Key Points
Question
Is intravenous tenecteplase as safe and effective as intravenous alteplase for patients with acute large vessel occlusion (LVO) stroke?
Findings
In this prespecified secondary analysis of the ACT randomized clinical trial including 520 patients with LVO stroke, the rates of modified Rankin scale score 0-1 and 0-2 at 90 days and safety outcomes were similar between tenecteplase and alteplase groups.
Meaning
Given the ease of administration of tenecteplase vs alteplase and the comparable safety and efficacy between both thrombolytics shown in this study, tenecteplase could be used as a first-line thrombolytic agent for patients with LVO stroke.
This prespecified secondary analysis of the ACT randomized clinical trial evaluates the safety and efficacy of tenecteplase compared to alteplase among patients with large vessel occlusion stroke.
Abstract
Importance
It is unknown whether intravenous thrombolysis using tenecteplase is noninferior or preferable compared with alteplase for patients with acute ischemic stroke.
Objective
To examine the safety and efficacy of tenecteplase compared to alteplase among patients with large vessel occlusion (LVO) stroke.
Design, Setting, and Participants
This was a prespecified analysis of the Intravenous Tenecteplase Compared With Alteplase for Acute Ischaemic Stroke in Canada (ACT) randomized clinical trial that enrolled patients from 22 primary and comprehensive stroke centers across Canada between December 10, 2019, and January 25, 2022. Patients 18 years and older with a disabling ischemic stroke within 4.5 hours of symptom onset were randomly assigned (1:1) to either intravenous tenecteplase or alteplase and were monitored for up to 120 days. Patients with baseline intracranial internal carotid artery (ICA), M1-middle cerebral artery (MCA), M2-MCA, and basilar occlusions were included in this analysis. A total of 1600 patients were enrolled, and 23 withdrew consent.
Exposures
Intravenous tenecteplase (0.25 mg/kg) vs intravenous alteplase (0.9 mg/kg).
Main Outcomes and Measures
The primary outcome was the proportion of modified Rankin scale (mRS) score 0-1 at 90 days. Secondary outcomes were an mRS score from 0 to 2, mortality, and symptomatic intracerebral hemorrhage. Angiographic outcomes were successful reperfusion (extended Thrombolysis in Cerebral Infarction scale score 2b-3) on first and final angiographic acquisitions. Multivariable analyses (adjusting for age, sex, National Institute of Health Stroke Scale score, onset-to-needle time, and occlusion location) were carried out.
Results
Among 1577 patients, 520 (33.0%) had LVO (median [IQR] age, 74 [64-83] years; 283 [54.4%] women): 135 (26.0%) with ICA occlusion, 237 (45.6%) with M1-MCA, 117 (22.5%) with M2-MCA, and 31 (6.0%) with basilar occlusions. The primary outcome (mRS score 0-1) was achieved in 86 participants (32.7%) in the tenecteplase group vs 76 (29.6%) in the alteplase group. Rates of mRS 0-2 (129 [49.0%] vs 131 [51.0%]), symptomatic intracerebral hemorrhage (16 [6.1%] vs 11 [4.3%]), and mortality (19.9% vs 18.1%) were similar in the tenecteplase and alteplase groups, respectively. No difference was noted in successful reperfusion rates in the first (19 [9.2%] vs 21 [10.5%]) and final angiogram (174 [84.5%] vs 177 [88.9%]) among 405 patients who underwent thrombectomy.
Conclusions and Relevance
The findings in this study indicate that intravenous tenecteplase conferred similar reperfusion, safety, and functional outcomes compared to alteplase among patients with LVO.
Introduction
Intravenous thrombolysis with or without endovascular treatment is the standard of care for the treatment of acute ischemic stroke within 4.5 hours of symptom onset.1,2 Recently, intravenous tenecteplase (0.25 mg/kg) has proven to be a safe and effective alternative to alteplase for patients with acute ischemic stroke who are eligible for thrombolysis.3,4
Fast and complete recanalization of cerebral arteries is a strong predictor for good functional outcome in stroke. In a multicenter prospective cohort study with 575 participants, the probability of successful recanalization with alteplase was less than one-third of patients, defined by repeated imaging within 6 hours of baseline assessment.5 Recanalization was 13% during the first 60 minutes in patients with proximal occlusions. Although the Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) phase 2 study6 of 202 patients suggested that early recanalization rates may be higher with tenecteplase, real-world data from a French study7 suggest comparable recanalization rates among patients who received thrombolysis in primary centers and were then transferred for endovascular therapy (EVT).
The Intravenous Tenecteplase Compared With Alteplase for Acute Ischaemic Stroke in Canada (ACT) randomized clinical trial enrolled patients who were routinely considered for intravenous thrombolysis and showed noninferiority of tenecteplase compared to alteplase on the primary outcome of 90-day modified Rankin scale (mRS) score of 0 to 1. The risk of symptomatic intracerebral hemorrhage was similar between the 2 treatment arms.3
However, the risks, economic cost, and benefits of intravenous thrombolysis use in patients with large vessel occlusion (LVO) planned for EVT continue to be debated.8,9 The individual patient data meta-analysis of 6 randomized clinical trials on combined intravenous thrombolysis and EVT (the Improving Reperfusion strategies in Ischemic Stroke [IRIS] collaboration) included 2314 patients and failed to show noninferiority with direct EVT compared to intravenous thrombolysis followed by EVT with higher chance of early successful reperfusion with combination therapy at the cost of increased intracranial bleeding.10 With increasing adoption of tenecteplase as an alternative to alteplase in clinical practice,11,12 and with the results of several trials on the utility of intravenous thrombolysis in patients otherwise eligible for EVT, a key question is whether intravenous tenecteplase is as safe and efficacious as alteplase in patients with LVO stroke and whether this treatment effect differs by site of occlusion. We compared the safety and efficacy of intravenous tenecteplase vs alteplase in patients with acute stroke with LVO.
