Introduction
First described by Randall et al.,1 monoclonal Ig deposition disease (MIDD) is a rare condition because of B-cell clonal disorders and defined by nonorganized monoclonal Ig fragments deposits in most organs, with prominent renal manifestations.2 MIDD comprises light chain (LC) deposition disease, heavy chain (HC) deposition disease (HCDD), and light and heavy chain deposition disease (LHCDD), which are characterized by tissue deposition of LC, HC, or both light and heavy chains fragments respectively, leading to organ dysfunction.3,4 In the kidney, MIDD is characterized by Ig deposits predominantly in and around tubular and glomerular basement membranes with a linear pattern, and also commonly observed in the mesangium and around arteriolar myocytes. Using light microscopy, the most common lesions are diffuse thickening of the tubular basement membranes and nodular glomerulosclerosis. Unlike LC amyloidosis, monoclonal Ig deposits in MIDD do not stain for Congo red, and ultra structurally display a typical nonorganized “powdery punctate” appearance. In addition, deposits in LC deposition disease are most often composed of kappa LCs, whereas the fibrils in LC amyloidosis usually derive from the variable region of monoclonal lambda LCs.2,3 Of note, HCDD results from the deposition of a truncated monoclonal Ig HC lacking the first constant domain, which is mandatory for the secretion of free HC by plasma cells.4 Most HCDD cases involve deposition of a truncated gamma HC, but rare cases of alpha, mu, or delta monoclonal HCDD have been described.5 HCDD and LHCDD most often complicate monoclonal gammopathy of unknown significance defined as monoclonal gammopathy of renal significance, and to a lesser extent multiple myeloma or other-B-cell disorders.3 In this study, we report the first case of Randall-type monoclonal IgE kappa LHCDD.
Results
A 65-year-old woman was referred to our nephrology department for renal impairment and low-grade proteinuria. Her past medical history consisted of hypertension and IgE monoclonal gammopathy of undetermined significance, diagnosed 6 years prior and characterized by stable monoclonal spike (about 1.5 g/l) on serum protein electrophoresis. Of note, no evidence, even remotely in the clinical history, of parasitic or allergic manifestation related to an IgE response was present in our patient.
On admission, the results of physical examination were unremarkable. Laboratory investigations showed progressive kidney failure with serum creatinine level of 130 μmol/l as compared to 90 μmol/l one year prior. The urinary protein-to-creatinine ratio was at 0.9 g/g with 0.3 g/g of albumin. Serum electrophoresis and immunofixation confirmed the presence of IgE kappa monoclonal gammopathy at 0.8 g/l with low residual gamma globulin level of 3.6 g/l and urine immunofixation revealed kappa LC Bence Jones proteinuria. Serum free LC testing showed increased kappa LC levels at 559 mg/l with lambda LC level of 3.72 mg/l and increased kappa to lambda LC ratio of 150. Full blood count revealed mild normocytic anemia at 9 g/dl with normal leukocyte and platelet counts. Serum calcium level was within the normal range.
