Abstract
The stoichiometric water splitting using a solar-driven Z-scheme approach is an emerging field of interest to address the increasing renewable energy demand and environmental concerns. So far, the reported Z-scheme must comprise two populations of photocatalysts. In the present work, only tungsten oxides are used to construct a robust Z-scheme system for complete visible-driven water splitting in both neutral and alkaline solutions, where sodium tungsten oxide bronze (Na0.56WO3–x) is used as a H2 evolution photocatalyst and two-dimensional (2D) tungsten trioxide (WO3) nanosheets as an O2 evolution photocatalyst. This system efficiently produces H2 (14 μmol h–1) and O2 (6.9 μmol h–1) at an ideal molar ratio of 2:1 in an aqueous solution driven by light, resulting in a remarkably high apparent quantum yield of 6.06% at 420 nm under neutral conditions. This exceptional selective H2 and O2 production is due to the preferential adsorption of iodide (I–) on Na0.56WO3–x and iodate (IO3–) on WO3, which is evidenced by both experiments and density functional theory calculation. The present liquid Z-scheme in the presence of efficient shuttle molecules promises a separated H2 and O2 evolution by applying a dual-bed particle suspension system, thus a safe photochemical process.
Keywords: green hydrogen, tungsten oxide, Z-scheme, visible photocatalysis, water splitting, DFT calculation
1. Introduction
Hydrogen (H2) production from earth-abundant water using sustainable solar energy is imperative to solve global energy demand and environmental issues.1 Besides, solar H2 is an alternative to gray H2 derived from fossil fuels to be used in many industrial processes as feedstock including ammonia synthesis.2,3 Solar-driven overall water splitting using semiconductor materials is one of the promising approaches to achieve sustainable production of H2 in an economically viable manner.4 However, the simultaneous production of H2 and O2 as an ideal process is extremely challenging in photocatalytic water splitting in the absence of an electric bias. A single photocatalyst with appropriate cocatalysts for pure water splitting has thus met with very limited success due to stringent bandgap requirements.5,6 In parallel, there are many studies on the half-reaction of either proton reduction or water oxidation in the presence of an efficient but costly chemical scavenger. Typically, BiVO47 or WO38 for water oxidation and C3N49 or Rh dope SrTiO310 for proton reduction were reported. A Z-scheme, also known as a dual photoexcitation system, akin to natural photosynthesis comprising H2 evolution photocatalyst (HEP) and O2 evolution photocatalyst (OEP), should be more efficient and economical for overall water splitting than the single photocatalyst with complicated cocatalysts.11,12 It offers an extended choice of semiconductor materials with a narrow bandgap for both half-reactions, enabling it to achieve high solar-to-hydrogen conversion efficiency (STH). Furthermore, it has been predicted that a maximum of 12% STH can be achieved using a single absorber whereas it can be upraised significantly to 22% for Z-scheme-based systems due to much better visible light harvesting.13 Typically, HEPs with the more negative conduction band (CB) potential concerning proton reduction (0.0 V vs NHE) and OEPs with the more positive valence band (VB) potential concerning water oxidation (1.23 V vs NHE) are suitable for H2 and O2 evolutions, respectively. The semiconductor particulate suspension-based Z-scheme system utilizing soluble redox mediator has been highly focused as it is much more simple and cost-effective than the solid Z-scheme,14 and more importantly promises to produce H2 and O2 separately in a dual-bed particle suspension system on a large scale, guaranteeing a safer chemical process compared with others, e.g., a solid Z-scheme.13,15−17
Several reports have successfully demonstrated the concept of dual semiconductor photocatalysts suspended in an aqueous solution containing redox couples such as Fe3+/2+, IO3– / I–, I3–/I–,18,19 etc., as we have summarized in our recent review for light-assisted water splitting.11 For example, Abe et al. accomplished an effective overall water splitting using Pt-TiO2 (anatase) and Pt-TiO2 (rutile) for H2 and O2 evolutions, respectively, in the presence of IO3–/I– redox mediator, while under ultraviolet (UV) light.20 Subsequently, several narrow-bandgap semiconductors viz. cation-doped SrTiO3, graphitic carbon nitride (g-C3N4), Sm2Ti2S2O5, etc., as HEPs, and BiVO4, WO3, H2WO4, AgNbO3, TaON, etc., as OEPs have been explored and implemented in a Z-scheme system to split water into stoichiometric amounts of H2 and O2.4,21,22 Notably, surface-modified oxynitrides were described to improve the water splitting significantly by absorbing visible light effectively and suppressing the charge carrier recombination; however, oxynitrides are self-photocorrosive, leading to poor photostability.23 In another report, a heterojunction based on oxynitrides (Pt-loaded MgTa2O6–x Ny/TaON) as a HEP was shown to suppress charge carrier recombination, resulting in a drastic enhancement in overall water splitting with a benchmark apparent quantum yield (AQY) of 3.4% at 420 nm when considering a 2-electron process for H2 production, in combination with PtOx/WOx as an OEP in the presence of IO3–/I– as redox mediator.24 Similarly, the AQY of a Z-scheme photocatalytic system at 420 nm was often measured.10,25−29 Though substantial advances have been made so far, a significant breakthrough is yet to come. Hence, intensive research is underway by developing new materials30 with special surface properties, high crystallinity, fewer defects,31 spatial separation of reduction and oxidation sites, cocatalyst loading,32 controlled morphology, heterojunction formation, etc., to achieve a high AQY.
Herein, we have developed a new tungsten oxide-only Z-scheme (WOZ) system for overall water splitting, in which both the HEP and OEP are tungsten oxides, i.e., sodium tungsten oxide bronze (Na0.56WO 3–x) as the HEP and two-dimensional (2D) WO3 nanosheets as the OEP in the presence of IO3–/I– redox couple. Tungsten oxide-based materials have been widely studied but only as photoanodes or water oxidation photocatalysts due to their conduction band being too positive to meet proton reduction.8,33,34 Moreover, WO3 absorbs ∼12% of the solar spectrum and possesses a moderate hole-diffusion length (∼150 nm) in addition to impressive electron mobility (∼12 cm2 V–1 s–1).34 Numerous stable nonstoichiometric WO3 materials could be realized by altering the lattice oxygen vacancies to attain interesting optoelectronic/catalytic properties. To the best of our knowledge, a Z-scheme system composed of one type of oxide photocatalysts for visible-driven-pure water splitting has not been reported. Very recently upraising the CB position of WOx to a more negative potential was shown, e.g., by creating oxygen vacancies35 or surface defects engineering.36 Here, we prepared a new and stable WOx-based HEP, Na0.56WO3–x. Interestingly, it can be coupled with WO3 nanosheets as an OEP to form a robust Z-scheme, which demonstrates efficient pure water splitting under light irradiation. Compared with the bulk materials, WO3 nanosheets provide more exposed active sites due to their sheet-like morphology. Such a novel Z-scheme generates H2 and O2 stoichiometrically, demonstrating a high AQY of 6.06% at 420 nm, under light irradiation and ambient conditions.
2. Results and Discussion
2.1. Characterization of the Photocatalysts
2.1.1. Hydrogen Evolution Photocatalyst (HEP)
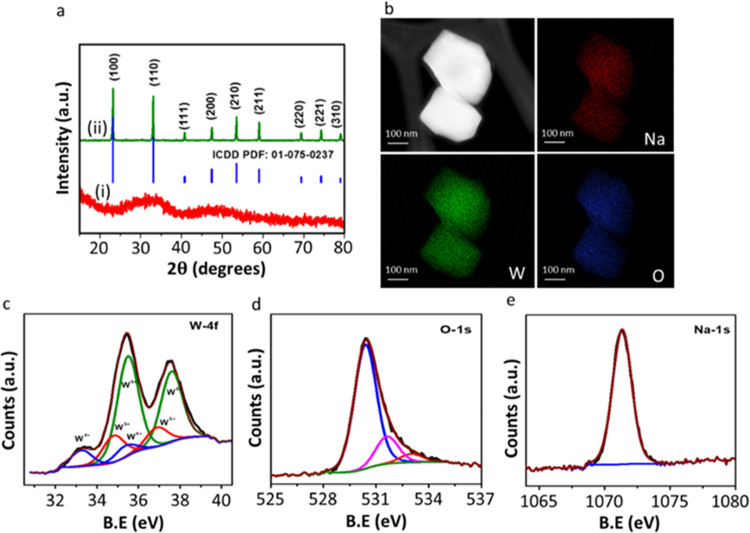
NaxWO3–x was prepared under ambient conditions by treating sodium tungstate with sodium borohydride (see details in the Experimental Section). The obtained powders were characterized using various physicochemical techniques. Figure 1a shows the powder XRD patterns of the as-synthesized samples and samples annealed at 800 °C under a N2 atmosphere. It is clearly seen that the as-synthesized sample is amorphous as it has very broad diffraction peaks. However, the annealed sample exhibits sharp strong peaks, indicating that annealing imparted crystallinity to the sample, as expected. These peaks can be assigned to the cubic NaxWO3–x (Pm-3m) (Figure S1), matching well with the standard pattern (PDF No. 01-75-0237). The Na content present in the annealed NaxWO3–x was determined to be around 0.56 using a microwave plasma-atomic emission spectrometer, which is also verified using the experimentally (XRD) measured lattice parameter (ao) according to the eq 1,37,38 so this sample is denoted Na0.56WO3–x.
| 1 |
Figure 1.
