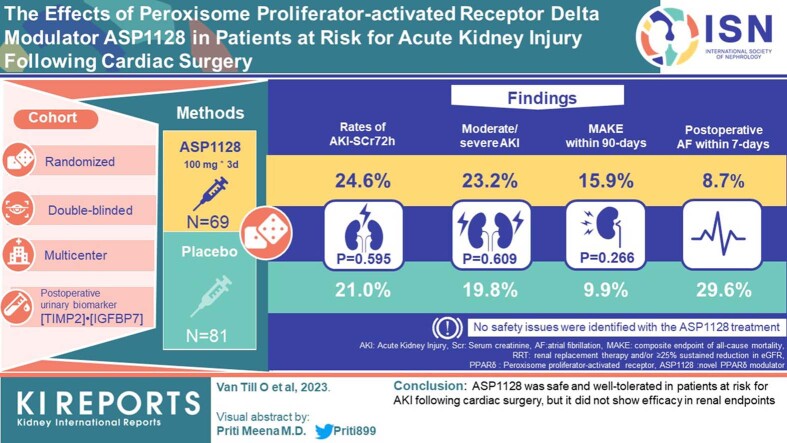
Abstract
Introduction
Peroxisome proliferator-activated receptor δ (PPARδ) plays a central role in modulating mitochondrial function in ischemia-reperfusion injury. The novel PPARδ modulator, ASP1128, was evaluated.
Methods
A randomized, double-blind, placebo-controlled, biomarker assignment-driven, multicenter study was performed in adult patients at risk for acute kidney injury (AKI) following cardiac surgery, examining efficacy and safety of a 3-day, once-daily intravenous dose of 100 mg ASP1128 versus placebo (1:1). AKI risk was based on clinical characteristics and postoperative urinary biomarker (TIMP2)•(IGFBP7). The primary end point was the proportion of patients with AKI based on serum creatinine within 72 hours postsurgery (AKI-SCr72h). Secondary endpoints included the composite end point of major adverse kidney events (MAKE: death, renal replacement therapy, and/or ≥25% reduction of estimated glomerular filtration rate [eGFR]) at days 30 and 90).
Results
A total of 150 patients were randomized and received study medication (81 placebo, 69 ASP1128). Rates of AKI-SCr72h were 21.0% and 24.6% in the placebo and ASP1128 arms, respectively (P = 0.595). Rates of moderate/severe AKI (stage 2/3 AKI-SCr and/or stage 3 AKI-urinary output criteria) within 72 hours postsurgery were 19.8% and 23.2%, respectively (P = 0.609). MAKE occurred within 30 days in 11.1% and 13.0% in the placebo and ASP1128 arms (P = 0.717), respectively; and within 90 days in 9.9% and 15.9% in the placebo and ASP1128 arms (P = 0.266), respectively. No safety issues were identified with ASP1128 treatment, but rates of postoperative atrial fibrillation were lower (11.6%) than in the placebo group (29.6%).
Conclusion
ASP1128 was safe and well-tolerated in patients at risk for AKI following cardiac surgery, but it did not show efficacy in renal endpoints.
Keywords: acute kidney injury, cardiac surgical procedures, controlled trial, PPAR delta, randomized, reperfusion injury
Graphical abstract
See Commentary on Page 1287
AKI is a clinical syndrome characterized by rapid loss of kidney function and is associated with increased short-term and long-term morbidity and mortality. AKI etiology is often multifactorial, but renal ischemia and reperfusion injury is a common cause of AKI.1,2 Renal tubular cells rely on oxidative phosphorylation to provide energy, and mitochondrial dysfunction has been recognized as a key factor in the progression of tubular damage in AKI.3,4 In preclinical studies, PPARδ modulation has been demonstrated to increase the expression of mitochondrial associated genes, resulting in an increase in mitochondrial function through increased fatty acid oxidation, as well as decreases in inflammation and fibrosis.5,6 ASP1128, also known as MA-0217, is a new molecular entity and a potent and selective PPARδ modulator. The nonclinical pharmacology data generated with ASP1128 show that it prevents and reduces the impact of ischemia or reperfusion-induced AKI and increases expression of PPARδ target genes, including mitochondrial function-related genes, in the blood and kidney.7,8
ASP1128 had a favorable safety profile and pharmacokinetics in the healthy human volunteer phase-1 study (NCT04742517) with doses up to 100 mg, and showed consistent treatment-related and dose-related upregulation of PPARδ/fatty acid oxidation target genes at least 24 hours after administration.9 The current clinical trial was designed to examine the effects of postsurgery treatment with ASP1128 in patients at risk for AKI following cardiac surgery to prevent and reduce severity of AKI.
Methods
Study Design, Patients, and Outcomes
The study was a double-blind, placebo-controlled, randomized, biomarker-driven, multicenter trial (1128-CL-0201; NCT03941483),10 and was conducted between November 1, 2019 and October 20, 2021 in 32 sites in the USA. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by an independent ethics committee at each study site. All enrolled patients provided signed informed consent before enrollment.
Patients in this study were adult patients undergoing nonemergent coronary artery bypass grafting and/or valve surgery within 4 weeks of screening and had an increased risk for developing AKI postsurgery (selection criteria are detailed in Supplementary Table S1). At screening or baseline, patients were required to have at least 1 AKI risk factor (age ≥70 years, eGFR <60 ml/min per 1.73 m2, congestive heart failure, diabetes mellitus, and/or proteinuria). And for randomization after surgery, an increased level of a tubular stress urinary biomarker, T2•I7, which is associated with increased risk for AKI, was required. T2•I7 is the product of the concentrations of TIMP2 and IGFBP7. T2•I7 was measured using NephroCheck® test on ASTUTE140 Meter platform (bioMérieux SA, Marcy l’Etoile, France), and labeled as Investigational Use Only to reflect its usage for patient selection in an interventional study. For T2•I7, a cutoff of 0.3 (ng/ml)2/1000 yielded good sensitivity for increased risk for developing moderate to severe AKI after cardiopulmonary bypass pump (CPB).11,12 To enrich the event rate of AKI endpoints, patients with a T2•I7 >0.3 within 2 to 22 hours after CPB were randomized to study the treatment of an intravenous dose of 100 mg ASP1128 or a matching placebo once daily for 3 days (first dose: <24 hours post-CPB; second dose: 24 to <48 hours post-CPB; third dose: 48–72 hours post-CPB). Randomization (1:1) was stratified for eGFR at screening <45 ml/min per 1.73 m2. Patients with a T2•I7 ≤0.3 at the postsurgery assessment were enrolled in a parallel observational cohort to allow for further evaluation of risk factors for AKI and MAKE (composite end point of all-cause mortality, renal replacement therapy and/or ≥25% sustained reduction in eGFR compared with baseline). The design of the study is detailed in Supplementary Figure S1.
