Abstract
Background
Childhood immunization remains one of the most cost-effective public health interventions. Globally, millions of children are not being reached with safe and effective vaccines and Nigeria has the highest number of unprotected children.
Objective
The effects of locally adapted interventions on vaccination timeliness and completeness were studied amongst Fulani populations across 6 health facilities in 2 districts of Bauchi State, Nigeria.
Methods
The intervention group consisted of newborns who received 5-color-coded bracelets representing different immunization contacts, while the control group had no bracelets. Vaccination rates across contacts were followed for 11 months. In addition, mothers of children in the intervention group were voluntarily recruited as peer-to-peer mobilizers (PPM).
Results
In this study, 435 children were studied. Vaccination completeness was higher in the intervention group compared to the control group at all contacts during follow-up. The difference was most noticeable at the fifth contact, with 158/256 (62%) children in the intervention group completing, compared to 73/179 (41%) in the control group (P<0.0001). Vaccination timeliness was better in the intervention group compared to the control one, which reached statistical significance at the second and third vaccination contacts (P<0.05). 68% of women volunteered as PPM and recruited 82 additional children for vaccination.
Conclusion
This study demonstrated the feasibility of a composite intervention (bracelets and PPM) to increase the completeness and timeliness of childhood immunization and provided preliminary evidence for its efficacy among Fulani populations in Nigeria. Findings from this pilot study should be confirmed through a larger cluster randomized controlled trial.
Key words: childhood vaccinations, vaccination timeliness, vaccination completeness, vaccination reminders
Introduction
Routine childhood immunization is considered one of the most cost-effective public health interventions for the prevention of morbidity and mortality related to vaccine-preventable diseases (VPD).1-4 Despite this, coverage of childhood vaccination remains low in many sub-Saharan African countries including Nigeria.5-8 With a recent estimate of 3 million children that were not reached with the third dose of pentavalent vaccines (diphtheria-tetanus, pertussis, hepatitis B, and haemophilus influenzae type B), Nigeria accounts for the highest number of unprotected children reported globally, at exactly 19.5 million.8 In addition to immunization coverage, vaccine effectiveness for children that present for routine vaccination depends also on the timeliness of its administration.9-11 Thus, to achieve maximum protection against VPD, a child should receive all scheduled vaccinations within the recommended intervals.12-14
In 2012, Nigeria changed from the diphtheria-tetanus-pertussis vaccination scheme to the pentavalent vaccination scheme (Penta).15 According to the Nigeria Demographic and Health Survey 2018 report, only 32% of children aged 12-23 months received all routine vaccinations, with urban children twice as likely to receive them than rural ones.16 Thus, it is not surprising that VPD (in particular, pneumonia and diarrheal diseases) are the most common causes of death in children under 5 years old globally,17-19 and equally in Nigeria.20 Consistent with the recommendation of the Expanded Program of Immunization of the World Health Organization (WHO), the national routine childhood vaccination schedule in Nigeria consists of several vaccines given over five contacts (Figure 1). In 2019, this was extended to 6 contacts following the introduction of the second dose of the measles vaccine at 15 months.21 Innovative interventions are urgently needed to improve the overall coverage and effectiveness of childhood vaccination in Nigeria, especially among the nomadic ethnic populations. About 60% of the population in Bauchi State are Fulanis, a traditionally nomadic ethnic group widely distributed in the countries of the Sahel Zone with particularly low rates of vaccination coverage, due to high mobility, low education level, and cultural reasons.22-24
Study objectives
The overall objective of this study was to test the feasibility and the effects of a pilot intervention aimed at improving vaccination timeliness and completeness and, thus, its effectiveness among Fulani populations of Nigeria. Specific objectives were: i) to investigate the feasibility of a color-coded bracelet intervention among ethnic Fulani populations; ii) to assess and compare the vaccination completeness in the intervention and control populations; iii) to assess and compare the vaccination timeliness in the intervention and control populations; iv) to study the feasibility and the effects of adding peer-to-peer mobilizers (PPM) for vaccination scale up.
Materials and Methods
Ethical considerations
Ethical Approval was obtained from Bauchi State Health Research Ethics Committee under the Ministry of Health (protocol approval number: NREC/12/05/2-13/2018/03). Mothers/caretakers of study children were duly informed about the study details and their written consent was obtained.