Methods
Study Population
This is a prespecified substudy from the ACT trial. ACT was a pragmatic, open-label, registry-linked randomized clinical trial with blinded outcome assessment, assessing the noninferiority of intravenous tenecteplase compared to alteplase in patients with acute ischemic stroke who were eligible for intravenous thrombolysis.3 The ACT trial methods and inclusion and exclusion criteria have been reported previously.3,13 In brief, we included all patients presenting with acute ischemic stroke who met eligibility for intravenous thrombolysis—that is, 18 years and older with a diagnosis of acute ischemic stroke causing disabling neurological deficit and presenting within 4.5 hours of symptom onset, including those eligible for EVT. Eligible patients were randomly assigned (1:1) to intravenous tenecteplase (0.25 mg/kg of body weight) or intravenous alteplase (0.9 mg/kg of body weight).
Patients were monitored for up to 120 days after randomization. mRS scores were collected through standardized telephone interviews centrally by blinded trained research coordinators using the Rankin Focused Assessment.14 Return to baseline function was obtained by the same central masked raters.3
Only patients with LVO were included in this substudy. LVO was defined by baseline computed tomography angiography as patients with occlusions of the intracranial internal carotid artery, first segment of the middle cerebral artery (M1-MCA), proximal and dominant second segment (M2)–MCA, or basilar artery. Indications for intravenous thrombolysis and EVT followed the 2018 Canadian Stroke Best Practice Recommendations.15 The trial was regulated by Health Canada and approved by research ethics boards at each participating center. The trial used deferred consent procedures wherever approved by local research ethics boards.16 The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Imaging Analysis
All imaging data (baseline noncontrast computed tomography, computed tomography angiography, digital subtraction angiography, and follow-up images) were read by an independent imaging core laboratory (F.Bala, N.S., I.A., F. Benali, S.S., B.M., M.A., with 4-20 years of experience), blinded to treatment allocation and clinical outcomes. Core laboratory readers were also blinded to follow-up imaging while reading the baseline imaging.
Reperfusion grade was assessed on the first and final angiographic imaging series of the thrombectomy procedure using the expanded Thrombolysis in Cerebral Infarction (eTICI)17 and recanalization of intracranial occlusions was assessed using the revised arterial occlusion (rAOL) scale on the first angiographic imaging series of the thrombectomy procedure5 Standard of care imaging at 24 hours after thrombolysis administration was assessed for any intracranial hemorrhage and classified using the Heidelberg classification (eMethods in Supplement 2).18
Outcomes and Measures
The primary outcome was excellent functional outcome at 90 days (mRS score of 0-1), assessed up to 120 days after randomization. Secondary outcomes were mRS score of 0 to 2, change along the full mRS scale at 90 days, length of hospital stay, return to baseline function, proportion of patients receiving EVT, successful recanalization (rAOL score of 2b-3), and successful reperfusion (eTICI score of 2b-3) at first and final angiographic imaging series in patients taken for EVT. All outcomes were measured as close to 90 days after randomization as possible, with allowance of measurements being up to 120 days after randomization.
Key safety outcomes were 90-day all-cause mortality and symptomatic intracerebral hemorrhage, defined as any intracerebral hemorrhage that was temporally related to and directly responsible for worsening of the patient’s neurological condition and in the investigator’s opinion was the most important factor for the neurological worsening.
Statistical Analyses
Baseline characteristics and imaging variables were compared between the tenecteplase and alteplase groups using descriptive statistics. Categorical variables were expressed as frequencies and percentages and quantitative variables as medians and IQRs. Fisher exact test was used for categorical data and Wilcoxon rank sum test for continuous variables. Adjusted analyses for binary, ordinal, and continuous outcomes were carried out using mixed-effects Poisson regression, mixed-effects ordinal logistic regression, and mixed-effects linear regression, respectively. Adjustments were made for age, sex, baseline stroke severity, stroke onset-to-needle time, and occlusion location at baseline (intracranial internal carotid artery, M1-MCA, M2-MCA, and basilar occlusions). The enrolling site was included as a random-effects variable to account for clustering within each site. For angiographic outcomes, adjusted variables were age and occlusion location at baseline. Effect size estimates were reported as adjusted risk ratios (aRRs), adjusted common odds ratio, or adjusted β coefficients with 95% CIs.
To analyze whether the effect of drug type was different by site of occlusion, primary and secondary outcomes were compared between tenecteplase and alteplase in each occlusion location subgroup (intracranial internal carotid artery vs M1-MCA vs M2-MCA vs basilar artery) using descriptive statistics. Effect modification of occlusion location on the relationship between treatment arm and outcomes was assessed using 2-way multiplicative interaction terms (treatment × occlusion location) in the multivariable models.
Further, we compared baseline characteristics and primary and secondary outcomes between treatment arms in patients who did not undergo EVT using univariable and multivariable analyses. Adjustments were made for age and occlusion location.
No imputation was performed as missing data were minimal. No correction for multiple testing was done as all secondary analyses were considered exploratory.
Statistical significance was defined as 2-tailed P < .05. All analyses were performed using Stata/MP version 17.0 (Stata Corp).
