Abstract
Background:
Psoriasis is an inflammatory skin disease associated with increased cardiovascular (CV) risk, whose pathogenesis is not fully known.
Objective:
We identified a transcriptomic signature in psoriasis and investigated its association with prevalent and future risk of a CV event to understand the connection between psoriasis and CV disease (CVD).
Methods:
Psoriasis patients (n=37) with a history of moderate-severe skin disease without CVD and 11 matched controls underwent whole blood RNA sequencing. This transcriptomic signature in psoriasis vs controls was evaluated in two CVD cohorts: Women referred for cardiac catheterization with (n=76) vs without (n=97) myocardial infarction (MI), and patients with peripheral artery disease (n=106) followed over 2.5 years for major adverse CV or limb events (MACLE). The association between genes differentially expressed in psoriasis and prevalent and incident CV events was assed.
Results:
In psoriasis, median age was 44 (IQR; 34 – 51) years, 49% male, and ACC/AHA ASCVD Risk Score of 1.0% (0.6 – 3.4) with no significant difference vs controls. The median psoriasis area and severity index score (PASI) was 4.0 (IQR 2.9 – 8.2) with 36% on biologic therapy. Overall, 247 whole blood genes were upregulated and 228 downregulated in psoriasis vs controls (p<0.05), and 1,302 genes positively and 1,244 genes negatively correlated with PASI (p<0.05). Seventy-three genes overlapped between psoriasis prevalence and PASI with key regulators identified as IL-6, IL-1β, and interferon gamma. In the CVD cohorts, 50 of 73 genes (68%) identified in psoriasis associated with prevalent MI, and 29 (40%) with incident MACLE. Key regulator transcripts identified in psoriasis and CVD cohorts included SOCS3, BCL3, OSM, PIM2, PIM3, and STAT5A.
Conclusions:
A whole blood transcriptomic signature of psoriasis diagnosis and severity associated with prevalent MI and incident MACLE. These data have implications for better understanding the link between psoriasis, systemic inflammation, and CVD.
Keywords: Psoriasis, Endothelial Dysfunction, Cardiovascular Risk
Introduction:
Enhanced CV risk in psoriasis is driven, in part, via upregulation of the systemic immune response including a contribution of leukocytes and pro-inflammatory cytokines.1 Previously, we and others have shown that biomarkers of systemic inflammation correlate with atherosclerosis burden in psoriasis subjects, including C-reactive protein and the neutrophil-to-leukocyte ratio.1 We aimed to identify the inflammatory and biomarker signature uniquely expressed in psoriasis and its overlap and association with CV events.
RNA sequencing allows for assessment of the systemic immune transcriptome and can provide mechanistic insight into pathophysiological responses. Previously, using whole blood RNA sequencing (to identify a transcriptomic signature), we uncovered a link between psoriasis prevalence and upregulated systemic pro-atherosclerotic processes which correlated with vascular endothelial inflammation, a key step in atherosclerosis initiation.2 However, the association between this systemic transcriptomic signature in psoriasis and prevalence and risk of a future CV event remains incompletely understood. Therefore, the purpose of this investigation was to define a blood inflammatory transcriptomic signature in psoriasis and investigate potential clinical implications via comparison with, 1) those having an acute myocardial infarction (MI), and 2) subsequent future risk of a CV event.
Methods:
Patients with a history of moderate-to-severe psoriasis, and age-, sex-matched controls were recruited as part of an ongoing clinical trial to assess vascular health in psoriasis (NCT03228017), the results of which are previously published.2–4 Exclusion criteria included those with a history of CV or cerebrovascular disease, prescribed aspirin or lipid-lowering (e.g. statin) therapy, and recent changes to immune-modulating therapy (within 1 month).2 Enrolled subjects underwent a fasting early morning assessment, including a blood draw with plasma, serum, and PAXgene® Blood RNA.
