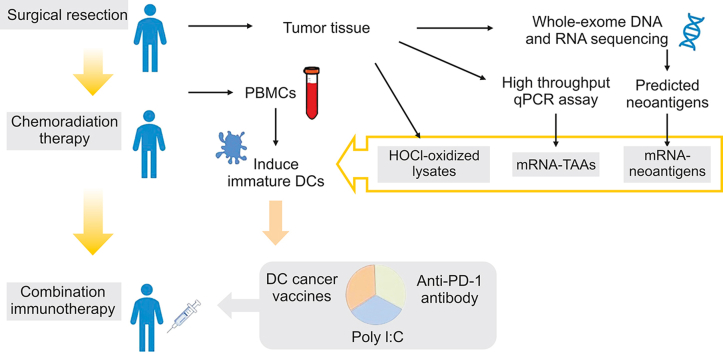
Abstract
Glioblastoma (GBM) is a lethal cancer with limited therapeutic options. Dendritic cell (DC)-based cancer vaccines provide a promising approach for GBM treatment. Clinical studies suggest that other immunotherapeutic agents may be combined with DC vaccines to further enhance antitumor activity. Here, we report a GBM case with combination immunotherapy consisting of DC vaccines, anti-programmed death-1 (anti-PD-1) and poly I:C as well as the chemotherapeutic agent cyclophosphamide that was integrated with standard chemoradiation therapy, and the patient remained disease-free for 69 months. The patient received DC vaccines loaded with multiple forms of tumor antigens, including mRNA-tumor associated antigens (TAA), mRNA-neoantigens, and hypochlorous acid (HOCl)-oxidized tumor lysates. Furthermore, mRNA-TAAs were modified with a novel TriVac technology that fuses TAAs with a destabilization domain and inserts TAAs into full-length lysosomal associated membrane protein-1 to enhance major histocompatibility complex (MHC) class I and II antigen presentation. The treatment consisted of 42 DC cancer vaccine infusions, 26 anti-PD-1 antibody nivolumab administrations and 126 poly I:C injections for DC infusions. The patient also received 28 doses of cyclophosphamide for depletion of regulatory T cells. No immunotherapy-related adverse events were observed during the treatment. Robust antitumor CD4+ and CD8+ T-cell responses were detected. The patient remains free of disease progression. This is the first case report on the combination of the above three agents to treat glioblastoma patients. Our results suggest that integrated combination immunotherapy is safe and feasible for long-term treatment in this patient. A large-scale trial to validate these findings is warranted.
Keywords: Glioblastoma multiforme, DC vaccine, Tumor-associated antigens, Neoantigens
Graphical abstract
Highlights
-
•
Combination immunotherapy consisting of DC vaccines, anti-PD-1 and poly I:C treating.
-
•
The DC vaccines were loaded with multiple forms of tumor antigens.
-
•
mRNA-TAAs were modified with a TriVac technology to enhance antigen presentation.
-
•
No immunotherapy related AEs were observed during the 69 months treatment.
-
•
The patient after surgery has been disease progression free for 69 months.
1. Introduction
Glioblastoma (GBM) remains one of the most lethal cancer types with limited effective treatment options [1]. Standard treatment for newly diagnosed GBM consisting of surgical resection and chemoradiotherapies yields a median overall survival of only 13.5 months [2]. Dendritic cell (DC)-based cancer vaccines provide a promising approach for GBM treatment [3]. Autologous DCs loaded with different forms of tumor antigens, such as mRNAs encoding tumor associated antigens (TAAs), tumor cell lysates, or synthetic peptides derived from TAAs, produced encouraging clinical outcomes [[4], [5], [6], [7]]. Recently, neoantigen-based cancer vaccines have also been tested in GBM patients. Neoantigen peptides induce potent antigen-specific T-cell responses and some clinical activities in GBM patients [8,9].
Several other immunotherapeutic agents have been investigated for GBM treatment. The anti-programmed death-1 (anti-PD-1) antibody nivolumab was combined with radiotherapy or chemoradiotherapy to treat newly diagnosed GBM with or without a methylated O-6-methylguanine-DNA methyltransferase (MGMT) promoter in two phase III trials [10,11]. The combination did not meet the primary endpoint of improving overall survival. However, combination therapy of anti-PD-1 and personalized cancer vaccines in other cancer types, including non-small cell lung cancer (NSCLC), melanoma, and bladder cancer, has shown promising clinical activities [4,12,13], suggesting a need to test this combination in GBM treatment. Multiple clinical trials have tested the safety and efficacy of the adjuvant poly-ICLC, a stabilized form of poly I:C, in combination with chemoradiotherapy or cancer vaccines to treat GBM [[14], [15], [16], [17], [18]]. These trials have shown good safety profiles and some promising clinical efficacy. Nevertheless, the above combinations are likely to need further addition of anti-PD-1 agents such as poly I:C to stimulate both programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) expression on primary human GBM cells and PD-L1 blockade to invigorate the immune stimulatory effect of poly I:C [19].
Here, we report a GBM case with integrated combination immunotherapy consisting of DC-based cancer vaccines, anti-PD-1 and poly I:C treatment. To maximize the possibility of cure, the patient received autologous DC vaccines loaded with multiple forms of tumor antigens, including mRNA-TAAs, mRNA-neoantigens, and hypochlorous acid (HOCl)-oxidized tumor lysates. The TAAs were modified with a novel TriVac technology that fuses TAAs with a destabilization domain (DD) or inserts TAAs into full-length lysosomal associated membrane protein 1 (LAMP-1) to enhance major histocompatibility complex (MHC) class I and II antigen presentation [20]. HOCI oxidation of whole tumor cells induces necrosis and increases the immunogenicity of tumor cells [21]. Personalized cancer vaccine trials showed that autologous DCs pulsed with HOCI-oxidized tumor lysates were safe and effective in eliciting broad antitumor immunity in patients with advanced-stage ovarian cancer [21,22]. To further enhance the vaccine-induced antitumor-specific T-cell responses, the patient also received low-dose cyclophosphamide for depletion of regulatory T cells [23,24]. No immunotherapy-related adverse events were observed during 69 months of treatment and follow-up observation. The patient had robust antitumor CD4+ and CD8+ T-cell responses and remained disease progression-free in the latest magnetic resonance imaging (MRI) scan. Our results suggest that integrated combination immunotherapy is safe and feasible for long-term treatment. A large-scale trial to validate these findings is warranted.