Results
Among 1577 patients in the ACT trial, 520 met the specified definition of LVO in this study, 263 (50.6%) in the tenecteplase group and 257 (49.4%) in the alteplase group (eFigure 1 in Supplement 2). All patients in this substudy received the assigned treatment. Overall, the median (IQR) age was 74 (64-83) years, and 283 participants (54.4%) were female. There were no significant differences in baseline characteristics between groups (Table 1). Among the 520 patients with LVO, 408 (78.5%) underwent EVT (207 [78.8%] in the tenecteplase group and 201 [78.2%] in the alteplase group. Compared to patients who underwent EVT, patients who did not undergo EVT were older (median [IQR] age, 77.5 [66-90] years vs 73 [63-81] years), had lower National Institute of Health Stroke Scale scores (median [IQR], 14 [6-20] vs 18 [12-22]), longer delays from stroke symptom onset to thrombolysis initiation (median [IQR], 134 [95-189] minutes vs 105 [83-150] minutes), more often had M2-MCA (34 [30.4%] vs 83 [20.3%]) and basilar occlusions (13 [11.6%] vs 18 [4.4%]), and had a higher prevalence of poor collateral grade at baseline (19 [19.6%] vs 44 [11.3%]) (eTable 1 in Supplement 2).
Table 1. Baseline Characteristics of Patients With Large Vessel Occlusion.
| No. (%) | P value | ||
|---|---|---|---|
| IV tenecteplase (n = 263) | IV alteplase (n = 257) | ||
| Baseline characteristics | |||
| Age, median (IQR), y | 74 (65-84) | 73 (63-83) | .38 |
| Female | 143 (54.4) | 140 (54.5) | .99 |
| Male | 120 (46.6) | 117 (46.5) | |
| Baseline NIHSS score, median (IQR) | 17 (11-22) | 17 (12-22) | .60 |
| Workflow times, min | |||
| Onset to hospital arrival (n = 518) | 72 (47-115) | 75 (52-129) | .29 |
| Onset to needle (n = 515) | 106 (84-150) | 111 (86-177) | .18 |
| Imaging to arterial access (in patients undergoing EVT [n = 408]) | 57 (40-84) | 55 (40-79) | .42 |
| Arterial access to successful reperfusion (in patients undergoing EVT [n = 345]) | 35 (22-57) | 33.5 (20-49) | .10 |
| Needle to reperfusion assessment (in patients undergoing EVT [n = 473]) | 57 (39-83) | 52 (35-77) | .17 |
| Needle to first successful reperfusion (in patients undergoing EVT [n = 342]) | 80.5 (57-114) | 75.5 (53.5-103.5) | .23 |
| Type of enrolling center | |||
| Primary stroke | 15 (5.7) | 15 (5.8) | .99 |
| Comprehensive stroke | 248 (94.3) | 242 (94.2) | |
| EVT utilization | 207 (78.7) | 201 (78.2) | .92 |
| Imaging characteristics | |||
| Occlusion location | |||
| ICA | 69 (26.2) | 66 (25.7) | .26 |
| M1-MCA | 118 (44.9) | 119 (46.3) | |
| M2-MCA | 65 (24.7) | 52 (20.2) | |
| Basilar artery | 11 (4.2) | 20 (7.8) | |
| Carotid tandem lesion (n = 517) | 61 (23.5) | 47 (18.3) | .16 |
| Collateral grade (anterior circulation occlusions [n = 486]), No. | 251 | 235 | .54 |
| Poor | 32 (12.7) | 31 (13.2) | |
| Intermediate | 142 (56.6) | 122 (51.9) | |
| Good | 77 (30.7) | 82 (34.9) | |
| ASPECTS, median (IQR) | 8 (7-10) | 8 (7-10) | .43 |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; EVT, endovascular thrombectomy; ICA, intracranial internal carotid artery; IV, intravenous; M1-MCA, first segment of the middle cerebral artery; M2-MCA, second segment of the middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale.
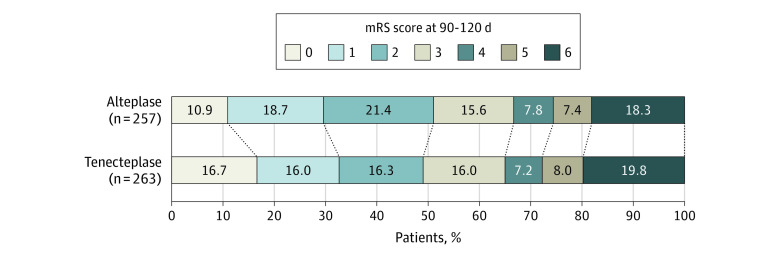
The proportion of patients with an mRS score 0 to 1 at 90 to 120 days was 86 (32.7%) in the tenecteplase group compared to 76 (29.6%) in the alteplase group (aRR, 1.15; 95% CI, 0.98-1.35 (Table 2 and Figure 1). Rates of functional independence (mRS 0-2) and return to baseline function were not different between groups (Table 2).