The clinical implications of the transcriptomic signatures in psoriasis were assessed in the ongoing clinical trials; 1) Women’s Heart Attack Research Program (HARP, NCT03022552) and, 2) Platelet Activity in Vascular Surgery and Cardiovascular Events (PACE, NCT02106429). HARP includes women with an acute MI caused by obstructive coronary artery disease, confirmed by coronary angiography enrolled within 24 hours of diagnosis, and controls referred for elective coronary angiography.5 PACE enrolled subjects with peripheral arterial disease (PAD) who underwent phenotyping just prior to lower extremity revascularization and followed for a major adverse cardiovascular or limb events (MACLE) over a median of 2.5 years.6,7
Blood was collected in PAXgene Blood RNA tubes and analyzed using an Illumina Hiseq 4000 sequencer. Those without adequate quality and quantity of RNA were excluded and all downstream analyses were performed in R environment (v3.1.1) (http://www.r-project.org/) and via Ingenuity Pathway Analysis (IPA, Qiagen Bioinformatics, Redwood City, CA). Adjustments were made for age, sex, body mass index, and biologic status. Kaplan-Meier analysis for the various transcripts in the PAD cohort comparing the upper half (Q4/Q3) to the lower (half Q1/Q2) was conducted using the survminer package in R. For baseline, anthropometric, and laboratory data, normality was assessed and non-parametric, parametric and chi2 tests used as appropriate. An exploratory p-value <0.05 was determined statistical significance. These methodology are all previously published.2,5,7
Results:
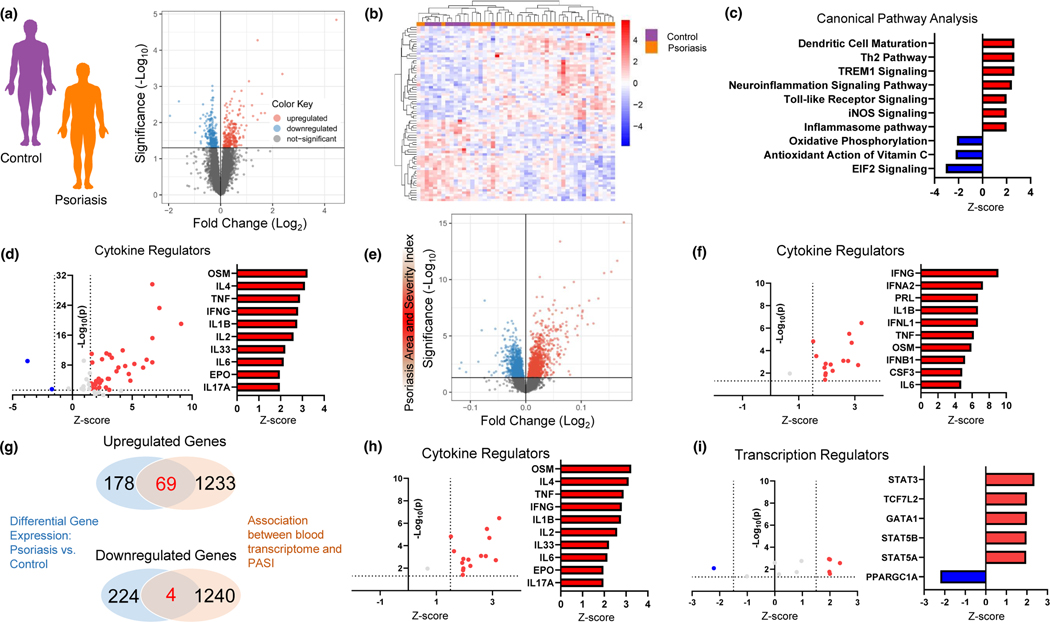
In the psoriasis cohort free of clinical CV disease (n=37), the median age was 44 (IQR; 34 – 51) years, 49% male, and low CV risk (ACC/AHA ASCVD Risk Score of 1.0% [0.6 – 3.4]) with no significant difference between controls (n=11, Table 1). The median psoriasis area and severity index score (PASI) was 4.0 (IQR 2.9 – 8.2), psoriasis duration 15 (IQR; 9 – 25) years, with 36% on biologic therapy. As expected, psoriasis subjects had higher circulating IL-17A than controls, but were otherwise well matched (Table 1). Unbiased whole blood RNA sequencing in psoriasis vs controls revealed 247 genes upregulated and 228 downregulated (Fig 1a, nominal p<0.05) with excellent unsupervised clustering separation (Fig 1b). Pathway analyses (via IPA) centered upon dendritic cell maturation, Th2, iNOS and inflammasome signaling (Fig 1c) while key enriched cytokine and transcription regulators included interferon signaling, IL-1β, TNF, and IL-6 related proteins (Fig 1d, eFig 1a).