2. Materials and methods
2.1. Clinical study and case description
A combination immunotherapy study for GBM was approved by the Scientific Review Committee and the Ethics Committee of Xijing Hospital as a compassionate use protocol based on our previous phase I trial (NCT02709616) [4]. The study was carried out in accordance with the ethical principles of the Declaration of Helsinki. The protocol numbers of the study are 201708152377 and 20202014-F-1. The primary objective was to test the safety and tolerability of personalized DC cancer vaccines pulsed with different types of tumor antigens combining anti-PD-1 and poly I:C in patients with GBM. The secondary objectives were to investigate the clinical efficacy of an integrated combination immunotherapy and the degree of DC cancer vaccine-induced CD4+ and CD8+ T-cell responses with the goal of maximizing the possibility of a cure.
A 37-year-old patient with glioblastoma multiforme (World Health Organization (WHO) grade IV) was enrolled in this study at Xijing Hospital, Xi'an, China. The patient presented with a lack of verbal fluency and word-finding difficulty on July 17, 2017. MRI revealed a 3.8 cm × 5.8 cm × 3.6 cm contrast-enhancing lesion in the left frontal lobe. Histopathology showed that the patient had GBM (WHO IV) with an isocitrate dehydrogenase 1 (IDH1) R132H mutation, chromosome loss of heterozygosity 1p/19q−/− and 52% promoter methylation of MGMT. The patient underwent surgical resection on July 20, 2017, followed by a six-week standard chemoradiotherapy and 12 cycles of adjuvant chemotherapy. The first leukapheresis to collect peripheral blood mononuclear cells (PBMCs) for autologous DC generation was performed before chemoradiotherapy. Tumor tissues from surgical resection were stored at −80 °C for subsequent analysis of TAA/neoantigen expression and generation of HOCI-oxidized lysates. Conformal radiotherapy was performed with a 6 MV X-ray fractionated dose of 2.0 Gy up to a total dose of 60 Gy in 30 fractions. Concurrent chemotherapy was administered with temozolomide (TMZ) at a dose of 75 mg/m2 per day. Twelve cycles of chemotherapy with TMZ at 200 mg/m2 orally per day for five consecutive days per 28-day treatment cycle were completed in September 2018. Oxcarbazepine was prescribed in March 2018 at an initial dose of 0.9 g/day, gradually increased to 1.35 g/day, and then tapered and withdrawn in December 2021 to control postoperative seizures. Oxiracetam was used to improve memory impairments from March 2018 to May 2019. No steroids were used during the entire treatment. The process of the integrated combination immunotherapy is described in Section 3. The patient was evaluated every three months for treatment response and disease progression using immunotherapy response assessment for neuro-oncology criteria. Adverse events were evaluated and reported using common terminology criteria for adverse events v4.
2.2. Generation of autologous DC cancer vaccines
Autologous DC cancer vaccines were generated as previously described in our personalized cancer vaccine phase I trials [4]. Briefly, PBMCs were obtained via leukapheresis, further purified by density gradient centrifugation, and cryopreserved. The cells were thawed for each DC vaccine preparation one week before vaccination. PBMCs were cultured in 30 mL of AIM-V containing 800 IU/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 500 IU/mL interleukin 4 (IL-4) to induce immature DCs (iDCs). On day 6, iDCs were harvested and transfected with the individual mRNA-TAAs and mRNA-neoantigens by electroporation or pulsed with HOCl-oxidized tumor lysates. The antigen-loaded iDCs were matured in AIM-V containing 800 IU/mL GM-CSF, 500 IU/mL IL-4, 160 ng/mL IL-6, 5 ng/mL IL-1β, 5 ng/mL tumor necrosis factor alpha (TNF-α), and 1 μg/mL prostaglandin E2 (PGE2) for 18−20 h and pooled for quality control before injection. The DC cancer vaccines were infused intradermally and intravenously at a 1:5 route ratio. Detailed information on the tumor antigens used for each immunization and the number of infused DCs is shown in Table S1. The sources for all reagents, including cytokines, antibodies, and therapeutic agents, used in this study are listed in Table S2.
2.3. Preparation of autologous HOCl-oxidized tumor lysates and antigen loading of DCs
HOCl-oxidized tumor lysate was prepared as previously described [21]. The HOCl concentration was determined by spectrophotometry at an absorbance of 252 nm. A final concentration of 60 M HOCl solution was prepared by diluting the stock NaOCl reagent (Sigma-Aldrich, St. Louis, MO, USA) with phosphate-buffered saline (Gibco, Jenks, OK, USA). HOCl solution was added immediately to the tumor cells to a final density of 106 cells/mL. The tumor cell suspension was incubated at 37 °C and 5% CO2 for 1 h with gentle agitation every 30 min to induce oxidation-dependent tumor cell death. After incubation, tumor cells were harvested, washed twice with phosphate-buffered saline and frozen/thawed for 6 cycles. The oxidized tumor lysates were tested for complete removal of the HOCl solution before loading into DCs. Aliquoted HOCl-oxidized tumor lysates were stored at −80 °C. HOCl-oxidized tumor cell lysates were cocultured with normal donor DCs (at a 1:1 ratio of original tumor cells to normal donor DCs) for 20−24 h at 37 °C for antigen loading. The antigen-loaded DCs were harvested, washed, and injected.
2.4. Whole-exome DNA and RNA sequencing
Genomic DNA and total RNA were extracted from paired tumor and paratumor samples for whole-exome sequencing and transcriptome sequencing (Anoroad Genome Ltd., Beijing, China). Genomic DNA was fragmented to a target size of 150–200 bp. Exons were captured using the Agilent SureSelect Human All Exon V6 Kit (Santa Clara, CA, USA) and subsequently sequenced on a HiSeq2500 (Illumina, San Diego, CA, USA) in paired-end sequencing of 150 bp forward and 150 bp reverse. Sequencing was completed using the HiSeq SBS Kit v4 (Illumina) with a target coverage of 100× for tumor samples and 80× for paired para-tumor samples. Sequence data were analyzed with the Genome Analysis Toolkit Best Practice Pipeline. Total RNA from paired tumor and paratumor samples was extracted and sequenced by a HiSeq2500 system. RAW data were analyzed with the MapSplice-RSEM-EBSeq pipeline.
