Abstract
Objectives
Glioma is one of the most aggressive brain tumours with poor overall survival despite advanced technology in surgical resection, chemotherapy and radiation. Progression and recurrence are the hinge causes of low survival. Our aim is to explain the concrete mechanism in the proliferation and progression of tumours based on tumour microenvironment (TME). The main purpose is to illustrate the mechanism of proton pump inhibitors (PPIs) in affecting acidity, hypoxia, oxidative stress, inflammatory response and autophagy based on the TME to induce apoptosis and enhance the sensitivity of chemoradiotherapy.
Findings
TME is the main medium for tumour growth and progression. Acidity, hypoxia, inflammatory response, autophagy, angiogenesis and so on are the main causes of tumour progress. PPIs, as a common clinical drug to inhibit gastric acid secretion, have the advantages of fast onset, long action time and small adverse reactions. Nowadays, several kinds of literature highlight the potential of PPIs in inhibiting tumour progression. However, long‐term use of PPIs alone also has obvious side effects. Therefore, till now, how to apply PPIs to promote the effect of radio‐chemotherapy and find the concrete dose and concentration of combined use are novel challenges.
Conclusions
PPIs display the potential in enhancing the sensitivity of chemoradiotherapy to defend against glioma based on TME. In the clinic, it is also necessary to explore specific concentrations and dosages in synthetic applications.
Pump proton inhibitors (PPIs) produce a marked effect on the pump proton on stomach. However, nowadays, PPIs have been discovered the potential in anti‐tumour. Many kinds of cytokines and signal pathways in tumour microenvironment play an essential role in fight tumour. Oxidative stress, inflammation and autophagy are also main factors inducing proliferation and metastasis of tumour. PPIs have the ability in anti‐tumour through acting on oxidative stress, inflammation and autophagy based on cytokines and signal pathways to induce tumour cells damage. Anti‐cancer is a long process. However, using PPIs alone for a long time will have strong side effects. Therefore, at present, using PPIs to sensitize radiotherapy and chemotherapy is a valuable way. Most studies have shown the effect of PPIs on gastric cancer and lung cancer, but there are few studies on glioma. This research analyzes the concrete mechanism that PPIs can be used to treat glioma, so as to provide basis for clinical practice in the future.
1. BACKGROUND
Glioma is one of the most aggressive brain tumours. Histologically, the World Health Organization (WHO) classified the tumours of the central nervous system (CNS) into astrocytomas, oligodendrogliomas and ependymoma. In addition, gliomas are classified into four grades according to the degree of malignancy. Types I and II are low‐grade gliomas and types III and IV are high‐grade gliomas. 1 , 2 , 3 The clinical cure rate and 5‐year survival rate of patients are all very low. The prognosis is very dismal and the average survival period is only about 1 year. One of the main reasons for the poor prognosis of patients is that glioma is prone to drug resistance to chemoradiation therapy resistance. 4 , 5 Therefore, it is urgent to explore new strategies for glioma treatment.
Pump proton inhibitors (PPIs) are a drug of choice for inhibiting gastric acid secretion. In clinical, they are the first‐line option to treat peptic ulcers, gastroesophageal reflux disease, zoai syndrome and upper gastrointestinal bleeding. 6 , 7 It has become the first‐line drug for abnormal gastric acid secretion and related diseases combined with amoxicillin, clarithromycin and other drugs to treat Helicobacter pylori infection. 8 , 9 PPIs have the advantages of fast onset, strong acid inhibition, long action time, low blood drug concentration and low adverse reactions. 10 The first generation includes omeprazole, lansoprazole and pantoprazole; the second generation includes iprazole, rabeprazole and esomeprazole. Compared with the first‐generation PPIs, in terms of drug properties, the second‐generation PPIs have the advantages of higher bioavailability, less affected by food and anti‐acid drugs, slow plasma clearance, less first‐pass effect after oral administration, higher stability, less adverse reactions and longer half‐life. In clinical, the second‐generation PPIs have better effects in inhibiting gastric acid secretion and H. pylori. Besides, they are more effective for relieving pain and the treatment of ulcers, especially, the coalescence of duodenal bulb ulcers. While combined with other medicines, the second‐generation PPIs revealed higher security and effectiveness. 11 , 12 , 13
Progression and invasion are the main causes of death in malignant tumour patients. 14 The tumour microenvironment (TME) is a multi‐complex environment regulated by various cytokines, transcription factors and pathways. 15 One major cause of the progression and invasion is set off by the intimate relationship of glioma cells with the microenvironment. Recent therapeutic anti‐tumour approaches focus on such critical components of TME. 16 , 17
Thus, we searched Pubmed and Cnki conducting for this review. Our aim is to explain the specific relationship between TME and the progression of glioma. PPIs, the main force for treating the stomach, were found the proficiency to promote the sensitivity of glioma to chemoradiotherapy. Thus, associating with the mechanism of PPIs, another crucial purpose is to make clear the mechanism of PPIs in hypersensitization to inhibit proliferation and progression of glioma based on TME. 18
2. EPIDEMIOLOGY OF GLIOMA
Glioma is the most common and malignant primary brain tumour in the CNS caused by canceration of glial cells in the brain and spinal cord. Up to the present, glioma is the most invasive and incurable cancer. 19 , 20 In the worldwide, about 100,000 cases of diffuse glioma are recorded every year. The annual incidence rate is about 3–8 cases per 10,000 persons. 21 However, the incidence of glioma is increasing gradually. The peak incidence of primary glioma is between the ages of 55 and 60 years and the incidence rate is higher in men than women. Secondary GBMs tend to affect younger individuals about age 40 years old. 22 , 23 The main median survival is 15 months under treatment and 5 months without treatment, respectively. 24 , 25 However, the patients affected by low‐grade gliomas may survive for more than 20 years. 26
Up to date, the mainstay of treatment methods contains surgical excision, chemotherapy and radiotherapy. 24 According to the fifth edition of the WHO Classification of Tumours of the CNS (WHO CNS5), the standard of care and prognosis of glioma, circumscribed gliomas are usually benign and recommended for early complete resection, associated with chemotherapy if necessary. Diffuse gliomas and other high‐grade gliomas according to their molecule subtype are slightly intractable, with the necessity of chemotherapy. However, for glioblastoma, feasible resection followed by radiotherapy and temozolomide chemotherapy is contained in the current standard of care. 27 , 28
Glioma stem cells with the characteristics of stem cells and heterogeneous resident nerve cell spheres can promote the recurrence and progression of glioma. 29 In terms of progression, there are two main methods of recurrence after surgical resection. The first is to convert the apoptosis‐related factor ligand (FasL) in astrocytes into the paracrine death signal pathway of cancer cells, or effect by inhibiting the axon Pathfinder L1 cell adhesion molecule (L1CAM) to promote the growth of glioma. Serine protease inhibitors (serpins) in glioma progression can inhibit the production of fibrinolytic enzymes and ensure the survival of cancer cells by protecting cancer cells from the biological process of death and promoting the growth of vessels. 30
Moreover, with the extension of treatment time, chemoresistance occurs frequently. Also, it is inevitable for normal tissues adjacent to cancer to be damaged. 31 Due to the resistance to chemoradiotherapy and the aggravation of glioma, local recurrence and distant progression are prone to occur. 32 Moreover, because the glioma is prone to pass across the blood–brain barrier (BBB) adhering to the surface of capillaries and growing around capillaries, the delivery and penetration of therapeutic drugs passed into the brain are restricted resulting in the weakened therapeutic effects. 33
As a result, the clinical therapeutic effect of glioma is deficient. Nowadays, it is an urgent problem to explore novel drugs with better curative effects to overcome the resistance and the physiological barriers.
3. THE CLINICAL APPLICATION OF PPIS
PPIs are kinds of stomach medicine with minimal side effects, generally. 34 Clinically, they are used to remedy peptic ulcers, gastroesophageal reflux disease, zoai syndrome and upper gastrointestinal bleeding having become the first‐line drugs for abnormal gastric acid secretion and related diseases. 35 , 36 , 37 , 38 However, different PPIs have different clinical application tactics and blood–brain barrier penetration (Table 1). 39 , 40 , 41 , 42
TABLE 1.
Molecular weight, dose in clinic, blood–brain barrier (BBB) permeability of pump proton inhibitors (PPIs)
| PPIs | Omeprazole | Lansoprazole | Pantoprazole | Rabeprazole | Esomeprazole |
|---|---|---|---|---|---|
| Molecular weight | 345.416 | 369.362 | 383.370 | 359.44 | 367.398 |
| Dose in clinic | 20 mg/day | 15–30 mg/day | 40 mg/day | 10–20 mg/day | 0–40 mg/day |
| BBB permeability |
Omeprazole was able to penetrate the BBB and the time to reach the maximum concentrations is about 60 min in brain. Lansoprazole could penetrate the BBB and is similar with omeprazole. The time to reach the maximum concentrations is about 40–60 min in brain. Pantoprazole is under trail. Rabeprazole is under trail. Esomeprazole is able to cross the BBB. The peak serum concentration is reached 90–180 min after oral administration. |
||||
PPIs are mostly derivatives of benzimidazole compounds. 43 They are mostly weakly basic drugs with low original drug activity. After being absorbed into the blood, it is transported to the gastric mucosal parietal cells and finally reaches the acidic cavity of the secretory tube where the pH < 4. 44 The technical drug is easy to be ionized and positively charged in this environment to play a preferable role. 45 Due to the low membrane penetration, the drug is continuously aggregated and converted into the form of bioactive hyposulfonic acid and hyposulfonamide under the catalysis of acid, and then mixed with the sulfhydryl (sh) of vacuolar ATPase (V‐ATPase) dehydrating and coupling to produce an irreversible covalent disulfide bond to inhibit the H+ transport mechanism of the enzyme and acid secretion. 46
PPIs are also H+‐K+ ATPase inhibitors which may act on the acid‐secreting tubules on parietal cells. The acid‐secreting tubules secrete acid in the way of H+ and K+ exchanging through H+‐K+ ATPase on the membrane to pump H+ out of the cells. PPIs cannot transfer the hydrogen of parietal cells to the gastric cavity and inhibit the formation of gastric acid. 44 , 47 Besides, PPIs bind to cysteine residues nearby the nucleotide‐binding domain of subunit A covalently resulting in the inactivation of V‐ATPase to inhibit acid content secreted. 48 Only as new pump molecules are synthesized and inserted into the cell membrane can gastric acid secretion restart. 49 Besides the application in gastrosia, PPIs has the potential in curing tumour such as epithelial ovarian and breast cancer nowadays. 50 , 51 , 52 , 53 Till now, the most used PPIs are to treat late‐stage tumours. Such as omeprazole could inhibit the progression of early colorectal adenoma to colorectal cancer and distal metastasis of advanced breast cancer. 54 , 55 However, there has been a study reporting PPIs administration could decrease the occurrence of the early‐stage gastric cancer induced by ulcerative differentiation. 54 In addition to being used alone, PPIs are often used in combination with conventional chemotherapy drugs for cancer.
Omeprazole has the ability to inhibit V‐ATPase to change the acidic microenvironment and enhance the sensitivity of drug‐resistant cells to taxol. Besides, omeprazole combined with paclitaxel can significantly reduce the tumour volume in animal models with epithelial ovarian cancer. 50 V‐ATPase is overexpressed in epithelial ovarian cancer compared with normal ovarian epithelial cells. YAP (Yes‐associated protein) is one of the major transcription activation factors playing an important role in regulating cell proliferation and organ development. YAP is overexpressed and is connected with the progression and multi‐drug resistance of the tumour. V‐ATPase D1 also known as ATP6V0D1, is the D subunit of the V0 domain. YAP is associated with V‐ATPase D1 to promote progression and MDA of the tumour. Esomeprazole could inhibit the expression of YAP and V‐ATPase D1, also the combination of YAP and V‐ATPase D1 to promote the sensitivity of tumour cells to conventional anticancer drugs such as PTX. 51 In terms of breast cancer, as the effective chemotherapeutic drugs for breast cancer, raloxifene and doxorubicin combined with omeprazole, lansoprazole and pantoprazole, the viability of breast cancer cells is decreased and the apoptosis is enhanced obviously, compared with raloxifene and doxorubicin solely. 52 , 53
4. TUMOUR MICROENVIRONMENT IS THE MAIN MEDIUM FOR THE PROLIFERATION AND PROGRESSION OF TUMOUR
Progression is the most common phenomenon for patients with glioma which may lead to death. Progression occurs when tumour cells spread from the site of origin to another part of the brain. Progression is a multifactorial process that depends on metabolic changes, gene mutations and TME. Tumour cells, cancer stem cells, cancer‐associated fibroblasts (CAFs) and cytokines secreted by these cells, extracellular matrix proteins, blood vessels and various extracellular substances form a complex environment consisting of TME. 56 , 57 TME is a recognized significant key element affecting tumour occurrence, growth and progression considered as a unit so as to generate a dynamic communication with tumour cells. 58 , 59 Tumour cells can change and maintain their own survival and development conditions through autocrine and paracrine, so as to promote the growth and development of tumours. 60 TME not only includes the structure, function and metabolism of tumour tissues but is also related to the internal (nuclear and cytoplasmic) and external environment of tumour cells. 61 The main characteristics of the tumour microenvironment are acidification and hypoxia because of the imbalanced steady‐state. 62 Therefore, tumours have intracellular alkaline pH and lower extracellular pH ranging from 7.2 to 7.4 and 6.5 to 7.1, respectively. 63 , 64 The acidic microenvironment strongly contributes to tumour progression by stimulating invasion and progression, inhibiting the immune surveillance of cancers and conferring chemoresistance. 65 The acidic extracellular environment is conducive to tissue damage and the activation of destructive enzymes in the extracellular matrix (ECM) increasing the potential for tumour progression and acquiring cell phenotype of multidrug resistance (MDR). 66 In order to maintain intracellular pH (pHi), the tumour cells have evolved powerful mechanisms to counteract cytoplasmic acidification and expel accumulated protons from cells, including Na+/H+ exchanger (NHE), carbonic anhydrase, monocarboxylic acid transporter (MCT) and proton pumps. 67
Pump proton could keep the stability of H+ in and outside the microenvironment, and evade the change of intracellular acidity caused by bioenergy conversion. 68 Proton pumps such as V‐ATPase, NHE and the carbonic anhydrase are upregulated in cancer cells. 69 In tumours, V‐ATPase can pump protons out of cells, alkalize the intracellular environment and acidify the extracellular environment, so as to resist apoptosis and promote tumour aggressiveness. 70 Increased activity of these pH regulators protects cells from changes in cell phenotype caused by pHi fluctuations by squeezing H+ in the extracellular space. 71
The metastatic potential of tumour cells is affected by the relationship between tumour cells and ECM. Low pH activates and triggers the secretion of proteolytic enzymes including matrix metalloproteinase‐2 (MMP‐2), MMP‐9, a tissue serine protease, adamalysin‐related membrane protease, cysteine protease, cathepsin and gelatinase leading to the degradation and remodelling of ECM, so as to promote tumour invasion and progression. 72
It is reported that the acidic environment after the metabolism of tumours is not conducive to normal cells and results in the immune escape of tumour cells. 73 PPIs can directly change the quantity of T‐cell receptors (TCR) or major histocompatibility complex (MHC) so as to improve the recognition and elimination of tumour cells by T cells. 74 Alkalizing the acidic environment of tumour cells can also improve the activity of other effector cells such as natural killer cells (NK) or natural killer T cells (NKT). 75 Under the acidic environment, tumour cells can use the nutrition provided by autophagy of normal cells to meet the needs of tumour cell growth. 76 PPIs can also inhibit autophagy resulting in the lack of nutritional supply for tumour cell proliferation and the death of the tumour. 77 Compared with normal cells, the tumour cells could be more adaptable to the imbalanced pH environment. 78 Because the V‐ATPase complex is mainly located at the edge of tumour cells which may induce the acidification of TME and furtherly promote the growth of cancer. 79 In addition, the acidic TME provides extremely suitable living conditions for the proteolytic activity of cathepsins especially the lysosomal cathepsins, since the activity of most of the cathepsins could be enhanced in an acidic condition. 80 And they could activate growth factors and proteases or degrade components of the extracellular matrix to promote progression. For instance, Cathepsin B (Cat B) can upregulate the function of MMPs which leads to the detachment of cells leading to the initiation of the cell migration. 81
Akt (protein kinase B[PKB]) accumulates in mitochondria and phosphorylates pyruvate dehydrogenase kinase 1 (PDK1) on phospho‐NDRG1 (THR346) to inactivate the pyruvate dehydrogenase complex. And then this pathway turns tumour metabolism to glycolysis to antagonize apoptosis, inhibit oxidative stress and maintain the proliferation of tumour cells. 82 Simultaneously, low pH could increase glycolysis to inspire progression. Glycolytic metabolites are the synthetic raw materials of biological macromolecules and the indispensable structural elements of new tumour cells. The increase of lactic acid produced by glycolysis will decompose and destroy ECM and promote progression. 83
EMT is pivotal for wound healing, processes and its occurrence in cancer is known to aggravate invasion, migration and drug resistance in tumours. The occurrence of EMT causes the loss of epithelial characters of tumour cells and the transformation into mesenchymal‐like cells and thus enhancing tumour cell proliferation and motility and decreasing cell apoptosis. 84 Transforming growth factor‐β (TGF‐β) has played a key role in deciding EMT. 85 V‐ATPase may promote EMT induced by TGF‐β. What is more, low pH is more conducive for tumour cells to produce more TGF‐β and promote EMT which implies the growth of cancer. 86
Hypoxia is another major feature of TME associated with malignant progression, therapy resistance and poor prognosis of glioblastomas (Figure 1). Hypoxia is a pathophysiological condition that generally arises as a consequence of the rapid proliferation of cancer cells as they outgrow their blood supply, therefore, depleting the cells of nutrients and available oxygen. Hypoxia always happens in late‐stage tumours. Hypoxic tumours are found to be highly aggressive and resistant to chemoradiotherapy because hypoxic tumours require triple the normal radiation dose to achieve the desirable cell death effect as a normal irradiating dose which means that hypoxia is also a key factor in determining the growth of tumour‐induced by MDR. 87 , 88 Previous studies have revealed that oxygenation in glioma is 10 mmHg compared with normal brain tissue which is 40 mmHg and which may arise from radiation resistance.
FIGURE 1.
