Take Home Message
We looked at outcomes of photodynamic diagnosis (PDD)-guided transurethral resection of bladder tumour (TURBT) in individuals with the first diagnosis of non–muscle-invasive bladder cancer suspected to be of intermediate or high risk of recurrence in a UK population. We found that introduction of PDD is unlikely to improve the cost effectiveness of TURBT.
Keywords: Non–muscle-invasive bladder cancer, Surgery, Photodynamic diagnosis, Randomised trial, Transurethral resection of bladder tumour, Urinary bladder neoplasms, Hexvix, Cost effectiveness
Abstract
Background
Recurrence of non–muscle-invasive bladder cancer (NMIBC) is common after transurethral resection of bladder tumour (TURBT). Photodynamic diagnosis (PDD) may reduce recurrence. PDD uses a photosensitiser in the bladder that causes the tumour to fluoresce to guide resection. PDD provides better diagnostic accuracy and allows more complete tumour resection.
Objective
To estimate the economic efficiency of PDD-guided TURBT (PDD-TURBT) in comparison to white light–guided TURNT (WL-TURBT) in individuals with a suspected first diagnosis of NMIBC at intermediate or high risk of recurrence on the basis of routine visual assessment before being scheduled for TURBT.
Design, setting, and participants
This is a health economic evaluation alongside a pragmatic, open-label, parallel-group randomised trial from a societal perspective. A total of 493 participants (aged ≥16 yr) were randomly allocated to PDD-TURBT (n = 244) or WL-TURBT (n = 249) in 22 UK National Health Service hospitals.
Outcome measurements and statistical analysis
Cost effectiveness ratios were based on the use of health care resources associated with PDD-TURBT and WL-TURBT and quality-adjusted life years (QALYs) gained within the trial. Uncertainties in key parameters were assessed using sensitivity analyses.
Results and limitations
On the basis of the use of resources driven by the trial protocol, the incremental cost effectiveness of PDD-TURBT in comparison to WL-TURBT was not cost saving. At 3 yr, the total cost was £12 881 for PDD-TURBT and £12 005 for WL-TURBT. QALYs at three years were 2.087 for PDD-TURBT and 2.094 for WL-TURBT. The probability that PDD-TURBT is cost effective was never >30% above the range of societal cost-effectiveness thresholds.
Conclusions
There was no evidence of a difference in either costs or QALYs over 3-yr follow-up between PDD-TURBT and WL-TURBT in individuals with suspected intermediate- or high-risk NMIBC. PDD-TURBT is not supported for the management of primary intermediate- or high-risk NMIBC.
Patient summary
We assessed overall costs for two approaches for removal of bladder tumours in noninvasive cancer and measured quality-adjusted life years gained for each. We found that use of a photosensitiser in the bladder was not more cost effective than use of white light only during tumour removal.
1. Introduction
Bladder cancer is the tenth most common cancer worldwide. It is the sixth most common cancer among men and the 17th most common among women, accounting for approximately 573 000 new cases and 213 000 deaths in 2020. Approximately 80% of individuals diagnosed with bladder cancer have non–muscle-invasive bladder cancer (NMIBC), which is routinely managed with adjuvant treatments, cystoscopic surveillance, and endoscopic transurethral resection of bladder tumour (TURBT) [1], [2]. NMIBC has high recurrence rate associated with high economic costs for society, with UK National Health Service (NHS) costs estimated at more than £210 million [3], [4], [5]. The estimated annual total health care costs for bladder cancer US$4.0 billion in the US in 2010 and €2.9 billion in Europe in 2012 [4], [6].
The aim of a high-quality TURBT is to completely eradicate Ta and T1 tumours to prevent recurrence and progression to the higher stage of life-threatening muscle-invasive bladder cancer (MIBC). However, incomplete resection during the initial TURBT has been associated with staging errors. Evidence suggests that failure to identify satellite tumours or to appreciate the full extent of the tumours visualised during resection using standard white light (WL)-guided cystoscopy may be a factor in 20–40% of recurrences [7], [8]. Furthermore, lesions missed during cystoscopy and TURBT can lead to receipt of suboptimal treatment for known bladder cancer, such as failure to remove an occult lesion or to institute a therapy if the patient’s risk category is underestimated [3].
Photodynamic diagnosis (PDD) uses an intravesical photosensitiser to cause tumours to fluoresce under blue light and guide TURBT. It has been argued that this offers better diagnostic accuracy and therefore may reduce subsequent recurrence [9]. Previous trials have considered this issue, but, to the best of our knowledge, no good-quality cost-effectiveness analysis (CEA) of PDD-guided TURBT (PDD-TURBT) has been reported [10]. Here we present cost-effectiveness results for PDD-TURBT in comparison to WL-guided TURBT (WL-TURBT).
2. Patients and methods
2.1. PHOTO trial
The PHOTO trial (ISRCTN registry no. ISRCTN84013636) was a multicentre, pragmatic, open-label, parallel-group, randomised controlled trial that compared PDD-TURBT and WL-TURBT for participants with a first diagnosis of NMIBC suspected to be at intermediate or high risk of recurrence according to visual characteristics. In total, 538 participants were recruited from November 2014 to February 2018 and followed up to September 2020 in 22 UK NHS hospitals. Eligible individuals were aged ≥16 yr with a suspected first diagnosis of intermediate- or high-risk NMIBC according to the European Organisation for Research and Treatment of Cancer (EORTC)/European Association of Urology and National Institute for Health and Care Excellence (NICE) risk tables. Exclusion criteria were: (1) low-risk NMIBC; (2) imaging evidence of MIBC; (3) upper-tract involvement; (4) another life-threatening malignancy in the previous 2 yr; (5) evidence of metastases; (6) porphyria or known porphyrin hypersensitivity; (7) pregnancy; and (8) any other contraindications to PDD or WL surgery.
The trial found no evidence of a difference in the interval to the first bladder cancer recurrence (NMIBC or MIBC) between the PDD-TURBT and WL-TURBT groups. All analyses and comparisons were performed on the basis of intention to treat (ITT). Full details of the trial design can be found in the study protocol [11] and the main outcome paper [12].
2.2. Type of evaluation and perspective
As part of PHOTO, we performed incremental CEA with a 3-yr time horizon from the perspective of the health care system, social care, and participants and their families. The sample size was based on all participants randomised to either PDD-TURBT or WL-TURBT. We calculated the net costs and net effectiveness of PDD-TURBT in comparison to WL-TURBT and expressed these as a ratio. The economic evaluation was conducted and reported in agreement with the Consolidated Health Economic Evaluation Reporting Standards statement [13] and International Society For Pharmacoeconomics and Outcomes Research guidelines [14].
2.3. Resource data
For each participant in the study, resource-use data from questionnaires or case report forms were routinely collected either on an ongoing basis by the clinical investigators or via self-reports by the individuals in the trial at the initial procedure or a preset follow-up period (3, 6, 12, 18, 24, 30, and 36 mo after randomisation). These included NHS and social-care resource use and participant resource utilisation in the PHOTO trial.