Table 2. Primary and Secondary Outcomes of Included Patients.
| No./total No. (%) | Unadjusted risk difference (95% CI) | Adjusted risk ratio (95% CI)a | Adjusted common odds ratioa,b | P valuec | ||
|---|---|---|---|---|---|---|
| Tenecteplase (n = 263) | Alteplase (n = 257) | |||||
| Primary outcome | ||||||
| mRS score 0-1 at 90-120 d (n = 520) | 86/263 (32.7) | 76/257 (29.6) | 3.1 (−4.8 to 11.1) | 1.15 (0.98 to 1.35) | NA | .08 |
| Secondary clinical outcomes | ||||||
| mRS 0-2 at 90 d at 90-120 d (n = 520) | 129/263 (49.0) | 131/257 (51.0) | −1.9 (−10.5 to 6.7) | 0.98 (0.84 to 1.14) | NA | .78 |
| Actual mRS score at 90-120 d (n = 520), median (IQR) | 3 (1 to 5) | 2 (1 to 5) | NA | NA | 0.91 (0.66 to 1.23) | .53 |
| Return to baseline function (n = 471) | 64 (27.0) | 49 (20.9) | 6.1 (−1.6 to 13.7) | 1.25 (0.90 to 1.73) | NA | .18 |
| Length of hospital stay in days (n = 482), median (IQR) | 6 (3 to 13) | 6 (3 to 13) | NA | 1.11 (0.92 to 1.35) | NA | .26 |
| Secondary procedural Outcomes (in patients undergoing EVT) | ||||||
| First acquisition eTICI score 2b-3 (n = 405)d | 19/206 (9.2) | 21/199 (10.5) | −1.3 (−7.1 to 4.5) | 0.89 (0.37 to 2.12) | NA | .80 |
| First acquisition rAOL 2b-3 (n = 405)d,e | 36/206 (17.5) | 29/199 (14.6) | 2.9 (−4.2 to 10.0) | 1.21 (0.75 to 1.93) | NA | .43 |
| Final eTICI scale score 2b-3 (n = 405)d | 174/206 (84.5) | 177/199 (88.9) | −4.5 (−11.0 to 2.1) | 0.95 (0.89 to 1.02) | NA | .16 |
| Procedural complications (n = 408) | 15/207 (7.2) | 7/201 (3.5) | 3.8 (−0.5 to 8.2) | NA | NA | .49 |
| Vessel perforation, median (IQR) | 7 (3.4) | 2 (1.0) | NA | NA | NA | |
| Intracranial dissection, median (IQR) | 0 | 0 | NA | NA | NA | |
| Extracranial dissection, median (IQR) | 2 (1.0) | 1 (0.5) | NA | NA | NA | |
| Emboli to new territory, median (IQR) | 3 (1.5) | 2 (1.0) | NA | NA | NA | |
| Access site complication, median (IQR) | 3 (1.5) | 2 (1.0) | NA | NA | NA | |
Abbreviations: eTICI, extended Thrombolysis in Cerebral Infarction; EVT, endovascular thrombectomy; mRS, modified Rankin scale; NA, not applicable; rAOL, revised arterial occlusive lesion score.
Clinical outcomes were adjusted for age, sex, baseline stroke severity, occlusion location, and time from stroke symptom onset to needle time as fixed-effects variables and site as a random-effects variable. Procedural outcomes were adjusted for age and occlusion location.
Common odds ratio is the odds ratio for a 1-unit increase in the modified Rankin scale score for tenecteplase vs alteplase.
P value for the adjusted effect size or the unadjusted risk difference if the former was not calculated.
Three patients did not have initial intracranial endovascular thrombectomy images.
Scored as follows: 0, primary occlusive thrombus remains same; 1, debulking of proximal part of the thrombus but without any recanalization; 2a, partial or complete recanalization of the primary thrombus with occlusion in major distal vascular branch; 2b, partial or complete recanalization of the primary thrombus with occlusion in minor distal vascular branch or partial recanalization of the primary thrombus with no thrombus in the vascular tree at or beyond the primary occlusive thrombus; and 3, complete recanalization of the primary occlusive thrombus with no clot in the vascular tree beyond.
Figure 1. Distribution of Modified Rankin Scale (mRS) Scores at 90 to 120 Days.
There was no significant difference between the tenecteplase and alteplase groups in the ordinal analysis of the mRS score, adjusted for age, sex, baseline stroke severity, occlusion location, and time from stroke symptom onset to needle as fixed-effects variables and site as a random-effects variable (adjusted common odds ratio, 0.91; 95% CI, 0.66-1.23). The mRS score ranges from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death.
Angiographic outcomes were available in 405 of 408 patients (99.3%). No differences were noted in successful reperfusion on first and final angiographic images. eTICI score 2b or higher on first angiographic acquisition was seen in 19 patients (9.2%) in the tenecteplase group vs 21 (10.5%) in the alteplase group (aRR, 0.89; 95% CI, 0.37-2.12). Successful arterial recanalization rates (rAOL score ≥2b) on first angiographic images were comparable between treatment groups: 36 (17.5%) in the tenecteplase group vs 29 (14.6%) in the alteplase group (aRR, 1.21; 95% CI, 0.75-1.93). Successful reperfusion (eTICI score ≥2b) on final angiographic images was observed in 174 patients (84.5%) in the tenecteplase group vs 177 (88.9%) in the alteplase groups (aRR, 0.95; 95% CI, 0.89-1.02) (Table 2).
Safety outcomes were similar between the 2 groups. Symptomatic intracerebral hemorrhage occurred in 16 patients in the tenecteplase group (6.1%) and 11 (4.3%) in the alteplase group (unadjusted risk difference, 1.8; 95% CI, −2.0 to 5.6) while death within 90 days of randomization occurred in 52 patients in the tenecteplase group (19.9%) and 46 (18.1%) in the alteplase group (unadjusted risk difference, 1.8; 95% CI, −5.0 to 8.6) (Table 3).