2.5. Neoantigen prediction and selection
Human leukocyte antigen (HLA) typing was performed using the polymerase chain reaction (PCR) sequencing-based typing method. The reads are aligned to reference HLA sequences from the IPD-IMGT/HLA Database version 3.16. Somatic mutations of the tumor tissue were identified by MuTect. Missense mutation expression was identified using transcriptome sequencing and validated with quantitative reverse transcription PCR (qRT-PCR). Expressed mutations were selected for immunogenicity prediction. First, all mutated sequences and wild-type sequences were translated to strings of 27 amino acids, with the mutated amino acid centrally situated. Second, all mutant and wild-type MHC-I and MHC-II epitope binding affinities were evaluated by NetMHCpan 4.0 (http://tools.immuneepitope.org/mhci/) and the consensus method (http://tools.immuneepitope. org/mhcii/), respectively. All mutations with binding scores below 500 nM were defined as neoantigens and subsequently selected for mRNA and peptide synthesis. A total of 49 somatic mutations were identified in the tumor sample, including 21 missense mutations and 28 nonsense mutations. The mRNA expression of all mutated genes was examined by quantitative PCR (qPCR), and 10 of the mutated genes were detected in the patient's tumor sample. The 10 putative neoantigens and their corresponding wild-type peptides were synthesized (Genscript, Piscataway, NJ, USA) and tested for in vitro T-cell stimulation. Four neoantigens with equal or greater T-cell stimulation capability compared to their wild-type counterparts were selected to generate mRNA-neoantigens for immunization.
2.6. Measurement of mRNA expression of TAAs
Total RNA was extracted from tumor samples obtained by resection and reverse transcribed. The cDNAs were analyzed by qPCR for the expression of TAAs. All reactions were performed in triplicate. The primers for the tested TAA genes were previously described [4]. The mRNA expression levels of TAAs in the tumor samples were compared with those in paratumor samples and are shown as the mean fold changes.
2.7. Tumor antigen DNA constructs and good manufacturing practices (GMP) mRNA production
To enhance tumor antigen presentation by MHC class I and class II pathways, full-length TAAs were modified with a novel TriVac technology [20]. The TAAs were either inserted between the sorting signal and the luminal domain of full-length LAMP-1 or fused with a DD as described. Mutations in human FK506-binding protein 12 created unstable proteins that subject the proteins fused with the mutants to rapid proteasomal degradation, therefore functioning as a destabilizing domain [25]. We tested antigen presentation by MHC class I complexes with TAAs fused with DD, which resulted in rapid proteasomal degradation and enhanced MHC class I antigen presentation (unpublished data). Modification of TAAs with TriVac technology also enhanced the antitumor activities of cancer vaccines in mouse models (unpublished data). For neoantigen constructs, a string of 27 amino acids with the mutation residue centrally situated was inserted between the sorting signal and the luminal domain of a full-length LAMP-1. Plasmids were all linearized by digestion with SpeI-HF (NEB, Ipswich, MA, USA) and used as templates in in vitro transcription reactions. In vitro transcription was performed with T7 RNA polymerase (mMESSAGE mMACHINE T7 Transcription kit, Ambion, Carlsbad, CA, USA) according to the manufacturer's instructions. The transcribed RNA was purified after TURBO DNase (Ambion) digestion on RNeasy columns (Qiagen, Venlo, the Netherlands) according to the manufacturer's instructions. RNA quality was verified by the Agilent 2100 Bioanalyzer to ensure that the levels of endotoxin contamination were below 0.25 EU/μg. RNA concentration was measured by Nanodrop. Manufactured mRNA-TAAs or mRNA-neoantigens were stored at −80 °C in aliquots. All mRNAs encoding full-length TAAs were premanufactured, while mRNAs encoding neoantigens were manufactured after their identification and selection in a GMP facility.
2.8. In vitro stimulation of PBMCs to select neoantigens
For in vitro T-cell stimulation to select neoantigens, day 6 iDCs were pulsed with 10 neoantigen or wild-type peptides for 24 h at 2 μg/mL and matured for 18−20 h. Then, the nonadherent T cells and antigen-loaded DCs were cocultured at an effector-to-target (E:T) ratio of 10:1 in the presence of 10 ng/mL IL-7. Three days after stimulation, fresh culture medium containing 100 IU/mL IL-2 and 10 ng/mL IL-7 was added every two to three days. After 12−14 days of stimulation, cells were restimulated with peptide-pulsed DCs and analyzed by flow cytometry.
2.9. Detection of tumor antigen-specific T cells after DC vaccination
PBMCs collected at different times plated at 5 × 105/well in 48-well plates were stimulated with autologous TAA-mRNA- or neoantigen-mRNA-transfected mature DCs (mDCs) at a 10:1 ratio in the presence of 10 ng/mL IL-7. On day 12, nonadherent T cells were restimulated with tumor antigen mRNA-transfected DCs for 6 h in RPMI 1640 with 10% fetal bovine serum (FBS) and GolgiStop (BD Biosciences, Franklin Lakes, NJ, USA). Tumor antigen-specific T cells were determined using intracellular staining of interferon-gamma (IFN-γ) and TNF-α. The in vitro stimulated T cells were stained with LIVE/DEAD-AmCyan and a panel of cell surface markers, including CD3-FITC, CD4-PE/Cy7, and CD8-PerCP, followed by fixation/permeabilization and staining with PE-anti–IFN–γ and APC-anti-TNF-α.
