Take Home Message
Artificial urinary sphincter in elderly male patients is as an effective option to treat stress urinary incontinence with few early postoperative risks. Yet, we observed more explantations due to erosions, which reminds us to do a careful patient selection.
Keywords: Geriatrics, Lower urinary tract symptoms, Urinary incontinence, Stress, Urinary sphincter, Artificial
Abstract
Background
Artificial urinary sphincter (AUS) is a gold standard treatment in male stress urinary incontinence but remains poorly used in elderly patients.
Objective
To assess the efficacy, safety, and reoperation-free survival of AUS implantation in male patients over 75 yr of age.
Design, setting, and participants
We retrospectively reviewed the charts of all 1233 non-neurological male AUS implantations between 2005 and 2020 at 13 French centers. We compared 330 patients ≥75 yr old (GROUP75+) with 903 patients <75 yr old (GROUP75–) at the time of AUS implantation.
Outcome measurements and statistical analysis
Our primary endpoint was social continence at 3 mo defined as the use of one or fewer pad daily. We used Kaplan-Meier analyses to assess reoperation-free survival. We sought factors of erosion using logistic regression.
Results and limitations
Early postoperative continence was comparable in both groups (74.4% vs 80.1%, p = 0.114). We observed a higher rate of postoperative complications in GROUP75+ (18.8% vs 12.6%, p = 0.014), but the complications were more frequently of low grade in GROUP75+ (p = 0.025). The overall reoperation-free survival was similar (p = 0.076) after a median follow-up of 2 yr. However, patients in GROUP75+ had poorer explantation-free survival (p < 0.0001). A history of radiotherapy was a predictive factor of erosion (odds ratio [OR] = 5.31, p < 0.01), but age was not (OR = 1.08, p = 0.87). Unfortunately, our dataset did not include a systematic geriatric evaluation.
Conclusions
AUS in elderly patients appears to be an effective option to treat stress urinary incontinence. However, we observed more postoperative complications and explantations, although age was not associated with the onset of erosion. A prospective study is required to determine whether a geriatric evaluation would be an effective strategy to select patients before surgery.
Patient summary
In this study, we looked at outcomes of artificial urinary sphincter in elderly men in a large population. We found satisfying efficacy but slightly more postoperative complications and device infections.
1. Introduction
Over the past decades [1], artificial urinary sphincter (AUS) has emerged as the gold standard treatment for moderate to severe male stress urinary incontinence (SUI) [2] showing consistent data in terms of efficacy [3] and safety [4].
Urinary incontinence (UI) is highly prevalent in elderly patients and has been identified as an important risk factor for falls [5], depression, and anxiety [6], and it has frequently been cited as the main precipitating factor for institutionalization [7] in the geriatric population.
In 2007, O’Connor et al [8] were the first to publish a series of AUS implantations in elderly men. They described promising functional results with few complications, supported later by another single-center study that showed no association between age and reoperations after AUS [9]. Conversely, advanced age was found to be associated with early complications and explantations in a recent series [10].
Overall, while AUS may pose specific challenges in elderly patients, such as poorer tissue quality or difficulties in manipulation of the pump due to cognitive dysfunction or poor manual dexterity, its use in elderly men has been evaluated only in a handful of small-sample retrospective series.
The main objective of the present study was to assess the functional, perioperative, and device-related outcomes of the AUS in elderly men, defined as those ≥75 yr old.
2. Patients and methods
2.1. Study design
The charts of all male patients who underwent AUS implantation—primary or not—between 2005 and 2020 at 13 French centers were reviewed retrospectively. A common template for data collection was provided to all participating centers. The indication for AUS was always moderate to severe SUI. We excluded patients with bladder neck cuff implantation and/or neurogenic SUI.
An AMS-800 (Boston Scientific, Marlborough, MA, USA) device was used in all cases. Owing to the retrospective and multicentric design of our study, pre- and postoperative workup as well as surgical technique was not standardized. A total of 103 different surgeons were involved in the study across 13 institutions: most of the procedures were performed by 24 experienced surgeons, while the others were usually done under the supervision of an experienced surgeon. In most cases, postoperative follow-ups included a clinical interview with physical examination, uroflowmetry, and postvoid residual. Preoperative urodynamic evaluation was performed electively at the surgeon’s discretion. The device was activated 4–6 wk after implantation.
The patients were divided into two groups based on their age at the first AUS implantation in the participating center: ≥75 yr old (GROUP75+) and <75 yr old (GROUP75–). They could not be part of both groups.
The study was conducted following the principles of the Declaration of Helsinki and received an institutional review board approval (IRB no. CNIL2216559).
