This study evaluates patterns in disease-modifying therapy initiations between 2001 and 2020 among commercially insured US adults and children with multiple sclerosis.
Key Points
Question
Has increasing availability of disease-modifying therapies for multiple sclerosis changed real-world prescribing patterns (2001 through 2020)?
Findings
In this cross-sectional study of 153 846 and 583 initiation episodes among adults and children, respectively, platform injectable therapies were most commonly initiated early in the study period. However, their use declined after 2010 with the introduction of oral agents, which became the most frequently initiated therapies by 2020; infusion therapy initiations remained low overall (less than 5%).
Meaning
Disease-modifying therapy prescribing patterns shifted substantially over the 20-year period; the increasing use of oral therapies may have been due to multiple factors, including convenience, insurance restrictions, or direct-to-consumer advertising.
Abstract
Importance
Many disease-modifying therapies (DMTs) have been approved for multiple sclerosis (MS) in the past 2 decades. Research evaluating how these approvals have changed real-world prescribing patterns is scarce.
Objective
To evaluate patterns in DMT initiations between 2001 and 2020 among commercially insured US adults and children with MS.
Design, Setting, and Participants
This serial cross-sectional study was conducted from 2001 through 2020 (mean patient enrollment duration, 4.8 years) and used US commercial claims data (MarketScan). Analysis took place between January 2022 and March 2023. Of 287 084 patients with MS identified, 113 583 patients (113 095 adults and 488 children) with MS newly initiated at least 1 DMT.
Exposure
New initiation episode of a DMT, defined as no claim for the same DMT in the previous year.
Main Outcome Measure
The proportion of total DMT initiations per year attributable to each DMT. Trends in initiations were evaluated annually.
Results
The study team identified 153 846 DMT initiation episodes among adults (median age, 46 [IQR, 38-53) years]; 86 133 female [76.2%]) and 583 among children (median age, 16 (IQR, 14-17) years; 346 female [70.9%]). Among adults, use of platform injectables showed an absolute decline of 73.8% over the study period, driven by a 61.2% reduction in interferon β initiations (P < .001 for trend). In contrast, the 2010 introduction of oral DMTs led to a rise in their use from 1.1% (2010) to 62.3% (2020) of all DMT initiations (P = .002 for trend). Infusion therapy initiations remained relatively low, accounting for 3.2% of all initiations since their introduction in 2004 but increased modestly annually after ocrelizumab was introduced (2017), reaching 8.2% of all initiations in 2020 (P < .001 for trend). Children showed similar initiation patterns, except for preferred oral therapy. Between 2019 and 2020, dimethyl fumarate was the most commonly initiated DMT in adults (23.3% to 27.2% of all initiations), while in children fingolimod was the most commonly initiated (34.8% to 68.8%).
Conclusions and Relevance
Current MS treatment guidelines emphasize shared decision-making between patients and clinicians to balance treatment efficacy, safety, cost, and convenience. This study found that oral DMTs were the predominant DMT type initiated by 2020. The cause of this shift cannot be determined from this study, but may reflect several factors, including convenience of administration, direct-to-consumer advertising, or insurance restrictions.
Introduction
Improved understanding of multiple sclerosis (MS) pathophysiology has led to the development and approval of new disease-modifying therapies (DMTs), which have substantially shifted the treatment paradigm for MS. In the past 2 decades alone, more than 10 new DMTs have been approved for MS treatment in the US, introducing new drug classes and routes of administration to the market.1 Compared with their older counterparts, many of the newer agents are more effective but present novel challenges related to safety and treatment costs.2,3,4,5,6 Specifically, oral DMTs became available in 2010 and have provided an alternative, more convenient form of administration compared with injectable and infusion therapies. As available treatment options for managing MS increase, treatment decisions revolve around shared decision-making between patients and clinicians regarding the need to balance convenience, safety and tolerability, efficacy, and costs.7
Despite the increasing availability of newer therapies to manage MS, there is very little research evaluating long-term trends in the patterns of their use in the US, especially among children. Furthermore, high-efficacy DMTs are now increasingly recommended earlier in the disease course to improve long-term outcomes.8,9,10 However, it is unclear how the availability of treatments and treatment guidelines have affected the use of DMTs in the US recently.11 The clinical utility of prior studies examining this topic are limited by several factors, including their short time horizons or cross-sectional designs, inclusion of only a small subset of available DMTs, or grouping DMTs based on common characteristics (eg, route of administration) rather than examining them individually. Additionally, there is limited research that includes children with MS, a small but unique and vulnerable population.12,13,14,15,16 Taken together, these factors limit the ability to draw conclusions regarding trends in the use of individuals DMTs in the US. Accordingly, the objective of this study was to evaluate trends and patterns in the initiation of DMTs between 2001 and 2020 among commercially insured adults and children with MS in the US.
