristaltic wave.
Data are presented as mean ± SD.
Statistical tests performed: Wilcoxon rank-sum test; chi-square test of independence; Fisher's exact test. P < 0.05 was statistically significant.
Univariate analysis highlighted basal respiratory minimum LES pressure, number of acid episodes, and total AET for further investigation with a multivariate analysis. On a stepwise multiple logistic regression analysis, only the basal respiratory minimum LES pressure was a significant predictor of GERD with OR = 0.95 (0.90-1.00, P = 0.040) (Table 4).
Table 4.
Predictors Across pH Impedance, High-resolution Manometry and Endoscopy for Patients With a Positive Diagnosis of Gastroesophageal Reflux Disease on Wireless pH Monitoring
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Basal respiratory LES pressure minimum (mmHg) | 0.95 | 0.90, 1.00 | 0.040 |
| Number of Acid Episodes | 1.03 | 0.99, 1.07 | 0.223 |
| AET | 1.27 | 0.88, 1.85 | 0.222 |
| Esophagitis | 3.56 | 0.75, 25.6 | 0.144 |
LES, lower esophageal sphincter; AET, acid exposure time.
P < 0.05 was statistically significant.
With regards to symptoms and endoscopy, the SAP did not differ for any of the individual or grouped symptoms between the 2 groups, while esophagitis was more common in the WPM GERD-Positive group compared to the WPM GERD-negative group (16.4% vs 1.7%; P < 0.001) on both average and worst day analysis (Table 1).
Discussion
The current study represents the largest cohort of pH impedance GERD-negative patients who subsequently underwent WPM testing. This cohort is unique for two reasons: firstly, this is the first study to use validated WPM thresholds for GERD.9 Secondly, the increased positive diagnosis yield made with wireless pH studies was determined for the first time as compared with MII-pH as opposed to standard pH studies.2
We found that a significant number of WPM studies were positive after a negative impedance study (33.7% and 34.2% on average and worst day analysis, respectively). In these patients, negative findings on MII-pH were possibly related to discomfort and altered behavior which have been shown to reduce reflux events.16 These findings are consistent with those of a previous work by Sweis and colleagues2 in which WMP increased sensitivity and diagnostic yield for GERD in patients with continuing esophageal symptoms despite negative 24-hour pH reflux monitoring without impedance. The reasons for the increased yield of WPM compared to MII-pH may be multifactorial including increased patient tolerability,17 better detection of intermittent reflux episodes, and accommodation of day to day variability.18 Accordingly, in this study, of the WPM GERD positive, the number of patients who were GERD negative in the first 24 hours was 31.1%. A further 18.0% were still negative after 48 hours, indicating that even a 48-hour pH study may be insufficient. In addition, 33.3% of patients positive for GERD on WPM were only positive on 1 day only. Because the first day of WPM was the worst day recorded for most patients, we compared the distribution of mean AET between each day in each of the worst day groups and found that a diagnosis of GERD on the first day of WPM is unlikely to be a false positive. Importantly, we also found a high degree of concordance between average and worst day analysis for positive diagnoses on WPM.
In this study, we also sought to identify possible predictors of an increased diagnostic yield on WPM in patients with a negative MII-pH. Although we examined clinical, endoscopic, HRM, and MII-pH variables, only the basal respiratory minimum LES pressure on HRM was a significant predictor of GERD (OR = 0.95). This finding is consistent with previous reports in which a reduced basal respiratory minimum pressure of the LES was associated to GERD.19,20 However, although impaired esophageal motility abnormalities have been shown to increase in parallel with GERD severity,20 our results did not show motility differences between patients positive and negative for GERD on WPM, possibly due to the low number of patients with severe esophagitis.20
It is noteworthy that neither the PSPW index nor the MNBI was able to segregate patients with and without GERD on WPM. However, although baseline impedance values inversely correlate with AET on impedance studies21 and PSPW index directly correlates with baseline impedance11 in our cohort, all included patients had a normal AET on MII-pH, which is consistent with normal MNBI and PSPW index values.6
Although typical GERD symptoms such as regurgitation and heartburn are well documented to be better predictors of esophagitis than atypical symptoms,22 in this study, the type of symptoms failed to segregate patients with and without GERD on WPM. This finding may be related to symptoms being user-defined as they were recorded by the patient and to a proportion of symptoms being unlabeled by patients.
The study has limitations that should be considered. First, this was an analysis of retrospective data. Second, patients selected for WPM were those who were judged to be suspicious for a false negative diagnosis on MII-pH. Although it may be argued that patients more likely to have GERD might have been selected, this cohort of patients is representative of those commonly encountered in real clinical practice scenarios, where the decision to undertake further GERD investigations following a negative MII-pH is complex and takes into account several factors, including physicians’ suspicion of a positive diagnostic yield of WPM. Third, data regarding response to treatment were not available for this study, and the impact of the increased diagnostic yield on clinical outcomes could not be assessed.
In conclusion, we showed that WPM increases GERD diagnostic yield in patients with a negative MII-pH selected for further testing based on clinical suspicion, and that basal respiratory minimum LES pressure predicts a positive WPM in this setting. It is difficult to determine which patients may benefit from prolonged esophageal pH monitoring in the context of a negative 24-hour pH impedance test; however, the increased diagnostic yield suggests that further investigation of WPM as a first line investigation is needed. This would ideally be done by comparing the diagnostic yield of both investigations in the same patient which the current study was not designed to do. Further studies are also needed to identify predictors of a positive WPM study.
Funding Statement
Financial support: None.
Footnotes
Conflicts of interest: None.