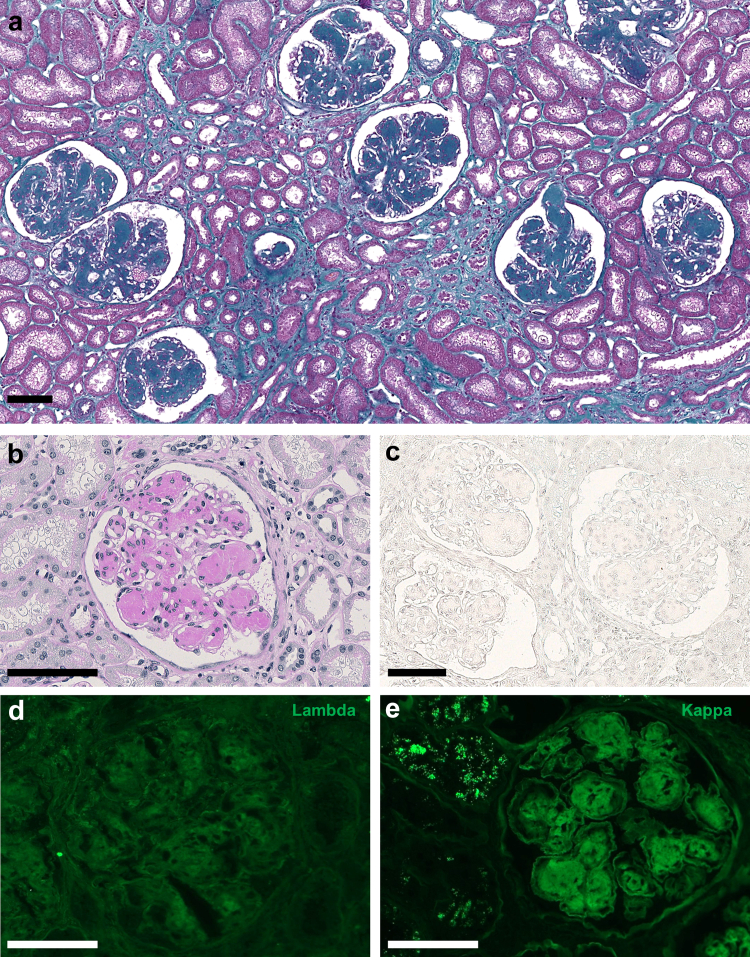
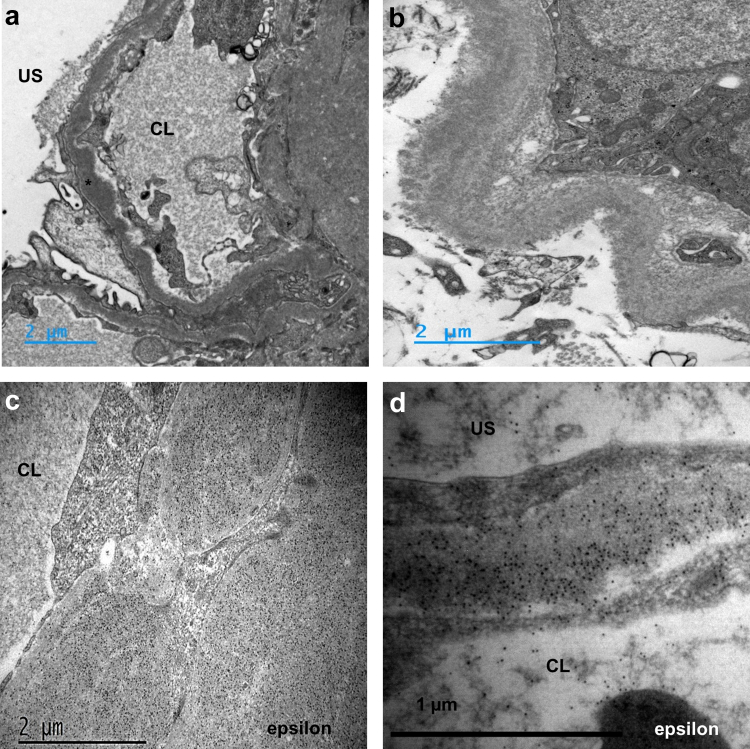
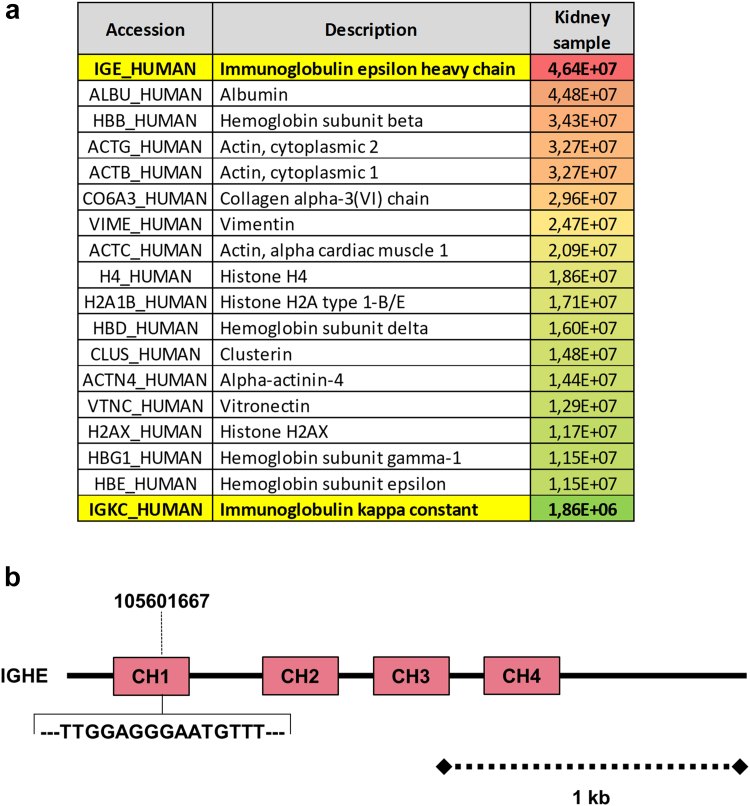
A kidney biopsy was performed (Supplementary Methods). Using light microscopy, the most prominent lesions were diffuse glomerular basement membrane thickening associated with PAS-positive and Congo red-negative, as well as global and diffuse nodular glomerulosclerosis (Figure 1a–c). The tubulointerstitial compartment revealed mild tubulointerstitial fibrosis (20% of cortical area) with no evidence of tubular basement membrane thickening. Immunofluorescence studies showed positive staining for kappa LC on tubular and glomerular basement membranes with linear distribution, and in the mesangium. No staining was observed with anti-lambda LC, IgA, IgG or IgM HC antibodies (Figure 1d and e). Electron microscopy confirmed the diagnosis of MIDD with osmiophilic powdery punctate deposits located in the mesangium, on the inner aspect of the glomerular basement membranes and the outer aspect of tubular basement membranes (Figure 2a and b). Immunogold studies showed strong positive staining of glomerular and tubular deposits with gold-conjugated anti-Epsilon antibody (Figure 2c and d) and mild positivity with gold-conjugated anti-kappa antibody (not shown). Laser