Key Points
Question
In an integrated care setting with preexisting deprescribing workflows, does an additional pharmacist-led, bundled hyperpolypharmacy deprescribing intervention reduce medication count or geriatric syndrome among patients using 10 or more medications?
Findings
In this randomized clinical trial of 2470 patients 76 years and older using 10 or more prescription medications, no association of the intervention with the primary outcomes of medication count or geriatric syndrome was found.
Meaning
Additional research is needed in other health care integrated settings and in more targeted populations.
This randomized clinical trial investigates the effectiveness of a pharmacist-administered intervention to reduce unnecessary hyperpolypharmacy in older primary care patients using 10 or more medications.
Abstract
Importance
Older patients using many prescription drugs (hyperpolypharmacy) may be at increased risk of adverse drug effects.
Objective
To test the effectiveness and safety of a quality intervention intended to reduce hyperpolypharmacy.
Design, Setting, and Participants
This randomized clinical trial allocated patients 76 years or older who used 10 or more prescription medications to a deprescribing intervention or to usual care (1:1 ratio) at an integrated health system with multiple preexisting deprescribing workflows. Data were collected from October 15, 2020, to July 29, 2022.
Intervention
Physician-pharmacist collaborative drug therapy management, standard-of-care practice recommendations, shared decision-making, and deprescribing protocols administered by telephone over multiple cycles for a maximum of 180 days after allocation.
Main Outcomes and Measures
Primary end points were change in the number of medications and in the prevalence of geriatric syndrome (falls, cognition, urinary incontinence, and pain) from 181 to 365 days after allocation compared with before randomization. Secondary outcomes were use of medical services and adverse drug withdrawal effects.
Results
Of a random sample of 2860 patients selected for potential enrollment, 2470 (86.4%) remained eligible after physician authorization, with 1237 randomized to the intervention and 1233 to usual care. A total of 1062 intervention patients (85.9%) were reached and agreed to enroll. Demographic variables were balanced. The median age of the 2470 patients was 80 (range, 76-104) years, and 1273 (51.5%) were women. In terms of race and ethnicity, 185 patients (7.5%) were African American, 234 (9.5%) were Asian or Pacific Islander, 220 (8.9%) were Hispanic, 1574 (63.7%) were White (63.7%), and 257 (10.4%) were of other (including American Indian or Alaska Native, Native Hawaiian, or >1 race or ethnicity) or unknown race or ethnicity. During follow-up, both the intervention and usual care groups had slight reductions in the number of medications dispensed (mean changes, −0.4 [95% CI, −0.6 to −0.2] and −0.4 [95% CI, −0.6 to −0.3], respectively), with no difference between the groups (P = .71). There were no significant changes in the prevalence of a geriatric condition in the usual care and intervention groups at the end of follow-up and no difference between the groups (baseline prevalence: 47.7% [95% CI, 44.9%-50.5%] vs 42.9% [95% CI, 40.1%-45.7%], respectively; difference-in-differences, 1.0 [95% CI, −3.5 to 5.6]; P = .65). No differences in use of medical services or adverse drug withdrawal effects were observed.
Conclusions and Relevance
In this randomized clinical trial from an integrated care setting with various preexisting deprescribing workflows, a bundled hyperpolypharmacy deprescribing intervention was not associated with reduction in medication dispensing, prevalence of geriatric syndrome, utilization of medical services, or adverse drug withdrawal effects. Additional research is needed in less integrated settings and in more targeted populations.
Trial Registration
ClinicalTrials.gov Identifier: NCT05616689
Introduction
Hyperpolypharmacy, or the use of 10 or more prescription drugs, is prevalent in 5% to 15% of patients 65 years or older.1,2 Hyperpolypharmacy has been associated with frailty, physical and cognitive dysfunction, medication interactions, unfavorable benefit-risk trade-offs, and increased health care costs.3 Deprescribing is the supervised withdrawal of drugs with the goal of providing guideline-concordant care and improved outcomes.4 In recent years, health systems have started bundling deprescribing of multiple drug classes into a single intervention.5,6 However, the safety and effectiveness of these programs are unclear because of heterogeneity in study settings, designs of tested interventions, and outcome definitions.6,7 Evaluations generally have focused on counts of drugs and inappropriate medications and the rare outcome of death, with fewer examining adverse drug effects (ADEs) or adverse drug withdrawal effects (ADWEs).8,9,10,11,12,13,14,15,16,17,18,19
Barriers to deprescribing in the primary care setting are numerous,20 and primary care physicians could benefit from deprescribing in partnership with pharmacists. In our integrated care system, leaders and front-line clinicians across administrative and clinical departments have worked for several years to design an intervention to reduce unnecessary hyperpolypharmacy in older primary care patients using a pharmacist-administered intervention. Guidance was developed with a focus on the American Geriatrics Society Choosing Wisely recommendations21 and commonly used medications that have relatively high harm-to-benefit ratio among older adults with hyperpolypharmacy.
This report describes the intervention and the results of a randomized clinical trial that was developed to evaluate safety and effectiveness. As detailed in later paragraphs, the 2 primary effectiveness end points included mean within-person change in the number of medications dispensed and the diagnosis of at least 1 component of geriatric syndrome, a composite measure of ADEs. The number of health care encounters and ADWEs were examined as secondary end points.
Methods
Kaiser Permanente Northern California uses a Research Determination Committee to determine human participants research status of proposed projects based on a written application. The Research Determination Committee for the Kaiser Permanente Northern California region reviewed this project’s application prior to its initiation and notified the project team in writing of its determination that the quality improvement project did not meet the regulatory definition of research involving human participants per 45 CFR 46.102(d) because the work was performed as a quality improvement project that was being tested for effectiveness in the health plan membership and not to create generalizable knowledge. The application included our intent to publish the results.22 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Setting
Our health system provides care to persons 65 years or older largely through its capitated Medicare Advantage program. The health system owns its pharmacies, and pharmacy information is integrated into the electronic health record (EHR). More than 95% of patients use the health system’s pharmacies to obtain their prescribed medications. Over-the-counter medications may be recorded in the EHR when purchased in our pharmacies; however, patients often purchase them outside the system.
Usual Care and Intervention
Regarding usual care, patients have deprescribing opportunities through several care pathways. Pharmacists working in various settings have collaborative practice agreements with physicians to provide deprescribing services at various touchpoints and transitions using single drug approaches that are triggered through a variety of mechanisms. Pharmacists also provide deprescribing as part of Medicare’s targeted Medication Therapy Management service. Physicians may deprescribe as well, although they often lack time during clinical encounters.