2.3.1. Initial procedure
The resources associated with the initial procedure included all the resources used until discharge. The operative details were recorded at the time of surgery (eg, time in theatre, grade of the operating surgeon). Resources used after the TURBT procedure but before discharge were collected on a case report form, which recorded the length of hospital stay for the initial TURBT (based on admission and discharge dates) and medical procedures and medical events that could occur during the treatment phase. Since there were only a small number of missing values for time in theatre and length of hospital stay, and the data available for these variables were already highly accurate with negligible variations, we decided to use group means for imputation of missing data. We believe that this approach is sufficient and there is no need for any other imputation method to add any additional informative value.
2.3.2. Subsequent use of services following discharge for the index procedure
After participants were discharged, their resource use was captured at 3, 6, 12, 18, 24, and 36 mo. Resources include all secondary care (WL, flexible cystoscopy, mitomycin, bacillus Calmette-Guérin, computed tomography scans, cystectomy, palliative care, inpatient admissions, day admissions, hospital doctor consultation, outpatient consultations, and accident and emergency consultations) and primary care contacts with health professionals (eg, consultations with general practitioners, practice and district nurses, and other health professionals). All visits with these health professionals could occur at the health care practice, at the participant’s home, or via telephone. We distinguished between the different types of consultation to account for the different costs associated with each consultation type.
2.3.3. Participant and family resource use
Resource use by participants and their families was collected 30 mo after randomisation and comprised three main elements: (1) costs for accessing and using health services (eg, travel costs including fares, parking, or use of their own or health service transport); (2) the time needed to access and use health services (eg, time away from usual activities or work); and (3) indirect costs due to ill health.
For our analysis, we assumed that any participant who completed the questionnaire only in part left questions blank because they considered that the questions did not apply to them. For participants who died during the follow-up period, their resource use was automatically imputed as zero from the start of a given data collection period; for example, if they died at month 5 of follow-up, we assumed a zero cost for the 3–6-mo data collection period. This does cause underestimation of our total costs, as it is most likely that these participants would have used some services within the data collection period before they died. We believe that this is a reasonable approach in general, given that it was difficult to accurately estimate resource use by those who died because of the limited data available.
2.4. Costs
We obtained unit costs for all resources used by trial participants from a variety of sources (Table 1). For each participant, the total use for each resource was multiplied by the unit cost to calculate the cost for each resource for that participant. For example, the initial length of admission was multiplied by the NHS cost per night for an inpatient stay on a general ward to obtain the cost of hospitalisation. We calculated mean net costs and associated 95% confidence intervals (CIs) per participant for each study group. The cost for each year beyond the first year was discounted at a rate of 3.5% per annum, as recommended by NICE guidelines [15]. Discounting converts future costs and effects to present values, reflecting the conventional view that individuals put a higher value on resources used today than at some point in the future. The total discounted costs from the health service perspective were calculated by summing all intervention treatment and follow-up discounted costs for each participant in the data set. All costs were inflated to 2018–2019 prices in UK pounds (£) [16].
Table 1.
Main unit costs (2018–2019) used in cost-effectiveness/utility analysis and sources of information
| Item | Unit cost (£) | Source |
|---|---|---|
| Intervention | ||
| TURBT | ||
| Hexvix | 347.00 | Dindyal et al 2008 [32]a |
| Operating surgeon | ||
| Consultant | 108.00 | Unit costs of health and social care, 2018 [33], VI. Hospital-based health care staff: 14. Hospital-based doctors, Consultant surgical |
| Registrar | 43.00 | Unit costs of health and social care, 2018 [33], VI. Hospital-based health care staff: 14. Hospital-based doctors, Registrar |
| Non-consultant | 105.00 | Unit costs of health and social care, 2018 [33], VI. Hospital-based health care staff: 14. Hospital-based doctors, Associate specialist |
| MMC dose (cost per 40-mg vial) | 135.00 | BNF 76th edition [34], NHS indicative price: Mitomycin 40 mg powder and solvent for intravesical solutions vials (medac UK) |
| MMC deliver in the theatre | ||
| Mito-In system (Laboratorios Inibsa SA, Barcelona, Spain) | 4.33 | £4.00 at 2012, NICE guideline [35]a |
| Surgical consultant time (estimate of 2 min) | 5.06 | £4.67 at 2012, NICE guideline [35]a |
| Secondary care | ||
| WL-guided cystoscopy | 1072.76 | £937 HTA report, 2010 [3], [9]a |
| PDD-guided cystoscopy | 1569.65 | £1371 HTA report, 2010 [3], [9]a |
| Narrow-band imaging | 1120.00 | NICE, Narrow band imaging for Barrett’s oesophagus [36] |
| Flexible cystoscopy (day case) | 467.58 | NHS reference costs 2018–2019 [37], HRG (day case) code LB72 A, Diagnostic flexible cystoscopy, 19 yr and over |
| Flexible cystoscopy (outpatient) | 186.79 | NHS reference costs 2018–2019 [37], HRG (outpatient) code LB72 A, Diagnostic flexible cystoscopy, 19 yr and over |
| Induction BCG drug cost (6 doses) | 429.66 | BNF 76th edition [34], NHS indicative price (hospital only), OncoTICE 12.5 mg powder for reconstitution for instillation vials (Merck Sharp and Dohme Ltd.) |
| Induction BCG delivery cost | 1464.88 | £1324.42 at 2012, NICE guideline [35]a |
| Maintenance BCG drug cost (3 doses, 1 every 6 mo) | 214.83 | £1324.42 at 2012, NICE guideline [35]a |
| Maintenance BCG delivery cost | 732.44 | £662.21 at 2012, NICE guideline [35]a |
| Computed tomography scan | 83.23 | NHS reference costs 2018–2019 [37], HRG (diagnostic imaging) code RD20 A, Computerised tomography scan of one area, without contrast, 19 yr and over |
| Magnetic resonance imaging scan | 136.00 | NHS reference costs 2018–2019 [37], HRG (diagnostic imaging) code RD01 A, Magnetic resonance imaging scan of one area, without contrast, 19 yr and over |
| Neoadjuvant chemotherapy | 1207.30 | £1091.54 at 2012, NICE guideline [35]a |
| Radical cystectomy | 10 416.00 | NHS reference costs 2018–2019 [37], HRG (elective inpatient) code LB39D, Cystectomy with urinary diversion and reconstruction, with CC score 0–2 |
| Blood tests (kidney and PSA tests) | 22.12 | £20 at 2012, NICE guideline [35]a |
| Urethroscopy | 961.73 | NHS reference costs 2018–2019 [37], HRG (day case) code LB55 A, Minor or intermediate, urethra procedures, 19 yr and over |
| Urology consultant | 110.82 | NHS reference costs 2018–2019 [37], code 101 (consultant-led) |
| Radical radiotherapy | 1156.00 | HTA report [3]a |
| A&E visit | 168.00 | NHS reference costs 2018–2019 [37], HRG (total unit cost) service code 180 |