Methods
This study was approved by the Rutgers University institutional review board and the requirement for informed consent was waived; appropriate data use agreements were in place. The study followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.17
Data Source and Study Population
The data source for this study was IBM MarketScan Commercial Claims and Encounters (IBM Watson Health), a health insurance database comprising individuals in the US with employer-based insurance. MarketScan provides patient-level information on sociodemographic characteristics, medical and pharmacy enrollment status, pharmacy dispensing, as well as outpatient and inpatient medical encounters (including infusions), which are coded using International Classification of Disease Ninth and Tenth Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) codes, Current Procedural Terminology Fourth Edition codes, Healthcare Common Procedure Coding System codes, and National Drug Codes.18
We identified a cohort of adults (18 to 64 years old) and children (younger than 18 years old) with MS (ICD-9 code 340.xx or ICD-10 code G35 in any position and a DMT claim within 365 days thereafter) who newly initiated a DMT between 2001 and 2020 (most recently available data at the time of analysis).19,20 New treatment initiation was defined as nonuse of the initiated DMT during the baseline period (365-day period prior to treatment initiation). The index date was defined as the date of the first claim for a qualifying DMT that was considered a new initiation. Patients were allowed to contribute more than 1 initiation episode as long as the eligibility criteria were met at the time of initiation. Cohort membership was restricted to those with continuous health care and pharmacy eligibility and to those with an MS diagnosis during the baseline period.
DMTs
We evaluated 19 DMTs individually and classified them into 5 mutually exclusive categories: (1) platform injectables (interferon β and glatiramer); (2) oral therapies (dimethyl fumarate, monomethyl fumarate, diroximel fumarate, fingolimod, siponimod, teriflunomide, and cladribine); (3) infusion therapies (natalizumab, ocrelizumab, and alemtuzumab); (4) newer injectable therapies (daclizumab and ofatumumab); and (5) other DMTs including off-label therapies (rituximab, azathioprine, cyclophosphamide, mitoxantrone, and mycophenolate).
Patient Characteristics
To describe the study population, we assessed patient characteristics, including sociodemographics (eg, age at index date, biological sex) and select comorbid conditions (cancer, depression, neuropathic pain). Medical conditions were assessed during the baseline period (365 days prior to index date).
Statistical Analysis
The study period was segmented into 20 calendar-year intervals and each initiation episode was assigned to 1 calendar year according to the index date. For each calendar year, we described the proportion of DMT initiations attributable to each individual DMT (numerator) over all DMT initiations (denominator). Temporal trends in DMT initiation patterns were evaluated visually through graphical presentation and statistically using regression models to assess for the presence of linear secular trends for each DMT.
We conducted 2 sensitivity analyses. First, patients with evidence of an alternative indication for DMT use (malignancies or rheumatic diseases) were excluded. This analysis was conducted to exclude initiations that may have been attributable to a non-MS indication and to evaluate whether off-label use of DMTs exhibited temporal trends throughout the study period. Second, in addition to examining new initiation episodes of DMTs, we also reported secular trends in prevalent use of DMTs where the requirement for new use was waived. All analyses were performed using SAS version 9.4 (SAS Institute).
Results
Of 287 084 patients identified with MS and a DMT claim, 113 095 adults and 488 children newly initiated at least 1 DMT and were included. A total of 154 429 new initiation episodes were identified among these patients—153 846 among adults and 583 among children. The mean (SD) duration of enrollment of eligible patients with MS in our cohort was 4.8 (SD, 3.6) years. Baseline characteristics of patients at their first eligible initiation episode are described in the Table (eTable 1 in Supplement 1 describes characteristics for all initiation episodes). In terms of geographical distribution, approximately one-third of the study cohort resided in the southern US, a quarter in the Midwest, one-fifth in the Northeast, and 18% in the West. Among adults, the median age was 46 (IQR, 38-53) years, 86 133 were female (76.2%), and approximately 14% had a diagnosis of depression during the baseline period. Among children, the median age was 16 (IQR, 14-17) years and 346 were female (70.9%). The baseline prevalence of many comorbid conditions (eg, depression) was similar among children and adults.
Table. Baseline Characteristics for the First Qualifying Episode for Adults (18 to 64 Years) and Children (Younger Than 18 Years) Who Newly Initiated at Least 1 Disease-Modifying Therapy for Multiple Sclerosis Between 2001 and 2020a.
| Characteristic | Adults (n = 113 095) No. (%) | Children (n = 488) No. (%) |
|---|---|---|
| Total No. of new use episodes | 153 846 | 583 |
| New use episodes per patient | ||
| 1 | 83 558 (73.9) | 319 (65.4) |
| ≥2 | 29 537 (26.1) | 169 (34.6) |
| Age, y, median (IQR) | 46 (38-53) | 16 (14-17) |
| Sex | ||
| Female | 86 133 (76.2) | 346 (70.9) |
| Male | 26 962 (23.8) | 142 (29.1) |
| Regionb | ||
| Northeast | 22 283 (19.7) | 90 (18.5) |
| Midwest | 28 087 (24.8) | 127 (26.0) |
| South | 38 245 (33.8) | 169 (34.6) |
| West | 19 740 (17.5) | 88 (18.0) |
| Unknown/missing | 4740 (4.2) | 14 (2.9) |
| Comorbiditiesc | ||
| Any cancer | 3263 (2.9) | 13 (2.7) |
| Metastatic cancer | 377 (0.3) | 4 (0.8) |
| Depression | 16 200 (14.3) | 47 (9.6) |
| Neuropathic pain | 2935 (2.6) | 11 (2.3) |
In patients with multiple initiation episodes during the study period, only the first initiation episode is included for the characteristics summarized in this Table.