2.2. Data collection
The following preoperative data were collected for all patients: age, body mass index, Charlson index, American Society of Anesthesiologists (ASA) score, antiplatelet and anticoagulant intake, history of pelvic radiotherapy, previous pelvic or incontinence surgery, preoperative pad test, urodynamic data, and etiology of incontinence (categorized as postprostatectomy, post–benign prostatic obstruction surgery, or postradiotherapy incontinence, or other). Elderly patients underwent AUS implantation if they were deemed fit by the implanting surgeon, but this evaluation was neither standardized nor based on validated tools. The following surgical data were also collected: surgical approach (penoscrotal vs perineal), cuff size and position (transcorporal vs bulbar urethra), and type of pressure regulating balloon. The institution caseload was determined for each procedure on a year-by-year basis.
2.3. Outcomes of interest
The primary endpoint was the continence status at 3 mo categorized as social continence (use of zero to one pad per day) or persistent SUI (use of more than one pad per day). The other outcomes of interest were the 30-d postoperative complication rate grading according to the Clavien-Dindo classification, length of hospital stay, and rates of AUS revision, replacement, explantation, and reoperation, as well as the reoperation-free survival. Reoperation was defined as any revision, replacement, or explantation.
A revision was defined as any replacement or repositioning of one or several components of the device, while a replacement was the change of the whole device. The indications for AUS reoperation were categorized as infection/erosion, mechanical failure, nonmechanical failure, and other. Mechanical failure was referred to as fluid loss or defect of any component of the AUS. Nonmechanical failure was defined as persistent or recurrent SUI despite a normally functioning device. Other indications included pump or balloon malposition.
2.4. Statistical analysis
Medians and quartiles were reported for quantitative variables. Proportions were reported for qualitative variables. The Wilcoxon test was used to compare quantitative variables, the Fisher exact test was used to compare dichotomous variables, and the Kruskal-Wallis test was used when there were more than two groups.
Patients were censored at the time of AUS reoperation or at the time of the last follow-up visit in the absence of an event. The Kaplan-Meier method was used to estimate the probability of reoperation-free survival. We plotted the overall reoperation-free survival and the survival for each reoperation type in both groups. We also plotted the reoperation-free survival in patients with a history of radiotherapy.
Multivariable logistic regression was used to identify the predictors of persistent SUI and infection or erosion. A Cox proportional hazard model was used to seek the predictors of reoperation-free survival. An assessment of the collinearities was performed in the initial model, and the factors known from the existing literature were included in the model. The R statistical software [11] was used for all statistical analyses. All tests were two sided with a significance level at p < 0.05.
3. Results
3.1. Patient characteristics
After the exclusion of patients with bladder neck cuff implantation (n = 8) and neurogenic SUI (n = 61), 1233 patients were included in the current analysis, with 903 patients in GROUP75– (73.2%) and 330 in GROUP75+ (26.8%).
The patients’ characteristics are presented in Table 1. Elderly patients were significantly more comorbid, with a higher median Charlson’s index (5 vs 4, p < 0.001), a higher proportion having ASA score ≥3 (45.7% vs 22.1%, p < 0.001), and more patients being under anticoagulant (16.2% vs 8%, p < 0.001) and antiplatelet (29.4% vs 19.8%, p = 0.002) therapy. There were more irradiated patients in GROUP75+ (40.5% vs 31.5%, p = 0.009). The SUI was significantly more severe in the elderly group (median 24-h pad test: 400 vs 300 ml, p = 0.007), and the rate of postprostatectomy SUI was higher in GROUP75– (88.6% vs 77.5%, p < 0.001).
Table 1.
Patients characteristics by group of age
| GROUP75– (n = 903) | GROUP75+ (n = 330) | p value a | |
|---|---|---|---|
| Age, median (Q1, Q3) | 68 (64, 71) | 79 (77, 81) | <0.001 |
| BMI (kg/m2), median (Q1, Q3) | 27.0 (24.6, 29.9) | 26.5 (24.4, 28.7) | 0.055 |
| Charlson index, median (Q1, Q3) | 4 (3, 5) | 5 (4, 6) | <0.001 |
| ASA score, n (%) | <0.001 | ||
| 1 | 39 (11.0) | 6 (4.3) | |
| 2 | 237 (66.9) | 70 (50.0) | |
| 3 | 77 (21.8) | 57 (40.7) | |
| 4 | 1 (0.3) | 6 (4.3) | |
| 5 | 0 (0) | 1 (0.7) | |
| Antiplatelet drug intake, n (%) | 128 (19.8) | 77 (29.4) | 0.002 |
| Anticoagulant drug intake, n (%) | 58 (8.0) | 47 (16.2) | <0.001 |
| History of pelvic radiotherapy, n (%) | 239 (31.5) | 109 (40.5) | 0.009 |
| Previous pelvic surgery, n (%) | 748 (97.5) | 271 (96.8) | 0.66 |
| Previous incontinence surgery, n (%) | 150 (16.6) | 49 (14.8) | 0.51 |
| Previous continence physiotherapy, n (%) | 431 (92.3) | 147 (82.1) | <0.001 |
| Preoperative 24-h pad test (ml), median (Q1, Q3) | 300 (150, 600) | 400 (275, 1000) | 0.007 |
| No. of Preoperative 24-h pads, median (Q1, Q3) | 3 (2, 5) | 3 (2, 5) | 0.99 |
| Incontinence etiologies, n (%) | <0.001 | ||
| Radical prostatectomy | 789 (88.6) | 251 (77.5) | |
| Endoscopic surgery | 56 (6.3) | 44 (13.6) | |
| Pelvic surgery | 28 (3.1) | 14 (4.3) | |
| Radiotherapy | 15 (1.7) | 9 (2.8) | |
| HIFU | 2 (0.2) | 4 (1.2) | |
| Brachytherapy | 1 (0.1) | 2 (0.6) | |
| Preoperative urodynamic data | |||
| Detrusor overactivity, n (%) | 70 (23.9) | 34 (27.4) | 0.52 |
| Bladder capacity (ml), median (Q1, Q3) | 328 (243, 400) | 362 (245, 438) | 0.074 |
| Maximal closure pressure (mmHg), median (Q1, Q3) | 43 (29, 60) | 39 (28, 57) | 0.50 |
ASA = American society of anesthesiologists; BMI = body mass index; GROUP75– = patients <75 yr old; GROUP75+ = patients ≥75 yr old; HIFU = high-intensity focalized ultrasound.