Table 1:
Baseline Characteristics Characteristics
| Control (n=11) | Psoriasis (n=37) | p-value | |
|---|---|---|---|
|
| |||
| Age, y | 42 (31 – 48) | 44 (34 – 51) | 0.19 |
| Male sex, n (%) | 7 (64) | 18 (49) | 0.38 |
| Body mass index, kg/m2 | 27 (23 – 30) | 27 (24 – 30) | 0.51 |
| Caucasian, n (%) | 6 (55) | 28 (76) | 0.35 |
| Systolic blood pressure (mmHg) | 119 (108 – 133) | 124 (109 – 139) | 0.49 |
| Diastolic blood pressure (mmHg) | 69 (61 – 73) | 73 (68 – 82) | 0.05 |
| ACC/AHA ASCVD Risk Score, % | 0.6 (0.3 – 3.6) | 1 (0.6 – 3.4) | 0.62 |
| Psoriasis | |||
| PASI score | 4.0 (2.9 – 8.2) | ||
| Psoriasis duration, years | 15 (9 – 25) | ||
| Psoriatic arthritis, n (%) | 8 (42) | ||
| Biologic therapy, n (%) | 13 (36) | ||
| Biomarkers | |||
| WBC, × 103 cells/mm3 | 6.3 (4.9 – 6.9) | 6.5 (5.5 – 7.7) | 0.36 |
| hs-CRP, mg/L | 1.1 (0.2 – 1.8) | 1.6 (0.7 – 3.6) | 0.31 |
| IL-17A, NPX | 1.8 (1.2 – 3.0) | 2.7 (1.9 – 4.6) | 0.03 |
| IL-6, NPX | 3.1 (2.7 – 3.7) | 3.4 (3.1 – 4.3) | 0.18 |
| TNF-α, NPX | 3.4 (3.3 – 3.8) | 3.7 (3.3 – 4.1) | 0.28 |
Figure 1: A circulating transcriptomic signature in psoriasis centers around pro-inflammatory processes.
A) Study design and volcano plot (nominal p<0.05), B) Heat map of top differentially expressed genes (nominal p<0.01), C) Top differentially expressed canonical pathways, and D) Enriched cytokine regulators of whole blood gene expression comparing psoriasis (n=37) to controls (N=11). E) Study design and volcano plot (nominal p<0.05) and F) Enriched cytokine regulators of differentially expressed blood transcripts assessing the association between psoriasis area and severity index and whole blood gene expression (nominal p<0.05). G) Overlap in upregulated and downregulated gene expression (genes = 73) between psoriasis vs. control (A) and association between psoriasis severity and whole blood gene expression (E). Enriched cytokine H) and transcript regulators I) of gene overlap from G. All pathways assessed using Ingenuity Pathway Analysis with differential gene expression adjusted for age, gender, body mass index, and active biologic use.
Assessment of the blood transcriptome with PASI yielded 1,302 genes positively and 1244 genes negatively associated with PASI (nominal p<0.05, Fig 1e). Consistent with data from psoriasis vs controls, similar canonical pathways (eFig 1b), enriched cytokine (Fig 1f), and transcription regulators (eFig 1c) were associated with psoriasis severity. To identify a circulating transcriptomic signature in psoriasis, we assessed transcripts that were (1) differentially expressed between psoriasis and controls (Fig 1a), and (2) associated with psoriasis severity (Fig 1e). Resultantly, 73 differentially expressed genes were noted; 69 upregulated and 4 downregulated (Fig 1g) between psoriasis and controls. IPA pathway analysis revealed enriched cytokine and transcription regulators of these 73 genes which centered upon interferon, TNF, IL-1B, and STAT-related proteins (Fig 1h and Fig 1i).
To determine the clinical utility and association with adverse CV events of this pro-inflammatory transcriptomic signature identified in psoriasis, we investigated these 73 genes in 2 separate cohorts: (1) women with acute MI versus women referred for angiography without MI (HARP), and (2) PAD patients enrolled at the time of lower extremity revascularization and followed a median of 2.5 years for cardiac and limb events (PACE). In the MI cohort, (HARP, eTable 1), 50 out of these 73 psoriasis-related genes (68%) were significantly upregulated in those having an acute MI compared to those without MI (Fig 2a). In the PAD cohort (PACE, eTable 2), 29 of the 73 psoriasis related genes (40%) were significantly associated with a future CV or limb event (MACLE, Fig 2a).