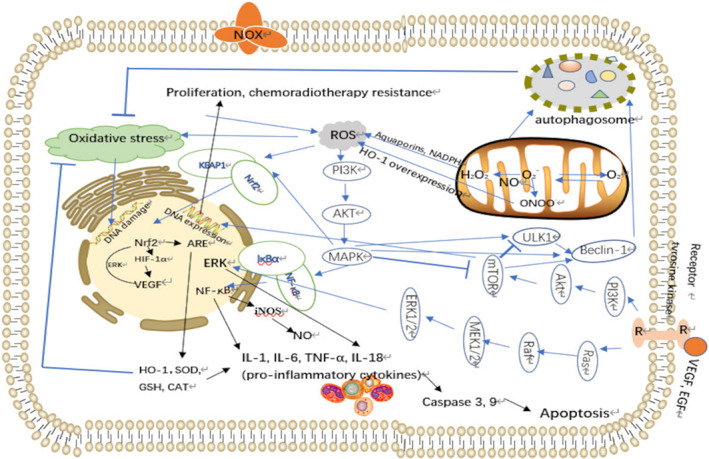
Schema depicting sources of ROS and antioxidant defences reported for glioma cells
Moreover, compared with normal cells, the expression of hypoxia‐inducible factor‐1α (HIF‐1α) and vascular endothelial growth factor (VEGF) in adjacent tissues was significantly higher. 89 , 90 Stably expressed HIF‐1α drives gene expression of two metabolic enzymes lactate dehydrogenase A (LDH‐A) and PDK1 which are vital in the conversion of pyruvate into lactate and inactivating pyruvate dehydrogenase (PDH) and subsequently prevent pyruvate oxidation in the mitochondria to promote glycolysis to improve the tolerance to hypoxia. This process could increase glucose to glycolysis while suppressing OXPHOS and mitochondrial respiration by decreasing the input into mitochondria to avoid apoptosis. 91 It is the classical Warburg effect. The hydrogen ions removed from sugar will not be oxidized into the water through the respiratory chain, but will accumulate in large quantities in the cells, such as lactic acid. 92 This altered glucose metabolism not only enables tumour cells to use glucose‐derived carbons for the synthesis of essential cellular ingredients but also rapidly provides ATP to fuel cellular activities. In addition, this metabolic shift contributes significantly to chemoradiotherapy resistance. Tumour cells can promote the Warburg effect, enhance acid secretion and increase extracellular pH (pHe) furtherly promoting the survival of tumour cells. 93 Lactate accumulation also promotes cancer cell migration by facilitating the interaction of fibroblasts and escaping immune surveillance. 94 At the same time, a large number of metabolites such as lactic acid and pyruvate can further improve the transcription and expression activity of HIF‐1α, and finally, form a positive feedback expression of HIF‐1α in a hypoxic environment (Figure 1). 95 HIF‐1α degrades extracellular matrix and upregulates collagen expression genes promoting collagen fibre synthesis. HIF‐1α promotes the reconstruction of ECM under hypoxia based on proline 4‐hydroxylase A1 (P4HA1), P4HA2 and procollagen lysine oxoglutarate dioxygenase 2 (PLOD2) to increase directional migration. 96 HIF‐1α regulates VEGF, TGF‐β and prospero homeobox‐1 (Prox‐1) to mediate the proliferation and progression of lymphatic endothelial cells and to disrupt the cellular barrier around existing blood vessels, pulling endothelial cells away to form new capillaries with fenestrations and fewer tight junctions. This results in an enhanced infiltration of cellular and plasma components into the brain, further inducing the reconstruction of TME, as well as maintaining the stem cell phenotype of tumour cells promoting progression and drug resistance. 97 , 98
In addition, nitric oxide synthases (NOS), widely expressed in gliomas, use l‐arginine to produce primary RNS type NO that interacts with O2−, generating ONOO−. NO is more effective in quenching superoxide and reacts with O2 to form other nitrogen oxides such as NO2, as a free radical, which in turn may react with NO to yield N2O3 reacting with biomolecules (lipids, proteins and DNA), potentially leading to cell death (Figure 1). 18 , 99 NO plays a role in the activation of NF‐κB which is a major transcription factor in glioma progression. IκB kinase‐independent NF‐κB activation may involve NO‐induced IκB nitration. RNS may disrupt the Keap1‐Nrf2 complex which can prolong the activation of Nrf2 and promote the antioxidant state inducing survival of tumour cells. 100 RNS also inhibits wild‐type p53 through cysteine (Cys) oxidation and tyrosine (Tyr) nitration contributing to glioma genesis furtherly. 101
Another reason promoting progression and recurrence is MDR. A formidable obstacle for MDR is the blood‐tumour barrier (BTB) and blood–brain barrier (BBB), filtering barriers of capillaries. They exclude most compounds except highly lipidized small molecules of less than 400 daltons, rendering potentially powerful anti‐cancer drugs impotent for GBM treatment. Thus, breaking the BTB or BBB will significantly impact GBM treatment. 98 Actually, the BBB is composed of non‐fenestrated brain endothelial cells (BECs) of the capillary wall, which is surrounded by pericytes, astrocytes, perivascular neurons, a basal membrane and an extracellular matrix, forming the highly organized neurovascular unit. 102 Besides the nitrosourea compounds carmustine (BCNU) and lomustine (CCNU) as well as the platinum agents cisplatin and carboplatin, the most widely used chemotherapeutic drug is temozolomide (TMZ), an imidazotetrazine derivative of dacarbazine. TMZ could penetrate into the CNS and has 96%–100% bioavailability. 103 However, the concentration of TMZ is far away efficient. TMZ is one of the substrates of P‐glycoprotein (P‐gp), an important efflux pump which locates on the apical membrane side of endothelial cells forming BBB and serves as a maintainer of the integrity and the polarity of BBB. P‐gp not only hinders brain entry of a large number of xenobiotics including potentially toxic substances and therapeutic agents but also transports the compounds that have crossed the BBB back into the circulation (Figure 2). 104 Due to the overexpression of P‐gp at the BBB of glioma, only 20% of TMZ regarding a systemic dose is able to enter the cerebral parenchyma. 105 In addition, BBB also accounts for the limited efficacy of other chemotherapeutic agents in GBM, such as etoposide, irinotecan, vincristine and cisplatin. Undeniably, BBB and BTB greatly contribute to the MDR. 106 The mechanisms of MDR in tumour cells which is in the TME include four aspects: (1) The amplification or overexpression of transmembrane transporter gene leads to the high expression of encoded transmembrane transporter, so as to promote drug efflux and the change of drug subcellular distribution, resulting in the decrease of drug concentration in cells 107 (2) the change of metabolic transformation, such as the change of some proteases in cells, result in the enhancement of cell detoxification (3) the change of drug action target, such as the decrease topoisomerase (TOPO) content or the change of property leads to the resistance to anti‐tumour drugs targeting TOPO (4) other mechanisms include the change of apoptosis‐related pathways, cell proliferation speed, the enhancement of damage repair and the change of pharmacokinetic factors. 108 Several transmembrane transporter proteins may hinder drugs from reaching the target by reducing the concentration of drugs in tumour cells or changing the distribution of drugs in cells, such as drug efflux P‐glycoprotein (P‐gp), multidrug resistance protein (MRP). P‐gp is an efflux drug transporter associated with multidrug resistance gene‐1 (MDR‐1) expression in the cell membrane, which is responsible for expelling various drugs from tumour cells to form multidrug resistance. 109 Weakly alkaline drugs can lead to capturing ions based on being protonated in an acidic extracellular environment, which hinders the effects of anti‐cancer chemotherapy drugs. In general, most chemotherapy drugs are weakly alkaline and the characteristics are prone to ionize in an acidic environment and with high polarity, so it is not suitable to pass through the cell membrane. 110 , 111 Once entering tumour cells, drugs are encapsulated in acidic organelles and the efficacy will be reduced or ineffective. Even several chemotherapy drugs induce the production of V‐ATPase in tumours maintaining the acid environment. Lysosomal vesicle or endosomes style structures in the acid environment can further expel drugs out of cells or eliminate drugs by activating relative secretory pathways. And then these structures can be further reused to enhance drug resistance limiting the drug's effects on its molecular targets (mainly DNA). Therefore, the concentration of chemotherapeutic drugs that can play a toxic and anti‐cancer role in cancer cells is still very low. 112 In the hypoxia and glucose deficient tumour microenvironment, the expression of mRNA and protein of Topo is decreased and associated with decreased DNA breakage leading to the decreased cytotoxicity of chemotherapy drugs. At the same time, the content of complexes and chemotherapy drugs‐TOPO‐DNA is decreased resulting in MDR. 113
FIGURE 2.
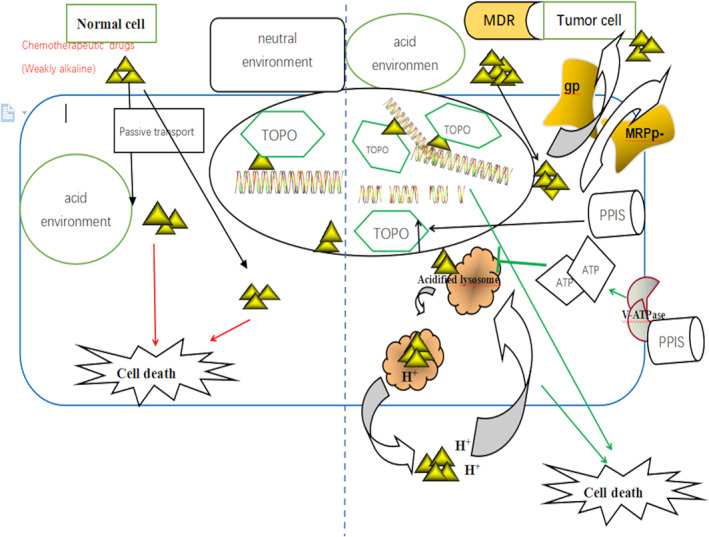
The mechanism of pump proton inhibitors (PPIs) for defending multidrug resistance
The immune system's response to the tumour can impact the glioma's survival, proliferation and invasiveness. 114 Tumour‐associated macrophages (TAMs) are formed by peripheral blood monocytes infiltrating into solid tumour tissues accounting for a large proportion of tumour stromal cells. TAMs are an important component of TME. It has been reported that macrophages are related to immunosuppression and immune escape. 115 In the early stages, tumour cells release chemokines to attract macrophages and other inflammatory cells reaching the extracellular matrix. Then TAMs can penetrate the basement membrane so that tumour cells escape the bondage of the basement membrane and reach the surrounding normal tissue matrix. 116 At the same time, TAMs and tumour cells can promote angiogenesis by releasing enzymes to generate angiogenesis, such as MMP2, MMP‐7, MMP‐9, MMP‐12 and cyclooxygenase‐2 (COX‐2) to improve the invasiveness and motility of cells. 117 , 118 Neovascularization can provide nutrition and oxygen for tumour growth and provide a path for tumour cell progression. 119 TAMs also could release cytokines and growth factors directly to promote growth, such as VEGF, tumour necrosis factor‐α (TNF‐α) interleukin‐8 (IL‐8) and so on. 120 Hypoxia in TME can further induce macrophages to produce HIF‐1α and promote angiogenesis. 121
CAFs exist in the stroma of tumour cells which are associated with EMT and angiogenesis. 122 Fibroblasts activate HIF‐1α and NF‐κB signalling pathways to stimulate oxidative stress, autophagy and glycolysis. These decomposition products create nutrient support for tumour growth. 123 Besides, CAFs produce a variety of cytokines and extracellular matrix proteins including stromal cell‐derived factor 1 derived factor 1 (SDF1), hepatocyte growth factor (HGF), VEGF, platelet‐derived growth factor (PDGF) and TGF‐β to provide a basis for the progress and progression. 124
Mast cells (MC) secret fibroblast growth factor 2 (FGF‐2), VEGF and TGF‐β to promote the progress and progression. 125 A variety of cytokines produced by tumour cells can induce the proliferation of myeloid‐derived suppressor cells (MDSCs), such as COX‐2, IL‐6 and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) and VEGF can further enhance the immunosuppression of myeloid suppressor cells and enhance immune escape together. 126 , 127
This kind of neuro‐inflammatory TME can lead to the loss of BBB integrity. 128 The consequences of a compromised BBB are deleteriously exposing the brain to potentially harmful concentrations of substances from the peripheral circulation, adversely affecting neuronal signalling and abnormal immune cell infiltration. 129 All of these can lead to disruption of brain homeostasis.
Autophagy also displays a key role in the growth of glioma. Autophagy is an important catabolic process of substances in cells. 130 It wraps the wrong proteins or damaged organelles through autophagy vesicles with double‐layer membrane structure, fuses with lysosomes and hydrolyzes with lysosomal acid hydrolases to produce biological molecules such as amino acids which are finally reused by cells to realize the circulation of substances in cells. 131 In the early stage of tumour progression, autophagy will inhibit tumour progression. In the middle and late stages of tumour progression, autophagy will protect the tumour from stimulating and anoikis apoptosis so as to promote progression. 132 Anoikis is a special form of programmed cell death induced by the loss of contact between cells and extracellular matrix and other cells. Usually, tumour cells gather together and closely adhere to the extracellular matrix to form a ‘home’ for self‐function and survival. When they break away from the cell adhesion matrix and lose the connection between cells, anoikis apoptosis will generate. 133 Moreover, tumours will produce extensively damaged proteins, organelles and other harmful components after chemotherapy and radiotherapy. At this time, the activity of autophagy is improved to remove harmful substances in time and provide emergency and raw materials for DNA damage repairing which may result in a poor prognosis of tumour treatment. 134
With the long‐term administration of chemistry, autophagy could gather the tumour cells to assemble into blocks promoting progression. Moreover, autophagy may induce the movement of damaged proteins, mitochondria and stressors including ROS to maintain the activation of advanced glioma. 135 Chemotherapy could activate the autophagy genes like autophagy‐related protein 5 (Atg5), LC3 and others involved in autophagic pathways inducing progression. 136 , 137 PI3K‐Akt‐mTOR is a key signalling pathway that is related to autophagy. 137 mTOR acts as the main regulator of autophagy containing two complexes called mammalian target of rapamycin complex 1 (mTORC1) and mammalian target of rapamycin complex 2 (mTORC2). MAPK inhibits mTORC1 activity, which leads to suppression of autophagy activating unc‐51‐like kinase 1 (ULK1). ULK1 induces the Beclin‐1 phosphorylation, which can result in autophagy. 138 , 139 , 140 At the same time, autophagy is an adaptable response under the stimulation of the endoplasmic reticulum (ER). Under ER stress, Ca2+ is released into the cytoplasm and triggers autophagy by activating the MAPK‐TOR signal pathway. In addition, in the tumour microenvironment, cancer cells experience hypoxia resulting in the exposure of hydrophobic regions of misfolded/unfolded proteins and accumulation largely in the ER inducing the upregulation of unfolded protein response (UPR) and the expression of autophagy genes inducing invasion and progression. In addition to canonical UPR, proteotoxicity also stimulates the selective, autophagy‐dependent, removal of discrete ER domains loaded with misfolded proteins to further alleviate ER stress. These mechanisms can favour the progression and long‐term survival of advanced glioma cells. 141 , 142
It has been evidenced that autophagy could increase the expression of NF‐κB to activate MMP inducing invasion and progression. 143 MMP plays a key role in the invasion process by degrading many elements of ECM, including collagens, fibronectin and laminin. 144 It was reported that MMP is localized in vasculature cells and tumour cells of malignant astrocytomas. 145 MMP inhibition significantly decreases invasion, migration and tumour progression in advanced glioma cells. 146 All of the tumour cells would upregulate glycolysis to reduce the energy supplied by mitochondrial. Autophagy provides a source of energy in this process to promote the survival and progression of advanced glioma. 76 , 147
In terms of the pathological of gliomas, some mutations will indeed cause cells to continuously enter the cell cycle for mitosis, escape apoptosis, contact inhibition and immunosuppression and make cells continuously obtain energy so as to cause metabolic abnormalities and induce angiogenesis, hypoxia and necrosis of brain tumours, such as IDH mutation, H3K27M mutation, TERT mutation, MGMT mutation and so on. These mutations will activate the expression of various signal pathways and form the basis for the occurrence and development of glioma. 148
4.1. PPIs can induce apoptosis by changing the acidic tumour microenvironment
More and more studies have proved that the acidic environment outside the tumour cells plays an important role in the development, infiltration, dissemination and drug resistance of the tumour (Figure 3). 149 PPIs inhibit the activity of V‐ATPase in the acidic environment outside the gastric cancer cells to hinder the proton transport, so as to change the pH gradient of gastric cancer cells and cause cell inactivation (Figure 3). The changed pH affects the structure and activity of almost every enzyme, then, these active enzymes affect various signals and pathways of cells. 150 For example, V‐ATPase is involved in the Wnt/β‐Catenin signalling pathway which promotes tumour development and progression. Pantoprazole can inhibit V‐ATPase so that this pathway is blocked. 151
FIGURE 3.