The primary efficacy end point was the proportion of patients developing AKI (any severity stage) based on serum creatinine (SCr) Kidney Disease Improving Global Outcomes criteria;13 that is, increase in SCr ≥ 0.3 mg/dl (≥26.5 μmol/l) within any 48 hours, or increase in SCr to ≥1.5 times baseline13 within 72 hours after end of CPB (AKI-SCr72h).
The secondary or other endpoints included additional AKI definitions (with or without urinary output criteria) and timing (at 7 days postsurgery), AKI severity (stage 1, 2, and 3, i.e., mild, moderate, and severe AKI13), AKI duration, and proportion of patients with MAKE at 30 days and 90 days postsurgery. Serum levels of creatinine, cystatin-C, and derived eGFRs were monitored to examine kidney function, as well as the kidney damage biomarker, kidney injury molecule-1. Distribution of AKI risk factors at baseline as defined by the Kidney Disease Improving Global Outcomes AKI guideline13 were examined.
Safety and tolerability of ASP1128 were evaluated using the nature, frequency, and severity of adverse events (AEs; Medical Dictionary for Regulatory Activities [MedDRA] version 23.0), vital signs, and safety laboratory tests (biochemistry, hematology, and urinalysis). Treatment-emergent AEs (TEAEs) were defined as AEs with onset after the first dose and up to the day 30 visit.
ASP1128 plasma concentrations and pharmacodynamics (target gene expression) were assessed. The following 7 PPARδ and fatty acid oxidation target genes were examined in mRNA samples extracted from whole blood predose and postdose at baseline (visit 2) and day 3 of dosing (visit 4) using NanoString technology:14 (i) acetyl-coenzyme A acyltransferase 2, (ii) adenosine triphosphate binding cassette subfamily A member 1, (iii) acyl-CoA dehydrogenase very long chain, (iv) catalase, (v) carnitine palmitoyltransferase 1A, (vi) pyruvate dehydrogenase lipoamide kinase isozyme 4, and (vii) solute carrier family 25 member 20.
Dose Rationale
The 100 mg dose was considered safe and well tolerated in the phase-1 study 1128-CL-0101 with up to 7 days multiple dosing (3–100 mg) in healthy nonelderly and elderly patients, and ASP1128 plasma concentration increased dose proportionally.9 PPARδ or fatty acid oxidation target gene upregulation revealed optimal results for 100 mg at all time points in single and multiple dosing.9 The treatment period up to 72 hours postsurgery and related gene upregulation was assumed to cover the period when AKI usually occurs in relation to injury sustained during cardiac surgery, and the peak of SCr and related biomarkers.15,16 Start of treatment was related to the increase of T2•I7, marking the start of renal stress when kidney function is affected but may still recover without permanent injury to the organ,17 that is, hypothetically preventing sustained cell/tissue damage. It was presumed that treatment should also not start too early, that is, before the tubular cells adapt naturally to the ischemic condition, because forestalling this adaptive advantage with induction of PPARδ is known to impede recovery.18, 19, 20, 21
Statistical Methodology
The planned sample size of the randomized cohort was 220 patients (110 patients/arm), to provide 80% power to detect a 30% reduction of the primary end point between study groups, assuming AKI-SCr72h proportion is 60% in the placebo group and 42% in the ASP1128 group. Assumed dropout rate was 10% and 1-sided significance level was 0.05. The assumed placebo group AKI event rate was based on results of a comparable interventional study (50% AKI-SCr in placebo group)22; the T2•I7 >0.3 randomization requirement was assumed to increase the event rate conservatively by 10%.17 The maximum sample size for the observational cohort was capped at 440 patients. An interim futility analysis by a data monitoring committee was planned when 60% of patients with primary end point data were enrolled, utilizing futility stop criteria as follows: when conditional power to detect the difference between ASP1128 and placebo on the primary end point of AKI-SCr72h proportion is <10%, that is, the 2-sided P-value is >0.518 and/or the common risk ratio (ASP1128/placebo) is >1, the study may stop.
The efficacy endpoint proportions were analyzed in the full analysis set (all randomized patients who received at least 1 dose of study treatment) using a chi-square test. The hypothesis testing on the primary analysis was performed at a 2-sided 0.10 significance level to test the null hypothesis that the AKI-SCr72h proportion is equal between the 2 study arms.
The estimate of the unadjusted risk ratio and 2-sided 90% confidence interval for the efficacy endpoints were calculated for the following prespecified subgroups: age (<70 years or ≥70 years), diabetes mellitus type 1 or 2, sex, kidney failure (eGFR at presurgery baseline [<45 or ≥45, <60 or ≥60]), surgery type (lower risk vs. higher risk surgery), T2•I7 at randomization (>0.3–0.7, >0.7 [alternative cutoff associated with stage 2 or 3 AKI]23), IV radio-contrast, peripheral arterial disease, cardiac function (ejection fraction% <40 or ≥40), duration of aortic cross-clamp, duration of CPB (<median or ≥median), and time of first dose (< 8 h or ≥8 h postsurgery).