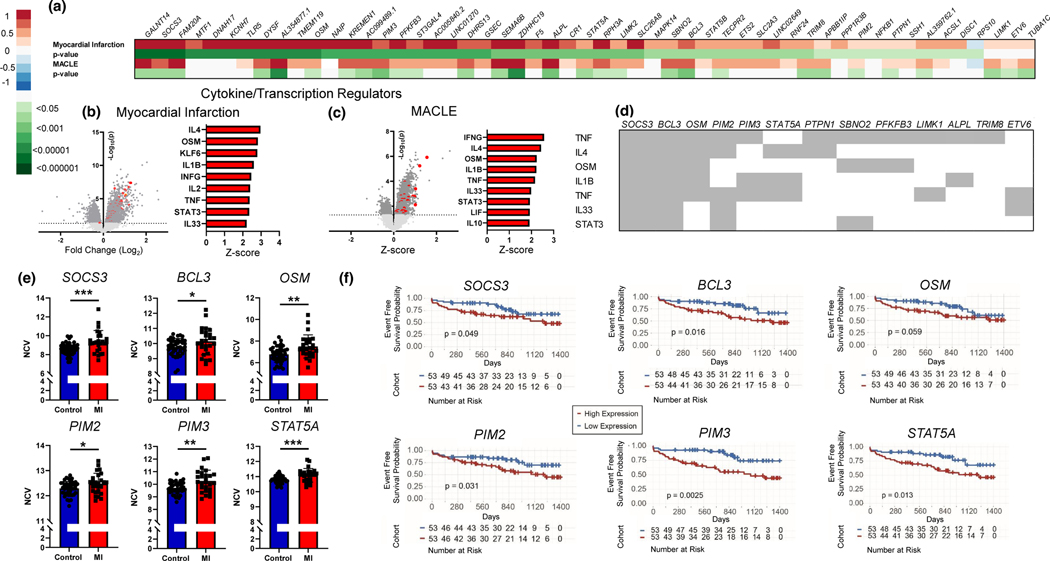
Figure 2. A circulating transcriptomic signature in psoriasis associates with prevalent and future cardiovascular events.
A) Heat map (Log2 fold change) of differential expressed psoriasis genes (vs. control) identified in Figure 1G also upregulated in a cohort of women (HARP cohort) referred for a coronary angiogram with myocardial infarction (MI) versus controls without MI, and a second cohort of patients with established cardiovascular disease (PACE-cohort, peripheral artery disease) at baseline followed longitudinally for a major adverse cardiovascular or limb event (MACLE) over a median follow-up of 2.5 years (all nominal p<0.05). Cytokine and transcription regulators of differentially expressed genes (nominal p<0.05) from Figure 2A in the HARP cohort B), and the PACE cohort C). D) Individual genes which compile cytokine and transcript regulator analyses from Ingenuity Pathway Analysis. E) Individual transcripts of those that did (MI) vs did not (controls) have an acute MI in HARP cohort (t-test). F) Kaplan-Meier curve and log-rank P value for select genes and the outcome of MACE in PACE cohort. HARP: Women’s Heart Attack Research Program. MACE/MACLE: Major adverse cardiovascular (or limb) events. MI; Myocardial infarction. NCV; Normalized count value. PACE; Platelet Activity and Vascular Surgery and cardiovascular events. (Mann-Whitney U-test) *<0.05, **<0.01, ***<0.001.
The differentially expressed psoriasis related genes that validated in both acute MI and incident MACLE were regulated via pro-atherosclerotic pathways, including interferon, IL-1B, IL-6, and STAT related proteins (Fig 2b and Fig 2c). Of the over-represented genes within these pro-atherosclerotic pathways (Fig 2d), a high CV risk signature emerged whereby SOCS3, BCL3, OSM, PIM2, PIM3, and STAT5A were all higher in psoriasis (eFig 2), MI (Fig 2e) and those with a future major adverse cardiac or limb event (Fig 2f). Similar cytokine and transcription regulator findings were observed after analyzing all genes upregulated in psoriasis (vs. control) and associated with MACLE (n=124, eFig 2C) but were not seen when restricting analyses to just genes upregulated in psoriasis vs control, associated with MALCE, but not associated with PASI (n=95, eFig 2D), highlighting the importance of both psoriasis skin severity and prevalence in determining a high CV risk signature.