Mechanism of pump proton inhibitors (PPIs) inducing glioma cell damage
The concrete mechanism of V‐ATPase is to maintain the balance of abnormal pH gradient inside and outside the cell. 152 After being acidified outside the cell, V‐ATPase can activate proteolytic enzymes, including MMPs, tissue serine proteases and bone morphogenetic protease type 1 metalloproteinase (BMP‐1), to degrade ECM. V‐ATPase can also remove various toxic molecules, such as H+ and ROS. 153 , 154 , 155 In addition, M‐ATPase and P‐ATPase play a similar role to V‐ATPase. These all exist on the cell membrane, mitochondrial membrane, lysosomal membrane, endosomal membrane and Golgi membrane, so as to maintain an acidic environment outside the cell membrane and inside the organelle membrane in gastric tumours. 156 Omeprazole could cause the disorder of the lysosomal function of gastric tumour cells, further causing the activation of caspase‐3. 157 The activated caspase‐3 cleaves the corresponding cytoplasmic and nuclear substrates, resulting in the apoptosis of the tumour finally. 158 One research report that the rate of immune cell infiltration (M1 macrophages, neutrophils, CD103 cells and NK cells) is high, and the antitumor effectors (iNOS, INF‐γ, IL‐1α) are enhanced after using the inhibitor of V‐ATPase. 159 At the same time, the number of cancer cell‐positive cells and the activity of caspase are all decreased. 155 The role of PPIs is exactly similar to the inhibitor of V‐ATPase in suppressing the progression of the tumours. Likewise, esomeprazole, as an inhibitor of V‐ATPase, can reverse EMT by inhibiting IκB protein and furtherly inhibiting the expression of TGF‐β and thus countering tumour. 86
The balance of acidity inside and outside cells can maintain the survival of normal cells. However, the acid–base imbalance is the main induced element of cell canceration. The main pH value is 7.2 and 7.4 in and outside the normal cells, respectively. However, the main pH value in and outside the tumour is 7.4 and 6.3, respectively. 85 The tumour could balance this kind of microenvironment through the functional expression of several different molecular types of machinery. The migration and growth of solid tumours rely on the supplement of glucose. 160 In the short term, the tumour cells could adapt to the decreation of glucose. But the long‐term reduction of glucose would increase the apoptosis of cancer cells. In order to combat the reduction of glucose, the change in the microenvironment may produce more lactic acid which may reduce the death of cells induced by a shortage of glucose. 161 The increase of lactic acid could downregulate the G1/S transition process in the cell cycle and inhibit tumour cells in the G0 phase, so as to reduce the demand of tumour cells for energy and nutrients. At the transcriptional level, the G2/M checkpoint was also down‐regulated which could further aggregate tumour cells in the G0 phase. 162 Lactic acid could also activate the autophagy process of cells to reuse intracellular substances to maintain cell survival. 163 , 164 At the same time, several studies illustrate that lactic acid also inhibits the apoptosis of tumour cells by maintaining the content of NADPH and high expression of anti‐apoptotic protein. 165 , 166 In a word, lactic acid can be the main reason leading to tumour cells escaping apoptosis. PPIs could harbour acid production to invert the proliferation induced by lactic acid. 167
4.2. PPIs can induce apoptosis by changing the anoxic tumour microenvironment
Oxidative stress is a popular research factor to recover the tumour even the glioma. In general, the damage induced by oxidative stress is mainly due to the imbalance between the antioxidant defence system and the excessive formation of ROS. 168 In the normal functional states, the production and removal of ROS are in a dynamic balance. However, if the production of ROS exceeds the removal as given an acute stimulation, then, the excessive free radicals will cause irreversible oxidation damage to the body. 169 The main resources of ROS are mitochondria and cytoplasm. 170 , 171 Mitochondria mainly produce oxygen‐free radicals and non‐oxygen‐free radicals, such as O2 − and H2O2. 172 Of course, other cells are the secondary source of ROS in TME. Macrophages, neutrophils and tumours are the main generated resources of ROS. ROS generated by macrophages is considered as defending and phagocytosing tumours. 173 Studies also show that activated monocytes contacting with tumours in TME could generate a high quantity of ROS producing more impact on DNA damage, metabolic activity, membrane structure and relative protein synthesis. 174 A low level of ROS may activate cell proliferation and inhibit cell senescence and death. The high quantity of ROS may lead to the oxidative modifications of DNA, protein and lipid, finally, harming cells. 175 A large amount of nitric oxide (NO) will produce peroxynitrite with a stronger oxidation effect with a superoxide anion. ONOO− can irreversibly damage mitochondria by inhibiting the mitochondrial respiratory function and the activity of Na+/K+ ATPase and reduce the biological activity of NO participating in the enhancement of cell adhesion, proliferation, vasoconstriction accelerating the injury of arteriosclerosis. 18 ROS oxidizes or peroxidases unsaturated fatty acids of which the function is to support the fluidity of the cell membrane damaging the permeability of cell membranes, such as membrane receptors, membrane proteins and ion channels. 176 Furthermore, the nutrients absorbed by cells are reduced, such as vitamins, amino acids and inorganic salts, resulting in immunopathologic injury. ROS can break the N‐glycosidic bond on nucleotides and produce base‐free sites leading to the breaking of the main chain. 177 It could also promote DNA to produce pyrimidine dimer. 178 In addition, modification of base groups occurs, such as acetylation which further affects protein synthesis, cytoskeleton and DNA damage repair. 179 When taking protein into consideration, ROS can break and crosslink protein or polypeptide chains resulting in protease inactivation and metabolic disorder. 180 Besides, due to the high expression of SOD and catalase enzymes in glioma; there is an accelerated conversion of superoxide to hydrogen peroxide in tumour cells which makes astrocytes particularly sensitive to damage induced by ROS. 181
PPIs have been used to modulate the pHi, disturb the mitochondrial membrane potential and produce excessive ROS leading to apoptosis (Figure 3). 182 Several studies implied the ROS in the TME may promote more macrophages to inhibit the progression of tumour. 183 In addition, PPIs have been applied to enhance the secretion of gastrin, and then enhance the secretion of insulin by islet cells, further inhibiting glycolysis and strengthening oxidative stress. 183
Studies concluded that P‐ATPase plays a crucial role in defending against ROS. 184 Glycolysislar V‐ATPase is overexpressed in tumour cells and metastatic cells to inhibit the generation of ROS in the tumour acidic microenvironment. 50 PPIs could inhibit P‐ATPase and V‐ATPase to enhance the sensitivity of tumour cells to oxidative stress. 185 Mild up regulation of mammalian target of rapamycin (mTOR) pathway activity can promote the production of cellular antioxidants, in turn, while over‐activation will promote the production of ROS. PPIs, such as esomeprazole, could reduce the over‐transduction of mTOR signals furtherly inhibiting the growth of cancer cells and prolonging lives. 186
The activity of cytochrome c oxidase (COX) is associated with the survival period of glioma. The median survival time of patients with low tumour COX activity is short, while the median survival time of patients with high tumour COX activity is long. 187 Because COX promotes the switch from glycolytic to OXPHOS metabolism. Increased COX activity in tumours has been associated with tumour progression after chemotherapy failure. 188 , 189 Recently, a variety of studies have considered the activity of COX as a prognostic indicator of glioma. 190 , 191 Pantoprazole blocked the level of COX inducing the increased depolarized mitochondria (Δψ m) and ROS levels. 149 Moreover, PPIs, as the inhibitor of pump proton, have the potential of inhibiting the activity of mitochondrial electron transport complex, electron transport to reduce mitochondrial respiration and energy supplement so that promoting tumour cell death. 192
Different PPIs own different mechanisms of inducing apoptosis. Bax and Bcl‐2 genes play an important role in apoptosis. 193 Pantoprazole can inhibit the activation of the STAT3 pathway and downregulate its downstream cyclin D1 and Bcl‐2 accordingly so as to inhibit the proliferation of tumour cells and induce apoptosis. 194 Omeprazole is different from pantoprazole. Experimental research shows omeprazole cannot induce the apoptosis of SGC‐7901 (from lymph node progression from a 56‐year‐old female patient with gastric adenocarcinoma) through the expression of Bax and Bcl‐2 related genes, but, through the decreased mitochondrial membrane potential after the action of ROS and caspase pathway. Apoptosis signalling pathways related to caspase activation include mitochondrial/cytochrome c (Cyt‐c) pathway, death receptor pathway and ER pathway. After the action of omeprazole, the expression of caspase‐3 in SGC‐7901 cells increased as time goes on indicating that omeprazole may activate caspase‐3 through the mitochondrial/cyt‐c pathway to induce apoptosis. 195
4.3. PPIs can enhance oxidative stress based on NF‐κB, MAPK, Keap1/NRF/ARE, PI3K/Akt signal pathways
ROS affects metabolism mainly based on NF‐κB, mitogen‐activated protein kinases (MAPK), Keap1/NRF/ARE and PI3K/Akt signal pathways (Figure 1). 71 At first, NF‐κB and MAPK pathways exert an essential implication in oxidative stress. 196 Under no stimulations, the main components P50/65 and IκBα are active in the cytoplasm with the limitation of an inhibitor of κB (IκB) protein. 197 While accepting a stimulation, IκB is phosphorylated by IκB kinase (IKK) and IκB detaches from NF‐κB, enabling NF‐κB dimers to enter the nucleus and express relative target genes actively, such as cytokines, COX‐2 and pro‐inflammatory proteins. 24
The three main subfamilies of MAPKs are extracellular signal‐regulated kinase (ERK), c‐Jun N‐terminal kinase (JNK) and p38 MAPK which may modulate gene expression of nuclear Nrf2 and antioxidants enzymes mediated by ARE. 198 , 199 Moreover, ERK could be activated in cell growth and differentiation. P38 was involved in cell apoptosis and stress signal pathway. 200 , 201 PPIs selectively inhibited the phosphorylation of ERK and stimulated the phosphorylation of p38 in a time and dose‐dependent manner to sensitize apoptosis. 201 When the above three enzymes are activated, they can further promote the phosphorylation of NF‐κB and stimulate the release of TNF‐α and IL‐6 which would promote the proliferation of tumours. 202 Omeprazole has been proved to inhibit the activity of MAPK and NF‐κB and subside with the downregulation of TNF‐α, IL‐6 and SOD2 which may suppress the growth of tumours. 195 MAPK pathways are downstream pathways of different growth factor receptors such as epidermal growth factor (EGF). EGF activates protein kinase C (PKC) thereby activating the Ras/Raf/MEK/ERK pathway. It has been reported that the Ras/Raf/MEK/ERK pathway is involved in mediating H2O2‐induced apoptosis in human glioma cells. 203 , 204 , 205 It is well known that KRAS mutations contribute to cell proliferation and survival in numerous cancers, including glioma. One pathway through which mutant KRAS acts is an inflammatory pathway that involves the kinase IKK and activates the transcription factor NF‐κB. BRAF is a kinase that is downstream of KRAS and is predictive of poor prognosis and therapeutic resistance. 206 However, there has been evidence that the inhibitor of V‐ATPase can inhibit the mutation of B‐Raf and the subsequent MAPK–ERK pathway to promote tumour apoptosis. 207 IDH (Isocitrate dehydrogenase) mutations were mainly distributed in II, and III‐grade gliomas and secondary gliomas defined by WHO. It is closely related to methylguanine methyltransferase (MGMT) promoter methylation and TP53 mutation. 208 IDH mutations can prevent cells from resisting γ Radiation, singlet oxygen, UVB radiation and other emergency damage, promoting the progression of glioma. In terms of mechanism, IDH mutations will affect the affinity of the enzyme, resulting in the decrease of the affinity between the enzyme and substrate. IDH mutants will compete with wild‐type IDH for substrate, and mutant IDH is more likely to combine with the substrate to form a dimer, leading to the accumulation of HIF‐ α and activation of downstream target genes including VEGF so as to promote tumour progression. 209 IDH mutations are often used to predict early glioma. In classifying lower‐grade gliomas with IDH mutation, the V‐ATPase is overexpressed. It is also mentioned that the use of V‐ATPase inhibitors can inhibit the expression of the neurodevelopmental core transcription factor POU3F2, thereby reducing IDH mutations and inhibiting tumour proliferation and anti‐radiation. 210 Therefore, PPIs have the possibility of inhibiting IDH mutation in glioma, thus reducing the occurrence of glioma. Till now, a clinical epidemiological survey has shown that esomeprazole can inhibit the methylation of tumour suppressor gene APC and increase the expression of APC in oesophageal cancer. 211 With regard to glioma, some studies have shown that PPIs can inhibit MGMT promoter methylation, thereby increasing the sensitivity of glioma to radiotherapy and chemotherapy. 212 Mutations in H3.3 often occur in glioma, resulting in decreased H3 histone methylation and increased H3 histone acetylation with subsequent activation of transcription promoting progression. 213 Studies have shown that acetylation inhibitors can inhibit the pump protons and down‐regulate the effective components of the MAPK–ERK‐BRaf pathway so as to inhibit tumour proliferation. However, in terms of tumours resistant to acetylation inhibitors, the activity of related drug metabolic enzymes increases so as to promote anti‐tumour, after the administration of omeprazole, lansoprazole and pantoprazole. These further indicate that PPIs have the potential to inhibit tumour progression. 214 , 215 , 216 One of the serious risks of glioma is epilepsy. Up to 75% of LGG patients have seizures during the course of the disease. 217 Epilepsy is a transient interruption of normal electroencephalogram activity, which significantly affects the quality of life. The mass effect of glioma mainly refers to the excessive proliferation of brain white matter, which leads to the gradual increase of tumour volume. 218 With the increased volume of tumours, it is easy to compress the surrounding brain tissue, and it is easy to cause the loss of stability and uncoordinated discharge of neurons during the growth of glioma. The two main reasons are IDH mutation and abnormal activation of the mTOR pathway. 219 However, PPIs have the potential to suppress the IDH mutation and mTOR pathway. Besides, it has been proved by an experiment that the proportion of myelinated axons increased after omeprazole treatment. In vitro incubation with omeprazole significantly promoted the differentiation and maturation of oligodendrocyte precursor cells. In vivo, Omeprazole treatment (10 mg/kg) for 2 weeks significantly improved the motor coordination function of demyelinated mice. 220 PPIs also could prolong action potential. 221 Thus, PPIs may play a crucial role in preventing seizures.
Nrf2 is a key transcription factor regulating gene expressions of several antioxidant enzymes, such as NAD(P)H quinone acceptor oxidoreductase 1 (NQO1), SOD, catalase, glutathione peroxidase (GPX), glutathione reductase (GR) and haeme oxygenase‐1 (HO‐1), which play important roles in protecting cells against oxidative damage. 222 Nrf2 also plays an important role in the tumour environment to promote the proliferation of glioma and protect glioma from anti‐tumour therapies. 223 Tumour cells lose the heterozygosity of gene through Nrf2 or Keap1 mutation so that Nrf2 and Keap1 cannot be combined normally resulting in Nrf2 accumulation in tumour cells and then activating downstream genes increasing the level of detoxifying enzymes in tumour cells, promoting the formation and growth of tumour cells, enhance the resistance of tumour cells to radiotherapy and chemotherapy. Thus, blocking‐up Nrf2 could suppress glioma. 224 , 225 The microenvironment serves as the basis for indirect mechanisms of Nrf2 in the treatment of glioma. The mechanism of downregulating Nrf2 is mainly about two aspects: direct and indirect ways. Indirect mechanisms include three main aspects of the microenvironment: perivascular, hypoxic and immune microenvironment. 226 Angiogenesis plays a key role in providing energy so as to activate the proliferation of glioma. Nrf2 was found to significantly increase microvessel density (MVD) and expression of small vessel marker CD31. 227 Nrf2 could also regulate angiogenesis based on HIF‐1α and VEGFs. 228 HIF‐1α is a downstream molecule of Nrf2 to regulate hypoxia. Activation of HIF‐1α could activate numerous perivascular compounds, such as angiopoietin, endothelin‐1, inducible nitric oxide synthase (iNOS), adrenomedullin and erythropoietin. Furtherly, in turn, VEGF can also activate Nrf2 according to activate ERK1/2 and induce the production of antioxidative enzymes. 229 , 230 The glioma is addicted to an anoxic environment. The overexpression of Nrf2 exerts antioxidant function and further promotes the expression of HIF‐1α and HO‐1(HO‐1 is a molecule to resists hypoxia) to inhibit the migration and invasion of tumours in a hypoxic microenvironment finally. 231 HO‐1 also plays a key role in fighting against inflammation via ERK/Nrf2 signal cascade induced by oxidative stress. 232
Glioma could evade immune surveillance to decrease the response between the tumour cells and immune surveillance cells. Nrf2/ARE pathway regulates tumour immune surveillance based on regulating the secretion of cytokines and the function of immune cells. 233 Nrf2 regulates the secretion of a variety of cytokines, such as INF‐γ, IL‐5 and IL‐13. 234 INF‐γ induces the production of cytokines affected by immunosuppression and growth factors, which is conducive to the growth and progress of tumour cells. Besides, it may downregulate Alpha‐fetoprotein (AFP) and melanoma antigen (MAGE) to promote antigen modulation of tumour cells so as to escape immune surveillance. 235 At the same time, Nrf2 could induce the transformation of CD4 (+) T cells into the T helper cells 2 (Th2) to secret IL‐4 and IL‐10 in order to inhibit immune protection. 236 The above indirect mechanisms all rely on vascular endothelial cells, fibroblasts and immune cells which are all existed in TME. 19 Nrf2 may active proteasome protective genes such as Phase II detoxification enzyme gene, proteasome gene, ubiquitinase gene, antioxidant protein gene and multidirectional drug‐resistant protein 2 (MRP2) gene which have been proved to inactive external substances and detoxify and be associated with MDR. 237 Besides, under low oxidative stress, overexpressed Nrf2 will reduce the sensitivity to cytotoxic chemotherapeutic drugs by promoting the detoxification of anti‐cancer drugs and enhancing the antioxidant capacity. 238 , 239 Based on the above mechanisms, PPIs have been proven to inhibit the activation of Nrf2 and downregulate the expression of genes or enzymes regulated by Nrf2 to suppress the growth of glioma, such as SOD, GPx, catalase (CAT), HO‐1 which may inhibit antioxidation. 240
The acidic microenvironment can inhibit the production and function of CD4, CD25, factor Forkhead box P3 (FOXp3) and iT‐regs through the PI3K/Akt/mTOR signal pathway. 241 IL‐10 produced by iT‐regs decreased the production of ROS in the vascular wall, the activity of NADPH and improved vascular endothelial dysfunction. 242 However, it has been proven that omeprazole and pantoprazole have the ability to reduce the iT‐regs differentiation in an acidic microenvironment so as to suppress the glioma. 241
4.4. PPIs can inhibit tumour progression by inhibiting tumour‐related inflammation
Enhanced inflammation is a risk factor for many cancers. Immunosuppression caused by inflammation is one of the main reasons for poor prognosis and the short survival of glioma patients. 238 Macrophages are natural immune cells that can be found in most TMEs. 243 Research reported that macrophages produce a small number of tumorigenic factors in vitro, and their ability to inhibit T‐cell activation and proliferation is reduced and associated with the decreased of Dew sugar receptor‐1 (CD206), IL‐10, TGF‐β, the expression of arginase‐1, matrix metalloproteinase and vascular endothelial growth factor after using sh‐V‐ATPase. 244 , 245 Being similar to the inhibitor of V‐ATPase and sh‐V‐ATPase, PPIs may play a similar role in inhibiting tumour progression.
In different microenvironments, there are different macrophage subtypes containing pro‐inflammatory and anti‐inflammatory. M1 subtype could promote macrophage phenotypes to inhibit tumour. M2 subtype could suppress macrophage phenotypes to promote tumour. 246 , 247 Research has proved that pantoprazole may enhance recruitment of TAM in TME showing augmented expression of CD11c, phagocytosis and macrophage morphology. 248 , 249 Phagocytosis and expression of CD11c are considered important signature markers of macrophages with M1 phenotype. 250 Additionally, pantoprazole displays increased anti‐tumour activity with an augmented expression of anti‐tumour molecules, such as IL‐1, TNF‐α, IL‐2R and NO. Experiments show that pantoprazole could counter the tumour as exposed in vitro. Thus, PPIs may not directly enhance the anti‐tumour activity, but rely on the cytokines secreted by macrophages indirectly. 251
Inflammatory mediators can change the local environment of tumours, and chronic inflammation can lead to DNA damage. 252 Clinically, anti‐inflammatory drugs can reduce the incidence of cancer. 85 Especially, PPIs have been studied to target TNF‐α monoclonal antibodies, anti‐sensory targeting Smad7, and non‐steroidal anti‐inflammatory drugs to prevent cancer based on inflammation (Figure 3). 253 Some inflammatory mediators and chemokines are special inflammatory mediators. PPIs directly inhibit epithelial cells and neutrophils or the production of relative chemokines playing an anti‐inflammatory role through suppressing the secretion of INF‐α and proinflammatory mediators such as IL‐1 and translationally controlled tumour protein (TCTP) so as to inhibit cancer‐related symptoms and progress. 254 Besides, STAT3 plays an important role in maintaining and promoting the tumorigenic inflammatory environment, the transformation and progression of malignant tumours based on NF‐κB and IL‐6/GP130/JAK pathways. 255 There has been a research reporting that pantoprazole can inhibit the activity of the IL‐6/STAT3 pathway in gastric cancer cells. 192
TNF‐α and IL‐6 are two kinds of cytokines that play an important role in the process of cancerous cachexia. 256 , 257 IL‐6‐like cytokines independently mediate the excessive lipolysis and metabolism in cancerous cachexia. 258 TNF‐α downregulates the PLIN pathway by upregulating the MAPK pathway so as to promote lipolysis. Abnormal metabolism of fat has a lot to do with the progression of tumours. 259 Several relative experiments showed that TNF‐α and IL‐6 declined apparently in the serum of cancer cells in mice after intragastric omeprazole which implied that PPIs inhibit the tumour progression caused by fat metabolism. 254 Besides, PPIs may decrease the quality of IL‐4 directly and furtherly stimulate the production of TNF‐α. 260 Omeprazole and pantoprazole have been already proved to decrease pro‐inflammatory factors such as TNF and IL‐6, and increase anti‐inflammatory factors such as IL‐10 implying the potential for anti‐tumour. 193 As a result, PPIs may give play to anti‐inflammatory and cancer clearance. Furthermore, PPIs could transfer M2 macrophages to M1 macrophages to fight cancer. 261 Also, PPIs have a direct effect on neutrophils, monocytes, endothelial cells and epithelial cells, so as to exert an anti‐inflammatory effect and inhibit INF‐α, secretion of proinflammatory mediators such as IL‐1 and TCTP. 262 The anti‐cancer activity of PPIs is based on the fact that it can significantly reduce the production of acid‐active substances, IL‐6 and nitric oxide, especially TNF‐α, expression of inducible NOS and COX‐2. PPIs can act in an acidic environment by inhibiting inflammatory factors to further inhibit cancer. 263
4.5. PPIs display an anti‐tumour effect through autophagy
V‐ATPase is the main proton pump that acidifies vesicles such as lysosomes. PPIs can disrupt the lysosomal localization of V‐ATPase leading to lysosomal dysfunction, thus contributing to the lysosomal storage disorders in order to counter tumours. 264
The mammalian target of rapamycin (mTOR) is a highly conservative serine/threonine‐protein kinase that belongs to the PI3K‐related protein kinase family. 112 MTOR can phosphorylate Atg13 preventing it from interacting with complexes containing Atg1 and Atg17, and inhibiting autophagy assembly so as to reduce radiation resistance. 265 The activation of mTOR could induce aerobic glycolysis through upregulating Pyruvate kinase M2 (PKM2), as well as the activation of OXPHO. 266 MTOR can also stimulate the metabolism of lipids, nucleotides and other components to promote tumour progression. Moreover, mTOR can activate HIF‐1α promoting the regeneration of blood vessels. 267 MTORC2 can also directly promote tumorigenesis by activating Akt or Serum‐ and glucocorticoid‐inducible kinases (SGK) promoting the growth of tumours. 268
Hypoxia plays a crucial role in regulating autophagy since the absence of oxygen leads to inhibition of mTORC1 and subsequently decreased inhibition of the unc‐51 like Ulk1 complex finally resulting in activation of autophagy. 269 PPIs depolarize mitochondrial membrane potential by increasing the opening of mitochondrial permeability transition pore (MPTP), resulting in excessive production of ROS which further stimulates lipid peroxidation and reduces glutathione levels. 270 This process could generate excessive ROS leading to lysosomal chamber instability which will affect mitochondrial membrane potential and mitochondrial energy supplement to suppress hypoxia and autophagy in advanced glioma promoting the anti‐glioma. 270 In addition, more ROS will promote the release of pro‐apoptotic molecules into the cytoplasm and further promote autophagy. 271 It has been reported that PPIs can induce autophagy by activating ERK and JNK and inhibit mTOR signalling by activating MAPK. 201 , 272 There is also evidence showing that PPIs mediate the strong upregulation of Beclin‐1 by activating NF‐κB which is responsible for the ROS‐induced autophagy making the foundation for anti‐tumour. 273 , 274
Besides, PPIs could moderate the acidic microenvironment to inhibit the activation of lysosomal cathepsins to suppress MMP increasing sensitivity to TMZ. Finally, the progression of the tumour was inhibited. 275 PPIs may activate MAPK kinase, reduce blood glucose levels and block glycolysis induced by TNF‐α so as to invert the Warburg effect which could activate the autophagy promoting progression. 276
5. PPIS SENSITIZE CHEMORADIOTHERAPY PROMOTING APOPTOSIS
Chemoradiotherapy resistance is an important element to early recurrence, treatment failure and dismal prognosis of glioblastoma. 277 With growing amounts of evidence that chemoradiotherapy may induce the more normal cells to glioma leading to recurrence and progression. 278 , 279 Even if the concentration of drugs entering plasma and cerebrospinal fluid is enough to inhibit the progression of the tumour, the drug resistance and progression of glioma also happen from time to time. 280 Thus, hoping to make use of PPIs and chemoradiotherapy together to play a good effect.