Results
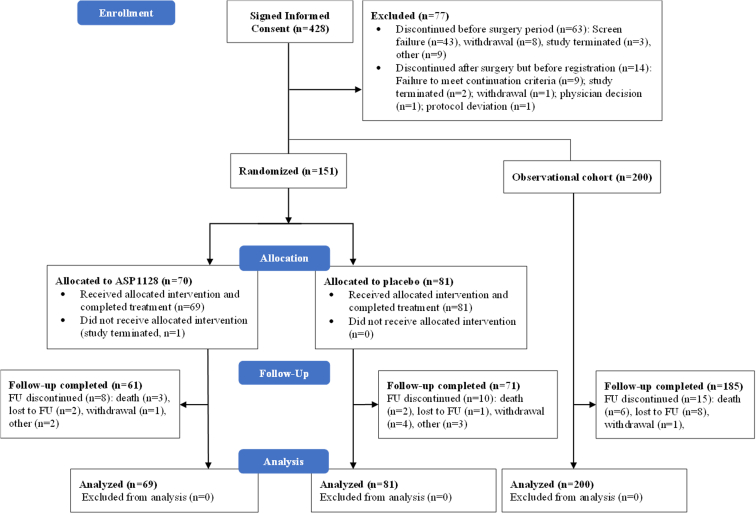
The interim analysis for futility was conducted with results from 133 randomized patients. The data monitoring committee recommended study discontinuation because the results met the futility stop criteria. Based on this, it was decided to stop enrollment and complete follow-up for the patients already enrolled. In total, 428 patients were screened, 151 patients were randomized, and 200 were enrolled in the observational cohort (Figure 1). The full analysis set comprised of 150 patients (69 in the ASP1128 arm and 81 in the placebo arm). The follow-up completion rate for all patients in the full analysis set was 88.4% (61/69) for the ASP1128 arm and 87.7% (71/81) for the placebo arm.
Figure 1.
Subject disposition, CONSORT flow diagram.
Demographic and baseline characteristics are detailed in Table 1. The percentage of female patients was higher in ASP1128 arm compared with placebo arm. At screening, chronic kidney disease was reported more often in the placebo arm, and peripheral arterial disease was reported more in the ASP1128 group. Surgical parameters were comparable, except that there were more patients undergoing mitral and tricuspid valve replacement in the ASP1128 group (Table 1). Baseline AKI risk factors were overall balanced between groups (Supplementary Table S2), except for female sex (8.6% placebo, 23.2% ASP1128) and chronic kidney disease (25.9% placebo, 8.7% ASP1128).
Table 1.
Baseline demographic and surgery characteristics
| Characteristics | Placebo (n = 81) | ASP1128 (n = 69) | Observational cohort (n = 200) |
|---|---|---|---|
| Demography/medical history | |||
| Age (yr) | 68.7 (9.4) | 68.0 (8.3) | 69.4 (8.8) |
| Sex (females) | 7 (8.6) | 16 (23.2) | 65 (32.5) |
| Weight (kg) | 95.9 (19.8) | 94.9 (22.4) | 92.1 (19.2) |
| LVEF (%) | 51.6 (11.5) | 53.8 (9.1) | 55.1 (10.3) |
| PAD at screening | 10 (12.3) | 15 (21.7) | 20 (10.0) |
| CKD at screening | 21 (25.9) | 6 (8.7) | 37 (18.5) |
| Surgery parameters | |||
| CABG (no valve surgery) | 49 (60.5) | 34 (49.3) | 122 (61.0) |
| CABG + valve surgery | 18 (22.2) | 12 (17.4) | 33 (16.5) |
| Valve surgery (no CABG) | 14 (17.3) | 23 (33.3) | 45 (22.5) |
| Mitral valve | 12 (14.8) | 21 (30.4) | 28 (14.0) |
| Aortic valve | 26 (32.1) | 17 (24.6) | 56 (28.0) |
| Tricuspid valve | 5 (6.2) | 11 (15.9) | 4 (2.0) |
| CPB (h) | 2.15 (0.93) | 2.24 (1.26) | 1.87 (0.72) |
| Aortic cross-clamping (h) | 1.52 (0.64) | 1.62 (0.87) | 1.40 (0.54) |
| Volume of blood loss during surgery (ml) | 481.7 (562.0) | 412.7 (455.2) | 351.9 (381.7) |
| Baseline kidney function laboratory values | |||
| T2.I7 ([ng/ml]2/1000) | 0.70 (0.66) | 0.69 (0.50) | 0.13 (0.07) |
| Serum creatinine (mg/dl) | 0.99 (0.25) | 0.90 (0.23) | 0.96 (0.26) |
| eGFR based on SCra (ml/min per 1.73 m2) | 76.9 (18.8) | 82.5 (16.7) | 75.9 (18.8) |
| eGFR based on cystatin-Cb (ml/min per 1.73 m2) | 66.2 (22.1) | 72.6 (22.6) | 62.2 (19.5) |
CABG, coronary artery bypass grafting; CKD, chronic kidney disease; CPB, cardio-pulmonary bypass pump; LVEF, left ventricular ejection fraction; PAD, peripheral arterial disease; SCr, serum creatinine; SD, standard deviation.
Mean (SD) for continuous parameters, N (%) for categorical parameters.
Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation13.
2012 CKD-EPI cystatin-C equation13.
The primary efficacy end point of AKI-SCr72h proportion was 24.6% for the ASP1128 arm and 21.0% for the placebo arm (Table 2), with a risk ratio of 1.174 (90% confidence interval: 0.715, 1.927; P = 0.595). No differences between groups were observed in prespecified subgroups. For example, for patients with early first dose (start <8 hours postsurgery) AKI-SCr72h rates were 25.6% and 21.7%; and for those with later first dose (start >8 hours postsurgery) 23.1% and 20.0% for ASP1128 and placebo, respectively. No statistically significant differences were observed in the secondary or other efficacy endpoints (Table 2).
Table 2.