3. Results
3.1. Integrated combination immunotherapy with DC vaccines plus anti-PD-1 and poly I:C
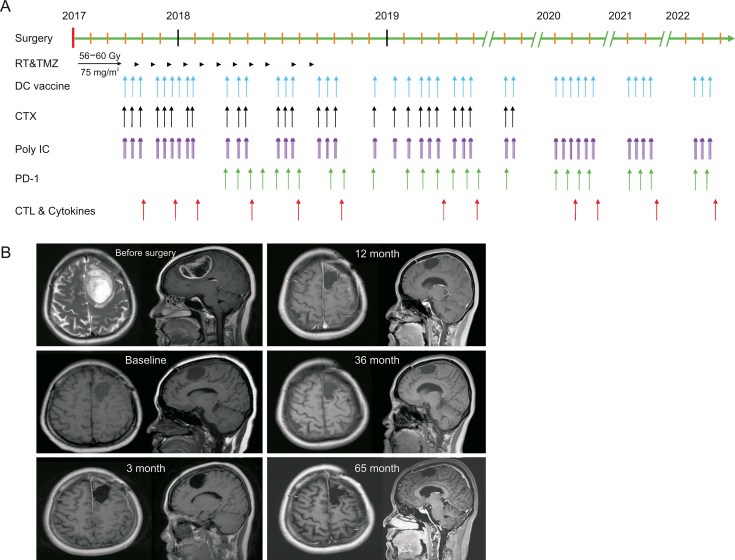
The overall combination immunotherapy strategy was based on our phase I trial of personalized cancer vaccine therapy [4] aiming to maximize the possibility of a cure. All immunotherapeutic interventions were integrated into the standard treatment procedure for GBM patients (Fig. 1A). DC vaccine therapy started one week after completion of the concurrent chemoradiation therapy and was administered every two to four weeks in the first two years with some long intervals in the last three years (Fig. 1A). The patient received a total of 42 DC cancer vaccines loaded with mRNA-TAAs, mRNA-neoantigens or HOCl-oxidized lysates from October 17, 2017 to May 4, 2022 (Fig. 1A and Table S1). The doses for the DC vaccines ranged from 2 × 106 to 3.5 × 107 cells per infusion, with most of the vaccinations at >1.5 × 107 cells (Table S1). Low-dose cyclophosphamide (200 mg/m2) was administered intravenously 24 h before each vaccination in the first 30 months (28 injections for a total of 8.4 g) to deplete Tregs [23,24] (Fig. 1A). Cyclophosphamide was discontinued at the discretion of treating physicians. To further enhance the clinical efficacy of DC cancer vaccines, the anti-PD-1 antibody nivolumab was administered from April 2018 to April 2022 at 100 mg (26 injections) during vaccinations. Poly I:C, an approved immune adjuvant in China [4], was injected at 3 mg intramuscularly 3 times every two days after each DC vaccination (Fig. 1A).
Fig. 1.
Schematic diagram of the treatment process and magnetic resonance imaging scan results. (A) The process of an integrated combination immunotherapy from 2017 to 2022. The arrow indicates the timing of the events. (B) Magnetic resonance imaging (MRI) results during the treatment. The labeled month refers to the time after surgery. RT: radiotherapy. TMZ: temozolomide; DC: dendritic cell; PD-1: programmed death-1; CTX: cyclophosphamide; CTL: cytotoxic T lymphocytes.
The patient was regularly monitored. MRI was performed every three months to monitor disease, and peripheral blood was drawn at 7 to 10 days after every three vaccine administrations to monitor immune responses. The patient's Karnofsky performance status (KPS) score was 70 immediately after the surgery, and the symptoms of speech and word-finding difficulty were gradually alleviated. The patient continued to live a normal life with a KPS of 90–100 at one year after the surgery. MRI results showed no signs of recurrence in November 2022 (Fig. 1B).
3.2. Safety of the combination immunotherapy
The combination of DC cancer vaccines, anti-PD-1 and poly I:C treatment was well tolerated. No reportable adverse events associated with DC vaccinations, anti-PD-1 and poly I:C treatment were observed. One temporary syncope and four periods of short-term lack of verbal fluency were recorded and attributed to postoperative seizures. The patient's peripheral lymphocyte counts gradually decreased during the first 20 months of treatment, which was attributed to chemoradiation and 12 cycles of TMZ.
3.3. Tumor antigen identification and modification
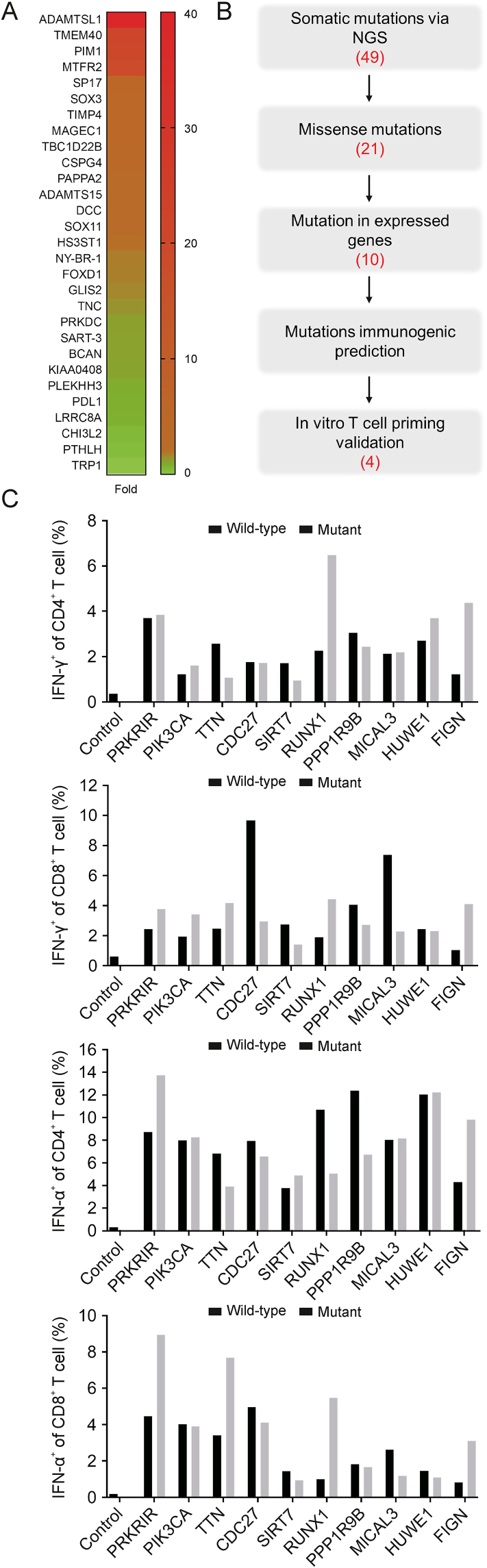
To maximize the possibility of disease cure, three types of tumor antigens, mRNA-TAAs, HOCl-oxidized lysates, and mRNA-neoantigens, were used to load autologous DCs. A high-throughput qPCR assay identified overexpressed TAAs in the patient's tumor tissue (Fig. 2A). The following criteria were used to select TAAs for DC vaccine: a >2-fold expression compared to that in para-tumor tissue, published studies reporting detection of TAA-specific T cells in cancer patients, and low relative expression in normal human tissues as reported in the Gencode site (www.gencodegenes.org). A total of 7 mRNA-TAAs, including ADAMTSL1, PIM1, MTFR2, SP17, SOX3, CSPG4, and SOX11, were used to load autologous DCs (Fig. 2A and Table S1). In addition, mRNA-CMVpp65 was included as a target antigen for GBM treatment in the first 3 immunizations based on previous clinical trials [26,27]. In the first phase of immunizations, nine DC vaccines with mRNA-TAAs fused with the sorting signal (transmembrane