The bundled hyperpolypharmacy deprescribing intervention was developed by the quality department, specialty physicians, Adult and Family Medicine, and the Pharmacy and Therapeutics Committee for primary care patients 76 years and older using at least 10 nontopical prescription drugs. The intervention used physician-pharmacist partnership to identify safe and effective deprescribing opportunities, including specific patient populations and drugs and drug classes that were well suited to pharmacist-led deprescribing. It engaged the primary care physician to approve patient selection, used collaborative drug therapy management, integrated pharmacist review of 27 drug classes into a single workflow, involved physician specialists when appropriate, and included pharmacist follow-up to ensure monitoring with the option to restart prescriptions. The deprescribing practice recommendations were standard of care for older, complex patients. For example, the recommendation for glycemic controls among older adults were promulgated by the American Geriatrics Society. Included drugs and drug classes were reviewed with subspecialty clinician leaders to assess appropriateness, establish specific contraindications to discontinuation, and identify potential adverse effects of discontinuation for monitoring.
Tools to support administration of the intervention included a detailed operational playbook, a Hyperpolypharmacy Program Tool, drug-specific deprescribing protocols, workflow guidance, and other resources. The tool (Figure 1) used the Agency for Healthcare Research and Quality SHARE (seek your patient’s participation; help your patient explore and compare treatment options; assess your patient’s values and preferences; reach a decision with your patient; and evaluate your patient’s decision) approach for shared decision-making and the CEASE (confirm, estimate, assess, sort, and eliminate) deprescribing framework.23,24,25,26,27,28 The deprescribing framework included medication reconciliation; assessment of benefits, harms, and adverse effects; consideration of individual factors; prioritization; changing, discontinuing, or adding medications; documentation of medication problems29; communication with physicians; and monitoring. The intervention was administered by ambulatory care pharmacists using collaborative drug therapy management, defined as a formal partnership between the pharmacist and physician to allow the pharmacist to manage a patient’s drug therapy as a physician extender.30
Figure 1. Hyperpolypharmacy Program Tool.
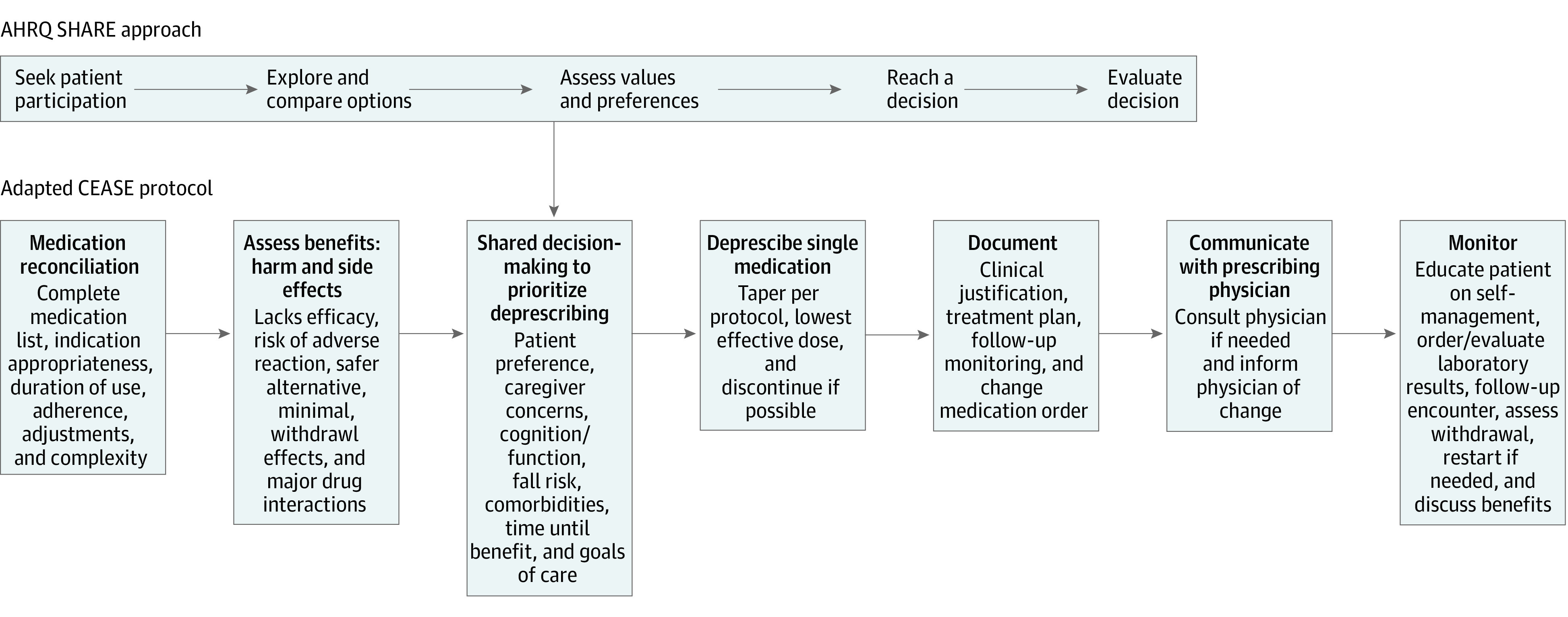
Tool uses the US Agency for Healthcare Research and Quality (AHRQ) SHARE (seek your patient’s participation; help your patient explore and compare treatment options; assess your patient’s values and preferences; reach a decision with your patient; and evaluate your patient’s decision) approach.23 Patients were able to decline or withdraw consent for participation at any point. CEASE indicates confirm, estimate, assess, sort, and eliminate.
Design of the Randomized Clinical Trial
The clinical trial was designed during 2018 and 2019. Because we sought to evaluate the effectiveness of an intervention in real-life routine practice conditions, we pursued a pragmatic design.31 In late 2019, the protocol was critiqued by an external Stakeholder Advisory Committee. The trial protocol is provided in Supplement 1. Accrual started on October 15, 2020, and ended on July 14, 2021, with follow-up through July 29, 2022.
Eligibility was determined using the EHR and included patients 76 years or older with at least 12 months of continuous enrollment, prescriptions for at least 10 nontopical drugs filled at least 2 times in the past year, and the most recent fill occurring within the 6 months before accrual. Patients with a transplant history, receiving dialysis, in hospice, with a new cancer diagnosis, with an oncology visit, or under active cancer treatment during the 12 months before accrual were ineligible because these complex conditions require frequent interventions by specialty care teams. Patients’ race and ethnicity data as listed in the EHRs were included to demonstrate the diversity and generalizability of the study population. We used translation services to include speakers of any language and included those with cognitive impairment by working with caregivers.
Patients were eligible for randomization only after physician authorization was obtained. To obtain physician authorization, a pharmacy technician sent a staff message to each eligible patient’s primary care physician. Physicians who did not respond within 1 week received a second message. The physician authorization process was closed at 2 weeks when the pharmacy technician sent a patient list to the research data analyst (M.A.), who rechecked eligibility and used simple random sampling to allocate physician-approved patients to intervention or usual care at a ratio of 1:1.
