Graphical abstract

Keywords: Loquat, Cell wall, Homogalacturonan, Demethylesterification, Egg-box, Texture
Highlights
-
•
Pectin metabolism patterns in lignified loquat fruit were different from other fruits.
-
•
Low-methylesterified HG accumulated in cell walls of loquat fruit during cold storage.
-
•
Low-methylesterified HG formed egg-box structure with Ca2+ in lignified loquat fruit.
-
•
LTC treatment inhibited the increase of low-methylesterified HG and egg-box structure.
-
•
Accumulation of pectin-involved egg-box structure was related to firmness increase.
Abstract
Introduction
Postharvest textural changes in fruit are mainly divided into softening and lignification. Loquat fruit could have severe lignification with increased firmness during postharvest storage. Pectin is mainly associated with the postharvest softening of fruit, but some studies also found that pectin could be involved in strengthening the mechanical properties of the plant.
Objectives
This study focused on characterizing the dynamics of pectin and its complexation in the cell wall of lignified loquat fruit during postharvest storage, and how these changes could influence fruit firmness.
Methods
The homogalacturonan (HG) pectin in the cell wall of loquat fruit was identified using monoclonal antibodies. An oligogalacturonide (OG) probe was used to label the egg-box structure formed by Ca2+ cross-linking with low-methylesterified HG. An exogenous injection was used to verify the role of egg-box structures in the firmness increase in loquat fruit.
Results
The JIM5 antibody revealed that low-methylesterified HG accumulated in the tricellular junctions and middle lamella of loquat fruit that had severe lignification symptoms. The pectin methylesterase (PME) activity increased during the early stages of storage at 0 °C, and the calcium-pectate content and flesh firmness constantly increased during storage. The OG probe demonstrated the accumulation of egg-box structures at the cellular level. The exogenous injection of PME and Ca2+ into the loquat flesh led to an increase in firmness with more low-methylesterified HG and egg-box structure signals.
Conclusion
PME-mediated demethylesterification generated large amounts of low-methylesterified HG in the cell wall. This low-methylesterified HG further cross-linked with Ca2+ to form egg-box structures. The pectin-involved complexations then contributed to the increased firmness in loquat fruit. Overall, besides being involved in fruit softening, pectin could also be involved in strengthening the mechanical properties of postharvest fruit. This study provides new ideas for obtaining a better texture of postharvest loquat fruits based on pectin regulation.
Introduction
Texture is an important quality index of fruit that determines the sensation experienced when eating it [1]. Fruit texture is often described as hard or soft, crisp or powdery, smooth or rough, which directly affects the preference and enjoyment of consumers [2]. Meanwhile, fruit texture is also an important factor to evaluate fruit maturity, choose appropriate packaging, and predict storage life [3], [4]. Therefore, dissecting the mechanisms underlying the changes in fruit texture after harvest is a core problem in the fruit industry [5].
Softening and lignification (hardening) are two typical forms of changes in fruit texture during postharvest storage [4], [6]. Fruit softening usually occurs in many fruits during postharvest storage, such as tomato [7], peach [8], and strawberry [9]. On the other hand, some fruits can experience lignification after harvest, including mangosteen [10], kiwifruit [11], and loquat [12].
Loquat (Eriobotrya japonica Lindl.) is a subtropical fruit originating from southeastern China. Based on the flesh color, loquat fruit can be categorized into two types: red-fleshed and white-fleshed cultivars [13]. Loquat fruit ripens at a high temperature in the rainy season, and the quality of loquat fruit deteriorates rapidly after harvest [14]. When using low temperature to delay fruit senescence and prolong the postharvest storage time, red-fleshed loquat fruit suffers severe chilling-induced lignification [15]. The lignification mainly manifests itself as a rapid deterioration in texture, including an increase in fruit firmness and lignin content, decrease in juice yield and taste, and difficulty in peeling [15]. The phenomenon of postharvest lignification significantly reduces the quality of loquat fruit [16]. Some postharvest treatments such as low-temperature conditioning (LTC) have been developed to alleviate the postharvest lignification of loquat fruit [17].
The texture of fruit mainly depends on the ability of the cell wall to maintain the turgor status and mediate cellular adhesion [18]. Cell walls contain pectin, hemicellulose, cellulose, lignin, structural proteins, and other components. The function of the cell wall is underpinned by the heterogeneities and dynamics of its components and architecture [19]. Fruit hardening during postharvest lignification is traditionally considered to be associated with the deposition of lignin in secondary cell walls [16], [20]. Particularly for loquat fruit, the cell wall mechanisms behind the increase in firmness during postharvest lignification have been investigated. Most of these investigations have centered on the lignin components of the cell wall and their associated changes at the physiological level, such as the lignin content and activity of related enzymes [14], [21]. Recently, genes and transcriptional networks controlling lignin biosynthesis genes in loquat fruit during postharvest storage have gradually been unraveled [13], [16], [22].
Unlike the deposition of lignin, which is responsible for the increase in fruit firmness, the degradation of pectin mainly plays an important role in fruit softening, and vice versa, treatments to enhance pectin in some fruits can extend the postharvest quality [23], [24]. Pectin is essentially a linear polysaccharide in which a few hundred to a thousand d-galacturonic acid (GalA) residues are linked via α-1,4-glycosidic bonds forming a backbone [25]. The backbone of pectin can be divided into two regions: ‘smooth’ regions composed of unbranched homogalacturonans (HGs), and ‘hairy’ regions that mainly contain highly branched rhamnogalacturonan I (RG-I) and RG-II domains as well as apiogalacturonan and xylogalacturonan in some species [26]. HG can be methylesterified at C-6 and demethylesterified by the action of pectin methylesterases (PMEs) in the wall [18]. PME can act in both random and blockwise patterns [27]. In the random pattern of demethylesterification, HG will become susceptible to the activation of polygalacturonases (PGs) and pectate lyases (PLs) for degradation. Meanwhile, in the blockwise pattern of demethylesterification, HG can further cross-link with Ca2+ to form a pectate gel. Pectin is mainly distributed in primary cell walls and cell wall lamella [25]. During the postharvest period, there is a series of modifications to the pectin components, along with degradation. These changes in pectin result in a loosening and weakening of the cell wall structure, which is a major contributor to fruit softening [6].
However, in recent years, numerous studies have found that pectin in the primary cell wall, especially the methylesterification state of HG, plays an important role in determining the mechanical properties of plant cell walls and tissues. Research published by Hongo, Sato, Yokoyama, and Nishitani [28] found that Arabidopsis PME35 mutants showed a pendant stem phenotype. Then, they demonstrated that PME35-mediated demethylesterification of HG in the primary cell wall directly regulates the mechanical strength of the Arabidopsis stem [28]. Peaucelle, Wightman, and Hofte [29] found that growth symmetry breaking in the Arabidopsis hypocotyl depends on asymmetry in cell wall mechanics, which is triggered by bipolar HG demethylesterification. Similarly, the mechanical heterogeneity of the cell wall in tomato and Arabidopsis leaves was found to be related to the methylesterification of HG [30]. In the guard cells, the methylesterified HG level and the demethylesterified HG level have been found to be important in the mechanical properties and dynamics of guard cell walls, which are required for stomatal function and plant response to external stimuli [31], [32]. However, it is unclear that the changes in the methylesterification state of HG during postharvest lignification of loquat fruit and whether it is associated with the increase in fruit firmness.
Previous works found that the changes in the pectin content of loquat fruit were the opposite to the typical depolymerization and solubilization during the softening of other fruit species. Goulas, Minas, Kourdoulas, Vicente and Manganaris [33] found that low-temperature storage inhibits normal pectin disassembly in 'Karantoki' loquats. In another study, the protopectin content in loquat fruit was found to increase gradually with postharvest storage at 1 °C, while the water-soluble pectin (WSP) content increased over the 14 d and then decreased [34]. It was also found that the PG/PME ratio of loquat fruit was relatively low, thus affecting the degradation of pectin [35]. However, the above studies were mainly based on chemical extraction of pectin fractions from homogenized bulk tissues and ignored the methylesterification state of HG and its distribution in the cell wall. Therefore, in order to obtain a comprehensive understanding of the texture variation in loquat fruit during its postharvest lignification process, it is necessary to access the spatiotemporal modifications to the cell wall components and structure in situ.
The aim of the present study was to characterize the dynamics of HG and the calcium-mediated HG complexation in loquat fruit during postharvest lignification and study how these changes could influence the firmness of loquat fruit. The specific objectives were to: (1) analyze the patterns of change in the structure, content, and spatial distribution of HG in loquat fruit during postharvest lignification; (2) measure the PME activity to determine the demethylesterification process of HG by PME; (3) measure the calcium-pectate content to determine whether the resulting low-methylesterified HG continued to cross-link with Ca2+ to form egg-box structures; (4) analyze the distribution of egg-box structures in the cell wall of loquat fruit; and (5) exogenously modulate the PME activity and Ca2+ content of loquat fruit to verify the contribution of the egg-box structures to firmness.
Materials and methods
Plant materials and treatment
Loquat fruits (Eriobotrya japonica Lindl. cv. Luoyangqing, ‘LYQ’) were picked from an orchard located in Taizhou, Zhejiang, China. After harvest, the loquat fruits were packed and transported to the laboratory within the same day. Fruits with a uniform size and color and without mechanical wounding or disease were selected and divided into two groups for storage at 0 °C or LTC. Loquat fruits in the LTC group were stored at 5 °C for 6 d, after which the storage temperature was lowered to 0 °C. Loquat fruits were sampled at 0 d, 3 d, 6 d, 18 d, and 30 d during postharvest storage. At each sampling point, fifteen peeled and pitted fruits were randomly divided into three replicates and cut into small pieces. Then, all the pieces were frozen in liquid nitrogen, ground into powder, and stored at −80 °C for further use.
Fruit firmness
The firmness of loquat fruit was measured at each sampling point by a TA-XT2i (Stable Micro Systems, England) texture analyzer. Fifteen individual fruits were randomly divided into three groups, and each fruit was measured twice on opposite sides after removing a small piece of peel. Each measurement was conducted using a probe with a 5 mm diameter to penetrate the loquat flesh at a depth of 4 mm in 4 s. The average of the two measurements represents the firmness of each fruit.
Lignin staining of loquat flesh
To acquire images of the lignin distribution in the loquat flesh, the Wiesner reaction method was used. On each sampling day, five fruits were cut in half at the equatorial plane. Then, 1 % phloroglucinol ethanol solution and concentrated HCl were successively dropped onto the surface of the equatorial-plane flesh at an interval of 30 s. Finally, the images of each equatorial plane after staining were collected using a binocular stereomicroscope and photographed (Carl Zeiss, Oberkochen, Germany).