The radiation may lead to the mutation of mitochondrion respiratory chain complex I and II and DNA which will lead to more ROS in glioma. 281 Excessive ROS could damage DNA containing mitochondrion DNA leading to respiratory chain dysfunction, even higher levels of free radicals in the cells, and the disbalance of O2 − in and outside the cells, and, further, block oxygen supplement. 282 In addition, ionizing radiation (IR) will produce more free radicals which combine with oxygen and further lead to permanent DNA damage. 283 Thus, radiotherapy can irreversibly damage DNA through reactive oxygen species. However, the tolerance of normal cells to radiation dose is limited, and the radiation dose cannot be increased continuously. 284 Hypoxic tumour cells continue to exist after radiation and transform into a more invasive phenotype. 19 PPIs such as esomeprazole could generate more ROS persistently to make up for the deficiency of oxidative stress caused by limited radiation. 285 The inhibitory effect of many chemotherapeutic drugs depends on the oxygen partial pressure of the TME. In general, the sensitivity of chemotherapy was the strongest at the peak of oxygen partial pressure. 286 , 287 Because of the ability to enhance oxygen partial pressure through enhancing oxidative stress, PPIs could increase the sensitivity to chemotherapy. However, we need to explore the dose and concentration of different PPIs with the maximum oxygen partial pressure in TME.
The inverted pH gradient of cell membrane inside and outside tumour is an important mechanism leading to drug resistance. 288 The acidic extracellular microenvironment makes a chemical and physical shelter for the anti‐tumour effect of weakly alkaline chemotherapeutic drugs. Once the chemotherapeutic drugs are protonated and alkalized, they cannot pass through the plasma membrane. 241 , 272 Chemotherapeutic drugs in tumour cells enter lysosomes, acidic organelles, and acidic vesicles through protonation and neutralization, and are further discharged out of cells. 289 PPIs changing cell microenvironment can inhibit the intracellular P13K/Akt/mTOR signal pathway and bypass the TSCL/2‐Rheb signal pathway to inhibit its downstream mTOR molecules, and then inhibit the expression of HIF‐1α, MDR protein and P‐gp. 290 , 291 In glioma, MDR protein and P‐gp gene are overexpressed. 292 P‐gp could combine with chemotherapeutic drugs to pump the substrate out of the cell by hydrolyzing ATP, so as to inhibit the effect of chemotherapeutic drugs. Being similar to P‐gp, overexpressed multidrug resistant‐associate protein‐1(MRP1) pumps the drug out of cells. 293 Research implied that PPIs may inhibit the expression of MRP1 and P‐gp to reverse chemoresistance. The excessive PPIs could be cultured in vitro by the alkalizate microenvironment of cancer cells so that increasing chemosensitivity. 294 PPIs can reverse the Warburg effect of tumours by inhibiting pyruvate dehydrogenase kinases and subsequently activating mitochondrial OXPHO or changing anaerobic digestion and ATP binding box (ABC) transcription factors, so as to increase the sensitivity of cancer cells to chemoradiotherapy inducing apoptosis. 86 , 295
PPIs can prevent the occurrence of EMT by interfering with the expression of the TGF‐β/Smad signal pathway and NF‐κB in the acidic microenvironment. 296 , 297 , 298 , 299 It also has been proved that rabeprazole administration induced cell death and reduced cell migration together with EMT by inhibiting Akt and Gsk‐3β phosphorylation, which in turn suppressed the EMT. In addition to molecular aberrations and hypoxia, pump protons may facilitate epithelial to mesenchymal transition by modulating the pH of the TME. We found rabeprazole could play a better role in the unbuffered acidic environment to inhibit EMT. 300 What is more, pantoprazole inhibits breast cancer resistance protein (BCRP) and has a chemo‐sensitizing activity, thereby contributing to improved delivery of imatinib (a kind of drug for treating leukaemia) into the CNS. 299 It has been proved that treatment of the cancer cells with the PPIs resulted in order of magnitude reduction in the half‐maximal inhibitory concentration (IC50) values. 301 Esomeprazole enhances radiosensitivity in radiation‐resistant squamous cell carcinoma of the head and neck. 285 PPIs could also increase chemotherapeutic drug uptake by the tumour cells. 302 PPIs, as the small molecules, could pass through BBB and BTB (Table 1).
In the clinic, PPIs have been proven the ability to overcome the resistance of conventional chemotherapeutic drugs for breast cancer and radiation. The use of PPIs in 6754 patients suffering from breast cancer significantly not only improved the overall survival rate of these patients and reduced the disease recurrence rate but overcome the resistance of conventional chemotherapy drugs and radiation. 303 Besides, based on clinical epidemiological comparative trials, PPIs can assist radiotherapy and chemotherapy to delay the overall survival of 206 patients with rectal cancer. 56 What is more, Omeprazole not only improved the effect of radiotherapy and chemotherapy but also delayed the recurrence and complications of 125 rectal cancer patients. 304 The combined use of PPIs in the treatment of laryngeal cancer can reduce the incidence of pharyngeal reflux and mucositis. 305 Although there has been no relative research studying the synergistic effect of chemoradiotherapy and PPIs in glioma, based on the above‐mentioned relevant clinical epidemiological study about the administration of PPIs in other cancers and preclinical pharmacologic or toxicologic metabolism and mechanism analysis. Of course, improper dosage selection of PPIs and chemoradiotherapy will also lead to the aggravation of adverse reactions. A clinical study showed that the inappropriate dose of PPIs led to the further aggravation of radiation pneumonia after radiotherapy for lung cancer. 306
In general, PPIs may be a potential valuable anti‐cancer drug to promote chemoradiotherapy sensitivity so as to block the growth of glioma.
6. THE ACUTE SIDE EFFECT OF PPIS AND THE NECESSITY OF COMBINING PPIS WITH CHEMORADIOTHERAPY
The basic side effects of PPIs contain nausea, 307 diarrhoea or headache, 308 and even more serious side effects such as subacute cutaneous lupus erythematosus, 309 and interstitial nephritis and pneumonia. 310 After a long period of the epidemiological follow‐up survey, patients with long‐term use of PPIs may get complications of suppurative liver abscess, pulmonary tuberculosis and inflammatory bowel disease. 311 Inflammatory mediators can cause metabolic disorders as being with PPIs for a long time, such as IL‐1, IL‐6, TNF‐α, INF‐γ and prostaglandin E2 (PGE2) which may induce an increase in muscle decomposition and a decrease of muscle synthesis lead to the decrease of muscle mass and muscle strength, even furtherly subside with decreased physical function, polymyositis and rhabdomyolysis. 312 Besides, mitochondrial would be impaired and intestinal microbial would change based on the proximal pH of the intestine increases. 313 It has been proved that PPIs elevate the pH of the stomach and upper gut causing more bacteria, even pathogenic bacteria, to survive in the gastrointestinal tract and enter the gut gradually. 314 Vitamin B12, vitamin C, vitamin D, magnesium, calcium, iron, β‐carotene and zinc will be reduced due to the decreased relative quality of food release and body absorption. 315 , 316 , 317 , 318 , 319 Suppressing magnesium may inhibit the absorption of Vitamin D implying the release of adipocytokine which is involved in the regulation of glucose levels and fatty acid breakdown inducing insulin resistance so that the appearance of obesity and contribute to an increase of the inflammatory state. 315 Based on a reported real case, PPIs may be associated with several mental diseases. 320 Thus, it is urgent to design specific and efficient combined medication or united anti‐glioma therapies to treat glioma.
7. CONCLUSIONS AND FUTURE DIRECTIONS
The tumour microenvironment is the main medium for proliferation, progression and multidrug resistance of tumours. Acidity and hypoxia, as well as the relative inflammatory response and autophagy, are the main influencing factors for tumour progression and progression in TME. PPIs could promote apoptosis and sensitize chemoradiotherapy through altering the release of inflammatory factors and cytokines of inflammatory cells, autophagy and promoting oxidative stress based on NF‐κB, MAPK, Keap1/NRF/ARE, PI3K/Akt signal pathways in TME. PPIs could be considered as an adjuvant treatment strategy to be combined with medication to fight glioma. In order to better predict the therapeutic effect, COX can be considered a prognostic indicator.
In terms of the practical application scope of PPIs nowadays, many kinds of PPIs have been used for different cancer, such as liver, and breast cancer. Nowadays, there is little known about its possible glioma protective effects and relatively few studies on practical application in glioma. Therefore, it is necessary to put PPIs into practice to determine the real advantages and disadvantages of PPIs.
AUTHOR CONTRIBUTIONS
Bihan Li collected, read relevant literature and wrote the article. Ying Liu collected relative literatures and guided this article. Shilong Sun collected relative literatures and guided the mentality and this article. [Correction added on 22 March 2023, after first online publication: Author Contributions have been amended.].
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
The authors acknowledge and appreciate all the collegues for their valuable efforts on this paper.
Li B, Liu Y, Sun S. Proton pump inhibitors display anti‐tumour potential in glioma. Cell Prolif. 2023;56(7):e13321. doi: 10.1111/cpr.13321
[Correction added on 22 March 2023, after first online publication: Ying Liu and Shilong Sun were added as authors and Liping Rao was removed as an author from the author list. Affiliations 1 and 2 were amended from, “1School of Public Health, Jilin University, Jilin, China” and “2School of Public Health, Nanchang University, Nanchang, Jiangxi, China” to “1Department of Toxicology, School of Public Health, Jilin University, Changchun, Jilin 130021, China” and “2NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun, Jilin 130021, China”. The correspondence section was amended from, “Bihan Li, School of Public Health, Jilin University, Changchun, Jilin 130000, China. Email: libh20200918@126.com” to “Shilong Sun, NHC Key Laboratory of Radiobiology, School of Public Health, Jilin University, Changchun, Jilin 130021, China. Email: slsun@jlu.edu.cn”.]
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Zhou YS, Wang W, Chen N, Wang LC, Huang JB. Research progress of anti‐glioma chemotherapeutic drugs (review). Oncol Rep. 2022;47(5):101. doi: 10.3892/or.2022.8312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hua T, Zhuo Z, Duan Y, et al. Prediction of H3 K27M‐mutant in midline gliomas by magnetic resonance imaging: a systematic review and meta‐analysis. Neuroradiology. 2022;64:1311‐1319. doi: 10.1007/s00234-022-02947-4 [DOI] [PubMed] [Google Scholar]
- 3. Grossen A, Smith K, Coulibaly N, et al. Physical forces in glioblastoma migration: a systematic review. Int J Mol Sci. 2022;23(7):4055. doi: 10.3390/ijms23074055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sisakht AK, Malekan M, Ghobadinezhad F, et al. Cellular conversations in glioblastoma progression, diagnosis and treatment. Cell Mol Neurobiol. 2022;101:31‐33. doi: 10.1007/s10571-022-01212-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ribatti D. The chick embryo chorioallantoic membrane as an experimental model to study in vivo angiogenesis in glioblastoma multiforme. Brain Res Bull. 2022;182:26‐29. doi: 10.1016/j.brainresbull.2022.02.005 [DOI] [PubMed] [Google Scholar]
- 6. Tyron JM, Eliasen A, Dalhoff K, Nørgaard MM. The efficacy and safety of proton pump inhibitors in infants with reflux. Ugeskr Laeger. 2022;184(15):V08210634. [PubMed] [Google Scholar]
- 7. Clarke K, Adler N, Agrawal D, et al. Indications for the use of proton pump inhibitors for stress ulcer prophylaxis and peptic ulcer bleeding in hospitalized patients. Am J Med. 2022;135(3):313‐317. doi: 10.1016/j.amjmed.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 8. Liou JM, Lee YC, Wu MS. Taiwan gastrointestinal disease and helicobacter consortium. Treatment of refractory helicobacter pylori infection‐tailored or empirical therapy. Gut Liver. 2022;16(1):8‐18. doi: 10.5009/gnl20330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sue S, Maeda S. Is a potassium‐competitive acid blocker truly superior to proton pump inhibitors in terms of Helicobacter pylori eradication? Gut Liver. 2021;15(6):799‐810. doi: 10.5009/gnl20242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarnaik MK, Modi S, Pisipati Y, et al. Proton pump inhibitors: exploring cardiovascular complications and prescription protocol. Cureus. 2021;13(7):e16744. doi: 10.7759/cureus.16744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gu L, Guan J, Huang Z, et al. β‐Cyclodextrin covalent organic framework supported by polydopamine as stationary phases for electrochromatographic enantioseparation. Electrophoresis. 2022;121:21‐25. doi: 10.1002/elps.202200029 [DOI] [PubMed] [Google Scholar]
- 12. Hammond CL, Roztocil E, Gupta V, Feldon SE, Woeller CF. More than meets the eye: the aryl hydrocarbon receptor is an environmental sensor, physiological regulator and a therapeutic target in ocular disease. Front Toxicol. 2022;3(4):791082. doi: 10.3389/ftox.2022.791082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang K, Lou D, Dai W, Fu R, Ma Z. Comparison of sequential therapy with concomitant therapy in first‐line treatment of Helicobacter pylori: an updated meta‐analysis. J Med Microbiol. 2022;71(1):845‐847. doi: 10.1099/jmm.0.001490 [DOI] [PubMed] [Google Scholar]
- 14. Chen Z, Zheng L, Chen Y, et al. Loss of ubiquitin‐specific peptidase 18 destabilizes 14‐3‐3ζ protein and represses lung cancer metastasis. Cancer Biol Ther. 2022;23(1):265‐280. doi: 10.1080/15384047.2022.2054242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X, Liu Q, Zhang T, et al. Bone‐targeted nanoplatform enables efficient modulation of bone tumor microenvironment for prostate cancer bone metastasis treatment. Drug Deliv. 2022;29(1):889‐905. doi: 10.1080/10717544.2022.2050845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang D, Wan Z, Yang Q, et al. Sonodynamical reversion of immunosuppressive microenvironment in prostate cancer via engineered exosomes. Drug Deliv. 2022;29(1):702‐713. doi: 10.1080/10717544.2022.2044937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang T, Zhang H, Qiu W, Han Y, Liu H, Li Z. Biomimetic nanoparticles directly remodel immunosuppressive microenvironment for boosting glioblastoma immunotherapy. Bioact Mater. 2022;5(16):418‐432. doi: 10.1016/j.bioactmat.2021.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostrowski RP, Pucko EB. Harnessing oxidative stress for anti‐glioma therapy. Neurochem Int. 2022. Mar;154:105281. doi: 10.1016/j.neuint.2022.105281 [DOI] [PubMed] [Google Scholar]
- 19. Uyar R. Glioblastoma microenvironment: the stromal interactions. Pathol Res Pract. 2022;232:153813. doi: 10.1016/j.prp.2022.153813 [DOI] [PubMed] [Google Scholar]
- 20. Salami R, Salami M, Mafi A, Vakili O, Asemi Z. Circular RNAs and glioblastoma multiforme: focus on molecular mechanisms. Cell Commun Signal. 2022;20(1):13. doi: 10.1186/s12964-021-00809-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu S, Kao HY, Yang T, Wang Y. Early and bi‐hemispheric seizure onset in a rat glioblastoma multiforme model. Neurosci Lett. 2022;766:136351. doi: 10.1016/j.neulet.2021.136351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sporikova Z, Slavkovsky R, Tuckova L, et al. IDH1/2 mutations in patients with diffuse gliomas: a single centre retrospective massively parallel sequencing analysis. Appl Immunohistochem Mol Morphol. 2022;30(3):178‐183. doi: 10.1097/PAI.0000000000000997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleischmann DF, Schön R, Corradini S, et al. Multifocal high‐grade glioma radiotherapy safety and efficacy. Radiat Oncol. 2021;16(1):165. doi: 10.1186/s13014-021-01886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Bao G, Zhang M, et al. CRB2 enhances malignancy of glioblastoma via activation of the NF‐κB pathway. Exp Cell Res. 2022;414(1):113077. doi: 10.1016/j.yexcr.2022.113077 [DOI] [PubMed] [Google Scholar]
- 25. Trivieri N, Visioli A, Mencarelli G, et al. Growth factor independence underpins a paroxysmal, aggressive Wnt5aHigh/EphA2Low phenotype in glioblastoma stem cells, conducive to experimental combinatorial therapy. J Exp Clin Cancer Res. 2022;41(1):139. doi: 10.1186/s13046-022-02333-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurokawa R, Baba A, Emile P, et al. Neuroimaging features of angiocentric glioma: a case series and systematic review. J Neuroimaging. 2022;32:389‐399. doi: 10.1111/jon.12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang K, Wu Z, Zhang H, et al. Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer. 2022;21(1):39. doi: 10.1186/s12943-022-01513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. 2022;128(1):47‐58. doi: 10.1002/cncr.33918 [DOI] [PubMed] [Google Scholar]
- 29. Lin Y, Sun H, Dang Y, Li Z. Isoliquiritigenin inhibits the proliferation and induces the differentiation of human glioma stem cells. Oncol Rep. 2018;39(2):687‐694. doi: 10.3892/or.2017.6154 [DOI] [PubMed] [Google Scholar]
- 30. Alanazi R, Nakatogawa H, Wang H, et al. Inhibition of TRPM7 with carvacrol suppresses glioblastoma functions in vivo. Eur J Neurosci. 2022;55(6):1483‐1491. doi: 10.1111/ejn.15647 [DOI] [PubMed] [Google Scholar]