| Day case | 752.00 | NHS reference costs 2018–2019 [37], Day case (unit cost) |
| Inpatient attendance | 468.00 | NHS reference costs 2018–2019 [37], Minor bladder procedures, age 19 yr and over (HRG code LB15E), excess bed-day for elective care |
| Outpatient attendance | 108.00 | NHS reference costs 2018–2019 [37], Urology outpatient attendance (service code 101), TOA |
| Primary care | ||
| GP | ||
| At practice | 33.30 | Unit costs of health and social care, 2018 [33] II. Community-based health care staff: 10.3. General practitioner |
| At home | 139.49 | Unit costs of health and social care, 2009 [38] (£120), home visit lasting 23.4 mina |
| Telephone | 15.10 | Unit costs of health and social care, 2018 [33] II. Community-based health care staff: 10.5. Telephone triage, GP-led and nurse-led |
| Out-of-hours | 72.91 | £68.30, out-of-hours GP services in England, Department of Health and Social Care and NHS England [39]a |
| Nurse | ||
| At hospital | 28.00 | Unit costs of health and social care, 2018 [33] VI. Hospital-based health care staff: 13. Hospital-based nurses, Band 2 |
| At practice | 36.00 | Unit costs of health and social care, 2018 [33] II. Community-based health care staff: 10.2. Nurse (GP practice) |
| At home | 23.25 | Unit costs of health and social care, 2009 [38] (£20)a |
| Telephone | 7.70 | Unit costs of health and social care, 2018 [33] II. Community-based health care staff: 10.5. Telephone triage – GP-led and nurse-led |
| Out-of-hours | 72.91 | Assumed to be the same as for GP out-of-hours |
| Hospital doctor | 43.00 | Unit costs of health and social care, 2018 [33]; VI. Hospital-based health care staff: 14. Hospital-based doctors, Registrar |
| Hospital doctor: telephone | 15.10 | Assumed to be the same as for GP-led phone triage |
| Participant and companion travel | ||
| Cost per mile travelled by car | 0.45 | HMRC travel – mileage and fuel rates and allowances [40] |
| Car parking charges | Various | Participant-reported data |
| Cost of public transport fares (e.g., bus, train, taxi) | Various | Participant-reported data |
| Cost of non-emergency participant transport service (via ambulance) | 47.67 | NHS reference costs 2009–2010 [41] (not included in reference costs since 2011)a |
| Participant and companion time | ||
| Paid work | 12.71 | ONS annual survey of hours and earnings time series of selected estimates, 2020 [42] (all employees: median hourly earnings excluding overtime) |
| Full employment | 14.31 | ONS annual survey of hours and earnings time series of selected estimates, 2020 [42] (full-time employees: mean hourly earnings excluding overtime) |
| Part-time employment | 9.34 | ONS annual survey of hours and earnings time series of selected estimates, 2020 [42] (part-time employees: median hourly earnings excluding overtime) |
| Housework | 11.24 | NHS Pay Review Body twenty-sixth report, 2012 [43]a |
| Child care | 12.71 | ONS annual survey of hours and earnings time series of selected estimates, 2020 [44] (as paid work) |
| Caring for someone | 12.71 | ONS annual survey of hours and earnings time series of selected estimates, 2020 [42] (as paid work) |
| Voluntary work | 12.71 | ONS annual survey of hours and earnings time series of selected estimates, 2020 [42] (as paid work) |
| Student | 5.20 | TAG data book v1.13.1 [44] (value of non–working time: other, 2010 valuesa) |
| Leisure activities | 5.20 | TAG data book v1.13.1 [44] (value of non–working time: other, 2010 valuesa) |
| Retired | 5.20 | TAG data book v1.13.1 [44] (value of non–working time: other, 2010 valuesa) |
| Unemployed | 5.20 | TAG data book v1.13.1 [44] (value of non–working time: other, 2010 valuesa) |
TURBT = transurethral resection of bladder tumour; MMC = mitomycin C; BCG = bacillus Calmette-Guérin; A&E: accident and emergency department; NHS = National Health Service; NICE = National Institute for Health and Care Excellence; PSA = prostate-specific antigen; CC = Charlson comorbidity; HTA = health technology assessment; HRG = health resource group; PSSRU = Personal Social Services Research Unit; BNF = British National Formulary; TOA = total outpatient attendance; GP = general practitioner; HMRC = HM Revenue and Customs; ONS = Office for National Statistics; TAG = transport analysis guidance.
Inflated to 2018–2019 prices using the Campbell and Cochrane Economics Methods Group/Evidence for Policy and Practice Information and Coordinating Centre inflation calculator [16].
2.5. Health outcome
The health outcome in the CEA was quality-adjusted life years (QALYs) based on the EQ-5D-3L instrument, which is the utility measure preferred by NICE [15]. The EQ-5D-3L measure divides health status into five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Each of these dimensions has three levels, so 243 possible combinations of health states exist. Each combination of levels across the dimensions is associated with an EQ-5D-3L index value [17]. Utility value data derived from the EQ-5D-3L were combined with mortality data from the trial, using the standard assumption that all participants who have died in the trial will have a utility value of 0 from the date of death to the end of follow-up. The QALY for each year was then calculated on the basis of these assumptions, using the area under the receiver operating characteristic curve approach, assuming linear extrapolation of utility between time points. QALYs for each year beyond the first year were discounted at a rate of 3.5% per annum. The total discounted QALYs for each participant were then calculated by summing the discounted QALYs over the trial follow-up period.
2.6. Handling of missing data
Missing data is a concern in this study because costs or health outcomes in individuals with missing data may be systematically different from those with fully observed information. A substantial proportion of missing data observed in the trial can pose significant problems for data analysis. The complete-case analysis is inefficient in the PHOTO study because all the information from participants with at least one assessment missing is discarded. In addition, the complete-case analysis cannot be considered “intention-to-treat” because some randomised participants with follow-up data are excluded [18]. Therefore, the multiple-imputed data analysis is used as the base-case analysis, and the complete-case analysis is conducted as a scenario analysis in our sensitivity analysis.
Multiple imputation using multivariate imputation by chained equations [19], [20], [21] was used to impute missing EQ-5D-3L utility values and total follow-up cost values for participants with data for at least one follow-up visit. When missing data are missing at random, valid conclusions can be drawn from the data available using the multiple imputation approach [22]. Missing values for total follow-up costs and EQ-5D-3L utility values at each time point were imputed using predictive mean matching by treatment allocation group. The imputation model included all the variables that were included in the analysis model, as well as the outcome variable [20], [23]
The imputation procedure predicted 50 plausible alternative imputed data sets, which was found to be sufficient to provide stable estimates [23], [24] An analysis of incremental costs and QALYs was undertaken across the 50 imputed data sets and combined to generate one imputed estimate of incremental costs and QALYs. We drew bootstrap samples from each of the 50 imputed data sets to simulate estimation uncertainty.