Characterization of HEP. (a) Powder XRD patterns of the (i) as-synthesized NaxWO3–x (NWO), a standard pattern of the cubic NaxWO3–x with PDF file no. 01-75-0237 (blue) and (ii) annealed NaxWO3–x (Na0.56WO3–x); (b) HAADF TEM image of Na0.56WO3–x and the corresponding elemental mapping of Na (red), W (green), and O (blue); and (c–e) XPS spectra of W-4f, O-1s, and Na-1s regions in Na0.56WO3–x, respectively.
Figure S2 represents transmission electron microscopy (TEM) images of the as-synthesized HEP, indicating that the sample is composed of interconnected spheres with dimensions around 100–150 nm. The high-resolution TEM (HRTEM) image of the as-synthesized sample shows the absence of crystallinity, demonstrating the amorphous nature. After annealing, the amorphous sample becomes crystalline, which is obvious from the TEM image (Figure S3), and the Pt nanoparticles distribution on the surface of the Na0.56WO3–x reveals the successful cocatalyst loading. Furthermore, the new crystalline Na0.56WO3–x lattice was identified as (100), and the photodeposited Pt nanoparticles show (200) lattice (Figure S4). The elemental mapping of Na0.56WO3–x shows the uniform distribution of Na, W, and O elements (Figure 1b). The surface oxidation states of Na0.56WO3–x were assessed using X-ray photoelectron spectroscopy (XPS). The survey spectrum is shown in Figure S5. Figure 1c–e represents the deconvoluted core-level XPS spectra of W-4f, O-1s, and Na-1s recorded for Na0.56WO3–x. As shown in Figure 1c, the presence of three doublets (4f7/2 and 4f5/2) in the W-4f spectra of Na0.56WO3–x unveils the presence of tungsten in multiple oxidation states. For instance, it shows peaks at binding energy (BE) values around 33.3 and 35.4 eV, 34.8 and 36.9 eV, and 35.5 and 37.6 eV. The first set of doublets corresponds to the presence of W4+, while the second and third doublets disclose the presence of W5+ and W6+ species, respectively. The deconvoluted O-1s XPS consists of three peaks at BE values 530.4, 531.6, and 533.1 eV37 (Figure 1d). The most intense peak at 530.4 eV is attributed to lattice oxygen (O2–) surrounded by W atoms in the Na0.56WO3–x lattice. The origin of the peak at 531.6 eV is due to oxygen (OH–) (the adsorbed OH–) in the regions of oxygen vacancies (due to W5+/W4+ species) within the Na0.56WO3–x matrix. In these oxygen-deficient sites, OH groups are known to bond with metal cations to compensate for the charge. The peak located at 533.1 eV could be due to the presence of adsorbed H2O on the surface of Na0.56WO3–x.37 The deconvoluted Na-1s XPS shows a single peak at 1071.2 eV (Figure 1e), in agreement with an earlier study.39 A similar set of peaks are observed after cocatalyst Pt loading, Pt- Na0.56WO3–x (see Table S1).
2.1.2. Oxygen Evolution Photocatalyst (OEP)
2D WO3 nanosheets as an OEP in the Z-scheme system were obtained by thermal annealing of 2D tungstic acid (WO3·H2O) under an air atmosphere at 500 °C (see the Experimental Section for details). The conversion of tungstic acid into tungsten trioxide was studied using XRD and Raman spectroscopy. The powder XRD pattern of WO3 nanosheets along with its precursor WO3·H2O are shown in Figure 2a. The as-synthesized WO3·H2O nanosheets are crystalline and the XRD pattern matches well with the standard pattern (PDF No: 01-084-0886) for orthorhombic (Pmnb) hydrated WO3 with strong diffraction peaks for (111), (020), and (131) planes, in agreement with the earlier report.40
Figure 2.

Characterization of OEP. (a) XRD patterns of (i) as-synthesized WO3·H2O and (ii) annealed (WO3 nanosheets) samples and their corresponding standard ICDD patterns; (b) Raman spectra of (i) WO3·H2O and (ii) WO3 nanosheets recorded using a 532 nm green laser; (c) TEM image of Pt-WO3 and the presence of Pt nanoparticles on the surface of WO3 is indicated with an arrow; and (d) XPS spectra corresponding to the W-4f region of WO3 nanosheets.
After annealing it in the air at 500 °C for 3 h, the formation of monoclinic (P21/n) WO3 nanosheets is observed, corresponding to the standard pattern (PDF No: 00-043-1035). It confirms the transformation of orthorhombic WO3·H2O into monoclinic WO3 due to annealing (Figure S6). This phenomenon is further verified by Raman spectroscopy, as shown in Figure 2b. The Raman spectra of WO3·H2O show three peaks at ∼642, ∼820, and ∼946 cm–1, corresponding to the first W–O–W, [ν1(W–O–W)], second W–O–W [ν2(W–O–W)], and terminal W=O stretching vibrations, respectively. The sharp peaks at ∼642 and ∼946 cm–1 confirm that the as-synthesized nanosheets are hydrated WO3 (WO3·H2O). The Raman spectra of WO3 nanosheets (Figure 2b) show two major peaks at ∼714 and ∼808 cm–1, corresponding to the W–O–W stretching modes, confirming the formation of monoclinic WO3. The other notable difference in WO3 nanosheet spectra is the absence of terminal W=O stretching mode at 946 cm–1 compared to WO3·H2O. Besides, a strong peak appears at 272 cm–1 along with two low intense peaks at around 192 and 325 cm–1, which are corresponding to W–O–W bending modes of WO3.41 The ratio of Iν2(W-O-W)/Iν(W=O) can provide information about x in WO3·xH2O as suggested earlier.40 As expected the ratio is lower for WO3·H2O, while it is higher for WO3 nanosheets, validating the dehydration of WO3·H2O. The layered morphology of WO3·H2O nanosheets is evident from the TEM images, as shown in Figure S7. The EDX spectra validate that the material is mainly made of W and O elements. Figure 2c shows the TEM image of Pt-WO3, which shows the presence of Pt nanoparticles on the surface of the WO3 mixture composed of nanosheets and nanoparticles (denoted WO3 nanosheets afterward). The deconvoluted W-4f XPS of WO3 shown in Figure 2d displays a doublet at BE values 37.5 and 37.6 eV suggesting that the W is present at +6 oxidation state only. These results suggest that W has a single oxidation state in WO3. The presence of W+6 also confirms the formation of WO3 upon annealing of WO3·H2O, complementing XRD, and Raman measurements.
2.1.3. Band Structure of HEP and OEP
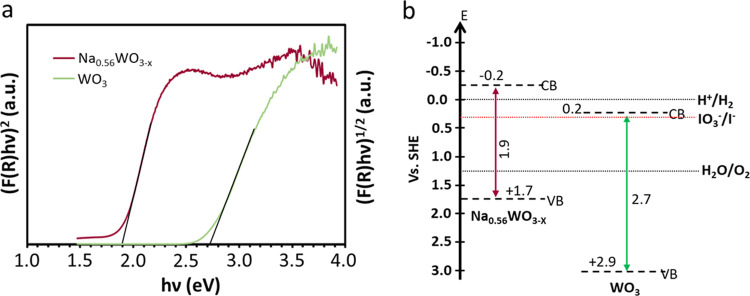
UV–Vis diffuse reflectance spectroscopy (DRS) was used to disclose the bandgap of the investigated photocatalysts. The optical absorbance spectra of Na0.56WO3–x and WO3 nanosheets are shown in Figure S8. It is clearly seen that both materials absorb visible light efficiently and the Tauc plots of Na0.56WO3–x and WO3 (Figure 3a) reveal a bandgap of 1.9 and 2.7 eV, respectively. To explicitly determine the band positions, we have measured the CB by photoelectrochemistry and Mott–Schottky measurement. The photocurrent onset potential measurement results are shown in Figure S9. The measured potential against Ag/AgCl was converted to SHE using E(SHE) = EAg/AgCl + EAg/AgCl0. The WO3 has a CB potential of +0.2 V, which is consistent with the reported42 and the Na0.56WO3–x has CB at −0.2 V. To validate it further, we have tested the CB potential of the Na0.56WO3–x and WO3 using the Mott–Schottky method (Figure S10), and the observed results agree with the photocurrent onset potential measurement. In addition, we have performed a control experiment to test the water oxidation property of Na0.56WO3–x in the presence of IO3– and observed a tiny amount of O2 evolved (0.3 μmol h–1), 60 times less active compared to WO3 (17.9 μmol h–1). Such a small O2 evolution rate of Na0.56WO3–x reveals the less positive VB potential of Na0.56WO3–x compared to WO3. Thus, it is essential to engineer a Z-scheme composed of Na0.56WO3–x and WO3 for complete water splitting. Accordingly, the energy band diagram was drawn, as shown in Figure 3b. The CB of WO3 is too positive to reduce protons into H2, which is essential to form an efficient Z-scheme system as it is close to the VB of the HEP when coupling Na0.56WO3–x with WO3. The deep VBM of WO3 nanosheets and negative CBM of Na0.56WO3–x suggest that it is highly suitable for water oxidation, and reduction reactions, respectively. More importantly, these well-matched band positions promise a new Z-scheme composed of tungsten oxides.