Discussion
Mechanisms and biomarkers to better understand the epidemiologic associations between psoriasis and cardiovascular risk are not fully clarified. In this study whole blood RNA sequencing in a psoriasis cohort free of clinical CVDC with mild to moderate psoriasis disease activity, centered upon pro-atherosclerotic processes including inflammasome signaling, TNF, and interferon related pathways. These same processes and the individual transcript components were upregulated not just in psoriasis, but also in those having an acute MI which also associated with future CV events.
We identified 73 differentially expressed transcripts in psoriasis (vs control) that were also associated with psoriasis skin severity with over half correlating with prevalent myocardial infarction and 40% with a future cardiovascular event. The cytokine and transcription pathway analyses identified several key genes including SOCS, BCL3, PIM2, OSM, PIM3, and STAT5A. SOCS3 and STAT5A are regulators of the JAK-STAT signaling pathway and inducible by IL-6 and interferon gamma. PIM2 and PIM3 are proto-oncogenes regulated by JAK-STAT signaling, while BLC3 is induced by NFkB, and OSM regulates IL-6, all processes upregulated in psoriasis and involved in the progression of atherosclerosis.8–12
Studies evaluating CV risk prediction in psoriasis are limited. Traditional CV risk score are inaccurate and prior biomarker studies of systemic inflammation in psoriasis (e.g. hs-CRP, neutrophil-to-lymphocyte ratio) correlate with degree of vascular inflammation and coronary plaque burden but are not necessarily outcomes based.13,14 Given the substantial overlap between psoriasis genes and prevalent and future CV events, our findings suggest candidate biomarkers to study. Furthermore, these identified biomarkers are not necessarily dependent on psoriasis skin disease activity and suggests a degree of “residual” inflammatory CV risk in psoriasis which deserves future investigation.
Our study has several limitations including our use of nominal p-values and that our psoriasis transcriptomic signature was validated in non-psoriasis cohorts. Additionally, the large majority of blood transcripts associated with PASI were not differentially expressed in psoriasis vs control. Whether this finding is due to study sample size and this difference would narrow when expanding enrollment (of psoriasis patients in general, and those with more severe skin disease) requires future investigation and study expansion. We are also not able to tell if reductions in gene expression (or psoriasis treatment) correlates with reductions in CV events. Nevertheless, our study is a first step towards developing more refined risk prediction tools in this at-risk population. In conclusion, a whole blood transcriptomic signature of psoriasis diagnosis and severity associates with prevalent MI and incident CV events. These data have implications for better understanding the link between psoriasis, systemic inflammation, and cardiovascular disease.
Supplementary Material
Sources of Support
This study was supported by a National Institutes of Health (NIH, Bethesda, MD) training grant T32HL098129, NIH CTSA at New York University (NYU) Awards (NY, NY)—UL1TR001445, KL2TR001446, and TL1TR001447, American Heart Association Career Development Grant (Dallas, TX) 18CDA34080540, Dermatology Foundation Research Grant, National Psoriasis Foundation Bridge Grant, and NIH K23HL152013 all awarded to Michael S. Garshick. J.S. Berger was supported, in part, by NIH (Bethesda, MD) grants R01HL139909, R01HL114978, R01HL144993, and R35HL144993. This research was also support, in part, by an American Heart Association (AHA) Go Red for Women Strategically Focused Research Network award grant No. 16SFRN28730002 to Dr Berger, grant No. 16SFRN28730004 to Dr Reynolds.
Abbreviations:
- CV
Cardiovascular
- CVD
Cardiovascular disease
- HARP
Women’s Heart Attack Research Program
- IL
Interleukin
- MACLE
Major Adverse Cardiovascular and Limb Event
- MI
Myocardial Infarction
- PACE
Platelet Activity in Vascular Surgery and Cardiovascular Events
- PAD
Peripheral Arterial Disease
- PASI
Psoriasis Area and Severity Index
Footnotes
Ethics statement
This study was approved by the NYU Langone Health Institutional Review Board and conducted in line with the Declaration of Helsinki
Conflicts of Interest
Dr. Garshick has received personal fees from Abbvie. Tessa J. Barrett, PhD, MacIntosh G. Cornwell, Kamelia Drenkova, Jessica Garelik, MD, Brittany N. Weber, MD, PhD, Florencia Schlamp, PhD, Caron Rockman, MD, Kelly V. Ruggles, PhD, Harmony R. Reynolds, MD, Jeffrey S. Berger, MD, MS all report no conflicts of interest.