The staining area ratios in the equatorial plane were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). First, partial images of the equatorial plane of each loquat were placed together to produce a complete panoramic image. Then, the staining images of the entire equatorial plane were converted to grayscale. After inverting black and white values, all the staining areas (S) in the images were selected from the grayscale images by manually adjusting thresholds and calculated using the software. On the other hand, the total areas of the equatorial plane (E) and core (C) were selected using the wand tool and calculated. Finally, the staining area ratio and the density of lignified cells in the equatorial plane were calculated using Function (1).
| (1) |
Chemical analysis of cell wall components
The cell wall material (CWM) of loquat flesh was obtained according to the method proposed by Renard [36]. The cell wall components were determined according to the method described by Figueroa, Opazo, Vera, Arriagada, Díaz, and Moya-León [37] and Vicente, Ortugno, Rosli, Powell, Greve, and Labavitch [38], with some modifications. The CWM was sequentially homogenized and extracted with deionized water, 50 mM sodium acetate buffer (pH 6.5) containing 50 mM cyclohexane trans-l,2-diamine tetra-acetate (CDTA), and 50 mM sodium carbonate (Na2CO3) containing 2 mM CDTA for 6 h. After each extraction, the supernatants were collected after centrifugation at 10,000g for 15 min and designated as water-soluble pectin (WSP), chelator-soluble pectin (CSP), and diluted alkali-soluble pectin (DASP). The content of GalA in the pectin fractions was determined by the carbazole method [39]. The colorimetric determination of GalA at 525 nm was conducted with a BioTek synergy4 multimode microplate reader (BioTek, USA). The results were expressed as the percentage of fresh weight (FW).
The lignin content was measured according to the methods described in our previous work [12]. Absorbance of the sample at 280 nm was measured using a Thermo Scientific Microplate Reader. The concentration of lignin was converted through standard curves measured in lignin (MWL, Arundo donax). The results were expressed as the percentage of FW.
Paraffin sections of loquat fruit
Six loquat fruits were taken on each sampling day and randomly divided into three groups composed of two fruits. About 1 cm3 of flesh from each loquat fruit was separated and marinated in formaldehyde–acetic acid–ethanol fixative (formalin: glacial acetic acid: 70 % ethanol = 5: 5: 90) for 48 h. Then, the fruit samples were dehydrated with gradients of ethanol (75 %, 85 %, 90 %, 95 %, 100 %) for 1 h in each gradient. Next, the fruit samples were placed in a gradient solution of xylene prepared in ethanol (30 %, 50 %, 75 %, 100 %) for 15 min at each concentration, and then the fruit samples were permeated with pure wax overnight. Finally, the fruit samples were placed in an embedding machine and cooled at −20 °C for solidification. The embedded flesh was sectioned at 6 μm thickness using an ultramicrotome.
Immunohistochemistry and confocal laser scanning microscopy
Paraffin sections of loquat fruit were deparaffinized in xylene for 10 min twice and hydrated in a graded series of ethanol (100, 95, 80, 70, 50, 30 %) for 2 min in each solution in the series. The sections were placed in EDTA antigen retrieval solution (pH = 9), boiled in an induction oven at high heat, and then heated at low heat for 20 min. When the temperature of the sections was restored to room temperature, sections of loquat fruit were then blocked with 5 % bovine serum albumin (BSA) in PBS buffer for at least 30 min at room temperature.
Primary antibodies JIM5 and JIM7 (Plantprobes, Leeds University, UK) were used in this experiment. Sections were incubated in primary antibody diluted (1:50) in 1 % BSA in PBS overnight at 4 °C. They were then washed with three changes of PBS for at least 5 min for each change before incubating with anti-rat secondary antibody conjugated with AlexaFluor 488 (diluted 1:200 in 1 % BSA in PBS) at room temperature for 2 h. Finally, the incubated sections were washed with three changes of PBS for at least 5 min for each change and enclosed in Dako Fluorescent Mounting Medium. Control reactions were carried out by omitting the primary antibody. All immunofluorescence images were acquired through laser scanning confocal microscopy (CLSM) (A1R; Nikon, Japan) with constant parameters (i.e., laser intensity, gain).
Measurement of PME activity and calcium-pectate content
For the measurement of PME activity, the method of Lin, Lin, Lin, Lin, Li, Yuan, Chen, and Xiao [40] was used, with some modifications. First, 0.5 g frozen flesh powder was mixed with 1.5 ml 40 mmol/L sodium acetate buffer (100 mmol/L NaCl, 5 % PVP, and 2 % mercaptoethanol, pH 5.2) and then centrifuged (12,000g, 20 min) at 4 °C to collect the supernatant as a crude enzyme for the measurement of PME activity. The reaction mixture contained the crude enzyme, 0.2 M sodium oxalate, and 1 % (w/v) pectin with a volume ratio of 3:5:10. Then, the reaction mixture was incubated in a constant-temperature incubator at 37 °C for 60 min, and its pH was adjusted to 7.8 using 0.1 M NaOH solution every 20 min. The total NaOH consumption was recorded, and one unit of PME activity was expressed as 1 μmol NaOH solution per min per g FW.
For the measurement of the calcium-pectate content, the method of Ohta, Yamamoto, and Deguchi [41] was used, with some modifications. Briefly, 3 g of frozen fruit flesh was homogenized and immersed in 10 ml deionized water for 1 h. Then, the homogenate was centrifuged at 4000g for 10 min to remove the supernatants. After repeated washing in deionized water three times, the residues were then extracted with NaCl (1 M, 10 ml) for 1 h, three times. A total of 30 ml of supernatant was collected, and the NaCl-Ca content was measured using atomic absorption spectrophotometers (Agilent Technologies, California, USA).
OG probe labeling
The oligogalacturonide (OG) probes were provided by Professor Jozef Mravec, University of Copenhagen. The synthesis of OG probes was prepared using the methods mentioned in Pontiggia, Ciarcianelli, Salvi, Cervone, De Lorenzo, and Mattei [42] and Mravec, Kračun, Rydahl, Westereng, Miart, Clausen, Fangel, Daugaard, Van Cutsem, De Fine Licht, Höfte, Malinovsky, Domozych, and Willats [43]. The use of OG probes was as described in Mravec, Kracun, Rydahl, Westereng, Pontiggia, De Lorenzo, Domozych, and Willats [44] with some modifications. Two consecutive paraffin sections of loquat fruit were used, one of which was directly labeled by the OG probes (OG labeling), while the other was labeled by the OG probes after pre-treatment with CDTA (CDTA + OG labeling). For OG labeling, the procedure was as follows: (1) The deparaffinization and antigen activation of paraffin sections of loquat fruit were carried out according to the method mentioned above. (2) The sections were washed 3 times with 50 mM 2-(N-morpholine)-ethanesulphonic acid (MES) buffer solution, 3 min each time. (3) The OG probes were diluted in a ratio of 1:500 in 50 mM MES buffer solution (pH = 5.7) containing 2 mM CaCl2, and then the sections were incubated in the OG probe dilution solution at 4 °C overnight. (4) The sections were washed 3 times with 50 mM MES buffer solution containing 2 mM CaCl2, 3 min each time. (5) The sections were covered with 50 mM MES buffer containing 10 % glycerol. For CDTA + OG labeling, sections were incubated with 50 mM CDTA solution overnight after step (1), in order to remove endogenous Ca2+ and destroy endogenous egg boxes. All subsequent steps were as described for OG labeling.
Exogenous injection of PME and Ca2+ solutions
The introduction of solutions into loquat fruit was carried out according to the injection method mentioned in Zhao, Dong, Zhu, Allan, Lin-Wang, and Xu [45]. The injected solution consisted of 15 U mL−1 PME (Sigma-Aldrich; P5400) and 1 % CaCl2. Considering the pressure inside the fruit, and in order to reduce the damage caused by the injection and the necessary depth required to measure fruit firmness, the volume of the injection was finally set to 30 μL per injection. Twelve individual freshly harvested loquat fruits were separately injected with 30 μL of 1 % (w/w) CaCl2 (Ca2+ group), 15 U mL−1 PME (PME group), 15 U mL−1 PME and 1 % (w/w) CaCl2 (PME + Ca2+ group), or double-distilled water (H2O group) at different locations on the same hemispheric surface of the fruit using a syringe. After the injection, the fruits were placed at room temperature for 1.5 h, and then fruit firmness at each location was measured in a completely random order. After measuring firmness, the flesh at the injection site was collected for subsequent staining with JIM5 and OG probe labeling.
Statistical analysis
Experiments were conducted using a completely randomized design, all with at least three replications, and data are presented as the mean ± standard error (mean ± SE). Statistical analyses were performed using SPSS 19.0. One-way ANOVA (Duncan's multiple difference test) was used to calculate the significance of differences within groups. An independent samples t-test was used to calculate the significance of differences between groups. A paired samples t-test was used to calculate the significance of differences in the OG probe staining, and the results of fruit firmness and JIM5 antibody and OG probe staining in the injection experiments. The results were analyzed at significance levels of P < 0.05 and P < 0.01.
Results
Postharvest pectin content
To determine the changes in the texture and degree of lignification of loquat fruit during postharvest storage, we measured the firmness, lignin staining images and staining area ratio of loquat flesh in the equatorial plane, and the lignin content at each sampling point.
The firmness of the loquat fruit increased continuously during storage for 30 d in both the 0 °C and LTC groups (Fig. 1A). Specifically, the loquat fruit had a firmness of 4.06 N at 0 d, and 4.8 N and 4.6 N at 30 d, in the 0 °C group and LTC group, respectively. The LTC treatment retarded the increase in the firmness of the loquat fruit during storage. The firmness of the loquat fruit in the 0 °C group increased rapidly over the first 6 d and stabilized in the later storage period.
Fig. 1.
Characteristics of physiological and biochemical changes in loquat fruit during postharvest storage: (A) firmness; (B) lignin staining images in the equatorial plane; (C) lignin staining area ratio in Fig. 1B; (D) lignin content; (E) diluted alkali-soluble pectin (DASP) content; (F) water-soluble pectin (WSP) content; (G) chelate-soluble pectin (CSP) content. Data are presented as the mean ± standard error from three independent biological replicates. Significant differences are indicated with asterisks above the bars (*, P < 0.05; **, P < 0.01). FW: fresh weight.