and cytoplasmic domains) of LAMP-1 were infused (Nos. 1−3 and 7−12, Table S1). In the second phase of immunizations, 22 DC vaccines with mRNA-TAAs modified with a new TriVac platform were infused [20] (Nos. 20−41, Table S1). Full-length TAAs were inserted between the luminal domain and sorting signal (transmembrane and cytoplasmic domains) of full-length LAMP-1, as this modification elicited stronger cellular and humoral immune responses than fusing antigen only to the LAMP-1 sorting signal [28]. Furthermore, we devised a new strategy to enhance MHC class I antigen presentation by targeting antigens for proteasomal degradation with fusion of TAAs to DD. The 7 TAAs were either inserted into a full-length LAMP-1 or fused with DD to generate individual mRNA-TAAs. DC cells were divided into equal portions for mRNA-TAA electroporation and pooled for infusion.
Fig. 2.
Identification and selection of tumor associated antigens and neoantigens. (A) Heatmap of mRNA expression of tumor associated antigens (TAAs) in the patient's tumor sample. The mRNAs of the indicated TAAs in tumor samples from resection were assayed by quantitative PCR (qPCR) in triplicate, and the expression levels of the measured genes were expressed as the mean fold changes. (B) Procedure for neoantigen identification and selection. The number of identified candidates is indicated for each step. (C) In vitro T-cell responses to synthesized neoantigen and wild-type peptides. Peripheral blood mononuclear cells (PBMCs) were stimulated with autologous dendritic cell (DC) pulsed with antigen peptides for 12 days and restimulated for 6 h. T cells were stained for intracellular expression of tumor necrosis factor alpha (TNF-α) and interferon-gamma (IFN-γ), and percentages of cytokine-expressing CD4+ or CD8+ T cells are shown. NGS: next generation sequencing.
To identify potential neoantigens as vaccine targets, we performed whole exome sequencing (WES) and RNAseq of the patient's tumor tissue. WES and RNAseq revealed 21 missense mutations with 10 of the mutated genes transcribed (Fig. 2B). To further select these tentative neoantigens, we stimulated the patient's PBMCs with synthesized wild-type and neoantigen peptides. Four of the 10 predicted neoantigens, PRKRIR, FLGN, HUWE1, and RUNX1, stimulated specific T-cell responses equal to or greater than those stimulated by wild-type antigenic peptides (Fig. 2C). mRNA-neoantigens for these four neoantigens were generated and electroporated into autologous DCs for immunization (Nos. 13−19, 24, and 42, Table S1).
Although encouraging results were reported on using HOCl-oxidized tumor lysates in DC-based cancer vaccine clinical trials [21,22], the antigens are limited by the amount of tumor tissue obtained from resection. The patient's HOCl-oxidized tumor lysates were only sufficient for use in three immunizations (Nos. 4−6, Table S1).
3.4. Specific T-cell responses to TAAs and neoantigens
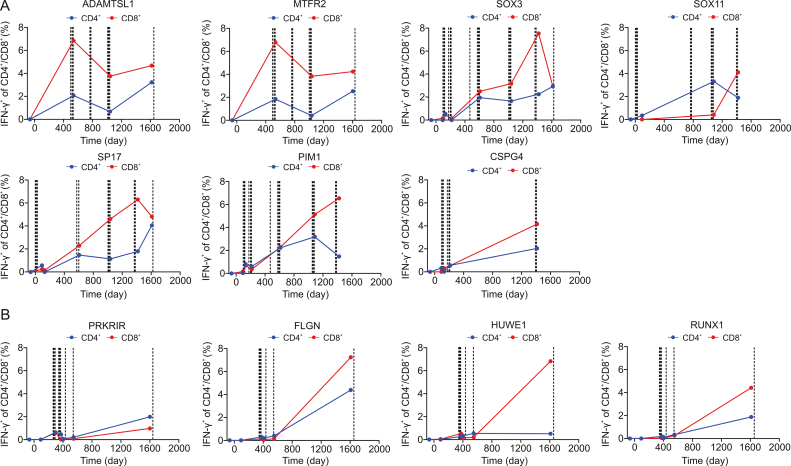
To examine tumor antigen-specific T-cell responses to the immunized TAAs and neoantigens, peripheral T cells taken at different time points during the treatment (Fig. 1A) were stimulated with mRNA-TAA- or mRNA-neoantigen-transfected autologous DCs in vitro and assayed for intracellular production of IFN-γ. Among the seven TAAs, five TAAs (SOX11, PIM1, SP17, SOX3, and CSPG4) modified with the sorting signal of LAMP-1 or full-length LAMP-1 and DD were used in the first and second phase immunizations, respectively (Fig. 3A and Table S1). Two TAAs (MTFR2 and ADAMTSL1) modified only with the new TriVac technology (full-length LAMP-1 and DD) were used in the second phase immunization (Fig. 3A and Table S1). DC vaccines induced strong antigen-specific CD4+ and CD8+ T-cell responses to all seven TAAs (Fig. 3A). The antigen-specific T-cell responses to the five TAAs were relatively weak after the first phase of immunization but substantially enhanced during the second phase of immunization (Fig. 3A). Interestingly, anti-MTFR2-and anti-ADAMTSL1-specific T-cell responses were strongly induced upon immunization in the second phase (Fig. 3A). Specific T-cell responses to these TAAs were maintained until the cutoff time of this study (Fig. 3A).