Estimates were given as median (first quartile, third quartile) or frequency (percentage). Bolding was used for p < 0.05.
The p values were calculated using the Wilcoxon test for quantitative variables, the Fisher exact test for dichotomous variables, and the Kruskal-Wallis test when there were more than two groups.
The surgical characteristics are summarized in Table 2. There was no statistically significant difference between both groups in terms of surgical approach, type of pressure regulating balloon, cuff size, or intraoperative complications. There was more transcorporal cuff positions in GROUP75+ (13.1% vs 5.5%, p < 0.001).
Table 2.
Surgical characteristics and perioperative data
| GROUP75– (n = 903) | GROUP75+ (n = 330) | p value a | |
|---|---|---|---|
| Institution caseload per year, median (Q1, Q3) | 9 (6, 14) | 10 [7, 14] | 0.48 |
| Surgical approach, n (%) | |||
| Perineal | 572 (65.7) | 203 (63.4) | 0.52 |
| Penoscrotal | 299 (34.3) | 117 (36.6) | |
| Cuff position, n (%) | <0.001 | ||
| Bulbar | 842 (94.5) | 279 (86.9) | |
| Transcorporal | 49 (5.5) | 42 (13.1) | |
| Pressure regulating balloon size (mmHg), n (%) | 0.92 | ||
| 51–60 | 10 (1.3) | 4 (1.4) | |
| 61–70 | 727 (98.1) | 283 (98.3) | |
| 71–80 | 4 (0.5) | 1 (0.3) | |
| Cuff size (mm), median (Q1, Q3) | 45 (40, 45) | 45 [40, 45] | 0.43 |
| Intraoperative complication, n (%) | 6 (0.8) | 4 (1.4) | 0.60 |
| Early postoperative complication, n (%) | 95 (12.6) | 54 (18.8) | 0.014 |
| Clavien-Dindo classification, n (%) | 0.025 | ||
| Grade I | 45 (47.4) | 23 (45.1) | |
| Grade II | 19 (20.0) | 15 (29.4) | |
| Grade IIIa | 4 (4.2) | 7 (13.7) | |
| Grade IIIb | 27 (28.4) | 6 (11.8) | |
| Grade IV-V | 0 (0) | 0 (0) | |
| Hospital stay (d), median (Q1, Q3) | 3 (2, 4) | 3 [2, 4] | 0.90 |
GROUP75– = patients <75 yr old; GROUP75+ = patients ≥75 yr old.
Estimates were given as median (first quartile, third quartile) or frequency (percentage). Bolding was used for p < 0.05.
The p values were calculated using the Wilcoxon test for quantitative variables, the Fisher exact test for dichotomous variables, and the Kruskal-Wallis test when there were more than two groups.
3.2. Functional and perioperative outcomes
The early postoperative social continence rate was lower in GROUP75+, but this difference was not statistically significant (74.4% vs 80.1%, p = 0.11; Table 3). The rate of 30-d postoperative complications was significantly higher in GROUP75+ (18.8% vs 12.6%, p = 0.014). The repartition of Clavien-Dindo grades was significantly different between groups (p = 0.025) as minor complications were proportionally more frequent in GROUP75+, but the rates of Clavien-Dindo grade III complications were similar in both groups. No Clavien-Dindo grade IV or V complications were reported. Grade III complications included local or abdominal hematomas, wound abscesses, and early explantations for sepsis or device mechanical dysfunction. The median hospital stay was comparable in both groups (3 vs 3 d, p = 0.90).
Table 3.