2.7. Statistical analysis
Economic analysis is fundamentally concerned with the mean value. Therefore, we report all results as mean values with standard deviation, and mean differences in costs and effects with 95% CIs. A seemingly unrelated regression approach was used to simultaneously estimate total discounted costs and total discounted QALYs at 3 yr, allowing for the likely correlation of costs and effects [25]. For the QALY outcome variables, baseline EORTC recurrence risk group, age at randomisation, sex, and baseline EQ-5D-3L utility value were included as covariates. For the cost outcome variables, the baseline EORTC recurrence risk group was included as a covariate.
To address the issue of sampling uncertainty in the data, we used nonparametric bootstrapping methods to estimate 95% CIs for the treatment effects on costs and QALYs, with 2000 repetitions [26]. This imprecision was then presented graphically as a cost-effectiveness plane. This shows a scatterplot of bootstrapped repetitions for incremental costs and incremental QALY pairs for PDD-TURBT in comparison to WL-TURBT [27]. The bootstrapped estimates of costs and QALYS were further used to produce cost-effectiveness acceptability curves (CEACs) [28] to demonstrate the probability of the treatment being cost effective given varying willingness-to-pay (WTP) thresholds. All analyses were conducted in Stata v16.1 (StataCorp LP, College Station, TX, USA).
2.8. Sensitivity analysis
The base-case analysis was conducted under the missing-at-random assumption, using multiple imputation to impute missing cost and health-related quality-of-life values. Sensitivity analysis explored both the imprecision in estimates of costs and QALYs, and costs falling on participants and their families and wider societal costs. We also explored the impact of varying the discount rate used for costs and QALYs following NICE best-practice recommendations, varying the discount rate from 0% to 6% per annum.
3. Results
The participants had a mean age of 70 yr (standard deviation 10), and most were men (80%). The median follow-up duration was 44 mo. Table 2 shows the associated mean cost per participant over the duration of the trial by category of cost and by trial arm (also summarised in Fig. 1). The standard deviation for some of these mean costs suggests skewness in the data.
Table 2.
Mean NHS costs and MCD for PDD-TURBT and WL-TURBT by cost category over 3 yr (2018–2019 values)
| Item | MCPP, £ (standard deviation) |
MCD per participant, £ (95% CI) | |
|---|---|---|---|
| PDD-TURBT | WL-TURBT | ||
| Total NHS costs | 12 927 (10 994) | 11 934 (8235) | 993 (−724 to 2709) |
| Intervention | |||
| First TURBT | |||
| Length of operation | 92 (45) | 84 (75) | 8 (−3 to 19) |
| Drugs in theatre: Hexvix | 323 (89) | 1 (22) | 321 (310 to 333) |
| Length of stay | 2914 (4189) | 2546 (2959) | 369 (−272 to 1009) |
| Postoperative instillation of MMC | 521 (446) | 554 (440) | −33 (−111 to 45) |
| Subtotal | 3851 (4153) | 3185 (2964) | 666 (28 to 1303) |
| Second TURBT | |||
| Length of operation | 89 (53) | 77 (51) | 11 (−5 to 28) |
| ubtotal | 89 (53) | 77 (51) | 11 (−5 to 28) |
| Total intervention costs | 3879 (4157) | 3210 (2967) | 669 (31 to 1308) |
| Follow-up management (1 yr) | |||
| Secondary care | |||
| Inpatient stay | 2363 (4970) | 2469 (4591) | −106 (−953 to 740) |
| Cystectomy | 854 (2,863) | 920 (2962) | −67 (−582 to 449) |
| Resection surgery | |||
| Length of operation | 11 (28) | 15 (32) | −4 (−10 to 1) |
| Length of stay | 168 (367) | 222 (402) | −54 (−122 to 14) |
| Cystoscopy | 1559 (1077) | 1437 (1011) | 122 (−63 to 307) |
| Hospital doctor consultation | |||
| Telephone | 1 (6) | 1 (7) | 0 (−1 to 1) |
| Out of hours | 4 (25) | 9 (46) | −5 (−11 to 2) |
| Outpatient consultations (face to face) | 787 (786) | 805 (829) | −18 (−161 to 125) |
| A&E consultations (face to face) | 57 (306) | 41 (125) | 16 (−25 to 57) |
| Subtotal | 5804 (6649) | 5920 (,323) | −116 (−1264 to 1032) |
| Primary care | |||
| Face to face | |||
| GP consultations | 34 (60) | 33 (58) | 1 (−10 to 11) |
| GP home visits | 14 (61) | 14 (114) | 0 (−16 to 16) |
| Nurse consultations | 24 (61) | 27 (70) | −3 (−15 to 8) |
| Nurse home visits | 22 (89) | 19 (76) | 3 (−12 to 18) |
| Telephone consultations | |||
| GP-led | 4 (14) | 5 (18) | −1 (−4 to 2) |
| Nurse-led | 5 (12) | 5 (15) | 0 (−2 to 2) |
| Other | 1 (5) | 2 (10) | −1 (−2 to 1) |
| Out-of-hours consultations | |||
| GP | 4 (31) | 3 (22) | 1 (−4 to 6) |
| Nurse | 6 (41) | 4 (31) | 2 (−4 to 9) |
| Other | 2 (17) | 1 (11) | 1 (−2 to 3) |
| Subtotal | 116 (195) | 114 (228) | 2 (−36 to 40) |
| Follow-up management (2–3 yr) | |||
| Secondary care | |||
| Inpatient stay | 955 (6438) | 552 (2995) | 403 (−483 to 1288) |
| Cystectomy | 245 (1545) | 280 (1651) | −36 (−319 to 248) |
| Resection surgery | |||
| Length of operation | 10 (26) | 4 (18) | 5 (1 to 9) |
| Length of stay | 139 (334) | 61 (225) | 78 (28 to 128) |
| Cystoscopy | 1279 (1287) | 1267 (1323) | 13 (−218 to 244) |
| Hospital doctor consultation | |||
| Telephone | 15 (44) | 12 (36) | 3 (−4 to 10) |
| Out of hours | 4 (45) | 14 (194) | −11 (−36 to 15) |
| Outpatient consultations (face to face) | 9 (38) | 15 (50) | −6 (−14 to 2) |
| A&E consultations (face to face) | 4 (21) | 3 (23) | 1 (−3 to 5) |
| Subtotal | 3089 (7492) | 2639 (4557) | 451 (−644 to 1546) |
| Primary care | |||
| Face to face | |||
| GP consultations | 15 (44) | 12 (36) | 3 (−4 to 10) |
| GP home visits | 4 (45) | 14 (194) | −11 (−36 to 15) |
| Nurse consultations | 9 (38) | 15 (50) | −6 (−14 to 2) |
| Nurse home visits | 4 (21) | 3 (23) | 1 (−3 to 5) |
| Telephone consultations | |||
| GP led | 2 (10) | 2 (23) | −1 (−4 to 3) |
| Nurse led | 2 (7) | 2 (8) | 0 (−2 to 1) |
| Other | 0 (3) | 0 (1) | 0 (0 to 1) |
| Out-of-hours consultations | |||
| GP | 1 (9) | 0 (4) | 0 (−1 to 2) |
| Nurse | 1 (9) | 1 (18) | 0 (−3 to 2) |
| Other | 1 (14) | 1 (12) | 0 (−3 to 2) |
| Subtotal | 38 (101) | 52 (257) | −14 (−48 to 21) |
| Total follow-up costs | 9048 (10 071) | 8724 (7677) | 323 (−1259 to 1906) |
A&E = accident and emergency department; GP = general practitioner; MMC = mitomycin C; NHS = National Health Service; TURBT = transurethral resection of bladder tumour; PDD-TURBT = photodynamic diagnosis–guided TURBT; WL-TURBT = white light–guided TURBT; MCD = mean cost difference; MCPP = mean cost per participant; SD = standard deviation; CI = confidence interval.