Based on US Census data from 2022, the geographic breakdown of the overall US population is as follows: Northeast (17.1%), Midwest (20.6%), South (38.6%), and West (23.6%).21
Comorbidities were evaluated in the year prior to the index date (date of claim associated with the initiation episode).
DMT Use in Adults
Trends in initiations of frequently prescribed DMTs in adults are presented in Figure 1 for individual DMTs, while Figure 2 describes these trends by route of administration (eTable 2 in Supplement 1 reports detailed information on all 19 DMTs and all routes of administration).
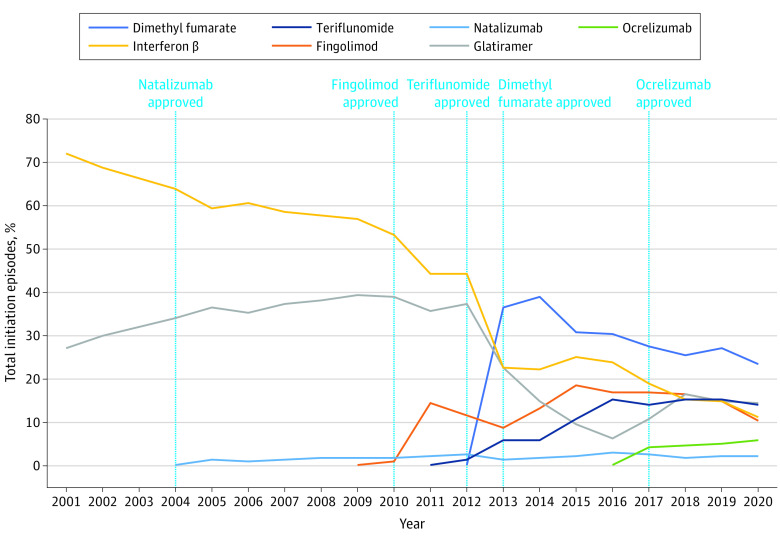
Figure 1. Initiation Episodes of Disease-Modifying Therapies (DMTs) in Adults (18 to 64 Years Old) With Multiple Sclerosis, 2001 Through 2020.
This figure displays the proportion of total new use episodes per year attributable to each individual DMT for adult patients. A DMT claim was considered a new use episode if a patient had no claim for the same DMT within 1 year prior to the current DMT claim. Only DMTs that were frequently used in our data are shown in this figure, so proportions presented here may not sum to 100%. Data for all DMTs are shown in eTable 2 in Supplement 1.
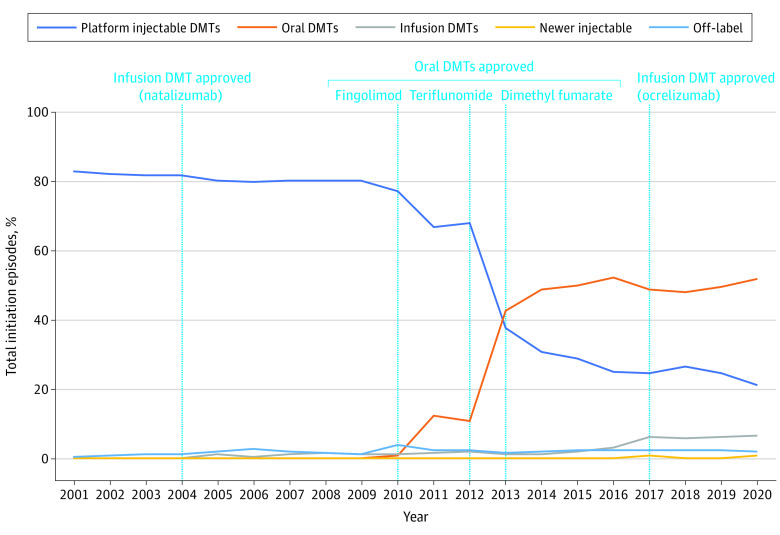
Figure 2. Initiation Episodes of Disease-Modifying Therapies (DMTs) by Route of Administration in Adults (18 to 64 Years Old) With Multiple Sclerosis, 2001 Through 2020.