Fig. 3.
Antigen-specific T-cell responses to tumor associated antigens (TAAs) and neoantigens. All timepoint data was normalized with the data that collected before DC vaccine treatment. (A) Kinetics of antigen-specific CD4+ and CD8+ T-cell responses against TAAs. (B) Kinetics of antigen-specific CD4+ and CD8+ T-cell responses against neoantigens. Dotted lines in each panel indicate DC immunizations for that specific TAA or neoantigen. IFN-γ: interferon-γ.
Antigen-specific CD4+ and CD8+ T cells to the immunized neoantigens were readily detectable upon DC vaccinations (Fig. 3B). The frequency of antigen-specific T cells increased markedly at the late phase (Fig. 3B), suggesting antigen-specific T-cell expansion during the interval.
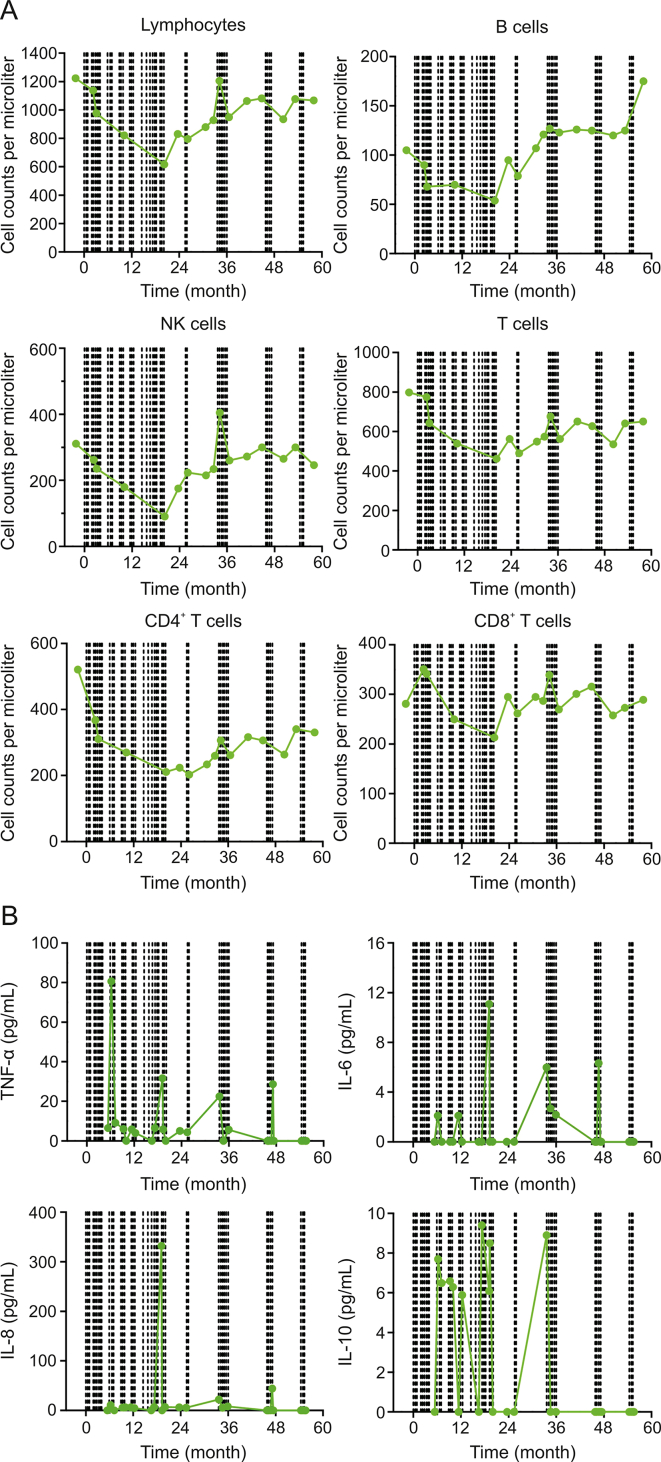
3.5. Immune cell and cytokine dynamics during the 5-year treatment
Circulating immune cell subsets and cytokine levels were monitored during the treatment. Total counts of peripheral lymphocytes, including CD4+ and CD8+ T cells, B cells and natural killer (NK) cells, gradually decreased during the first 20 months of treatment (Fig. 4A). The immune cell subsets began to increase at 20 months and stabilized at 26 months (Fig. 4A). Interestingly, these cells had a transient increase coincidental with 6 DC vaccine immunizations during this period (Fig. 4A and Table S1).
Fig. 4.
Dynamics of peripheral immune cell subsets and cytokine levels. (A) Cell counts of total lymphocytes and subsets during the course of the treatment. (B) Cytokine levels during the course of the treatment. Dotted lines in each panel indicate dendritic cell (DC) immunizations for any tumor associated antigens or neoantigens. NK: natural killer; TNF: tumor necrosis factor; IL: interleukin.
A total of 12 cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, IFN-α, IFN-γ, and TNF-α) in circulation were monitored. Four cytokines (TNF-α, IL-6, IL-8, and IL-10) displayed changes above the baseline levels (Fig. 4B), while the other eight cytokines did not (data not shown). Increased expression of these four cytokines was observed during therapy (Fig. 4B), suggesting that DC vaccination activated cytokine responses.
4. Discussion
In this report, we present a patient with GBM treated with long-term combination immunotherapy consisting of DC vaccines, anti-PD-1 and poly I:C as well as the chemotherapeutic agent cyclophosphamide. Several novel aspects were observed in this case study. First, this case represents the first study combining three immunotherapeutic agents in GBM treatment. Although all three immunotherapeutic agents have been individually tested in GBM, there has been no report of combining them. Second, combination immunotherapy can be performed in the long term and appears to be safe and effective. The study was originally designed to test the safety and tolerability of combination immunotherapy in an attempt to maximize the possibility of cure. The patient's 69 months of progression-free survival is very encouraging. Third, the different forms of tumor antigens, including mRNA-TAAs, mRNA-neoantigens and HOCl-oxidized tumor lysates, for priming antitumor T-cell responses may complement each other in promoting vaccine efficacy. Fourth, TriVac technology-based modification of tumor antigens appears to be safe and generates robust antitumor-specific CD4+ and CD8+ T-cell responses in the patient.