Results of primaryd, secondarye (as defined in the study protocol) and other efficacy outcomes, N (%)
| Outcome parameter | Placebo (N = 81) | ASP1128 (N = 69) | P-valuea | Risk ratio (90% CI)b | Observational cohort (N = 200) |
|---|---|---|---|---|---|
| AKI-SCr72hd | 17 (21.0) | 17 (24.6) | 0.595 | 1.174 (0.715, 1.927) | 70 (35.0) |
| AKI stage 1 | 16 (19.8) | 11 (15.9) | NDc | ND | 63 (31.5) |
| AKI stage 2 | 0 (0) | 4 (5.8) | NDc | ND | 7 (3.5) |
| AKI stage 3 | 1 (1.2) | 2 (2.9) | NDc | ND | 0 (0) |
| AKI-SCr72h stage 2/3 | 1 (1.2) | 6 (8.7) | 0.031 | 7.043 (1.217, 40.780) | 7 (3.5) |
| AKI-KDIGO72he | 63 (77.8) | 55 (79.7) | 0.773 | 1.025 (0.891, 1.179) | ND |
| AKI-UO72h stage 3 | 16 (19.8) | 14 (20.3) | 0.935 | 1.027 (0.600, 1.760) | ND |
| AKI-SCr72h stage 2/3 and/or AKI-UO72h stage 3 | 16 (19.8) | 16 (23.2) | 0.609 | 1.174 (0.701, 1.965) | ND |
| AKI-SCr7de | 19 (23.5) | 21 (30.4) | 0.335 | 1.297 (0.831, 2.026) | 74 (37.0) |
| AKI-KDIGO7de | 69 (85.2) | 61 (88.4) | 0.563 | 1.038 (0.935, 1.152) | ND |
| MAKE30, all criteriae | 9 (11.1) | 9 (13.0) | 0.717 | 1.174 (0.567, 2.429) | 14 (7.0) |
| All-cause mortality | 0 (0) | 2 (2.9) | 0.123 | ND | 1 (0.5) |
| RRT | 2 (2.5) | 2 (2.9) | 0.871 | 1.174 (0.232, 5.948) | 4 (2.0) |
| Sustained eGFR reduction | 8 (9.9) | 7 (10.1) | 0.956 | 1.027 (0.458, 2.303) | 11 (5.5) |
| MAKE90, all criteriae | 8 (9.9) | 11 (15.9) | 0.266 | 1.614 (0.789, 3.300) | 13 (6.5) |
| All-cause mortality | 2 (2.5) | 3 (4.3) | 0.523 | 1.761 (0.402, 7.713) | 5 (2.5) |
| RRT | 2 (2.5) | 2 (2.9) | 0.871 | 1.174 (0.232, 5.948) | 4 (2.0) |
| Sustained eGFR reduction | 5 (6.2) | 7 (10.1) | 0.371 | 1.643 (0.652, 4.143) | 5 (2.5) |
AKI, acute kidney injury; CI, confidence interval; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes; ND, not done; RRT, renal replacement therapy.
AKI-KDIGO72h, AKI as defined by all KDIGO criteria13 within 72 h after surgery.
AKI-KDIGO7d, AKI as defined by all KDIGO criteria13 within 7 days after surgery.
AKI-SCr7d, AKI as defined by KDIGO based on serum creatinine criteria13 within 7 days after surgery.
AKI-UO72h stage 3, AKI as defined by KDIGO based on urinary output criteria13 severity stage 3 within 72 h after surgery.
MAKE30, major adverse kidney events (all-cause mortality, RRT and/or sustained eGFR reduction [i.e., ≥25% reduction of eGFR-SCr compared with baseline]) within 30 days after surgery.
MAKE90, MAKE within 90 days after surgery.
2-sided P-value (chi-square test).
Risk ratio to placebo (2-sided 90% CI for risk ratio).
Wilcoxon-test distribution severity categories (no AKI, stage 1–3): P = 0.461.
Primary efficacy outcome.
Secondary efficacy outcomes.
Although the distribution of AKI-SCr72h severity stages (0–3) was not different between groups (P = 0.461), more patients with moderate/severe AKI-SCr72h (stage 2/3 Kidney Disease Improving Global Outcomes) were observed in the ASP1128 arm (8.7% [n = 6; stage 2 n = 4, stage 3 n = 2]) than in the placebo arm (1.2% [n = 1; stage 2 n = 0, stage 3 n = 1], P = 0.031). In patients with postsurgery T2•I7 value >0.7 (ng/ml)2/1000 (19 placebo, 22 ASP1128), stage 2/3 AKI-SCr72h were observed in 1 placebo-treated patient (stage 3) and in 2 ASP1128-treated patients (1 stage 2, 1 stage 3). Rates of clinically relevant moderate/severe AKI (stage 2/3 AKI-SCr and/or stage 3 AKI based on urinary output criteria) within 72 hours postsurgery24 were 23.2% in the ASP1128 treatment arm and 19.8% in the placebo treatment arm (P = 0.609). Most, if not all, patients with stage 2/3 AKI-SCr72h had confounders for AKI such as perioperative and postoperative complications of cardiac surgery (Supplementary Table S3). No relevant differences in rates of MAKE or the individual composites were observed between groups (Table 2).
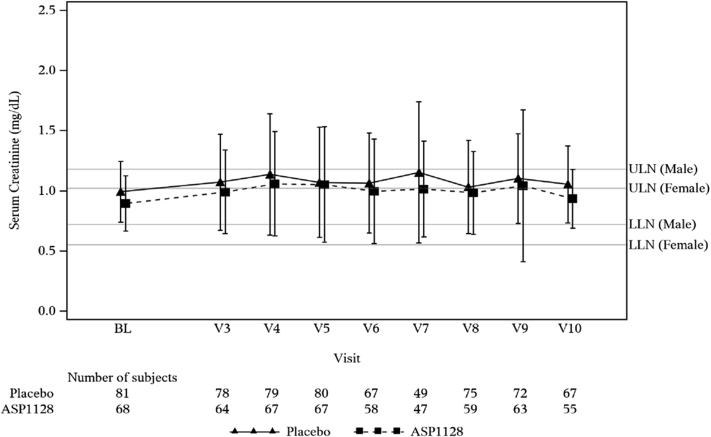
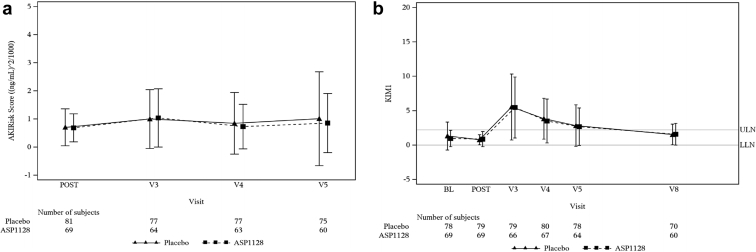
There were no significant differences between groups in SCr values (Figure 2). The area under the curve of SCr values from baseline to 72 hours after surgery was 3.05 (SD 1.03) ASP1128 versus 3.26 (SD 1.19) placebo; mean difference −0.21 (90% confidence interval: −0.52, 0.09; P = 0.255); at 7 days after surgery that was 5.69 (SD 2.35) versus 5.96 (SD 2.52), respectively; mean difference −0.27 (90% confidence interval: −0.93, 0.40); P = 0.508. Similarly, no relevant differences were observed between groups in eGFR, cystatin-C, and blood urea nitrogen levels. The number of patients with renal recovery (defined as SCr at Day 7 or discharge ≤ baseline) was 22 (31.9%) in the ASP1128 arm versus 21 (25.9%) in the placebo arm (P = 0.421). Values in kidney stress and damage biomarkers did not show differences (Figure 3).