This figure displays the proportion of total new use episodes per year attributable to any DMT within each group among adults. Platform injectable DMTs include interferon β and glatiramer. Oral DMTs include dimethyl fumarate, diroximel fumarate, fingolimod, ozanimod, siponimod, teriflunomide, and cladribine. Infusion DMTs include alemtuzumab, natalizumab, and ocrelizumab. Off-label DMTs and newer injectable DMTs are not displayed in this graph due low usage in adults. A DMT claim was considered a new use episode if a patient had no claim for the same DMT within 1 year prior to the current DMT claim. Data for all routes of administration are shown in eTable 2 in Supplement 1.
New initiations for platform injectable therapies as a proportion of overall DMT initiations declined from 2001 (99.3% of all initiations) to 2020 (25.5% of all initiations; P < .001 for trend). Among individual platform injectables, interferon β showed an absolute decline of 61.2% over the study period from 72.3% in 2001 to 11.1% in 2020 (P < .001 for trend). By contrast, initiations of glatiramer increased from 27.0% in 2001, peaked at 39.3% in 2009, and declined thereafter to 14.4% of initiations by 2020 (P < .001 for trend).
Infusion therapies accounted for a very small proportion of initiation episodes throughout the study period (3.2% of initiations since 2004), with a modest increase in 2017 with the introduction of ocrelizumab (P < .001 for trend). Initiations of natalizumab (approved in 2004) remained relatively stable throughout the study period with a peak at 3.0% in 2016 (P = .001). In contrast, ocrelizumab initiations accounted for 4.3% of DMT initiations in 2017, the year of its initial approval, and its use steadily increased through the end of the study period to 5.8% of initiations in 2020, though this increase was not statistically significant (P = .10 for trend).
The decline in initiations of platform injectables corresponded strongly with the market introduction of oral therapies in 2010, rather than the availability of infusion DMTs in 2004. Initiations of oral therapies greatly increased between 2010 (1.1% of initiations) and 2020 (62.3% of initiations) (P = .002 for trend). Among oral therapies, dimethyl fumarate (approved in 2013) was the most frequently initiated oral agent within its class. Dimethyl fumarate saw high uptake in its first year of use, peaking at 36.4% of all DMT initiations in 2013 and declining modestly thereafter to 23.3% of initiations (P = .001 for trend). In comparison, initiations of fingolimod (approved in 2010) did not exceed 20% during the study period, peaking at 18.4% in 2015 (P = .10 for trend). Similarly, initiations of teriflunomide (approved in 2012) increased steadily after its approval, peaking at 15.5% in 2018 (P = .002 for trend). Additionally, the increase in use of oral DMTs between 2019 and 2020 was driven largely by uptake of newer agents within the class, including cladribine (3.2% of initiations in 2020), diroximel fumarate (6.7%), ozanimod (1.1%), and siponimod (3.3%) (eTable 1 in Supplement 1). Off-label drug initiations (azathioprine, cyclophosphamide, mycophenolate, rituximab) and initiations of mitoxantrone were minimal (less than 2% annually) in adults throughout the study period.
DMT Use in Children
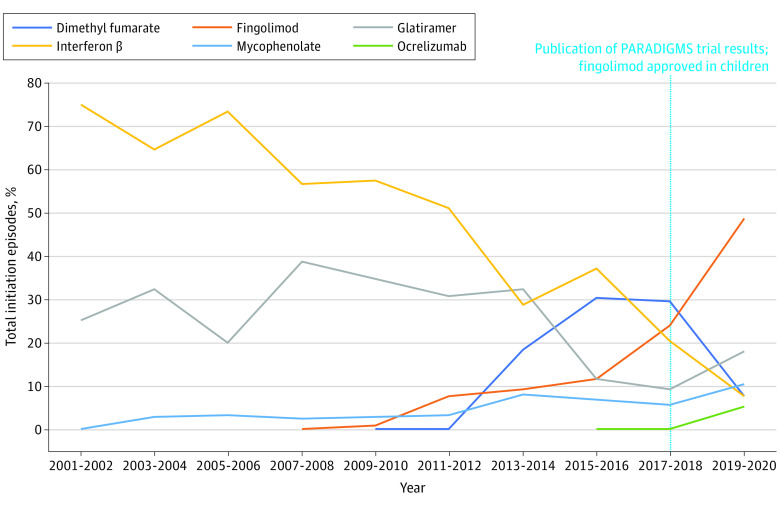
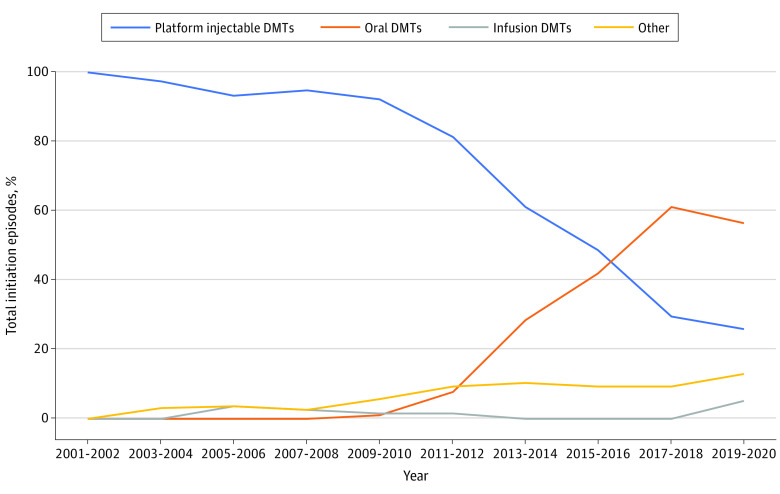
Due to the small number of initiation episodes identified in children, data for children were grouped into 2-year intervals for graphical presentation, but trend analysis was conducted annually. Trends in DMT initiations in children are presented by individual DMTs (Figure 3) and route of administration (Figure 4); eTable 3 in Supplement 1 contains detailed information on all DMTs and routes of administration in children. Overall, trends in DMT initiations in children reflected trends in adults with 1 major exception. While use of fingolimod slightly decreased in adults after 2015, its use in children increased significantly through the end of the study period (P = .002). Between 2019 and 2020, fingolimod accounted for 68.8% of all initiation episodes.