Fig. 1.
Mean cost per participant over median follow-up of 44 mo by cost category and allocation to PDD- or WL-guided TURBT. PDD = photodynamic diagnosis; TURBT = transurethral resection of bladder tumour; WL = white light.
3.1. Intervention costs
The total cost of the intervention per participant was £3879 for PDD-TURBT and £3210 for WL-TURBT. There was no evidence of differences between the groups in terms of staff time, length-of-stay costs, and postoperative mitomycin C instillation. The additional equipment cost for PDD-TURBT is the cost of the photosensitiser (Hexvix), which results in, on average, a higher total intervention cost of £669 (95% CI £31 to £1308) for PDD-TURBT in comparison to WL-TURBT.
3.2. Follow-up management costs
The most costly follow-up management was for cases involving hospitalisation. The mean cost of hospitalisation per participant was £2363 in the first year and £955 since the second year over the follow-up period in the PDD-TURBT group, in comparison to £2469 and £552 in the WL-TURBT group. The difference in costs between PDD-TURBT and WL-TURBT was not statistically significant, with wide CIs that include the possibility of no difference or a small difference in either direction in both the first year (−£106, 95% CI −£953 to £740) and second year (£403, 95% CI −£483 to £1288) of follow-up. When calculated over the whole trial period, PDD-TURBT was on average more costly than WL-TURBT (£9048 per participant for PDD-TURBT and £8724 for WL-TURBT). The difference was on average £323 (95% CI −£1259 to 1906) throughout the trial; however, the CI is sufficiently wide to include economically important differences favouring either treatment.
3.3. Costs directly incurred by participants and indirect costs
The mean costs for accessing and using inpatient, outpatient, and primary care appointments per participant were £204, £676, and £40 for PDD-TURBT, in comparison to £124, £713, and £51, respectively, for WL-TURBT. There was no evidence of differences between the groups except for participants using inpatient appointments, for which the mean cost difference was £80 (95% CI £55 to £105; Table 3). Furthermore, a small number of participants incurred direct costs for private health care or self-purchased medication. However, the majority did not and, as with the analyses above, there was no evidence of a difference between the groups (Table 3).
Table 3.
Mean participant, companion, and indirect costs, and MCD for PDD-TURBT and WL-TURBT over 3 yr (2018–2019 values)
| Participant and companion time and travel costs | MCPP, £ (standard deviation) |
MCD per participant, £ (95% CI) | |
|---|---|---|---|
| PDD-TURBT | WL-TURBT | ||
| Inpatient appointments | 204 (187) | 124 (79) | 80 (55 to 105) |
| Outpatient appointments | 676 (750) | 713 (1500) | −37 (−248 to 173) |
| Primary care appointments | 40 (78) | 51 (130) | −11 (−30 to 8) |
| Self-purchased health care and medication | 8 (67) | 19 (273) | −11 (−46 to 24) |
| Time off work | 222 (693) | 352 (1513) | −130 (−339 to 79) |
| Total indirect and participant costs | 1150 (1184) | 1259 (2737) | −109 (−484 to 265) |
| Total NHS costs | 12 927 (10 994) | 11 934 (8235) | 993 (−724 to 2709) |
| Overall NHS, participant and indirect costs | 14 077 (11 802) | 13 193 (9630) | 883 (−1024 to 2788) |
MCD = mean cost difference; TURBT = transurethral resection of bladder tumour; PDD-TURBT = photodynamic diagnosis–guided TURBT; WL-TURBT = white light–guided TURBT; MCPP = mean cost per participant; SD = standard deviation; CI = confidence interval; NHS = National Health Service.
Mean indirect costs for sick leave taken by participants over 3 yr for reasons related to clinical symptoms of recurrence or progression were £222 for PDD-TURBT and £352 for WL-TURBT. However, there was no evidence of a significant difference between the groups. The mean difference was −£130 (95% CI −£339 to £79).
3.4. Total NHS costs
The total NHS cost (including intervention and follow-up management costs) per participant was £12 927 for PDD-TURBT and £11 934 for WL-TURBT. The difference in costs between the groups over the 3 yr was £993 (95% CI −£724 to £2709); the wide CI indicates substantial uncertainty and the possibility of no difference or a small difference in either direction.
3.5. Total costs
The total NHS, personal health care, and productivity costs were £14 077 for PDD-TURBT and £13 193 for WL-TURBT. However, there was no evidence of a significant difference between the groups. The mean difference was £883 (95% CI −£1021 to £2788).
3.6. Outcomes
Table 4 shows descriptive data for mean utility scores and QALY outcomes and group differences. The EQ-5D-3L responses were similar in the PDD-TURBT and WL-TURBT groups at all time points. There was no evidence of a difference in QALYs gained per participant between the groups at 3 yr.
Table 4.
EQ-5D-3L index values at baseline, discharge, and follow-up for PDD-TURBT and WL-TURBT over 3 yr
| Time point | Mean EQ-5D-3L index (SD) |
MCD per participant, £ (95% CI) | |
|---|---|---|---|
| PDD-TURBT | WL-TURBT | ||
| Baseline | 0.823 (0.015) | 0.820 (0.015) | 0.003 (−0.038 to 0.045) |
| Discharge | 0.702 (0.019) | 0.691 (0.021) | 0.012 (−0.044 to 0.067) |
| 3 mo | 0.788 (0.017) | 0.780 (0.016) | 0.008 (−0.037 to 0.053) |
| 6 mo | 0.802 (0.017) | 0.792 (0.017) | 0.010 (−0.037 to 0.057) |
| 12 mo | 0.757 (0.022) | 0.763 (0.022) | −0.006 (−0.067 to 0.056) |
| 18 mo | 0.728 (0.023) | 0.761 (0.022) | −0.033 (−0.096 to 0.030) |
| 24 mo | 0.684 (0.026) | 0.717 (0.026) | −0.032 (−0.104 to 0.040) |
| 36 mo | 0.630 (0.035) | 0.610 (0.035) | 0.020 (−0.077 to 0.116) |
| QALYs gaineda | 2.112 (0.093) | 2.207 (0.084) | −0.096 (−0.342 to 0.151) |
TURBT = transurethral resection of bladder tumour; PDD-TURBT = photodynamic diagnosis–guided TURBT; WL-TURBT = white light–guided TURBT; SD = standard deviation; MCD = mean cost difference; CI = confidence interval; QALY = quality-adjusted life year.