Study design and area
This study was designed as a controlled pilot intervention trial. It took place in 2018 in 2 districts, Gamawa and Katagum in Bauchi State, north-eastern Nigeria (Figure 2). At the time of this study, Gamawa district had a projected population of 424,701, while Katagum district had 442,073 inhabitants. The two study district populations represent around 12% of the estimated 7 million people in the state.25 The study area is characterized by low education and high childhood and maternal mortality rates.16,26 Subsistence farming is the main economic activity in the area.26 Being the administrative headquarter of the Bauchi Northern Senatorial Zone, Katagum is categorized as an urban district, while Gamawa is a rural district. These districts and the respective study health facilities - one urban and two rural pairs - were selected purposively based on the number of births. Health facility pairs were selected by comparable characteristics and group assignments were done by lottery (Table 1).
Figure 1.

Entry and exit bracelets used in infants of northeastern Nigeria.
Figure 2.
Map of Africa, Nigeria, and Bauchi state, showing the study area (marked in red).
In the urban Katagum district, the Federal Medical Center Azare and the General Hospital Azare were chosen as the pair of health facilities. In the rural Gamawa district, 4 health facilities were chosen and paired, namely the Gamawa Town Maternity, the primary health care (PHC) center Gadiya, the PHC center Gololo, and the PHC center Wabu. These formed the 3 intervention and 3 control facilities (Table 1).
Study population
Newborns (0-14 days old) of Fulani families attending vaccination sessions in any of the six selected study health facilities in 2018 were invited to participate. The two weeks (14 days) maximum age of enrollment is related to the cut-off point of the hepatitis B 0 vaccination window in line with the national policy. Enrollment of the study population took place across sites for 3 months from February to May 2018.
The inclusion criteria were i) the mother or the father or both belong to the Fulani ethnic group; ii) children below 2 weeks of age that are under routine health care situation considered as eligible for vaccination based on the national immunization guidelines; iii) written informed consent of mothers/caregivers (acknowledging to have understood the study protocol and signing or thumbprinting the consent form); iv) intention of mothers/caregivers to remain for at least one year within the study area and with no intention to take the study child for routine immunization to other places.
Study procedures
All consenting mothers/caretakers attending the study health facilities with eligible newborns were recruited and followed up for 11 months. For each study child, the enrollment officer filled in a consent form signed or thumb printed by the mother/caregiver, before filling the enrollment form. Key variables collected in the enrolment form include the unique identification number, names of the study child and its caretaker, health facility record number, date and place of birth of study child, educational status of caretaker and their occupation, ethnicity, number of siblings and birth order, address, and telephone numbers. Telephone numbers and house addresses collected were used for reminder calls or follow-ups.
The children in the 3 intervention sites (bracelet group) were provided with locally produced 5-color-coded bracelets fixed together as single, but detachable units. The caregivers were informed about the meaning of the bracelet colors regarding vaccine types, the susceptibility to particular VPD (at each contact) in case of failure to meet up with scheduled appointments, and the timing of these vaccinations. Therefore, our study used these colorcoded bracelets as visible reminders for different contacts of vaccination due dates to enhance compliance with the immunization schedules (Figure 1 and Table 2). Bracelets are common cultural adornments, worn as part of normal dressing amongst the Fulani populations in Nigeria and elsewhere. Bright colors were purposely selected based on their affiliation and are common across most local markets. For our study, the sequencing of the color order (from red to green) was meant to connote risks associated with lack of vaccination. As for the exit bracelet, we used two colors (pink for girls and black for boys) as shown in Figure 1. To ensure uniformity of messaging at the intervention sites (bracelet group), a short audio recording in the 2 languages (Fulani and Hausa) was played to each mother/caregiver of the study children during every vaccination visit to the health facilities. Upon every vaccination, the corresponding unit of the bracelet was cut off with a pair of scissors. At the fifth contact, a different design exit bracelet (Figure 3) was issued to the child as a replacement gift to indicate his/her fully immunized status.
Table 1.