Figure 3. Initiation Episodes of Disease-Modifying Therapies (DMTs) in Children (Younger Than 18 Years Old) With Multiple Sclerosis, 2001 Through 2020.
This figure displays the proportion of total new use episodes attributable to each individual DMT for children, grouped into 2-year periods. A DMT claim was considered a new use episode if a patient had no claim for the same DMT within 1 year prior to the current DMT claim. Only DMTs that were frequently used in our data are shown in this figure, so proportions presented here may not sum to 100%. Data for all DMTs are shown in eTable 3 in Supplement 1.
Figure 4. Initiation Episodes of Disease-Modifying Therapies (DMTs) by Route in Children (Younger Than 18 Years Old) With Multiple Sclerosis, 2001 Through 2020.
This figure displays the proportion of total new use episodes per year attributable to any DMT within each group among children, grouped into 2-year periods. Platform injectable DMTs include interferon β and glatiramer. Oral DMTs include dimethyl fumarate, fingolimod, teriflunomide, azathioprine, and mycophenolate. Infusion DMTs include natalizumab and ocrelizumab. Other DMTs are not included in this graph because they were not used in children or because they fit into multiple route categories. A DMT claim was considered a new use episode if a patient had no claim for the same DMT within 1 year prior to the current DMT claim.
Sensitivity Analysis
Results for the 2 sensitivity analyses are described in eTables 4 and 5, as well as eFigures 1 and 2 in Supplement 1. Study results were consistent with the primary analysis when we excluded patients with potentially alternative indications for DMT use, as well as in the prevalent use analysis. Study results were consistent with the primary analysis when the study team excluded patients with potentially alternative indications for DMT use (eTables 4 and 5 in Supplement 1), as well as in the prevalent use analysis.
Discussion
We used a large US commercial claims database to evaluate trends in initiation patterns of DMTs in adults and children with MS from 2001 through 2020. At the beginning of the study period, few DMTs were available and platform injectables (interferon β and glatiramer) were the most commonly initiated DMTs, accounting for almost 100% of initiations in 2001. With the approval of new DMTs throughout the study period, initiations of platform injectable therapies exhibited a downward trend through 2020, accounting for only 25% of initiations by 2020. Although infusion therapies became available in 2004, their use remained relatively low throughout the study period. In contrast, initiations of oral therapies rose sharply after their introduction in 2010, accounting for most new initiations by 2020.
The treatment guidelines for MS emphasize shared decision-making between patients and clinicians.7 Because of the variety of DMTs currently available, several factors may be considered in these decisions, including efficacy, safety, tolerability and ease of administration, treatment costs, and insurance limitations. Understanding how real-world treatment patterns in MS have shifted can provide insight into patients’ and clinicians’ evolving priorities in making these decisions. The findings from our study suggest that, in recent years, patients with MS were more likely to initiate oral DMTs than older platform injectable or infusion DMTs. Platform injectable therapies have well-established safety profiles and may have a lower risk of certain serious but extremely rare adverse events compared with many newer agents. However, clinical trials have found some of the newer agents to be more effective than interferon β based on relapse rates, lesion burden, and/or disability scores.2,3,4,5,6,22 Initiations of both interferon β and glatiramer significantly declined throughout the study period, but glatiramer did not follow a strictly linear decline. We saw a modest increase in the use of glatiramer around 2015 through 2016, which may reflect the 2014 approval of a less frequent subcutaneous dosing schedule, allowing for glatiramer to be administered three times per week rather than once daily.23
Throughout the study period, infusion DMTs accounted for less than 10% of initiations. Infusion agents have been associated with certain very rare adverse events and these associations may have contributed to their limited adoption during the study period.11 Natalizumab was briefly removed from the market following reported cases of progressive multifocal leukoencephalopathy (PML), an extremely rare adverse event, in a few treated patients.24,25 However, while natalizumab initiations remained low throughout the study, initiations of ocrelizumab appeared to increase steadily after its approval in 2017. There were no reported cases of PML associated with ocrelizumab until 2020, perhaps in part due to proactive testing for John Cunningham virus that enhanced ocrelizumab's safety profile.26,27 This distinction may partly explain the divergent trends between natalizumab and ocrelizumab. However, PML is extremely rare and the impact of this risk on treatment decisions cannot be understood from this study. Other factors, including insurance formulary restrictions, may have also limited the use of high-efficacy DMTs (eg, natalizumab and ocrelizumab) as first-line options. However, their use may increase in the future as recommendations for their use early in the disease course are increasingly adopted.8
The decline in use of platform injectable therapies was more strongly linked to the increasing availability and use of oral therapies, introduced in 2010, rather than infusion therapies. By the end of the study period, oral therapies were the most commonly initiated DMTs overall. While this study is unable to attribute this observed trend to any particular cause, there may be several reasons that oral medications may have been preferred to infusion or injectable therapies. First, they are more convenient to take and do not require a visit to clinicians or infusion centers. Such considerations may have played a more outsized role early in the COVID-19 pandemic. Second, their increasing popularity may also be driven by direct-to-consumer advertising, as well as potential coverage limitations imposed by insurance companies, including formulary restrictions, prior authorization requirements, and cost sharing.
