Abstract
Co-expression of immune checkpoint (IC) molecules can exacerbate T cell exhaustion in patients with hematological malignancies (HMs) and contribute to the immune escape of tumor cells, which is related to poor clinical outcome. It is worth establishing and optimizing an ideal prediction model based on the co-expression patterns of IC molecules to evaluate the immune status of HM patients and predict their clinical outcome. In this perspective, we summarize the co-expression patterns of IC molecules and their importance as biomarkers that predict the prognosis of patients with different HMs, providing new insights for designing dual IC blockades (ICBs).
Keywords: Immune checkpoint, T cell exhaustion, co-expression pattern, prognosis, hematological malignancy
Introduction
Immune escape is an important factor leading to progression and poor clinical outcomes (1-4). The primary reason for immune escape is downregulation of immune cell function, which is mainly manifested as immune cell exhaustion (2,5-7). This exhaustion included high expression of immune checkpoint (IC) molecules as well as high expression of IC ligands on tumor cells (2,8,9). Therefore, the immune suppression status can be evaluated by detecting the expression levels of IC molecules, which may be potential immune biomarkers that predict the prognosis of patients with hematological malignancies (HMs) and guide the design of immunotherapies (2,10-12). IC molecules include programmed cell death 1 (PD-1), PD ligand 1 (PD-L1), PD ligand 2 (PD-L2), cytotoxic T-lymphocyte associated protein 4 (CTLA-4), T cell immunoglobulin domain and mucin domain-3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT), CD276, etc. (13-16). IC molecules have been confirmed to be up-regulated in acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), B-cell acute lymphocytic leukemia (B-ALL) and B-cell lymphoma (BCL) and have an association with clinical outcomes (2,10,17,18). Thus, the expression status of IC molecules may be an immune biomarker for HMs. Therefore, in this perspective, we comprehensively analyzed differences in IC molecule expression and their optimal combination for predicting the prognosis of patients with different HMs and discussed the advantages and limitations of different analyses and detection methods.
Clinical implications of abnormal IC molecule expression in HMs
Upregulation of IC proteins on immune cells or tumor cell surfaces will lead to an increase in the exhausted immune cell number and reduce effective anti-tumor immune responses in patients with HMs, thereby inducing immune tolerance (19-21). IC mRNA expression has been detected by quantitative real-time polymerase chain reaction (qRT-PCR), flow cytometry, and transcriptome sequencing, and its high expression was significantly associated with adverse clinical outcomes for patients with HMs (10,11,17,18). For example, higher PD-1 expression concurrent with exhausted CD4+/CD8+ T cells exhibits a reduced anti-leukemia response in patients with de novo AML, and bone marrow (BM) contains more exhausted T cells than peripheral blood (PB) (11,22,23). Moreover, high expression of PD-1, PD-L1, PD-L2, CTLA4, or LAG-3 is significantly associated with poor overall survival (OS) in AML patients (10,24). After allogeneic hematopoietic stem cell transplantation (allo-HSCT), IC proteins can serve a critical role in the anti-leukemic immune response in AML (25). For example, higher expression of TIGIT was associated with poor OS and progression-free survival (PFS) in AML after allo-HSCT (25). Moreover, up-regulated PD-1, PD-L1, PD-L2, CTLA4, TIGIT, or CD47 predicts adverse clinical outcomes for MDS patients (17,26). In patients with B-ALL, two heterogeneous exhausted T cell populations, characterized by upregulation of TIGIT, PD-1, HLADRA, LAG-3, and CTLA4, were identified, and this may be correlated with poor prognosis and the effectiveness of immune-based therapies targeting B-ALL (18). In addition, abnormal expression of PD-1, TIM-3, and CD244 in CD3+, CD4+, Treg and CD8+ T cells may contribute to T cell exhaustion and impair anti-tumor T cell activity in B-cell lymphoma (27). Similarly, high expression of PD-1, PD-L1, LAG-3, or TIM-3 may predict poor clinical outcomes for patients with peripheral T-cell lymphoma (PTCL) (28). These findings suggest that IC molecules can serve as biomarkers for prognostic prediction, and more importantly, they may further provide a reference for the application of immune checkpoint inhibitors (ICIs) based on the alteration of immunosuppressive receptors.