Data Availability
The data that support these findings are available from the corresponding author upon reasonable request.
References
- 1.Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular Risk in Patients With Psoriasis: JACC Review Topic of the Week. J Am Coll Cardiol. Apr 6 2021;77(13):1670–1680. doi: 10.1016/j.jacc.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garshick MS, Barrett T, Wechter T, et al. Inflammasome Signaling and Impaired Vascular Health in Psoriasis. Arterioscler Thromb Vasc Biol. Feb 14 2019:ATVBAHA118312246. doi: 10.1161/ATVBAHA.118.312246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garshick MS, Tawil M, Barrett TJ, et al. Activated Platelets Induce Endothelial Cell Inflammatory Response in Psoriasis via COX-1. Arterioscler Thromb Vasc Biol. May 2020;40(5):1340–1351. doi: 10.1161/ATVBAHA.119.314008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garshick MS, Drenkova K, Barrett TJ, et al. A Randomized Open Label Clinical Trial of Lipid-Lowering Therapy in Psoriasis to Reduce Vascular Endothelial Inflammation. J Invest Dermatol. Nov 19 2021;doi: 10.1016/j.jid.2021.07.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett TJ, Lee AH, Smilowitz NR, et al. Whole-Blood Transcriptome Profiling Identifies Women With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circ Genom Precis Med. Dec 2018;11(12):e002387. doi: 10.1161/CIRCGEN.118.002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dann R, Hadi T, Montenont E, et al. Platelet-Derived MRP-14 Induces Monocyte Activation in Patients With Symptomatic Peripheral Artery Disease. J Am Coll Cardiol. Jan 2 2018;71(1):53–65. doi: 10.1016/j.jacc.2017.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman JD, Cornwell MG, Zhou H, et al. Gene Expression Signature in Patients With Symptomatic Peripheral Artery Disease. Arterioscler Thromb Vasc Biol. Apr 2021;41(4):1521–1533. doi: 10.1161/ATVBAHA.120.315857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Jia J, Yu Z, et al. Inhibition of JAK2/STAT3/SOCS3 signaling attenuates atherosclerosis in rabbit. BMC Cardiovasc Disord. Mar 13 2020;20(1):133. doi: 10.1186/s12872-020-01391-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Ding X, Yan J, et al. STAT5 inhibitor attenuates atherosclerosis via inhibition of inflammation: the role of STAT5 in atherosclerosis. Am J Transl Res. 2021;13(3):1422–1431. [PMC free article] [PubMed] [Google Scholar]
- 10.Warfel NA, Kraft AS. PIM kinase (and Akt) biology and signaling in tumors. Pharmacol Ther. Jul 2015;151:41–9. doi: 10.1016/j.pharmthera.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Joyce BT, Hwang SJ, et al. Association of Cardiovascular Health Through Young Adulthood With Genome-Wide DNA Methylation Patterns in Midlife: The CARDIA Study. Circulation. Jul 12 2022;146(2):94–109. doi: 10.1161/CIRCULATIONAHA.121.055484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y, Ye D, Wang Z, et al. The Role of Interleukin-6 Family Members in Cardiovascular Diseases. Front Cardiovasc Med. 2022;9:818890. doi: 10.3389/fcvm.2022.818890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi AA, Lerman JB, Aberra TM, et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res. Nov 11 2016;119(11):1242–1253. doi: 10.1161/CIRCRESAHA.116.309637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey AK, Teague HL, Adamstein NH, et al. Association of neutrophil-to-lymphocyte ratio with non-calcified coronary artery burden in psoriasis: Findings from an observational cohort study. J Cardiovasc Comput Tomogr. Jul-Aug 2021;15(4):372–379. doi: 10.1016/j.jcct.2020.12.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support these findings are available from the corresponding author upon reasonable request.