When the lignin staining images of loquat flesh in the equatorial plane were examined (Fig. 1B), it was found that lignin staining areas were randomly distributed in the loquat flesh and constantly increased in both the 0 °C and LTC groups during postharvest storage. The lignin staining area ratios in the equatorial plane in Fig. 1B were calculated and are shown in Fig. 1C. In general, the staining area ratio of lignin in the 0 °C group was higher than that in the LTC group. During the whole storage period, the staining area ratio of the 0 °C group increased from 0.30 % to 1.39 %, while the staining area ratio of the LTC group increased from 0.30 % to 0.89 %. The LTC treatment significantly inhibited the increase in the lignin staining area ratio of the loquat fruit. During postharvest storage of the loquat fruit, there was a significant positive correlation between the ratio of the lignin staining area ratio and fruit firmness (Supplemental Table 1).
Table 1.
Pearson correlation matrix for firmness, average fluorescence intensity of JIM5 in parenchyma cells, vascular bundles, and exocarps, and calcium-pectate content in loquat flesh during lignification.
| Variables | Firmness | JIM5 signal intensity in parenchyma cells | JIM5 signal intensity in vascular bundles | JIM5 signal intensity in exocarps | Calcium-pectate content |
|---|---|---|---|---|---|
| Firmness | 1 | ||||
| JIM5 signal intensity in parenchyma cells | 0.867** (R2 = 0.7509) |
1 | |||
| JIM5 signal intensity in vascular bundles | 0.820** (R2 = 0.6728) |
0.916** (R2 = 0.8396) |
1 | ||
| JIM5 signal intensity in exocarps | 0.886** (R2 = 0.7851) |
0.898** (R2 = 0.8066) |
0.884** (R2 = 0.7812) |
1 | |
| Calcium-pectate content | 0.946** (R2 = 0.7851) |
0.912** (R2 = 0.8322) |
0.765** (R2 = 0.5847) |
0.887** (R2 = 0.7874) |
1 |
The values with two stars ** represent significant correlation at the significance level alpha = 0.01. R2 in brackets stands for regression analysis values.
The lignin content of the loquat fruit also constantly increased in both the 0 °C and LTC groups during postharvest storage (Fig. 1D). At 0 d, the lignin content of the loquat fruit was 0.41 % FW, and at 30 d, the lignin contents of the 0 °C group and LTC group were 0.71 % FW and 0.54 % FW, respectively. The lignin content was significantly correlated with fruit firmness (R2 = 0.848) and the lignin staining area ratio (R2 = 0.742) (Supplemental Table 1). These results indicate that loquat fruit in the 0 °C group suffered severe chilling-induced lignification, including increases in firmness and lignin content, and that the LTC treatment retarded the lignification of the loquat fruit.
The contents of different pectin fractions in the loquat fruit during postharvest storage were measured based on their solubility properties, including DASP, CSP, and WSP. The DASP content of loquat fruit in both the 0 °C and LTC groups showed no significant changes during the whole postharvest storage, except for a significant decrease in the LTC group at the end of storage (Fig. 1E). Meanwhile, there was no significant difference in the DASP content between the 0 °C group and LTC group. Similarly, the WSP pectin content of loquat fruit remained stable during the whole postharvest storage period, except for a significant increase in the LTC group at the end of storage (Fig. 1F). There was no significant difference between the WSP content of the 0 °C group and the WSP content of the LTC group.
The CSP content of loquat fruit showed an increasing trend during postharvest storage in both the 0 °C and LTC groups (Fig. 1G). At 0 °C, the CSP content increased rapidly over the first 6 d and tended to remain stable during the later storage period. In the LTC group, the CSP content showed a slow upward trend during the entire storage period, although there was no significant change. In addition, the CSP content at 0 °C was significantly higher than that of the LTC group at 18 d. Previous results found that CSP decreased with fruit softening during storage [46], [47], which is contrary to the trend we found for loquat fruit. Therefore, it is speculated that some pectin fractions may be related to the firmness increase in loquat fruit during postharvest storage. However, the different pectin fractions, such as DASP, WSP, and CSP, were fractionated by different solvents and quantified by galacturonic acid content, and they cannot be directly matched to the reactants and products of the catalytic enzymes involved in pectin metabolism, such as high- and low-methylesterified HG. Meanwhile, the pectin content measured based on the solvent extraction method lacked in situ spatial information. For this reason, it is necessary to study the patterns of change in the structure, content, and spatial distribution of pectin substances occurring in the cell wall during pectin metabolism to clarify their mechanism of action in the process of the firmness increase in postharvest loquat fruit.
Low-methylesterified HG in the cell wall
To further analyze the modifications to the pectin structure and distribution in situ, immunolabeling assays on loquat fruit during postharvest storage were performed. JIM5 and JIM7 monoclonal antibodies were used to identify low- and high-methylesterified domains of HG, respectively. Three areas in loquat were mainly observed: parenchyma cells, vascular bundles, and exocarps (Supplemental Fig. 1). As shown in Supplemental Fig. 2, in the control group with the primary antibody omitted, the cuticle of the exocarps presented autofluorescence, and the epidermis of the exocarps also presented weak autofluorescence, while autofluorescence was barely detectable in the remaining part of the observed areas, which did not affect the observation of the subsequent antibody labeling results.
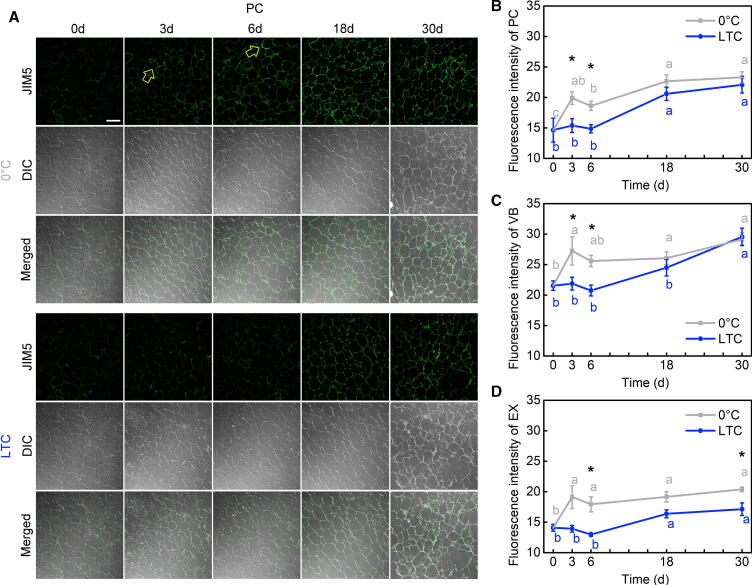
Fig. 2.
Low-methylesterified homogalacturonan (HG) accumulation in parenchyma cells of loquat fruit during postharvest storage. (A) Low-methylesterified HG in representative parenchyma cells in loquat flesh section during postharvest storage was immunolabeled with the JIM5 antibody. (B) The average fluorescence intensity of the JIM5 antibody in parenchyma cells in Fig. 2A, vascular bundles in Fig. 3A, and exocarps in Fig. 3B of loquat fruits during postharvest storage. Yellow arrows indicate cellular areas with signal heterogeneity. Scale bar = 200 μm. Data are presented as the mean ± standard error from three independent biological replicates. Significant differences are indicated with asterisks above the bars (*, P < 0.05; **, P < 0.01). PC: parenchyma cells; VB: vascular bundles; EX: exocarps; DIC: differential interference contrast. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the parenchyma cells, the JIM5 antibody signal was rarely found at 0 d with respect to the control conditions (Fig. 2A; Supplemental Fig. 2), which indicates that the freshly harvested loquat fruit hardly contained any low-methylesterified HG. As the storage time increased, the tricellular junctions and middle lamella of loquats in both the 0 °C and LTC groups continued to accumulate the JIM5 antibody signal. The JIM5 signal in the 0 °C group accumulated more quickly than in the LTC group. The distribution of the JIM5 signal in the 0 °C group was more continuous and extensive, accompanied by a significantly higher signal intensity than in the LTC group at 3 d and 6 d (Fig. 2 B). These results indicate that loquat fruit with lignification symptoms largely accumulated low-methylesterified HG in both the tricellular junctions and middle lamella of the parenchyma cells, especially during the early stages of lignification.
In the vascular bundles, the JIM5 signal was already present in both the tricellular junctions and middle lamella, in contrast to the parenchyma cells (Fig. 3A). During postharvest storage, the JIM5 signal in the vascular bundles of both the 0 °C and LTC groups showed an increasing trend during storage. Meanwhile, the intensity of the JIM signal in the 0 °C group was significantly higher than that in the LTC group at 3 d and 6 d (Fig. 2C). Overall, the signal intensity in the vascular bundles was higher than that in the parenchyma cells. The above results show that vascular bundles are also involved in the accumulation of low-methylesterified HG during the postharvest lignification of loquat fruit.
Fig. 3.
Low-methylesterified homogalacturonan (HG) accumulation in vascular bundles and exocarps of loquat fruit during postharvest storage. Low-methylesterified HG in representative vascular bundles (A) and exocarps (B) in loquat flesh sections during postharvest storage was immunolabeled with the JIM5 antibody. Yellow arrows indicate cellular areas with signal heterogeneity. Scale bars = 200 μm. VB: vascular bundles; EX: exocarps; DIC: differential interference contrast. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the exocarps, the trend of the JIM5 signal was similar to that in the parenchyma cells (Fig. 3B). Specifically, the JIM5 signal was barely detected in loquat fruit at 0 d. As the storage time increased, loquat fruit in the 0 °C group rapidly accumulated large amounts of JIM5 signals in both the tricellular junctions and middle lamella, accompanied by an increased signal intensity (Fig. 2D). The LTC treatment significantly inhibited the accumulation of low-methylesterified HG labeled by JIM5 in the exocarps. The above results show that loquat fruits with lignification symptoms also accumulate low-methylesterified HG in the exocarps.
In addition, the accumulation of the JIM5 signal showed cell heterogeneity (Fig. 2, Fig. 3, yellow arrows). The intensity of the JIM5 signal in some cells was significantly higher than that in other surrounding cells, and the collective accumulation rate of a population of cells cannot represent the accumulation rate of individual cells. This phenomenon was found in different tissues at different sampling points.
Correlation analysis between the JIM5 signal intensity of different areas and the firmness of loquat fruit during postharvest storage was carried out, and the results are shown in Table 1. There were significant correlations between firmness and the JIM5 signal intensities of the parenchyma cells, vascular bundles, and exocarps. The results above suggest that HG demethylesterification could cause an increase in the firmness of loquat.
On the other hand, Supplemental Fig. 3A and 4A,B show that the JIM7 antibody signal in the parenchyma cells, vascular bundles, and exocarps was barely observed in the freshly harvested loquat fruit and in loquat fruit during postharvest storage in both the 0 °C and LTC groups. Only sporadic signals were present in both the tricellular junctions and middle lamella. To further examine the changes in the JIM7 signal of loquat fruit in the 0 °C and LTC groups, the intensity of the JIM7 signal was calculated, and the results show no significant difference in high-methylesterified HG between these two groups (Supplemental Fig. 3B–D).