QALYs were discounted at a rate of 3.5% per annum and the total QALYs were based on individuals with complete data over 3 yr. QALYs gained were based on an analysis of the area under the receiver operating characteristic curve [45].
Caution is required in interpreting Table 4, as the results are presented only for participants who completed the EQ-5D-3L at each time point. The number of participants providing utility data in each treatment arm decreased by approximately 47% from randomisation to the 1-yr visit, with a further 21% decrease between the 1-yr and 3-yr visits.
3.7. Missing data
Missing data were mostly driven by missing EQ-5D data. Utilities were completed by 90% of the individuals at baseline and by 52% at 36 mo. Data completeness for QALYs at 3 yr was evenly distributed between the groups, with missing data for 176 of 265 (66%) in the PDD-TURBT group and 180 of 268 (67%) in the WL-TURBT group. We investigated the underlying mechanism by exploring the impact of baseline covariates on missing EQ-5D data. Missing EQ-5D data differed significantly by EORTC risk category (p = 0.007) and age group (p = 0.008).
Complete resource-use data were available for 100% of participants at the initial procedure and for 46–96% of participants in the ITT population at follow-up visits. Resource-use data at follow-up visits were complete for 98% of participants (all of the health care data were missing for the remaining 2% at follow-up). Further analysis showed that the average 3-yr cost is less in the PDD-TURBT arm than in the WL-TURBT arm for participants without complete QALY data. This finding suggests that the complete-case analyses may overestimate the true follow-up costs for PDD-TURBT, and that the cost difference between the two groups may be smaller after multiple imputation.
3.8. Cost effectiveness
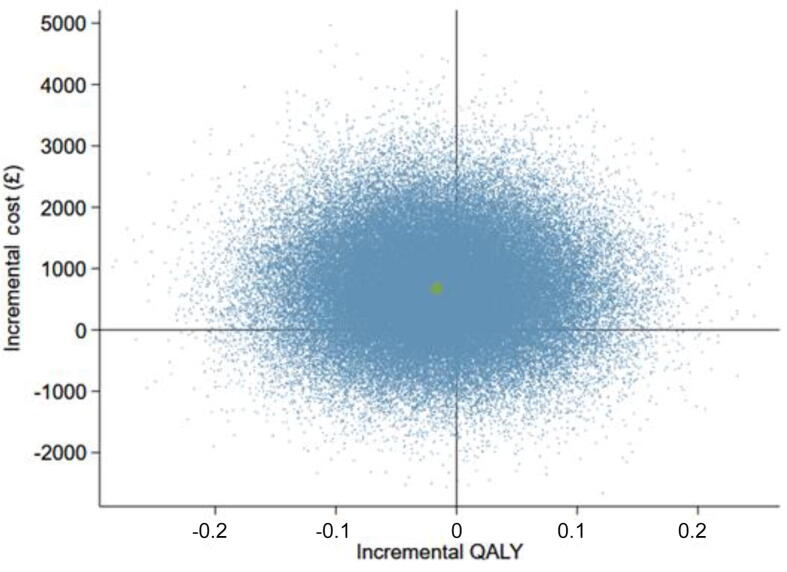
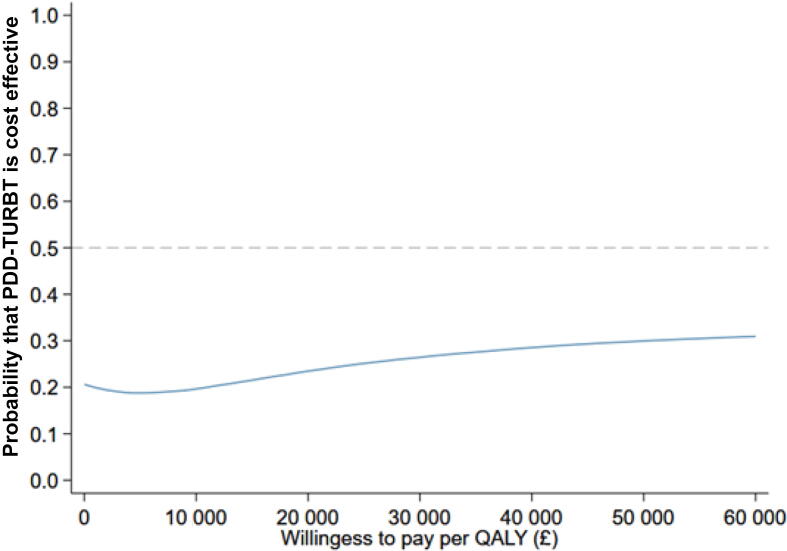
The primary CEA of the trial was conducted under the missing-at-random assumption, using multiple imputation to impute missing follow-up cost and QALY values. Table 5 presents the results of the base-case analysis from an NHS and personal social services perspective over the 3-yr time horizon. On average, PDD-TURBT is more costly and less effective; therefore, an incremental cost-effectiveness ratio (ICER) is not presented. Figure 2 shows a scatterplot of incremental costs and incremental QALYs for this analysis. It is evident that there is substantial uncertainty in the number of QALYs gained, and that PDD-TURBT is more costly than WL-TURBT. The CEAC in Figure 3 shows that the chance of PDD-TURBT being considered cost effective in comparison to WL-TURBT is 23% at an ICER threshold of £20 000 per QALY gained and 26% at an ICER threshold of £30 000 per QALY gained. Thus, WL-TURBT is far more likely to be cost effective than PDD-TURBT.
Table 5.
