Abstract
Corneal epithelium can resist the invasion of external pathogenic factors to protect the eye from external pathogens. Sodium hyaluronate (SH) has been confirmed to promote corneal epithelial wound healing. However, the mechanism by which SH protects against corneal epithelial injury (CEI) is not fully understood. CEI model mice were made by scratching the mouse corneal epithelium, and in vitro model of CEI were constructed via curettage of corneal epithelium or ultraviolet radiation. The pathologic structure and level of connective tissue growth factor (CTGF) expression were confirmed by hematoxylin and eosin staining and immunohistochemistry. CTGF expression was detected by an immunohistochemistry assay. The levels of CTGF, TGF-β, Col1A1, FN, LC3B, Beclin1, and P62 expression were monitored by RT-qPCR, ELISA, Western blotting or immunofluorescence. Cell proliferation was detected by the CCK-8 assay and EdU staining. Our results showed that SH could markedly upregulate CTGF expression and downregulate miR-18a expression in the CEI model mice. Additionally, SH could attenuate corneal epithelial tissue injury, and enhance the cell proliferation and autophagy pathways in the CEI model mice. Meanwhile, overexpression of miR-18a reversed the effect of SH on cell proliferation and autophagy in CEI model mice. Moreover, our data showed that SH could induce the proliferation, autophagy, and migration of CEI model cells by downregulating miR-18a. Down-regulation of miR-18a plays a significant role in the ability of SH to promote corneal epithelial wound healing. Our results provide a theoretical basis for targeting miR-18a to promote corneal wound healing.
Key words: miR-18a, sodium hyaluronate, corneal epithelial injury, proliferation, autophagy
Introduction
Corneal epithelial injury (CEI) refers to a pathological condition in which the function and integrity of the corneal epithelial barrier are destroyed, leading to partial or complete loss of the corneal epithelial cell layer. CEI is a very common ocular surface disease diagnosed by ophthalmologists, and patients often present with dry eyes, swelling, pain, itching, eye fatigue, and other symptoms,1 The cornea is the transparent front part of the eye, and plays a key role in refraction of the anterior segment.2 Corneal epithelium is the outermost layer of the ocular surface, and most likely layer to be injured by external stimuli, such as mechanical wear, chemical burns, and ophthalmic surgery.3 The proper repair of damaged corneal epithelium is key for restoring the barrier function of corneal epithelial cells, inducing wound healing, and maintaining good eyesight.4 At present, CEI is mainly treated with ocular surface lubricants and antiinflammatory drugs; however, those treatments can sometimes damage the cornea of patients, and the improvements they produce are limited.1 Moreover, CEI can also cause corneal edema and ulceration, and obviously affect the visual acuity of patients. Therefore, it is imperative to search for new drugs that can improve CEI.
Sodium hyaluronate (SH) is an acid mucopolysaccharide molecule and the main glycosaminoglycan found in the fibrous space of the corneal cross-linked collagen matrix.5 SH is a natural polysaccharide with high adhesive, elasticity, and water retention properties, and a colorless transparency; it has no antigenicity and no colloid permeability.6A previous study found that SH can stimulate cell proliferation, migration, and adhesion during the corneal epithelial wound healing process.7 Currently, SH is commonly used to promote corneal wound healing, and its therapeutic effect mainly depends on its adhesion, elasticity, and pharmacological properties. 8,9 However, the mechanism by which SH heals of CEI remains controversial, and requires further investigation.
MicroRNAs (miRNAs) are non-coding single-stranded RNA molecules that contain 20-25 nucleotides, and the genes that code for miRNAs comprise one the largest gene families known.10,11 Currently, >600 miRNA-coding genes have been identified in the human genome.12 Study has demonstrated that miRNAs play key regulatory roles in early development, proliferation, differentiation, apoptosis, and signal transduction.13 Abnormal expression of miRNAs can cause multiple diseases, such as cancer, developmental abnormalities, cardiovascular abnormalities, and inflammatory diseases.14,15 Specific miRNAs have also been found to play functional roles in corneal epithelial development, such as miR-145,16 miR-204,17 miR-184,18 miR-205-3p,19 miR-155-5p,20 and miR- 21.21 Previously, we used microarray methods to explore the latent miRNAs in corneal epithelial cells that enhanced wound healing stimulated by SH. We discovered that miR-18a was expressed at significantly lower levels in SH-treated human corneal epithelial cells (HCECs), indicating that miR-18a was correlated to some extent with the stimulatory effect that SH exerted on the healing of human corneal epithelium wounds. As far as we know, a miR-18a plays carcinogenic and tumor angiogenic roles in multiple tumors, including breast cancer,22 leukemia,23 and gastric cancer.24 In colorectal cancer, miR-18a exerts an anti-tumor effect by inhibiting the CDC42 and PI3K pathways.25 However, the role and mechanism of miR-18a in SH-stimulated HCEC wound healing remain unclear.
