To the Editor:
In this journal, we recently reported a novel form of renal tubular acidosis (RTA), which we termed Type V RTA, associated with immune checkpoint inhibitors (ICIs).1 We demonstrated that ICI-associated RTA was a unique immune-related adverse event.
Rechallenge with an ICI in more common kidney-limited immune-related adverse events, such as interstitial nephritis, has been debated but may be acceptable on the basis of risks and benefits.2 However, little is known about rechallenge in a rare immune-related adverse event, such as ICI-associated RTA. Here, to the best of our knowledge, we report the first case of ICI rechallenge in ICI-associated RTA.
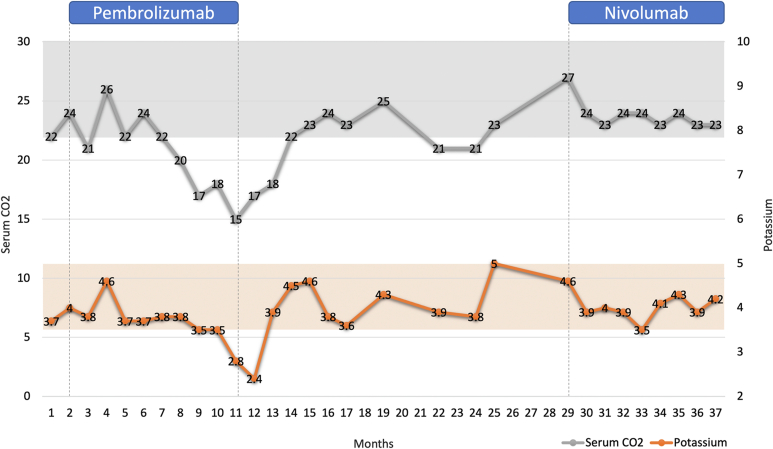
A 46-year-old woman with metastatic lung cancer and squamous cell cancer of the tonsil developed RTA after 6 months of anti-programmed death-1 (PD1) immunotherapy, pembrolizumab, that resolved after pembrolizumab discontinuation, as reported previously.1 One year later, the patient developed a progression of her disease. Because of the excellent antitumor response to ICI therapy previously, the decision was made to treat her with a different ICI anti-PD1 immunotherapy, nivolumab. During 8 months of follow-up, she has not developed recurrent Type V RTA (Figure 1).
Figure 1.
Rechallenge with a different immune checkpoint inhibitor (ICI) anti-PD1 immunotherapy, nivolumab, did not lead to a recurrence of ICI-associated renal tubular acidosis (ICI-RTA).
Our findings highlight 2 important findings. First, ICI rechallenge with a different ICI may be safe and not result in the recurrence of the RTA. Second, the finding that one ICI, but not another, both of which had anti-PD1 actions, induced RTA suggests that this effect may be an idiosyncratic effect and not a direct effect of the anti-PD1 action on kidney tubules. We speculated previously that interference of the proximal tubule ammoniagenesis as the likely mechanism of ICI-induced Type V RTA.1 Still, the factors leading to such a condition that only affects certain patients remain unidentified. Further studies are warranted to better understand the pathophysiological mechanism of this condition.
References
- 1.Shah C.V., Lee H.W., Clapp W.L., Weiner I.D. A novel form of renal tubular acidosis associated with immune checkpoint inhibitors. Kidney Int Rep. 2023;8:197–201. doi: 10.1016/j.ekir.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirali A.C. Is rechallenge appropriate in patients that develop immune checkpoint inhibitor-associated AKI?: commentary. Kidney360. 2022;3:806–808. doi: 10.34067/KID.0005592021. [DOI] [PMC free article] [PubMed] [Google Scholar]