Assignment of health facilities in urban Katagum district and rural Gamawa district of northeastern Nigeria into intervention and control groups.
| S/N | Facility | Grouping | Setting | Average monthly institutional delivery | Allocated monthly sample target for the study |
|---|---|---|---|---|---|
| 1 | General Hospital Azare, Katagum district | Intervention (bracelet) | Urban 1 | 45 | 20 |
| 2 | Federal Medical Center Azare, Katagum district | Control (no bracelet) | Urban 2 | 135 | 30 |
| 3 | Gamawa town maternity, Gamawa district | Intervention (bracelet) | Rural 1 | 35 | 25 |
| 4 | PHC center Gololo, Gamawa district | Control (no bracelet) | Rural 1 | 22 | 15 |
| 5 | PHC center Wabu, Gamawa district | Intervention (bracelet) | Rural 2 | 36 | 25 |
| 6 | PHC center Gadiya, Gamawa district | Control (no bracelet) | Rural 2 | 23 | 15 |
PHC, primary health care.
Table 2.
Color coding of bracelets for the five vaccination contacts.
| Vaccination contact | Vaccinations | Bracelet color |
|---|---|---|
| (1) At birth | BCG, HepB0, OPV0 | red |
| (2) At 6 weeks | Penta1, PCV1 and OPV1 | yellow |
| (3) At 10 weeks | Penta2, PCV2, OPV2 | white |
| (4) At 14 weeks | Penta3, PCV3, and IPV | blue |
| (5) At 9 months | Measles & Yellow fever | green |
BCG, bacille Calmette-Guerin; HepB0, hepatitis B; OPV0, poliomyelitis.
Children who came on the vaccination dates with damaged bracelets got them replaced according to their vaccination status. At the 3 control sites, no bracelets were issued to any child, but the mothers/caregivers received the standard information provided at routine immunization services including the 6 basic immunization messages: i) what vaccines were given; ii) the number of vaccination visits the child still needed to complete; iii) side effects that may occur and how to manage them; iv) place and time of the next immunization; v) instruction to bring the child back if sick; vi) taking good care of vaccination card and always bring it along for subsequent visits. During follow-up, the dates study participants visited the study sites were recorded for each of the 5 vaccination contacts according to Nigeria’s immunization policy.
At the intervention sites (bracelet group), mothers were also voluntarily enrolled for PPM to motivate other women to bring their children to promote vaccination uptake. The enrollment officer indicated in the enrollment form whether the mother/caregiver agreed to participate as PPM. The health worker in charge of vaccination provided key mobilization messages as well as specific PPM cards to the enrolled PPM mother/caregiver. PPM messages emphasized the importance of getting as many children as possible vaccinated. The key messages from the PPM to the peer include: i) the vaccination of a child is a basic right; ii) the vaccination of as many children as possible also protects other children; iii) I have been with my child to a given health facility for his/her vaccinations; iv) if you go with your child to this health facility, please take the PPM card with you; v) if you have further questions, feel free to ask the health worker, who will be willing to address your concerns. The interventions employed in the study and control group are summarized in Table 3.
Recruitment and training of health workers
Before the beginning of the enrollment, a 3-day training was conducted at Azare (Katagum District) from 16-18 February 2018. Attendees were 2 routine immunization service providers from each of the 6 health facilities and 1-2 enrollment officers per health facility. The training was facilitated by 5 study team members and the state immunization officer and focused on: i) the enrollment process and orientation on how to prepare health facility and immunization site before clients’ arrival; ii) effective communication with caregivers during sessions; iii) assessment of infants; iv) correct technique of vaccination; v) vaccine and cold chain management; vi) good data recording and archiving; vii) health education messages; viii) concept of voluntary PPM. For health workers in the intervention sites, the training focused additionally on the bracelet introduction, including the purpose of the intervention, the color connotation for each contact, how to fix the bracelets to babies, when and how to change bracelets, and documentation in the register. Recruited health workers were provided with a monthly stipend for 12 months to cover study activities.
Monitoring and supervision
During supervision by the research and state immunization teams, a process checklist was administered, and on-the-job training was conducted at the health facilities with identified gaps. The focus of the supervision was to monitor service provision, and adherence to the study’s standard operating procedures and to correctly identify deficiencies. After each site visit, the supervisors documented observations and the corrective measures applied in a special supervision book. While most of the monitoring and supervisory feedback were satisfactory, key identified and resolved gaps include data entry problems, inadequate adverse events following immunization tools, kits and PPM cards, and low session turnout.
Data management and quality assurance
The study data consisted of enrollment forms and vaccination data. The vaccination data were collected using a special child research register. From the 450 records of enrollees, 15 records (8 from intervention sites and 7 from control facilities) were excluded from the study following data cleaning which revealed that they were older than 2 weeks. 8 mothers in this study (7 from intervention and 1 from control facilities) had twins enrolled in the study.