Trial-based cost-effectiveness analysis results for PDD-TURBT versus WL-TURBT
| Analysis | Adjusted mean (95% CI) |
Incremental mean (95% CI) |
ICER (£/QALY) | Probability that PDD-TURBT is cost effective by WTP threshold (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | £0 | £20 000 | £30 000 | £50 000 | ||
|
Base case analysis Imputed data analysis: 3 yr, NHS/PSS perspective | |||||||||
| WL-TURBT | 12 005 (10 845–13 166) | 2.094 (2.010–2.178) | WL-TURBT dominates |
||||||
| PDD-TURBT | 12 881 (11 713–14 049) | 2.087 (1.996–2.179) | 876 (−766 to 2518) | −0.007 (−0.133 to 0.119) | PDD-TURBT | 21 | 23 | 26 | 30 |
|
Sensitivity analyses Complete-case analysis: 3 yr, NHS/PSS perspective | |||||||||
| WL-TURBT | 12 265 (10 131–14 399) | 2.146 (2.030–2.261) | 95 606 | ||||||
| PDD-TURBT | 15 089 (12 577–17 602) | 2.168 (2.032–2.305) | 3236 (−1081 to 6554) | 0.034 (−0.146 to 0.213) | 2 | 16 | 26 | 38 | |
| Imputed data analysis: 3 yr, wider economic perspective | |||||||||
| WL-TURBT | 13 249 (11 954–14 545) | 2.098 (2.015–2.182) | WL-TURBT dominates |
||||||
| PDD-TURBT | 14 012 (12 719–15 306) | 2.095 (2.005–2.186) | 763 (−1048 to 2574) | −0.003 (−0.123 to 0.116) | PDD-TURBT | 28 | 27 | 30 | 32 |
| Complete-case analysis: 3 yr, wider economic perspective | |||||||||
| WL-TURBT | 14 147 (11 554–16 740) | 2.146 (2.030–2.261) | |||||||
| PDD-TURBT | 16 583 (13 657–19 508) | 2.168 (2.032–2.305) | 2715 (−1101 to 5630) | 0.035 (−0.145 to 0.214) | 78 682 | 2 | 15 | 25 | 38 |
| Imputed data analysis: 3 yr, 0% discount rate | |||||||||
| WL-TURBT | 12 165 (10 975–13 356) | 2.169 (2.083–2.255) | WL-TURBT dominates |
||||||
| PDD-TURBT | 13 055 (11 843–14 266) | 2.168 (2.072–2.264) | 889 (−787 to 2566) | −0.001 (−0.130 to 0.127) | PDD-TURBT | 21 | 26 | 29 | 33 |
| Imputed data analysis: 3 yr, 6% discount rate | |||||||||
| WL-TURBT | 11 879 (10 739–13 019) | 2.047 (1.969–2.126) | WL-TURBT dominates |
||||||
| PDD-TURBT | 12 745 (11 603–13 887) | 2.044 (1.958–2.131) | 886 (−733 to 2465) | −0.003 (−0.119 to 0.113) | PDD-TURBT | 20 | 24 | 27 | 31 |
TURBT = transurethral resection of bladder tumour; PDD-TURBT = photodynamic diagnosis–guided TURBT; WL-TURBT = white light–guided TURBT; CI = confidence interval; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year; NFS = National Health Service; PSS = personal social services; WTP = willingness to pay for 1 additional QALY.
Fig. 2.
Scatterplot of incremental costs and QALYs for PDD-guided TURBT in comparison to WL-guided TURBT (base case). PDD = photodynamic diagnosis; QALY = quality-adjusted life year; TURBT = transurethral resection of bladder tumour; WL = white light.
Fig. 3.
Cost-effectiveness acceptability curves (base case) showing the probability that PDD-guided TURBT is cost effective in comparison to WL-guided TURBT. PDD = photodynamic diagnosis; QALY = quality-adjusted life year; TURBT = transurethral resection of bladder tumour; WL = white light.
3.9. Sensitivity analysis
Results for the complete-case analysis (Table 5) show that QALYs are similar between the groups over 3 yr, but PDD-TURBT is more costly. The point estimate for the incremental cost per QALY gained for PDD-TURBT in comparison to WL-TURBT is £95 606. However, this estimate should be interpreted in light of the considerable uncertainty. The probability of PDD-TURBT being the preferred treatment option is substantially lower; it never reaches a cost-effectiveness probability of >40% at threshold values up to £50 000 per QALY gained.
Widening the perspective of costs to include those falling on participants and families and wider societal costs changed the incremental cost to £763 (95% CI −£1048 to £2574). PDD-TURBT remained unlikely to be considered cost effective in comparison to WL-TURBT over the range of societal cost-effectiveness WTP thresholds per QALY gained that we considered, compared with WLC-guided TURBT. Our results were also consistent across alternative discount rates applied to costs and QALYs (Table 5).
4. Discussion
We present a comprehensive economic analysis of PDD-TURBT in individuals with a first diagnosis of NMIBC suspected to be at intermediate or high risk of recurrence. This economic analysis, conducted as part of the PHOTO trial, shows that there is no difference in QALY values between the two groups, and PDD-TURBT was on average a more costly procedure, driven by the cost of drugs in theatre, in comparison to WL-TURBT. However, it is important to note that the cost effectiveness of an intervention is determined not only by the primary outcome but also by the balance between the costs and the health outcomes achieved. Therefore, in the absence of a difference in the primary outcome between the two groups, we looked at the balance between the costs and QALYs, and found that the introduction of PDD was unlikely to improve the cost effectiveness of TURBT over the range of societal cost-effectiveness WTP thresholds per QALY gained that we considered. We also conducted sensitivity analyses to explore the robustness of our results to changes in key assumptions and parameters.
Although PDD showed promise on the basis of modelled predictions of its cost effectiveness when introduced for TURBT in an earlier evidence synthesis, it was acknowledged that the evidence was not conclusive given the need to splice data from multiple sources and the assumptions made about the mechanism by which costs and QALYs would be generated [9]. The end-to-end evaluation reported in this paper is based directly on information obtained from a clinical trial, and therefore uses data on the effectiveness and use of resources that are not prone to the sources of bias, confounding, and uncertainty that are likely to affect nonrandomised studies. Our analysis presents for the first time evidence suggesting that the promise seen in the modelling was not realised in practice. We applied a microcosting approach to provide a more accurate, transparent, and detailed estimate of the actual resource use in delivering interventions in comparison to previous studies based on an aggregate-level costing approach [15], [29], [30]. For instance, the number of resections was higher in the PDD-TURBT group than in the WL-TURBT group, resulting in more travel time. In addition, incorporation of a wider economic perspective on costs adds value in terms of the broader economic perspective and an understanding of the non–healthcare costs for participants and their families and the economy. The analysis of QALYs on the basis of EQ-5D-3L participant-level responses follows best practice.
Despite the strengths outlined, when interpreting the results it has to be considered that the study was not powered to statistically test health economic differences. Second, costs were assessed via self-reports. Third, the costs and effects were evaluated over 3 yr. Therefore, no conclusions regarding the long-term cost effectiveness can be drawn, although the data available from the trial suggest that longer-term data would not change the conclusions drawn from this study. Finally, some data were missing for costs and QALY outcomes. We used multiple imputation techniques that are frequently recommended [31], as was done in the base-case analysis.
5. Conclusions
Over 3-yr follow-up, our analysis found no evidence of a difference in costs or QALYs between PDD-TURBT and WL-TURBT. Therefore, it is unlikely that PDD-TURBT would be considered cost effective in comparison to WL-TURBT over the range of societal cost-effectiveness WTP thresholds per QALY gained we considered. The results remained unchanged over a range of plausible assumptions and suggest that the PDD-TURBT use may not be cost effective in the UK health care system. Given the potential variation in the cost effectiveness of PDD-TURBT across different health care systems and European countries, further research is needed to assess the generalisability of our findings and to explore the optimal use of this technology in different contexts.
Author contributions: Ge Yu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Yu, Vale.
Acquisition of data: Mariappan, Penegar.
Analysis and interpretation of data: Yu, Rice, Heer, Vale.
Drafting of the manuscript: Yu.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Lewis, Vadiveloo.
Obtaining funding: Heer.
Administrative, technical, or material support: Penegar, Clark, Tandogdu, Hall.
Supervision: None.
Other: None.
Financial disclosures: Ge Yu certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: The article processing charge was funded by the PHOTO project, which was funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme (11/142/02). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The funding body had no direct role in the study.