microdissection and tandem mass spectrometry analysis of glomerular deposits identified Ig epsilon HC as the highest enriched component in glomerular deposits together with kappa LC deposits (Figure 3a). Altogether, these results confirmed the diagnosis of IgE kappa LHCDD.
Figure 1.
(a) Kidney biopsy with Masson Trichrome staining showing global and diffuse nodular mesangial deposits. (b) Kidney biopsy with Periodic Acid Schiff (PAS) staining, showing PAS-positive nodular mesangial deposits and glomerular basement membrane thickening (c) Kidney biopsy with Congo red staining showing Congo red-negative mesangial deposits. (d) and (e) Immunofluorescence analyses of the kidney biopsy using anti-kappa and anti-lambda antibodies showing mild monotypic kappa light chain staining on glomerular deposits. Scale bars 100 μm.
Figure 2.
(a) and (b) Electron microscopy analysis of the kidney biopsy showing homogeneous microgranular osmiophilic deposits (∗) located in the mesangium, the glomerular basement membranes (a) and in the tubular basement membranes (b). (c) and (d) Immunogold studies with anti-epsilon antibody showing a significant concentration of gold beads on glomerular deposits in (c) mesangium and (d) in glomerular basement membranes. CL: capillary lumen US: urinary space.
Figure 3.