Our preliminary data support the notion that combination therapy with different immunotherapeutic agents may be needed to treat GBM. Although the recent two phase III trials failed to demonstrate improved overall survival with nivolumab combined with radiotherapy or chemoradiotherapy in treating newly diagnosed GBM [10,11], the addition of anti-PD-1 to personalized cancer vaccines has shown promising clinical activities in NSCLC, melanoma, and bladder cancer [4,12,13]. In our study, the addition of nivolumab serves two purposes. First, mature DCs, the final products for infusion, upregulate both PD-L1 and PD-L2 (unpublished data). Second, poly I:C stimulates both PD-L1 and PD-L2 expression on primary human GBM cells [19]. Thus, nivolumab blocks the inhibitory signals of PD-L1/2 on mDCs and GBM tumor cells from being delivered to T cells.
This case study shows a promising disease progression-free status and an excellent safety profile in the patient upon long-term combination immunotherapy. DC vaccination was initiated at one week after the completion of concurrent chemoradiation and continued for five years, achieving a cure status. Consistent with our previous phase I trial safety profile [4], no immunotherapy-related adverse events were observed. Cyclophosphamide was discontinued out of precaution by the physician team based on an 8.4 g cumulative dose. Although the results are encouraging, other favorable factors, including young age, IDH1 mutation, and MGMT promoter methylation, may also contribute to improved survival. Therefore, a large trial to validate the efficacy of this combination immunotherapy in newly diagnosed GBM is needed.
Three approaches for preparing the tumor antigens were used in this case study to induce a wide array of antitumor T cells required to cover the heterogeneous expression of tumor antigens in GBM [4,29]. These approaches, each with its edges and limitations, may complement each other to serve as targets for long-term cancer vaccinations. Although HOCl-oxidized tumor lysates with enhanced immunogenicity have a major advantage as a source of tumor antigens containing both TAAs and neoantigens, procurement of a sufficient amount of tumor tissue for long-term vaccination is not feasible. In contrast, in vitro synthesized mRNA-encoded TAAs and neoantigens provide unlimited supplies. However, the use of TAAs is hampered by their poor immunogenicity compared to neoantigens due to host tolerance mechanisms [20]. Modification of TAAs with TriVac technology enhances both MHC class I and II antigen presentation and T-cell stimulation [20]. Neoantigen-based cancer vaccines induce a potent antigen-specific T-cell response and may have some clinical activities in GBM patients [8,9]. Nevertheless, two major issues exist for the use of neoantigens in GBM treatment. First, GBM has a low tumor mutation burden with few neoantigens to select, and second, manufacturing GMP grade mRNA-neoantigens takes time. Therefore, the combined use of mRNA-TAAs and neoantigens in this case is necessary to resolve the above issues.
Our data support further investigation into the clinical application of TriVac technology [20]. We previously tested the capacity of mRNA-TAAs modified with the sorting signal of LAMP-1 to induce antigen-specific T cells in cancer patients [4]. The new TriVac technology platform uses full-length LAMP-1 and a DD to modify tumor antigens to enhance antigen presentation to both MHC class I and II complexes. Consistent with this notion, two TAAs (MTFR2 and ADAMTSL1) modified only with this new technology induced robust antigen-specific T-cell responses. In contrast, five TAAs (SOX11, PIM1, SP17, SOX3, and CSPG4) modified with the sorting signal of LAMP-1 in the first phase of immunization induced relatively weak antigen-specific T-cell responses, while these five TAAs induced much stronger T-cell responses in the second phase of immunization after modification with TriVac technology. Alternatively, the enhanced antigen-specific T-cell responses could result from a general elevated systemic immune activation status derived from nivolumab and poly I:C treatment. These possibilities need to be examined in more detailed clinical studies. The mRNA-neoantigen vaccines also induced significant levels of antigen-specific T-cell responses. Whether these antigen-specific T cells play any roles in the patient's disease-free status remains to be determined.
5. Conclusions
In conclusion, we report here a GBM case with long-term integrated combination immunotherapy characterized by good safety and 69 months of disease progression-free survival, suggesting that integrated combination immunotherapy is safe and feasible for long-term treatment in GBM patient. Future large-scale studies are warranted to validate our findings.
CRediT author statement
Ping Zhu: Funding acquisition, Project administration, Conceptualization, Supervision, Investigation, Resources, Formal analysis, Writing - Reviewing and Editing; Shi-You Li: Supervision, Formal analysis, Investigation; Jin Ding: Formal analysis, Investigation, Writing - Original draft preparation, Reviewing and Editing, Visualization; Zhou Fei: Resources, Investigation; Sheng-Nan Sun: Formal analysis, Investigation, Writing - Original draft preparation, Reviewing and Editing, Visualization; Zhao-Hui Zheng: Resources, Investigation; Ding Wei: Visualization, Writing - Reviewing and Editing; Jun Jiang: Formal analysis; Jin-Lin Miao: Investigation, Data curation; San-Zhong Li: Resources, Investigation; Xing Luo, Kui Zhang, Bin Wang, Kun Zhang, Su Pu, Qian-Ting Wang, and Xin-Yue Zhang: Investigation; Gao-Liu Wen: Data curation; Jun O. Liu: Methodology, Writing - Reviewing and Editing; John Thomas August: Methodology; Huijie Bian: Funding acquisition, Project administration, Supervision, Writing - Reviewing and Editing; Zhi-Nan Chen: Funding acquisition, Project administration, Conceptualization, Formal analysis, Writing - Reviewing and Editing; You-Wen He: Project administration, Conceptualization, Methodology, Formal Analysis, Visualization, Writing - Original draft preparation, Reviewing and Editing.