Figure 2.
Mean (± SD) values of serum creatinine (mg/dl). BL, Day 1 of surgery presurgery baseline; LLN, lower limit of normal; V3, visit 3/day 2 (24 h after surgery); V4, visit 4/day3 (48 h after surgery); V5, visit 5/day 4 (72 h after surgery); V6, visit 6/day 5; V7, visit 7/day 6; V8, visit 8/day 7/day of discharge; V9, visit 9/day 30; V10, visit 10/day 90; ULN, upper limit of normal.
Figure 3.
Mean (± SD) values of (a) kidney stress (T2•I7 AKIRisk score), and (b) damage (KIM-1) biomarker levels. BL, Day 1 of surgery presurgery baseline; LLN, lower limit of normal; POST, day 1 of surgery postsurgery assessment pretreatment; V3, visit 3/day 2 (24 h after surgery); V4, visit 4/day 3 (48 h after surgery); V5, visit 5/day 4 (72 h after surgery); V8, visit 8/day 7/day of discharge. KIM-1, kidney injury molecule-1; ULN, upper limit of normal.
The percentage of patients with AKI-SCr72h in the observational cohort was 35.0% (Table 2); 7 (3.5%) had stage 2/3 AKI and 63 (31.5%) had stage 1 AKI, compared with 7 of 150 (4.7%) and 27 of 150 (18.0%) randomized patients, respectively. The rate of MAKE was 7.0% (Day 30) and 6.5% (Day 90), compared with 18 of 150 (12.0%) and 19 of 150 (12.7%) randomized patients.
Pharmacokinetics and Pharmacodynamics
ASP1128 plasma concentrations at the end of infusion for the first, second, and third administrations were similar. ASP1128 plasma concentrations decreased rapidly from the end of infusion to 4 to 12 hours postdose. Plasma exposures of 100 mg dose in the current patient population were comparable with 100 mg dose in phase-1 healthy volunteers (Data not shown).
Pharmacodynamic analyses showed that ASP1128 treatment significantly increased target gene expression from (postsurgery pretreatment) baseline at 1 or more timepoints of adenosine triphosphate binding cassette subfamily A member 1, acetyl-coenzyme A acyltransferase 2, acyl-CoA dehydrogenase very long chain, carnitine palmitoyltransferase 1A, pyruvate dehydrogenase lipoamide kinase isozyme 4, and solute carrier family 25 member 20 compared with the placebo-treated patients. There were no treatment-related trends for changes in expression of catalase (Supplementary Figure S2).
Safety
The frequency of TEAEs (overall, drug-related, serious events) and severity of AEs were similar between treatment arms; 75.4% ASP1128 versus 72.8% placebo reported at least 1 TEAE, and 49.3% ASP1128 versus 51.9% placebo reported serious TEAEs. In 1 patient, treatment (ASP1128) was discontinued because of a TEAE of hepatic failure in the context of multiple organ failure. There were 5 patient deaths during the study period (multiple organ dysfunction [ASP1128; Day 5], hypotension [ASP1128; Day 28], sepsis [placebo; Day 42], unknown etiology [ASP1128; Day 48], and cardiac/respiratory failure [placebo; Day 66]). None of the deaths were considered related to the study drug. There were no clinically meaningful findings in the vital sign measurements or safety labs in this study. The most common TEAEs, occurring in at least 5% of patients in any randomized group, are presented in Table 3. The most relevant differences between groups were seen in atrial fibrillation, AKI, pleural effusion, and thrombocytopenia. Of these events, 1 atrial fibrillation (placebo) and 3 AKI (2 placebo, 1 ASP1128) were regarded as possibly related to the study drug by the investigator; all others were regarded as not related. A post hoc analysis was undertaken to evaluate TEAEs occurring during the early in-hospital period overlapping with study drug administration events reported within 7 days after surgery (Supplementary Table S4): atrial fibrillation (6 [8.7%] ASP1128 vs. 24 [29.6%] placebo), AKI (11 [15.9%] ASP1128 vs. 9 [11.1%] placebo), pleural effusion (4 [5.8%] ASP1128 vs. 2 [2.5%] placebo), and thrombocytopenia (6 [8.7%] vs. 2 [2.5%]). No differences between study groups were observed in mean/median levels of platelets (Supplementary Figure S3).
Table 3.
The most common treatment-emergent adverse events and serious adverse events, occurring in ≥5% of subjects in any randomized group
| Adverse event (MedDRA V23.0) preferred term | Placebo (N = 81) n (%) | ASP1128 (N = 69) n (%) | Total (N = 150) n (%) |
|---|---|---|---|
| TEAEs | |||
| Overall | 59 (72.8) | 52 (75.4) | 111 (74.0) |
| Atrial fibrillation | 24 (29.6) | 8 (11.6) | 32 (21.3) |
| Constipation | 13 (16.0) | 13 (18.8) | 26 (17.3) |
| Acute kidney injury | 10 (12.3) | 14 (20.3) | 24 (16.0) |
| Nausea | 8 (9.9) | 5 (7.2) | 13 (8.7) |
| Acute respiratory failure | 6 (7.4) | 6 (8.7) | 12 (8.0) |
| Pleural effusion | 2 (2.5) | 9 (13.0) | 11 (7.3) |
| Anemia | 7 (8.6) | 3 (4.3) | 10 (6.7) |
| Pneumonia | 5 (6.2) | 4 (5.8) | 9 (6.0) |
| Thrombocytopenia | 2 (2.5) | 7 (10.1) | 9 (6.0) |
| Hypokalemia | 3 (3.7) | 5 (7.2) | 8 (5.3) |
| Hypocalcemia | 2 (2.5) | 5 (7.2) | 7 (4.7) |
| Hypotension | 5 (6.2) | 2 (2.9) | 7 (4.7) |
| Blood loss anemia | 3 (3.7) | 4 (5.8) | 7 (4.7) |
| Insomnia | 5 (6.2) | 1 (1.4) | 6 (4.0) |
| Decreased appetite | 1 (1.2) | 4 (5.8) | 5 (3.3) |
| Treatment-emergent SAEs | |||
| Atrial fibrillation | 24 (29.6) | 8 (11.6) | 32 (21.3) |
| Acute kidney injury | 10 (12.3) | 14 (20.3) | 24 (16.0) |
| Acute respiratory failure | 6 (7.4) | 6 (8.7) | 12 (8.0) |
MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event; TEAE, treatment-emergent adverse events.