Our results were largely consistent between children and adults overall. However, the most commonly initiated oral DMT in 2020 was dimethyl fumarate among adults but fingolimod among children. This distinction coincides with the 2018 publication of the PARADIGMS trial results and subsequent 2018 US Food and Drug Administration approval of fingolimod in children 10 to 17 years old.28,29 The trial showed that, compared with interferon β, fingolimod was associated with significantly fewer relapses and lower rates of new/newly enlarged lesions on magnetic resonance imaging. The pediatric labeling of fingolimod makes it the first and only currently US Food and Drug Administration–approved DMT for children. Of note, results from the CONNECT trial were published in 2022, which found dimethyl fumarate to be superior to interferon β in children with MS based on similar end points.30 How these new findings will affect future trends in DMT use in children remains to be seen. Trends in use of oral DMTs differed between adults and children in other ways as well. Initial uptake of oral DMTs was higher in children: by 2009, oral DMTs accounted for 5.7% of initiations in children but less than 0.1% of initiations in adults. However, subsequent uptake of oral DMTs appeared to be faster in adults than children: oral DMTs became the most commonly initiated DMTs in adults by 2013, 2 to 3 years before this occurred for children.
The findings from our study were generally consistent with the limited available literature on trends in DMT use for MS. A 2021 article comparing new use of fingolimod with platform injectable DMTs among commercially insured US adults at least 18 years found that, between 2010 and 2011, platform injectables accounted for 84.4% of new use episodes, while fingolimod accounted for 15.6%.31 Our study found similar results for 2011. A 2020 study that evaluated MS treatment patterns by route of administration among commercially insured or Medicare-insured adults in the US found that, in 2011, platform injectable, oral, and infusion DMTs accounted for about 77.8%, 13.1%, and 9.1% of DMT initiations, respectively.15 By 2016, platform injectable, oral, and infusion DMTs accounted for 53.9%, 32.4%, and 13.7% of DMT initiations, respectively. Our findings were similar for 2011 but were slightly different for 2016. These discrepancies may be attributed to the different study populations, respective definitions of new use, and/or DMTs evaluated.
Our study used a large and diverse sample of commercially insured patients with MS to evaluate trends in DMT use in the US over a 20-year period. Our study also included children with MS, a vulnerable and understudied population. The findings from this study contribute to a greater understanding of MS treatment patterns and how they have changed over time with the approval of several new DMTs that have increased patients’ and clinicians’ options for the treatment of MS.
Limitations
Some study limitations are noted. We evaluated a sample of adults and children in the US with commercial health insurance (approximately 55% of the US population has commercial health insurance), limiting our ability to evaluate DMT use in the elderly or in patients with public or no insurance. Additionally, MarketScan data modestly oversample patients in the Northeast (18.5% to 19.7% in our study vs 17.1% from 2022 US Census) and Midwest (24.8% to 26.0% in our study vs 20.6% from 2022 US Census) of the US—regions where MS is more prevalent.21,32 We were also unable to evaluate race, ethnicity, or socioeconomic status, which may have roles in prescribing patterns and disease severity. As MS is rare in children, very few children met the inclusion criteria for this study, which affected the precision of our estimates for specific MS therapies in this population. As with previous claims-based studies, ICD-based definitions of MS are unable to distinguish MS subtypes.19,20 As our study ended in 2020 due to lag time in data availability, we were unable to study recent trends (2021 onward) in the uptake of newer agents (eg, ponesimod and ofatumumab) that were approved in 2019 or later. However, similar to other DMTs studied, it is expected that it may take several years to evaluate their full impact at the population level. Lastly, we were unable to adequately evaluate the impact of the COVID-19 pandemic on treatment disruptions/switches.33
Conclusions
In regard to this observational study, our investigation offers an important step in understanding the evolving treatment landscape for MS among US adults and children. The declining use of platform injectable DMTs corresponded largely with the availability of oral alternatives rather than the introduction of infusion DMTs. The exact reasons for these observed patterns cannot be determined from this study, but likely reflect several factors, including the convenience of administration, insurance limitations, and advertising strategies. As new DMTs continue to enter the market, future research should evaluate the impact that these approvals will have on MS treatment patterns.