Table 3. Safety Outcomes in Patients Reported as Treated and Who Received at Least Some Dose of Either Thrombolytic Agent.
| No./total No. (%) | Risk difference (95% CI) | P value | ||
|---|---|---|---|---|
| IV tenecteplase (n = 261) | IV alteplase (n = 254) | |||
| Death within 90 d of randomization | 52/261 (19.9) | 46/254 (18.1) | 1.8 (−5.0 to 8.6) | .60 |
| Symptomatic intracerebral hemorrhage | 16/261 (6.1) | 11/254 (4.3) | 1.8 (−2.0 to 5.6) | .43 |
| Imaging identified intracranial hemorrhage | 71/259 (27.4) | 83/249 (33.3) | −5.9 (−13.9 to 2.1) | .15 |
| Subarachnoid hemorrhage | 32/259 (12.4) | 31/249 (12.4) | 0.0 (−5.8 to 5.6) | .99 |
| Subdural hemorrhage | 1/259 (0.4) | 2/249 (0.8) | −0.4 (−1.8 to 0.9) | .62 |
| Intraventricular hemorrhagea | 12/259 (4.6) | 9/249 (3.6) | 1.0 (−2.5 to 4.5) | .66 |
| HI1 (scattered small petechiae) | 5/259 (1.9) | 13/249 (5.2) | −3.3 (−6.6 to 0.1) | .06 |
| HI2 (confluent petechiae) | 26/259 (10.0) | 35/249 (14.1) | −4.0 (−9.6 to 6.3) | .17 |
| PH1 (hematoma occupying <30% of infarct with no substantive mass effect) | 10/259 (3.9) | 12/249 (4.8) | −1.0 (−4.6 to 2.6) | .67 |
| PH2 (hematoma occupying ≥30% of infarct with obvious mass effect) | 13/259 (5.0) | 11/249 (4.4) | 0.6 (−3.1 to 4.2) | .84 |
| Remote PH-1 | 4/259 (1.5) | 2/249 (0.8) | 0.7 (−1.1 to 2.6) | .69 |
| Remote PH-2 | 1/259 (0.4) | 2/249 (0.8) | −0.4 (−1.8 to 0.9) | .62 |
| Any PH | 27/259 (10.4) | 26/249 (10.4) | 0.0 (−5.3 to 5.3) | .99 |
Abbreviations: HI, hemorrhagic infarction; PH, parenchymal hematoma.
Imaging-identified intracranial hemorrhages were assessed using the Heidelberg classification.
Effect Modification of Treatment Outcome Association by Occlusion Location
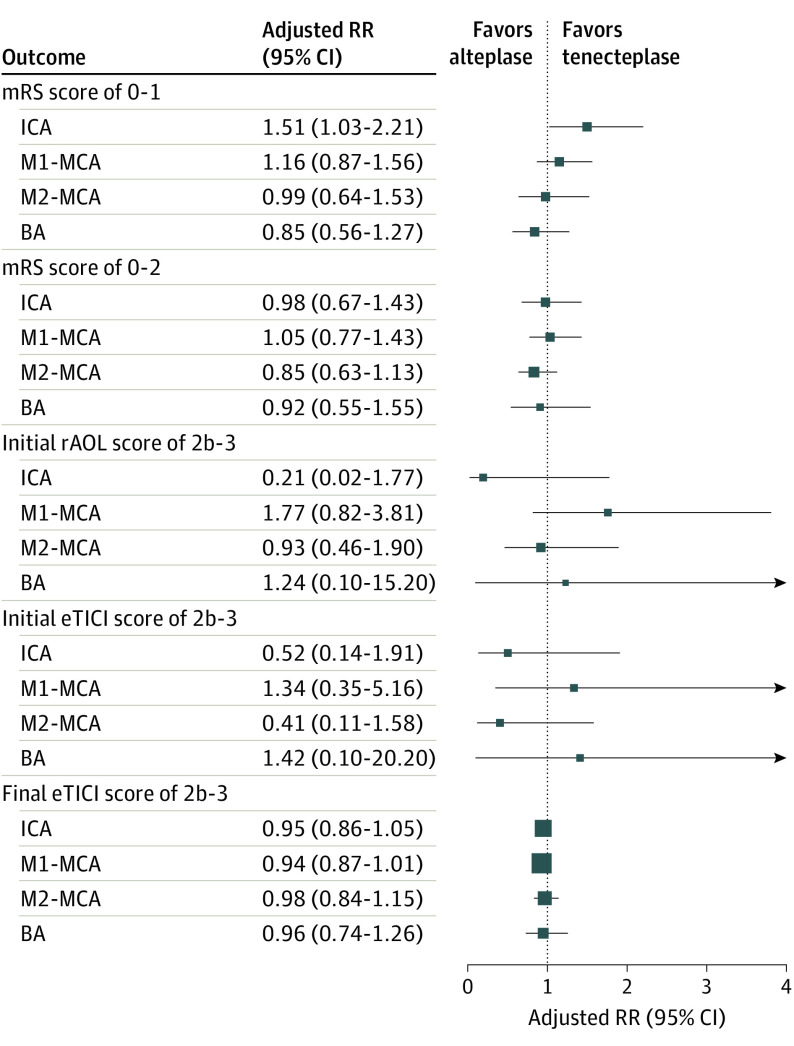
Among the 520 patients with LVO, 135 (26.0%) had an intracranial internal carotid artery occlusion, 237 (45.6%) an M1-MCA occlusion, 117 (22.5%) an M2-MCA occlusion, and 31 (6.0%) a basilar occlusion. The proportion of patients who achieved mRS 0 to 1 at 90 to 120 days was 26.1% vs 18.2% in intracranial internal carotid artery occlusion (P = .30) and 34.7% vs 30.2% in M1-MCA occlusion (P = .49) in the tenecteplase and alteplase groups, respectively. In patients with M2-MCA and basilar occlusions, similar proportions of mRS 0-1 were achieved in both groups (33.9% vs 36.5% in M2-MCA [P = .85] and 45.4% vs 45.0% in basilar occlusion [P = .99]) (Figure 2; eTable 2 and eFigure 2 in Supplement 2).