Analysis
Completeness of vaccination was defined as receipt of all recommended vaccines at respective contacts (e.g. bacille Calmette- Guerin, hepatitis B, and poliomyelitis at contact 1) regardless of the timing of administration.
Vaccination timeliness was defined as the time when a child received the vaccine in comparison to the time recommended. Therefore, vaccination time can be early (before the day it is due), on the due date (the actual date recommended by the national vaccination schedule), after the date (up to 2 weeks after the due date), and late (after 2 weeks of recommended date). All vaccinations that occurred on due dates and after dates (up to 2 weeks after the due date) are considered timely based on the national guideline and WHO recommendations. The dependent variables (completeness and timeliness) were analyzed against some independent variables such as the study group, age of the child, rural/urban categorization of study sites and health facilities, and child gender. Data were entered in Excel tables and analyzed with Epi Info version 7 (CDC, Atlanta Georgia, USA) and Statistical Package for Social Science (SPSS) version 20 (IBM, New York, USA) with respect to descriptive parameters such as proportions and means. Mann- Whitney/Wilcoson two-sample test as obtained from Epi Info version 7 was used in calculating the P values.
Results
Most mothers (82%) recruited in this study were housewives and aged under 30 years (67%). There were no major differences regarding socio-demographic characteristics between the two groups. Recruitment of study children started on February 21, 2018 and was finalized on May 27, 2018. All children were followed up across the 5 contacts for 11 months. A total of 435 (256 intervention group, 179 control group) infants were finally followed up (Table 4). At the end of the study, 231/435 (53%) study children completed their vaccination schedule at the fifth contact; 158/256 (62%) were from the intervention group compared to 73/179 (41%) from the control group, a highly significant difference (P=0.0001).
Table 3.
Description of intervention and control procedures.
| Type of Intervention | Intervention group | Control group |
|---|---|---|
| Due vaccines given | Yes | Yes |
| Wearing/cutting of bracelets | Yes | No |
| Six key immunization messages | Yes | Yes |
| Appointment cards with the date of the next visit | Yes | Yes |
| Monitoring for adverse events following immunization (AEFI) | Yes | Yes |
| Targeted one-on-one messaging surrounding the bracelets | Yes | No |
Completeness of vaccination
Figure 3 shows the completeness of vaccination at different contacts in both the intervention and control groups by vaccination contact and vaccine type. Completeness of vaccinations was significantly (P<0.05) higher in the intervention group compared to the control one across all contacts. The difference was most pronounced at the fifth contact (P<0.0001).
Vaccination timeliness
Figure 4 shows the timeliness of the vaccinations by vaccination contact according to the study groups. Vaccination timeliness was consistently reduced over time in both groups and was consistently higher in the intervention one compared to the control one at number 2-5 follow-up vaccination contacts. The difference between groups was significant at vaccination contact 2 and 3 (P<0.05).
Vaccination compliance and bracelet retention
None of the mothers/caregivers who came back with their child to a study health facility refused vaccinations during followup. The bracelet retention rate (proportion of children whose bracelet was still intact after the fifth contact) was 99% during the 11-month follow-up period.
Effects of peer-to-peer mobilizers
164/240 (68%) mothers and caregivers volunteered as PPM. PPM were able to recruit and refer 82 children for vaccination, all of whom were enrolled and thus part of the study population. Rural mobilizers recruited roughly 2 times the number of children than their urban peers (Figure 5).
Discussion
The main findings from this study are that a bracelet intervention appears to be feasible and well-accepted in Fulani populations of Nigeria and that there is preliminary evidence for this intervention being effective. Infants in the intervention group showed significantly higher rates of routine vaccination timeliness and completeness compared to control infants. Although vaccination completeness remained a challenge in both intervention and control settings, these findings are promising and support comparable findings from other studies with similar methodological approaches, such as vaccine indicator bands to serve as electronic colorcoded change reminders.27
Figure 3.