Recently, increasing numbers of anti-tumor immunotherapy clinical trials based on the expression of IC molecules are being performed (9,29-31). PD-1/PD-L1 or CTLA-4 blockade has shown clinical benefit in only certain types of HMs, such as Hodgkin lymphoma (HL) and follicular lymphoma (FL) with an objective response rate (ORR) of 64%−87% (32-36). However, patients with relapsed or refractory PTCL, multiple myeloma (MM), AML, diffuse large B cell lymphoma (DLBCL), mycosis fungoides, and Sèzary syndrome have a limited reactivity response to PD-1/PD-L1 or CTLA-4 blockade with an ORR less than 45% in phase I/II trials (32,37-41). Furthermore, in a phase 1/1b multicenter trial, 23% of patients had a complete response, 9% had a partial response, and 27% had decreased tumor burden when CTLA-4 blockade was used in patients with relapsed HMs after allo-HSCT (40). Therefore, beyond PD-1 and CTLA-4 blockade, clinical trials are ongoing to evaluate the efficacy and safety of LAG-3, TIM-3, and TIGIT blockade in HMs (32). Because ICI monotherapy in HMs has limited clinical benefit, co-expression of IC proteins and the anti-tumor activity of ICI combinations are currently being explored. Therefore, the detection of the IC protein expression pattern may guide the design of combined immunotherapy.
Co-expression of IC molecules predicts clinical outcome for patients with HMs
The detection of co-expressing IC molecules mainly includes analysis of RNA and protein levels (10,11). The mRNA of IC genes is detected by qRT-PCR and transcriptome sequencing, and IC protein levels on the surface of T cells are mainly detected by flow cytometry and immunohistochemistry (IHC) (Table 1) (10,18,42,43). qRT-PCR assesses the mRNA expression level of co-expressing IC proteins by detecting preserved RNA or cDNA and has the advantage of being fast, convenient, and cost-effective, but it cannot accurately determine which T cell subpopulation expresses each IC RNA (10). Transcriptome sequencing can comprehensively identify the internal co-expression of all IC genes or co-expression relationships with other genes, while its disadvantages include a longer time to results, requiring subsequent qRT-PCR validation, and it also cannot identify which cell subpopulation expresses the IC genes (10,17,24). Flow cytometry and IHC can relatively accurately detect the co-expression levels of IC proteins on T cell subpopulations, but the disadvantages of flow cytometry include high cost and the requirement of fresh samples (11,42,44). The disadvantages of IHC are mainly applicable to BCL and T cell lymphoma (TCL) (Table 1) (45-47). Overall, detection of the mRNA and protein expression levels of IC genes can complement and verify each other.
Table 1. Advantages and disadvantages of qRT-PCR, flow cytometry, and IHC.
| Methods | Advantages | Disadvantages |
| qRT-PCR, quantitative real-time polymerase chain reaction; IHC, immunohistochemistry; IC, immune checkpoint; BCL, B-cell lymphoma; TCL, T-cell lymphoma. | ||
| qRT-PCR | 1. Fast 2. Convenient 3. Cost-effective |
Cannot identify the T cell subpopulation |
| Transcriptome sequencing | 1. Comprehensively observe the internal co-expression of all IC genes 2. Co-expression IC genes with other genes |
1. Longer time to results 2. Requires qRT-PCR validation 3. Cannot identify the T cell subpopulation |
| Flow cytometry | 1. Detect the co-expression of IC proteins on T cell subpopulations |
1. High cost 2. Fresh sample requirement |
| IHC | Detect the co-expression of IC proteins on T cell subpopulations | Mainly applicable to BCL and TCL |
The co-expression levels of IC molecules may be different in patients with newly diagnosed disease or in patients with HMs after treatment, and this may guide the risk stratification and prediction of clinical outcomes through their detection at different treatment stages. For example, increased co-expression of PD-1/CTLA-4 or PD-L2/CTLA-4 correlated with poor OS in patients newly diagnosed with AML by detecting the co-expression levels of IC mRNA in BM samples using transcriptome sequencing and qRT-PCR (Table 2) (10). These findings are further supported by an ongoing phase 2 clinical trial on PD-1/CTLA-4 dual blockade in which the data demonstrated that the combination of nivolumab and ipilimumab can improve OS for relapsed or refractory AML patients with a median OS time of 7.6 months (48,49). Moreover, increased PD-1+TIM-3+, PD-1+TIGIT+ or CTLA-4+LAG-3+ exhausted T cells in newly diagnosed patients detected by flow cytometry were associated with adverse clinical outcomes, and these might be biomarkers for designing dual blockade strategies (Table 2) (42,44,50,51). In addition, cells with an exhausted phenotype, such as BM CD8+ T cells with PD-1, CTLA-4, and TIM-3 upregulation, were detected in a relapsed AML subgroup, indicating that combining ICIs with HSCT is a strategy for preventing recurrence based on the dynamic detection of IC protein expression in AML patients after allo-HSCT (52,53). PD-1/TIGIT, PD-1/CD47, or TIGIT/CD47 as detected by qRT-PCR in BM samples from newly diagnosed MDS patients was optimal combinations for predicting poor OS in patients (Table 2) (17). These findings provide novel insights into designing combination ICI strategies. Similarly, V-domain Ig suppressor of T cell activation (VISTA) and PD-L1 synergistically predicts poor OS and PFS in patients with extranodal natural killer/T-cell lymphoma (NK/TCL) and provides a rationale for designing dual blockade strategies for targeting VISTA and PD-L1 (Table 2) (43).