To enroll the patient, the pharmacy technician telephoned each patient to offer a medication review by the pharmacist, which the patient could decline. If the technician did not reach the patient, they followed up with a secure electronic message through the patient portal and placed another telephone call 1 week later.
Prior to the encounter, the pharmacist reviewed current and past medications, including refill history and any documentation of intolerance or ADEs, appropriate indications, and recent encounters. During the encounter, the pharmacist performed a full medication review with the patient, discussing medication adherence and the patient’s experience with the drug, including ADEs. Next, they discussed the patient’s preferences and made a shared decision to prioritize deprescribing. The patient was asked whether they wanted to accept or decline the specific recommendation. Depending on appropriateness and patient understanding, medications were deprescribed 1 or more at a time over a window of 180 days.
The pharmacist used their discretion to communicate with primary care and specialist physicians if the medication change was outside the scope defined in the practice recommendations, if the patient requested confirmation from the physician, or to confirm a treatment plan. Based on the drug-specific practice recommendation, the pharmacist scheduled a follow-up appointment in 2 to 4 weeks to assess ADWEs, disease management, the results of laboratory monitoring, and disposal of unused pills and to consider additional deprescribing.
All encounters between the pharmacist and study participants were documented as clinical notes. Patients were discharged from the program when all possible deprescribing was completed and when the patient and primary care physician received a written summary of medication changes and a list of current medications.
Outcomes
Consistent with the pragmatic design, outcomes were obtained from clinical information routinely recorded from telephone and video encounters, as well as clinic, emergency department, and hospital visits. The 2 primary effectiveness end points included mean within-person change in (1) the number of medications dispensed and (2) the diagnosis of 1 or more components of geriatric syndrome (a composite measure of ADEs) recorded in the EHR from 181 to 365 days after allocation. Geriatric syndrome was used as a concept based on the work of Inouye et al32 and Vasilevskis et al.33 It was defined simply as the composite of falls, cognition, urinary incontinence, or pain, coded as any or none. These components of geriatric syndrome were selected by clinicians and investigators with specialty expertise in pharmacy, family medicine, geriatrics, cardiology, gastroenterology, and pulmonology. Operational definitions of geriatric syndrome are provided in the trial protocol in Supplement 1.
Secondary end points included change in the number of outpatient visits, in the prevalence of 1 or more emergency department visits, and in the prevalence of 1 or more inpatient visits from 181 to 365 days after allocation. Adverse drug withdrawal effects possibly resulting from loss of disease control included emergency department visits and hospital discharges for lower respiratory tract, cardiovascular, and gastrointestinal tract disease; hyperuricemia; and elevated blood glucose level. The internal Data Safety Monitoring Committee34 included a biostatistician (S.E.A.), cardiologist (A.K.), gastroenterologist, and pulmonologist who met every 6 months to compare the rates of ADWEs in the intervention and usual care groups.
Statistical Analysis
The trial was designed to accrue 1000 intervention and 1000 usual care patients, providing statistical power to detect differences of 0.3 in the number of medications and 8% in the prevalence of at least 1 component of geriatric syndrome. Differences in the 2 primary outcomes between the usual care and intervention groups were evaluated using difference-in-differences models.35,36 Baseline status was computed from the information recorded in the EHR during the 180-day period before allocation (baseline). Changes in outcomes were measured from 181 to 365 days after randomization (with the intervention administered during days 1-180). Intention-to-treat analysis included all randomized patients, including those who were not reached, had no deprescribing recommendation identified, chose not to pursue deprescribing, or were represcribed medications during follow-up. Because the intervention could have no effect on patients for whom no drug was deprescribed, we also performed an analysis restricted to patients who received an intervention (as treated) and a second analysis to those whose intervention resulted in deprescribing (accepted deprescribing). After allocation, some patients continued their enrollment but did not fill their medications in the health plan during their follow-up period. Patients who had no medications dispensed during follow-up or who died or disenrolled were assumed to have no change in outcomes between baseline and follow-up.
When assessing the statistical significance of the 2 primary outcomes, we used a Bonferroni correction to account for testing 2 outcomes (α = .025). We further adjusted the α level for a single interim analysis after the 500th intervention patient completed their follow-up. This analysis used the O’Brien-Fleming bounds for sequential testing with a 2-sided test.37 The Bonferroni correction with O’Brien-Fleming bounds results in a significant level of α = .0015 for the interim analysis and α = .0244 for the final analysis. Consistent with the CONSORT reporting guideline for randomized clinical trials, we did not adjust analyses based on statistically significant baseline differences that were not clinically significant. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
The number of eligible patients at the start of the study was 10 012 (Figure 2). Of these, 2860 patients (28.6%) were sampled, of whom physician authorization was obtained for 2687 (94.0%). The number of patients randomized was 2470, with 1237 allocated to the intervention and 1233 to usual care. The median age of eligible patients was 80 (range, 76-104) years; 1197 (48.5%) were men and 1273 (51.5%) were women. In terms of race and ethnicity as recorded in EHRs, 185 (7.5%) were African American, 234 (9.5%) were Asian or Pacific Islander, 220 (8.9%) were Hispanic, 1574 (63.7%) were White, and 257 (10.4%) were other (including American Indian or Alaska Native, Native Hawaiian, or >1 race or ethnicity) or unknown. Nearly half (1153 [46.7%]) had a Charlson Comorbidity Index score of 5 or more. Baseline characteristics of the usual care and intention-to-treat arms were balanced, although intervention patients were slightly older (Table 1).
Figure 2. Study Flow Diagram.