eTable 1. Baseline characteristics for all new use episodes in adults (18-64) and children (<18) who newly initiated at least one disease-modifying therapy for multiple sclerosis between 2001-2020
eTable 2. New use episodes of individual disease-modifying therapies in adults (18-64 years old) with multiple sclerosis between 2001-2020
eTable 3. New use episodes of individual disease-modifying therapies in children (<18 years old) with multiple sclerosis between 2001-2020
eTable 4. New use episodes of individual disease-modifying therapies in adults (18-64 years old) with multiple sclerosis, excluding those with a diagnosis of malignancy or rheumatic disease during the baseline period, between 2001-2020
eTable 5. New use episodes of individual disease-modifying therapies in children (<18 years old) with multiple sclerosis, excluding those with a diagnosis of malignancy or rheumatic disease during the baseline period, between 2001-2020
eFigure 1. Prevalent use of disease-modifying therapies in adults (18-64 years old) with multiple sclerosis, 2001-2020
eFigure 2. Prevalent use of disease-modifying therapies in children (<18 years old) with multiple sclerosis, 2001-2020
Data sharing statement
References
- 1.National Multiple Sclerosis Society . Disease-modifying therapies for MS. Accessed May 31, 2023. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Managing-MS/Disease-Modification
- 2.Faulkner M. Risk of progressive multifocal leukoencephalopathy in patients with multiple sclerosis. Expert Opin Drug Saf. 2015;14(11):1737-1748. doi: 10.1517/14740338.2015.1093620 [DOI] [PubMed] [Google Scholar]
- 3.Coles AJ, Compston DA, Selmaj KW, et al. ; CAMMS223 Trial Investigators . Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786-1801. doi: 10.1056/NEJMoa0802670 [DOI] [PubMed] [Google Scholar]
- 4.Hauser SL, Bar-Or A, Comi G, et al. ; OPERA I and OPERA II Clinical Investigators . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 5.Cohen JA, Comi G, Selmaj KW, et al. ; RADIANCE Trial Investigators . Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021-1033. doi: 10.1016/S1474-4422(19)30238-8 [DOI] [PubMed] [Google Scholar]
- 6.Comi G, Kappos L, Selmaj KW, et al. ; SUNBEAM Study Investigators . Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009-1020. doi: 10.1016/S1474-4422(19)30239-X [DOI] [PubMed] [Google Scholar]
- 7.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777-788. doi: 10.1212/WNL.0000000000005347 [DOI] [PubMed] [Google Scholar]
- 8.Simpson A, Mowry EM, Newsome SD. Early aggressive treatment approaches for multiple sclerosis. Curr Treat Options Neurol. 2021;23(7):19. doi: 10.1007/s11940-021-00677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019;76(5):536-541. doi: 10.1001/jamaneurol.2018.4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He A, Merkel B, Brown JWL, et al. ; MSBase study group . Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307-316. doi: 10.1016/S1474-4422(20)30067-3 [DOI] [PubMed] [Google Scholar]
- 11.Jalkh G, Abi Nahed R, Macaron G, Rensel M. Safety of newer disease modifying therapies in multiple sclerosis. Vaccines (Basel). 2020;9(1):12. doi: 10.3390/vaccines9010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg B, Kolodny S, Wang M, Deshpande C. Utilization and treatment patterns of disease-modifying therapy in pediatric patients with multiple sclerosis in the United States. Int J MS Care. 2021;23(3):101-105. doi: 10.7224/1537-2073.2019-095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonafede MM, Johnson BH, Wenten M, Watson C. Treatment patterns in disease-modifying therapy for patients with multiple sclerosis in the United States. Clin Ther. 2013;35(10):1501-1512. doi: 10.1016/j.clinthera.2013.07.330 [DOI] [PubMed] [Google Scholar]
- 14.Bowen J, Mehta R, Pelletier C, et al. Treatment patterns among patients with multiple sclerosis initiating second-line disease-modifying therapy. Adv Ther. 2020;37(7):3163-3177. doi: 10.1007/s12325-020-01367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantor D, Mehta R, Pelletier C, et al. Treatment patterns and relapses among newly treated multiple sclerosis patients from a retrospective claims analysis. Clin Ther. 2020;42(11):2136-2147.e3. doi: 10.1016/j.clinthera.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 16.Yan K, Balijepalli C, Desai K, Gullapalli L, Druyts E. Epidemiology of pediatric multiple sclerosis: a systematic literature review and meta-analysis. Mult Scler Relat Disord. 2020;44:102260. doi: 10.1016/j.msard.2020.102260 [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 18.IBM Watson Health . IBM MarketScan research databases for life sciences researchers. Accessed June 5, 2023. https://www.ibm.com/downloads/cas/0NKLE57Y