Figure 3.
(a) Tauc plots of WO3 nanosheets, an indirect bandgap semiconductor, and Na0.56WO3–x, a direct bandgap semiconductor; (b) the proposed band diagram for Na0.56WO3–x- and WO3-based Z-scheme photocatalytic water splitting system vs SHE.
2.2. Photocatalytic H2 Evolution
To verify the photocatalytic ability of Na0.56WO3–x-based materials for H2 production, experiments were performed in aqueous solutions containing NaI (pH ∼7.0) as a hole scavenger under Xe lamp irradiation, and the results are shown in Figure 4a. The compelling observation from Figure 4a is that the NWO and Na0.56WO3–x can produce H2 without any cocatalysts, which is otherwise difficult with WO3-based materials due to the inappropriate CB position concerning the H2 evolution potential, suggesting that both NWO and Na0.56WO3–x can be used as a photocatalyst for H2 production from water. Both NWO (amorphous) and Na0.56WO3–x (crystalline) photocatalysts produce H2 at a rate of 2.2 and 2.5 μmol h–1, respectively. Subsequently, the effect of pH on H2 evolution was studied to optimize the condition at which NWO-based materials yield higher rates of H2 production. As shown in Figure S11, the Na0.56WO3–x shows better activity than NWO from neutral to weakly alkaline solution, where the H2 evolution rate was obtained from the first 6 h run. Furthermore, we have tested the chemical stability of Na0.58WO3–x for H2 evolution at pH 8.5 and 10.5 by running four 6 h reaction cycles (Figure S12). The initial rate of H2 evolution does not change significantly at both pH values, but from the third cycle onward, the H2 evolution rate starts to reduce and saturates in the fourth cycle only at pH 10.5. This suggests that the present HEP is highly stable under a weakly alkaline condition but not stable at a strong alkaline condition for a prolonged run. Hence, well-crystallized Na0.56WO3–x was selected for further studies. To improve the activity, 3wt%-Pt (cocatalyst) particles were loaded on the surface of Na0.58WO3–x by photodeposition (see Experimental Section for details), which significantly improves the H2 evolution to a rate of 16.8 μmol h–1 at pH 7.0. It is worth noting that after loading the cocatalyst (3%Pt-Na0.56WO3–x), the best activity was observed at pH 7.0. This is anticipated as the cocatalysts extract the electron efficiently and minimize the recombination with holes. The H2 evolution reaction in the presence of I– ions at pH 7.0 can be represented by the following equations.
| 2 |
| 3 |
| 4 |
| 5 |
Figure 4.
Photocatalytic H2 and O2 evolution half-reactions. (a) H2 evolution from water (pH 7.0) using 10 mg of NWO-based materials (NWO, Na0.56WO3–x, and 3wt%Pt-Na0.56WO3–x) containing 5 mM NaI as a hole scavenger under a full-arc condition for 6 h; (b) O2 evolution from water (pH 8.5) using 10 mg of WO3-based materials (WO3·H2O, WO3 nanosheets, and 0.5%Pt-WO3 nanosheets) containing 5 mM NaIO3 as an electron scavenger under a full-arc condition for 6 h; (c) the H2 evolution stability study on 3%Pt-Na0.56WO3–x in an aqueous solution (pH 7.0) containing 5 mM NaI under a full-arc condition for three consecutive 7 h runs; and (d) the O2 evolution stability study on 0.5%Pt-WO3 nanosheets in an aqueous solution (pH 8.5) containing 5 mM NaIO3 under a full-arc condition for three 7 h runs; Ar gas was purged after each run.
The stability of the 3%Pt-Na0.56WO3–x for prolonged H2 evolution was investigated by measuring the H2 evolution for 21 h (composed of three consecutive 7 h runs) and no significant change in the H2 evolution rate is observed, as shown in Figure 4c, suggesting the robustness of Na0.56WO3–x under neutral pH. We further validated the stability of the HEP by testing the compositional changes using XPS after three consecutive photocatalytic runs, as shown in Figure S13. No obvious changes were observed in the deconvoluted spectra of O-1S and Na-1S; however, a small reduction in the W4+ signal was observed, while the signals for W5+ and W6+ are stable. The identical H2 evolution suggests that W5+ and W6+ are the species dominating water reduction under the present experimental conditions. Furthermore, the XRD spectra of the 3%Pt-Na0.56WO3–x before and after the photocatalysis show (Figure S14a) no significant changes, which again confirms the robustness of the HEP under neutral pH.
2.3. Photocatalytic O2 Evolution
Subsequently, we investigated the photocatalytic O2 evolution half-reaction using WO3 under a full-arc condition in water containing IO3– ions as a sacrificial electron acceptor. As expected, a significant amount of O2 evolution was observed at ∼pH 8.5 using both WO3·H2O and WO3, but monoclinic WO3 nanosheets show better activity than orthorhombic WO3·H2O (Figure 4b). Thereafter, WO3 nanosheets were loaded with 0.5 wt % Pt cocatalyst (Pt-WO3), leading to an O2 evolution rate of 17.9 μmol h–1, which is superior to cocatalyst-free WO3 (3.8 μmol h–1) and WO3·H2O (2.2 μmol h–1). The present Pt-WO3 water oxidation activity is comparable to the best activity reported previously,43−45 thanks to the advantages of nanosheet morphology. The O2 evolution using Pt-WO3 from weakly alkaline water containing IO3– ions can be represented by the following equations.
| 6 |
| 7 |
| 8 |
In the absence of a photocatalyst or an electron acceptor (IO3–), no O2 evolution is observed. The H2 evolution activity of the Pt-WO3 was also monitored using I– as the hole scavenger to examine the suitability of WO3 for a potentially efficient Z-scheme for water splitting. As anticipated, negligible H2 evolution is observed (Figure S15), in agreement with the CB position of WO3 measured in Figure 3d, which is not appropriate for water reduction, suggesting that the Pt-WO3 is highly selective for the water oxidation half-reaction. The stability of Pt-WO3 nanosheets for prolonged O2 evolution at pH 8.5 was examined for 21 h (composed of 3 consecutive 7-h runs), and no significant decrease in O2 evolution rate is observed, as shown in Figure 4d, suggesting that WO3 is a robust photocatalyst under weakly alkaline conditions. In addition, no changes were observed in the XRD of Pt-WO3 before and after photocatalysis (Figure S14b), which further ensures stability. Furthermore, we also tested the stability of WO3 at pH 10.5 and observed no change in the beginning of the reaction, similar to the HEP, but over a prolonged run, the O2 evolution rate was significantly reduced (Figure S16). This strongly suggests that the present OEP is stable only under weak alkaline pH and not stable in strong alkaline solutions.