- 31. Valiente M, Obenauf AC, Jin X, et al. Serpins promote cancer cell survival and vascular co‐option in brain metastasis. Cell. 2014;156(5):1002‐1016. doi: 10.1016/j.cell.2014.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crotty EE, Smith SMC, Brasel K, et al. Medulloblastoma recurrence and metastatic spread are independent of colony‐stimulating factor 1 receptor signaling and macrophage survival. J Neurooncol. 2021;153(2):225‐237. doi: 10.1007/s11060-021-03767-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang R, Wang X, Li J, Di L, Zhou J, Ding Y. Lipoprotein‐biomimetic nanostructure enables tumor‐targeted penetration delivery for enhanced photo‐gene therapy towards glioma. Bioact Mater. 2021;2(13):286‐299. doi: 10.1016/j.bioactmat.2021.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gable A, Fiore B, Cheatham J. Clarification of eosinophilic esophagitis treatment in the DoD retention standards. Mil Med. 2022;187(1–2):35‐36. doi: 10.1093/milmed/usab359 [DOI] [PubMed] [Google Scholar]
- 35. Arnoux A, Bailhache M, Tetard C, et al. Proton pump inhibitors are still overprescribed for hospitalized children. Arch Pediatr. 2022;29(4):258‐262. doi: 10.1016/j.arcped.2022.02.004 [DOI] [PubMed] [Google Scholar]
- 36. Singh G, Haileselassie Y, Briscoe L, et al. The effect of gastric acid suppression on probiotic colonization in a double blinded randomized clinical trial. Clin Nutr ESPEN. 2022;47:70‐77. doi: 10.1016/j.clnesp.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 37. Chung CS, Chen CC, Chen KC, et al. Randomized controlled trial of early endoscopy for upper gastrointestinal bleeding in acute coronary syndrome patients. Sci Rep. 2022;12(1):5798. doi: 10.1038/s41598-022-09911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saven H, Zhong L, McFarlane IM. Co‐prescription of dual‐antiplatelet therapy and proton pump inhibitors: current guidelines. Cureus. 2022;14(2):e21885. doi: 10.7759/cureus.21885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng FC, Ho YF, Hung LC, Chen CF, Tsai TH. Determination and pharmacokinetic profile of omeprazole in rat blood, brain and bile by microdialysis and high‐performance liquid chromatography. J Chromatogr A. 2002;949(1–2):35‐42. doi: 10.1016/s0021-9673(01)01225-0 [DOI] [PubMed] [Google Scholar]
- 40. Rojo LE, Alzate‐Morales J, Saavedra IN, Davies P, Maccioni RB. Selective interaction of lansoprazole and astemizole with tau polymers: potential new clinical use in diagnosis of Alzheimer's disease. J Alzheimers Dis. 2010;19(2):573‐589. doi: 10.3233/JAD-2010-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miner P Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five‐way crossover study. Am J Gastroenterol. 2003;98(12):2616‐2620. doi: 10.1111/j.1572-0241.2003.08783.x [DOI] [PubMed] [Google Scholar]
- 42. Grattagliano I, Portincasa P, Mastronardi M, Palmieri VO, Palasciano G. Esomeprazole‐induced central fever with severe myalgia. Ann Pharmacother. 2005;39(4):757‐760. doi: 10.1345/aph.1E377 [DOI] [PubMed] [Google Scholar]
- 43. Papp LA, Hancu G, Kelemen H, Tóth G. Chiral separation in the class of proton pump inhibitors by chromatographic and electromigration techniques: an overview. Electrophoresis. 2021;42(17–18):1761‐1789. doi: 10.1002/elps.202100032 [DOI] [PubMed] [Google Scholar]
- 44. Bruno G, Zaccari P, Rocco G, et al. Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J Gastroenterol. 2019;25(22):2706‐2719. doi: 10.3748/wjg.v25.i22.2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang L, Chai Y, Zhu W, Pan Y, Sun C, Zeng S. Doubly charged trimeric cluster ions: effective in mutual chiral recognition of tadalafil and three proton pump inhibitors. Analyst. 2017;142(5):745‐751. doi: 10.1039/c6an02666d [DOI] [PubMed] [Google Scholar]
- 46. Seidel T, Scholl S, Krebs M, et al. Regulation of the V‐type ATPase by redox modulation. Biochem J. 2012;448(2):243‐251. doi: 10.1042/BJ20120976 [DOI] [PubMed] [Google Scholar]
- 47. Makunts T, Alpatty S, Lee KC, Atayee RS, Abagyan R. Proton‐pump inhibitor use is associated with a broad spectrum of neurological adverse events including impaired hearing, vision, and memory. Sci Rep. 2019;9(1):17280. doi: 10.1038/s41598-019-53622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen YC, Backus KM, Merkulova M, et al. Covalent Modulators of the Vacuolar ATPase. J Am Chem Soc. 2017;139(2):639‐642. doi: 10.1021/jacs.6b12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yuan J, Liu W, Karvar S, et al. Potassium channel KCNJ15 is required for histamine‐stimulated gastric acid secretion. Am J Physiol Cell Physiol. 2015;309(4):C264‐C270. doi: 10.1152/ajpcell.00012.2015 [DOI] [PubMed] [Google Scholar]
- 50. He J, Shi XY, Li ZM, et al. Proton pump inhibitors can reverse the YAP mediated paclitaxel resistance in epithelial ovarian cancer. BMC Mol Cell Biol. 2019;20(1):49. doi: 10.1186/s12860-019-0227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee YY, Jeon HK, Hong JE, et al. Proton pump inhibitors enhance the effects of cytotoxic agents in chemoresistant epithelial ovarian carcinoma. Oncotarget. 2015;6(33):35040‐35050. doi: 10.18632/oncotarget.5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ihraiz WG, Ahram M, Bardaweel SK. Proton pump inhibitors enhance chemosensitivity, promote apoptosis, and suppress migration of breast cancer cells. Acta Pharm. 2020;70(2):179‐190. doi: 10.2478/acph-2020-0020 [DOI] [PubMed] [Google Scholar]
- 53. Altundag K. Letter comments on: drug‐drug interactions between palbociclib and proton pump inhibitors may significantly affect clinical outcome of patients with metastatic breast cancer. ESMO Open. 2022;7(1):100382. doi: 10.1016/j.esmoop.2022.100382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang J, Shan F, Li S, Li Z, Wu Q. Effect of administration of a proton pump inhibitor for ulcerative differentiated early gastric cancer prior to endoscopic submucosal dissection. Dig Endosc. 2021;33(6):939‐947. doi: 10.1111/den.13892 [DOI] [PubMed] [Google Scholar]
- 55. Jin UH, Lee SO, Pfent C, Safe S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC Cancer. 2014;9(14):498. doi: 10.1186/1471-2407-14-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang CJ, Li D, Danielson JA, et al. Proton pump inhibitors suppress DNA damage repair and sensitize treatment resistance in breast cancer by targeting fatty acid synthase. Cancer Lett. 2021;509:1‐12. doi: 10.1016/j.canlet.2021.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Menter T, Tzankov A, Dirnhofer S. The tumor microenvironment of lymphomas: insights into the potential role and modes of actions of checkpoint inhibitors. Hematol Oncol. 2021;39(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 58. Asadirad A, Baghaei K, Hashemi SM, et al. Dendritic cell immunotherapy with miR‐155 enriched tumor‐derived exosome suppressed cancer growth and induced antitumor immune responses in murine model of colorectal cancer induced by CT26 cell line. Int Immunopharmacol. 2022;104:108493. doi: 10.1016/j.intimp.2021.108493 [DOI] [PubMed] [Google Scholar]
- 59. Morgan D, Berggren KL, Spiess CD, et al. Mitogen‐activated protein kinase‐activated protein kinase‐2 (MK2) and its role in cell survival, inflammatory signaling, and migration in promoting cancer. Mol Carcinog. 2022;61(2):173‐199. doi: 10.1002/mc.23348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Annese T, Tamma R, Bozza M, Zito A, Ribatti D. Autocrine/paracrine loop between SCF+/c‐kit+ mast cells promotes cutaneous melanoma progression. Front Immunol. 2022;24(13):794974. doi: 10.3389/fimmu.2022.794974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang H, Yung MMH, Ngan HYS, Chan KKL, Chan DW. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci. 2021;22(12):6560. doi: 10.3390/ijms22126560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Demuynck R, Efimova I, Naessens F, Krysko DV. Immunogenic ferroptosis and where to find it? J Immunother Cancer. 2021;9(12):e003430. doi: 10.1136/jitc-2021-003430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Campion KL, McCormick WD, Warwicker J, et al. Pathophysiologic changes in extracellular pH modulate parathyroid calcium‐sensing receptor activity and secretion via a histidine‐independent mechanism. J Am Soc Nephrol. 2015;26(9):2163‐2171. doi: 10.1681/ASN.2014070653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weijenborg PW, Smout AJ, Bredenoord AJ. Esophageal acid sensitivity and mucosal integrity in patients with functional heartburn. Neurogastroenterol Motil. 2016;28(11):1649‐1654. doi: 10.1111/nmo.12864 [DOI] [PubMed] [Google Scholar]
- 65. Cao L, Li W, Yang X, et al. Inhibition of host Ogr1 enhances effector CD8+ T‐cell function by modulating acidic microenvironment. Cancer Gene Ther. 2021;28(10–11):1213‐1224. doi: 10.1038/s41417-021-00354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B‐cell non‐Hodgkin lymphoma. J Clin Oncol. 2015;33(25):2803‐2811. doi: 10.1200/JCO.2014.59.5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spugnini EP, Sonveaux P, Stock C, et al. Proton channels and exchangers in cancer. Biochim Biophys Acta. 2015;1848(10 Pt B):2715‐2726. doi: 10.1016/j.bbamem.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 68. Xu X, Fei J, Xu Y, et al. Boric acid‐fueled ATP synthesis by Fo F1 ATP synthase reconstituted in a supramolecular architecture. Angew Chem Int Ed Engl. 2021;60(14):7617‐7620. doi: 10.1002/anie.202016253 [DOI] [PubMed] [Google Scholar]
- 69. Liu J, Wang Y, Zhang M, et al. Color fading in lotus (Nelumbo nucifera) petals is manipulated both by anthocyanin biosynthesis reduction and active degradation. Plant Physiol Biochem. 2022;15(179):100‐107. doi: 10.1016/j.plaphy.2022.03.021 [DOI] [PubMed] [Google Scholar]
- 70. Halcrow P, Datta G, Ohm JE, Soliman ML, Chen X, Geiger JD. Role of endolysosomes and pH in the pathogenesis and treatment of glioblastoma. Cancer Rep. 2019;2(6):e1177. doi: 10.1002/cnr2.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Asgharzadeh MR, Barar J, Pourseif MM, et al. Molecular machineries of pH dysregulation in tumor microenvironment: potential targets for cancer therapy. Bioimpacts. 2017;7(2):115‐133. doi: 10.15171/bi.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yu C, Liu H, Guo C, et al. Dextran sulfate‐based MMP‐2 enzyme‐sensitive SR‐A receptor targeting nanomicelles for the treatment of rheumatoid arthritis. Drug Deliv. 2022;29(1):454‐465. doi: 10.1080/10717544.2022.2032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wi DH, Cha JH, Jung YS. Mucin in cancer: a stealth cloak for cancer cells. BMB Rep. 2021;54(7):344‐355. doi: 10.5483/BMBRep.2021.54.7.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ali A, Biswas R, Bhattacharjee S, Nath P, Pan S, Bagchi A. Comparative analyses of the relative effects of various mutations in major histocompatibility complex I‐a way to predict protein‐protein interactions. Appl Biochem Biotechnol. 2016;180(1):152‐164. doi: 10.1007/s12010-016-2090-z [DOI] [PubMed] [Google Scholar]
- 75. Choi SJ, Cho H, Yea K, Baek MC. Immune cell‐derived small extracellular vesicles in cancer treatment. BMB Rep. 2021;54:335‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chadet S, Allard J, Brisson L, et al. P2x4 receptor promotes mammary cancer progression by sustaining autophagy and associated mesenchymal transition. Oncogene. 2022;9(8):432‐445. doi: 10.1038/s41388-022-02297-8 [DOI] [PubMed] [Google Scholar]
- 77. Cao Y, Chen M, Tang D, et al. The proton pump inhibitor pantoprazole disrupts protein degradation systems and sensitizes cancer cells to death under various stresses. Cell Death Dis. 2018;9(6):604. doi: 10.1038/s41419-018-0642-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nam LB, Keum YS. Regulation of NRF2 by Na+/K+‐ATPase: implication of tyrosine phosphorylation of Src. Free Radic Res. 2020;54(11–12):883‐893. doi: 10.1080/10715762.2020.1735633 [DOI] [PubMed] [Google Scholar]
- 79. Santos‐Pereira C, Rodrigues LR, Côrte‐Real M. Emerging insights on the role of V‐ATPase in human diseases: therapeutic challenges and opportunities. Med Res Rev. 2021;41(4):1927‐1964. doi: 10.1002/med.21782 [DOI] [PubMed] [Google Scholar]
- 80. Chiang YR, Wang LC, Lin HT, Lin JH. Bioactivity of orange‐spotted grouper (Epinephelus coioides) cathepsin L: proteolysis of bacteria and regulation of the innate immune response. Fish Shellfish Immunol. 2022;122:399‐408. doi: 10.1016/j.fsi.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 81. Krueger F, Kappert K, Foryst‐Ludwig A, et al. AT1‐receptor blockade attenuates outward aortic remodeling associated with diet‐induced obesity in mice. Clin Sci (Lond). 2017;131(15):1989‐2005. doi: 10.1042/CS20170131 [DOI] [PubMed] [Google Scholar]
- 82. Chae YC, Vaira V, Caino MC, et al. Mitochondrial Akt regulation of hypoxic tumor reprogramming. Cancer Cell. 2016;30(2):257‐272. doi: 10.1016/j.ccell.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ordway B, Gillies RJ, Damaghi M. Extracellular acidification induces lysosomal dysregulation. Cells. 2021;10(5):1188. doi: 10.3390/cells10051188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nath B, Bidkar AP, Kumar V, et al. Deciphering hydrodynamic and drug‐resistant behaviors of metastatic EMT breast cancer cells moving in a constricted microcapillary. J Clin Med. 2019;8(8):1194. doi: 10.3390/jcm8081194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang G, Zhou X, Guo Z, et al. The anti‐fibrosis drug Pirfenidone modifies the immunosuppressive tumor microenvironment and prevents the progression of renal cell carcinoma by inhibiting tumor autocrine TGF‐β. Cancer Biol Ther. 2022;23(1):150‐162. doi: 10.1080/15384047.2022.2035629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cao X, Yang Q, Qin J, et al. V‐ATPase promotes transforming growth factor‐β‐induced epithelial‐mesenchymal transition of rat proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2012;302(9):F1121‐F1132. doi: 10.1152/ajprenal.00278.2011 [DOI] [PubMed] [Google Scholar]
- 87. Zhang Y, Yang F, Peng X, et al. Hypoxia constructing the prognostic model of colorectal adenocarcinoma and related to the immune microenvironment. Front Cell Dev Biol. 2021;20(9):665364. doi: 10.3389/fcell.2021.665364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lefebvre TL, Brown E, Hacker L, et al. The potential of photoacoustic imaging in radiation oncology. Front Oncol. 2022;3(12):803777. doi: 10.3389/fonc.2022.803777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang M, Yan J, Cao X, Hua P, Li Z. Hydrogen sulfide modulates epithelial‐mesenchymal transition and angiogenesis in non‐small cell lung cancer via HIF‐1α activation. Biochem Pharmacol. 2020;172:113775. doi: 10.1016/j.bcp.2019.113775 [DOI] [PubMed] [Google Scholar]
- 90. Campbell EJ, Dachs GU, Morrin HR, Davey VC, Robinson BA, Vissers MCM. Activation of the hypoxia pathway in breast cancer tissue and patient survival are inversely associated with tumor ascorbate levels. BMC Cancer. 2019;19(1):307. doi: 10.1186/s12885-019-5503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Trivlidis J, Aloufi N, Al‐Habeeb F, et al. HuR drives lung fibroblast differentiation but not metabolic reprogramming in response to TGF‐β and hypoxia. Respir Res. 2021;22(1):323. doi: 10.1186/s12931-021-01916-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Man CH, Mercier FE, Liu N, et al. Proton export alkalinizes intracellular pH and reprograms carbon metabolism to drive normal and malignant cell growth. Blood. 2022;139(4):502‐522. doi: 10.1182/blood.2021011563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Michl J, Wang Y, Monterisi S, et al. CRISPR‐Cas9 screen identifies oxidative phosphorylation as essential for cancer cell survival at low extracellular pH. Cell Rep. 2022;38(10):110493. doi: 10.1016/j.celrep.2022.110493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Goetze K, Fabian CG, Siebers A, et al. Manipulation of tumor metabolism for therapeutic approaches: ovarian cancer‐derived cell lines as a model system. Cell Oncol (Dordr). 2015;38(5):377‐385. doi: 10.1007/s13402-015-0237-5 [DOI] [PubMed] [Google Scholar]
- 95. Gao L, Xu QH, Ma LN, et al. Trophoblast‐derived lactic acid orchestrates Decidual macrophage differentiation via SRC/LDHA signaling in early pregnancy. Int J Biol Sci. 2022;18(2):599‐616. doi: 10.7150/ijbs.67816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Morimoto C, Takedachi M, Kawasaki K, et al. Hypoxia stimulates collagen hydroxylation in gingival fibroblasts and periodontal ligament cells. J Periodontol. 2021;92(11):1635‐1645. doi: 10.1002/JPER.20-0670 [DOI] [PubMed] [Google Scholar]
- 97. Ji RC. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;346(1):6‐16. doi: 10.1016/j.canlet.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 98. Gorick CM, Saucerman JJ, Price RJ. Computational model of brain endothelial cell signaling pathways predicts therapeutic targets for cerebral pathologies. J Mol Cell Cardiol. 2022;164:17‐28. doi: 10.1016/j.yjmcc.2021.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cantó A, Olivar T, Romero FJ, Miranda M. Nitrosative stress in retinal pathologies: review. Antioxidants (Basel). 2019;8(11):543. doi: 10.3390/antiox8110543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pagnotta S, Tramutola A, Barone E, et al. CAPE and its synthetic derivative VP961 restore BACH1/NRF2 axis in down syndrome. Free Radic Biol Med. 2022;183:1‐13. doi: 10.1016/j.freeradbiomed.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 101. Zhou W, Chen C, Shi Y, et al. Targeting glioma stem cell‐derived Pericytes disrupts the blood‐tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell. 2017;21(5):591‐603.e4. doi: 10.1016/j.stem.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Daneman R, Prat A. The blood‐brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Prados MD. Future directions in the treatment of malignant gliomas with temozolomide. Semin Oncol. 2000;27(3 Suppl 6):41‐46. [PubMed] [Google Scholar]
- 104. Munoz JL, Walker ND, Scotto KW, Rameshwar P. Temozolomide competes for P‐glycoprotein and contributes to chemoresistance in glioblastoma cells. Cancer Lett. 2015;367(1):69‐75. doi: 10.1016/j.canlet.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 105. Park SH, Kim MJ, Jung HH, et al. Safety and feasibility of multiple blood‐brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg. 2020;3:1‐9. doi: 10.3171/2019.10.JNS192206 [DOI] [PubMed] [Google Scholar]
- 106. Declèves X, Amiel A, Delattre JY, Scherrmann JM. Role of ABC transporters in the chemoresistance of human gliomas. Curr Cancer Drug Targets. 2006;6(5):433‐445. doi: 10.2174/156800906777723930 [DOI] [PubMed] [Google Scholar]