(a) Mass spectrometry analysis results showing Ig epsilon heavy chain as the dominant component of glomerular deposits associated with kappa light chain. (b) Schematic representations of the patient’s immunoglobulin heavy constant epsilon gene structure with 5 codons deletion (15 base pairs) in the exon 1 (chr14, 105601667, CH1).
Bone marrow smears revealed 14% infiltration with dystrophic kappa-positive plasma cells. Of particular interest, next-generation sequencing analysis of the immunoglobulin heavy constant epsilon gene revealed a partial deletion (15 base pairs, 5 codons) of the exon 1 (chr14, 105601667) corresponding to the first constant domain of the IgE HC protein (Figure 3b). Molecular studies also showed a t(11;14) translocation and a KRAS subclonal mutation. No copy number alteration, del(17p), and t(4;14) translocation were detected. Overall, next-generation sequencing results were considered favorable (Supplementary Methods).
The patient was treated with 4 cycles of daratumumab, bortezomib, lenalidomide, and dexamethasone followed by high-dose melphalan (140 mg/m2) with autologous stem cell transplantation 5 months after diagnosis. Thereafter, she received 2 additional cycles of daratumumab, bortezomib, lenalidomide, and dexamethasone as consolidation followed by lenalidomide maintenance. At last follow-up (16 months after diagnosis), the patient is still on lenalidomide maintenance, and at least in very good partial response with negative immunofixation on the serum and urine, normal free LC levels, and normal free LC ratio. Serum creatine remained stable at 130 μmol/l and no proteinuria was detected.
Discussion
LHCDD and HCDD are rare forms of MIDD characterized by tissue deposits of a truncated monoclonal immunoglobulin HC with or without an LC.2,3 These 2 entities account for less than 10% of all MIDD and are predominantly composed of monoclonal kappa LC and gamma HC deposits (>80%) with an overrepresentation of the gamma-1 HC subclass.3,6
Plasma cell dyscrasias resulting in the production of monoclonal IgE is a very rare condition with IgE multiple myeloma accounting for only 0.01% of all patients with myeloma.7 This very low prevalence of IgE secreting plasma cell clonal disorders might explain the fact that IgE-associated HCDD and LHCDD, to the best our knowledge, have not been reported since the first report of HCDD 30 years ago.8
The diagnosis of LHCDD relies on detailed studies of kidney biopsy samples, the most important steps being immunofluorescence and electron microscopy.2 In difficult cases, more sensitive techniques, such as immunoelectron microscopy and proteomic analysis after laser microdissection should be considered. Tissue proteomics have emerged as a new investigative tool for renal biopsy analysis and allow to refine the diagnosis of a number of renal diseases and to deepen their pathogenesis.9 In our case, laser microdissection and mass spectrometry analysis of the glomerular deposits further allowed us to confirm the precise composition of the observed deposits by identifying Ig epsilon HC as the highest enriched component in glomerular deposits. Immunoelectron microscopy confirmed these data with clear demonstration that staining of gold-conjugated anti-epsilon and anti-kappa antibodies was restricted to glomerular and tubular deposits. These data are in line with the high diagnostic performance rate reported with the combined use of proteomics and immunoelectron microscopy for identifying amyloid deposits.10
The deposits in HCDD are composed of the Ig HC, which typically lacks the first constant domain.4,6 Deletion of this first constant domain results in failure to bind with the chaperone protein BiP, leading to the secretion and renal deposition of truncated monoclonal free HCs.S3 Consistent with Bridoux et al.4 report, the observed 5 codons deletion in our patient, was probably sufficient to allow secretion of the nephrotoxic free epsilon HC by plasma cells.
For MIDD, as in other monoclonal gammopathy of renal significance-related renal diseases, clone-directed therapy with bortezomib-based regimens has been shown to produce high rates of hematologic responses resulting in favorable patient and renal outcomes. In the largest cohort of MIDD reported by Joly et al.,3 among 169 patients who received chemotherapy based on novel antimyeloma agents, 52% achieved a very good partial hematological response whereas renal response occurred in 62 patients (36%). In this study, renal survival at 36 months was 86% in patients with MIDD and was associated with the quality of hematological response and with renal response.