Efficacy and reoperations
| GROUP75– (n = 903) | GROUP75+ (n = 330) | p value a | |
|---|---|---|---|
| Social continence, n (%) | 446 (80.1) | 148 (74.4) | 0.11 |
| No. of postoperative 24-h pads, median (Q1, Q3) | 0 (0, 1) | 0 (0, 1) | 0.61 |
| Overall reoperation rate, n (%) | 314 (34.8) | 126 (38.2) | 0.30 |
| Revision rate, n (%) | 140 (16.9) | 41 (13.1) | 0.14 |
| Replacement rate, n (%) | 90 (13.3) | 32 (12.4) | 0.79 |
| Explantation rate, n (%) | 183 (22.6) | 97 (31.7) | 0.002 |
| Reoperation indication, n (%) | 0.003 | ||
| Infection and/or erosion | 129 (41.9) | 74 (57.8) | |
| Mechanical failure | 69 (22.4) | 21 (16.4) | |
| Nonmechanical failure | 91 (29.5) | 21 (16.4) | |
| Other | 19 (6.2) | 12 (9.4) | |
AUS = artificial urinary sphincter; GROUP75– = patients <75 yr old; GROUP75+ = patients ≥75 yr old; SUI = stress urinary incontinence.
Revision was defined as any reoperation consisting in replacement or repositioning of one or several components of the device. Mechanical failures were defined as any fluid loss or defect of any components of the AUS. Nonmechanical failures were defined as recurrence or persistence of SUI despite normally functioning devices. The other indications included pump or balloon malposition.
Estimates were given as median (first quartile, third quartile) or frequency (percentage). Bolding was used for p < 0.05.
The p values were calculated using the Wilcoxon test for quantitative variables, the Fisher exact test for dichotomous variables, and the Kruskal-Wallis test when there were more than two groups.
3.3. Reoperation rates and survival
There were similar overall reoperation rates in both groups (38.2% and 34.8%, p = 0.30) as well as revision and replacement rates (13.1% vs 16.9%, p = 0.14 and 12.4% vs 13.3%, p = 0.79, respectively), but the explantation rate was significantly higher in GROUP75+ (31.7% vs 22.6%, p = 0.002). Regarding reoperation indications, there was a larger proportion of infection/erosion in GROUP75+ (57.8% vs 41.9%, p = 0.003), although it was the main indication in both groups.
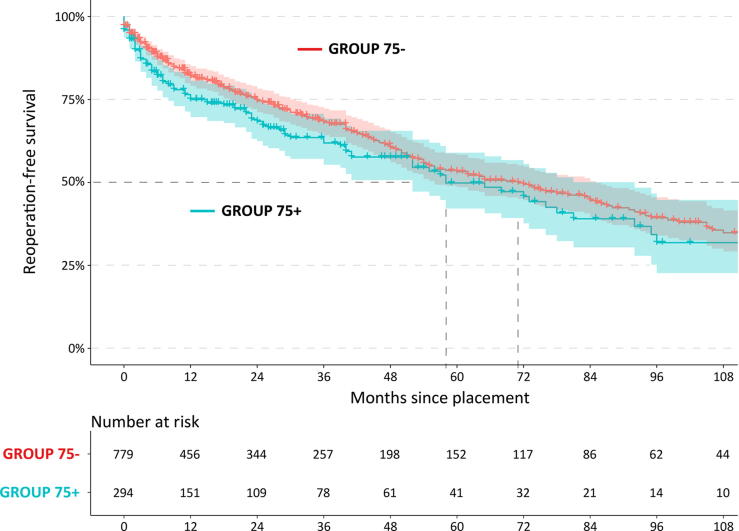
After a median follow-up of 24 and 19 mo in GROUP75– and GROUP75+, respectively, the estimated median overall reoperation-free survival was 5 yr for GROUP75+ and 6 yr for GROUP75–, with no statistically significant difference (p = 0.076; Fig. 1).
Fig. 1.
Kaplan-Meier curve for reoperation-free survival after AUS placement in patients over 75 yr of age (GROUP75+; blue line) versus patients under 75 yr of age (GROUP75–, red line), with 95% confidence interval illustrated with corresponding shaded area (p = 0.076). AUS = artificial urinary sphincter; GROUP75– = patients <75 yr old; GROUP75+ = patients ≥75 yr old.