2.4. Z-Scheme Overall Water Splitting
The overall water splitting activity of the new Z-scheme system composed of only tungsten oxides, i.e., 3%Pt-Na0.56WO3–x as the HEP and 0.5%Pt-WO3 as the OEP was then tested under full-arc as well as visible light conditions. When each photocatalyst is tested independently, only H2 or O2 evolution was observed (Table 1, run 1 and 2). When the system is composed of both the HEP and OEP in an aqueous solution containing I– as a redox mediator, the successful evolution of H2 and O2 was observed simultaneously (Table 1, run 6). In the case of an aqueous solution containing IO3– as a redox mediator, the simultaneous evolution of H2 and O2 was not observed. Instead, only O2 evolution was noted (Table 1, run 3), suggesting that the reduction of IO3– to I– is slower, either because I– cannot desorb easily from the OEP or IO3– reduction strongly competes with proton reduction to H2, which will be discussed later. Both H2 and O2 production rates are highly dependent on the pH of the solution, which is shown in Table 1 that at pH ∼3.0 acidic condition, poor H2 and no O2 evolutions were observed in the I– aqueous solution (Table 1, run 4). This could be due to the oxidation of I– to mainly I3–, which is a poor electron scavenger compared to IO3– at pH 3.0, as listed in eq 3 against eq 4, and is preferable in a neutral/alkaline solution and in turn hinders the establishment of the IO3–/I– redox system.46 Similar results were observed while using Fe2+ as a redox mediator (Table 1, run 5). At pH ∼7.0, in the presence of I–, the evolution of both H2 and O2 gases was attained (Table 1, run 6); however, the stoichiometric ratio of H2 to O2 has not been achieved. Further, when the reactions were carried out at pH ∼8.5 (Table 1, run 7), simultaneous productions of H2 and O2 were observed; however, yet again in nonstoichiometric quantities. Subsequently, we performed optimization studies by varying the weight ratio of HEP to OEP at pH ∼7.0. A weight ratio of 1:1 (HEP:OEP, by weight) has been found to produce H2 and O2 gases simultaneously with a rate of 14 μmol h–1 (2800 μmol h–1 g–1) and 6.9 μmol h–1 (1380 μmol h–1 g–1), respectively, at a stoichiometric molar ratio of 2:1 (Table 1, run 8).
Table 1. Photocatalytic Water Splitting over the WOx Catalysts (Runs 3 to 7, and 10: 10 mg of HEP and 5 mg of OEP, runs 8, 9, and 11: 5 mg of HEP and 5 mg of OEP) Suspended in an Aqueous Solution Performed under Different Experimental Conditions.
| run | HEP | OEP | mediator | pH | weight ratio | HER (μmol h–1) | OER (μmol h–1) |
|---|---|---|---|---|---|---|---|
| 1 | 3%Pt-Na0.56WO3–x | - | NaI | 7.0 | - | 16.8 | - |
| 2 | - | 0.5%Pt-WO3 | NaIO3 | 8.5 | - | - | 17.9 |
| 3 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | NaIO3 | 7.0 | 2:1 | - | 11.3 |
| 4 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | NaI | 3.0 | 2:1 | 3.0 | - |
| 5 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | FeCl2 | 3.0 | 2:1 | 2.8 | - |
| 6 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | NaI | 7.0 | 2:1 | 14.6 | 4.8 |
| 7 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | NaI | 8.5 | 2:1 | 13.9 | 5.3 |
| 8 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | NaI | 7.0 | 1:1 | 14.0 | 6.9 |
| 9 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | NaI | 10.5 | 1:1 | 10.7 | 7.9 |
| 10 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | NaI | 10.5 | 2:1 | 9.6 | 4.9 |
| 11 | 3%Pt-Na0.56WO3–x | 0.5%Pt-WO3 | - | 7.0 | 1:1 | - | - |
The temporal gas evolution is shown in Figure 5a and the consecutive run is represented in Figure 5b, suggesting that the present system is not only efficient but also rather stable under the present experimental conditions. We also tested the present WOx-based Z-scheme (WOZ) activity at pH 10.5 and observed no stoichiometric gas evolution at a 1:1 mass ratio (Table 1, run 9), whereas at a mass ratio of 2:1, stoichiometric gas evolution was observed (Table 1, run 10), proving that the tungsten oxide-based Z-scheme can split water at both neutral and weakly alkaline conditions. In the absence of a redox mediator (Table 1, run 11), no gas evolution was observed, indicating that the I– is crucial for the Z-scheme. The performance of the present Z-scheme water splitting system under visible-light irradiation (λ ≥ 420 nm) was also measured, as shown in Figure S17. Again, the simultaneous production of H2 and O2 with a rate of 7.4 and 3.6 μmol h–1 is monitored, and the gas amount produced remains almost linear increase with time. The H2 production activity of the Na0.56WO3–x has been tested under half-reaction conditions at higher wavelengths using 600 and 650 nm monochromatic filters. The obvious H2 evolution was observed under half-reaction conditions with a rate of 0.9 and 0.27 μmol h–1, respectively (Figure S18), but under Z-scheme working conditions, pure water splitting was not observed (generation of both H2 and O2) as WO3 is silent at these very long wavelengths. The AQY for H2 production from water using the proposed WOZ system was assessed three times and the average was reported. At 420 nm, an AQY of 6.06% (Figure S19) for water splitting was determined by considering the 2-electron process involved for one molecule of H2 evolution (see Experimental Section for details, and in some works of the literature, the 4-electron process was used to calculate the AQY.47 Using that method, the AQY is 12% herein). The H2 and O2 evolution rates under 420 nm monochromatic irradiation are shown in Figure S20. The Z-scheme photocatalytic activity of the present works was compared with the reported representative studies (Table S2).
Figure 5.
Stoichiometric water splitting with error bar after three measurements. (a) WOZ water splitting system composed of 10 mg of 3wt%Pt-Na0.56WO3–x as the HEP and 10 mg of 0.5wt%Pt-WO3 nanosheets as the OEP under the full-arc condition at pH 7.0 and 5 mM NaI as a redox mediator; (b) the stability study on 3wt%Pt-Na0.56WO3–x–NaI–0.5wt%Pt-WO3 under the same photocatalytic conditions for three successive 6 h runs.
2.5. Adsorption Studies
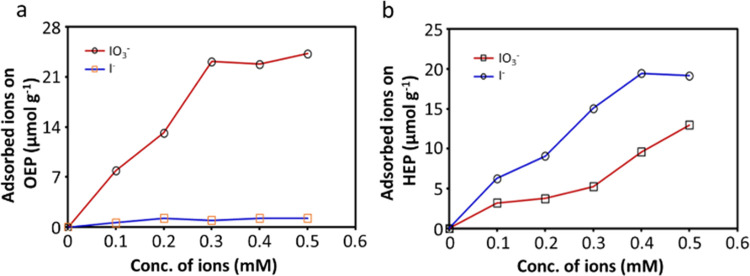
As the current Z-scheme works very efficiently, we investigated the shuttle molecule′s function in detail. First, both HEP and OEP were tested for H2 production in the presence of hole scavenger I– (Figure S15). One can see that the H2 is produced on the 3%Pt-Na0.56WO3–x but not on the 0.5%Pt-WO3 nanosheets, proving that the CB of WO3 nanosheets is not appropriate for proton reduction while the CB of Na0.56WO3–x is negative enough to reduce the proton to H2 (Figure 3d). The selectivity of the OEP, 0.5%Pt-WO3 for the O2 evolution half-reaction is then verified, where I– oxidation (I– → IO3–) might compete with water oxidation by consuming the photogenerated holes from the OEP. We examined this by adding 1 mM I– anions at 3 h during the ongoing water oxidation half-reaction in an aqueous solution (pH ∼8.5) containing 5 mM IO3. Interestingly, no influence was observed by the extra I– anions on the water oxidation reaction (Figure S21), strongly indicating that the nanosheets OEP can produce O2 highly selectively even in the presence of efficient hole scavenger I–. To disclose the reason behind this, we have studied the adsorption behavior of IO3– and I– anions onto the OEP by measuring their concentrations before and after the addition of 0.5%Pt-WO3 powder to the solution under dark conditions (see Experimental Section for details).
As shown in Figure 6a, the IO3– ions are adsorbed preferentially onto the OEP surface whereas I– shows poor adsorption, confirming that the water oxidation is dominant on the OEP rather than the oxidation of I– to IO3–. On the other hand, the crucial IO3– ions reduction is guaranteed due to their strong adsorption. Therefore, the couple of IO3–/I– is the ideal charge mediator for a Z-scheme containing the WO3 nanosheets. Similarly, we also tested the competitive reaction between water reduction and IO3– reduction (IO3– → I–) driven by the photogenerated electrons on 3%Pt-Na0.56WO3–x, HEP. As shown in Figure S22, we added two distinct concentrations of IO3– (100 μM and 1 mM) after 3 h during the ongoing water reduction half-reaction and noticed no significant change upon the addition of 100 μM of IO3–. However, a substantial deceleration of H2 evolution was observed when adding 1 mM IO3–. These results suggest that at higher IO3– concentrations, the photogenerated electrons from the HEP are also consumed by IO3– ions, in good agreement with the earlier report20 where a similar phenomenon was observed.