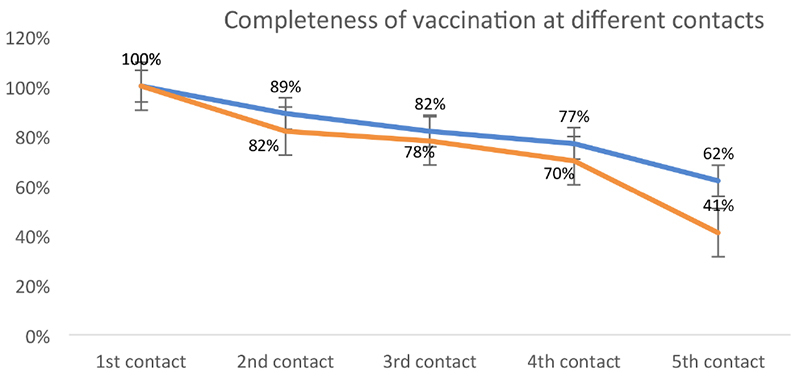
Completeness of vaccination across different contacts for intervention (n=256) and control group (n=179) children.
Figure 4.
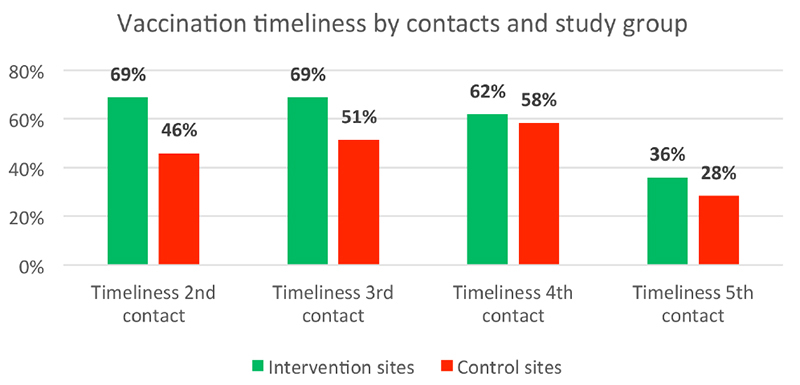
Timelines of vaccinations during follow-up by vaccination date and study group.
Figure 5.
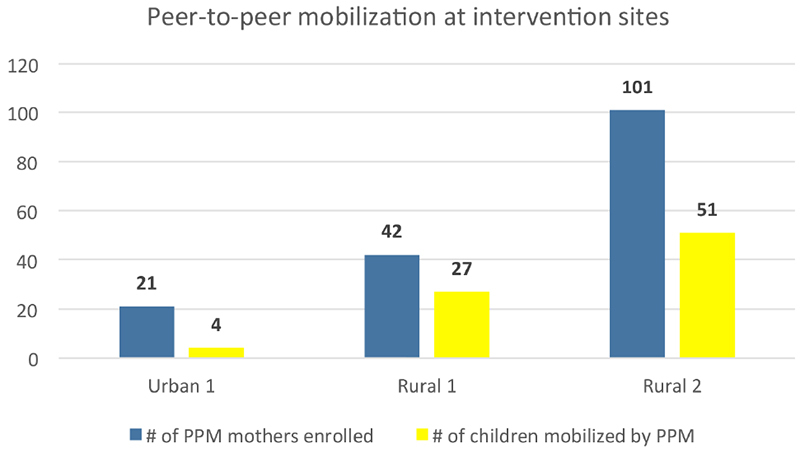
Effects of peer-to-peer mobilization on the number of additionally recruited children in the rural compared to the urban study area.
Table 4.
Proportion of children recruited in each facility.
| Facility name | Type | Type | Frequency | Percent |
|---|---|---|---|---|
| Federal Medical Center Azare (Katagum district) | No Bracelet | Urban | 83 | 19 |
| PHC Gadiya (Gamawa district) | No Bracelet | Rural | 50 | 11 |
| PHC Gololo (Gamawa district) | No Bracelet | Rural | 46 | 11 |
| General Hospital, Azare (Katagum district) | Bracelet | Urban | 63 | 14 |
| Gamawa Town Maternity (Gamawa district) | Bracelet | Rural | 86 | 20 |
| PHC Wabu (Gamawa district) | Bracelet | Rural | 107 | 25 |
| Total | 435 | 100 |
Specific reminders have been used in national vaccination programs across different settings. These include home visits, appointment dates on immunization cards, reminder calls, text messaging, and financial incentives, but with variable results.28-33 Text messaging appears to be the most popular, but its applicability is limited in many countries by cost considerations, poor network coverage, and low literacy levels, as also shown in Nigeria.33,34 Additionally, transmission delays due to power cuts,31 and messaging (data entry) errors were reported as constraints.35 In our study only about a third (153/427) of mothers/caregivers had basic Western education and only 35% (154/435) owned a personal phone. Vaccine indicator and reminder ankle bands for infants have successfully been tried in Pakistan,36,37 and there are first experiences with such an intervention in Nigeria.27 Bracelets are quite common among Fulani populations of West Africa, and this is to our knowledge the first study on the effects of such a local adornment regarding adherence to immunization schedules in Africa.