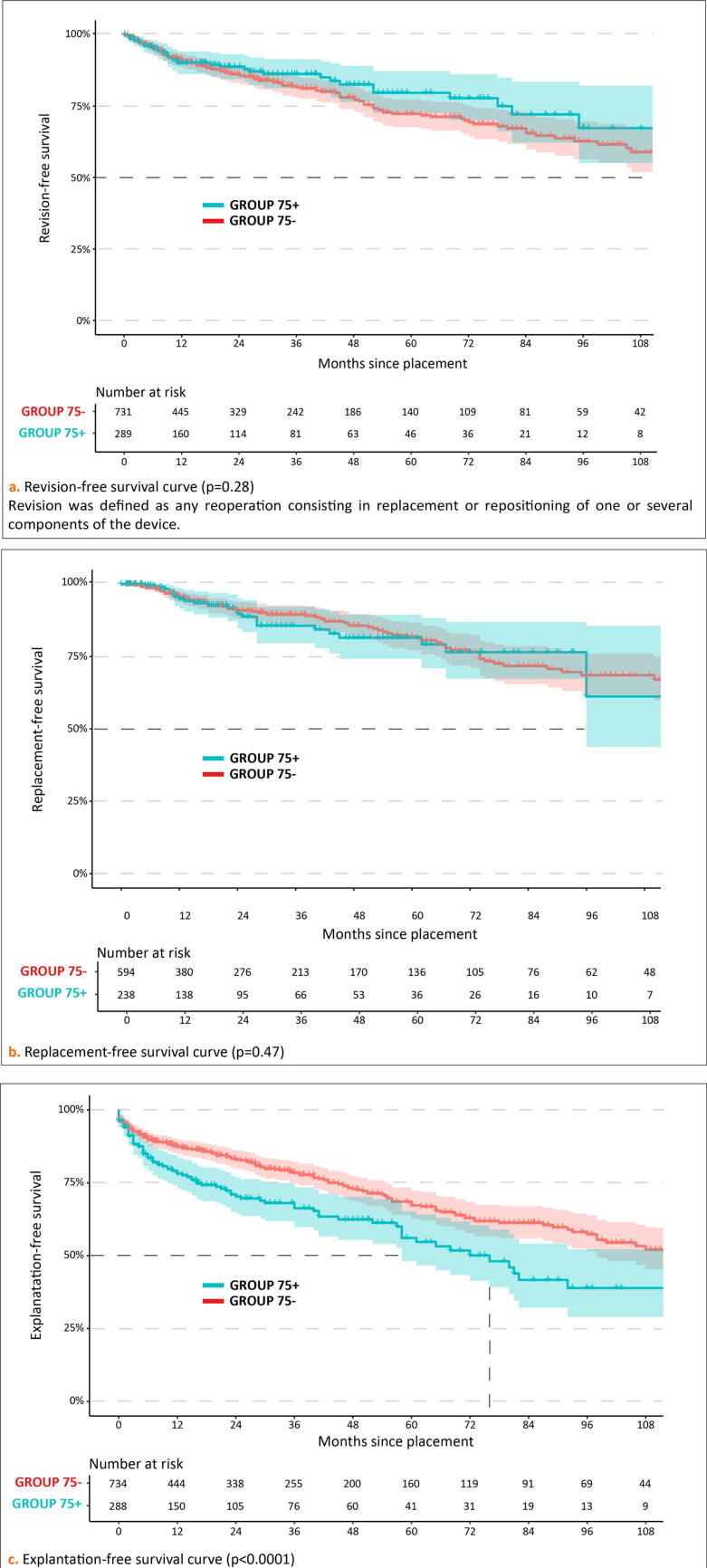
However, there was a tendency toward a higher occurrence of early (<1 yr) reoperations in GROUP75+. Revision-free survival and replacement-free survival were comparable in both groups (Fig. 2). However, explantation-free survival was significantly poorer in GROUP75+ (p < 0.0001).
Fig. 2.
Kaplan-Meier curves for each type of reoperation after AUS placement in patients over 75 yr (GROUP75+, blue line) versus patients under 75 yr (GROUP75–, red line), with 95% confidence interval illustrated with corresponding shaded area. (A) Revision-free survival curve (p = 0.28). Revision was defined as any reoperation consisting in replacement or repositioning of one or several components of the device. (B) Replacement-free survival curve (p = 0.47). (C) Explantation-free survival curve (p < 0.0001). AUS = artificial urinary sphincter; GROUP75– = patients <75 yr old; GROUP75+ = patients ≥75 yr old.
3.4. Predictors of persistent SUI, reoperation-free survival, and infection/erosion
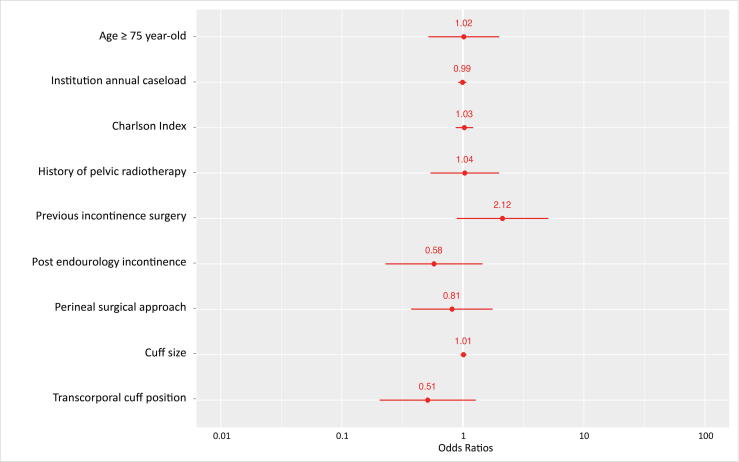
In a multivariate analysis, age ≥75 yr was not associated with postoperative SUI (odds ratio [OR] = 1.02 [0.53–2.03], p = 0.96; Fig. 3). No studied parameter was significantly associated with persistent SUI.
Fig. 3.