- 107. Yang G, Xu S, Peng L, Li H, Zhao Y, Hu Y. The hypoxia‐mimetic agent CoCl₂ induces chemotherapy resistance in LOVO colorectal cancer cells. Mol Med Rep. 2016;13(3):2583‐2589. doi: 10.3892/mmr.2016.4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kachalaki S, Ebrahimi M, Mohamed Khosroshahi L, Mohammadinejad S, Baradaran B. Cancer chemoresistance; biochemical and molecular aspects: a brief overview. Eur J Pharm Sci. 2016;30(89):20‐30. doi: 10.1016/j.ejps.2016.03.025 [DOI] [PubMed] [Google Scholar]
- 109. Zhou X, Jin N, Chen B. Tetrandrine overcomes drug resistance mediated by bone marrow microenvironment by regulating the expression of P‐glycoprotein in acute leukemia. Hematology. 2022;27(1):274‐279. doi: 10.1080/16078454.2022.2034256 [DOI] [PubMed] [Google Scholar]
- 110. Uprety B, Chandran R, Arderne C, Abrahamse H. Anticancer activity of urease mimetic cobalt (III) complexes on A549‐lung cancer cells: targeting the acidic microenvironment. Pharmaceutics. 2022;14(1):211. doi: 10.3390/pharmaceutics14010211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Charifson PS, Walters WP. Acidic and basic drugs in medicinal chemistry: a perspective. J Med Chem. 2014;57(23):9701‐9717. doi: 10.1021/jm501000a [DOI] [PubMed] [Google Scholar]
- 112. Hraběta J, Belhajová M, Šubrtová H, Merlos Rodrigo MA, Heger Z, Eckschlager T. Drug sequestration in lysosomes as one of the mechanisms of chemoresistance of cancer cells and the possibilities of its inhibition. Int J Mol Sci. 2020;21(12):4392. doi: 10.3390/ijms21124392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tsuruo T, Naito M, Tomida A, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003. Jan;94(1):15‐21. doi: 10.1111/j.1349-7006.2003.tb01345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kanwore K, Kanwore K, Adzika GK, et al. Cancer metabolism: the role of immune cells epigenetic alteration in tumorigenesis, progression, and metastasis of glioma. Front Immunol. 2022;22(13):831636. doi: 10.3389/fimmu.2022.831636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhao Z, Wang Z, Wu Y, Liao D, Zhao B. Comprehensive analysis of TAMs marker genes in glioma for predicting prognosis and immunotherapy response. Mol Immunol. 2022;144:78‐95. doi: 10.1016/j.molimm.2022.02.012 [DOI] [PubMed] [Google Scholar]
- 116. Strepkos D, Markouli M, Klonou A, Piperi C, Papavassiliou AG. Insights in the immunobiology of glioblastoma. J Mol Med (Berl). 2020;98(1):1‐10. doi: 10.1007/s00109-019-01835-4 [DOI] [PubMed] [Google Scholar]
- 117. Mummolo S, Botticelli G, Quinzi V, Giuca G, Mancini L, Marzo G. Implant‐safe test in patients with peri‐implantitis. J Biol Regul Homeost Agents. 2020;34(3 Suppl. 1):147‐153. [PubMed] [Google Scholar]
- 118. Rong X, Huang B, Qiu S, Li X, He L, Peng Y. Tumor‐associated macrophages induce vasculogenic mimicry of glioblastoma multiforme through cyclooxygenase‐2 activation. Oncotarget. 2016;7(51):83976‐83986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhu D, Li Y, Zhang Z, et al. Recent advances of nanotechnology‐based tumor vessel‐targeting strategies. J Nanobiotechnology. 2021;19(1):435. doi: 10.1186/s12951-021-01190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Okikawa S, Morine Y, Saito Y, et al. Inhibition of the VEGF signaling pathway attenuates tumor‐associated macrophage activity in liver cancer. Oncol Rep. 2022;47(4):71. doi: 10.3892/or.2022.8282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yang Y, Zhang Y, Chen X, Su Z, Deng Y, Zhao Q. Khasianine ameliorates psoriasis‐like skin inflammation and represses TNF‐α/NF‐κB axis mediated transactivation of IL‐17A and IL‐33 in keratinocytes. J Ethnopharmacol. 2022;28(292):115124. doi: 10.1016/j.jep.2022.115124 [DOI] [PubMed] [Google Scholar]
- 122. Tamai S, Ichinose T, Tsutsui T, et al. Tumor microenvironment in glioma invasion. Brain Sci. 2022;12(4):505. doi: 10.3390/brainsci12040505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ermakov MS, Nushtaeva AA, Richter VA, Koval OA. Cancer‐associated fibroblasts and their role in tumor progression. Vavilovskii Zhurnal Genet Selektsii. 2022;26(1):14‐21. doi: 10.18699/VJGB-22-03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang Y, Bian Y, Wang Y, et al. HIF‐1α is necessary for activation and tumour‐promotion effect of cancer‐associated fibroblasts in lung cancer. J Cell Mol Med. 2021;25(12):5457‐5469. doi: 10.1111/jcmm.16556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zimmerlin L, Park TS, Zambidis ET, Donnenberg VS, Donnenberg AD. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95(12):2235‐2245. doi: 10.1016/j.biochi.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Czekay RP, Cheon DJ, Samarakoon R, Kutz SM, Higgins PJ. Cancer‐associated fibroblasts: mechanisms of tumor progression and novel therapeutic targets. Cancers (Basel). 2022;14(5):1231. doi: 10.3390/cancers14051231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Paolino G, Corsetti P, Moliterni E, et al. Mast cells and cancer. G Ital Dermatol Venereol. 2019. Dec;154(6):650‐668. doi: 10.23736/S0392-0488.17.05818-7 [DOI] [PubMed] [Google Scholar]
- 128. Abiko K, Hayashi T, Yamaguchi K, Mandai M, Konishi I. Potential novel ovarian cancer treatment targeting myeloid‐derived suppressor cells. Cancer Invest. 2021;39(4):310‐314. doi: 10.1080/07357907.2020.1871487 [DOI] [PubMed] [Google Scholar]
- 129. Alghamri MS, McClellan BL, Hartlage MS, et al. Targeting neuroinflammation in brain cancer: uncovering mechanisms, pharmacological targets, and Neuropharmaceutical developments. Front Pharmacol. 2021;18(12):680021. doi: 10.3389/fphar.2021.680021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Abbas S, Singh SK, Saxena AK, Tiwari S, Sharma LK, Tiwari M. Role of autophagy in regulation of glioma stem cells population during therapeutic stress. J Stem Cells Regen Med. 2020;16(2):80‐89. doi: 10.46582/jsrm.1602012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rakesh R, LC PD, Sakthivel KM, Rasmi RR. Role and regulation of autophagy in cancer. Biochim Biophys Acta Mol Basis Dis. 2022;1868(7):166400. doi: 10.1016/j.bbadis.2022.166400 [DOI] [PubMed] [Google Scholar]
- 132. Yu Y, Liu B, Li X, et al. ATF4/CEMIP/PKCα promotes anoikis resistance by enhancing protective autophagy in prostate cancer cells. Cell Death Dis. 2022;13(1):46. doi: 10.1038/s41419-021-04494-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kim J, Chee WY, Yabuta N, Kajiwara K, Nada S, Okada M. Atg5‐mediated autophagy controls apoptosis/anoikis via p53/Rb pathway in naked mole‐rat fibroblasts. Biochem Biophys Res Commun. 2020;528(1):146‐153. doi: 10.1016/j.bbrc.2020.05.083 [DOI] [PubMed] [Google Scholar]
- 134. Li Y, Gao S, Du X, Ji J, Xi Y, Zhai G. Advances in autophagy as a target in the treatment of tumours. J Drug Target. 2022;30(2):166‐187. doi: 10.1080/1061186X.2021.1961792 [DOI] [PubMed] [Google Scholar]
- 135. He C, Lu S, Wang XZ, et al. FOXO3a protects glioma cells against temozolomide‐induced DNA double strand breaks via promotion of BNIP3‐mediated mitophagy. Acta Pharmacol Sin. 2021;42(8):1324‐1337. doi: 10.1038/s41401-021-00663-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yuan T, Wang J, Shi C, et al. Downregulation of FAPP2 gene induces cell autophagy and inhibits PI3K/AKT/mTOR pathway in T‐cell acute lymphoblastic leukemia. Hematol Oncol. 2022;40(2):249‐257. doi: 10.1002/hon.2948 [DOI] [PubMed] [Google Scholar]
- 137. Duggan MR, Weaver M, Khalili K. PAM (PIK3/AKT/mTOR) signaling in glia: potential contributions to brain tumors in aging. Aging (Albany NY). 2021;13(1):1510‐1527. doi: 10.18632/aging.202459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Trelford CB, Di Guglielmo GM. Canonical and non‐canonical TGFβ signaling activate autophagy in an ULK1‐dependent manner. Front Cell Dev Biol. 2021;25(9):712124. doi: 10.3389/fcell.2021.712124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lu G, Wu Z, Shang J, Xie Z, Chen C, Zhang C. The effects of metformin on autophagy. Biomed Pharmacother. 2021. May;137:111286. doi: 10.1016/j.biopha.2021.111286 [DOI] [PubMed] [Google Scholar]
- 140. Park JW, Jeong J, Bae YS. Protein kinase CK2 is upregulated by calorie restriction and induces autophagy. Mol Cells. 2022;45(3):112‐121. doi: 10.14348/molcells.2021.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li Y, Li S, Wu H. Ubiquitination‐proteasome system (UPS) and autophagy two Main protein degradation machineries in response to cell stress. Cell. 2022;11(5):851. doi: 10.3390/cells11050851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chipurupalli S, Ganesan R, Martini G, et al. Cancer cells adapt FAM134B/BiP mediated ER‐phagy to survive hypoxic stress. Cell Death Dis. 2022;13(4):357. doi: 10.1038/s41419-022-04813-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Qin X, Wang J, Chen S, et al. Astrocytic p75NTR expression provoked by ischemic stroke exacerbates the blood‐brain barrier disruption. Glia. 2022;70(5):892‐912. doi: 10.1002/glia.24146 [DOI] [PubMed] [Google Scholar]
- 144. Izdebska M, Zielińska W, Krajewski A, et al. Downregulation of MMP‐9 enhances the anti‐migratory effect of cyclophosphamide in MDA‐MB‐231 and MCF‐7 breast cancer cell lines. Int J Mol Sci. 2021;22(23):12783. doi: 10.3390/ijms222312783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ahir BK, Engelhard HH, Lakka SS. Tumor development and angiogenesis in adult brain tumor: glioblastoma. Mol Neurobiol. 2020;57(5):2461‐2478. doi: 10.1007/s12035-020-01892-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cheng B, Hong X, Wang L, et al. Curzerene suppresses progression of human glioblastoma through inhibition of glutathione S‐transferase A4. CNS Neurosci Ther. 2022;28(5):690‐702. doi: 10.1111/cns.13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yang B, Ding L, Chen Y, Shi J. Augmenting tumor‐starvation therapy by cancer cell autophagy inhibition. Adv Sci (Weinh). 2020;7(6):1902847. doi: 10.1002/advs.201902847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405‐417. doi: 10.1038/s41582-019-0220-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jiang L, Liu J. Immunological effect of tyrosine kinase inhibitors on the tumor immune environment in non‐small cell lung cancer. Oncol Lett. 2022;23(5):165. doi: 10.3892/ol.2022.13285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Geeviman K, Babu D, Prakash BP. Pantoprazole induces mitochondrial apoptosis and attenuates NF‐κB signaling in glioma cells. Cell Mol Neurobiol. 2018;38(8):1491‐1504. doi: 10.1007/s10571-018-0623-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shen W, Zou X, Chen M, et al. Effect of pantoprazole on human gastric adenocarcinoma SGC7901 cells through regulation of phospho‐LRP6 expression in Wnt/β‐catenin signaling. Oncol Rep. 2013;30(2):851‐855. doi: 10.3892/or.2013.2524 [DOI] [PubMed] [Google Scholar]
- 152. Ehrenfeld J, Klein U. The key role of the H+ V‐ATPase in acid‐base balance and Na+ transport processes in frog skin. J Exp Biol. 1997;200(Pt 2):247‐256. doi: 10.1242/jeb.200.2.247 [DOI] [PubMed] [Google Scholar]
- 153. Lu Y, Zhang R, Liu S, Zhao Y, Gao J, Zhu L. ZT‐25, a new vacuolar H(+)‐ATPase inhibitor, induces apoptosis and protective autophagy through ROS generation in HepG2 cells. Eur J Pharmacol. 2016;15(771):130‐138. doi: 10.1016/j.ejphar.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 154. Brisson L, Reshkin SJ, Goré J, Roger S. pH regulators in invadosomal functioning: proton delivery for matrix tasting. Eur J Cell Biol. 2012;91(11–12):847‐860. doi: 10.1016/j.ejcb.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 155. Kulshrestha A, Katara GK, Ibrahim SA, et al. In vivo anti‐V‐ATPase antibody treatment delays ovarian tumor growth by increasing antitumor immune responses. Mol Oncol. 2020;14(10):2436‐2454. doi: 10.1002/1878-0261.12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Battistone MA, Merkulova M, Park YJ, et al. Unravelling purinergic regulation in the epididymis: activation of V‐ATPase‐dependent acidification by luminal ATP and adenosine. J Physiol. 2019. Apr;597(7):1957‐1973. doi: 10.1113/JP277565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fontecha‐Barriuso M, Martín‐Sanchez D, Martinez‐Moreno JM, et al. Molecular pathways driving omeprazole nephrotoxicity. Redox Biol. 2020;32:101464. doi: 10.1016/j.redox.2020.101464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mashimo M, Onishi M, Uno A, et al. The 89‐kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF‐mediated apoptosis. J Biol Chem. 2021;296:100046. doi: 10.1074/jbc.RA120.014479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Qi C, Lei L, Hu J, Wang G, Liu J, Ou S. Identification of a five‐gene signature deriving from the vacuolar ATPase (V‐ATPase) sub‐classifies gliomas and decides prognoses and immune microenvironment alterations. Cell Cycle. 2022;10:1‐22. doi: 10.1080/15384101.2022.2049157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shiratori R, Furuichi K, Yamaguchi M, et al. Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism‐dependent manner. Sci Rep. 2019;9(1):18699. doi: 10.1038/s41598-019-55296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Watson MJ, Delgoffe GM. Fighting in a wasteland: deleterious metabolites and antitumor immunity. J Clin Invest. 2022;132(2):e148549. doi: 10.1172/JCI148549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Dkhil MA, Al‐Quraishy S, Al‐Khalifa MS. The effect of Babesia divergens infection on the spleen of Mongolian gerbils. Biomed Res Int. 2014;2014:483854. doi: 10.1155/2014/483854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Fedotova EI, Dolgacheva LP, Abramov AY, Berezhnov AV. Lactate and pyruvate activate autophagy and mitophagy that protect cells in toxic model of Parkinson's disease. Mol Neurobiol. 2022;59(1):177‐190. doi: 10.1007/s12035-021-02583-8 [DOI] [PubMed] [Google Scholar]
- 164. Kardideh B, Samimi Z, Norooznezhad F, Kiani S, Mansouri K. Autophagy, cancer and angiogenesis: where is the link? Cell Biosci. 2019;13(9):65. doi: 10.1186/s13578-019-0327-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ding Y, Zhu W, Sun R, et al. Diphenylene iodonium interferes with cell cycle progression and induces apoptosis by modulating NAD(P)H oxidase/ROS/cell cycle regulatory pathways in Burkitt's lymphoma cells. Oncol Rep. 2015;33(3):1434‐1442. doi: 10.3892/or.2015.3726 [DOI] [PubMed] [Google Scholar]
- 166. Zheng J, Dai Y, Lin X, et al. Hypoxia‐induced lactate dehydrogenase a protects cells from apoptosis in endometriosis. Mol Med Rep. 2021;24(3):637. doi: 10.3892/mmr.2021.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749‐756. doi: 10.1136/gutjnl-2015-310861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Liu K, Hua S, Song L. PM2.5 exposure and asthma development: the key role of oxidative stress. Oxid Med Cell Longev. 2022;2022:3618806. doi: 10.1155/2022/3618806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Mendes S, Sá R, Magalhães M, Marques F, Sousa M, Silva E. The role of ROS as a double‐edged sword in (in)fertility: the impact of cancer treatment. Cancers (Basel). 2022;14(6):1585. doi: 10.3390/cancers14061585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bai J, Xie N, Hou Y, et al. The enhanced mitochondrial dysfunction by cantleyoside confines inflammatory response and promotes apoptosis of human HFLS‐RA cell line via AMPK/Sirt 1/NF‐κB pathway activation. Biomed Pharmacother. 2022;149:112847. doi: 10.1016/j.biopha.2022.112847 [DOI] [PubMed] [Google Scholar]
- 171. Tang X, Steinman AD, Xue Q, Xu Y, Xie L. Simultaneous electrochemical removal of Microcystis aeruginosa and sulfamethoxazole and its ecologic impacts on Vallisneria spiralis. Sci Total Environ. 2022;1(815):152769. doi: 10.1016/j.scitotenv.2021.152769 [DOI] [PubMed] [Google Scholar]
- 172. Zhang B, Pan C, Feng C, et al. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022;27(1):45‐52. doi: 10.1080/13510002.2022.2046423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Li Y, Yang L, Hou Y, et al. Polydopamine‐mediated graphene oxide and nanohydroxyapatite‐incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;22(18):213‐227. doi: 10.1016/j.bioactmat.2022.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Han CY, Pichon TJ, Wang X, et al. Leukocyte activation primes fibrinogen for proteolysis by mitochondrial oxidative stress. Redox Biol. 2022. May;51:102263. doi: 10.1016/j.redox.2022.102263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Xi X, Wang J, Qin Y, You Y, Huang W, Zhan J. The biphasic effect of flavonoids on oxidative stress and cell proliferation in breast cancer cells. Antioxidants (Basel). 2022;11(4):622. doi: 10.3390/antiox11040622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhuo M, Ma J, Quan X. Cytotoxicity of functionalized CeO2 nanoparticles towards Escherichia coli and adaptive response of membrane properties. Chemosphere. 2021;281:130865. doi: 10.1016/j.chemosphere.2021.130865 [DOI] [PubMed] [Google Scholar]
- 177. Park JH, Troxel AB, Harvey RG, Penning TM. Polycyclic aromatic hydrocarbon (PAH) o‐quinones produced by the aldo‐keto‐reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8‐oxo‐dGuo via reactive oxygen species. Chem Res Toxicol. 2006;19(5):719‐728. doi: 10.1021/tx0600245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kalegari P, Leme DM, Disner GR, et al. High melanin content in melanoma cells contributes to enhanced DNA damage after rose Bengal photosensitization. Photochem Photobiol. 2022;33(2)192‐196. doi: 10.1111/php.13632 [DOI] [PubMed] [Google Scholar]
- 179. Khurana H, Hazari PP, Mishra AK. Radioprotective efficacy of GSH based peptidomimetic complex of manganese against radiation induced damage: DT(GS)2Mn(II). Free Radic Biol Med. 2019;145:161‐174. doi: 10.1016/j.freeradbiomed.2019.09.023 [DOI] [PubMed] [Google Scholar]
- 180. Luciani A, Villella VR, Vasaturo A, et al. Lysosomal accumulation of gliadin p31‐43 peptide induces oxidative stress and tissue transglutaminase‐mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa. Gut. 2010;59(3):311‐319. doi: 10.1136/gut.2009.183608 [DOI] [PubMed] [Google Scholar]