Table 2. Optimal combination of IC proteins in patients with HMs.
| IC combination | Stage | Detection method | HM patients |
| IC, immune checkpoint; HM, hematological malignancy; PD-1, programmed cell death 1; CTLA-4, cytotoxic T-lymphocyte associated protein 4; PD-L2, PD ligand 2; TIM-3, T cell immunoglobulin domain and mucin domain-3; TIGIT, T cell immunoreceptor with Ig and ITIM domains; LAG-3, lymphocyte-activation gene 3; VISTA, V-domain Ig suppressor of T cell activation; qRT-PCR, quantitative real-time polymerase chain reaction; IHC, immunohistochemistry; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; NK/TCL, natural killer/T-cell lymphoma. | |||
| PD-1/CTLA-4 | De novo | qRT-PCR | AML |
| PD-L2/CTLA-4 | De novo | qRT-PCR | AML |
| PD-1/TIM-3 | De novo | Flow cytometry | AML |
| PD-1/TIGIT | De novo | Flow cytometry | AML |
| CTLA-4/LAG-3 | De novo | Flow cytometry | AML |
| PD-1/TIGIT | De novo | qRT-PCR | MDS |
| PD-1/CD47 | De novo | qRT-PCR | MDS |
| TIGIT/CD47 | De novo | qRT-PCR | MDS |
| VISTA/PD-L1 | De novo | IHC | Extranodal NK/TCL |
Although the IC co-expression patterns detected by qRT-PCR, flow cytometry, and single cell or bulk transcriptome sequencing provide evidence for predicting clinical outcomes for patients with HMs, another validation dataset is needed to improve the accuracy and feasibility of prediction (10,54). However, different validation methods have different credibility of the results. Validation using multiple datasets is more conclusive than that using data from a single clinical center. Importantly, validation results from multicenter clinical trials, particularly randomized controlled studies from multiple countries, have great value for clinical application and guide clinicians to accurately perform risk stratification and choice-making for patients with HMs (32,54).
Conclusions and further directions
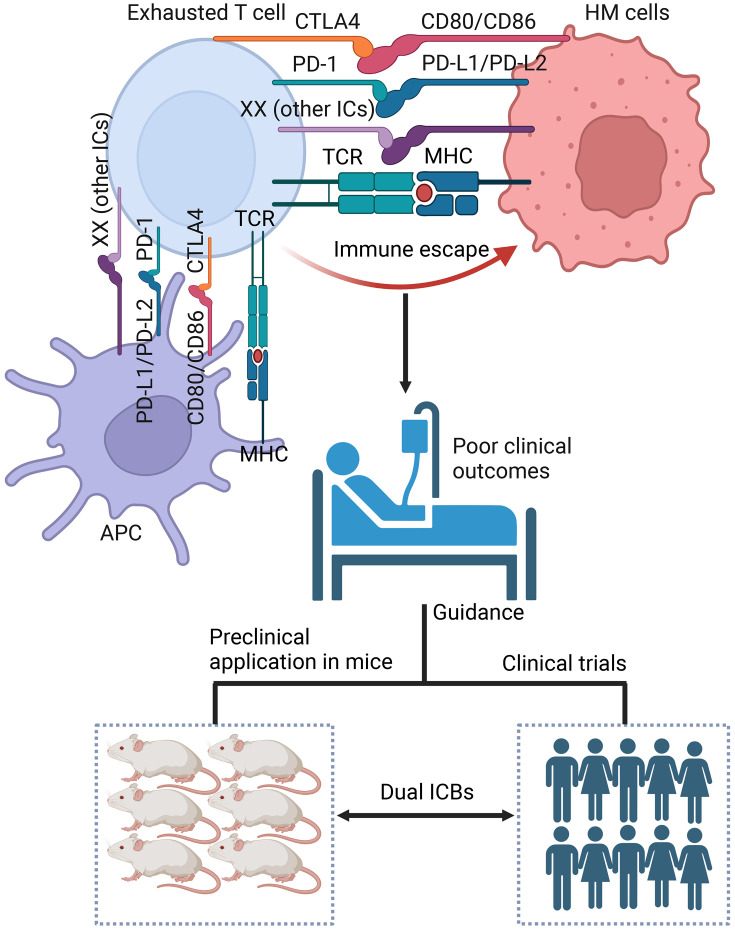
Increasing co-expression of IC molecules can exacerbate T cell exhaustion, which is related to poor clinical outcomes in patients with HMs. Different models for co-expression patterns are establishing and modifying for predicting clinical outcomes and potential for disease relapse in different HMs and guide the development of a ICB combination in clinical trials (48). However, clinical trials for the synergistic application of ICB in MDS and extranodal NK/TCL as well as B-ALL, T-ALL and BCL are little known. To better guide the clinical application of dual ICBs and select the best IC combinations from prognostic information, it is important to provide evidence from preclinical application in mice and clinical trials involving dual ICBs in patients with HMs (Figure 1).
Figure 1.
IC molecule co-expression predicts poor clinical outcomes for patients with HMs and guides preclinical application in mice and clinical trials for dual ICBs. HM, hematological malignancy; PD-1, programmed cell death 1; PD-L1, PD ligand 1; PD-L2, PD ligand 2; CTLA-4, cytotoxic T-lymphocyte associated protein 4; IC, immune checkpoint; ICB, IC blockade; TCR, T cell receptor; MHC, major histocompatibility complex.
Acknowledgements
This work is supported by grants from the National Natural Science Foundation of China (No. 82293630, No. 82293632 and No. 82070152); the Guangdong Natural Science Foundation (No. 2023A1515012968) and Medical Scientific Research Foundation of Guangdong Province (No. A2023330).
References