Logistic regression analysis of the association between social continence after AUS placement in male patients with non-neurogenic stress urinary incontinence and possible risk factors. Age ≥75 yr is not statistically associated with persistent stress urinary incontinence (OR = 1.02 [0.53–2.03], p = 0.96) as any of the other studied factors. AUS = artificial urinary sphincter; OR = odds ratio.
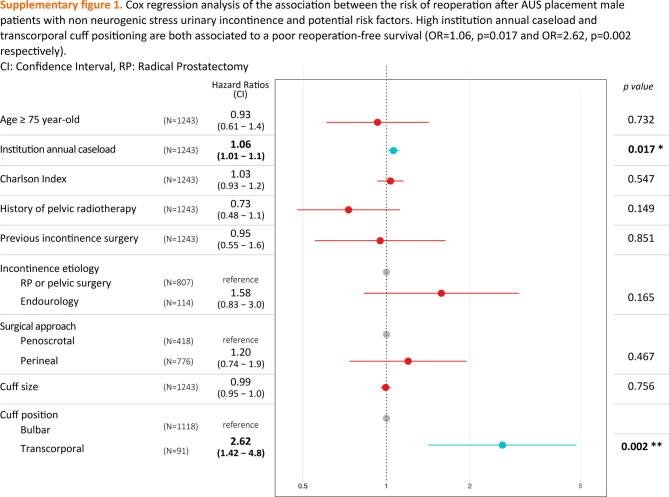
In a Cox multivariate analysis, age ≥75 yr was not associated with reoperation-free survival (hazard ratio [HR] = 0.93 [0.61–1.40], p = 0.73; Supplementary Fig. 1). The only predictors associated with reoperation-free survival were institution annual caseload and transcorporal cuff position (HR = 1.06 [1.01–1.10], p = 0.017 and HR = 2.62 [1.42–4.80], p = 0.002, respectively).
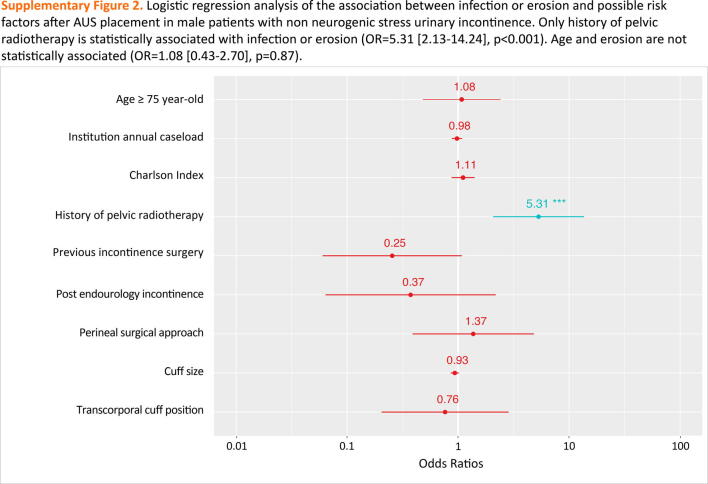
Age ≥75 yr was not associated with the onset of erosion in the multivariate analysis (OR = 1.08, [0.43–2.70], p = 0.87). Only a history of pelvic radiotherapy was a predictor of device infection or erosion (OR = 5.31 [2.13–14.24], p < 0.001; Supplementary Fig. 2).
3.5. Efficacy, security, and reoperation-free survival in irradiated patients
Irradiated patients had a similar early complication rate (15.3% vs 14.4%, p = 0.8) and early social continence rate (79.1% vs 78.8%, p = 1.0). However, they had a higher explantation rate (31.3% vs 20.3%, p < 0.001). Nevertheless, patients with a history of pelvic radiotherapy showed a similar overall reoperation-free survival curve to unirradiated patients (p = 0.35).
3.6. Outcomes in patients aged ≥80 yr at AUS implantation
In GROUP75+, 36% of patients (n = 120) were octogenarians at surgery. Of them, 47.4% had a history of radiotherapy (n = 45); 74.7% reached early social continence (n = 59). The early complication rate was 19.0% (n = 20). The reoperation and explantation rates were, respectively, 36.7% (n = 44) and 31.0% (n = 35) after a median follow-up of 15 mo.
4. Discussion
In the present series, which is, to the best of our knowledge, the first multicenter series to date focused on AUS in elderly men, we found equivalent early postoperative continence in patients ≥75 yr old with few early complications.
In both groups, fewer than one in five patients had early postoperative complications. Although the rate was slightly higher in GROUP75+, it had no impact on the length of hospital stay, and the vast majority of complications were of Clavien-Dindo grade <III. These findings are consistent with the study of Medendorp et al [10], which described more minor complications in elderly patients.