Comedication that could affect postoperative atrial fibrillation (POAF) occurrence was evaluated in post hoc analyses; the percentage of patients receiving prophylactic treatment with amiodarone (on Day 1) was comparable between groups (55.1% ASP1128 vs. 45.7% placebo). Occurrence of POAF within 7 days in patients without prophylactic amiodarone was 5 of 31 (16.1%) ASP1128 versus 20 of 44 (45.5%) placebo. The percentage of patients who used betablockers or calcium channel blockers within 72 hours after surgery was comparable between study groups as follows: 59.4% ASP1128 versus 60.5% placebo, and 53.6% ASP1128 versus 51.9% placebo, respectively.
Discussion
The current study examined the novel PPAR-δ modulator, ASP1128 in adult patients at risk for AKI following cardiac surgery defined by clinical criteria and biomarker testing. It was successfully completed despite the challenges caused by the COVID-19 pandemic.
Overall, ASP1128 did not reduce the rates or severity of AKI, or related long-term endpoints such as MAKE, in the enrolled patient population. In general, the AKI(-SCr)event rate was low in the study population, that is, approximately half of the rates in control groups of previous drug trials in cardiac surgery patients.22,25 In addition, the overall severity of AKI was low; rates of moderate/severe (stage 2/3) AKI(SCr) , which are associated with long-term outcomes,24 were 1.2% in the placebo group (11.5% in a previous trial22) versus 8.7% in the ASP1128 group, which are on par with rates reported in trials using a similar T2•I7 cutoff for inclusion (2.9–12.0%).11,26 The trend of difference in numbers of moderate/severe AKI between groups may be due to the effect of confounding postoperative events associated with the development of AKI and variability because of the smaller than anticipated sample size and event rate. An effect of the drug, that was safe and effective in preclinical studies,7,8 is less likely. The clinical relevance of this trend was low because it did not translate into any difference in longer-term outcomes on kidney function, such as rates of renal recovery, renal replacement therapy, and sustained eGFR reduction between groups. Regardless, the numbers of stage 2/3 AKI and MAKE were too small to make an informed conclusion, which is a key limitation of the current study.
In designing the study, it was anticipated that the postoperative T2•I7 requirement would enrich the AKI rate in the randomized population. However, overall AKI rates were surprisingly lower in the randomized cohort than in the observational cohort. This difference was limited to stage 1 AKI. The rates of stage 2/3 AKI and MAKE (especially sustained eGFR loss) were higher in the randomized cohort (stage 2/3 of all AKI: 7/34 randomized vs. 7/70 observational cohort). The negative predictive value for stage 2/3 AKI of the T2•I7 using a cutoff of 0.3 was high (96.5%: 193/200 observational cohort patients), which is consistent with the previous literature.23 Therefore, using the biomarker for enrollment did exclude patients at lower risk. To increase the positive predictive value, a higher T2•I7 cutoff value may be required (e.g., 0.7).23 However, the numbers in the subgroup of patients with prerandomization T2•I7 >0.7 were too small to allow a meaningful evaluation.
Except for the postoperative T2•I7 criterion, patients in the randomized and observational cohorts met the same selection criteria; therefore selection bias cannot explain the difference in AKI rates. A disbalance in sex proportions could also not explain this because a post hoc multiple logistic regression analysis did not indicate (female) sex to be associated with developing AKI. In the observational cohort, AEs and comedication were not recorded beyond 24 hours after surgery; therefore these could not be verified for confounding effects. The T2•I7 values used for randomization were known to the investigators; this information may have introduced bias because of differences in assumed risk for AKI affecting patient management. Previous studies showed that applying directed treatment in patients with a ‘positive’ T2•I7 signal (i.e., >0.3) reduced AKI rates following cardiac surgery.23,26 If treatment bias occurred in the current study, that is, supportive treatment to prevent AKI was provided (consciously or unconsciously) to ‘positive T2•I7’ at-risk patients and not to “negative T2•I7” lower-risk patients, it may explain the relatively overall lower rates of AKI in the randomized cohort. Unfortunately, this is difficult to verify because the study was not designed to evaluate this.
Based on the positive preclinical and phase-1 results,7, 8, 9 treatment effect was assumed for 100 mg ASP1128 in the clinical setting. Reasons for an absence of this treatment effect on renal outcomes of ASP1128 can be related to issues with the compound (dosing, timing of treatment), the study (disbalance in confounding factors, population, and sample size), or both. Unequal distribution between groups of baseline AKI risk factors (chronic kidney disease, female sex) was observed, but subgroup analyses correcting for these factors did not reveal any efficacy signals. Postbaseline factors in the ASP1128 group relating to postoperative complications may have confounded an AKI efficacy signal. Plasma exposures and target gene expression of the 100 mg dose in the study patients were comparable with those in phase-1 healthy volunteers, so the dose has shown significant target engagement. The 72-hour period of drug administration started at the onset of injury, as related to T2•I7 increase, and covered the peaks of kidney injury biomarkers; and most AKI occurred in the first 72 hours postsurgery. In subgroup analyses, no differences in AKI rates were observed if treatment started early or later. It is unlikely that the exposure, timing, and effect of the drug was insufficient to attenuate injury leading to AKI. However, in the study population, this injury may not have been sufficient quantitatively and/or qualitatively to allow an attenuative effect of ASP1128.