In the current study, we constructed CEI animal and cell models and then used them to verify whether SH could accelerate corneal epithelial wound healing via miR-18a. Additionally, we confirmed the mechanism by which miR-18a improves a CEI following treatment with SH. Our results will not only help investigators to understand the mechanism of SH in CEI, but also expand our understanding of miRNA-targeted therapy for corneal wounds.
Materials and Methods
Animals
BALB/c mice (male, 10-weeks-old) were purchased from the Department of Laboratory Animals of Central South University. All mice were fed a standard diet and housed in a room maintained at 22-24°C with a 2 h light/dark cycle. The study protocol was approved by the Medical Ethics Committee of Hunan Provincial People’s Hospital, and all procedures were performed in compliance with relevant guidelines (approval No. 2018-33).
Establishment and treatment of the CEI model mice
All mice were anesthetized by intramuscular injection of chlorpromazine and ketamine. Next, the eyes and peripheral hair of each mouse were disinfected, and the central cornea was labelled and removed with an electric epithelial curettage device (without damaging the corneal stroma or marginal tissue). Mice were randomly divided into groups with 8 mice per group. After surgery, the mice were given a local administration or subconjunctival injection of SH (local administration: local drop of 0.6 mg/mL SH, 5 times per day; subconjunctival injection: 6 μL of miR-18a mimics or the inhibitor was injected into the temporal sub-conjunctiva of mice at two points; after which, the antibiotic oculentum was applied), Corneal epithelial wound healing in mice was observed under a microscope (XTL-168, Phenix, Jiangxi, China). The animal grouping and corresponding treatments were as follows:
Blank: mice did not undergo corneal injury.
Injury + PBS: mice received corneal injury and injection with PBS.
Injury + SH: mice received corneal injury and injection with SH.
Injury + SH+ miRNA NC: mice received corneal injury and injection with SH and miRNA negative control.
Injury + SH+ miR-18a inhibitor: mice received corneal injury and injection with SH and miR-18a inhibitor.
Injury + SH+ miR-18a mimics: mice received corneal injury and injection with SH and miR-18a mimics.
If the eyeball is infected, the animal should be excluded from this experiment and not be tested. After anesthesia with sodium pentobarbital, the animals were euthanized quickly by cervical dislocation.
Cell culture and treatment
Damage caused by ultraviolet radiation is an important factor in corneal injury.26 HCECs (CP-H128; Procell, Austin, TX, USA) were grown in DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA) containing 6% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA), 7 g/mL fetal bovine insulin (Sigma-Aldrich), and 7 ng/mL recombinant human epithelial growth factor (Sigma- Aldrich) at 37°C in a 5% CO2 atmosphere. After referring to relevant literature,27 HCECs were exposed to UV light (5.73 W/m2) at a distance of 30 cm for 5 min at 37°C in a 5% CO2 atmosphere in a special apparatus. The UV intensity used for the control group was 0.1719 J/cm². An miR-18 inhibitor, negative control (NC), and miR-18a mimics were purchased from GenePharma (Suzhou, China). HCECs were seeded into the wells of a 6-well plate (1 . 105 cells/well). The cells were then treated with SH and transfected for 48 h with miR-18a mimics by using Lipofectamine 3000 (Invitrogen) as described in the manufacturer’s instructions.
Hematoxylin and eosin staining
The cornea was quickly rinsed with PBS, and the eyeball was removed. Next, a portion of corneal epithelial tissue with a range of 4 mm, which included the wound surface, was removed under a microscope. After being rinsed with PBS, the tissue was immediately fixed in 4% paraformaldehyde (room temperature, 24 h); after which, it was dehydrated, embedded, and cut into 4 μm-thick sections, which were subsequently heated in a 60°C oven for storage. Next, the tissue sections were sequentially immersed in xylene I, xylene II, gradient alcohol, and distilled water. The sections were then stained with Harris hematoxylin for 5 min, differentiated with 1% alcohol hydrochloride, immersed in 0.6% ammonia, and stained with eosin for 2 min. After dehydration and transparency, the slices were sealed with neutral gum and examined under a microscope (E100, magnification 100X; Nikon, Tokyo, Japan).
Immunohistochemistry assay
After dehydration and transparency, the tissue sections were placed in a microwave oven and heated in EDTA antigen repair buffer (pH=9.0) for antigen repair; after which, they were placed in 3% hydrogen peroxide solution for 25 min. Next, the tissue sections were sealed with 3% BSA for 30 min, incubated overnight with anti-CTGF (1:800; Abcam, Cambridge, MA, USA) at 4°C, and then incubated with the corresponding secondary antibody (1:1000; Boster Bio, Wuhan, China) for 50 min at room temperature. The sections were then stained with fresh DAB, re-stained with Harris hematoxylin for 3 min, differentiated with 1% alcohol hydrochloride, and returned to blue with ammonia. Next, the sections were dehydrated, made transparent, sealed, and the results were visualized under a light microscope (E100, magnification 100X; Nikon). The sample, which omitted the primary antibody incubation, was used as a negative control (Figure S1).