- 181. Коbylinska LI, Boiko NM, Panchuk RR, et al. Putative anticancer potential of novel 4‐thiazolidinone derivatives: cytotoxicity toward rat C6 glioma in vitro and correlation of general toxicity with the balance of free radical oxidation in rats. Croat Med J. 2016;57(2):151‐163. doi: 10.3325/cmj.2016.57.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chueca E, Apostolova N, Esplugues JV, García‐González MA, Lanas Á, Piazuelo E. Proton pump inhibitors display antitumor effects in Barrett's adenocarcinoma cells. Front Pharmacol. 2016;25(7):452. doi: 10.3389/fphar.2016.00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Boj‐Carceller D. Proton pump inhibitors: impact on glucose metabolism. Endocrine. 2013;43(1):22‐32. doi: 10.1007/s12020-012-9755-3 [DOI] [PubMed] [Google Scholar]
- 184. Nakagawa S, Arai Y, Kishida T, et al. Lansoprazole inhibits nitric oxide and prostaglandin E(2) production in murine macrophage RAW 264.7 cells. Inflammation. 2012;35(3):1062‐1068. doi: 10.1007/s10753-011-9412-7 [DOI] [PubMed] [Google Scholar]
- 185. Wang Y, Floor E. Hydrogen peroxide inhibits the vacuolar H+‐ATPase in brain synaptic vesicles at micromolar concentrations. J Neurochem. 1998. Feb;70(2):646‐652. doi: 10.1046/j.1471-4159.1998.70020646.x [DOI] [PubMed] [Google Scholar]
- 186. Gu S, Zhou C, Pei J, et al. Esomeprazole inhibits hypoxia/endothelial dysfunction‐induced autophagy in preeclampsia. Cell Tissue Res. 2022;388(1):181‐194. doi: 10.1007/s00441-022-03587-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Griguer CE, Cantor AB, Fathallah‐Shaykh HM, et al. Prognostic relevance of cytochrome C oxidase in primary glioblastoma multiforme. PLoS One. 2013;8(4):e61035. doi: 10.1371/journal.pone.0061035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Eldridge RC, Uppal K, Hayes DN, et al. Plasma metabolic phenotypes of HPV‐associated versus smoking‐associated head and neck cancer and patient survival. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1858‐1866. doi: 10.1158/1055-9965.EPI-21-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ieraci L, Eberg M, Forster K, et al. Development of population‐level colon cancer pathway concordance measures and association with survival. Int J Cancer. 2022;150(12):2046‐2057. doi: 10.1002/ijc.33964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wang QW, Bao ZS, Jiang T, Zhu YJ. Tumor microenvironment is associated with clinical and genetic properties of diffuse gliomas and predicts overall survival. Cancer Immunol Immunother. 2022;71(4):953‐966. doi: 10.1007/s00262-021-03058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Han Y, Zou C, Zhu C, et al. The systematic landscape of nectin family and nectin‐like molecules: functions and prognostic value in low grade glioma. Front Genet. 2021;1(12):718717. doi: 10.3389/fgene.2021.718717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yoneda M, Aklima J, Ohsawa I, Ohta Y. Effects of proton pumping on the structural rigidity of cristae in mitochondria. Arch Biochem Biophys. 2022;15(720):109172. doi: 10.1016/j.abb.2022.109172 [DOI] [PubMed] [Google Scholar]
- 193. Flores‐Romero H, Hohorst L, John M, et al. BCL‐2‐family protein tBID can act as a BAX‐like effector of apoptosis. EMBO J. 2022;41(2):e108690. doi: 10.15252/embj.2021108690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Huang S, Chen M, Ding X, Zhang X, Zou X. Proton pump inhibitor selectively suppresses proliferation and restores the chemosensitivity of gastric cancer cells by inhibiting STAT3 signaling pathway. Int Immunopharmacol. 2013;17(3):585‐592. doi: 10.1016/j.intimp.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 195. Xie L, Guo YL, Chen YR, et al. A potential drug combination of omeprazole and patchouli alcohol significantly normalizes oxidative stress and inflammatory responses against gastric ulcer in ethanol‐induced rat model. Int Immunopharmacol. 2020;85:106660. doi: 10.1016/j.intimp.2020.106660 [DOI] [PubMed] [Google Scholar]
- 196. Zhao X, Shi X, Liu Q, Li X. Tea polyphenols alleviates acetochlor‐induced apoptosis and necroptosis via ROS/MAPK/NF‐κB signaling in Ctenopharyngodon idellus kidney cells. Aquat Toxicol. 2022;246:106153. doi: 10.1016/j.aquatox.2022.106153 [DOI] [PubMed] [Google Scholar]
- 197. López‐Posadas R, Ballester I, Mascaraque C, et al. Flavonoids exert distinct modulatory actions on cyclooxygenase 2 and NF‐kappaB in an intestinal epithelial cell line (IEC18). Br J Pharmacol. 2010;160(7):1714‐1726. doi: 10.1111/j.1476-5381.2010.00827.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kciuk M, Gielecińska A, Budzinska A, Mojzych M, Kontek R. Metastasis and MAPK pathways. Int J Mol Sci. 2022;23(7):3847. doi: 10.3390/ijms23073847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kong L, Barber T, Aldinger J, et al. ROS generation is involved in titanium dioxide nanoparticle‐induced AP‐1 activation through p38 MAPK and ERK pathways in JB6 cells. Environ Toxicol. 2022;37(2):237‐244. doi: 10.1002/tox.23393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lommen J, Sus M, Berr K, et al. Analysis of spontaneous and induced osteogenic differentiation in 3D‐micromasses of human multipotent stem cells. In Vivo. 2022. May‐Jun;36(3):1067‐1076. doi: 10.21873/invivo.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Rao Z, Jordan PM, Wang Y, et al. Differential role of vacuolar (H+)‐ATPase in the expression and activity of cyclooxygenase‐2 in human monocytes. Biochem Pharmacol. 2020;175:113858. doi: 10.1016/j.bcp.2020.113858 [DOI] [PubMed] [Google Scholar]
- 202. Tian J, Tang C, Wang X, Zhang X, Xiao L, Li W. Supramolecular structure features and immunomodulatory effects of exopolysaccharide from Paecilomyces cicadae TJJ1213 in RAW264.7 cells through NF‐κB/MAPK signaling pathways. Int J Biol Macromol. 2022;15(207):464‐474. doi: 10.1016/j.ijbiomac.2022.03.029 [DOI] [PubMed] [Google Scholar]
- 203. Kanai M, Mullen C, Podolsky DK. Intestinal trefoil factor induces inactivation of extracellular signal‐regulated protein kinase in intestinal epithelial cells. Proc Natl Acad Sci U S A. 1998;95(1):178‐182. doi: 10.1073/pnas.95.1.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lee SH, Na SI, Heo JS, et al. Arachidonic acid release by H2O2 mediated proliferation of mouse embryonic stem cells: involvement of Ca2+/PKC and MAPKs‐induced EGFR transactivation. J Cell Biochem. 2009;106(5):787‐797. doi: 10.1002/jcb.22013 [DOI] [PubMed] [Google Scholar]
- 205. Choi S, Yeum CH, Kim YD, et al. Receptor tyrosine and MAP kinase are involved in effects of H(2)O(2) on interstitial cells of Cajal in murine intestine. J Cell Mol Med. 2010;14(1–2):257‐266. doi: 10.1111/j.1582-4934.2008.00403.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Margalef P, Colomer C, Villanueva A, et al. BRAF‐induced tumorigenesis is IKKα‐dependent but NF‐κB‐independent. Sci Signal. 2015;8(373):ra38. doi: 10.1126/scisignal.2005886 [DOI] [PubMed] [Google Scholar]
- 207. Mazzio EA, Soliman KFA. Whole‐transcriptomic profile of SK‐MEL‐3 melanoma cells treated with the histone deacetylase inhibitor: trichostatin A. Cancer Genomics Proteomics. 2018;15(5):349‐364. doi: 10.21873/cgp.20094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kessler T, Sahm F, Sadik A, et al. Molecular differences in IDH wildtype glioblastoma according to MGMT promoter methylation. Neuro Oncol. 2018;20(3):367‐379. doi: 10.1093/neuonc/nox160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Huang J, Yu J, Tu L, Huang N, Li H, Luo Y. Isocitrate dehydrogenase mutations in glioma: from basic discovery to therapeutics development. Front Oncol. 2019;12(9):506. doi: 10.3389/fonc.2019.00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Terrasi A, Bertolini I, Martelli C, et al. Specific V‐ATPase expression sub‐classifies IDHwt lower‐grade gliomas and impacts glioma growth in vivo. EBioMedicine. 2019;41:214‐224. doi: 10.1016/j.ebiom.2019.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wronska E, Polkowski M, Orlowska J, Mroz A, Wieszczy P, Regula J. Argon plasma coagulation for Barrett's esophagus with low‐grade dysplasia: a randomized trial with long‐term follow‐up on the impact of power setting and proton pump inhibitor dose. Endoscopy. 2021;53(2):123‐132. doi: 10.1055/a-1203-5930 [DOI] [PubMed] [Google Scholar]
- 212. Lombardi G, Rumiato E, Bertorelle R, et al. Clinical and genetic factors associated with severe hematological toxicity in glioblastoma patients during radiation plus Temozolomide treatment: a prospective study. Am J Clin Oncol. 2015;38(5):514‐519. doi: 10.1097/COC.0b013e3182a790ea [DOI] [PubMed] [Google Scholar]
- 213. Camelo‐Piragua S, Kesari S. Further understanding of the pathology of glioma: implications for the clinic. Expert Rev Neurother. 2016;16(9):1055‐1065. doi: 10.1080/14737175.2016.1194755 [DOI] [PubMed] [Google Scholar]
- 214. Crettol S, Petrovic N, Murray M. Pharmacogenetics of phase I and phase II drug metabolism. Curr Pharm des. 2010;16(2):204‐219. doi: 10.2174/138161210790112674 [DOI] [PubMed] [Google Scholar]
- 215. Prasad H, Rao R. Histone deacetylase‐mediated regulation of endolysosomal pH. J Biol Chem. 2018;293(18):6721‐6735. doi: 10.1074/jbc.RA118.002025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Scheuch E, Walter R, Hadasová E, Amon I, Siegmund W. Influence of H2‐receptor‐ and proton pump inhibitors on some functions of the oxydative and conjugative drug metabolism. Pharmazie. 1996;51(7):493‐497. [PubMed] [Google Scholar]
- 217. Piotrowski AF, Blakeley J. Clinical Management of Seizures in patients with low‐grade glioma. Semin Radiat Oncol. 2015;25(3):219‐224. doi: 10.1016/j.semradonc.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Moliterno JA, Patel TR, Piepmeier JM. Neurosurgical approach. Cancer J. 2012;18(1):20‐25. doi: 10.1097/PPO.0b013e3183243f6e3 [DOI] [PubMed] [Google Scholar]
- 219. Huberfeld G, Vecht CJ. Seizures and gliomas—towards a single therapeutic approach. Nat Rev Neurol. 2016;12(4):204‐216. doi: 10.1038/nrneurol.2016.26 [DOI] [PubMed] [Google Scholar]
- 220. Zhu K, Sun J, Kang Z, et al. Repurposing of omeprazole for oligodendrocyte differentiation and remyelination. Brain Res. 2019;1(1710):33‐42. doi: 10.1016/j.brainres.2018.12.037 [DOI] [PubMed] [Google Scholar]
- 221. Yenisehirli A, Onur R. Positive inotropic and negative chronotropic effects of proton pump inhibitors in isolated rat atrium. Eur J Pharmacol. 2005;519(3):259‐266. doi: 10.1016/j.ejphar.2005.06.040 [DOI] [PubMed] [Google Scholar]
- 222. Chen YY, Tsai CF, Tsai MC, Hsu YW, Lu FJ. Inhibitory effects of rosmarinic acid on pterygium epithelial cells through redox imbalance and induction of extrinsic and intrinsic apoptosis. Exp Eye Res. 2017;160:96‐105. doi: 10.1016/j.exer.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 223. Sun W, Zhang W, Yu J, Lu Z, Yu J. Inhibition of Nrf2 might enhance the anti‐tumor effect of temozolomide in glioma cells via inhibition of Ras/Raf/MEK signaling pathway. Int J Neurosci. 2021;131(10):975‐983. doi: 10.1080/00207454.2020.1766458 [DOI] [PubMed] [Google Scholar]
- 224. Kim MJ, Jeon JH. Recent advances in understanding Nrf2 Agonism and its potential clinical application to metabolic and inflammatory diseases. Int J Mol Sci. 2022;23(5):2846. doi: 10.3390/ijms23052846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Li N, Zhan X. Machine learning identifies Pan‐cancer landscape of Nrf2 oxidative stress response pathway‐related genes. Oxid Med Cell Longev. 2022;17(2022):8450087. doi: 10.1155/2022/8450087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhou J, Li XY, Liu YJ, et al. Full‐coverage regulations of autophagy by ROS: from induction to maturation. Autophagy. 2021;18:1‐16. doi: 10.1080/15548627.2021.1984656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ji XJ, Chen SH, Zhu L, et al. Knockdown of NF‐E2‐related factor 2 inhibits the proliferation and growth of U251MG human glioma cells in a mouse xenograft model. Oncol Rep. 2013;30(1):157‐164. doi: 10.3892/or.2013.2476 [DOI] [PubMed] [Google Scholar]
- 228. Fan J, Lv H, Li J, et al. Roles of Nrf2/HO‐1 and HIF‐1α/VEGF in lung tissue injury and repair following cerebral ischemia/reperfusion injury. J Cell Physiol. 2019;234(6):7695‐7707. doi: 10.1002/jcp.27767 [DOI] [PubMed] [Google Scholar]
- 229. Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow‐derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206‐220. doi: 10.1016/j.ccr.2008.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yang Y, Wang X, Zhang J, et al. Abnormal phenotype of Nrf2 is associated with poor prognosis through hypoxic/VEGF‐A‐Rap1b/VEGFR2 pathway in gastric cancer. Aging (Albany NY). 2022;14(7):3293‐3312. doi: 10.18632/aging.204013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Zhao J, Niu X, Yu J, et al. Poria cocos polysaccharides attenuated ox‐LDL‐induced inflammation and oxidative stress via ERK activated Nrf2/HO‐1 signaling pathway and inhibited foam cell formation in VSMCs. Int Immunopharmacol. 2020;80:106173. doi: 10.1016/j.intimp.2019.106173 [DOI] [PubMed] [Google Scholar]
- 232. Foresti R, Bains SK, Pitchumony TS, et al. Small molecule activators of the Nrf2‐HO‐1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res. 2013;76:132‐148. doi: 10.1016/j.phrs.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 233. Seelige R, Washington A Jr, Bui JD. The ancient cytokine IL‐17D is regulated by Nrf2 and mediates tumor and virus surveillance. Cytokine. 2017;91:10‐12. doi: 10.1016/j.cyto.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Huang M, Mehrabi Nasab E, Athari SS. Immunoregulatory effect of mesenchymal stem cell via mitochondria signaling pathways in allergic asthma. Saudi J Biol Sci. 2021. Dec;28(12):6957‐6962. doi: 10.1016/j.sjbs.2021.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Grauer O, Pöschl P, Lohmeier A, Adema GJ, Bogdahn U. Toll‐like receptor triggered dendritic cell maturation and IL‐12 secretion are necessary to overcome T‐cell inhibition by glioma‐associated TGF‐beta2. J Neurooncol. 2007;82(2):151‐161. doi: 10.1007/s11060-006-9274-2 [DOI] [PubMed] [Google Scholar]
- 236. Li J, Zhao L, Zhang Y, et al. Imbalanced immune responses involving inflammatory molecules and immune‐related pathways in the lung of acute and subchronic arsenic‐exposed mice. Environ Res. 2017;159:381‐393. doi: 10.1016/j.envres.2017.08.036 [DOI] [PubMed] [Google Scholar]
- 237. Lee S, Hur EG, Ryoo IG, Jung KA, Kwak J, Kwak MK. Involvement of the Nrf2‐proteasome pathway in the endoplasmic reticulum stress response in pancreatic β‐cells. Toxicol Appl Pharmacol. 2012;264(3):431‐438. doi: 10.1016/j.taap.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 238. Garufi A, Pistritto G, D'Orazi V, Cirone M, D'Orazi G. The impact of NRF2 inhibition on drug‐induced colon cancer cell death and p53 activity: a pilot study. Biomolecules. 2022;12(3):461. doi: 10.3390/biom12030461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Catanzaro E, Calcabrini C, Turrini E, Sestili P, Fimognari C. Nrf2: a potential therapeutic target for naturally occurring anticancer drugs? Expert Opin Ther Targets. 2017;21(8):781‐793. doi: 10.1080/14728222.2017.1351549 [DOI] [PubMed] [Google Scholar]
- 240. Zhang HF, Wang JH, Wang YL, et al. Salvianolic acid a protects the kidney against oxidative stress by activating the Akt/GSK‐3β/Nrf2 signaling pathway and inhibiting the NF‐κB signaling pathway in 5/6 Nephrectomized rats. Oxid Med Cell Longev. 2019;18(2019):2853534. doi: 10.1155/2019/2853534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Gan X, Zhang R, Gu J, et al. Acidic microenvironment regulates the severity of hepatic ischemia/reperfusion injury by modulating the generation and function of Tregs via the PI3K‐mTOR pathway. Front Immunol. 2020;9(10):2945. doi: 10.3389/fimmu.2019.02945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Gravano DM, Vignali DA. The battle against immunopathology: infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci. 2012;69(12):1997‐2008. doi: 10.1007/s00018-011-0907-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rolim GB, Dantas Lima AJP, Dos Santos Cardoso VI, et al. Can inflammasomes promote the pathophysiology of glioblastoma multiforme? A view about the potential of the anti‐inflammasome therapy as pharmacological target. Crit Rev Oncol Hematol. 2022;172:103641. doi: 10.1016/j.critrevonc.2022.103641 [DOI] [PubMed] [Google Scholar]
- 244. Zhang Y, Feng Z, Liu J, et al. Polarization of tumor‐associated macrophages by TLR7/8 conjugated radiosensitive peptide hydrogel for overcoming tumor radioresistance. Bioact Mater. 2022;3(16):359‐371. doi: 10.1016/j.bioactmat.2021.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Wedekind H, Walz K, Buchbender M, et al. Head and neck tumor cells treated with hypofractionated irradiation die via apoptosis and are better taken up by M1‐like macrophages. Strahlenther Onkol. 2022;198(2):171‐182. doi: 10.1007/s00066-021-01856-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Katara GK, Kulshrestha A, Jaiswal MK, Pamarthy S, Gilman‐Sachs A, Beaman KD. Inhibition of vacuolar ATPase subunit in tumor cells delays tumor growth by decreasing the essential macrophage population in the tumor microenvironment. Oncogene. 2016;35(8):1058‐1065. doi: 10.1038/onc.2015.159 [DOI] [PubMed] [Google Scholar]
- 247. Davuluri GVN, Chan CH. Regulation of intrinsic and extrinsic metabolic pathways in tumor‐associated macrophages. FEBS J. 2022;105(33):111‐113. doi: 10.1111/febs.16465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Zhang W, Shi Y, Li H, et al. In situ injectable nano‐complexed hydrogel based on chitosan/dextran for combining tumor therapy via hypoxia alleviation and TAMs polarity regulation. Carbohydr Polym. 2022;15(288):119418. doi: 10.1016/j.carbpol.2022.119418 [DOI] [PubMed] [Google Scholar]