PME activity and calcium-pectate content
HG is demethylesterified by PME, yielding low-methylesterified HG. Then, low-methylesterified HG can either be further modified by pectin-degrading enzymes, causing cell wall loosening, or cross-linked with other low-methylesterified HGs through Ca2+, forming a rigid gel, thereby affecting both the cell wall mechanical properties and intercellular adhesion via the tricellular junctions and middle lamella. We further measured the total PME activity and calcium-pectate content in loquat fruit.
PME activity in the 0 °C group increased rapidly by 3 d, while PME activity in the LTC group was significantly lower at 3 d (Fig. 4A). From 0 d to 18 d, the 0 °C group had higher PME activity than the LTC group, indicating that LTC treatment can inhibit the PME activity of loquat fruits during early storage. At the end of storage, the PME activity of the LTC group increased significantly, while the PME activity of the 0 °C group showed a downward trend.
Fig. 4.
Egg-box structure of homogalacturonan (HG) accumulation in loquat fruit during postharvest storage. Changes in pectin methylesterase (PME) activity (A) and pectate-calcium content (B) in loquat fruit during postharvest storage. (C) Scheme of egg-box structure. Long stretches of HG form calcium-mediated HG complexation after being demethylesterified by PME. (D–F) The signal distribution of oligogalacturonide (OG) probes in loquat fruit at 0 d (D), 30 d stored at 0℃ (E), and 30 d stored after LTC (F) by direct OG labeling or prior pre-treatment with the chelating agent 1,2-diaminocyclohexanetetraacetic acid and OG (CDTA+OG) labeling. (G–I) The average fluorescence intensity of OG probes in exocarps (G); parenchyma cells (H), and vascular bundles (I) in Fig 4D–F. Scale bar = 200 μm. Data are presented as the mean ± standard error from three independent biological replicates. Significant differences are indicated with asterisks above the bars (*, P < 0.05; **, P < 0.01). FW: fresh weight; PC: parenchyma cells; VB: vascular bundles; EX: exocarps; DIC: differential interference contrast.
The calcium-pectate content in the 0 °C group increased quickly during postharvest storage, while the rate of increase in the calcium-pectate content in the LTC group was relatively slow, with no significant difference over the first 6 d (Fig. 4B). The calcium-pectate content was significantly correlated with the firmness and JIM5 signal intensities of the parenchyma cells, vascular bundles, and exocarps.
The above results indicate that the PME activity of loquat fruit in the 0 °C group was increased significantly at the early stage, resulting in a large accumulation of demethylesterified HG that could cross-link with Ca2+, forming an egg-box structure (Fig. 4C), while the LTC treatment slowed down the accumulation of low-demethylesterified HG and the formation of the egg-box structure by inhibiting the PME activity.
Calcium-mediated pectin complexation in the cell wall
OG probes can directly label low-methylesterified HG in samples that have not yet formed egg-box structures with exogenous Ca2+. Pre-treatment of samples with CDTA can destroy existing endogenous egg-box structures, exposing more binding sites for OG probes. To gain an insight into the distribution of egg-box structures in the cell walls of loquat fruit, in the present study, we used these techniques with OG probes to measure the presence and distribution of these epitopes in the native tissue. By comparing the results of the direct OG probe labeling with those of the OG probe labeling after CDTA pre-treatment, the distribution of the endogenous egg-box structures of the samples was analyzed in situ.
Fig. 4D shows the OG labeling and CDTA + OG labeling results for loquat fruit at 0 d with respect to the control condition (Supplemental Fig. 5). Through OG labeling, it was found that the low-methylesterified HG in the loquat fruit that had not yet formed an egg-box structure was distributed in both the tricellular junctions and middle lamella of the parenchyma cells, vascular bundles, and exocarps. The signal intensity in the region of the vascular bundles was higher than that in the parenchyma cells and exocarps. The distribution of the OG probe signal after the CDTA + OG labeling of parenchyma cells, vascular bundles, and exocarps showed only a marginal change, and there was no significant difference in intensity (Fig. 4G–I). These results suggest that the egg-box structure is rarely distributed in freshly harvested loquat fruit.
Fig. 4E shows the results obtained through the OG and CDTA + OG labeling of loquat fruit stored for 30 d at 0 °C. According to the OG labeling, the low-methylesterified HG that had not yet formed an egg-box structure was abundantly distributed with a strong signal intensity in both the tricellular junctions and middle lamella of the parenchyma cells, vascular bundles, and exocarps (Fig. 4G–I). According to the CDTA + OG labeling, after CDTA destroyed the endogenous egg-box structures, more binding sites for OG probes were exposed in the tricellular junctions and middle lamella corners of the parenchyma cells, vascular bundles, and exocarps. Additionally, the signal intensity was significantly higher (Fig. 4G–I). The above results indicate that for the loquat fruit that had increased firmness during postharvest storage at 0 °C, the distribution of egg-box structures accumulated in the cell walls of these fruits, especially in the parenchyma cells and exocarps.
Fig. 4F shows the results obtained with OG and CDTA + OG labeling of loquat fruit stored for 30 d in the LTC group. According to the OG labeling, the distribution of the low-methylesterified HG in the loquat fruit that had not yet formed an egg-box structure was less than that at 30 d in the 0 °C group. After CDTA + OG labeling, the distribution of OG probes in both the tricellular junctions and middle lamella of the parenchyma cells, vascular bundles, and exocarps increased, and the signal intensity also increased, with a significant difference in the parenchyma cells and exocarps, but the signal intensity was lower than that of loquat fruit at 30 d in the 0 °C group (Fig. 4G–I). Therefore, LTC treatment can inhibit the formation of the egg-box structure in loquat fruit.
Firmness change after injection of exogenous PME and Ca2+
The above results reveal that low-methylesterified HG is significantly accumulated and further cross-linked with Ca2+, forming egg-box structures, in loquat fruit that have increased firmness during postharvest lignification. However, it was difficult to establish whether these HG alterations directly caused the firmness increase in loquat fruit. To gain an insight into this question, the HG methylesterification status of loquat fruit was exogenously modulated by injection with PME and Ca2+ in vivo to investigate how the PME-introduced HG demethylesterification and the ion cross-linking influenced the firmness of the loquat fruit. The results show that the firmness of loquats treated with PME + Ca2+ was the highest, followed by the PME group and the Ca2+ group, while the firmness of the control group (H2O group) was the lowest (Fig. 5A). The firmness of loquats in the PME + Ca2+ group was significantly higher than that of the control group (both p < 0.05 according to a paired t-test).
Fig. 5.
Exogenous modulation of pectin methylesterase (PME) activity and Ca2+ content contributes to the firmness increase and low-methylesterified homogalacturonan (HG) accumulation in loquat flesh. Loquat fruit firmness (A), distribution (B) and average fluorescence intensity (C) of low-methylesterified HG identified by the JIM5 antibody in parenchyma cells, and distribution (D) and average fluorescence intensity (E) of low-methylesterified HG identified by the JIM5 antibody in vascular bundles in the PME + Ca2+ group, PME group, Ca2+ group, and H2O group. Scale bar = 200 μm. Data are presented as the mean ± standard error from three independent biological replicates. Significant differences are indicated with asterisks above the bars (*, P < 0.05; **, P < 0.01). PC: parenchyma cells; VB: vascular bundles; DIC: differential interference contrast.
To further illustrate how PME accelerated the accumulation of low-methylesterified HG after injection, loquat flesh from the injection sites was sampled and sectioned, and JIM5 was used to identify low-methylesterified HG in these sections. As shown in Fig. 5B,D, sections of the flesh areas from the PME + Ca2+ and PME groups had more JIM5 epitopes distributed in the tricellular junctions and middle lamella than those from the Ca2+ and control groups. The intensity of the JIM5 signal in the PME + Ca2+ and PME groups was significantly higher than that of the Ca2+ and control groups (Fig. 5C,E). These results suggest that the injection of PME into the loquat flesh resulted in the accumulation of low-methylesterified HG.
The egg-box structure in the loquat flesh after exogenous injection of PME and Ca2+ was labeled by OG probes (Fig. 6A–D). The OG signal obtained through CDTA + OG labeling was more widely distributed in the parenchyma cells and vascular bundles of the loquat flesh in the PME + Ca2+ group compared with that found through OG labeling. Meanwhile, the signal intensity obtained through CDTA + OG labeling was higher than that obtained through OG labeling (Fig. 6E,F). These results suggest that after the injection of PME and Ca2+ into the loquat flesh, egg-box structures accumulated.
Fig. 6.
Exogenous modulation of pectin methylesterase (PME) activity and Ca2+ content contributes to egg-box structure accumulation in loquat flesh. (A–D) Distribution of oligogalacturonide (OG) probes in parenchyma cells and vascular bundles of loquat flesh sections from four exogenous injection groups by direct OG labeling or pre-treatment with the chelating agent 1,2-diaminocyclohexanetetraacetic acid and OG (CDTA+OG) labeling: (A) PME + Ca2+ group; (B) PME group; (C) Ca2+ group; (D) H2O group. (E–F) Average fluorescence intensity of OG probes in parenchyma cellls (E) and vascular bundles (F) of loquat flesh sections from four exogenous injection groups in Fig. 6A–D. Scale bar = 200 μm. Data are presented as the mean ± standard error from three independent biological replicates. Significant differences are indicated with asterisks above the bars (*, P < 0.05; **, P < 0.01). PC: parenchyma cells; VB: vascular bundles; DIC: differential interference contrast.
The results above suggest that the exogenous injection of PME + Ca2+ into the loquat fruit led to the accumulation of low-methylesterified HG and egg-box structures, which contributed to the firmness increase in the loquat fruit.