Elderly patients presented a tendency to undergo early reoperations compared with the younger group. However, this association was not statistically significant in the multivariate analysis, suggesting that it may partly be related to cofounders such as the higher rate of transcorporal cuff in the elderly group. The higher explantation rate in this group was largely driven by the higher rate of infection/erosion, as the latter takes a larger part in reoperation etiologies in GROUP75+. This association was previously described by Medendorp et al [10], who found increasing odds of having AUS removal according to age (OR = 2.9 [1.6–5.4], p < 0.01 for age 75–84 yr and OR = 9.8 [4.3–22.2], p < 0.01 for age ≥85 yr). Several assumptions could be made to explain this finding. One may hypothesize that elderly patients have poorer blood supply to the urethral wall, which may favor erosions. In addition, impairment of the immune system with age may be a cause of the higher infection rates [12]. Although we found more frequent explantations in the elderly population, one in two patients will benefit from a normally functioning device for >5 yr, which should be put in perspective with their life expectancy at this age. Thus, the possibly shorter device survival in these patients should be interpreted cautiously and may not prevent them from receiving this effective therapy.
The transcorporal cuff position that is commonly used in patients with a fragile urethra was associated with poorer reoperation-free survival. However, one may argue that it could be a confusion bias as our multivariate model did not include other factors of fragile urethra than radiotherapy (eg, a history of previous erosion and a history of urethroplasty). Literature is highly controversial on this subject, but the largest published series [13] found that transcorporal cuff was neither a risk nor a protective factor of erosion, once adjusted for all known factors of fragile urethra.
Unfortunately, our study did not allow the specific risk factors of AUS failures to be determined in elderly male patients. Indeed, patients of GROUP75+ in the present series are not representative of the elderly male population as they all have been deemed fit enough to undergo AUS implantation by their urologist. Moreover, no validated geriatric tools were used to assess patients’ individual health statuses preoperatively. Thus, in our series, octogenarians showed similar results to the rest of GROUP75+ in terms of efficacy, security, and reoperation-free survival in spite of their higher rate of radiation exposure.
To ensure reliable patient selection, it could be interesting for elderly men with SUI to undergo a systematic specialized geriatric evaluation before AUS implantation. Indeed, during such evaluations, gerontologists will be able to assess patient life expectancy, cognitive status, and fine motricity skills. They will collect signs of evolutive conditions that could threaten AUS usage over time, as well as the presence of any other known risk factors that could lead to AUS failure, due to either erosion or the inability of these patients to use the device properly with more accuracy than any urologist or anesthesiologist evaluation alone.
In recent years, systematic specialized geriatric evaluations have become more integrated in the preoperative workup of various pathologies. Indeed, they proved their efficacy to assess postoperative risk prediction before undergoing transcatheter aortic valve implantation [14], [15]. They are also recommended for all men aged 75 yr and older with prostate cancer before treatment decision-making [16], on the basis that the bladder should be managed according to their individual health status and not according to chronological age. Indeed, gerontologists could also identify the presence of a frailty syndrome that could lead to a contraindication for surgery, as it has been associated with poorer postoperative prognosis [17]. Additionally, they could set actions up—such as rehabilitation or nutritional support—in order to improve their postoperative prognosis. A prospective study is needed to prove the potential benefits of a systematic specialized geriatric evaluation before implanting AUS in elderly men.
On another note, a serious limitation of the present series was the relatively short follow-up. This prevented the assessment of a proportion of elderly patients who had to stop using their AUS in the long term because they developed cognitive dysfunction, impaired manual dexterity, visual problems, or other conditions with an occurrence that increases with age, which can prevent them from using AUS properly. This might be another possible benefit of a specialized geriatric evaluation preoperatively: screening for possible causes of difficulties with handling the device over time following implantation and trying to evaluate the inherent individual prognosis of the AUS in each elderly man.
Our study had several other limitations that should be acknowledged. First, it has all the biases inherent to its retrospective nature with no standardized surgical techniques or follow-up protocols across centers. Owing to the multicenter study design, many surgeons were involved, which is a source of heterogeneity, for instance, in terms of treatment decision-making. Continence status was measured only by plain questions during clinical interviews, which are not as accurate as validated questionnaires. One of the main limitations of this study was the lack of data on patient comorbidities in order to refine the multivariate model. Indeed, several studies described other risk factors of AUS failure such as type 2 diabetes [18], cognitive dysfunction, impaired dexterity [9], low testosterone levels [19], and fragility [10], which are frequent in elderly patients and may lead to a confusion bias in our model. Unfortunately, these patient characteristics were not reported in our dataset. In addition, we did not report whether patients were included for a primary or secondary implantation because information was missing in some centers. However, when available, the rate of secondary implantation was <2%. Finally, the lack of validated geriatric frailty scores prevented the investigation of specific risk factors for failure in the elderly population.
5. Conclusions
AUS implantation in male patients aged ≥75 yr appears to be an effective option for the treatment of non-neurogenic SUI.
However, we observed higher rates of postoperative complications and early explantations among elderly patients, probably driven by the increased proportion of device infection or erosion.
These findings may help with preoperative patient counseling and stress the importance of careful patient selection, which could be improved through a systematic specialized preoperative geriatric evaluation with validated tools.