- 249. Vishvakarma NK, Singh SM. Augmentation of myelopoiesis in a murine host bearing a T cell lymphoma following in vivo administration of proton pump inhibitor pantoprazole. Biochimie. 2011;93(10):1786‐1796. doi: 10.1016/j.biochi.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 250. Vishvakarma NK, Singh SM. Immunopotentiating effect of proton pump inhibitor pantoprazole in a lymphoma‐bearing murine host: implication in antitumor activation of tumor‐associated macrophages. Immunol Lett. 2010;134(1):83‐92. doi: 10.1016/j.imlet.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 251. Jindal A, Bruzzì S, Sutti S, et al. Fat‐laden macrophages modulate lobular inflammation in nonalcoholic steatohepatitis (NASH). Exp Mol Pathol. 2015;99(1):155‐162. doi: 10.1016/j.yexmp.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 252. Rentscher KE, Carroll JE, Polsky LR, Lamkin DM. Chronic stress increases transcriptomic indicators of biological aging in mouse bone marrow leukocytes. Brain Behav Immun Health. 2022;12(22):100461. doi: 10.1016/j.bbih.2022.100461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lee HJ, Park JM, Han YM, et al. The role of chronic inflammation in the development of gastrointestinal cancers: reviewing cancer prevention with natural anti‐inflammatory intervention. Expert Rev Gastroenterol Hepatol. 2016;10(1):129‐139. doi: 10.1586/17474124.2016.1103179 [DOI] [PubMed] [Google Scholar]
- 254. Kotsuka M, Hashimoto Y, Nakatake R, et al. Omeprazole increases survival through the inhibition of inflammatory mediaters in two rat sepsis models. Shock. 2022;57(3):444‐456. doi: 10.1097/SHK.0000000000001897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Gao H, Hoesel LM, Guo RF, Rancilio NJ, Sarma JV, Ward PA. Adenoviral‐mediated overexpression of SOCS3 enhances IgG immune complex‐induced acute lung injury. J Immunol. 2006;177(1):612‐620. doi: 10.4049/jimmunol.177.1.612 [DOI] [PubMed] [Google Scholar]
- 256. Ishibashi K, Koguchi T, Matsuoka K, et al. Interleukin‐6 induces drug resistance in renal cell carcinoma. Fukushima J Med Sci. 2018;64(3):103‐110. doi: 10.5387/fms.2018-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Sehgal A. Molecular changes during the genesis of human gliomas. Semin Surg Oncol. 1998;14(1):3‐12. doi: [DOI] [PubMed] [Google Scholar]
- 258. Rupert JE, Narasimhan A, Jengelley DHA, et al. Tumor‐derived IL‐6 and trans‐signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J Exp Med. 2021;218(6):e20190450. doi: 10.1084/jem.20190450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Rydén M, Arvidsson E, Blomqvist L, Perbeck L, Dicker A, Arner P. Targets for TNF‐alpha‐induced lipolysis in human adipocytes. Biochem Biophys Res Commun. 2004;318(1):168‐175. doi: 10.1016/j.bbrc.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 260. Li J, Chen XL, Shaker A, et al. Contribution of immunomodulators to gastroesophageal reflux disease and its complications: stromal cells, interleukin 4, and adiponectin. Ann N Y Acad Sci. 2016;1380(1):183‐194. doi: 10.1111/nyas.13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Thomas L, Rao Z, Gerstmeier J, et al. Selective upregulation of TNFα expression in classically‐activated human monocyte‐derived macrophages (M1) through pharmacological interference with V‐ATPase. Biochem Pharmacol. 2017;15(130):71‐82. doi: 10.1016/j.bcp.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 262. Kedika RR, Souza RF, Spechler SJ. Potential anti‐inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54(11):2312‐2317. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Kim YJ, Lee JS, Hong KS, Chung JW, Kim JH, Hahm KB. Novel application of proton pump inhibitor for the prevention of colitis‐induced colorectal carcinogenesis beyond acid suppression. Cancer Prev Res (Phila). 2010;3(8):963‐974. doi: 10.1158/1940-6207.CAPR-10-0033 [DOI] [PubMed] [Google Scholar]
- 264. Lee H, Koh JY. Roles for H+ /K+‐ATPase and zinc transporter 3 in cAMP‐mediated lysosomal acidification in bafilomycin A1‐treated astrocytes. Glia. 2021;69(5):1110‐1125. doi: 10.1002/glia.23952 [DOI] [PubMed] [Google Scholar]
- 265. Ye WF, Liu J, Wan L, et al. Effect of Xinfeng capsule on AS patients and their serum immunoglobulin subtypes and peripheral lymphocyte autophagy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36(3):310‐316. [PubMed] [Google Scholar]
- 266. Li T, Han J, Jia L, Hu X, Chen L, Wang Y. PKM2 coordinates glycolysis with mitochondrial fusion and oxidative phosphorylation. Protein Cell. 2019;10(8):583‐594. doi: 10.1007/s13238-019-0618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Wang Y, Han X, Fu M, et al. Qiliqiangxin attenuates hypoxia‐induced injury in primary rat cardiac microvascular endothelial cells via promoting HIF‐1α‐dependent glycolysis. J Cell Mol Med. 2018;22(5):2791‐2803. doi: 10.1111/jcmm.13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Chen Z, Dong H, Jia C, et al. Activation of mTORC1 in collecting ducts causes hyperkalemia. J Am Soc Nephrol. 2014;25(3):534‐545. doi: 10.1681/ASN.2013030225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Li J, Zhang T, Ren T, et al. Oxygen‐sensitive methylation of ULK1 is required for hypoxia‐induced autophagy. Nat Commun. 2022;13(1):1172. doi: 10.1038/s41467-022-28831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Basit F, van Oppen LM, Schöckel L, et al. Mitochondrial complex I inhibition triggers a mitophagy‐dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8(3):e2716. doi: 10.1038/cddis.2017.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Sanfelice RADS, Bortoleti BTDS, Tomiotto‐Pellissier F, et al. Biogenic silver nanoparticles (AgNp‐bio) reduce toxoplasma gondii infection and proliferation in HeLa cells, and induce autophagy and death of tachyzoites by apoptosis‐like mechanism. Acta Trop. 2021;222:106070. doi: 10.1016/j.actatropica.2021.106070 [DOI] [PubMed] [Google Scholar]
- 272. Lu ZN, Shi ZY, Dang YF, et al. Pantoprazole pretreatment elevates sensitivity to vincristine in drug‐resistant oral epidermoid carcinoma in vitro and in vivo. Biomed Pharmacother. 2019. Dec;120:109478. doi: 10.1016/j.biopha.2019.109478 [DOI] [PubMed] [Google Scholar]
- 273. Wang H, Wang A, Wang X, Zeng X, Xing H. AMPK/PPAR‐γ/NF‐κB axis participates in ROS‐mediated apoptosis and autophagy caused by cadmium in pig liver. Environ Pollut. 2022;294:118659. doi: 10.1016/j.envpol.2021.118659 [DOI] [PubMed] [Google Scholar]
- 274. Yue X, Wen S, Long‐Kun D, et al. Three important short‐chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5‐FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022;23(1):19. doi: 10.1186/s12865-022-00495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Bosnjak T, Solberg R, Hemati PD, Jafari A, Kassem M, Johansen HT. Lansoprazole inhibits the cysteine protease legumain by binding to the active site. Basic Clin Pharmacol Toxicol. 2019;125(2):89‐99. doi: 10.1111/bcpt.13230 [DOI] [PubMed] [Google Scholar]
- 276. Ebrahimpour A, Wang M, Li L, et al. Esomeprazole attenuates inflammatory and fibrotic response in lung cells through the MAPK/Nrf2/HO1 pathway. J Inflamm (Lond). 2021;18(1):17. doi: 10.1186/s12950-021-00284-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Schnöller LE, Albrecht V, Brix N, et al. Integrative analysis of therapy resistance and transcriptomic profiling data in glioblastoma cells identifies sensitization vulnerabilities for combined modality radiochemotherapy. Radiat Oncol. 2022;17(1):79. doi: 10.1186/s13014-022-02052-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Ma C, Nguyen HPT, Jones JJ, et al. Extracellular vesicles secreted by glioma stem cells are involved in radiation resistance and glioma progression. Int J Mol Sci. 2022;23(5):2770. doi: 10.3390/ijms23052770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Yu Y, Wang A, Wang S, et al. Efficacy of temozolomide‐conjugated gold nanoparticle photothermal therapy of drug‐resistant glioblastoma and its mechanism study. Mol Pharm. 2022;19(4):1219‐1229. doi: 10.1021/acs.molpharmaceut.2c00083 [DOI] [PubMed] [Google Scholar]
- 280. Ortiz R, Perazzoli G, Cabeza L, et al. Temozolomide: an updated overview of resistance mechanisms, nanotechnology advances and clinical applications. Curr Neuropharmacol. 2021;19(4):513‐537. doi: 10.2174/1570159X18666200626204005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Averbeck D, Rodriguez‐Lafrasse C. Role of mitochondria in radiation responses: epigenetic, metabolic, and signaling impacts. Int J Mol Sci. 2021;22(20):11047. doi: 10.3390/ijms222011047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Tomita K, Kuwahara Y, Igarashi K, et al. Mitochondrial dysfunction in diseases, longevity, and treatment resistance: tuning mitochondria function as a therapeutic strategy. Genes (Basel). 2021;12(9):1348. doi: 10.3390/genes12091348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Tričković JF, Šobot AV, Joksić I, Joksić G. Telomere fragility in radiology workers occupationally exposed to low doses of ionising radiation. Arh Hig Rada Toksikol. 2022;73(1):23‐30. doi: 10.2478/aiht-2022-73-3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Lo Greco MC, Milazzotto R, Liardo RLE, et al. Relapsing high‐grade glioma from peritumoral zone: critical review of radiotherapy treatment options. Brain Sci. 2022;12(4):416. doi: 10.3390/brainsci12040416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Hebert KA, Jaramillo S, Yu W, et al. Esomeprazole enhances the effect of ionizing radiation to improve tumor control. Oncotarget. 2021;12(14):1339‐1353. doi: 10.18632/oncotarget.28008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Cao L, Zhu Y, Wang W, Wang G, Zhang S, Cheng H. Emerging nano‐based strategies against drug resistance in tumor chemotherapy. Front Bioeng Biotechnol. 2021;7(9):798882. doi: 10.3389/fbioe.2021.798882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhang Q, Ding J, Wang Y, He L, Xue F. Tumor microenvironment manipulates chemoresistance in ovarian cancer (review). Oncol Rep. 2022;47(5):102. doi: 10.3892/or.2022.8313 [DOI] [PubMed] [Google Scholar]
- 288. Hsu HH, Chen MC, Baskaran R, et al. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J Cell Physiol. 2018;233(7):5458‐5467. doi: 10.1002/jcp.26406 [DOI] [PubMed] [Google Scholar]
- 289. Xue YY, Lu YY, Sun GQ, et al. CN‐3 increases TMZ sensitivity and induces ROS‐dependent apoptosis and autophagy in TMZ‐resistance glioblastoma. J Biochem Mol Toxicol. 2022;36(3):e22973. doi: 10.1002/jbt.22973 [DOI] [PubMed] [Google Scholar]
- 290. Lustig SD, Kodali SK, Longo SL, Kundu S, Viapiano MS. Ko143 reverses MDR in glioblastoma via deactivating P‐glycoprotein, sensitizing a resistant phenotype to TMZ treatment. Anticancer Res. 2022;42(2):723‐730. doi: 10.21873/anticanres.15530 [DOI] [PubMed] [Google Scholar]
- 291. Liu M, Tang R, Jiang Y. Pantoprazole induces apoptosis of leukemic cells by inhibiting expression of P‐glycoprotein/multidrug resistance‐associated Protein‐1 through PI3K/AKT/mTOR signaling. Indian J Hematol Blood Transfus. 2017;33(4):500‐508. doi: 10.1007/s12288-017-0808-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Bianchi G, Ravera S, Traverso C, et al. Curcumin induces a fatal energetic impairment in tumor cells in vitro and in vivo by inhibiting ATP‐synthase activity. Carcinogenesis. 2018;39(9):1141‐1150. doi: 10.1093/carcin/bgy076 [DOI] [PubMed] [Google Scholar]
- 293. Kang R, Tang D, Schapiro NE, et al. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33(5):567‐577. doi: 10.1038/onc.2012.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Rodón J, Carducci M, Sepulveda‐Sánchez JM, et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor‐β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs. 2015;33(2):357‐370. doi: 10.1007/s10637-014-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Peppicelli S, Bianchini F, Toti A, Laurenzana A, Fibbi G, Calorini L. Extracellular acidity strengthens mesenchymal stem cells to promote melanoma progression. Cell Cycle. 2015;14(19):3088‐3100. doi: 10.1080/15384101.2015.1078032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Gao H, Wang X, Qu X, et al. Omeprazole attenuates cisplatin‐induced kidney injury through suppression of the TLR4/NF‐κB/NLRP3 signaling pathway. Toxicology. 2020;440:152487. doi: 10.1016/j.tox.2020.152487 [DOI] [PubMed] [Google Scholar]
- 297. Babu D, Mudiraj A, Yadav N, Y B V K C, Panigrahi M, Prakash Babu P. Rabeprazole has efficacy per se and reduces resistance to temozolomide in glioma via EMT inhibition. Cell Oncol (Dordr). 2021. Aug;44(4):889‐905. doi: 10.1007/s13402-021-00609-w [DOI] [PubMed] [Google Scholar]
- 298. Hada N, Netzer WJ, Belhassan F, Wennogle LP, Gizurarson S. Nose‐to‐brain transport of imatinib mesylate: a pharmacokinetic evaluation. Eur J Pharm Sci. 2017;1(102):46‐54. doi: 10.1016/j.ejps.2017.02.032 [DOI] [PubMed] [Google Scholar]
- 299. Kulkarni P, Haldar MK, Katti P, et al. Hypoxia responsive, tumor penetrating lipid nanoparticles for delivery of chemotherapeutics to pancreatic cancer cell spheroids. Bioconjug Chem. 2016;27(8):1830‐1838. doi: 10.1021/acs.bioconjchem.6b00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Matsumoto S, Kishimoto S, Saito K, et al. Metabolic and physiologic imaging biomarkers of the tumor microenvironment predict treatment outcome with radiation or a hypoxia‐activated prodrug in mice. Cancer Res. 2018;78(14):3783‐3792. doi: 10.1158/0008-5472.CAN-18-0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Ouar Z, Bens M, Vignes C, et al. V andewalle a. inhibitors of vacuolar H+‐a TPase impair the preferential accumulation of daunomycin in lysosomes and reverse the resistance to anthracyclines in drug‐resistant renal epithelial cells. Biochem J. 2003;370:185‐193. doi: 10.1042/BJ20021411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779‐786. doi: 10.2217/14796694.1.6.779 [DOI] [PubMed] [Google Scholar]
- 303. Menon A, Abraham AG, Mahfouz M, et al. Concomitant use of proton pump inhibitors with Capecitabine based Neoadjuvant Chemoradiotherapy for locally advanced rectal cancer: is it Safe? Am J Clin Oncol. 2021;44(9):487‐494. doi: 10.1097/COC.0000000000000850 [DOI] [PubMed] [Google Scholar]
- 304. Zhang JL, Liu M, Yang Q, et al. Effects of omeprazole in improving concurrent chemoradiotherapy efficacy in rectal cancer. World J Gastroenterol. 2017;23(14):2575‐2584. doi: 10.3748/wjg.v23.i14.2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Eguchi K, Suzuki M, Ida S, et al. Successful treatment of radiation‐induced mucositis with proton pump inhibitor administration: a report of two laryngeal cancer cases. Auris Nasus Larynx. 2017;44(1):122‐125. doi: 10.1016/j.anl.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 306. Su Q, Wang D, Yuan B, Liu F, Lei Y, Li S. Effects of proton pump inhibitors on lung cancer precise radiotherapy‐induced radiation pneumonitis. Cell Biochem Biophys. 2014;70(2):1113‐1117. doi: 10.1007/s12013-014-0030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Xun X, Yin Q, Fu Y, He X, Dong Z. Proton pump inhibitors and the risk of community‐acquired pneumonia: an updated meta‐analysis. Ann Pharmacother. 2022;56(5):524‐532. doi: 10.1177/10600280211039240 [DOI] [PubMed] [Google Scholar]
- 308. Naghibzadeh N, Salmani F, Nomiri S, Tavakoli T. Investigating the effect of quadruple therapy with saccharomyces boulardii or lactobacillus reuteri strain (DSMZ 17648) supplements on eradication of helicobacter pylori and treatments adverse effects: a double‐blind placebo‐controlled randomized clinical trial. BMC Gastroenterol. 2022;22(1):107. doi: 10.1186/s12876-022-02187-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Bataille P, Chasset F, Monfort JB, et al. Cutaneous drug‐induced lupus erythematosus: clinical and immunological characteristics and update on new associated drugs. Ann Dermatol Venereol. 2021;133:56‐62. [DOI] [PubMed] [Google Scholar]
- 310. Kamal F, Khan MA, Molnar MZ, Howden CW. The association between proton pump inhibitor use with acute kidney injury and chronic kidney disease. J Clin Gastroenterol. 2018;52(6):468‐476. doi: 10.1097/MCG.0000000000001035 [DOI] [PubMed] [Google Scholar]
- 311. Rameau A, Andreadis K, Bayoumi A, Kaufman M, Belafsky P. Side effects of proton pump inhibitors: what are Patients' concerns? J Voice. 2021;35(5):809.e15‐809.e20. doi: 10.1016/j.jvoice.2020.01.018 [DOI] [PubMed] [Google Scholar]
- 312. Jakubowski JK, Patel R, Buddharaju V. Polymyositis presenting as rhabdomyolysis after the initiation of omeprazole. Cureus. 2020;12(5):e8125. doi: 10.7759/cureus.8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Gamaletsou MN, Denning DW. Gastroesophageal reflux disease and pulmonary diseases associated with Aspergillosis: is there a connection? Mycopathologia. 2017;182(11–12):1125‐1129. doi: 10.1007/s11046-017-0176-y [DOI] [PubMed] [Google Scholar]
- 314. Jimenez L, Campos Codo A, Sampaio VS, et al. Acid pH increases SARS‐CoV‐2 infection and the risk of death by COVID‐19. Front Med (Lausanne). 2021;8:637885. doi: 10.3389/fmed.2021.637885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Vinke P, Wesselink E, van Orten‐Luiten W, van Norren K. The use of proton pump inhibitors may increase symptoms of muscle function loss in patients with chronic illnesses. Int J Mol Sci. 2020;21(1):323. doi: 10.3390/ijms21010323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Shikh EV, Makhova AA, Chemeris AV, Tormyshov IA. Iatrogenic deficits of micronutrients. Vopr Pitan. 2021;90(4):53‐63. Russian. doi: 10.33029/0042-8833-2021-90-4-53-63 [DOI] [PubMed] [Google Scholar]
- 317. Dries LS, Haefliger R, Seibert BS, de Lima AG, Cardoso CO, Perassolo MS. Cognition, oxidative stress and vitamin B12 levels evaluation on patients under long‐term omeprazole use. J Pharm Pharmacol. 2022;74(4):547‐555. doi: 10.1093/jpp/rgab001 [DOI] [PubMed] [Google Scholar]
- 318. Liew JW, Peloquin C, Tedeschi SK, et al. Proton pump inhibitors and risk of calcium pyrophosphate deposition in a population‐based study. Arthritis Care Res (Hoboken). 2022;35(12):371‐378. doi: 10.1002/acr.24876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Douwes RM, Vinke JSJ, Gomes‐Neto AW, et al. Type of proton‐pump inhibitor and risk of iron deficiency in kidney transplant recipients ‐ results from the TransplantLines biobank and cohort study. Transpl Int. 2021;34(11):2305‐2316. doi: 10.1111/tri.14110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Vanwing V, Schevenels S, Klockaerts C, Danckaerts M. Psychotische symptomen als bijwerking van omeprazol [psychotic symptoms as a side‐effect of omeprazole]. Tijdschr Psychiatr. 2018;60(12):834‐837. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