Discussion
Pectin metabolism patterns in lignified loquat fruit
Fruit texture is considered a principal quality indicator. The changes in fruit texture during postharvest storage are a crucial factor for the fruit industry to optimize the preharvest practice, assess ripeness, and determine transportability, storability, and shelf life. Red-fleshed loquat is a chilling-sensitive fruit and exhibits severe lignification symptoms when stored at low temperature (Fig. 1A–D). The firmness of the loquat fruit quickly increased during postharvest storage, accompanied by an increase in the lignin content, which led to a decrease in the merchantability of the loquat fruit. Similar symptoms have been found in several other fruits, such as kiwifruit and zucchini [48]. Thus far, the increase in the firmness of loquat fruit during postharvest lignification has been mainly considered to be related to the accumulation of lignin in the secondary cell wall. Research on postharvest lignification of loquat fruit has focused on the biochemical and molecular mechanisms of lignin synthesis [13], [14], [22]. However, an increasing amount of evidence shows that pectin plays an important role in regulating the mechanical properties of plant cell walls and tissues [28], [31], [32]. Therefore, in the present study, we focused on the role of cell wall pectin fractions in the texture changes of loquat fruit during postharvest lignification. Based on chemical analysis, it was found that there was an upward trend in the CSP content (Fig. 1G) and no significant change in the DASP and WSP contents of the loquat fruit (Fig. 1E,F), which is not consistent with the trend observed in most fruits softened after harvest [6]. Abnormal metabolism of pectin has also been found in other fruits with chilling injury, due to an imbalance in the activities of pectin-degrading enzymes, such as tomato [49], peach [50], apricot [51], and plum [52]. Therefore, the metabolism of pectin may be related to postharvest lignification symptoms in loquat fruit, which gives us a new target substance to study the mechanism of postharvest lignification of fruit.
Low-methylesterified HG in the tricellular junctions and middle lamella
Immunohistochemistry was used to measure the distribution and structure changes of pectin in loquat fruit during postharvest lignification. Low-methylesterified HG epitopes identified by the JIM5 antibody were mainly distributed in both the tricellular junctions and middle lamella and accumulated in the parenchyma cells, vascular bundles, and exocarps of the loquat fruit during postharvest storage. Meanwhile, based on quantitative analysis of the gray value, the signal intensity of low-methylesterified HG epitopes was found to increase continuously during storage. The results of the immunofluorescence experiments were consistent with the CSP content measured in the chemical extraction analysis, since the main part of low-methylesterified HG is CSP [6]. In a study on tomato, low-methylesterified HG epitopes were also found to be distributed in intercellular spaces and tricellular junctions during fruit growth [53], which is consistent with our results. The softening of tomato fruit was considered to be associated with the disaggregation and depolymerization of these demethylesterified HGs in intercellular spaces and tricellular junctions by pectin-degrading enzymes acting in a hierarchy [7], [54]. The distribution of low-methylesterified HG in apple fruit during postharvest storage has also been studied at the cellular level through immunohistochemistry [55], and the results showed that low-methylesterified HG was also located at the tricellular junctions and middle lamella of apple fruit. However, the trend of the changes in low-methylesterified HG in apple and loquat was opposite. During postharvest storage, demethylesterified HG gradually decreased and was visible only in the cell well corners in apple fruit after 3 months of storage, while loquat fruit constantly accumulated demethylesterified HG over 30 d of storage. The accumulation of demethylesterified HG was also found in chilling-injured peaches due to the imbalance influence of pectin-degrading enzyme activity [56]. Therefore, unlike the disaggregation and depolymerization of low-methylesterified HG in postharvest softened fruit, the accumulation of low-methylesterified HG may be related to the occurrence of a firmness increase in lignified loquat fruit. Reducing the accumulation of low-methylesterified HG could be beneficial in preventing the texture deterioration in postharvest fruits.
PME-mediated low-methylesterified HG and Ca2+ cross-linked HG
The demethylesterification of HG by PME is divided into two possible patterns—random and linear—leading to two distinct HG domains and biological consequences [57], [58]. Demethylesterification of HG during fruit softening usually follow the former pattern, which subsequently permits PGs and PLs to catalyze the degradation of the HG backbone, resulting in the loosening and disintegration of the cell wall [7], [59]. The latter pattern, however, generates large blocks of demethylesterified GalpA residues on the HG chain, which can cross-link Ca2+ to form a strengthening pectate gel—the so-called ‘egg box’ structure—thereby causing an increase in cellular adhesion and cell wall stiffening [60]. Therefore, in the present study, we focused on the PME activity and calcium-pectate content of loquat fruits during postharvest storage. The PME activity of loquat fruits stored at 0 °C increased significantly during the early storage period, which indicated the accumulation of low-methylesterified HG pectin related to the rise in PME enzyme activity. The increased calcium-pectate content during the lignification of loquat suggests that the accumulated low-methylesterified HG combines with Ca2+ to form egg-box structures in the cell wall. Based on the OG probes, the presence of egg-box structures was confirmed to be abundantly distributed in the parenchyma cells, vascular bundles, and exocarps of loquat fruit with severe lignification symptoms. Previous studies have found that temperature can dynamically regulate the content and methylesterification degree of pectin in plants [61], [62]. The PME activity in Arabidopsis increased when subjected to cold stress [63]. When rapeseed underwent cold acclimation, the pectin content and the PME activity increased, accompanied by a decrease in the degree of HG methylesterification and an increase in the tensile strength of the cell wall [64]. Studies on peas, onions, etc., have also found evidence of pectin accumulation during low-temperature acclimation [62], [65]. In the study of cold stress in vegetables and fruits, the enzymatic activity of PME was also found to increase [48], [66]. In peach fruits with chilling injury, demethylesterified HG accumulated without being depolymerized and interacted with Ca2+, forming calcium pectate complexation, which has the capacity to capture apoplastic water from the flesh [50], [67]. The regulation of PME during the postharvest storage of fruits could be a key way to reduce postharvest chilling injury losses and maintain the market value of fruits.
Contribution of egg-box structures to fruit firmness
Traditionally, the degradation of pectin is generally considered to be the main cause of fruit softening [6], [18], while the deposition of lignin is considered to be the main factor in fruit hardening during postharvest lignification [16], [20]. However, in recent years, there has been evidence that the egg-box structure formed by cross-linking with Ca2+ after HG demethylesterification can greatly change the hydration and physical properties of the cell wall, thereby changing the mechanical strength at the single-cell and tissue levels, affecting the related physiological processes of plants [68]. The Arabidopsis mutant alleles of PME genes (vgd1, atppme1) result in the reduction in or loss of Ca2+ pectate gel formation in the pollen tube wall, leading to a loss of or reduction in the pollen tube wall strength [60], [69] In tomato fruit, PME1 and PME2 generated block-wise demethylesterified HG that cross-linked with Ca2+ to form calcium-pectate gel, resulting in slow fruit softening [70], [71]. Recently, SlBES1 was found to inhibit PMEU1-related pectin demethylesterification, resulting in less egg-box structure formation, which led to tomato fruit softening, while the SlBES1 mutant with more egg-box structure formation was related to firmer tomato fruits and a longer shelf life during postharvest storage [72]. The mechanism of action of preharvest and postharvest calcium or boron treatments on the shelf-life increase is through higher pectin production or hardening [24]. In vitro tests showed that the egg-box structure formed by Ca2+ cross-linking with HG was associated with increased firmness and decreased elasticity [73], [74]. Exogenous injection of PME has been used to study the role of PME activity in Arabidopsis defenses against aphids [75]. Therefore, to demonstrate the relationship between low-methylesterified HG and loquat firmness, the present study further injected exogenous PME and Ca2+ into freshly harvested loquat fruit to simulate the status of low-methylesterified HG accumulation. The combination of PME and Ca2+ injection has also been used to improve the firmness of fresh-cut fruits, such as jujube [76], mango [77], strawberry [78], apple [79], pineapple [80], and papaya [81], mainly preventing them from softening too fast during postharvest storage. In the present study, the firmness of the loquat fruit in the PME + Ca2+ group was the highest. At the same time, loquat fruit in the PME + Ca2+ group had more low-methylesterified HG and egg-box structures. The results of the injection experiment suggest that low-methylesterified HG cross-linked with Ca2+ contributes to the firmness increase in loquat fruit. Appropriate regulation of pectin-involved egg-box structures is expected to be used to reduce postharvest losses of fruits with undesired hardening and softening.
Role of HG in the reduced juice yield and peeling difficulty
In addition to increasing the stiffness of the cell wall, the egg-box structure is a stable pectin gel, which can hold large amounts of free water from the flesh. In seed mucilage, the addition of Ca2+ leads to a denser mucilage as the Ca2+ cross-links with low-methylesterified HG, while the addition of a Ca2+ chelator leads to less dense and more voluminous mucilage as the number of network cross-links between Ca2+ and low-methylesterified HG is reduced [82]. In peach fruit with chilling injury, the decrease in juice yield is related to the strong water binding by the formation of calcium-pectate gels, although the amount of water in fruit with chilling injury and healthy fruit is the same [56]. This may be related to the juiceless pulp of loquat fruit with severe lignification symptoms. It is speculated that the reduced juice yield in the pulp of loquat fruit after postharvest lignification is due to the formation of calcium-pectate gels.
Moreover, the lignification symptoms of loquat fruit also include difficulty in peeling. The low-methylesterified HG and egg-box structure were also found to be abundantly distributed in both the tricellular junctions and middle lamella of the exocarps in the loquat fruit. Pectin is a major component of the middle lamella that connects the cell walls of adjacent plant cells and plays an important role in intercellular adhesion [18]. The egg-box structure formed by low-methylesterified HG cross-linking with Ca2+ is important for cell–cell adhesion [68]. The mesophyll of pme3 mutants with high-methylesterified HG was found to be more susceptible to cell-wall-degrading enzymes and could separate the cells more easily [83]. The loss of low-methylesterified HG in the middle lamella contributes to leaf abscission in Impatiens (Balsaminaceae), which is necessary for the plant to shed its leaves [84]. Therefore, the low-methylesterified HG and the egg-box structure may contribute to the increase in intercellular adhesion, which results in the difficulty in peeling loquat fruit. Thus far, factors affecting loquat fruit peeling remain unclear. For loquat fruit to have a better taste and quality with increased market value, further evidence is needed on the effect of the role of low-methylesterified HG and the egg-box structure on the reduced juice yield and difficulty of peeling of loquat fruit.
Heterogeneity in the accumulation of low-methylesterified HG
During postharvest lignification of loquat fruit, it is found that there is cell heterogeneity in the low-methylesterified HG accumulation. The signal intensity of low-methylesterified HG in some cells or cell populations is also significantly higher than that of other surrounding cells (Fig. 2A and 3A,B). In our previous works, we found that there was also heterogeneity in the process of lignin accumulation in loquat fruit. Some parenchyma cells were the first to accumulate lignin until they became solid lignified cells and then seemed to transmit the ‘lignification signal’ to neighboring parenchyma cells to become lignified, which could explain the formation of lignified cell clusters [12], [85]. Whether cells or cell clusters with a higher signal intensity of low-methylesterified HG in loquat fruit first accumulate demethylesterified HG and subsequently affect the pattern distribution of HG or influence the initiation, deposition, or location of lignin in the cell walls of loquat fruit is unclear. Recently, high-resolution spatiotemporal transcriptomes have been used to provide metabolic and regulatory specialization during fruit development and ripening [86], [87]. In future works, details of the molecular mechanism governing the spatiotemporal heterogeneity of pectin in loquat fruit should be investigated. The cells or cell clusters with a higher low-methylesterified HG signal than other cells may be the key to reducing the postharvest lignification of loquat fruit. This result provides target cells for further research to improve the postharvest quality, marketability, and storability of loquat fruit.