Declaration of competing interest
You-Wen He and Shi-You Li are co-founders of Tricision Biotherapeutics Inc., and the contribution of You-Wen He in this project was through his scientific advisor to tricision Biotherapeutic Inc. Sheng-Nan Sun, Jun Jiang, Qian-Ting Wang, and Shi-You Li are full-time employees of Tricision Biotherapeutics Inc. You-Wen He and Jun O. Liu are co-inventors of the TriVac technology patented by Duke University and Johns Hopkins University and co-founders of TriVac Inc. TriVac technology used by Tricision Biotherapeutics Inc. was licensed by Duke/JHU through TriVac Inc. Other authors declare that there are no conflicts of interest.
Acknowledgments
We thank the patient for many contributions to this study. We thank Dr. Edward F. Patz Jr. and Dr. Elizabeth B. Gottlin for critical reading of the manuscript. The study was supported by Natural Science Foundation of Shaanxi Province (Grant No.: 2019ZY-CXPT-03-01) to Ping Zhu and Key Research and Development Program of Shaanxi Province (Grant No.: 2020ZDLSF03-02) to Zhi-Nan Chen and Huijie Bian as well as Tricision Biotherapeutics Inc.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
This article has been published as preprint in Research Sequare (https://www.researchsquare.com/). The DOI of the preprint is https://doi.org/10.21203/rs.3.rs-2199151/v1.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.04.012.
Contributor Information
Ping Zhu, Email: zhuping@fmmu.edu.cn.
Huijie Bian, Email: znchen@fmmu.edu.cn.
Zhi-Nan Chen, Email: hjbian@fmmu.edu.cn.
You-Wen He, Email: youwen.he@duke.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.A. Rodríguez-Camacho, J.G. Flores-Vázquez, J. Moscardini-Martelli, et al., Glioblastoma treatment: State-of-the-art and future perspectives, Int. J. Mol. Sci. 23 (2022), 7207. [DOI] [PMC free article] [PubMed]
- 2.Marenco-Hillembrand L., Wijesekera O., Suarez-Meade P., et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neurooncol. 2020;147:297–307. doi: 10.1007/s11060-020-03451-6. [DOI] [PubMed] [Google Scholar]
- 3.Lv L., Huang J., Xi H., et al. Efficacy and safety of dendritic cell vaccines for patients with glioblastoma: A meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106336. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q.-T., Nie Y., Sun S.-N., et al. Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients. Cancer Immunol. Immunother. 2020;69:1375–1387. doi: 10.1007/s00262-020-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liau L.M., Ashkan K., Tran D.D., et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018;16:142. doi: 10.1186/s12967-018-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J.L., Omofoye O.A., Rudnick J.D., et al. A phase I study of autologous dendritic cell vaccine pulsed with allogeneic stem-like cell line lysate in patients with newly diagnosed or recurrent glioblastoma. Clin. Cancer Res. 2022;28:689–696. doi: 10.1158/1078-0432.CCR-21-2867. [DOI] [PubMed] [Google Scholar]
- 7.Wen P.Y., Reardon D.A., Armstrong T.S., et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin. Cancer Res. 2019;25:5799–5807. doi: 10.1158/1078-0432.CCR-19-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilf N., Kuttruff-Coqui S., Frenzel K., et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 9.Keskin D.B., Anandappa A.J., Sun J., et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omuro A., Brandes A.A., Carpentier A.F., et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro Oncol. 2023;25:123–134. doi: 10.1093/neuonc/noac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omuro A., Reardon D.A., Sampson J.H., et al. Nivolumab plus radiotherapy with or without temozolomide in newly diagnosed glioblastoma: Results from exploratory phase I cohorts of CheckMate 143, Neurooncol. Adv. 2022;4 doi: 10.1093/noajnl/vdac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott P.A., Hu-Lieskovan S., Chmielowski B., et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. 2020;183:347–362.e24. doi: 10.1016/j.cell.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 13.Awad M.M., Govindan R., Balogh K.N., et al. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell. 2022;40:1010–1026.e11. doi: 10.1016/j.ccell.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Butowski N., Chang S.M., Junck L., et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: A North American Brain Tumor Consortium (NABTC01-05) J. Neurooncol. 2009;91:175–182. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenfeld M.R., Chamberlain M.C., Grossman S.A., et al. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12:1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada H., Kalinski P., Ueda R., et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Migliorini D., Dutoit V., Allard M., et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro Oncol. 2019;21:923–933. doi: 10.1093/neuonc/noz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boydell E., Marinari E., Migliorini D., et al. Exploratory study of the effect of IMA950/poly-ICLC vaccination on response to bevacizumab in relapsing high-grade glioma patients. Cancers (Basel) 2019;11 doi: 10.3390/cancers11040464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Waele J., Marcq E., Van Audenaerde J.R., et al. Poly(I:C) primes primary human glioblastoma cells for an immune response invigorated by PD-L1 blockade. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2017.1407899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu L., Jiang J., Zhan M., et al. Targeting tumor-associated antigens in hepatocellular carcinoma for immunotherapy: Past pitfalls and future strategies. Hepatology. 2021;73:821–832. doi: 10.1002/hep.31502. [DOI] [PubMed] [Google Scholar]
- 21.Chiang C.L., Kandalaft L.E., Tanyi J., et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin. Cancer Res. 2013;19:4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanyi J.L., Bobisse S., Ophir E., et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao5931. [DOI] [PubMed] [Google Scholar]
- 23.Walter S., Weinschenk T., Stenzl A., et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 24.Emens L.A., Asquith J.M., Leatherman J.M., et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: A chemotherapy dose-ranging factorial study of safety and immune activation. J. Clin. Oncol. 2009;27:5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banaszynski L.A., Chen L.-C., Maynard-Smith L.A., et al. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batich K.A., Reap E.A., Archer G.E., et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin. Cancer Res. 2017;23:1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell D.A., Batich K.A., Gunn M.D., et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marques E.T.A., Chikhlikar P., de Arruda L.B., et al. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine Chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J. Biol. Chem. 2003;278:37926–37936. doi: 10.1074/jbc.M303336200. [DOI] [PubMed] [Google Scholar]
- 29.Li J.-Q., Wang Q.-T., Nie Y., et al. A multi-element expression score is a prognostic factor in glioblastoma multiforme. Cancer Manag. Res. 2019;11:8977–8989. doi: 10.2147/CMAR.S228174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.