Figure 2. Forest Plot of Adjusted Risk Ratio (RR) for the Clinical and Procedural Outcomes Stratified by Vessel Occlusion Location.
Clinical outcomes (modified Rankin scale [mRS] score 0-1 and mRS 0-2) were adjusted for age, sex, baseline stroke severity, occlusion location, and time from stroke symptom onset to needle time as fixed-effects variables and site as a random-effects variable. Procedural outcomes were adjusted for age as a fixed-effects variable and site as a random-effects variable. P values for interaction were not significant for all outcomes (P > .05). BA indicates basilar artery; eTICI, expanded thrombolysis in cerebral infarction; ICA, intracranial internal carotid artery; M1-MCA, first segment of middle cerebral artery; M2-MCA, second segment of the middle cerebral artery; rAOL, revised arterial occlusion scale.
No effect modification of treatment outcome association by occlusion location was noted in the adjusted analysis. The proportion of 90-day mRS 0 to 2 or mortality was not different between treatment groups across any occlusion subgroups (Figure 2; eTable 2 and eFigure 2 in Supplement 2).
Successful reperfusion (eTICI score 2b-3) on first and final angiographic acquisitions and successful recanalization (rAOL score 2b-3) on first angiographic acquisition were similar between treatment arms across all occlusion location subgroups. No interaction between occlusion location and treatment group was found (eTable 2 in Supplement 2; Figure 2).
There were no differences in the proportion of symptomatic intracerebral hemorrhage (intracranial internal carotid artery subgroup: 7.2% vs 3.0, P = .44, M1-MCA subgroup: 2.5% vs 4.2%, P = .72, M2-MCA subgroup 10.7% vs 5.8%, P = .51, basilar subgroup: 9.1% vs 5.0%, P = .99) and PH2 rates in all subgroups between patients who received tenecteplase and alteplase (eTable 2 in Supplement 2). No interaction between occlusion location and treatment group was found.
Subgroup of Patients With LVO Who Did Not Undergo EVT
There were 112 patients treated with intravenous thrombolysis alone, 56 in each arm. Baseline characteristics were similar between treatment arms (eTable 3 in Supplement 2). Functional and safety outcomes were similar between the treatment groups in the univariable analysis; however, after adjustment for confounders, patients who received tenecteplase had higher odds of mRS 0 to 1 at 90 days (aRR, 2.01; 95% CI, 1.21-3.30) compared to the alteplase group (eTable 4 in Supplement 2).
Discussion
In this substudy of patients with LVO in the ACT trial, we found similar early reperfusion and recanalization, final reperfusion, clinical efficacy, and safety outcomes when patients were administered intravenous tenecteplase at a dose of 0.25 mg/kg vs alteplase (0.9 mg/kg) within 4.5 hours from stroke symptom onset. Treatment outcome association was not modified by baseline occlusion site.
Tenecteplase is a genetically modified molecule of alteplase. The 3 variants in alteplase molecules result in 15-fold greater fibrin specificity, 80-fold higher resistance to plasminogen activator inhibitor, and prolonged half-life.19 These properties allow for bolus administration of tenecteplase, which has multiple advantages, including rapid transfer to thrombectomy-capable centers in patients arriving to thrombolysis-only centers. The ACT trial, along with multiple previous phase 2 studies, showed that tenecteplase at a dose of 0.25 mg/kg was similar to alteplase as an intravenous thrombolytic agent in all patients presenting early after acute ischemic stroke.3,4 The current study further substantiates evidence from the ACT trial by showing that in patients presenting with LVO who were candidates for EVT, tenecteplase at a dose of 0.25 mg/kg showed similar safety and efficacy compared to alteplase. The rates of excellent and functional independence reported in this study are similar to real-world data, thus attesting to the generalizability of results from the ACT trial.20
This study reports early recanalization (thrombolysis of the occluded artery measured using the rAOL scale) and early reperfusion (perfusion to ischemic territory achieved because of recanalization and measured using the eTICI scale rates after thrombolysis administration). A numerically higher but statistically nonsignificant recanalization rate was seen with tenecteplase (0.25 mg/kg) vs alteplase (17.5% vs 14.6%), while early reperfusion rates (9% vs 10%) were similar for both thrombolytic agents before EVT. Early recanalization rates with alteplase in this study are similar to those reported in the Identifying New Approaches to Optimize Thrombus Characterization for Predicting Early Recanalization and Reperfusion With IV Alteplase and Other Treatments Using Serial CT Angiography (INTERRSECT) study5 (ie, 13%). These findings are also consistent with a French multicenter study7 of 262 patients and the pooled analysis21 of EXTEND IA and EXTEND IA TNK trials and the Melbourne Stroke Registry, which found similar rates of early reperfusion in both thrombolytic agents (21% vs 18% and 21% vs 19%, respectively). A large proportion of patients in these studies were transferred from a primary stroke center (drip-and-ship paradigm) and had long times from thrombolytic administration to reperfusion assessment. This has potentially contributed to the higher reperfusion rates overall for both thrombolytic agents and for the reported difference between treatment groups.7,21 Of note, median (IQR) time from thrombolysis to reperfusion assessment in our study was 53 (35-77) minutes (Table 1) vs 76 (48-125) minutes in the pooled Australian data.21 The differences in early recanalization vs early reperfusion rates in this study are expected, as thrombolysis could result in thrombus migration to major distal branches without corresponding improvement in reperfusion, especially soon after lytic administration.22 The fact that tenecteplase showed numerically higher recanalization rates than alteplase in our study may suggest a small but higher likelihood of early thrombus debulking and lysis with tenecteplase that was not substantial enough to influence reperfusion early on after intravenous lytic administration. Final successful reperfusion rates with tenecteplase and alteplase in this study were comparable to those reported in the EXTEND-IA TNK trial.6
Ninety-day clinical outcomes showed a trend toward increased benefit with tenecteplase over alteplase for excellent 90-day functional outcome (mRS 0-1). This effect was less apparent when assessing higher grades of disability and 90-day mortality (Figure 1). This effect on 90-day mRS with tenecteplase vs alteplase may have been influenced by the small increase in early recanalization rates in patients with LVO and good collaterals. Good collaterals are associated with smaller thrombus burden and potentially more exposure of the thrombus to intravenous thrombolytics compared to patients with moderate to poor collaterals.23,24 Such an effect may not be as obvious in patients with more moderate collaterals. Of interest, this differential effect on the 90-day mRS was accentuated in patients with intracranial internal carotid artery occlusions where the influence of collaterals and thrombus burden on early recanalization rates and final clinical outcomes may be most seen. Analysis of collateral status, thrombus burden, and recanalization rates were not within the scope of this analysis.