RT-qPCR
TRIzol reagent (Invitrogen) was used to extract the total RNA from ground corneal tissue and processed HCECs. cDNA was synthesized using a BestarTM qPCR RT kit (Cat. No. 2220; DBI Bioscience, Germany) based on 10 ng of total RNA. The levels of miRNA or mRNA expression was quantified by Real-time PCR that was performed using BestarTM qPCR Master Mix (Cat. No. 2043; DBI Bioscience), The expression levels were normalized to those of U6 (for miRNA) or GAPDH (for mRNA) using 2-ΔΔCt. The primer sequences used for RT-PCR are shown in Table 1.
ELISA
The levels of CTGF and TGF-β were detected using CTGF ELISA kit (E-EL-M0340c; Elabscience, China) and TGF-β ELISA kit, respectively (E-EL-0162c; Elabscience), as described in the manufacturer’s instructions.
Western blotting
The ground corneal tissue and processed HCECs were added to NP-40 buffer containing 1 mM PMSF (Genebase, Vancouver, Canada) and incubated on ice for 30 min. After high-speed centrifugation, the supernatants (total proteins) were collected, and the protein concentration in each supernatant was determined using a PierceTM BCA Protein Assay Kit (Cat. No. 23227). Next, a 40 μg sample of total protein from each supernatant was denatured and separated by 10% SDS-PAGE; after which, the protein bands were transferred onto PVDF membranes (Millipore, Burlington, MA, USA), which were subsequently blocked. The membranes were then incubated overnight at 4°C with primary antibodies, followed by a 1 h incubation with a secondary antibody (anti-rabbit IgG, ab6721, 1:15000; Abcam). Finally, the immunostained protein bands were detected with chemiluminescence reagent (Millipore), and photographed using X-ray film. The primary antibodies used were Col1A1 (BA0325, 1:800; Boster), FN (A00564-1, 1:1000; Boster), LC3B (ab192890, 1:2000; Abcam), Beclin1 (BA3123-2, 1:1500; Boster), P62 (BA2849, 1:1000; Boster), and GAPDH (ab8245, 1:5000; Abcam).
CCK-8 assay
Processed HCECs were seeded into the wells of 96-well plates at a density of 1 x 104 cells/well and then treated with 15 μL of CCK-8 solution (CK04; Dojindo, Tokyo, Japan) for 0, 24, 48, and 72 h, respectively. After incubation for 3 h at 37°C, the absorbance of each well at 450 nm was recorded using a microplate reader (Bio-Tek Winooski, VT, USA).
EdU staining
Processed HCECs were treated with 50 μM EdU solution (RiboBio, Guangzhou, China) for 2 h, and harvested afterwards. After washing, the HCECs were fixed with 4% paraformaldehyde (30 min), made transparent with Triton X-100, and treated with 2 mg/mL glycine (5 min). The HCECs were then stained with Apollo and Hoechst 33342. Finally, the EdU positive cells were observed using a fluorescence microscope (LSM800, magnification 100X; Zeiss, Jena, Germany).
Transwell assay
Briefly, processed HCECs (100 μL, 1 . 105 cells/well) and medium containing 10% FBS (500 μL) were placed into the upper and lower chambers, respectively, of a Transwell plate (Costar®; Sigma-Aldrich), After 48 h of incubation at 37°C, the non-migrated cells were removed with a swab and the migrated cells were fixed and stained with 0.2% crystal violet (C0775; Sigma- Aldrich). The migrated cells were then counted and photographed under a light microscope (E100, magnification 100X; Nikon).
Immunofluorescence staining
The treated HCECs were harvested, and then fixed with 4% paraformaldehyde for 30 min, followed by treatment with 0.2% Triton X-100 for 5 min. After being blocked with 10% normal goat serum for 30 min, the HCECs were incubated overnight at 4°C with anti-LC3B (1:50, ab192890; Abcam), and then incubated with a fluorescent secondary antibody (1:200; Abcam) for 1 h at room temperature. After treatment with DAPI, the fluorescence intensity of the cells was observed under a fluorescence microscope (LSM800, magnification 100X; Zeiss).
Table 1.
Primers used for RT-PCR.
| Primer name | Sequence (5’- 3’) |
|---|---|
| β-actin F | CATTGCTGACAGGATGCAGA |
| β-actin R | CTGCTGGAAGGTGGACAGTGA |
| CTGF F | AGACCCAACTATGATGCGAG |
| CTGF R | AAAGCTCAAACTTGACAGGC |
| TGF-β F | TACAGGGCTTTCGATTCAGC |
| TGF-β R | TTCATGTCATGGATGGTGCC |
| Col1A1 F | AATGGTGAGACGTGGAAACC |
| Col1A1 R | TGGGACAGTCCAGTTCTTCA |
| FN F | CCCCAATCTTCATGGACCAG |
| FN R | TGTGCCAGGAAGCTGAATAC |
| U6 F | CTCGCTTCGGCAGCACA |
| U6 R | AACGCTTCACGAATTTGCGT |
| All R | CTCAACTGGTGTCGTGGA |
| miR-18a RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTATCTG |
| miR-18a F | ACACTCCAGCTGGGTAAGGTGCATCTAGTGCAG |
Statistical analysis
All statistical data were analyzed using IBM SPSS Statistics for Windows, v. 21 software (IBM Corp, Armonk, NY, USA). Statistical results are presented as a mean value ±SD. Student’s ttest was used to analyze differences between individual groups, one-way ANOVA was used to analyze differences between multiple groups, and the Kruskal-Wallis test was used to analyze differences between multiple groups with uneven variance. A p<0.05 was considered to be statistically significant.