Figure 6.
Adsorption study. (a) Adsorption behavior of IO3– and I– anions on the surface of 0.5wt%Pt-WO3 (OEP) powder suspended in an aqueous solution at pH ∼8.5 under dark conditions; (b) adsorption behavior of IO3– and I– anions on 3wt%Pt-Na0.56WO3–x (HEP) powder suspended in an aqueous solution at pH ∼7.0 under dark conditions.
To understand further, we observed the competitive adsorption behavior of I– and IO3– on 3%Pt- Na0.56WO3–x. As shown in Figure 6b, both I– and IO3– anions are adsorbed on the surface of the HEP, but I– ions show relatively high preference, which is also proved by our XPS measurement of the used photocatalyst (Figure S13a) and is crucial for the Z-scheme water splitting. The adsorption behavior of I– and IO3– anions on bare Na0.56WO3–x was also undertaken (Figure S23). It is obvious that the I– ions are preferentially adsorbed especially at low concentrations. Therefore, the sufficient adsorption of I– on Na0.56WO3–x enables to accept the photogenerated holes, reducing the recombination with photogenerated electrons and hence promoting the selective H2 evolution. However, beyond 1 mM concentration, IO3– adsorption is dominated. Hence, in the presence of excess IO3– ions, H2 evolution is limited, which agrees well with our control experiment (Figure S22). We also found out that only O2 is produced in the WOZ with only the IO3– mediator (Table 1, run 3), wherein one can see the reason that IO3– can relatively easily adsorb on the HEP (Figure 6b) and its reduction strongly competes with proton reduction (Figure S22). Therefore, controlling the concentration of IO3– ions in the WOZ is one key issue, which from another angle proves that the initial addition of only I– mediator instead of both I– and IO3– mediators is of importance as demonstrated in our experimental design. We also found that continuous production of H2 and O2 for a prolonged time in our WOZ is stable and reproducible and the concentration of I– and pH were observed to be constant, demonstrating a highly selective and efficient Z-scheme system. A similar experimental study was reported previously48 in which the selective adsorption of IO3– anion on WO3 (OEP) was observed, leading to the selective O2 evolution reaction.
To consolidate the adsorption behavior observed experimentally, the redox molecules′ adsorption on photocatalysts was also studied by DFT calculation. The description of the model for WO3Pt4 and Na0.625WO2.875Pt4 is detailed in the Supporting Information. IO3– and I– were adsorbed onto each catalyst at two sites: over the platinum cluster and directly onto the surface of the catalyst. The optimized catalyst models, as well as the adsorption models, can be seen in Figures S24–S31. Adsorption energies are calculated using eq 9, where E represents the enthalpy, A is the adsorbent, B is the adsorbate, and AB is the complex structure.
| 9 |
The calculated adsorption energies for each tested adsorption site are shown in Figure 7. It can be seen that, for the catalyst WO3Pt4, iodate is more easily adsorbed than iodide (in good agreement with experimental results). For the Na0.625WO2.875Pt4 catalyst, it was found that both iodate and iodide were adsorbed onto the catalyst; however, iodide was adsorbed to a greater degree onto the catalyst. In each case, iodate adsorbed more preferentially onto the Pt cluster for subsequent reduction reactions, while iodide adsorbed more easily onto the catalyst surface for subsequent oxidation reactions. It should be noted that for both catalysts, the relaxation of iodate led to the stretching of the I=O bonds in every case, even when constraints were applied and, in most cases, the O atom dissociated. Overall, the DFT calculation results suggest that the preferential adsorption of IO3– on OEP and I– on HEP would be the reason for the enhanced selective H2 and O2 evolution using the present Z-scheme.
Figure 7.
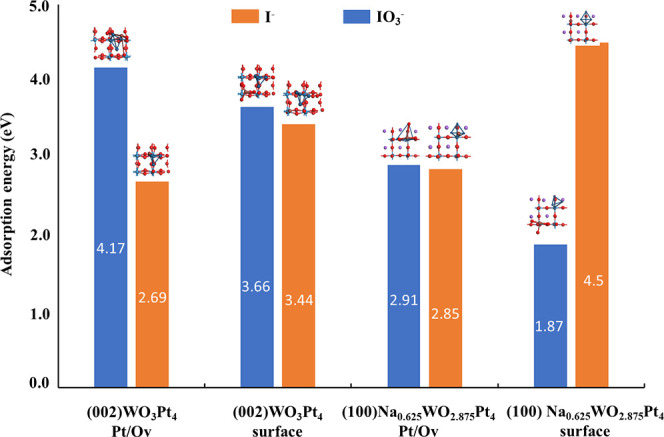
Adsorption energies and selected configurations of IO3 and I adsorbed onto the surface of WO3Pt4 and Na0.625WO2.875Pt4. Pt/Ov represents adsorption onto the Pt cluster, while surface represents adsorption onto the catalyst surface.
We also attempted to realize a direct Z-scheme (in the absence of a redox couple, maybe a solid Z-scheme concept) by mixing 3%Pt-Na0.56WO3–x and 0.5%Pt-WO3 powders in an aqueous solution to produce H2 and O2, simultaneously. However, no gas evolution is noted (Table 1, run 11), suggesting that the simple physical mixing of the HEP and OEP powders cannot work well for water splitting herein, which is also useful for the separation of H2 and O2 production in two compartments based on the particle suspension Z-scheme rather than solid Z-scheme that produce mixed H2 and O2 in one cell.
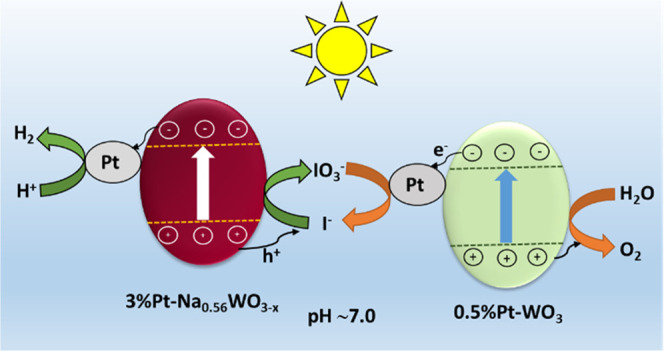
Based on the above results, we proposed the reaction pathway for the new WOZ system schematically in Scheme 1, which is supported by the above investigation. The stoichiometric overall water splitting at pH 7.0 occurs in the presence of a redox mediator IO3–/I–. Upon irradiation, photoexcited electrons from Pt-Na0.56WO3–x transfer to Pt active sites, where proton reduction occurs to produce H2, and the corresponding holes oxidize I– ions into IO3–, which gets regenerated back to I– by the photogenerated electrons on Pt-WO3, while photogenerated holes on Pt-WO3 perform water oxidation to generate O2, resulting into a complete cycle.
Scheme 1. Proposed Reaction Pathway for the New WOZ Water Splitting System.

3. Conclusions
In summary, we have demonstrated the original tungsten oxides-only suspension Z-scheme for efficient photocatalytic pure water splitting under the full-arc and visible light conditions in the presence of the IO3–/I– redox couple. Such a design enables separately and readily optimization of each photocatalyst and the cocatalyst. So the stepwise independent H2 and O2 evolution half-reactions optimization was carried out to minimize/eliminate undesirable reactions. The band position measurements indicate a matched electronic structure for the new Z-scheme. Then, for the first time, the WO3-based Z-scheme has been validated for visible-driven complete water splitting by a double excitation mechanism. This novel particulate WOZ exhibits 6.06% AQY at 420 nm when considering the electron number for H2 production as 2. Furthermore, I– was found to be favorably adsorbed on Na0.56WO3–x and IO3– on WO3 nanosheets by both experimental runs and theoretical modeling, being crucial for an efficient Z-scheme system. The low cost and ensured stability of tungsten oxides are also highly appropriate to subseqent scaling up.
4. Experimental Section
4.1. Materials and Reagents
Sodium tungstate dihydrate (Na2WO4·2H2O), sodium borohydride (NaBH4), sodium iodide (NaI), and sodium iodate (NaIO3) were purchased from Sigma Aldrich and used as received. All solutions were prepared using deionized (DI) water. Other chemicals used for experiments were purchased from commercial sources and used without further purification.