Author contributions: Camille Girard had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Girard, Bentellis, El-Akri, Peyronnet.
Acquisition of data: El-Akri, Durand, Cornu, Brierre, Cousin, Gaillard, Dupuis, Tricard, N. Hermieu, Leon, Chevallier, Bruyere, Biardeau, J.-F. Hermieu, Lecoanet, Capon, Game, Saussine, Peyronnet, Bentellis.
Analysis and interpretation of data: Girard, Bentellis, Durand, Peyronnet.
Drafting of the manuscript: Girard.
Critical revision of the manuscript for important intellectual content: Durand, Peyronnet, Guérin, Rambaud, Tricard.
Statistical analysis: Bentellis.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bentellis, Peyronnet.
Other: None.
Financial disclosures: Camille Girard certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: D. Chevallier: expert consultant for Boston Scientific. B. Peyronnet: consultant for Boston Scientific. X. Biardeau: speaker for Boston Scientific.
Funding/Support and role of the sponsor: None.
Associate Editor: Véronique Phé
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.03.014.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- 1.Scott F.B., Bradley W.E., Timm G.W. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology. 1973;1:252–259. doi: 10.1016/0090-4295(73)90749-8. [DOI] [PubMed] [Google Scholar]
- 2.Sandhu J.S., Breyer B., Comiter C., et al. Incontinence after prostate treatment: AUA/SUFU guidelines. J Urol. 2019;202:369–378. doi: 10.1097/JU.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 3.Dupuis H.G.A., Bentellis I., El-Akri M., et al. Early efficacy and safety outcomes of artificial urinary sphincter for stress urinary incontinence following radical prostatectomy or benign prostatic obstruction surgery: results of a large multicentric study. Eur Urol Focus. 2022;8:1053–1059. doi: 10.1016/j.euf.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Yafi F.A., DeLay K.J., Stewart C., Chiang J., Sangkum P., Hellstrom W.J.G. Device survival after primary implantation of an artificial urinary sphincter for male stress urinary incontinence. J Urol. 2017;197:759–765. doi: 10.1016/j.juro.2016.08.107. [DOI] [PubMed] [Google Scholar]
- 5.Moon S., Chung H.S., Kim Y.J., et al. The impact of urinary incontinence on falls: a systematic review and meta-analysis. PLoS One. 2021;16:e0251711. doi: 10.1371/journal.pone.0251711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S, Lin D, Hu T, et al. Association of urinary incontinence and depression or anxiety: a meta-analysis. J Int Med Res 2020;48:300060520931348. [DOI] [PMC free article] [PubMed]
- 7.Heller B.R., Whitehead W.E., Johnson L.D. Incontinence. J Gerontol Nurs. 1989;15:16–23. doi: 10.3928/0098-9134-19890501-05. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor R.C., Nanigian D.K., Patel B.N., Guralnick M.L., Ellision L.M., Stone A.R. Artificial urinary sphincter placement in elderly men. Urology. 2007;69:126–128. doi: 10.1016/j.urology.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Raup V.T., Eswara J.R., Marshall S.D., Vetter J., Brandes S.B. Artificial urinary sphincters for treatment of urinary incontinence in elderly males. Urol Int. 2016;97:200–204. doi: 10.1159/000445254. [DOI] [PubMed] [Google Scholar]
- 10.Medendorp A.R., Anger J.T., Jin C., et al. The impact of frailty on artificial urinary sphincter placement and removal procedures. Urology. 2019;129:210–216. doi: 10.1016/j.urology.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R Core Team. The R project for statistical computing. https://www.r-project.org/.
- 12.Sadighi Akha A.A. Aging and the immune system: an overview. J Immunol Methods. 2018;463:21–26. doi: 10.1016/j.jim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 13.El-Akri M., Bentellis I., Tricard T., et al. Transcorporal vs. bulbar artificial urinary sphincter implantation in male patients with fragile urethra. World J Urol. 2021;39:4449–4457. doi: 10.1007/s00345-021-03783-6. [DOI] [PubMed] [Google Scholar]
- 14.Stortecky S., Schoenenberger A.W., Moser A., et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489–496. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Massussi M., Adamo M., Rosato S., et al. Functional and metabolic frailty predicts mortality in patients undergoing TAVI: insights from the OBSERVANT II study. Eur J Intern Med. 2022;106:90–96. doi: 10.1016/j.ejim.2022.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Boyle H.J., Alibhai S., Decoster L., et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019;116:116–136. doi: 10.1016/j.ejca.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 18.Kaiho Y., Masuda H., Takei M., et al. Surgical and patient reported outcomes of artificial urinary sphincter implantation: a multicenter, prospective, observational study. J Urol. 2018;199:245–250. doi: 10.1016/j.juro.2017.08.077. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe A.R., Ortiz N.M., Baumgarten A.S., et al. Most men with artificial urinary sphincter cuff erosion have low serum testosterone levels. Neurourol Urodyn. 2021;40:1035–1041. doi: 10.1002/nau.24663. [DOI] [PubMed] [Google Scholar]