Results
SH markedly attenuated corneal epithelial tissue injury and upregulated CTGF expression in the CEI model mice
To investigate the effect of SH on CEI, we first established a mouse model of CEI. Hematoxylin and eosin staining results showed that in the blank group, the corneal structure was clear and complete, there was no edema and thickening in the stroma layer. In CEI group, the corneal tissue was thickened due to edema, the matrix fibers were disordered. In the CEI+SH group, the corneal epithelium had a normal appearance, and the amount of stroma edema was decreased (Figure 1A). Additionally, immunohistochemistry data indicated that CTGF expression was markedly higher in the CEI group than in the blank group, and the degree of CTGF upregulation could be further increased by SH in the CEI model mice (Figure 1 B,b). Overall, these data verified that SH could attenuate CEI and upregulate CTGF in the corneal tissues of mice with CEI.
SH downregulated miR-18a expression and accelerated cell proliferation and autophagy in mice with CEI
Subsequently, we found that miR-18a was highly expressed in the CEI model mice when compared to mice in the blank group; however, the increase in miR-18a expression in the CEI model mice was reversed by SH after 24, 48, and 72 h (Figure 2A). RTqPCR results showed that CTGF and TGF-β were notably upregulated in the CEI group relative to expression in the blank group, and SH could also further increase the levels of CTGF and TGF-β in the CEI mice (Figure 2B). ELISA results indicated that SH could markedly increase the concentrations of CTGF and TGF-β in mice with CEI (Figure 2C). Moreover, Western blot data showed that CEI treatment could result in a remarkable upregulation of FN, and P62 expression, and a significant downregulation of LC3B and Beclin1 expression. Moreover, the changes in expression of those proteins could also be further increased by SH in the CEI model mice, along with a decreased P62 expression (Figure 2 D,E).
Figure 1.

SH markedly attenuated corneal epithelial tissue injury and upregulated CTGF in CEI model mice. A) Hematoxylin and eosin staining was used to observe pathological changes in the corneal epithelium of CEI model mice after treatment with SH. B,b) HIC was used to confirm the effect of SH on CTGF expression in mice with CEI. Magnification: B), x100; b) x200.
Overexpression of miR-18a attenuated the effects of SH on miR-18a, as well as cell autophagy-related pathways, CTGF and TGF-β level in CEI model mice
Next, we further explored whether the effects of SH on corneal epithelial cell autophagy, CTGF and TGF-β level in the CEI model mice were realized by regulating miR-18a. After SH treatment, the CEI model mice were injected with miR-18a mimics or the inhibitor at the conjunctiva. We first discovered that the downregulation of miR-18a mediated by SH could be markedly reversed by miR-18a overexpression and further enhanced by miR-18a inhibition in the CEI model mice (Figure 3A). Additionally, the data showed that overexpression of miR-18a could markedly reduce CTGF and TGF-β levels, and inhibition of miR-18a could dramatically increase CTGF and TGF-β levels, which were induced by SH in the conjunctival tissues of CEI model mice (Figure 3 B,C). Simultaneously, our data indicated that the SH-mediated up-regulation of LC3B and Beclin1 and down-regulation of p62 could be reversed by miR-18a overexpression and dramatically enhanced by miR-18a inhibitor in the CEI model mice (Figure 3D). When taken together, our findings indicated that SH could enhance cell autophagy by reducing miR-18a expression in mice with CEI.
Introduction of miR-18a dramatically reversed the SHinduced effect of CEI model cells
Based on the effects of SH and miR-18a on CEI CTGF and TGF-β level, cell viability in mice, we further examined their roles in corneal epithelial cells. HCECs were used to establish a CEI cell model by exposing the cells to UV light. RT-qPCR data showed that SH could markedly downregulate miR-18a expression in UVtreated HCECs, and the downregulation could be notably reversed by transfection with miR-18a mimics (Figure 4A). CCK-8 assay results revealed that cell viability was markedly inhibited in the UV group relative to that in the control (PBS) group, while cell viability could be facilitated by SH in the UV-treated model cells. Additionally, the promotion of cell viability mediated by SH could also be significantly attenuated by miR-18a overexpression in the CEI model cells (Figure 4B). ELISA results showed that CTGF and TGF-β levels were markedly elevated in the UV+SH group relative to their levels in the UV group, and those increases mediated by SH could be attenuated by miR-18a overexpression in CEI model cells (Figure 4C). Similarly, EdU staining of HCECs showed that miR-18a overexpression reversed the promotion of viability induced by SH in corneal epithelial cells (Figure 4D). In summary, these data verified that SH could induce the viability of UV-treated HCECs by downregulating miR-18a.