Improvements in vaccination timeliness were also achieved through text messaging and automated calls in Pakistan and Nigeria.38,39 However, even with high mobile phone coverage in both countries, disparities of access by geography and gender as well as low literacy levels need to be considered as major barriers to an effective large-scale application of such a phone-based intervention. As mobile coverage will increase also in rural areas, a combination of a bracelet intervention together with a specific text messaging intervention could be tried in future trials, particularly in reducing the observed high drop-out rates between vaccination contact 4 and 5, as shown in our study. The 2018 reported districtwide dropout rates from Penta 1 to Penta 3 (15.3% for Gamawa and 7.6% for Katagum) were comparable to the calculated dropout rates for our intervention sites (13.4%) and control health facilities (14.6%).40
Another relevant finding from our study is that PPM was shown to increase the number of children coming for vaccination to respective study health centers. PPM represent an effective intervention for many public health challenges, such as community- based peer education on HIV/AIDS,41,42 and peer-to-peer mobilization for HIV testing services.43 Regarding timeliness and completeness of vaccinations, a study on social signaling (a form of PPM using differently colored wrist bracelets) has shown positive effects in African countries.44 Moreover, a study on the effects of using religious peer leaders in Iraq has also given some evidence for this intervention to improve the uptake of routine childhood vaccination.45
Finally, the fact that PPM effects were much more pronounced in the rural as compared to the urban intervention areas of our study could be explained by the close and interconnected nature of the rural settings, having largely similar patterns of social behavior and beliefs.
Our study has some limitations. Firstly, it is a small-scale pilot study in a selected population with limited generalizability. Secondly, the assignment for the intervention and control arm was not random, thus, the findings can be biased. Thirdly, dropout rates towards the end of the follow-up period were rather high in both the intervention and the control group. Lastly, for obvious reasons, the study was not blinded.
Conclusions
This study has demonstrated the feasibility of a composite intervention (bracelets accompanied by PPM) in the ethnic minority populations of Fulani in northeastern Nigeria. Bracelets may become a sustainable intervention as they are generally accepted as a traditional adornment across many cultural groups in Nigeria and their cost (~0.3 USD/bracelet) is very moderate. The data from this study provide the first evidence for the effectiveness of this intervention in improving the completeness and timeliness of infant vaccination in a major ethnic minority group of sub-Saharan African countries. The findings from this study need to be confirmed through a larger cluster randomized control trial.
Acknowledgments
The authors would like to thank the mothers and caregivers for their participation and those of their enrolled children in this study. The authors also appreciate the support from the Bauchi State Ministry of Health and the State Primary Health Care Development Agency, and thank the staff of the six study facilities and the members of the research teams, the National Primary Health Care Development Agency (NPHCDA), and the University of Heidelberg, Germany.
Funding Statement
Funding: this study was funded by the Bill & Melinda Gates Foundation (BMGF) through a Grand Challenge Exploration phase 1 award to the NPHCDA (ID number OPP1182555).