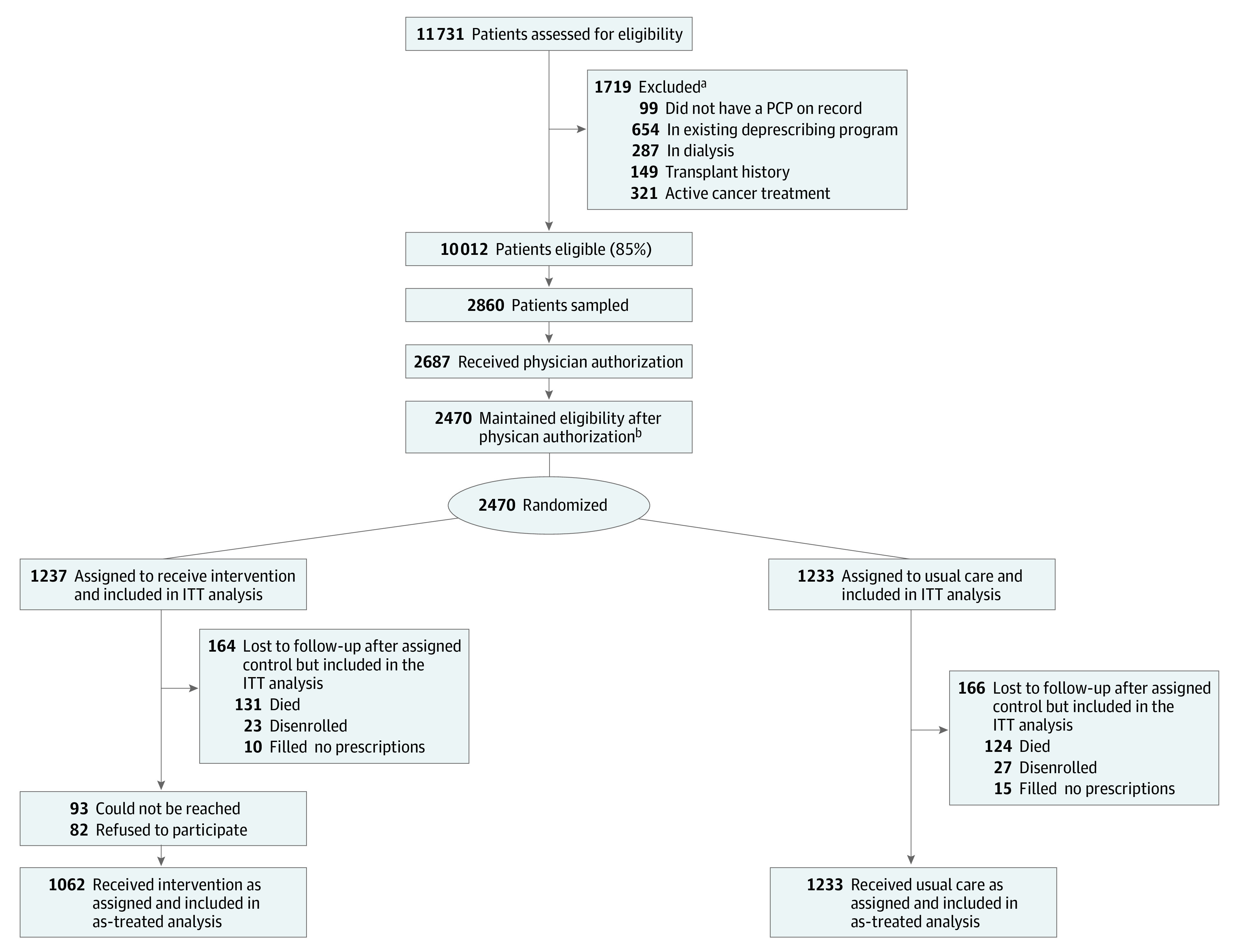
ITT indicates intention to treat; PCP, primary care physician.
aSome patients were excluded for multiple reasons.
bOf the 2687 patients, a total of 216 lost eligibility after physician authorization: 202 reduced their medication count below 10, 24 had active cancer, 11 entered hospice, and 8 died, with some patients excluded for multiple reasons.
Table 1. Baseline Characteristics of Older Adults With Hyperpolypharmacy Allocated to Bundled Hyperpolypharmacy Intervention or Usual Care.
| Characteristic in the 6 months prior to recruitment | Treatment group, No. (%) of patientsa | ||||
|---|---|---|---|---|---|
| Usual care | Intervention | ||||
| Intention to treat (n = 1233) | Missing and lost to follow-up (n = 166) | Intention to treat (n = 1237) | Reached, agreed, treated, and alive (n = 1062) | Missing and lost to follow-up (n = 164) | |
| Age, y | |||||
| 76-79 | 524 (42.5) | 58 (34.9) | 507 (41.0) | 442 (41.6) | 48 (29.3) |
| 80-84 | 431 (35.0) | 53 (31.9) | 399 (32.3) | 348 (32.8) | 53 (32.3) |
| ≥85 | 278 (22.5) | 55 (33.1) | 331 (26.8) | 272 (25.6) | 63 (38.4) |
| Sex | |||||
| Men | 605 (49.1) | 88 (53.0) | 592 (47.9) | 515 (48.5) | 78 (47.6) |
| Women | 628 (50.9) | 78 (47.0) | 645 (52.1) | 547 (51.5) | 86 (52.4) |
| Race and ethnicity | |||||
| African American | 100 (8.1) | 8 (4.8) | 85 (6.9) | 77 (7.3) | 10 (6.1) |
| Asian or Pacific Islander | 121 (9.8) | 11 (6.6) | 113 (9.1) | 88 (8.3) | 13 (7.9) |
| Hispanic | 108 (8.8) | 13 (7.8) | 112 (9.1) | 93 (8.8) | 11 (6.7) |
| White | 780 (63.3) | 118 (71.1) | 794 (64.2) | 695 (65.4) | 116 (70.7) |
| Otherb | 114 (9.2) | NS | 124 (10.0) | 100 (9.4) | 14 (8.5) |
| Unknown | 10 (0.8) | NS | 9 (0.7) | 9 (0.8) | 0 |
| Body mass indexc | |||||
| ≤18.4 | 9 (0.7) | NS | 20 (1.6) | 15 (1.4) | 6 (3.7) |
| 18.5-24.9 | 253 (20.5) | 52 (31.3) | 227 (18.4) | 195 (18.4) | 45 (27.4) |
| 25.0-29.9 | 363 (29.4) | 46 (27.7) | 350 (28.3) | 298 (28.1) | 47 (28.7) |
| ≥30.0 | 441 (35.8) | 53 (31.9) | 465 (37.6) | 409 (38.5) | 47 (28.7) |
| Not recorded | 167 (13.5) | NS | 175 (14.1) | 145 (13.7) | 19 (11.6) |
| Charlson Comorbidity Index score | |||||
| 0 | 136 (11.0) | 16 (9.6) | 172 (13.9) | 136 (12.8) | 23 (14.0) |
| 1-2 | 196 (15.9) | 22 (13.3) | 212 (17.1) | 189 (17.8) | 17 (10.4) |
| 3-4 | 308 (25.0) | 29 (17.5) | 293 (23.7) | 255 (24.0) | 28 (17.1) |
| 5-6 | 327 (26.5) | 45 (27.1) | 314 (25.4) | 271 (25.5) | 45 (27.4) |
| ≥7 | 266 (21.6) | 54 (32.5) | 246 (19.9) | 211 (19.9) | 51 (31.1) |
| No. of drugs prescribed | |||||
| 10 | 451 (36.6) | 57 (34.3) | 457 (36.9) | 397 (37.4) | 49 (29.9) |
| 11 | 320 (26.0) | 42 (25.3) | 317 (25.6) | 271 (25.5) | 34 (20.7) |
| 12 | 197 (16.0) | 25 (15.1) | 203 (16.4) | 172 (16.2) | 39 (23.8) |
| ≥13 | 265 (21.5) | 42 (25.3) | 260 (21.1) | 222 (20.9) | 42 (25.6) |
| No. of ambulatory visits | |||||
| 0-2 | 143 (11.6) | 17 (10.2) | 173 (14.0) | 141 (13.3) | 21 (12.8) |
| 3-4 | 157 (12.7) | 24 (14.5) | 135 (10.9) | 109 (10.3) | 19 (11.6) |
| 5-7 | 197 (16.0) | 18 (10.8) | 195 (15.8) | 172 (16.2) | 24 (14.6) |
| ≥8 | 736 (59.7) | 107 (64.5) | 734 (59.3) | 640 (60.3) | 100 (61.0) |
| Prior emergency department visit | |||||
| No | 782 (63.4) | 80 (48.2) | 791 (63.9) | 686 (64.6) | 78 (47.6) |
| Yes | 451 (36.6) | 86 (51.8) | 446 (36.1) | 376 (35.4) | 86 (52.4) |
| Prior hospitalization | |||||
| No | 1031 (83.6) | 124 (74.7) | 1048 (84.7) | 907 (85.4) | 123 (75.0) |
| Yes | 202 (16.4) | 42 (25.3) | 189 (15.3) | 155 (14.6) | 41 (25.0) |
Abbreviation: NS, not shown.