Figure 2.
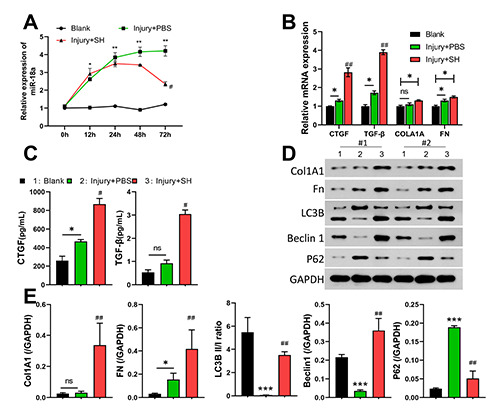
SH markedly downregulated miR-18a expression and mediated autophagy-related protein expression in mice with CEI. CEI model mice were established and then treated with SH. A) Changes in miR-18a expression in mice were identified by using qPCR at 0, 12, 24, 48, and 72 h. B) RT-qPCR analyses of CTGF, TGF-β, Col1A1, and FN expression. C) The concentrations of CTGF and TGF-β were detected with ELISA kits at 72 h post injury. D,E) Western blotting was used to monitor changes in Col1A1, FN, LC3B, Beclin 1, and P62 expression in corneal tissues at 72 h post injury.
SH induced autophagy and migration by downregulating miR-18a in CEI model cells
In addition, we found that SH could promote the migration of UV-treated HCECs, and that promotional effect could be attenuated by miR-18a overexpression (Figure 5A). Western blot data showed that the levels of LC3B and Beclin1 were dramatically increased, and the levels of P62 were decreased in the UV+SH group relative to those in the UV group, indicating that SH induced changes in autophagy-related proteins. We also found that miR- 18a overexpression suppressed autophagy in UV-treated HCECs by inhibiting LC3B II and Beclin 1 expression and promoting P62 expression (Figure 5 B,C). When taken together, these findings indicated that SH promoted migration and autophagy in the CEI model cells by lowering miR-18a expression in the cells.
Discussion
Corneal epithelial wound healing involves a number of highly coordinated events, including cell proliferation, migration, adhesion, and differentiation that help to maintain the integrity of the corneal epithelial surface.28,29 SH has been confirmed to exert a positive pharmacological effect on corneal wound healing,30,31 and also protect the corneal epithelium and endothelium. For example, SH can form a protective film on the surface of eye tissue and surgical instruments to prevent mechanical irritation and injury; SH can protect the corneal epithelium from desiccation by its water retention; SH could apply its elasticity to buffer mechanical pressures and shear forces; SH might also effectively support the anterior chamber during intraocular surgery, thus maintaining the proper depth.32,33 Furthermore, SH can enhance the moisture retention of the corneal surface due to its excellent water-retention property, delay tear film breakup time, effectively relieve the symptoms of dry eye, and serve as an artificial tear in treatment of dry eye.34 SH is widely used in contact lenses and nursing systems.35 However, the pharmacological mechanism by which SH promotes corneal epithelial wound healing remains unclear. In our study, we verified that SH could improve corneal epithelial tissue injury, and induce the proliferation of corneal epithelial cells in CEI model mice. We also found that SH could accelerate the proliferation and migration of CEI model cells. These results are consistent with previous reports.31,36 More importantly, our results also confirmed that SH could markedly enhance autophagy in CEI model cells.
An understanding of the regulatory role played by miRNA is crucial for studying eye development and pathology.37 Abnormal miRNA expression plays a key role in corneal epithelial development, suggesting the therapeutic potential of miRNA.20,38 Past studies have confirmed that several miRNAs play significant roles in corneal epithelial wound healing. For instance, miR-21 can induce wound healing in corneal epithelial cells via SPRY2;21 miR-155-5p was found to decrease corneal epithelial permeability during corneal wound healing;20 decreased miR-129-5p expression was shown to alleviate CEI via EGFR.39 Our previous research showed that miR-18a could mediate the cell viability and migration of corneal epithelial cells treated with SH.40While, in our current study, we discovered that miR-18a can help to stimulate the effects of SH on corneal epithelial wound healing both in vivo and in vitro. We found that autophagy of cells may play an important role in corneal injury and is regulated by miR-18a.
Autophagy is a cellular process in which the cells utilize their own enzyme system to degrade and recycle discarded proteins, organelles and metabolic products . Autophagy can eliminate cellular waste and provide nutrient substrates to support cellular survival and repair. There is a close relationship between autophagy and tissue repair.41 Briefly, autophagy participates in the clearance of intracellular waste and the recycling of metabolic products, which can provide necessary nutrients for damaged tissues and promote tissue repair.42 Ma and colleagues have found that inducing autophagy leads to more labeled MSCs migrating to the site of injury. Inducing autophagy in MSCs promotes functional recovery after spinal cord injury, while inhibiting autophagy in MSCs results in weaker functional recovery.43 In this study, it was found that autophagy was impaired in both the CEI animal model and cell model, accompanied by an upregulation of miR-18a expression. After inhibiting miR-18a, there was a significant increase in autophagy levels. Therefore, the role of autophagy in corneal injury deserves further investigation.