- 1.Liang Y, Liu X, Li K, et al Current situation of programmed cell death protein 1/programmed cell death ligand 1 inhibitors in advanced triple-negative breast cancer. Chin J Cancer Res. 2022;34:117–30. doi: 10.21147/j.issn.1000-9604.2022.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian Y, Yang T, Liang H, et al Myeloid checkpoints for cancer immunotherapy. Chin J Cancer Res. 2022;34:460–82. doi: 10.21147/j.issn.1000-9604.2022.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu Y, Chen T, Hu R, et al Next frontier in tumor immunotherapy: macrophage-mediated immune evasion. Biomark Res. 2021;9:72. doi: 10.1186/s40364-021-00327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang P, Chen Y, Long Q, et al Increased coexpression of PD-L1 and TIM3/TIGIT is associated with poor overall survival of patients with esophageal squamous cell carcinoma. J Immunother Cancer. 2021;9:e002836. doi: 10.1136/jitc-2021-002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin-Acevedo JA, Kimbrough EO, Lou Y Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14:45. doi: 10.1186/s13045-021-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai M, Liu M, Yang H, et al New insights into epigenetic regulation of resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms and therapeutic opportunities. Exp Hematol Oncol. 2022;11:101. doi: 10.1186/s40164-022-00356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong M, Gao R, Zhao R, et al BET bromodomain inhibition rescues PD-1-mediated T-cell exhaustion in acute myeloid leukemia. Cell Death Dis. 2022;13:671. doi: 10.1038/s41419-022-05123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Yu X, Xu L, et al Current insight into the regulation of PD-L1 in cancer. Exp Hematol Oncol. 2022;11:44. doi: 10.1186/s40164-022-00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Ma L, Zhang X, et al Targeting PD-1/PD-L1 pathway in myelodysplastic syndromes and acute myeloid leukemia. Exp Hematol Oncol. 2022;11:11. doi: 10.1186/s40164-022-00263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Liang C, Wang S, et al Expression patterns of immune checkpoints in acute myeloid leukemia. J Hematol Oncol. 2020;13:28. doi: 10.1186/s13045-020-00853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan J, Chen S, Lu Y, et al Higher PD-1 expression concurrent with exhausted CD8+ T cells in patients with de novo acute myeloid leukemia. Chin J Cancer Res. 2017;29:463–70. doi: 10.21147/j.issn.1000-9604.2017.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang XY, Xiong YL, Shi XG, et al IGSF11 and VISTA: a pair of promising immune checkpoints in tumor immunotherapy. Biomark Res. 2022;10:49. doi: 10.1186/s40364-022-00394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Cai YY, Herold T, et al An immune risk score predicts survival of patients with acute myeloid leukemia receiving chemotherapy. Clin Cancer Res. 2021;27:255–66. doi: 10.1158/1078-0432.CCR-20-3417. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Chen S, Chi P, et al Survival prediction optimization of acute myeloid leukaemia based on T-cell function-related genes and plasma proteins. Br J Haematol. 2022;199:572–86. doi: 10.1111/bjh.18453. [DOI] [PubMed] [Google Scholar]
- 15.Zhao SJ, Muyayalo KP, Luo J, et al Next generation of immune checkpoint molecules in maternal-fetal immunity. Immunol Rev. 2022;308:40–54. doi: 10.1111/imr.13073. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Li H, Xia Y, et al Immune checkpoint of B7-H3 in cancer: from immunology to clinical immunotherapy. J Hematol Oncol. 2022;15:153. doi: 10.1186/s13045-022-01364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong M, Chen C, Zhao W, et al High co-expression of PDCD1/TIGIT/CD47/KIR3DL2 in bone marrow is associated with poor prognosis for patients with myelodysplastic syndrome. J Oncol. 