4.2. Synthesis of HEP
Sodium tungsten oxide bronze (NaxWO3–x) was synthesized by reducing the sodium tungstate dihydrate (Na2WO4·2H2O) using sodium borohydride (NaBH4).49 Briefly, 25 mL of 2.4 M NaBH4 prepared in ice-cold water at pH >10 (essential to suppress the hydrogen evolution from NaBH4 for upholding the reducing power) was added dropwise to 25 mL of 0.24 M Na2WO4·2H2O solution (pH decreased to 6.5) under stirring. Simultaneously, 5 M HCl was added dropwise to the stirring solution to maintain the pH of ∼6.5 because the addition of NaBH4 would raise the pH due to the formation of NaOH and NaBO2. This mixture was stirred continuously for 2 h, resulting in a dark brown precipitate, which was kept undisturbed for 2 h for settling down the precipitate. Then, the suspension was centrifuged to separate the precipitate for 10 min at 9000 rpm (each run) and the residue was washed several times with water to remove unreacted precursors, followed by drying at 70 °C for 2 h in a vacuum oven. To obtain crystalline NaxWO3–x, the synthesized sample was annealed at 800 °C for 3 h (denoted Na0.56WO3–x) under a N2 atmosphere in a tube furnace.
4.3. Synthesis of OEP
Two-dimensional (2D) tungstic acid (WO3·H2O) nanosheets were synthesized as per the earlier report.40 Briefly, 120 mM Na2WO4·2H2O was prepared in 40 mL of DI water, and 4 mL of HCl was added in a dropwise manner while stirring for 1 h. The obtained pale green suspension was then centrifuged for 10 min at 9000 rpm (each run) to separate the precipitate, and the residue was washed several times with water to remove the unreacted precursors, followed by drying in an air oven at 70 °C overnight. The as-synthesized WO3·H2O nanosheets were annealed at 500 °C for 3 h in a muffle furnace at a heating rate of 10 °C min–1 to obtain monoclinic WO3 nanosheets.
4.4. Platinum Cocatalyst Loading
A 3wt%Pt cocatalyst was loaded onto the Na0.56WO3–x (HEP) surface and 0.5wt%Pt was loaded on WO3 nanosheets (OEP) using a photodeposition method by continuous full-arc irradiation of the 10% aqueous methanol solution containing a H2PtCl6·6H2O precursor for 1 h using a 300 W Xe lamp (TrusTech PLS–SXE 300/300UV). Later, the reaction mixture was centrifuged, followed by washing several times to remove the unreacted Pt precursors. Then, the residue was dried at 80 °C for 12 h under vacuum. The HEP photocatalyst was immediately used after the centrifugation.
4.5. Characterization
The X-ray diffraction (XRD) patterns were obtained using a STOE STADI-P diffractometer with Mo Kα as the X-ray radiation source. UV–Vis diffuse reflectance spectra (DRS) of the HEP and OEP powders were collected using an Agilent Cary 5000 spectrophotometer fitted with an integrating sphere using standard barium sulfate powder as a reference. TEM measurements were performed using a JEOL2100 TEM, operated at 200 kV. Samples for TEM measurements were prepared by drop-casting the dilute dispersions of the photocatalysts onto the carbon-coated copper grids. X-ray photoelectron spectroscopy (XPS) measurements were undertaken using a Kratos Axis SUPRA machine using monochromated Al-Kα irradiation as a source of X-rays. XPS data analysis was performed using Casa XPS software. Shirley/Touguard methods were used for background corrections. Raman spectra were measured on a Renishaw inVia Raman microscope, using a 532 nm excitation laser. The photoelectrochemical onset potential measurements were recorded using linear sweep voltammetry by sweeping the potential from +0.8 to −0.5 V vs Ag/AgCl in a conventional three-electrode (photocatalyst film-deposited electrode as the working electrode, Ag/AgCl as the reference electrode, and a platinum mesh as the counter electrode) cell using an electrochemical analyzer (IVIUM Technologies). A 0.1 M Na2SO4 (pH ∼7.0) solution was used as the electrolyte in the presence of 10% methanol as a hole scavenger. The photocatalyst film was prepared by dispersing 20 mg of the photocatalyst in a 5.4 mL solution comprising water:ethanol at a 4:1 (v/v) ratio and 0.4 mL of Nafion (5% solution), followed by 1 h ultrasonication. 50 μL of the obtained slurry was drop-cast onto the precleaned fluorine-doped tin oxide (FTO) conducting substrate, followed by drying at 60 °C in an oven before the electrochemical tests. The area of the photocatalyst thin film was 1 × 1 cm2, whereas the size of the FTO substrate was 1 × 2 cm2. The catalyst loading amount was ca. 3.7 mg mL–1.
4.6. Photocatalytic Studies
The H2 and O2 evolution half-reactions and their simultaneous production in a Z-scheme system were carried out in a custom-made glass batch reactor with a top quartz window. The known amount of photocatalysts was loaded in the reactor containing 70 mL of water and 5 mM NaI and dispersed well by ultrasonication for 30 min. The pH of the solution was controlled by adding dilute solutions of H2SO4 and NaOH. The reactor was sealed and purged with high-purity Ar gas for 1 h to remove air/dissolved oxygen in the solution and headspace. After baseline measurement (0 h), the reactor was irradiated using a 300 W Xe lamp (Newport). The reactor was placed in a water bath during irradiation to maintain the reaction temperature. The production of H2 and O2 gases was quantified at regular intervals using gas chromatography (Varian 430-GC, TCD, argon carrier gas) equipped with a molecular 5A column using Ar as the carrier gas and N2 as the internal reference. The apparent quantum yield (AQY) was determined by performing photocatalytic experiments at specific wavelengths by using an appropriate bandpass filter. For AQY measurements, 60 mg of HEP and 30 mg of OEP were dispersed in 70 mL of water containing 5 mM NaI. The reactor was irradiated through a 2 cm diameter aperture, enabling the central beam through, which is very reliable for the AQY analysis as highlighted by Domen et al.50 The light intensity of the lamp was measured at five different points to obtain an average intensity using a calibrated photodiode coupled with an optical power meter (Newport, Model 1908-R). The measured light intensity with a 420 nm bandpass filter was 0.5 mW cm–2. The AQY was calculated (see Supporting Information for detailed calculation) using the following equation
| 10 |
where α = 2 and 4 for H2 and O2 evolution reactions provided that there are 2 electrons required for one H2 molecule production and 4 holes for one O2 molecule production, respectively. The GC calibration curves for H2 and O2 are provided in Figure S32. Furthermore, the raw GC chromatograms for the Z-scheme water splitting, under a full-arc condition, corresponding to the gas evolution shown in Figure 5a, are given in Figure S33.
4.7. Adsorption Study
The adsorption of I– and IO3– ions on the surface of the HEP and OEP was studied by measuring their concentrations using UV–Vis absorption spectroscopy. The determination of the I– ion concentration is straightforward as it can show the absorption peak at 225 nm without adding any reagent. However, IO3– shows absorption peaks at 288 and 352 nm only in the presence of an excess of I– and 0.2 M H2SO4. The calibration curves for I– and IO3– are shown in Figures S34 and S35, respectively. We monitored the peak at 352 nm to determine the concentration of IO3–.
4.8. Computational Details
Density functional theory (DFT) calculations were carried out to investigate the adsorption of IO3– and I– onto both WO3Pt4 and Na0.625WO2.875Pt4. The structure of Na0.625WO2.875Pt4, through XRD analysis, was deemed to be structurally similar enough to the experimentally investigated Na0.56WO3–x. For the adsorption calculations, BIOVIA Materials Studio (MS) package was used with the generalized gradient approximation (GGA) and Perdew–Burke–Ernzerhof (PBE) functional to account for the exchange-correlation energy.51 All calculations were spin-polarized, with the projector augmented wave (PAW) pseudopotentials and the Van der Waals interactions were described by the Grimme DFT-D3 method.52 The Kohn–Sham equations were solved with a convergence criterion of 2.0e-5 eV/atom for energy and 0.05 eV Å–1. Additionally, k-point sampling was carried out using the Monkhorst–Pack scheme with a (1 ×1 × 1) grid, and a cutoff energy of 489.8 eV was used.
Acknowledgments
The authors acknowledge the UK EPSRC (EP/S018204/2), Leverhulme Trust (RPG-2017-122), Royal Society Newton Advanced Fellowship grant (NAF\R1\191163), and Royal Society Leverhulme Trust Senior Research Fellowship (SRF\R1\21000153). XPS data collection was performed at the EPSRC National Facility for XPS (“HarwellXPS”), operated by Cardiff University and UCL, under Contract No. PR16195. The authors also thank Dr Jijia Xie, Ms Hui Wang, and Jianfeng Ye for constructive discussion on the experimental design.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.3c01312.