Figure 3.

Overexpression of miR-18a attenuated the effects of SH on miR-18a, and autophagy-related pathways, CTGF and TGF-β level in CEI model mice. CEI model mice were treated with SH and injected with miR-18a mimics or an inhibitor, respectively. A) MiR-18a levels were determined by RT-qPCR. The levels of CTGF and TGF-β in each group were detected using RT-qPCR (B) and ELISA kits. C,D) Changes in LC3B, Beclin 1, and P62 expression were detected by Western blotting.
CTGF (connective tissue growth factor) and TGF-β (transforming growth factor-β) are two important proteins that play crucial roles in tissue repair.44,45 CTGF promotes cell proliferation, differentiation, and collagen synthesis, which are essential factors for maintaining normal physiological functions of tissues. After tissue injury, the expression of CTGF gradually increases, which can attract and activate various cell types required for the repair process.46 TGF-β also plays a crucial role in tissue repair by regulating differentiation, cell proliferation, extracellular matrix synthesis, and inflammatory responses, among others, after tissue injury. TGF-β can stimulate fibroblast proliferation and induce them to produce collagen, fibronectin, and other important biomolecules, thereby playing an essential role in tissue repair.47 In the present study, it was observed that following corneal injury, the expression of miR-18a in the cornea was markedly upregulated, along with an upregulation of CTGF and TGF-β. This upregulation can be attributed to the body’s stress response to the inflicted damage. Intriguingly, after undergoing SH treatment, miR-18a exhibited a downregulation, resulting in the further elevation of CTGF and TGF-β expression. This finding implies that miR-18a plays a detrimental role in the tissue repair process. However, the precise manner in which miR-18a affects the expression levels of CTGF and TGF-β remains undetermined in this study, warranting further investigation to fully unravel the underlying mechanisms. We used bioinformatics to predict the latent target genes of miR-18a that might induce SH-stimulated corneal epithelial wound healing. Those results showed that the predicted target genes of miR-18a were enriched in the downstream pathway of Arf6. The Arf6 pathway mainly contains 22 predicted target genes, including cell cycle-related genes (e.g., CDC42 and CCND2) and cell growth-related genes (e.g., FRS2 and CTGF). CDC42 and CCND2 are key components of the cell cycle and cell proliferation pathways. FRS2 and CTGF are not only related to cell proliferation but also to cell migration. It was also discovered that CTGF can induce Col1A1 synthesis in corneal fibroblasts by mediating TGF-β, thus promoting corneal wound healing.48 Our study verified that SH could upregulate CTGF and TGF-β expression by downregulating miR-18a expression in the CEI model mice and cells. We therefore concluded that CTGF might be the target gene of miR-18a, and miR-18a might regulate biological processes by downregulating CTGF. This hypothesis is a limitation of our research and needs to be confirmed in a future study.
Figure 4.

Treatment with miR-18a dramatically reversed the induction effect of SH on the cell viability, CTGF and TGF-β of CEI model cells. CEI cell model was constructed using HCECs that were exposed to UV radiation. Next, the HCECs were treated with SH and miR-18a mimics. A) RT-qPCR analysis of miR-18a. B) CCK8 assays were performed to assess changes in cell viability. C) ELISA kits were used to detect the concentrations of CTGF and TGF-β. D) Edu assays revealed changes in cell proliferation. Magnification: x100.
Figure 5.

SH induced autophagy and cell migration by downregulating miR-18a in CEI model cells. CEI model cells were treated with SH and miR-18a mimics, respectively. A) Cell migration was analyzed by the Transwell assay, and the numbers of migrated cells in each group were counted. B) Western blotting showed changes in LC3B, Beclin1, and P62 expression. C) LC3B expression was examined by IF staining. Magnification: x200.
In conclusion, our study results confirmed that miR-18a was involved in the accelerated rates of cell proliferation, autophagy, and migration detected in SH-treated corneal epithelial cells and CEI model mice. Moreover, miR-18a was found to affect the production of both CTGF and TGF-β. Therefore, our study provides theoretical support for the mechanism of corneal epithelial wound healing and suggests new therapeutic targets for treating CEIs. This study establishes the expression profile of miR-18a in corneal injury and elucidates a preliminary mechanism, providing foundational insights for further in-depth investigations.
Funding Statement
Funding: this work was supported by the Natural Science Foundation of Hunan Province of China (No.2019JJ50325) and Ren Shu Foundation of Hunan Provincial People's Hospital (No.RS201817), and Research and Development Program in Key areas of Hunan Province (No.2020SK2118), and Department of Education of Hunan Province (No. 22A0044).