Author contributions: Sebastian S Zeki: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, and writing the original draft; Ismail Miah: investigation and methodology; Anna Wolak: investigation and methodology; Pierfrancesco Visaggi: data curation, formal analysis, methodology, visualization, and writing review and editing; Minerva deSilva: investigation and methodology; Jason M Dunn: conceptualization; Andrew Davies: conceptualization writing (review and editing); James Gossage and Abrie Botha: writing (review and editing); Guiping Sui: formal analysis and methodology; Jafar Jafari: methodology, project administration, and writing (review and editing); and Terry Wong: conceptualization, formal analysis, and writing (review and editing). All authors approved the final draft.
References
- 1.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweis R, Fox M, Anggiansah A, Wong T. Prolonged, wireless pH-studies have a high diagnostic yield in patients with reflux symptoms and negative 24-h catheter-based pH-studies. Neurogastroenterol Motil. 2011;23:419–426. doi: 10.1111/j.1365-2982.2010.01663.x. [DOI] [PubMed] [Google Scholar]
- 3.Sweis R, Fox M, Anggiansah R, et al. Patient acceptance and clinical impact of Bravo monitoring in patients with previous failed catheter-based studies. Aliment Pharmacol Ther. 2009;29:669–676. doi: 10.1111/j.1365-2036.2008.03923.x. [DOI] [PubMed] [Google Scholar]
- 4.Tseng D, Rizvi AZ, Fennerty MB, et al. Forty-eight-hour pH monitoring increases sensitivity in detecting abnormal esophageal acid exposure. J Gastrointest Surg. 2005;9:1043–1051. discussion 1051–1042. doi: 10.1016/j.gassur.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Scarpulla G, Camilleri S, Galante P, Manganaro M, Fox M. The impact of prolonged pH measurements on the diagnosis of gastroesophageal reflux disease: 4-day wireless pH studies. Am J Gastroenterol. 2007;102:2642–2647. doi: 10.1111/j.1572-0241.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 6.Visaggi P, Mariani L, Svizzero FB, et al. Clinical use of mean nocturnal baseline impedance and post-reflux swallow-induced peristaltic wave index for the diagnosis of gastro-esophageal reflux disease. Esophagus. 2022;19:525–534. doi: 10.1007/s10388-022-00933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredenoord AJ, Weusten BL, Timmer R, Conchillo JM, Smout AJ. Addition of esophageal impedance monitoring to pH monitoring increases the yield of symptom association analysis in patients off PPI therapy. Am J Gastroenterol. 2006;101:453–459. doi: 10.1111/j.1572-0241.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 8.Karamanolis G, Kotsalidis G, Triantafyllou K, et al. Yield of combined impedance-pH monitoring for refractory reflux symptoms in clinical practice. J Neurogastroenterol Motil. 2011;17:158–163. doi: 10.5056/jnm.2011.17.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusu RI, Fox MR, Tucker E, et al. Validation of the Lyon classification for GORD diagnosis: acid exposure time assessed by prolonged wireless pH monitoring in healthy controls and patients with erosive oesophagitis. Gut. 2021;70:2230–2237. doi: 10.1136/gutjnl-2020-323798. [DOI] [PubMed] [Google Scholar]
- 10.Frazzoni M, Bertani H, Manta R, et al. Impairment of chemical clearance is relevant to the pathogenesis of refractory reflux oesophagitis. Dig Liver Dis. 2014;46:596–602. doi: 10.1016/j.dld.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Martinucci I, de Bortoli N, Savarino E, et al. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. 2014;26:546–555. doi: 10.1111/nmo.12299. [DOI] [PubMed] [Google Scholar]
- 12.Hoshikawa Y, Sawada A, Sonmez S, et al. Measurement of Esophageal nocturnal baseline impedance: a simplified method. J Neurogastroenterol Motil. 2020;26:241–247. doi: 10.5056/jnm19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bortoli N, Gyawali CP, Frazzoni M, et al. Bile reflux in patients with nerd is associated with more severe heartburn and lower values of mean nocturnal baseline impedance and chemical clearance. Neurogastroenterol Motil. 2020;32:e13919. doi: 10.1111/nmo.13919. [DOI] [PubMed] [Google Scholar]
- 14.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fass R, Hell R, Sampliner RE, et al. Effect of ambulatory 24-hour esophageal pH monitoring on reflux-provoking activities. Dig Dis Sci. 1999;44:2263–2269. doi: 10.1023/A:1026608804938. [DOI] [PubMed] [Google Scholar]
- 17.Wong WM, Bautista J, Dekel R, et al. Feasibility and tolerability of transnasal/per-oral placement of the wireless pH capsule vs. traditional 24-h oesophageal pH monitoring--a randomized trial. Aliment Pharmacol Ther. 2005;21:155–163. doi: 10.1111/j.1365-2036.2005.02313.x. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]
- 19.van Hoeij FB, Smout AJ, Bredenoord AJ. Predictive value of routine esophageal high-resolution manometry for gastro-esophageal reflux disease. Neurogastroenterol Motil. 2015;27:963–970. doi: 10.1111/nmo.12570. [DOI] [PubMed] [Google Scholar]
- 20.Savarino E, Gemignani L, Pohl D, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:476–486. doi: 10.1111/j.1365-2036.2011.04742.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhong C, Duan L, Wang K, et al. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol. 2013;48:601–610. doi: 10.1007/s00535-012-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estores DS. Symptom predictability in gastroesophageal reflux disease and role of proton pump inhibitor test. Gastroenterol Clin North Am. 2014;43:27–38. doi: 10.1016/j.gtc.2013.11.002. [DOI] [PubMed] [Google Scholar]