Structural representation of materials; SEM, TEM, and EDX of HEP and OEP; XRD, and XPS data before and after photocatalysis; UV–Vis of HEP and OEP; photoelectrochemical onset potential measurements; Mott–Schottky measurements; pH optimization; H2 evolution activity at longer wavelengths; control experiments; AQY comparison with optical spectra of HEP and OEP; DFT results; GC calibration and raw data; and adsorption of I– and IO3– (PDF)
Author Present Address
§ School of Chemistry, University of Nottingham, Nottingham NG7 2RD, UK
Author Present Address
∥ Department of Chemistry, Birla Institute of Science & Technology, Pilani, K. K. Birla Goa campus, Goa 403726, India
Author Contributions
⊥ M.T. and K.V. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
This paper was originally published ASAP on June 27, 2023, with an error in Figure 7. The corrected version was reposted on July 7, 2023.
Supplementary Material
References
- Pareek A.; Dom R.; Gupta J.; Chandran J.; Adepu V.; Borse P. H. Insights into Renewable Hydrogen Energy: Recent Advances and Prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. 10.1016/j.mset.2019.12.002. [DOI] [Google Scholar]
- Smith C.; Hill A. K.; Torrente-Murciano L. Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. 10.1039/C9EE02873K. [DOI] [Google Scholar]
- Sobrino F. H.; Monroy C. R.; Pérez J. L. H. Critical Analysis on Hydrogen as an Alternative to Fossil Fuels and Biofuels for Vehicles in Europe. Renewable Sustainable Energy Rev. 2010, 14, 772–780. 10.1016/j.rser.2009.10.021. [DOI] [Google Scholar]
- Wang Q.; Domen K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. 10.1021/acs.chemrev.9b00201. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Vogel A.; Sachs M.; Sprick R. S.; Wilbraham L.; Moniz S. J. A.; Godin R.; Zwijnenburg M. A.; Durrant J. R.; Cooper A. I.; Tang J. Current Understanding and Challenges of Solar-Driven Hydrogen Generation Using Polymeric Photocatalysts. Nat. Energy 2019, 4, 746–760. 10.1038/s41560-019-0456-5. [DOI] [Google Scholar]
- Maeda K.; Teramura K.; Lu D.; Takata T.; Saito N.; Inoue Y.; Domen K. Photocatalyst Releasing Hydrogen from Water. Nature 2006, 440, 295. 10.1038/440295a. [DOI] [PubMed] [Google Scholar]
- Tan H. L.; Amal R.; Ng Y. H. Alternative Strategies in Improving the Photocatalytic and Photoelectrochemical Activities of Visible Light-Driven BiVO4: A Review. J. Mater. Chem. A 2017, 5, 16498–16521. 10.1039/C7TA04441K. [DOI] [Google Scholar]
- Quan H.; Gao Y.; Wang W. Tungsten Oxide-Based Visible Light-Driven Photocatalysts: Crystal and Electronic Structures and Strategies for Photocatalytic Efficiency Enhancement. Inorg. Chem. Front. 2020, 7, 817–838. 10.1039/C9QI01516G. [DOI] [Google Scholar]
- Moniz S. J. A.; Shevlin S. A.; Martin D. J.; Guo Z.-X.; Tang J. Visible-Light Driven Heterojunction Photocatalysts for Water Splitting – a Critical Review. Energy Environ. Sci. 2015, 8, 731–759. 10.1039/C4EE03271C. [DOI] [Google Scholar]
- Kato H.; Sasaki Y.; Shirakura N.; Kudo A. Synthesis of Highly Active Rhodium-Doped SrTiO3 Powders in Z-Scheme Systems for Visible-Light-Driven Photocatalytic Overall Water Splitting. J. Mater. Chem. A 2013, 1, 12327–12333. 10.1039/c3ta12803b. [DOI] [Google Scholar]
- Wang Y.; Suzuki H.; Xie J.; Tomita O.; Martin D. J.; Higashi M.; Kong D.; Abe R.; Tang J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. 10.1021/acs.chemrev.7b00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatomi T.; Domen K. Reaction Systems for Solar Hydrogen Production via Water Splitting with Particulate Semiconductor Photocatalysts. Nat. Catal. 2019, 2, 387–399. 10.1038/s41929-019-0242-6. [DOI] [Google Scholar]
- Pinaud B. A.; Benck J. D.; Seitz L. C.; Forman A. J.; Chen Z.; Deutsch T. G.; James B. D.; Baum K. N.; Baum G. N.; Ardo S.; Wang H.; Miller E.; Jaramillo T. F. Technical and Economic Feasibility of Centralized Facilities for Solar Hydrogen Production via Photocatalysis and Photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. 10.1039/c3ee40831k. [DOI] [Google Scholar]
- Wang Q.; Okunaka S.; Tokudome H.; Hisatomi T.; Nakabayashi M.; Shibata N.; Yamada T.; Domen K. Printable Photocatalyst Sheets Incorporating a Transparent Conductive Mediator for Z-Scheme Water Splitting. Joule 2018, 2, 2667–2680. 10.1016/j.joule.2018.08.003. [DOI] [Google Scholar]
- Idriss H. The Elusive Photocatalytic Water Splitting Reaction Using Sunlight on Suspended Nanoparticles: Is There a Way Forward?. Catal.: Sci. Technol. 2020, 10, 304–310. 10.1039/C9CY01818B. [DOI] [Google Scholar]
- Maeda K. Z-Scheme Water Splitting Using Two Different Semiconductor Photocatalysts. ACS Catal. 2013, 3, 1486–1503. 10.1021/cs4002089. [DOI] [Google Scholar]
- Chen S.; Takata T.; Domen K. Particulate Photocatalysts for Overall Water Splitting. Nat. Rev. Mater. 2017, 2, 17050. 10.1038/natrevmats.2017.50. [DOI] [Google Scholar]
- Abe R.; Shinmei K.; Koumura N.; Hara K.; Ohtani B. Visible-Light-Induced Water Splitting Based on Two-Step Photoexcitation between Dye-Sensitized Layered Niobate and Tungsten Oxide Photocatalysts in the Presence of a Triiodide/Iodide Shuttle Redox Mediator. J. Am. Chem. Soc. 2013, 135, 16872–16884. 10.1021/ja4048637. [DOI] [PubMed] [Google Scholar]
- Nishioka S.; Hojo K.; Xiao L.; Gao T.; Miseki Y.; Yasuda S.; Yokoi T.; Sayama K.; Mallouk T. E.; Maeda K. Surface-Modified, Dye-Sensitized Niobate Nanosheets Enabling an Efficient Solar-Driven Z-Scheme for Overall Water Splitting. Sci. Adv. 2023, 8, eadc9115 10.1126/sciadv.adc9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe R.; Sayama K.; Domen K.; Arakawa H. A New Type of Water Splitting System Composed of Two Different TiO2 Photocatalysts (Anatase, Rutile) and a IO3–/I– Shuttle Redox Mediator. Chem. Phys. Lett. 2001, 344, 339–344. 10.1016/S0009-2614(01)00790-4. [DOI] [Google Scholar]
- Konta R.; Ishii T.; Kato H.; Kudo A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. 10.1021/jp049556p. [DOI] [Google Scholar]
- Martin D. J.; Reardon P. J. T.; Moniz S. J. A.; Tang J. Visible Light-Driven Pure Water Splitting by a Nature-Inspired Organic Semiconductor-Based System. J. Am. Chem. Soc. 2014, 136, 12568–12571. 10.1021/ja506386e. [DOI] [PubMed] [Google Scholar]
- Maeda K.; Lu D.; Domen K. Solar-Driven Z-Scheme Water Splitting Using Modified BaZrO3–BaTaO2N Solid Solutions as Photocatalysts. ACS Catal. 2013, 3, 1026–1033. 10.1021/cs400156m. [DOI] [Google Scholar]
- Chen S.; Qi Y.; Hisatomi T.; Ding Q.; Asai T.; Li Z.; Ma S. S. K.; Zhang F.; Domen K.; Li C. Efficient Visible-Light-Driven Z-Scheme Overall Water Splitting Using a MgTa2O6–xNy /TaON Heterostructure Photocatalyst for H2 Evolution. Angew. Chem., Int. Ed. 2015, 54, 8498–8501. 10.1002/anie.201502686. [DOI] [PubMed] [Google Scholar]