2023;2023:1972127. doi: 10.1155/2023/1972127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Chen Y, Li Z, et al Single-cell RNA-Seq of T cells in B-all patients reveals an exhausted subset with remarkable heterogeneity. Adv Sci (Weinh) 2021;8:e2101447. doi: 10.1002/advs.202101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowshanravan B, Halliday N, Sansom DM CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu-Monette ZY, Zhou J, Young KH PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascual M, Mena-Varas M, Robles EF, et al PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphomas. Blood. 2019;133:2401–12. doi: 10.1182/blood.2018889931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia B, Wang L, Claxton DF, et al Bone marrow CD8 T cells express high frequency of PD-1 and exhibit reduced anti-leukemia response in newly diagnosed AML patients. Blood Cancer J. 2018;8:34. doi: 10.1038/s41408-018-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Tan J, Chen Y, et al A skewed distribution and increased PD-1+Vβ+CD4+/CD8+ T cells in patients with acute myeloid leukemia. J Leukoc Biol. 2019;106:725–32. doi: 10.1002/JLB.MA0119-021R. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Xu L, Gao R, et al Transcriptome-based co-expression of BRD4 and PD-1/PD-L1 predicts poor overall survival in patients with acute myeloid leukemia. Front Pharmacol. 2020;11:582955. doi: 10.3389/fphar.2020.582955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori N, Kawaguchi Y, Sasaki Y, et al Monitoring TIGIT/DNAM-1 and PVR/PVRL2 immune checkpoint expression levels in allogeneic stem cell transplantation for acute myeloid leukemia. Biol Blood Marrow Transplant. 2019;25:861–67. doi: 10.1016/j.bbmt.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Bueso-Ramos C, DiNardo C, et al Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–8. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Liang C, Zhao Y, et al Increased TOX expression concurrent with PD-1, Tim-3, and CD244 in T cells from patients with non-Hodgkin lymphoma. Asia Pac J Clin Oncol. 2022;18:143–49. doi: 10.1111/ajco.13545. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Wu W, Wei W, et al Immune checkpoint inhibitors in peripheral T-cell lymphoma. Front Pharmacol. 2022;13:869488. doi: 10.3389/fphar.2022.869488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu S, Zhang T, Zheng L, et al Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14:156. doi: 10.1186/s13045-021-01164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Qian W, Sun X, et al Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J Hematol Oncol. 2022;15:143. doi: 10.1186/s13045-022-01362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao W, Ma X, Fischer JV, et al Immunotherapy in endometrial cancer: rationale, practice and perspectives. Biomark Res. 2021;9:49. doi: 10.1186/s40364-021-00301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salik B, Smyth MJ, Nakamura K Targeting immune checkpoints in hematological malignancies. J Hematol Oncol. 2020;13:111. doi: 10.1186/s13045-020-00947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansell SM, Lesokhin AM, Borrello I, et al PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen R, Zinzani PL, Fanale MA, et al Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–32. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramchandren R, Domingo-Domènech E, Rueda A, et al Nivolumab for newly diagnosed advanced-stage classic hodgkin lymphoma: safety and efficacy in the phase II checkmate 205 study. J Clin Oncol. 2019;37:1997–2007. doi: 10.1200/JCO.19.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armand P, Engert A, Younes A, et al Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II checkmate 205 trial. J Clin Oncol. 2018;36:1428–39. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansell SM, Minnema MC, Johnson P, et al Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol. 2019;37:481–9. doi: 10.1200/JCO.18.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khodadoust MS, Rook AH, Porcu P, et al Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38:20–8. doi: 10.1200/JCO.19.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mateos MV, Orlowski RZ, Ocio EM, et al Pembrolizumab combined with lenalidomide and low-dose dexamethasone for relapsed or refractory multiple myeloma: phase I KEYNOTE-023 study. Br J Haematol. 2019;186:e117–21. doi: 10.1111/bjh.15946. [DOI] [PubMed] [Google Scholar]