The reported primary end point event rate in the placebo group was lower than anticipated in the sample size calculation; therefore the power to assess a reduction of AKI rates with ASP1128 against placebo could be too low to be reliable. But the interim analysis result indicated that such an effect was unlikely, even with a larger sample size. This relatively low event rate and severity may be due to applying comparatively mild selection criteria. Teprasiran, a small interfering RNA inhibiting p53-mediated apoptosis, showed positive results in the phase-2 study with AKI rates and severity levels that were more than twice as high as in the current study.22 That study had a lower eGFR threshold for enrollment than the current study (≥20 vs. ≥30 ml/min per 1.73 m2) and required 2 or more AKI risk factors at screening in low-risk surgeries,22 whereas the current study required 1 AKI risk factor. Overall, good renal reserve of the study population at baseline reduces the risk of AKI,27 negatively impacting the sensitivity of SCr as the defining parameter of AKI. The baseline median eGFR in the current study was approximately 80 ml/min per 1.73 m2, whereas this was approximately 60 ml/min per 1.73 m2 in the teprasiran study; in addition, the SCr levels (baseline and changes from baseline) were lower.22 The follow-up phase-3 study with teprasiran, which had selection criteria comparable to the current study, was terminated because the primary end point (reduction of MAKE 90%) and secondary endpoints (e.g., reduction of AKI%) were not achieved despite the large sample size of 1043 enrolled patients.28,29 A probable reason for this is a floor effect, that is, AKI events in the phase-3 were too few and/or not severe enough to replicate positive results of the phase-2 study. The Statin AKI Cardiac Surgery trial verified the retrospective observation that perioperative atorvastatin treatment reduced AKI% following cardiac surgery; however, it failed to replicate this.30 This may have been due to the lack of efficacy of the intervention, but AKI rate and severity were also quite low, comparable to the current study (placebo group: AKI rate 19.5%; stage 2/3 2.6%).30 Although even mild AKI (i.e., stage 1), including transient oliguria, is associated with adverse long-term consequences, mild AKI has a better prognosis than moderate/severe AKI;24 therefore a treatment effect on long-term outcomes will be less pronounced and therefore more difficult to observe if the overall disease severity of the study population is low. The subgroup analysis in patients with a limited baseline kidney function (eGFR <60) did not show any differences between groups, but the sample size was too small (16 placebo, 7 ASP1128) to be conclusive. Whether ASP1128 would be beneficial in a population with more severe kidney damage remains to be determined.
The safety of the compound was acceptable, although some numerical differences in TEAEs between groups were observed. The numerical difference in thrombocytopenia events did not translate into a difference in average platelet levels, and no relevant differences were seen for AKI and pleural effusion concurrently with drug administration, so a direct drug effect causing these were less likely. For POAF, a common and relevant complication of cardiac surgery, the difference between groups was relevant; during the first 7 postoperative days, a 71% reduction in incidence was observed (numbers needed to treat: 5). In comparison, in a meta-analysis this was 48% for (preventive) beta-blocker treatment.31 The incidence of POAF in the placebo group is in line with previous studies,22,25 and the cardiac surgery population in general.32 The effect could not be explained by differences between groups in confounding factors, such as risk factors or comedications (POAF treatment was not regulated in the study); an effect of ASP1128 in reducing the incidence of POAF could not be excluded. Impaired mitochondrial metabolic function in response to cardiac ischemia-reperfusion is implicated in the etiology of POAF.32 PPARδ modulation could be of interest as a drug target in such injury, like in POAF and myocardial infarction. However, the current study was not designed to evaluate POAF, so the findings need to be verified in dedicated clinical studies.
In conclusion, treatment with the PPARδ modulator ASP1128 was safe and well tolerated but lacked efficacy to reduce postoperative AKI and MAKE in biomarker-positive cardiac surgery patients. A possible effect on reducing POAF could not be excluded.
Disclosure
JWOvT is an employee of Mitobridge. CH is an employee of Astellas Pharma US Inc. HN, CK, and TS are employees of Astellas Pharma Inc. Japan. HN, CK, and TS report Astellas stock shares. BAM was an advisory board member on this study for Astellas. ADS was an advisory board member on this study for Astellas and an advisory board member for AM Pharma, Novartis, and FAST Biomedical; and was president for the Society of Cardiovascular Anesthesiologists and director for Perioperative Quality Initiative. DTE participated on an advisory board for Edward Lifesciences, Elimu, Rockwell Medical, Astellas, Alexion, Terumo, and Medela; and a consultant role for Premier Healthcare, Renibus Therapeutics, and Guard Therapeutics. TBW has nothing to disclose. JAK was an advisory board member on this study for Astellas and received consulting fees from bioMérieux unrelated to this study. He is currently an employee of Spectral Medical.
Acknowledgments
The authors are grateful to the investigators and their staff, and the patients who contributed to this study. This study was funded by Astellas Pharma Inc. and investigators received funding to conduct this study from the study sponsor.
Data Availability Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Author Contributions
Project development was done by JWOvT, HN, CK, CH, TS, BAM, ADS, DTE, and JAK. Data analysis was conducted by JWOvT, CH, and TS. Manuscript writing was done by JWOvT, HN, CK, CH, TS, TBW, BAM, ADS, DTE, and JAK. Data collection and study execution was by JWOvT, HN, CK, CH, TS, and TBW. Critical revision of the manuscript was done by JWOvT, HN, CK, CH, TS, TBW, BAM, ADS, DTE, and JAK.
Footnotes
Figure S1. Study design flow chart.
Figure S2. Box plots of fold change from baseline of gene expression data.
Figure S3. Blood platelet count.
Table S1. Selection criteria for 1128-CL-0201.
Table S2. AKI risk factors at baseline; based on KDIGO AKI-guidelines.13
Table S3. All adverse events in patients with AKI-SCr72h stage 2/3.
Table S4. Treatment emergent adverse events of atrial fibrillation, acute kidney injury, pleural effusion, thrombocytopenia in relation to onset time period after surgery, N (% of total group).
CONSORT Checklist.
Supplementary Material
Figure S1. Study design flow chart.
Figure S2. Box plots of fold change from baseline of gene expression data.
Figure S3. Blood platelet count.
Table S1. Selection criteria for 1128-CL-0201.
Table S2. AKI risk factors at baseline; based on KDIGO AKI-guidelines.13
Table S3. All adverse events in patients with AKI-SCr72h stage 2/3.
Table S4. Treatment emergent adverse events of atrial fibrillation, acute kidney injury, pleural effusion, thrombocytopenia in relation to onset time period after surgery, N (% of total group).
CONSORT Checklist.