- Kato T.; Hakari Y.; Ikeda S.; Jia Q.; Iwase A.; Kudo A. Utilization of Metal Sulfide Material of (CuGa)1–XZn2xS2 Solid Solution with Visible Light Response in Photocatalytic and Photoelectrochemical Solar Water Splitting Systems. J. Phys. Chem. Lett. 2015, 6, 1042–1047. 10.1021/acs.jpclett.5b00137. [DOI] [PubMed] [Google Scholar]
- Iwashina K.; Iwase A.; Ng Y. H.; Amal R.; Kudo A. Z-Schematic Water Splitting into H2 and O2 Using Metal Sulfide as a Hydrogen-Evolving Photocatalyst and Reduced Graphene Oxide as a Solid-State Electron Mediator. J. Am. Chem. Soc. 2015, 137, 604–607. 10.1021/ja511615s. [DOI] [PubMed] [Google Scholar]
- Miseki Y.; Fujiyoshi S.; Gunji T.; Sayama K. Photocatalytic Z-Scheme Water Splitting for Independent H2/O2 Production via a Stepwise Operation Employing a Vanadate Redox Mediator under Visible Light. J. Phys. Chem. C 2017, 121, 9691–9697. 10.1021/acs.jpcc.7b00905. [DOI] [Google Scholar]
- Qi Y.; Chen S.; Li M.; Ding Q.; Li Z.; Cui J.; Dong B.; Zhang F.; Li C. Achievement of Visible-Light-Driven Z-Scheme Overall Water Splitting Using Barium-Modified Ta3N5 as a H2-Evolving Photocatalyst. Chem. Sci. 2017, 8, 437–443. 10.1039/C6SC02750D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B.; Qi Y.; Cui J.; Liu B.; Xiong F.; Jiang X.; Li Z.; Xiao Y.; Zhang F.; Li C. Synthesis of BaTaO2N Oxynitride from Ba-Rich Oxide Precursor for Construction of Visible-Light-Driven Z-Scheme Overall Water Splitting. Dalton Trans. 2017, 46, 10707–10713. 10.1039/C7DT00854F. [DOI] [PubMed] [Google Scholar]
- Thangamuthu M.; Ruan Q.; Ohemeng P. O.; Luo B.; Jing D.; Godin R.; Tang J. Polymer Photoelectrodes for Solar Fuel Production: Progress and Challenges. Chem. Rev. 2022, 122, 11778–11829. 10.1021/acs.chemrev.1c00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Miao T. J.; Tang J. Charge Carrier Dynamics and Reaction Intermediates in Heterogeneous Photocatalysis by Time-Resolved Spectroscopies. Chem. Soc. Rev. 2022, 51, 5777–5794. 10.1039/D1CS01164B. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Chen E.; Tang J. Insight on Reaction Pathways of Photocatalytic CO2 Conversion. ACS Catal. 2022, 12, 7300–7316. 10.1021/acscatal.2c01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Liu T.; Li H.; Liu B.; Yang L. Tungsten-Based Photocatalysts with UV–Vis–NIR Photocatalytic Capacity: Progress and Opportunity. Tungsten 2019, 1, 247–257. 10.1007/s42864-020-00031-z. [DOI] [Google Scholar]
- Huang Z.-F.; Song J.; Pan L.; Zhang X.; Wang L.; Zou J.-J. Tungsten Oxides for Photocatalysis, Electrochemistry, and Phototherapy. Adv. Mater. 2015, 27, 5309–5327. 10.1002/adma.201501217. [DOI] [PubMed] [Google Scholar]
- Paik T.; Cargnello M.; Gordon T. R.; Zhang S.; Yun H.; Lee J. D.; Woo H. Y.; Oh S. J.; Kagan C. R.; Fornasiero P.; Murray C. B. Photocatalytic Hydrogen Evolution from Substoichiometric Colloidal WO3–x Nanowires. ACS Energy Lett. 2018, 3, 1904–1910. 10.1021/acsenergylett.8b00925. [DOI] [Google Scholar]
- Wang L.; Tsang C.-S.; Liu W.; Zhang X.; Zhang K.; Ha E.; Kwok W.-M.; Park J. H.; Suk Lee L. Y.; Wong K.-Y. Disordered Layers on WO3 Nanoparticles Enable Photochemical Generation of Hydrogen from Water. J. Mater. Chem. A 2019, 7, 221–227. 10.1039/C8TA09446B. [DOI] [Google Scholar]
- Azimirad R.; Akhavan O.; Moshfegh A. Z. Simple Method to Synthesize NaxWO3 Nanorods and Nanobelts. J. Phys. Chem. C 2009, 113, 13098–13102. 10.1021/jp902189h. [DOI] [Google Scholar]
- Brown B. W.; Banks E. The Sodium Tungsten Bronzes1,2. J. Am. Chem. Soc. 1954, 76, 963–966. 10.1021/ja01633a004. [DOI] [Google Scholar]
- Lee W. H.; Hwang H.; Moon K.; Shin K.; Han J. H.; Um S. H.; Park J.; Cho J. H. Increased Environmental Stability of a Tungsten Bronze NIR-Absorbing Window. Fibers Polym. 2013, 14, 2077–2082. 10.1007/s12221-013-2077-0. [DOI] [Google Scholar]
- Nayak A. K.; Lee S.; Choi Y. I.; Yoon H. J.; Sohn Y.; Pradhan D. Crystal Phase and Size-Controlled Synthesis of Tungsten Trioxide Hydrate Nanoplates at Room Temperature: Enhanced Cr(VI) Photoreduction and Methylene Blue Adsorption Properties. ACS Sustainable Chem. Eng. 2017, 5, 2741–2750. 10.1021/acssuschemeng.6b03084. [DOI] [Google Scholar]
- Liu J.; Han L.; An N.; Xing L.; Ma H.; Cheng L.; Yang J.; Zhang Q. Enhanced Visible-Light Photocatalytic Activity of Carbonate-Doped Anatase TiO2 Based on the Electron-Withdrawing Bidentate Carboxylate Linkage. Appl. Catal., B 2017, 202, 642–652. 10.1016/j.apcatb.2016.09.057. [DOI] [Google Scholar]
- Wang P.-Q.; Bai Y.; Luo P.-Y.; Liu J.-Y. Graphene–WO3 Nanobelt Composite: Elevated Conduction Band toward Photocatalytic Reduction of CO2 into Hydrocarbon Fuels. Catal. Commun. 2013, 38, 82–85. 10.1016/j.catcom.2013.04.020. [DOI] [Google Scholar]
- Kudo A.; Omori K.; Kato H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. 10.1021/ja992541y. [DOI] [Google Scholar]
- Dunkle S. S.; Helmich R. J.; Suslick K. S. BiVO4 as a Visible-Light Photocatalyst Prepared by Ultrasonic Spray Pyrolysis. J. Phys. Chem. C 2009, 113, 11980–11983. 10.1021/jp903757x. [DOI] [Google Scholar]
- Yang Y.; Chen J.; Liu X.; Qiu M.; Liu L.; Gao F. Oxygen Vacancy-Mediated WO3 Nanosheets by Etched {200} Facets and the Efficient Visible-Light Photocatalytic Oxygen Evolution. New J. Chem. 2019, 43, 16391–16395. 10.1039/C9NJ04286E. [DOI] [Google Scholar]
- Ohno T.; Saito S.; Fujihara K.; Matsumura M. Photocatalyzed Production of Hydrogen and Iodine from Aqueous Solutions of Iodide Using Platinum-Loaded TiO2 Powder. Bull. Chem. Soc. Jpn. 1996, 69, 3059–3064. 10.1246/bcsj.69.3059. [DOI] [Google Scholar]
- Wang Q.; Hisatomi T.; Jia Q.; Tokudome H.; Zhong M.; Wang C.; Pan Z.; Takata T.; Nakabayashi M.; Shibata N.; Li Y.; Sharp I. D.; Kudo A.; Yamada T.; Domen K. Scalable Water Splitting on Particulate Photocatalyst Sheets with a Solar-to-Hydrogen Energy Conversion Efficiency Exceeding 1%. Nat. Mater. 2016, 15, 611–615. 10.1038/nmat4589. [DOI] [PubMed] [Google Scholar]
- Abe R.; Higashi M.; Domen K. Overall Water Splitting under Visible Light through a Two-Step Photoexcitation between TaON and WO3 in the Presence of an Iodate–Iodide Shuttle Redox Mediator. ChemSusChem 2011, 4, 228–237. 10.1002/cssc.201000333. [DOI] [PubMed] [Google Scholar]
- Tsang C.; Lai S. Y.; Manthiram A. Reduction of Aqueous Na2WO4 by NaBH4 at Ambient Temperatures To Obtain Lower Valent Tungsten Oxides. Inorg. Chem. 1997, 36, 2206–2210. 10.1021/ic9610039. [DOI] [PubMed] [Google Scholar]
- Takata T.; Jiang J.; Sakata Y.; Nakabayashi M.; Shibata N.; Nandal V.; Seki K.; Hisatomi T.; Domen K. Photocatalytic Water Splitting with a Quantum Efficiency of Almost Unity. Nature 2020, 581, 411–414. 10.1038/s41586-020-2278-9. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.