Acknowledgments: This study is part of the PHOTO project. We thank the PHOTO team who contributed to the overall research project and are grateful to all the participants and facility staff who took part in the study.
Ethics approval: Favourable ethical opinion for this research was provided by the Newcastle & North Tyneside ethics committee (REC reference number: 14/NE/1062) in July 2014.
Associate Editor: M. Carmen Mir
References
- 1.Burger M., Zaak D., Stief C.G., et al. Photodynamic diagnostics and noninvasive bladder cancer: is it cost-effective in long-term application? A Germany-based cost analysis. Eur Urol. 2007;52:142–147. doi: 10.1016/j.eururo.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK. Bladder cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bladder-cancer.
- 3.Mowatt G., Zhu S., Kilonzo M., et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess. 2010;14:1–331. doi: 10.3310/hta14040. [DOI] [PubMed] [Google Scholar]
- 4.Leal J., Luengo-Fernandez R., Sullivan R., Witjes J.A. Economic burden of bladder cancer across the European Union. Eur Urol. 2016;69:438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Sangar V.K., Ragavan N., Matanhelia S.S., Watson M.W., Blades R.A. The economic consequences of prostate and bladder cancer in the UK. BJU Int. 2005;95:59–63. doi: 10.1111/j.1464-410X.2005.05249.x. [DOI] [PubMed] [Google Scholar]
- 6.Mariotto A.B., Robin Yabroff K., Shao Y., Feuer E.J., Brown M.L. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryan R.T., Collins S.I., Daykin M.C., et al. Mechanisms of recurrence of Ta/T1 bladder cancer. Ann R Coll Surg Engl. 2010;92:519–524. doi: 10.1308/003588410X12664192076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babjuk M., Burger M., Compérat E., et al. European Association of Urology; Arnhem, The Netherlands: 2017. EAU guidelines on non-muscle-invasive bladder cancer (Ta T1 and CIS) [DOI] [PubMed] [Google Scholar]
- 9.Mowatt G., N’Dow J., Vale L., et al. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: systematic review and meta-analysis. Int J Technol Assess Health Care. 2011;27:3–10. doi: 10.1017/S0266462310001364. [DOI] [PubMed] [Google Scholar]
- 10.Witjes J.A., Babjuk M., Gontero P., et al. Clinical and cost effectiveness of hexaminolevulinate-guided blue-light cystoscopy: evidence review and updated expert recommendations. Eur Urol. 2014;66:863–871. doi: 10.1016/j.eururo.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Tandogdu Z., Lewis R., Duncan A., et al. Photodynamic versus white light-guided treatment of non-muscle invasive bladder cancer: a study protocol for a randomised trial of clinical and cost-effectiveness. BMJ Open. 2019;9:e022268. doi: 10.1136/bmjopen-2018-022268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heer R., Lewis R., Vadiveloo T., et al. A randomized trial of PHOTOdynamic surgery in non–muscle-invasive bladder cancer. NEJM Evidence. 2022 doi: 10.1056/EVIDoa2200092. [DOI] [PubMed] [Google Scholar]
- 13.Husereau D., Drummond M., Petrou S., et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey S.D., Willke R.J., Glick H., et al. Cost-effectiveness analysis alongside clinical trials II—an ISPOR Good Research Practices Task Force report. Value Health. 2015;18:161–172. doi: 10.1016/j.jval.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. London, UK: NICE; 2013. https://www.nice.org.uk/process/pmg9/. [PubMed]
- 16.Shemilt I. CCEMG-EPPI-Centre cost converter; version 1.6. Campbell and Cochrane Economics Methods Group/Evidence for Policy and Practice Information and Coordinating Centre; 2019. https://eppi.ioe.ac.uk/costconversion/.
- 17.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 18.White I.R., Horton N.J., Carpenter J., Pocock S.J. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342 doi: 10.1136/bmj.d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Buuren S. CRC Press; New York, NY: 2018. Flexible imputation of missing data. [Google Scholar]
- 20.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 21.Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 22.Rubin D.B. John Wiley & Sons; New York, NY: 2004. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 23.Sterne J.A., White I.R., Carlin J.B., et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338 doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton N.J., Lipsitz S.R. Multiple imputation in practice: comparison of software packages for regression models with missing variables. Am Stat. 2001;55:244–254. [Google Scholar]
- 25.Greene W.H. Pearson Education; Harlow, UK: 2014. Econometric analysis: international edition. [Google Scholar]
- 26.Briggs A.H., Wonderling D.E., Mooney C.Z. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Briggs A.H., Gray A.M. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess. 1999;3:1–334. [PubMed] [Google Scholar]
- 28.Briggs A.H., O’Brien B.J., Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 29.Polsky D., Glick H. Costing and cost analysis in randomized controlled trials: caveat emptor. Pharmacoeconomics. 2009;27:179–188. doi: 10.2165/00019053-200927030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein M.C., Siegel J.E., Gold M.R., Kamlet M.S., Russell L.B. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 31.Faria R., Gomes M., Epstein D., White I.R. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32:1157–1170. doi: 10.1007/s40273-014-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dindyal S., Nitkunan T., Bunce C.J. The economic benefit of photodynamic diagnosis in non-muscle invasive bladder cancer. Photodiagn Photodyn Ther. 2008;5:153–158. doi: 10.1016/j.pdpdt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Curtis L., Burns A. Personal Social Services Research Unit, University of Kent; Canterbury, UK: 2018. Unit costs of health and social care. [Google Scholar]
- 34.Joint Formulary Committee JF. British national formulary. ed. 76. London, UK: BMJ Group and Pharmaceutical Press; 2018.
- 35.National Institute of Health and Care Excellence. Bladder cancer: diagnosis and management. NICE guideline NG2. London, UK: NICE; 2015. [PubMed]
- 36.National Institute of Health and Care Excellence. Narrow band imaging for Barrett’s oesophagus. Medtech innovation briefing MIB179. London, UK: NICE; 2019.
- 37.NHS Improvement. NHS reference costs 2018–19. London, UK: NHS Improvement; 2019.
- 38.Curtis L. Personal Social Services Research Unit, University of Kent; Canterbury, UK: 2009. Unit costs of health and social care. [Google Scholar]
- 39.National Audit Office . National Audit Office; London, UK: 2014. Out-of-hours GP services in England. [Google Scholar]
- 40.HM Revenue and Customs . UK Government; London, UK: 2019. Travel – mileage and fuel rates and allowances. [Google Scholar]
- 41.Department of Health and Social Care . Department of Health and Social Care; London, UK: 2011. NHS reference costs 2009–2010. [Google Scholar]
- 42.Office for National Statistics . ONS; Newport, UK: 2020. Annual survey of hours and earnings. Time series of selected estimates. [Google Scholar]
- 43.NHS Pay Review Body . The Stationery Office; London, UK: 2012. NHS Pay Review Body twenty-sixth report. [Google Scholar]
- 44.Department for Transport . Department for Transport; London, UK: 2018. Transport analysis guidance (TAG) data book v1.13.1. [Google Scholar]
- 45.Matthews J., Altman D.G., Campbell M., Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]