References
- 1.Ozawa S, Clark S, Portnoy A, et al. Estimated economic impact of vaccinations in 73 low- and middle-income countries, 2001-2020. Bull World Health Organ 2017;95:629-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet. Looking beyond the decade of vaccines. Lancet 2018;392:2139. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ. Immunizations and vaccines: a decade of successes and reversals, and a call for ‘vaccine diplomacy. Int Health 2019;11:331-3. [DOI] [PubMed] [Google Scholar]
- 4.Ozawa S, Stack ML, David MB, et al. During the 'decade of vaccines,' the lives of 6.4 million children valued at $231 billion could be saved. Health Aff (Millwood) 2011;30:1010-20. [DOI] [PubMed] [Google Scholar]
- 5.Peck M, Gacic-Dobo M, Diallo MS, et al. Global routine vaccination coverage, 2018. Morbidity & mortality Weekly Report (MMWR) 2019;68:937-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosser JF, Gagne-Maynard W, Rao PC, et al. Mapping diphtheria- pertussis-tetanus vaccine coverage in Africa, 2000-2016: a spatial and temporal modelling study. Lancet 2019;393:1843-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wariri O, Edem B, Nkereuwem E, et al. Tracking coverage, dropout and multidimensional equity gaps in immunisation systems in West Africa, 2000-2017. BMJ Global Health 2019;4:e001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO UNICEF. Progress and challenges with achieving universal immunization coverage; 2020. Available from: https://cdn.who.int/media/docs/default-source/immunization/coverage/who-immuniz.pdf?sfvrsn=72fd7237_2&download=true. Accessed: 17 September 2020. [Google Scholar]
- 9.Shrivastwa N, Gillespie BW, Lepkowski JM, Boulton ML. Vaccination timeliness in children under india's universal immunization program. Pediatr Infect Dis J 2016;35:955-60. [DOI] [PubMed] [Google Scholar]
- 10.Ateudjieu J, Yakum MN, Goura APet al. EPI immunization coverage, timeliness and dropout rate among children in a west cameroon health district: a cross sectional study. BMC Public Health 2020;20:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alrowaili GZR, Dar UF, Bandy AH. May we improve vaccine timeliness among children? A cross sectional survey in northern saudi arabia. J Family Community Med 2019;26:113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adokiya MN, Awoonor-Williams JK, Beiersmann C, Müller O. Reporting completeness and timeliness of the integrated disease surveillance and response system in northern ghana. Ghana Med J 2016;50:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbengue MAS, Mboup A, Deme IL, et al. Vaccination coverage and immunization timeliness among children aged 12-23 months in senegal: a kaplan-meier and cox regression analysis approach. Pan Afr Med J 2017;27:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzanne W, Mario C, Carol D, et al. Measuring the timeliness of childhood vaccinations: using cohort data and routine health records to evaluate quality of immunisation services. Vaccine 2017;35:7166-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GAVI. Nigeria launches pentavalent vaccines. Available from: https://www.gavi.org/news/media-room/nigeria-launches-pentavalent-vaccine. Accessed: 20 June 2020. [Google Scholar]
- 16.World Bank. Demographic and health survey 2018; 2019. Available from: https://microdata.worldbank.org/index.php/catalog/3540/related-materials. Accessed: 1 April 2020. [Google Scholar]
- 17.UNICEF. Committing to child survival. A promise renewed. Progress report 2013; 2013. Available from: https://data.unicef.org/resources/committing-to-child-survivala-promise-renewed-progress-report-2013/. [Google Scholar]
- 18.Our World in Data. Causes of death in children under 5, world, 2019. Available from: https://ourworldindata.org/grapher/causes-of-death-in-children-under-5. Accessed: 20 May 2020. [Google Scholar]
- 19.Hannah R, Spooner F, Roser M. Causes of death; 2018. Available from: https://ourworldindata.org/causes-ofdeath#citation. Accessed: 15 June 2020. [Google Scholar]
- 20.Our World in Data. Causes of death in children under 5, Nigeria, 2019. Available from: https://ourworldindata.org/grapher/causes-of-death-in-children-under-5?country=~NGA. Accessed: 20 May 2020. [Google Scholar]
- 21.National Primary Health Care Development Agency (NPHCDA). Available from: https://thewhistler.ng/fg-introduces-2ndmeasles-vaccination-at-15-months/ Accessed: 28 May 2020. [Google Scholar]
- 22.Sheik-Mohamed A, Velema JP. Where health care has no access: the nomadic populations of sub-saharan africa. Trop Med Int Health 1999;4:695-707. [DOI] [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention. Polio field census and vaccination of underserved populations — northern nigeria, 2012-2013. MMWR Morb Mortal Wkly Rep 2013;62:663-5. [PMC free article] [PubMed] [Google Scholar]