In conclusion, we report herein the first IgE kappa LHCDD case, adding to the spectrum of renal lesions associated with monoclonal gammopathy of renal significance. This case illustrates the importance of in-depth pathologic analysis combined with thorough hematological workup to accurately establish the diagnosis. In monoclonal gammopathy of renal significance, introduction of clone-targeted chemotherapy before the development of advanced kidney failure remains the current standard of care.
Disclosure
All the authors declared no competing interests
Author Contribution
PI analyzed histopathological findings, analysis, and interpretation of the data, and wrote the manuscript. NB, DS, and AK took care of the reported patient, analysis, and interpretation of the data, and editing of the manuscript. AR, GT, CO, SK, GT, FB and JMG performed electron microscopy and immunogold studies, analysis, and interpretation of the data, and editing of the manuscript. MC performed mass spectrometry-based proteomic analysis, analysis, and interpretation of the data, and editing of the manuscript. LDSF and HAL performed bone marrow molecular studies, analysis, and interpretation of the data, and editing of the manuscript. MR analyzed histopathologic findings, analysis, and interpretation of the data, and editing of the manuscript. All the authors facilitated the study and declare they have seen and approved the final version of the manuscript.
Footnotes
Supplementary Material
Supplementary Methods.
Supplementary References.
References
- 1.Randall R.E., Williamson W.C., Mullinax F., Tung M.Y., Still W.J. Manifestations of systemic light chain deposition. Am J Med. 1976;60:293–299. doi: 10.1016/0002-9343(76)90440-x. [DOI] [PubMed] [Google Scholar]
- 2.Leung N., Bridoux F., Nasr S.H. Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384:1931–1941. doi: 10.1056/nejmra1810907. [DOI] [PubMed] [Google Scholar]
- 3.Joly F., Cohen C., Javaugue V., et al. Randall-type monoclonal immunoglobulin deposition disease: novel insights from a nationwide cohort study. Blood. 2019;133:576–587. doi: 10.1182/blood-2018-09-872028. [DOI] [PubMed] [Google Scholar]
- 4.Bridoux F., Javaugue V., Bender S., et al. Unravelling the immunopathological mechanisms of heavy chain deposition disease with implications for clinical management. Kidney Int. 2017;91:423–434. doi: 10.1016/j.kint.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Royal V., Quint P., Leblanc M., et al. IgD heavy-chain deposition disease: detection by laser microdissection and mass spectrometry. J Am Soc Nephrol. 2015;26:784–790. doi: 10.1681/ASN.2014050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasr S.H., Valeri A.M., Cornell L.D., et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7:231–239. doi: 10.2215/CJN.08640811. [DOI] [PubMed] [Google Scholar]
- 7.Kyle R.A., Gertz M.A., Witzig T.E., et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins D., Wolever T., Rao A.V., et al. TThe new England journal of medicine nejm.org on March 29. N Engl J Med 1993. 2011;329:21–26. doi: 10.1056/NEJM199307013290104. https://www.nejm.org/doi/full/10.1056/NEJM199311043291905 For personal use only. No other uses without permission. [DOI] [PubMed] [Google Scholar]
- 9.Sedor J.R. Tissue proteomics: a new investigative tool for renal biopsy analysis. Kidney Int. 2009;75:876–879. doi: 10.1038/ki.2009.54. [DOI] [PubMed] [Google Scholar]
- 10.Abildgaard N., Rojek A.M., Møller H.E.H., et al. Immunoelectron microscopy and mass spectrometry for classification of amyloid deposits. Amyloid. 2020;27:59–66. doi: 10.1080/13506129.2019.1688289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.