From a safety perspective, we found similar risks of symptomatic and asymptomatic intracerebral hemorrhage between the tenecteplase and alteplase groups, similar to previous meta-analyses of randomized and nonrandomized studies.25 The rate of symptomatic intracerebral hemorrhage in the tenecteplase group was slightly higher than the rate reported in a meta-analysis of some previous phase 2 studies and the Norwegian Tenecteplase Stroke Trial (NOR-TEST).26,27 Patient selection strategies, the smaller sample sizes of the phase 2 studies, the pragmatic design of the ACT trial that enrolled all patients eligible for thrombolysis and EVT, and differences in the definition of symptomatic intracerebral hemorrhage between the studies may explain these differences.
From an emergency workflow perspective, times from thrombolysis administration to reperfusion assessment in patients en route to EVT were not different between the 2 thrombolytic agents. However, the reports of shorter workflow times in tenecteplase vs alteplase were mainly noted in patients from the drip-and-ship paradigm. For example, in the pooled EXTEND-IA trials, 64% of patients receiving alteplase and 42% of those receiving tenecteplase were transferred from a primary stroke center. In our substudy, only 30 patients (5.7%) were transferred from a primary stroke center.21 Because of its ease of administration, it is possible that tenecteplase may make acute stroke workflow more efficient, especially in patients who are candidates for EVT.28
Limitations
There are limitations to this study. First, this is a post hoc analysis of a large phase 3 randomized trial. However, this analysis was prespecified. Second, only patients treated within 4.5 hours from stroke symptom onset were included, so our results may not be generalizable to patients treated outside this window. Third, although to our knowledge this is the largest sample to date (n = 520) of patients with LVO administered tenecteplase vs alteplase, the sample size is still small for further subgroup analyses stratified by occlusion location or other clinical and imaging parameters of interest. Fourth, some patients with LVO did not undergo EVT. Although this is possible in the real-world for many reasons—including large infarct core, age, other preexisting comorbidities, workflow, and logistical issues, including long transport times—the specific reasons for exclusion from EVT were not collected.
Conclusions
In this preplanned subgroup analysis of the ACT randomized clinical trial comparing the safety and effectiveness of tenecteplase (0.25 mg/kg) vs alteplase (0.9 mg/kg) in patients with large vessel occlusion, tenecteplase conferred similar benefit and safety as alteplase. These results support the transition to tenecteplase (0.25 mg/kg) as a first-choice thrombolytic in patients with large vessel occlusion stroke.
Trial protocol
Statistical analysis plan
eMethods
eTable 1. Baseline characteristics in patient who underwent versus did not undergo endovascular therapy
eTable 2. Primary and secondary outcomes stratified by occlusion location and treatment type
eTable 3. Baseline characteristics of patients with large vessel occlusion who did not underwent endovascular therapy (n=112)
eTable 4. Univariable and multivariable analyses of association of drug type with primary and secondary outcomes in patients who did not undergo EVT
eFigure 1. CONSORT Flow diagram. CTA= computed tomography angiography. LVO=large vessel occlusion
eFigure 2. Distribution of modifies Rankin Scale score stratified by occlusion location
Data sharing statement
References
- 1.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 3.Menon BK, Buck BH, Singh N, et al. ; AcT Trial Investigators . Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. doi: 10.1016/S0140-6736(22)01054-6 [DOI] [PubMed] [Google Scholar]
- 4.Abuelazm M, Seri AR, Awad AK, et al. The efficacy and safety of tenecteplase versus alteplase for acute ischemic stroke: an updated systematic review, pairwise, and network meta-analysis of randomized controlled trials. J Thromb Thrombolysis. 2023;55(2):322-338. doi: 10.1007/s11239-022-02730-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon BK, Al-Ajlan FS, Najm M, et al. ; INTERRSeCT Study Investigators . Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018;320(10):1017-1026. doi: 10.1001/jama.2018.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell BCV, Mitchell PJ, Churilov L, et al. ; EXTEND-IA TNK Investigators . Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 7.Seners P, Caroff J, Chausson N, et al. ; PREDICT-RECANAL collaborators . Recanalization before thrombectomy in tenecteplase vs. alteplase-treated drip-and-ship patients. J Stroke. 2019;21(1):105-107. doi: 10.5853/jos.2018.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell BCV, Kappelhof M, Fischer U. Role of intravenous thrombolytics prior to endovascular thrombectomy. Stroke. 2022;53(6):2085-2092. doi: 10.1161/STROKEAHA.122.036929 [DOI] [PubMed] [Google Scholar]