- 40.Davids MS, Kim HT, Bachireddy P, et al Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375:143–53. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daver N, Garcia-Manero G, Basu S, et al Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov. 2019;9:370–83. doi: 10.1158/2159-8290.CD-18-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan J, Yu Z, Huang J, et al Increased PD-1+Tim-3+ exhausted T cells in bone marrow may influence the clinical outcome of patients with AML. Biomark Res. 2020;8:6. doi: 10.1186/s40364-020-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He HX, Gao Y, Fu JC, et al VISTA and PD-L1 synergistically predict poor prognosis in patients with extranodal natural killer/T-cell lymphoma. Oncoimmunology. 2021;10:1907059. doi: 10.1080/2162402X.2021.1907059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y, Tan J, Huang S, et al Higher frequency of the CTLA-4+ LAG-3+ T-cell subset in patients with newly diagnosed acute myeloid leukemia. Asia Pac J Clin Oncol. 2020;16:e12–8. doi: 10.1111/ajco.13236. [DOI] [PubMed] [Google Scholar]
- 45.Autio M, Leivonen SK, Brück O, et al Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma. Haematologica. 2021;106:718–29. doi: 10.3324/haematol.2019.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho J, Kim SJ, Park WY, et al Immune subtyping of extranodal NK/T-cell lymphoma: a new biomarker and an immune shift during disease progression. Mod Pathol. 2020;33:603–15. doi: 10.1038/s41379-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 47.Veldman J, Rodrigues Plaça J, Chong L, et al CD4+ T cells in classical Hodgkin lymphoma express exhaustion associated transcription factors TOX and TOX2: Characterizing CD4+ T cells in Hodgkin lymphoma. Oncoimmunology. 2022;11:2033433. doi: 10.1080/2162402X.2022.2033433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daver N, Basu S, Garcia-Manero G, et al. Azacitidine (AZA) with nivolumab (Nivo), and AZA with nivo plus ipilimumab (Ipi) in relapsed/refractory (R/R) acute myeloid leukemia: clinical and immune biomarkers of response. Blood 2020;136.
- 49.Broglie L, Gershan J, Burke MJ Checkpoint inhibition of PD-L1 and CTLA-4 in a child with refractory acute leukemia. Int J Hematol Oncol. 2019;8:Ijh10. doi: 10.2217/ijh-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, Bu J, Zhou M, et al CD8+T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clin Immunol. 2018;190:64–73. doi: 10.1016/j.clim.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 51.Xu L, Liu L, Yao D, et al PD-1 and TIGIT are highly co-expressed on CD8+ T cells in AML patient bone marrow. Front Oncol. 2021;11:686156. doi: 10.3389/fonc.2021.686156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noviello M, Manfredi F, Ruggiero E, et al Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat Commun. 2019;10:1065. doi: 10.1038/s41467-019-08871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh A, Barba P, Perales MA Checkpoint inhibitors in AML: are we there yet. Br J Haematol. 2020;188:159–67. doi: 10.1111/bjh.16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C, Zeng C, Li Y The importance of genomic predictors for clinical outcome of hematological malignancies. Blood Sci. 2021;3:93–5. doi: 10.1097/BS9.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]