References
- 1.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res 2015; 49:17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson SE, Torricelli AAM, Marino GK. Corneal epithelial basement membrane: Structure, function and regeneration. Exp Eye Res 2020;194:108002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma X, Wang Q, Song F, Li Y, Li J, Dou S, et al. Corneal epithelial injury-induced norepinephrine promotes Pseudomonas aeruginosa keratitis. Exp Eye Res 2020;195:108048. [DOI] [PubMed] [Google Scholar]
- 4.Raghunathan VK, Thomasy SM, Strom P, Yanez-Soto B, Garland SP, Sermeno J, et al. Tissue and cellular biomechanics during corneal wound injury and repair. Acta Biomater 2017; 58:291-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Shi XL, He XH, Mao YH, Li C, Dong N. Loteprednol combined with sodium hyaluronate in the treatment of dry eye disease and its effect on TNF-α and CXCL10 in tears. J Biol Regul Homeost Agents 2020;34:1825-9. [DOI] [PubMed] [Google Scholar]
- 6.Becker LC, Bergfeld WF, Belsito DV, Klaassen CD, Marks JG Jr., Shank RC, et al. Final report of the safety assessment of hyaluronic acid, potassium hyaluronate, and sodium hyaluronate. Int J Toxicol 2009;28:5-67. [DOI] [PubMed] [Google Scholar]
- 7.Wen Y, Zhang X, Chen M, Han D. Sodium hyaluronate in the treatment of dry eye after cataract surgery: a meta-analysis. Ann Palliat Med 2020;9:927-39. [DOI] [PubMed] [Google Scholar]
- 8.Carracedo G, Villa-Collar C, Martin-Gil A, Serramito M, Santamaria L. Comparison between viscous teardrops and saline solution to fill orthokeratology contact lenses before overnight wear. Eye Contact Lens 2018;44:S307-11. [DOI] [PubMed] [Google Scholar]
- 9.Camillieri G, Bucolo C, Rossi S, Drago F. Hyaluronan-induced stimulation of corneal wound healing is a pure pharmacological effect. J Ocul Pharmacol Ther 2004;20:548-53. [DOI] [PubMed] [Google Scholar]
- 10.Gumusoglu E, Tuba G, Hosseini MK, Seymen N, Senol T, Sezerman U, et al. The importance of dysregulated miRNAs on ovarian cysts and epithelial ovarian cancer. Eur J Gynaecol Oncol 2021;42:66-72. [Google Scholar]
- 11.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol 2019;234: 5451-65. [DOI] [PubMed] [Google Scholar]
- 12.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol 2018;141:1202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis 2015;35:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garofalo M, Leva GD, Croce CM. MicroRNAs as anti-cancer therapy. Curr Pharm Des 2014;20:5328-35. [DOI] [PubMed] [Google Scholar]
- 15.Kalayinia S, Arjmand F, Maleki M, Malakootian M, Singh CP. MicroRNAs: roles in cardiovascular development and disease. Cardiovasc Pathol 2021;50:107296. [DOI] [PubMed] [Google Scholar]
- 16.Lee SK, Teng Y, Wong HK, Ng TK, Huang L, Lei P, et al. MicroRNA-145 regulates human corneal epithelial differentiation. PLoS One 2011;6:e21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Di G, Dong M, Qu M, Zhao X, Duan H, et al. Epithelium-derived miR-204 inhibits corneal neovascularization. Exp Eye Res 2018;167:122-7. [DOI] [PubMed] [Google Scholar]
- 18.Cao Q, Xu W, Chen W, Peng D, Liu Q, Dong J, et al. MicroRNA-184 negatively regulates corneal epithelial wound healing via targeting CDC25A, CARM1, and LASP1. Eye Vis (Lond) 2020;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu JY, Yu XF, Wang HQ, Lan JW, Shao WQ, Huo YN. MiR- 205-3p protects human corneal epithelial cells from ultraviolet damage by inhibiting autophagy via targeting TLR4/NF-κB signaling. Eur Rev Med Pharmacol Sci 2020;24:6494-504. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Wang D, Song M, Zhou Q, Liao R, Wang Y. MiRNA- 155-5p reduces corneal epithelial permeability by remodeling epithelial tight junctions during corneal wound healing. Curr Eye Res 2020; 45:904-13. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Yuan F, Liu L, Chen Z, Ma X, Lin Z, et al. The role of the miR-21/SPRY2 axis in modulating proangiogenic factors, epithelial phenotypes, and wound healing in corneal epithelial cells. Invest Ophthalmol Vis Sci 2019;60:3854-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair MG, Prabhu JS, Korlimarla A, Rajarajan SPSH, Kaul R, et al. miR-18a activates Wnt pathway in ER-positive breast cancer and is associated with poor prognosis. Cancer Med 2020;9:5587-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Kong L, Yang Y. miR-18a Inhibitor suppresses leukemia cell proliferation by upregulation of PTEN expression. Med Sci Monit 2020;26:e921288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi B, Dong Y, Qiao XL. Effects of miR-18a on proliferation and apoptosis of gastric cancer cells by regulating RUNX1. Eur Rev Med Pharmacol Sci 2020;24:9957-64. [DOI] [PubMed] [Google Scholar]