Small cell sizes (usually <6) have been omitted to protect confidentiality.
Includes American Indian or Alaska Native, Native Hawaiian, or >1 race or ethnicity.
Calculated as weight in kilograms divided by height in meters squared.
Among patients randomized to the intervention, 1144 (92.5%) were reached; of these, 1062 (92.8%) agreed to enroll (Figure 2). Of these 1062 patients, the number with at least 1 medication discussed was 739 (69.6%), with 438 patients (41.2%) accepting at least 1 recommendation to deprescribe for 752 medications and 25 (2.4%) later restarting their medication (eTables 1 and 2 in Supplement 2). Regarding missing information and loss to follow-up among the intervention patients, 131 died, 23 disenrolled without a death report, and 10 had no medication dispensed during follow-up (total, 164 [13.3%]). Among usual care patients, 124 died, 27 disenrolled without a death report, and 15 had no medication dispensed during follow-up (total, 166 [13.5%]). Among those who were lost to follow-up, intervention patients were older (aged ≥85 years: 63 [38.4%] vs 55 [33.1%]) and more likely to be women (86 [52.4%] vs 78 [47.0%]) and White (116 [70.7%] vs 118 [71.1%]) compared with usual care patients (Table 1).
At baseline, the mean number of medications was 13.6 (95% CI, 13.4-13.8) in the usual care and intention-to-treat groups. After follow-up, the mean declined by 0.4 in each group (95% CI, −0.6 to −0.3 in the usual care group and −0.6 to −0.2 in the intention-to-treat group; P = .71), so that in the intention-to-treat analysis the difference-in-differences was 0.02 (95% CI, −0.3 to 0.3; P = .91) (Table 2). Regarding the prevalence of at least 1 geriatric syndrome condition, at baseline, intervention patients had a lower prevalence than usual care patients (42.9% [95% CI, 40.1%-45.7%] vs 47.7% [95% CI, 44.9%-50.5%]). During follow-up, the prevalence was similar in the intervention and usual care groups, with a difference-in-differences of 1.0 (95% CI, −3.5 to 5.6) that was not statistically significant (P = .65) (Table 2). We observed no clinically or statistically significant difference-in-differences in components of geriatric syndrome (eTable 3 in Supplement 2), secondary outcomes (Table 2), or ADWEs (Table 3). Restriction to the as-treated and accepted deprescribing groups altered these findings only negligibly (Tables 2 and 3). Finally, we found no difference in the risk of death during follow-up between patients in the intervention and usual care groups (eTable 4 in Supplement 2).
Table 2. Association of Bundled Hyperpolypharmacy Deprescribing Intervention vs Usual Care With Primary and Secondary Outcomesa.
| Group | Primary outcomes | Secondary outcomes | |||
|---|---|---|---|---|---|
| Mean No. of medications (95% CI) | Prevalence of geriatric syndrome, % (95% CI) | Mean No. of outpatient visits (95% CI) | Prevalence of an inpatient visit, % (95% CI) | Prevalence of an emergency department visit, % (95% CI) | |
| Usual care (n = 1233) | |||||
| Baseline | 13.6 (13.4 to 13.8) | 47.7 (44.9 to 50.5) | 11.6 (11.1 to 12.2) | 16.6 (14.6 to 18.7) | 37.1 (34.4 to 39.8) |
| Difference | −0.4 (−0.6 to −0.3) | 1.9 (−1.4 to 5.1) | −0.3 (−0.8 to 0.2) | 0.0 (−2.5 to 2.5) | 2.9 (−0.2 to 6.0) |
| Intention to treat (n = 1237) | |||||
| Baseline | 13.6 (13.4 to 13.8) | 42.9 (40.1 to 45.7) | 11.3 (10.7 to 11.8) | 15.4 (13.4 to 17.4) | 36.3 (33.6 to 38.9) |
| Difference | −0.4 (−0.6 to −0.2) | 2.9 (−0.3 to 6.1) | 0.1 (−0.4 to 0.6) | 1.6 (−0.9 to 4.1) | 4.3 (1.0 to 7.6) |
| Difference-in-differences | 0.02 (−0.3 to 0.3) | 1.0 (−3.5 to 5.6) | 0.4 (−0.3 to 1.1) | 1.6 (−1.9 to 5.1) | 1.4 (−3.1 to 5.9) |
| P valueb | .91 | .65 | .26 | .37 | .55 |
| As-treated (n = 1062) | |||||
| Baseline | 13.5 (13.3 to 13.7) | 42.6 (39.6 to 45.5) | 11.4 (10.8 to 11.9) | 14.6 (12.5 to 16.7) | 35.4 (32.5 to 38.3) |
| Difference | −0.4 (−0.6 to −0.2) | 3.2 (−0.3 to 6.7) | 0.2 (−0.4 to 0.7) | 2.2 (−0.6 to 4.9) | 4.0 (0.4 to 7.7) |
| Difference-in-differences | 0.1 (−0.2 to 0.3) | 1.3 (−3.4 to 6.1) | 0.5 (−0.3 to 1.2) | 2.2 (−1.5 to 5.9) | 1.1 (−3.6 to 5.9) |
| P valueb | .64 | .58 | .22 | .25 | .64 |
| Accepted deprescribing (n = 438) | |||||
| Baseline | 13.4 (13.1 to 13.7) | 42.2 (37.6 to 46.9) | 10.8 (10.0 to 11.7) | 12.6 (9.5 to 15.7) | 32.0 (27.6 to 36.3) |
| Difference | −0.6 (−0.9 to −0.3) | 0.7 (−4.8 to 6.2) | 0.4 (−0.5 to 1.2) | 2.2 (−2.0 to 6.6) | 3.6 (−1.9 to 9.2) |
| Difference-in-differences | −0.1 (−0.5 to 0.2) | −1.2 (−7.6 to 5.2) | 0.7 (−0.3 to 1.6) | 2.2 (−2.6 to 7.2) | 0.7 (−5.6 to 7.0) |
| P valueb | .48 | .72 | .16 | .37 | .82 |
Of the 1237 intervention patients, 1062 agreed to the deprescribing program, and 739 discussed a medication. For patients lost to follow-up (151 usual care and 154 intervention) or missing (15 usual care and 10 intervention), we assumed that medication count on day 365 equaled the medication count on day 0. The difference-in-differences compared the measure recorded during days 181 to 365 after allocation with the 180 days before allocation.