References
- 1.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre J.V., Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishimoto Y., Inagi R. Mitochondria: a therapeutic target in acute kidney injury. Nephrol Dial Transplant. 2016;31:1062–1069. doi: 10.1093/ndt/gfv317. [DOI] [PubMed] [Google Scholar]
- 4.Hall A.M., Schuh C.D. Mitochondria as therapeutic targets in acute kidney injury. Curr Opin Nephrol Hypertens. 2016;25:355–362. doi: 10.1097/MNH.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y.Z., Nikolić N., Bakke S.S., et al. PPARδ activation in human myotubes increases mitochondrial fatty acid oxidative capacity and reduces glucose utilization by a switch in substrate preference. Arch Physiol Biochem. 2014;120:12–21. doi: 10.3109/13813455.2013.829105. [DOI] [PubMed] [Google Scholar]
- 6.Sahebkar A., Chew G.T., Watts G.F. New peroxisome proliferator-activated receptor agonists: potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin Pharmacother. 2014;15:493–503. doi: 10.1517/14656566.2014.876992. [DOI] [PubMed] [Google Scholar]
- 7.Bracken C., Pulito K., Tozzo E. Modulation of PPARδ with MTB-2 post-reperfusion attenuates IR-induced AKI injury biomarkers and histopathology in rats. J Am Soc Nephrol. 2017 https://www.asn-online.org/education/kidneyweek/2017/program-abstract.aspx?controlId=2776254 28(Abstract Edition):165:[Abstract TH-PO243] [Google Scholar]
- 8.Bracken C., Stanwix J., Hoang H., Bell E., Tozzo E. PPARd modulator MTB-2 enhances FAO in vitro and attenuates IR-induced geneexpression changes in vivo, 48 hours and 14 days post AKI. Mitobridge. https://www.asn-online.org/education/kidneyweek/2017/program-abstract.aspx?controlId=2776316 Accessed April 21, 2023.
- 9.Taniuchi Y., van Till O., Wojtkowski T., et al. Single- and multiple-dose safety, tolerability, pharmacokinetics and pharmacodynamics of ASP1128, a novel peroxisome proliferator-activated receptor δ modulator, in healthy adult and elderly participants. Clin Pharmacol Drug Dev. Forthcoming. 2023 doi: 10.1002/cpdd.1236. [DOI] [PubMed] [Google Scholar]
- 10.van Till O., Renfurm R., Mulligan G., et al. Design of the 1128-CL-0201 study, a phase 2 proof of concept, double-blind, randomized, placebo-controlled study of ASP1128 in patients at risk for Acute Kidney Injury following cardiac surgery. Mitobridge. https://www.asn-online.org/education/kidneyweek/2019/program-abstract.aspx?controlId=3232970 Accessed April 21, 2023.
- 11.Meersch M., Schmidt C., Hoffmeier A., et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meersch M., Schmidt C., Van Aken H., et al. Urinary TIMP-2 and IGFBP7 as Early Biomarkers of Acute Kidney Injury and Renal Recovery following Cardiac Surgery. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 14.Geiss G.K., Bumgarner R.E., Birditt B., et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 15.Cummings J.J., Shaw A.D., Shi J., Lopez M.G., O’Neal J.B., Billings F.T. Intraoperative prediction of cardiac surgery–associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg. 2019;157:1545–1553.e5. doi: 10.1016/j.jtcvs.2018.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian Y., Che L., Yan Y., et al. Urine klotho is a potential early biomarker for acute kidney injury and associated with poor renal outcome after cardiac surgery. BMC Nephrol. 2019;20:268. doi: 10.1186/s12882-019-1460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashani K., Al-Khafaji A., Ardiles T., et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez J.M., Folkow L.P., Blix A.S. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu Rev Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- 19.Vaughn A.E., Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong D., Kim T.-S., Cho I.T., Kim I.Y. Modification of glycolysis affects cell sensitivity to apoptosis induced by oxidative stress and mediated by mitochondria. Biochem Biophys Res Commun. 2004;313:984–991. doi: 10.1016/j.bbrc.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Kishi S., Campanholle G., Gohil V.M., et al. Meclizine preconditioning protects the kidney against ischemia–reperfusion injury. EBiomedicine. 2015;2:1090–1101. doi: 10.1016/j.ebiom.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thielmann M., Corteville D., Szabo G., et al. Teprasiran, a small interfering RNA, for the prevention of acute kidney injury in high-risk patients undergoing cardiac surgery: a randomized clinical study. Circulation. 2021;144:1133–1144. doi: 10.1161/CIRCULATIONAHA.120.053029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelman D.T., Crisafi C., Germain M., et al. Using urinary biomarkers to reduce acute kidney injury following cardiac surgery. J Thorac Cardiovasc Surg. 2020;160:1235–1246.e2. doi: 10.1016/j.jtcvs.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Priyanka P., Zarbock A., Izawa J., Gleason T.G., Renfurm R.W., Kellum J.A. The impact of acute kidney injury by serum creatinine or urine output criteria on major adverse kidney events in cardiac surgery patients. J Thorac Cardiovasc Surg. 2021;162:143–151. doi: 10.1016/j.jtcvs.2019.11.137. [DOI] [PubMed] [Google Scholar]
- 25.Himmelfarb J., Chertow G.M., McCullough P.A., et al. Perioperative THR-184 and AKI after cardiac surgery. J Am Soc Nephrol. 2018;29:670–679. doi: 10.1681/ASN.2017020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarbock A., Küllmar M., Ostermann M., et al. Prevention of cardiac surgery–associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI-multicenter randomized controlled trial. Anesth Analg. 2021;133:292–302. doi: 10.1213/ANE.0000000000005458. [DOI] [PubMed] [Google Scholar]
- 27.Husain-Syed F., Ferrari F., Sharma A., et al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg. 2018;105:1094–1101. doi: 10.1016/j.athoracsur.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Quark Results of Phase III Clinical Trial of Acute Kidney Insufficiency (AKI) Preventative Drugs and Impact on Consolidated Business Performance. SBI Holdings Corporation. Published February 12, 2021. Accessed October 5, 2022. https://www.sbigroup.co.jp/news/2021/0212_12330.html
- 29.Quark Pharmaceuticals QPI-1002 Phase 3 for prevention of major adverse kidney events (MAKE) in subjects at high risk for AKI following cardiac surgery. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03510897
- 30.Hendricks P.A., Schildcrout J.S., et al. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. 2016;315:877–888. doi: 10.1001/jama.2016.0548. Billings FT 4th. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda Y., Luo H.D., Kang G.S., Teoh K.L.-K., Kofidis T. Meta-analysis of the benefit of beta-blockers for the reduction of isolated atrial fibrillation incidence after cardiac surgery. JTCVS Open. 2020;3:66–85. doi: 10.1016/j.xjon.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobrev D., Aguilar M., Heijman J., Guichard J.B., Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–436. doi: 10.1038/s41569-019-0166-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.