- 19.Capkun G, Lahoz R, Verdun E, et al. Expanding the use of administrative claims databases in conducting clinical real-world evidence studies in multiple sclerosis. Curr Med Res Opin. 2015;31(5):1029-1039. doi: 10.1185/03007995.2015.1014029 [DOI] [PubMed] [Google Scholar]
- 20.Oleen-Burkey M, Cyhaniuk A, Swallow E. Treatment patterns in multiple sclerosis: administrative claims analysis over 10 years. J Med Econ. 2013;16(3):397-406. doi: 10.3111/13696998.2013.764309 [DOI] [PubMed] [Google Scholar]
- 21.United States Census Bureau . United States population growth by region. Accessed May 31, 2023. https://www.census.gov/popclock/data_tables.php?component=growth
- 22.US Food and Drug Administration . Gilenya (fingolimod) (package insert). Accessed May 31, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022527s031lbl.pdf
- 23.US Food and Drug Administration . Copaxone (glatiramer) (package insert). Accessed May 31, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020622s057lbl.pdf
- 24.Berger JR. Natalizumab and progressive multifocal leucoencephalopathy. Ann Rheum Dis. 2006;65 Suppl 3(Suppl 3):iii48-53. doi: 10.1136/ard.2006.058404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clerico M, Artusi CA, Liberto AD, et al. Natalizumab in multiple sclerosis: long-term management. Int J Mol Sci. 2017;18(5):940. doi: 10.3390/ijms18050940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sriwastava S, Kataria S, Srivastava S, et al. Disease-modifying therapies and progressive multifocal leukoencephalopathy in multiple sclerosis: a systematic review and meta-analysis. J Neuroimmunol. 2021;360:577721. doi: 10.1016/j.jneuroim.2021.577721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamout B, Sahraian M, Bohlega S, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord. 2020;37:101459. doi: 10.1016/j.msard.2019.101459 [DOI] [PubMed] [Google Scholar]
- 28.Chitnis T, Arnold DL, Banwell B, et al. ; PARADIGMS Study Group . Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med. 2018;379(11):1017-1027. doi: 10.1056/NEJMoa1800149 [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration . FDA expands approval of Gilenya to treat multiple sclerosis in pediatric patients. Accessed May 31, 2023. https://www.fda.gov/news-events/press-announcements/fda-expands-approval-gilenya-treat-multiple-sclerosis-pediatric-patients
- 30.Vermersch P, Scaramozza M, Levin S, et al. Effect of dimethyl fumarate vs interferon β-1a in patients with pediatric-onset multiple sclerosis: the CONNECT randomized clinical trial. JAMA Netw Open. 2022;5(9):e2230439. doi: 10.1001/jamanetworkopen.2022.30439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earla JR, Hutton GJ, Thornton DJ, Chen H, Johnson ML, Aparasu RR. Comparative treatment effectiveness of oral fingolimod and conventional injectable disease-modifying agents in multiple sclerosis. Pharmacotherapy. 2021;41(5):440-450. doi: 10.1002/phar.2517 [DOI] [PubMed] [Google Scholar]
- 32.Wallin MT, Culpepper WJ, Campbell JD, et al. ; US Multiple Sclerosis Prevalence Workgroup . The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi: 10.1212/WNL.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salama S, Ahmed SF, Ibrahim Ismail I, Alroughani R. Impact of coronavirus disease (COVID-19) pandemic on multiple sclerosis care. Clin Neurol Neurosurg. 2020;197:106203. doi: 10.1016/j.clineuro.2020.106203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline characteristics for all new use episodes in adults (18-64) and children (<18) who newly initiated at least one disease-modifying therapy for multiple sclerosis between 2001-2020
eTable 2. New use episodes of individual disease-modifying therapies in adults (18-64 years old) with multiple sclerosis between 2001-2020
eTable 3. New use episodes of individual disease-modifying therapies in children (<18 years old) with multiple sclerosis between 2001-2020
eTable 4. New use episodes of individual disease-modifying therapies in adults (18-64 years old) with multiple sclerosis, excluding those with a diagnosis of malignancy or rheumatic disease during the baseline period, between 2001-2020
eTable 5. New use episodes of individual disease-modifying therapies in children (<18 years old) with multiple sclerosis, excluding those with a diagnosis of malignancy or rheumatic disease during the baseline period, between 2001-2020
eFigure 1. Prevalent use of disease-modifying therapies in adults (18-64 years old) with multiple sclerosis, 2001-2020
eFigure 2. Prevalent use of disease-modifying therapies in children (<18 years old) with multiple sclerosis, 2001-2020
Data sharing statement