The Bonferroni correction with O’Brien-Fleming bounds for the 2 primary outcomes was 2-sided α = .0244.
Table 3. Association of Bundled Hyperpolypharmacy Deprescribing Intervention vs Usual Care With Adverse Drug Withdrawal Effects.
| Group | Adverse drug withdrawal effect, % (95% CI)a | ||||
|---|---|---|---|---|---|
| Lower respiratory tract | Cardiovascular | Gastrointestinal tract | Hyperuricemia | Elevated blood glucose level | |
| Usual care (n = 1233) | |||||
| Baseline | 18.7 (16.5 to 20.8) | 31.8 (29.2 to 34.4) | 21.6 (19.3 to 23.9) | 42.9 (31.7 to 54.3) | 31.6 (21.9 to 41.4) |
| Difference-in-differences | −0.3 (−2.7 to 2.0) | 1.3 (−1.6 to 4.2) | −1.4 (−3.8 to 1.1) | 0.9 (−0.4 to 2.2) | −0.2 (−1.2 to 0.9) |
| Intention to treat (n = 1237)b | |||||
| Baseline | 19.6 (17.4 to 21.8) | 31.9 (29.3 to 34.5) | 20.6 (18.3 to 22.9) | 38.8 (28.0 to 49.5) | 34.7 (24.5 to 44.9) |
| Difference | 0.1 (−2.5 to 2.6) | −0.3 (−3.4 to 2.8) | 0.5 (−2.1 to 3.1) | 0.5 (−0.7 to 1.7) | 0.4 (−0.8 to 1.6) |
| Difference-in-differences | 0.4 (−3.0 to 3.9) | −1.6 (−5.9 to 2.7) | 1.9 (−1.8 to 5.5) | −0.4 (−2.2 to 1.4) | 0.6 (−1.1 to 2.2) |
| P valuec | .82 | .46 | .32 | .66 | .50 |
| As treated (n = 1062) | |||||
| Baseline | 19.6 (17.2 to 21.9) | 31.1 (28.4 to 33.9) | 19.6 (17.2 to 22.0) | 4.1 (3.0 to 5.3) | 3.8 (2.6 to 4.9) |
| Difference | 0.1 (−2.7 to 2.9) | −0.6 (−3.9 to 2.8) | 1.1 (−1.7 to 4.0) | 0.4 (−1.0 to 1.8) | 0.6 (−0.8 to 1.9) |
| Difference-in-differences | 0.4 (−3.2 to 4.1) | −1.9 (−6.4 to 2.6) | 2.5 (−1.3 to 6.3) | −0.5 (−2.4 to 1.4) | 0.7 (−1.0 to 2.4) |
| P valuec | .82 | .42 | .20 | .60 | .41 |
| Accepted deprescribing (n = 438) | |||||
| Baseline | 18.7 (15.1 to 22.4) | 28.1 (23.9 to 32.3) | 17.6 (14.0 to 21.1) | 2.7 (1.2 to 4.3) | 2.9 (1.4 to 4.6) |
| Difference | −1.4 (5.8 to 3.0) | −1.1 (−6.3 to 4.1) | 2.3 (−2.3 to 6.9) | 1.4 (−0.5 to 3.3) | 0.7 (−1.6 to 2.9) |
| Difference-in-differences | −1.1 (−6.0 to 3.9) | −2.4 (−8.4 to 3.5) | 3.7 (−1.6 to 8.9) | 0.5 (−1.8 to 2.8) | 0.9 (−1.6 to 3.3) |
| P valuec | .68 | .42 | .17 | .69 | .50 |
Information was obtained from emergency department visits or hospital discharges with a relevant International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis code for lower respiratory tract (J40-J47), cardiovascular (G45, I48, I49, I50, I63, I73, I74, R60, I10, I11, I16, I20-I25, R07.9, R00.0, I60, I61, I62, and I67), and gastrointestinal tract disease (K21-29, K29.71, K31.80, K31.88, K63.80, K63.88, K91.80, K55.8, K25.0, K25.2, K25.4, K25.6, K26.0, K26.2, K26.4, K26.6, K27.0, K27.2, K27.4, K27.6, K28.0, K28.2K28.4K28.6, K92.2, K92.0, and K92.1); hyperuricemia (E79.0 and M10); and elevated blood glucose level (R73).
Of the 1237 intervention patients, 1062 agreed to the deprescribing program and 739 discussed a medication. For patients lost to follow-up (151 controls and 154 intervention) or missing (15 controls and 10 intervention), we assumed that medication count on day 365 equaled the medication count on day 0. The difference-in-differences compared the measure from days 181 to 365 after allocation with the 180 days before allocation.
The Bonferroni correction with O’Brien-Fleming bounds for the 2 primary outcomes was 2-sided α = .0244.