Relationship between lignin and low-methylesterified HG
Lignification is generally conceived of as an uneven process moving from the middle lamella and primary cell wall, which contains a large amount of pectin, to the inner part of the cell wall [88], [89], [90]. Earlier studies have found that pectin, mainly composed of uronic acid, can form a covalent bond with lignin via ester or ether linkages formed during lignin polymerization [91], [92]. Pectin has been suggested to be involved in lignification of the cell wall, especially in the initiation steps [93], [94], [95]. The distribution pattern of pectin has been suggested to be related to the subsequent inhomogeneity of lignin deposition in alfalfa (Medicago sativa) [96]. The effect of pectic galactan on the cell wall architecture during the early events of secondary cell wall deposition was also studied in Arabidopsis [97]. In our previous work, the newly formed lignin in the loquat fruits during postharvest storage was also found to be specifically deposited in both the tricellular junctions and middle lamella of some parenchyma cells surrounding the vascular bundles [85]. In the present study, low-methylesterified HG also largely accumulated in both the tricellular junctions and middle lamella of the loquat fruit. The relationship between the accumulation of low-methylesterified HG and lignin accumulation in the tricellular junctions and middle lamella of loquat fruit during lignification requires further study.
Moreover, calcium in cambial tissue has also been found to be important for the lignification process of cell walls [98], [99]. In the present study, low-methylesterified HG in the tricellular junctions and middle lamella further cross-linked with Ca2+ to form egg-box structures, and egg-box structures significantly accumulated in the loquat fruit that had severe lignification symptoms during postharvest storage at 0 °C. Whether the formation of egg-box structures in the tricellular junctions and middle lamella interferes with the deposition of lignin remains to be determined. Elucidation of the relationship between pectin and lignin during the firmness increase could be beneficial to better reduce postharvest waste and loss of loquat fruit.
Conclusions
During postharvest storage at 0 °C, severe lignification symptoms were found in loquat fruit, accompanied by a constantly increasing firmness and lignin content. Moreover, it was also found that the CSP content measured through chemical extraction also increased in loquat fruit during postharvest storage. At the single-cell level, low-methylesterified HG labeled by the JIM5 antibody was found to constantly accumulate in both the tricellular junctions and middle lamella of the parenchyma cells, vascular bundles, and exocarps of the loquat fruit, and the accumulation trend was consistent with CSP. The LTC treatment inhibited the accumulation trend of low-methylesterified HG. These results show that loquat fruit with lignification symptoms had different metabolism patterns of pectin compared with other fruits. In the upstream of HG metabolism, the trend of PME activity suggests that PME mediated the demethylesterification process of HG in loquat fruit stored at 0 °C, yielding the accumulation of low-methylesterified HG. In the downstream of HG metabolism, the calcium pectate content and OG probe labeling indicate that low-methylesterified HG in the cell wall of parenchyma cells, vascular bundles, and exocarps could further cross-link with Ca2+ to form egg-box structures. In addition, loquat fruit with PME + Ca2+ exogenous injection had significantly higher firmness and more low-methylesterified HG and egg-box structure signals than the control group, showing that the egg-box structure contributed to the firmness increase in loquat fruit. Overall, the above results show that the PME-mediated HG demethylesterification activity increased during postharvest storage of loquat fruit, generating the accumulation of low-methylesterified HG and egg-box structures, resulting in the firmness increase in loquat fruit. Therefore, retarding the formation of low-methylesterified HG and egg-box structures could be a promising way to improve the quality of postharvest loquat fruit and other fruits that are susceptible to chilling injury.
CRediT authorship contribution statement
Weinan Huang: Conceptualization, Methodology, Investigation, Visualization, Writing – original draft, Writing – review & editing. Yanna Shi: Methodology, Investigation, Writing – review & editing. He Yan: Methodology. Hao Wang: Methodology, Writing – review & editing. Di Wu: Conceptualization, Supervision, Writing – review & editing. Donald Grierson: Writing – review & editing. Kunsong Chen: Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors acknowledge Professor Jozef Mravec, University of Copenhagen, for furnishing OG probes, as well as Jingyao Chen, Qiong Huang, and Shuangshuang Liu from the core facility plat form of School of Medicine, Zhejiang University for their technical support.
Funding
Zhejiang Provincial Natural Science Foundation of China (LR22C200004)
National Natural Science Foundation of China (31972117)
National Natural Science Foundation of China (32030082)
The 111 Project (B17039)
Fundamental Research Funds for the Central Universities (2021FZZX001-55).
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Data and materials availability:
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.09.009.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Harker F.R., Stec M.G.H., Hallett I.C., Bennett C.L. Texture of parenchymatous plant tissue: a comparison between tensile and other instrumental and sensory measurements of tissue strength and juiciness. Postharvest Biol Technol. 1997;11:63–72. doi: 10.1016/S0925-5214(97)00018-5. [DOI] [Google Scholar]
- 2.Toivonen P.M.A., Brummell D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol Technol. 2008;48:1–14. doi: 10.1016/j.postharvbio.2007.09.004. [DOI] [Google Scholar]
- 3.Tucker G., Yin X., Zhang A., Wang MiaoMiao, Zhu Q., Liu X., et al. Ethylene† and fruit softening. Food Qual Saf. 2017;1(4):253–267. [Google Scholar]
- 4.Li X., Xu C., Korban S.S., Chen K. Regulatory mechanisms of textural changes in ripening fruits. CRC Crit Rev Plant Sci. 2010;29:222–243. doi: 10.1080/07352689.2010.487776. [DOI] [Google Scholar]
- 5.Shi Y., Li B.-J., Su G., Zhang M., Grierson D., Chen K.-S. Transcriptional regulation of fleshy fruit texture. J Integr Plant Bioln/a. 2022 doi: 10.1111/jipb.13316. [DOI] [PubMed] [Google Scholar]
- 6.Brummell D.A. Cell wall disassembly in ripening fruit. Funct Plant Biol. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- 7.Uluisik S., Chapman N.H., Smith R., Poole M., Adams G., Gillis R.B., et al. Genetic improvement of tomato by targeted control of fruit softening. Nat Biotechnol. 2016;34:950–952. doi: 10.1038/nbt.3602. [DOI] [PubMed] [Google Scholar]
- 8.Huang W., Nie Y., Zhu N., Yang Y., Zhu C., Ji M., et al. Hybrid label-free molecular microscopies for simultaneous visualization of changes in cell wall polysaccharides of peach at single- and multiple-cell levels during postharvest storage. Cells. 2020;9:761. doi: 10.3390/cells9030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paniagua C., Ric-Varas P., García-Gago J.A., López-Casado G., Blanco-Portales R., Muñoz-Blanco J., et al. Elucidating the role of polygalacturonase genes in strawberry fruit softening. J Exp Bot. 2020;71:7103–7117. doi: 10.1093/jxb/eraa398. [DOI] [PubMed] [Google Scholar]
- 10.Mustafa M.A., Ali A., Seymour G., Tucker G. Delayed pericarp hardening of cold stored mangosteen (Garcinia mangostana L.) upon pre-treatment with the stress hormones methyl jasmonate and salicylic acid. Sci Hortic-Amsterdam. 2018;230:107–116. doi: 10.1016/j.scienta.2017.11.017. [DOI] [Google Scholar]
- 11.Suo J., Li H., Ban Q., Han Y., Meng K., Jin M., et al. Characteristics of chilling injury-induced lignification in kiwifruit with different sensitivities to low temperatures. Postharvest Biol Technol. 2018;135:8–18. doi: 10.1016/j.postharvbio.2017.08.020. [DOI] [Google Scholar]
- 12.Huang W., Zhu N., Zhu C., Wu D., Chen K. Morphology and cell wall composition changes in lignified cells from loquat fruit during postharvest storage. Postharvest Biol Technol. 2019;157 doi: 10.1016/j.postharvbio.2019.110975. [DOI] [Google Scholar]
- 13.Xu M., Zhang M.X., Shi Y.N., Liu X.F., Li X., Grierson D., et al. EjHAT1 participates in heat alleviation of loquat fruit lignification by suppressing the promoter activity of key lignin monomer synthesis gene EjCAD5. J Agr Food Chem. 2019;67:5204–5211. doi: 10.1021/acs.jafc.9b00641. [DOI] [PubMed] [Google Scholar]
- 14.Cai C., Chen K., Xu W., Zhang W., Li X., Ferguson I. Effect of 1-MCP on postharvest quality of loquat fruit. Postharvest Biol Technol. 2006;40:155–162. doi: 10.1016/j.postharvbio.2005.12.014. [DOI] [Google Scholar]
- 15.Xu Q., Wang W., Zeng J., Zhang J., Grierson D., Li X., et al. A NAC transcription factor, EjNAC1, affects lignification of loquat fruit by regulating lignin. Postharvest Biol Technol. 2015;102:25–31. doi: 10.1016/j.postharvbio.2015.02.002. [DOI] [Google Scholar]
- 16.Zhang M., Shi Y., Liu Z., Zhang Y., Yin X., Liang Z., et al. An EjbHLH14-EjHB1-EjPRX12 module is involved in methyl jasmonate alleviation of chilling-induced lignin deposition in loquat fruit. J Exp Bot. 2022;73:1668–1682. doi: 10.1093/jxb/erab511. [DOI] [PubMed] [Google Scholar]
- 17.Liu W., Zhang J., Jiao C., Yin X., Fei Z., Wu Q., et al. Transcriptome analysis provides insights into the regulation of metabolic processes during postharvest cold storage of loquat (Eriobotrya japonica) fruit. Hort Res. 2019;6:49. doi: 10.1038/s41438-019-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Yeats T.H., Uluisik S., Rose J.K.C., Seymour G.B. Fruit softening: revisiting the role of pectin. Trends Plant Sci. 2018;23:302–310. doi: 10.1016/j.tplants.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Hofte H., Voxeur A. Plant cell walls. Curr Biol. 2017;27:R865–R870. doi: 10.1016/j.cub.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Kamdee C., Imsabai W., Kirk R., Allan A.C., Ferguson I.B., Ketsa S. Regulation of lignin biosynthesis in fruit pericarp hardening of mangosteen (Garcinia mangostana L.) after impact. Postharvest Biol Technol. 2014;97:68–76. doi: 10.1016/j.postharvbio.2014.06.004. [DOI] [Google Scholar]
- 21.Cai C., Xu C., Li X., Ferguson I., Chen K. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol Technol. 2006;40:163–169. doi: 10.1016/j.postharvbio.2005.12.009. [DOI] [Google Scholar]
- 22.Zhang J., Yin X.R., Li H., Xu M., Zhang M.X., Li S.J., et al. ETHYLENE RESPONSE FACTOR39-MYB8 complex regulates low-temperature-induced lignification of loquat fruit. J Exp Bot. 2020;71:3172–3184. doi: 10.1093/jxb/eraa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.J. Obando, C. Miranda, M. M. Jowkar, E. Moreno, M. K. Sour, J. A. Martínez et al., in Advances in Plant Ethylene Research, A. Ramina et al., Eds. (Springer Netherlands, Dordrecht, 2007), pp. 197-205.