- 24.Gidado SO, Ohuabunwo C, Nguku PM, et al. Outreach to underserved communities in northern nigeria, 2012-2013. J Infect Dis 2014;210:S118-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Population Commission (NPC). Nigeria 2006 census data. Available from: https://nigeria.opendataforafrica.org/wytkbxb/populationstates. Accessed: 13 August 2020. [Google Scholar]
- 26.World Bank. Living standards survey (2018-2019). Available from: https://microdata.worldbank.org/index.php/catalog/3827/related-materials. Accessed: 10 June 2021. [Google Scholar]
- 27.Obi-Jeff C, Rakhshani NS, Bello-Malabu JI, et al. Vaccine indicator and reminder band to improve demand for vaccination in Northern Nigeria: a qualitative evaluation of implementation outcomes. Vaccine 2020;38:4191-9. [DOI] [PubMed] [Google Scholar]
- 28.Hofstetter Am, DuRivage N, Vargas CY, et al. Text message reminders for timely routine mmr vaccination: a randomized controlled trial. Vaccine 2015;33:5741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyo-Ita A, Wiysonge CS, Oringanje C, et al. Interventions for improving coverage of childhood immunisation in low- and middle-income countries. Cochrane Database Syst Rev 2016;7:CD008145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson DG, Ochieng B, Kagucia EW, et al. Mobile phonedelivered reminders and incentives to improve childhood immunisation coverage and timeliness in Kenya (M-SIMU): a cluster randomised controlled trial. Lancet Glob Health 2017;5:e428-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domek GJ, Contreras-Roldan IL, O’Leary ST, et al. SMS text message reminders to improve infant vaccination coverage in guatemala: a pilot randomized controlled trial. Vaccine 2016;34:2437-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vora S, Verber L, Potts S, et al. Effect of a novel birth intervention and reminder-recall on on-time immunization compliance in high-risk children. Hum Vaccin 2009;5:395-402. [DOI] [PubMed] [Google Scholar]
- 33.Eze GU, Adeleye OO. Enhancing routine immunization performance using innovative technology in an urban area of nigeria. West Afr J Med 2015;34:3-10. [PubMed] [Google Scholar]
- 34.Brown VB, Oluwatosin OA. Feasibility of implementing a cellphone-based reminder/recall strategy to improve childhood routine immunization in a low-resource setting: a descriptive report. BMC Health Serv Res 2017;4:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakadha H, Chandir S, Were EV, et al. The feasibility of using mobile phone-based sms reminders and conditional cash transfers to improve timely immunization in rural kenya. Vaccine 2013;31:987-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqi DA, Munir M, Shah MT, et al. Effect of vaccine reminder and tracker bracelets on routine childhood immunization coverage and timeliness in urban Pakistan: protocol for a randomized controlled trial. BMC Public Health 2019;19:1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakshani NA, Tahir R, Ali F, Khan MI. Increasing immunisation in karachi, pakistan: a feasibility and acceptability study of the vaccine indicator and reminder band community intervention; 2019. Available from: https://www.3ieimpact.org/sites/default/files/2019-04/FE-TW10.1030-VIR-band-Pakistan.pdf. [Google Scholar]
- 38.Kazi AM, Ali M, Zubair K, et al. Effect of mobile phone text message reminders on routine immunization uptake in pakistan: randomized controlled trial. JMIR Public Health Surveill 2018;4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekhaguere OA, Oluwafemi RO, Badejoko B, et al. Automated phone call and text reminders for childhood immunisations (primm): a randomised controlled trial in nigeria. BMJ Glob Health 2019;4:e001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DHIS2. Nigeria district health information software 2 (DHIS2) 2018 data. Available from: https://dhis2nigeria.org.ng/. Accessed: 28 May 2020. [Google Scholar]
- 41.Medley A, Kennedy C, O'Reilly K, Sweat M. Effectiveness of peer education interventions for hiv prevention in developing countries: a systematic review and meta-analysis. AIDS Educ Prev 2009;21:181-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menna T, Ali A, Worku A. Effects of peer education intervention on hiv/aids related sexual behaviors of secondary school students in addis ababa, ethiopia: a quasi-experimental study. Reprod Health 2015;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das A, George B, Ranebennur V, et al. Getting to the first 90: incentivized peer mobilizers promote hiv testing services to men who have sex with men using social media in mumbai, india. Glob Health Sci Pract 2019;7:469-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karing A. Social signalling and childhood immunization: a field experiment in sierra leone; 2021. Available from: https://novafrica.org/wp-content/uploads/2021/03/2021.03.03_Anne-Karing.pdf. 2018. [Google Scholar]
- 45.Abdul Rahman MA, Al Dabbagh SA, Al Habeeb QS. Health education and peer leaders-role in improving low vaccination coverage in akre district, kurdistan region, iraq. East Mediterr Health J 2013;19:125-9. [PubMed] [Google Scholar]