- 25.Humphreys KJ, McKinnon RA, Michael MZ. miR-18a inhibits CDC42 and plays a tumour suppressor role in colorectal cancer cells. PLoS One 2014;9:e112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H, Yu Y, Zhao Z, Cui L, Hou J, Shi H. Prdx6 is required to protect human corneal epithelial cells against ultraviolet B injury. Eur J Ophthalmol 2021;31:367-78. [DOI] [PubMed] [Google Scholar]
- 27.Xu M, Sivak JG, McCanna DJ. Ocular toxicology: synergism of UV radiation and benzalkonium chloride. Cutan Ocul Toxicol 2020;39:370-9. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal VB, Tsai RJ. Corneal epithelial wound healing. Indian J Ophthalmol 2003;51:5-15. [PubMed] [Google Scholar]
- 29.Wilson SE. Corneal wound healing. Exp Eye Res 2020;197:108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asena L, Gokgoz G, Helvacıoğlu F, Ozgun G, Deniz EE, Dursun Altinors D. Effects of topical coenzyme Q10, Xanthan gum and sodium hyaluronate on corneal epithelial wound healing. Clin Exp Optom 2022;105:378-84. [DOI] [PubMed] [Google Scholar]
- 31.Ozek D, Kemer OE. Effect of the bioprotectant agent trehalose on corneal epithelial healing after corneal cross-linking for keratoconus. Arq Bras Oftalmol 2018;81:505-9. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed YH, Uematsu M, Ueki R, Inoue D, Sasaki H, Kitaoka T. Safety of sodium hyaluronate eye drop with C12- benzalkonium chloride. Cutan Ocul Toxicol 2019;38:156-60. [DOI] [PubMed] [Google Scholar]
- 33.Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol 2004;88:821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mi Y, Miao Q, Cui J, Tan W, Guo Z. Novel 2-Hydroxypropyltrimethyl ammonium chitosan derivatives: synthesis, characterization, moisture absorption and retention properties. Molecules 2021;26:4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JS, Park JM, Cho HK, Kim SJ, Huh HD, Park YM. Influence of sodium hyaluronate concentration on corneal aberrations in soft contact lens wearers. Korean J Ophthalmol 2018;32:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bock U, Deylen DV, Jochner M, Doerr M, Stabler C, Reichl S. Development of in vitro methodologies to investigate binding by sodium hyaluronate in eye drops to corneal surfaces. Open Ophthalmol J 2018;12:226-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu CH, Huang S, Britton WR, Chen J. MicroRNAs in vascular eye diseases. Int J Mol Sci 2020;21:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan N, Liu M, Wang J, Yang W, Fiolek E, Peng H, et al. Eph signaling is regulated by miRNA-210: Implications for corneal epithelial repair. FASEB J 2022;36:e22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Gong B, Wu ZZ, Shuai P, Li DF, Liu LL, et al. Inhibition of microRNA-129-5p expression ameliorates ultraviolet ray-induced corneal epithelial cell injury via upregulation of EGFR. J Cell Physiol 2019;234:11692-707. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Lu X, Wang H. Downregulation of miR-18a induces CTGF and promotes proliferation and migration of sodium hyaluronate treated human corneal epithelial cells. Gene 2016. 591:129-36. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Wang J, Wu X, Simon N, Svensson CI, Yuan J, et al. eEF2 improves dense connective tissue repair and healing outcome by regulating cellular death, autophagy, apoptosis, proliferation and migration. Cell Mol Life Sci 2023;80:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei C, Pan Y, Zhang Y, Dai Y, Jiang L, Shi L, et al. Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells. Cell Death Dis 2020;11:935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma F, Li R, Tang H, Zhu T, Xu F, Zhu J. Regulation of autophagy in mesenchymal stem cells modulates therapeutic effects on spinal cord injury. Brain Res 2019;1721:146321. [DOI] [PubMed] [Google Scholar]
- 44.Kim E, Ham S, Jung BK, Park JW, Kim J, Lee JH. Effect of baicalin on wound healing in a mouse model of pressure ulcers. Int J Mol Sci 2022;24:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liarte S, Bernabe-Garcia A, Rodriguez-Valiente M, Moraleda JM, Castellanos G, Nicolas FJ. Amniotic Membrane restores chronic wound features to normal in a keratinocyte TGF-β- chronified cell model. Int J Mol Sci 2023;24:6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laursen SH, Hansen SG, Taskin MB, Chen M, Wogensen L, Nygaard JV, et al. Electrospun nanofiber mesh with connective tissue growth factor and mesenchymal stem cells for pelvic floor repair: Long-term study. J Biomed Mater Res B Appl Biomater 2023;111:392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang K, Li Y, Xiang C, Xiong Y, Jia J. Rebalancing SMAD7/SMAD3 signaling reduces adhesion formation during flexor tendon healing. J Microbiol Biotechnol 2023;33:339-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blalock TD, Duncan MR, Varela JC, Goldstein MH, Tuli SS, Grotendorst GR, et al. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci 2003;44:1879-87. [DOI] [PubMed] [Google Scholar]