- 24.Khalaj K., Ahmadi N., Souri M.K. Improvement of postharvest quality of asian pear fruits by foliar application of boron and calcium. Horticulturae. 2016;3:15. doi: 10.3390/horticulturae3010015. [DOI] [Google Scholar]
- 25.Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Atmodjo M.A., Hao Z., Mohnen D. Evolving views of pectin biosynthesis. Annu Rev Plant Biol. 2013;64:747–779. doi: 10.1146/annurev-arplant-042811-105534. [DOI] [PubMed] [Google Scholar]
- 27.Du J., Kirui A., Huang S., Wang L., Barnes W.J., Kiemle S.N., et al. Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in Arabidopsis. Plant Cell. 2020;32(11):3576–3597. doi: 10.1105/tpc.20.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hongo S., Sato K., Yokoyama R., Nishitani K. Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell. 2012;24:2624–2634. doi: 10.1105/tpc.112.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peaucelle A., Wightman R., Hofte H. The control of growth symmetry breaking in the Arabidopsis hypocotyl. Curr Biol. 2015;25:1746–1752. doi: 10.1016/j.cub.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Qi J., Wu B., Feng S., Lu S., Guan C., Zhang X., et al. Mechanical regulation of organ asymmetry in leaves. Nat Plants. 2017;3:724–733. doi: 10.1038/s41477-017-0008-6. [DOI] [PubMed] [Google Scholar]
- 31.Amsbury S., Hunt L., Elhaddad N., Baillie A., Lundgren M., Verhertbruggen Y., et al. Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr Biol. 2016;26:2899–2906. doi: 10.1016/j.cub.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo H., Xiao C., Liu Q., Li R., Yan Z., Yao X., et al. Two galacturonosyltransferases function in plant growth, stomatal development, and dynamics. Plant Physiol. 2021;187:2820–2836. doi: 10.1093/plphys/kiab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goulas V., Minas I.S., Kourdoulas P.M., Vicente A.R., Manganaris G.A. Phytochemical content, antioxidants and cell wall metabolism of two loquat (Eriobotrya japonica) cultivars under different storage regimes. Food Chem. 2014;155:227–234. doi: 10.1016/j.foodchem.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 34.Jin P., Duan Y., Wang L., Wang J., Zheng Y. Reducing chilling injury of loquat fruit by combined treatment with hot air and methyl jasmonate. Food Bioproc Tech. 2014;7:2259–2266. doi: 10.1007/s11947-013-1232-3. [DOI] [Google Scholar]
- 35.Cao S., Zheng Y., Wang K., Rui H., Tang S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2010;118:641–647. doi: 10.1016/j.foodchem.2009.05.047. [DOI] [Google Scholar]
- 36.Renard C.M. Variability in cell wall preparations: quantification and comparison of common methods. Carbohyd Polym. 2005;60:515–522. doi: 10.1016/j.carbpol.2005.03.002. [DOI] [Google Scholar]
- 37.Figueroa C.R., Opazo M.C., Vera P., Arriagada O., Díaz M., Moya-León M.A. Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chem. 2012;132:2014–2022. doi: 10.1016/j.foodchem.2011.12.041. [DOI] [Google Scholar]
- 38.Vicente A.R., Ortugno C., Rosli H., Powell A.L., Greve L.C., Labavitch J.M. Temporal sequence of cell wall disassembly events in developing fruits. 2. Analysis of blueberry (Vaccinium species) J Agr Food Chem. 2007;55:4125–4130. doi: 10.1021/jf063548j. [DOI] [PubMed] [Google Scholar]
- 39.Sun Q., Zhang N., Wang J., Zhang H., Li D., Shi J., et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J Exp Bot. 2015;66:657–668. doi: 10.1093/jxb/eru332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y., Lin Y., Lin H., Lin M., Li H., Yuan F., et al. Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chem. 2018;264:1–8. doi: 10.1016/j.foodchem.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Y. Ohta, K. Yamamoto, M. Deguchi, Chemical fractionation of calcium in the fresh rice leaf blade and influences of deficiency or oversupply of calcium and age of leaf on the content of each calcium fraction: chemical fractionation of calcium in some plant species (part 1). J. Soil Sci. Plant Nutr.41, 19-26 (1970). 10.20710/dojo.41.1_19.
- 42.Pontiggia D., Ciarcianelli J., Salvi G., Cervone F., De Lorenzo G., Mattei B. Sensitive detection and measurement of oligogalacturonides in Arabidopsis. Front Plant Sci. 2015;6:258. doi: 10.3389/fpls.2015.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mravec J., Kračun S.K., Rydahl M.G., Westereng B., Miart F., Clausen M.H., et al. Tracking developmentally regulated post-synthetic processing of homogalacturonan and chitin using reciprocal oligosaccharide probes. Development. 2014;141(24):4841–4850. doi: 10.1242/dev.113365. [DOI] [PubMed] [Google Scholar]
- 44.Mravec J., Kracun S.K., Rydahl M.G., Westereng B., Pontiggia D., De Lorenzo G., et al. An oligogalacturonide-derived molecular probe demonstrates the dynamics of calcium-mediated pectin complexation in cell walls of tip-growing structures. Plant J. 2017;91:534–546. doi: 10.1111/tpj.13574. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y., Dong W., Zhu Y., Allan A.C., Lin-Wang K., Xu C. PpGST1, an anthocyanin-related glutathione S-transferase gene, is essential for fruit coloration in peach. Plant Biotechnol J. 2020;18:1284–1295. doi: 10.1111/pbi.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Jin P., Wang J., Jiang L., Shan T., Zheng Y. Effect of β-aminobutyric acid on cell wall modification and senescence in sweet cherry during storage at 20°C. Food Chem. 2015;175:471–477. doi: 10.1016/j.foodchem.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y., Yu J., Lin H., Zheng Y., Fan Z., Wang H., et al. Phomopsis longanae Chi-induced longan pulp breakdown and softening in relation to cell wall polysaccharides disassembly. Postharvest Biol Technol. 2022;186 doi: 10.1016/j.postharvbio.2022.111837. [DOI] [Google Scholar]
- 48.Carvajal F., Palma F., Jamilena M., Garrido D. Cell wall metabolism and chilling injury during postharvest cold storage in zucchini fruit. Postharvest Biol Technol. 2015;108:68–77. doi: 10.1016/j.postharvbio.2015.05.013. [DOI] [Google Scholar]
- 49.Bai C., Zheng Y., Watkins C.B., Fu A., Ma L., Gao H., et al. Revealing the Specific Regulations of Brassinolide on Tomato Fruit Chilling Injury by Integrated Multi-Omics. 2021;8 doi: 10.3389/fnut.2021.769715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lurie S. Genomic and transcriptomic studies on chilling injury in peach and nectarine. Postharvest Biol Technol. 2021;174 doi: 10.1016/j.postharvbio.2020.111444. [DOI] [Google Scholar]
- 51.Li Y., Zhao Y., Zhang Z., He H., Shi L., X. Zhu et al., Near-freezing temperature storage improves shelf-life and suppresses chilling injury in postharvest apricot fruit (Prunus armeniaca L.) by regulating cell wall metabolism. Food Chem. 2022;387 doi: 10.1016/j.foodchem.2022.132921. [DOI] [PubMed] [Google Scholar]
- 52.Pan H., Wang L., Wang R., Xie F., Cao J. Modifications of cell wall pectin in chilling-injured ‘Friar’ plum fruit subjected to intermediate storage temperatures. Food Chem. 2018;242:538–547. doi: 10.1016/j.foodchem.2017.09.090. [DOI] [PubMed] [Google Scholar]
- 53.Guillon F., Moïse A., Quemener B., Bouchet B., Devaux M.-F., Alvarado C., et al. Remodeling of pectin and hemicelluloses in tomato pericarp during fruit growth. Plant Sci. 2017;257:48–62. doi: 10.1016/j.plantsci.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Wang D., Samsulrizal N.H., Yan C., Allcock N.S., Craigon J., Blanco-Ulate B., et al. Characterization of crispr mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 2019;179:544–557. doi: 10.1104/pp.18.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leszczuk A., Chylinska M., Zdunek A. Distribution of arabinogalactan proteins and pectins in the cells of apple (Malus x domestica) fruit during post-harvest storage. Ann Bot. 2019;123:47–55. doi: 10.1093/aob/mcy133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fruk G., Cmelik Z., Jemric T., Hribar J., Vidrih R. Pectin role in woolliness development in peaches and nectarines: A review. Sci Hortic-Amsterdam. 2014;180:1–5. doi: 10.1016/j.scienta.2014.09.042. [DOI] [Google Scholar]
- 57.Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6:414–419. doi: 10.1016/S1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- 58.G. A. Tucker, G. B. Seymour, in Pectins and Their Manipulation, G. Seymour, J. P. Knox, Eds. (FL: CRC Press, Boca Raton, 2002), pp. 150–173.