- 9.LeCouffe NE, Kappelhof M, Treurniet KM, et al. ; MR CLEAN–NO IV Investigators . A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833-1844. doi: 10.1056/NEJMoa2107727 [DOI] [PubMed] [Google Scholar]
- 10.14th World Stroke Congress, Singapore, 26-29 October 2022. Int J Stroke. 2022;17(3_suppl):3-288. doi: 10.1177/17474930221125973 [DOI] [Google Scholar]
- 11.Gerschenfeld G, Liegey J-S, Laborne F-X, et al. Treatment times, functional outcome, and hemorrhage rates after switching to tenecteplase for stroke thrombolysis: insights from the TETRIS registry. Eur Stroke J. 2022;7(4):358-364. doi: 10.1177/23969873221113729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahawish K, Gommans J, Kleinig T, Lallu B, Tyson A, Ranta A. Switching to tenecteplase for stroke thrombolysis: real-world experience and outcomes in a regional stroke network. Stroke. 2021;52(10):e590-e593. doi: 10.1161/STROKEAHA.121.035931 [DOI] [PubMed] [Google Scholar]
- 13.Sajobi T, Singh N, Almekhlafi MA, et al. Alteplase compared to tenecteplase in patients with Acute Ischemic Stroke (AcT) Trial: protocol for a pragmatic registry linked randomized clinical trial. Stroke Vasc Intervent Neurol. 2022;0:e12329. doi: 10.1161/SVIN.121.000447 [DOI] [Google Scholar]
- 14.Saver JL, Filip B, Hamilton S, et al. ; FAST-MAG Investigators and Coordinators . Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke. 2010;41(5):992-995. doi: 10.1161/STROKEAHA.109.571364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulanger JM, Lindsay MP, Gubitz G, et al. Canadian stroke best practice recommendations for acute stroke management: prehospital, emergency department, and acute inpatient stroke care, 6th Edition, update 2018. Int J Stroke. 2018;13(9):949-984. doi: 10.1177/1747493018786616 [DOI] [PubMed] [Google Scholar]
- 16.Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT Trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi: 10.1161/STROKEAHA.122.038760 [DOI] [PubMed] [Google Scholar]
- 17.Liebeskind DS, Bracard S, Guillemin F, et al. ; HERMES Collaborators . eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433-438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 18.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981-2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 19.Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41(15):1229-1245. doi: 10.2165/00003088-200241150-00001 [DOI] [PubMed] [Google Scholar]
- 20.Gerschenfeld G, Smadja D, Turc G, et al. ; TETRIS Study Group . Functional outcome, recanalization, and hemorrhage rates after large vessel occlusion stroke treated with tenecteplase before thrombectomy. Neurology. 2021;97(22):e2173-e2184. doi: 10.1212/WNL.0000000000012915 [DOI] [PubMed] [Google Scholar]
- 21.Yogendrakumar V, Beharry J, Churilov L, et al. Tenecteplase improves reperfusion across time in large vessel stroke. Ann Neurol. 2023;93(3):489-499. doi: 10.1002/ana.26547 [DOI] [PubMed] [Google Scholar]
- 22.Ohara T, Menon BK, Al-Ajlan FS, et al. ; for INTERRSeCT Study Investigators . Thrombus migration and fragmentation after intravenous alteplase treatment: the INTERRSeCT study. Stroke. 2021;52(1):203-212. doi: 10.1161/STROKEAHA.120.029292 [DOI] [PubMed] [Google Scholar]
- 23.Joundi RA, Menon BK. Thrombus composition, imaging, and outcome prediction in acute ischemic stroke. Neurology. 2021;97(20)(suppl 2):S68-S78. doi: 10.1212/WNL.0000000000012796 [DOI] [PubMed] [Google Scholar]
- 24.Qazi EM, Sohn SI, Mishra S, et al. Thrombus characteristics are related to collaterals and angioarchitecture in acute stroke. Can J Neurol Sci. 2015;42(6):381-388. doi: 10.1017/cjn.2015.291 [DOI] [PubMed] [Google Scholar]
- 25.Katsanos AH, Psychogios K, Turc G, et al. Off-label use of tenecteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(3):e224506. doi: 10.1001/jamanetworkopen.2022.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsanos AH, Safouris A, Sarraj A, et al. Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta-analysis. Stroke. 2021;52(1):308-312. doi: 10.1161/STROKEAHA.120.030220 [DOI] [PubMed] [Google Scholar]
- 27.Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781-788. doi: 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 28.Warach SJ, Dula AN, Milling TJ, et al. Prospective observational cohort study of tenecteplase versus alteplase in routine clinical practice. Stroke. 2022;53(12):3583-3593. doi: 10.1161/STROKEAHA.122.038950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
eTable 1. Baseline characteristics in patient who underwent versus did not undergo endovascular therapy
eTable 2. Primary and secondary outcomes stratified by occlusion location and treatment type
eTable 3. Baseline characteristics of patients with large vessel occlusion who did not underwent endovascular therapy (n=112)
eTable 4. Univariable and multivariable analyses of association of drug type with primary and secondary outcomes in patients who did not undergo EVT
eFigure 1. CONSORT Flow diagram. CTA= computed tomography angiography. LVO=large vessel occlusion
eFigure 2. Distribution of modifies Rankin Scale score stratified by occlusion location
Data sharing statement