Discussion
We conducted a pragmatic randomized clinical trial of a quality improvement initiative to reduce hyperpolypharmacy among older adults, observing no clinically important or statistically significant differences in medication counts, ADEs, or ADWEs between intervention and usual care patients. Since 2014, randomized clinical trials set in ambulatory populations in North America and Europe13,14,15,16,18,19,38,39,40,41,42,43,44 have compared various deprescribing interventions with usual care. Interventions have included education and training of patients and primary care clinicians,13,18 clinician-led medication review with or without shared decision-making,14,16,38,39,40,41,42 geriatric assessment,43,44 and clinical decision support.15,19 These studies have reported an increase in the number of pharmaceutical interventions to improve medication appropriateness38,43,45 and a reduction in the number of potentially inappropriate medications14,17,44 with no change or a reduction in the number of medications.13,14,15,17,19,40,42,44 Of 9 studies examining clinical outcomes (death, falls, fractures, and cognition), 7 found no difference at 3 to 24 months.14,16,19,38,40,43,44 Of the remaining 2, one focused on anticholinergic drugs, used medication review, and reported improvement in sedative side effects and cognition at 3 months,41 while the other used medication review by 3 experts per patient and found a reduction in falls at 24 months.39 Two studies16,43 reported improvement in self-reported health or quality of life but 2 others39,42 did not. Three studies of use of health care services reported no differences associated with the intervention.14,16,40 Settings, study populations, interventions, and outcome definitions have been heterogeneous across studies, and evidence is difficult to synthesize and limited. Last, it is important to note that interventions have not been linked to increased ADWEs.40,42
We suspect that our health system’s several pharmacy programs and deprescribing programs—which were designed for single drug classes, single diseases, and care transitions—minimized the opportunity to further decrease hyperpolypharmacy in our membership. Also, although it is widely believed that overprescribing is a problem, this may not be correct. The underlying medical condition of patients using 10 or more medications may be such that deprescribing is not feasible or appropriate in some settings.
Limitations
Study limitations may have hampered our ability to find a difference. Most importantly, in our integrated setting, few patients using 10 or more medications were identified by the pharmacists as using a medication that was appropriate for deprescribing, and the measure of potential benefit of the deprescribing program may have been reduced through the inclusion of these patients. It was for this reason that we performed an analysis restricted to patients who received an intervention (as treated) and a second analysis to those whose intervention resulted in deprescribing (accepted deprescribing), which also showed no benefit of the intervention. The benefits and harms of deprescribing may range from the short term to the long term, and misspecification of the timing of the intervention effect may have resulted in a spurious negative finding if the benefit occurred further out in time. However, given the broad range of drugs under study, it would be difficult to select a single optimal period of measurement. The routinely recorded information in the EHR may have lacked some specification for both geriatric syndrome and ADWEs, although this would not affect medication counts. Additionally, dose reductions and switching to lower-risk regimens may affect syndrome severity, but not necessarily ameliorate the syndrome completely, given that many geriatric syndromes are multifactorial; however, reduction in severity likely would have been reflected in outpatient visit counts, which we did not observe. Finally, we did not measure patient-reported outcomes.
Conclusions
In this randomized clinical trial in an integrated care setting with various preexisting deprescribing workflows, a bundled hyperpolypharmacy deprescribing intervention was not associated with the 2 primary outcomes of reduction in medication dispensing or prevalence of geriatric syndrome or with secondary outcomes of use of health care services or ADWEs. It will be critical in future studies of the effectiveness of hyperpolypharmacy deprescribing to provide detailed descriptions of study characteristics. It may be possible to identify subpopulations, such as those with symptoms of geriatric syndrome, for whom an intervention is more effective. However, given the evidence from this study for lack of improvement over usual care, Kaiser Permanente Northern California has not broadly implemented the bundled hyperpolypharmacy deprescribing intervention among its full membership. We believe that the rigorous information gained from this study is generalizable to other integrated settings that have well-established pharmacy management programs. However, additional research is needed about the potential benefit and harms of bundled deprescribing in less integrated systems with less developed pharmacy management capabilities.
Trial Protocol
eTable 1. Deprescribing Process Variables Among Those Who Agreed to the Intervention (n = 1062)
eTable 2. Medication Classes Discussed Among 739 Patients and Deprescribing Actions
eTable 3. Association of Bundled Hyperpolypharmacy Deprescribing Intervention vs Usual Care With Components of Geriatric Syndrome
eTable 4. Risk of Death During Follow-up, Usual Care Compared With Intervention Groups
Data Sharing Statement
References
- 1.Slater N, White S, Venables R, Frisher M. Factors associated with polypharmacy in primary care: a cross-sectional analysis of data from the English Longitudinal Study of Ageing (ELSA). BMJ Open. 2018;8(3):e020270. doi: 10.1136/bmjopen-2017-020270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson CA, Thomson WM, Smith MB, et al. Medication taking in a national sample of dependent older people. Res Social Adm Pharm. 2020;16(3):299-307. doi: 10.1016/j.sapharm.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 3.McCarthy LM, Visentin JD, Rochon PA. Assessing the scope and appropriateness of prescribing cascades. J Am Geriatr Soc. 2019;67(5):1023-1026. doi: 10.1111/jgs.15800 [DOI] [PubMed] [Google Scholar]
- 4.Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of “deprescribing” with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254-1268. doi: 10.1111/bcp.12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi: 10.1111/bcp.12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayliss EA, Albers K, Gleason K, et al. Recommendations for outcome measurement for deprescribing intervention studies. J Am Geriatr Soc. 2022;70(9):2487-2497. doi: 10.1111/jgs.17894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dills H, Shah K, Messinger-Rapport B, Bradford K, Syed Q. Deprescribing medications for chronic diseases management in primary care settings: a systematic review of randomized controlled trials. J Am Med Dir Assoc. 2018;19(11):923-935.e2. doi: 10.1016/j.jamda.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 8.Allard J, Hébert R, Rioux M, Asselin J, Voyer L. Efficacy of a clinical medication review on the number of potentially inappropriate prescriptions prescribed for community-dwelling elderly people. CMAJ. 2001;164(9):1291-1296. [PMC free article] [PubMed] [Google Scholar]
- 9.Beer C, Loh PK, Peng YG, Potter K, Millar A. A pilot randomized controlled trial of deprescribing. Ther Adv Drug Saf. 2011;2(2):37-43. doi: 10.1177/2042098611400332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalleur O, Boland B, Losseau C, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs Aging. 2014;31(4):291-298. doi: 10.1007/s40266-014-0157-5 [DOI] [PubMed] [Google Scholar]
- 11.Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428-437. doi: 10.1016/S0002-9343(97)89519-8 [DOI] [PubMed] [Google Scholar]