- 59.Paniagua C., Kirby A.R., Gunning A.P., Morris V.J., Matas A.J., Quesada M.A., et al. Unravelling the nanostructure of strawberry fruit pectins by endo-polygalacturonase digestion and atomic force microscopy. Food Chem. 2017;224:270–279. doi: 10.1016/j.foodchem.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 60.Jiang L., Yang S.-L., Xie L.-F., Puah C.S., Zhang X.-Q., Yang W.-C., et al. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell. 2005;17(2):584–596. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J., Chen X., Zhang Q., Zhang Y., Ou X., An L., et al. A cold-induced pectin methyl-esterase inhibitor gene contributes negatively to freezing tolerance but positively to salt tolerance in Arabidopsis. J Plant Physiol. 2018;222:67–78. doi: 10.1016/j.jplph.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Baldwin L., Domon J.-M., Klimek J.F., Fournet F., Sellier H., Gillet F., et al. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry. 2014;104:37–47. doi: 10.1016/j.phytochem.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 63.Qu T., Liu R., Wang W., An L., Chen T., Liu G., et al. Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress. Cryobiology. 2011;63(2):111–117. doi: 10.1016/j.cryobiol.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Solecka D., Zebrowski J., Kacperska A. Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann Bot. 2008;101:521–530. doi: 10.1093/aob/mcm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanino K.K., Kobayashi S., Hyett C., Hamilton K., Liu J., Li B., et al. Allium fistulosum as a novel system to investigate mechanisms of freezing resistance. Physiol Plant. 2013;147:101–111. doi: 10.1111/j.1399-3054.2012.01716.x. [DOI] [PubMed] [Google Scholar]
- 66.Khademi O., Besada C., Mostofi Y., Salvador A. Changes in pectin methylesterase, polygalacturonase, catalase and peroxidase activities associated with alleviation of chilling injury in persimmon by hot water and 1-MCP treatments. Sci Hortic-Amsterdam. 2014;179:191–197. doi: 10.1016/j.scienta.2014.09.028. [DOI] [Google Scholar]
- 67.Zhou H.W., Ben-Arie R., Lurie S. Pectin esterase, polygalacturonase and gel formation in peach pectin fractions. Phytochemistry. 2000;55:191–195. doi: 10.1016/S0031-9422(00)00271-5. [DOI] [PubMed] [Google Scholar]
- 68.Levesque-Tremblay G., Pelloux J., Braybrook S.A., Muller K. Tuning of pectin methylesterification: consequences for cell wall biomechanics and development. Planta. 2015;242:791–811. doi: 10.1007/s00425-015-2358-5. [DOI] [PubMed] [Google Scholar]
- 69.Tian G.-W., Chen M.-H., Zaltsman A., Citovsky V. Pollen-specific pectin methylesterase involved in pollen tube growth. Dev Biol. 2006;294:83–91. doi: 10.1016/j.ydbio.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 70.Wen B., Ström A., Tasker A., West G., Tucker G.A., Mendel R. Effect of silencing the two major tomato fruit pectin methylesterase isoforms on cell wall pectin metabolism. Plant Biology. 2013;15(6):1025–1032. doi: 10.1111/j.1438-8677.2012.00714.x. [DOI] [PubMed] [Google Scholar]
- 71.Wen B., Zhang F., Wu X., Li H. Characterization of the Tomato (Solanum lycopersicum) Pectin Methylesterases: Evolution, Activity of Isoforms and Expression During Fruit Ripening. Front Plant Sci. 2020;11:238. doi: 10.3389/fpls.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.H. Liu, L. Liu, D. Liang, M. Zhang, C. Jia, M. Qi et al., SlBES1 promotes tomato fruit softening through transcriptional inhibition of PMEU1. iScience24, 102926 (2021). 10.1016/j.isci.2021.102926. [DOI] [PMC free article] [PubMed]
- 73.Ngouémazong D.E., Tengweh F.F., Fraeye I., Duvetter T., Cardinaels R., Van Loey A., et al. Effect of de-methylesterification on network development and nature of Ca2+-pectin gels: Towards understanding structure–function relations of pectin. Food Hydrocoll. 2012;26:89–98. doi: 10.1016/j.foodhyd.2011.04.002. [DOI] [Google Scholar]
- 74.Gel microstructure and stiffness C. Kyomugasho, C. Munyensanga, M. Celus, D. Van de walle, K. Dewettinck, A. M. Van Loey et al., Molar mass influence on pectin-Ca2+ adsorption capacity, interaction energy and associated functionality. Food Hydrocoll. 2018;85:331–342. doi: 10.1016/j.foodhyd.2018.07.024. [DOI] [Google Scholar]
- 75.Silva-Sanzana C., Celiz-Balboa J., Garzo E., Marcus S.E., Parra-Rojas J.P., Rojas B., et al. Pectin methylesterases modulate plant homogalacturonan status in defenses against the aphid Myzus persicae. Plant Cell. 2019;31(8):1913–1929. doi: 10.1105/tpc.19.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L., Wang P., Chen F., Lai S., Yu H., Yang H. Effects of calcium and pectin methylesterase on quality attributes and pectin morphology of jujube fruit under vacuum impregnation during storage. Food Chem. 2019;289:40–48. doi: 10.1016/j.foodchem.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Sirijariyawat A., Charoenrein S., Barrett D.M. Texture improvement of fresh and frozen mangoes with pectin methylesterase and calcium infusion. J Sci Food Agric. 2012;92:2581–2586. doi: 10.1002/jsfa.5791. [DOI] [PubMed] [Google Scholar]
- 78.Langer S.E., Marina M., Burgos J.L., Martinez G.A., Civello P.M., Villarreal N.M. Calcium chloride treatment modifies cell wall metabolism and activates defense responses in strawberry fruit (Fragaria x ananassa, Duch) J Sci Food Agric. 2019;99:4003–4010. doi: 10.1002/jsfa.9626. [DOI] [PubMed] [Google Scholar]
- 79.Guillemin A., Guillon F., Degraeve P., Rondeau C., Devaux M.F., Huber F., et al. Firming of fruit tissues by vacuum-infusion of pectin methylesterase: Visualisation of enzyme action. Food Chem. 2008;109:368–378. doi: 10.1016/j.foodchem.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 80.Zhong L., Wang X., Fan L., Ye X., Li Z., Cui Z., et al. Characterization of an acidic pectin methylesterase from Paenibacillus xylanexedens and its application in fruit processing. Protein Expr Purif. 2021;179 doi: 10.1016/j.pep.2020.105798. [DOI] [PubMed] [Google Scholar]
- 81.Yang H., Wu Q., Ng L.Y., Wang S. Effects of vacuum impregnation with calcium lactate and pectin methylesterase on quality attributes and chelate-soluble pectin morphology of fresh-cut papayas. Food Bioproc Tech. 2017;10:901–913. doi: 10.1007/s11947-017-1874-7. [DOI] [Google Scholar]
- 82.Voiniciuc C., Dean G.H., Griffiths J.S., Kirchsteiger K., Hwang Y.T., Gillett A., et al. Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell. 2013;25(3):944–959. doi: 10.1105/tpc.112.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lionetti V., Cervone F., De Lorenzo G. A lower content of de-methylesterified homogalacturonan improves enzymatic cell separation and isolation of mesophyll protoplasts in Arabidopsis. Phytochemistry. 2015;112:188–194. doi: 10.1016/j.phytochem.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 84.Bowling A.J., Vaughn K.C. Leaf abscission in Impatiens (Balsaminaceae) is due to loss of highly de-esterified homogalacturonans in the middle lamellae. Am J Bot. 2011;98:619–629. doi: 10.3732/ajb.1000268. [DOI] [PubMed] [Google Scholar]
- 85.Zhu N., Zhao C., Wei Y., Sun C., Wu D., Chen K. Biosynthetic labeling with 3-O-propargylcaffeyl alcohol reveals in vivo cell-specific patterned lignification in loquat fruits during development and postharvest storage. Hort Res. 2021;8:61. doi: 10.1038/s41438-021-00497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shinozaki Y., Nicolas P., Fernandez-Pozo N., Ma Q., Evanich D.J., Shi Y., et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat Commun. 2018;9:364. doi: 10.1038/s41467-017-02782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin L.B.B., Nicolas P., Matas A.J., Shinozaki Y., Catalá C., Rose J.K.C. Laser microdissection of tomato fruit cell and tissue types for transcriptome profiling. Nat Protoc. 2016;11:2376–2388. doi: 10.1038/nprot.2016.146. [DOI] [PubMed] [Google Scholar]
- 88.Liu C.-J. Deciphering the enigma of lignification: precursor transport, oxidation, and the topochemistry of lignin assembly. Mol Plant. 2012;5:304–317. doi: 10.1093/mp/ssr121. [DOI] [PubMed] [Google Scholar]
- 89.Li M., Yoo C.G., Pu Y., Biswal A.K., Tolbert A.K., Mohnen D., et al. Downregulation of pectin biosynthesis gene GAUT4 leads to reduced ferulate and lignin-carbohydrate cross-linking in switchgrass. Commun Biol. 2019;2:22. doi: 10.1038/s42003-018-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cathala B., Chabbert B., Joly C., Dole P., Monties B. Synthesis, characterisation and water sorption properties of pectin-dehydrogenation polymer (lignin model compound) complex. Phytochemistry. 2001;56:195–202. doi: 10.1016/S0031-9422(00)00373-3. [DOI] [PubMed] [Google Scholar]
- 91.Minor J.L. Location of lignin-bonded pectic polysaccharides. J Wood Chem Technol. 1991;11:159–169. doi: 10.1080/02773819108050268. [DOI] [Google Scholar]
- 92.Jeffries T.W. in Physiology of Biodegradative microorganisms. (Springer. 1991:163–176. [Google Scholar]
- 93.Donaldson L.A. Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry. 2001;57:859–873. doi: 10.1016/S0031-9422(01)00049-8. [DOI] [PubMed] [Google Scholar]
- 94.Hao Z., Mohnen D. A review of xylan and lignin biosynthesis: foundation for studying Arabidopsis irregular xylem mutants with pleiotropic phenotypes. Crit Rev Biochem Mol Biol. 2014;49:212–241. doi: 10.3109/10409238.2014.889651. [DOI] [PubMed] [Google Scholar]
- 95.Lairez D., Cathala B., Monties B., Bedos-Belval F., Duran H., Gorrichon L. Aggregation during coniferyl alcohol polymerization in pectin solution: a biomimetic approach of the first steps of lignification. Biomacromolecules. 2005;6:763–774. doi: 10.1021/bm049390y. [DOI] [PubMed] [Google Scholar]
- 96.Wi S.G., Singh A.P., Lee K.H., Kim Y.S. The pattern of distribution of pectin, peroxidase and lignin in the middle lamella of secondary xylem fibres in alfalfa (Medicago sativa) Ann Bot. 2005;95:863–868. doi: 10.1093/aob/mci092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moneo-Sánchez M., Vaquero-Rodríguez A., Hernández-Nistal J., Albornos L., Knox P., Dopico B., et al. Pectic galactan affects cell wall architecture during secondary cell wall deposition. Planta. 2020;251:100. doi: 10.1007/s00425-020-03394-2. [DOI] [PubMed] [Google Scholar]
- 98.Fromm J. Wood formation of trees in relation to potassium and calcium nutrition. Tree Physiol. 2010;30:1140–1147. doi: 10.1093/treephys/tpq024. [DOI] [PubMed] [Google Scholar]
- 99.Österås A.H., Greger M. Interactions between calcium and copper or cadmium in Norway spruce. Biol Plant. 2006;50:647. doi: 10.1007/s10535-006-0101-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.