- 12.Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS One. 2016;11(3):e0149984. doi: 10.1371/journal.pone.0149984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayliss EA, Shetterly SM, Drace ML, et al. Deprescribing education vs usual care for patients with cognitive impairment and primary care clinicians: the OPTIMIZE pragmatic cluster randomized trial. JAMA Intern Med. 2022;182(5):534-542. doi: 10.1001/jamainternmed.2022.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campins L, Serra-Prat M, Gózalo I, et al. ; REMEI Group . Randomized controlled trial of an intervention to improve drug appropriateness in community-dwelling polymedicated elderly people. Fam Pract. 2017;34(1):36-42. doi: 10.1093/fampra/cmw073 [DOI] [PubMed] [Google Scholar]
- 15.Fried TR, Niehoff KM, Street RL, et al. Effect of the tool to reduce inappropriate medications on medication communication and deprescribing. J Am Geriatr Soc. 2017;65(10):2265-2271. doi: 10.1111/jgs.15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenander C, Elfsson B, Danielsson B, Midlöv P, Hasselström J. Effects of a pharmacist-led structured medication review in primary care on drug-related problems and hospital admission rates: a randomized controlled trial. Scand J Prim Health Care. 2014;32(4):180-186. doi: 10.3109/02813432.2014.972062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milos V, Rekman E, Bondesson Å, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs Aging. 2013;30(4):235-246. doi: 10.1007/s40266-013-0057-0 [DOI] [PubMed] [Google Scholar]
- 18.Quintana-Bárcena P, Lord A, Lizotte A, Berbiche D, Lalonde L. Prevalence and management of drug-related problems in chronic kidney disease patients by severity level: a subanalysis of a cluster randomized controlled trial in community pharmacies. J Manag Care Spec Pharm. 2018;24(2):173-181. doi: 10.18553/jmcp.2018.24.2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822. doi: 10.1136/bmj.m1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty AJ, Boland P, Reed J, et al. Barriers and facilitators to deprescribing in primary care: a systematic review. BJGP Open. 2020;4(3):bjgpopen20X101096. doi: 10.3399/bjgpopen20X101096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AGS Choosing Wisely Workgroup . American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622-631. doi: 10.1111/jgs.12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Office for Human Research Protections, US Department of Health and Human Services . Quality improvement activities FAQs: if I plan to carry out a quality improvement project and publish the results, does the intent to publish make my quality improvement project fit the regulatory definition of research? Revised October 13, 2022. Accessed May 11, 2023. https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/quality-improvement-activities/index.html
- 23.Agency for Healthcare Research and Quality . The SHARE approach. Reviewed March 2023. Accessed June 6, 2023. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- 24.Scott IA, Le Couteur DG. Physicians need to take the lead in deprescribing. Intern Med J. 2015;45(3):352-356. doi: 10.1111/imj.12693 [DOI] [PubMed] [Google Scholar]
- 25.Reeve E, Thompson W, Farrell B. Deprescribing: a narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. Eur J Intern Med. 2017;38:3-11. doi: 10.1016/j.ejim.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 26.Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi: 10.1503/cmaj.131873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott I, Anderson K, Freeman C. Review of structured guides for deprescribing. Eur J Hosp Pharm. 2017;24(1):51-57. doi: 10.1136/ejhpharm-2015-000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834. doi: 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 29.Pharmacy Quality Alliance . PQA Medication Therapy Problem Categories Framework. Updated March 5, 2021. Accessed October 25, 2021. https://www.pqaalliance.org/pqa-measures
- 30.Academy of Managed Care Pharmacy . Practice advisory on collaborative drug therapy management. February 2012. Accessed August 31, 2021. https://www.amcp.org/sites/default/files/2019-03/Practice%20Advisory%20on%20CDTM%202.2012_0.pdf
- 31.Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13(2):217-224. doi: 10.31887/DCNS.2011.13.2/npatsopoulos [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791. doi: 10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasilevskis EE, Shah AS, Hollingsworth EK, et al. ; Shed-MEDS Team . A patient-centered deprescribing intervention for hospitalized older patients with polypharmacy: rationale and design of the Shed-MEDS randomized controlled trial. BMC Health Serv Res. 2019;19(1):165. doi: 10.1186/s12913-019-3995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellenberg SS, Fleming TR, Demets DL. Data Monitoring Committees in Clinical Trials: A Practical Perspective. 2nd ed. John Wiley & Sons Ltd; 2019. doi: 10.1002/9781119512684 [DOI] [Google Scholar]
- 35.Warton EM, Parker MM. Oops, I D-I-D it again! advanced difference-in-differences models in SAS. Paper 25-2018. Accessed September 13, 2022. https://www.lexjansen.com/wuss/2018/25_Final_Paper_PDF.pdf
- 36.Warton EM, Parker MM, Karter AJ. How D-I-D you do that? basic difference-indifference models in SAS. September 5-7, 2016. Accessed September 13, 2022. https://www.lexjansen.com/wuss/2016/49_Final_Paper_PDF.pdf
- 37.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 38.Geurts MM, Stewart RE, Brouwers JR, de Graeff PA, de Gier JJ. Implications of a clinical medication review and a pharmaceutical care plan of polypharmacy patients with a cardiovascular disorder. Int J Clin Pharm. 2016;38(4):808-815. doi: 10.1007/s11096-016-0281-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahlknecht A, Wiedermann CJ, Sandri M, et al. Expert-based medication reviews to reduce polypharmacy in older patients in primary care: a northern-Italian cluster-randomised controlled trial. BMC Geriatr. 2021;21(1):659. doi: 10.1186/s12877-021-02612-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy C, Clyne B, Boland F, et al. ; SPPiRE Study Team . GP-delivered medication review of polypharmacy, deprescribing, and patient priorities in older people with multimorbidity in Irish primary care (SPPiRE Study): a cluster randomised controlled trial. PLoS Med. 2022;19(1):e1003862. doi: 10.1371/journal.pmed.1003862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open. 2018;8(7):e019042. doi: 10.1136/bmjopen-2017-019042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zechmann S, Senn O, Valeri F, et al. Effect of a patient-centred deprescribing procedure in older multimorbid patients in Swiss primary care—a cluster-randomised clinical trial. BMC Geriatr. 2020;20(1):471. doi: 10.1186/s12877-020-01870-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romskaug R, Skovlund E, Straand J, et al. Effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician for improving health-related quality of life in home-dwelling older patients receiving polypharmacy: a cluster randomized clinical trial. JAMA Intern Med. 2020;180(2):181-189. doi: 10.1001/jamainternmed.2019.5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zintchouk D, Gregersen M, Lauritzen T, Damsgaard EM. Impact of geriatrician-performed comprehensive geriatric care on medication use and cognitive function in older adults referred to a non–hospital-based rehabilitation unit. Am J Med. 2019;132(1):93-102.e2. doi: 10.1016/j.amjmed.2018.09.030 [DOI] [PubMed] [Google Scholar]
- 45.Bloomfield HE, Greer N, Linsky AM, et al. Deprescribing for community-dwelling older adults: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(11):3323-3332. doi: 10.1007/s11606-020-06089-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Deprescribing Process Variables Among Those Who Agreed to the Intervention (n = 1062)
eTable 2. Medication Classes Discussed Among 739 Patients and Deprescribing Actions
eTable 3. Association of Bundled Hyperpolypharmacy Deprescribing Intervention vs Usual Care With Components of Geriatric Syndrome
eTable 4. Risk of Death During Follow-up, Usual Care Compared With Intervention Groups
Data Sharing Statement


