Abstract
T follicular helper (TFH) cells are essential for developing protective antibody responses following vaccination. Greater understanding of the genetic program leading to TFH differentiation is needed. Chromatin modifications are central in the control of gene expression. However, detailed knowledge of how chromatin regulators (CRs) regulate differentiation of TFH cells is limited. We screened a large shRNA library targeting all known CRs in mouse and identified the histone methyltransferase Mll1 as a positive regulator of TFH differentiation. Loss of Mll1 expression reduced formation of TFH cells following acute viral infection or protein immunization. In addition, expression of the TFH lineage-defining transcription factor Bcl6 was reduced in the absence of Mll1. Transcriptomics analysis identified Lef1 and Tcf7 as genes dependent on Mll1 for their expression, which provides one mechanism for the regulation of TFH differentiation by Mll1. Taken together, CRs such as Mll1 substantially influence TFH differentiation.
INTRODUCTION
High-affinity antibodies are central to immune protection elicited following vaccination. Germinal center (GCs) are essential for the production of such antibodies (1). T follicular helper CD4 T cells (TFH) cells provide essential help signals to antigen-specific B cells for the formation of germinal center B cells (BGC) that culminate in the formation of long-lived antibody-secreting plasma cells and memory B cells (1). Differentiation of CD4 T cells into TFH cells is initiated upon interaction of activated CD4 T cell with antigen-presenting dendritic cells (1). Expression of the transcriptional repressor Bcl6 in CD4 T cells induces the TFH transcriptional program. Bcl6 directs TFH differentiation by suppressing alternative T helper cell fates and by promoting expression of gene products associated with TFH cells and function (1–5). Other transcription factors such as LEF-1/TCF-1 (Lef1/Tcf7) (6–8), Batf (9, 10), Maf (11, 12) or E proteins (13, 14) play critical roles in supporting TFH differentiation. These transcription factors are balanced by inhibitors of TFH differentiation such as Blimp-1 (5) and Klf2 (2, 15).
Differentiation of CD4 T cells into a wide variety of helper subsets is tightly controlled by chromatin modifications (16). Transcription factors driving TFH differentiation have been thoroughly characterized, but the role of epigenetics in controlling this process remains mostly unexplored. TFH cells possess cell type-specific enhancers identified by monomethylation of histone 3 lysine 4 (H3K4Me) and acetylation at lysine 27 of histone 3 (H3K27Ac) (17, 18). Additionally, TFH cells possess unique patterns of chromatin accessibility (2, 17). All of the above suggest an epigenetic program specific for TFH.
Bcl6 plays a central role in establishing the TFH-specific epigenetic program. The middle domain of Bcl6, which recruits the corepressor MTA3, is necessary for full activity of Bcl6 and proper formation of TFH cells (19). Additionally, depletion of the corepressor NCoR1, or deletion of BCoR, impairs TFH formation (2, 20). TCF-1 recruits the histone methyltransferase EZH2 to activate Bcl6 expression (21), and absence of EZH2 abrogates TFH differentiation (21, 22). The histone methyltransferase Nsd2 influences TFH differentiation by promoting expression of Bcl6 in the early stages of CD4 T cell activation (23). Bcl6, of course, is not the only TFH-associated target of CRs. The histone demethylase UTX impacts TFH differentiation by regulating Il6ra expression (24). We sought to identify novel CRs essential for TFH formation. To this end, we adapted our in vivo shRNA-based screening approach (25) to survey a library of shRNAmir targeting all known CRs in mouse. We identified Mll1 (Mixed Lineage Leukemia 1) as a positive regulator of TFH differentiation and function.
MATERIALS AND METHOD
Mice, LCMV infection and protein immunization.
SMARTA mice (transgenic expression of an I-Ab-restricted TCR specific for LCMV glycoprotein amino acids 66–77) (26) and CD45.1+ mice were on a full C57BL/6 background and bred in-house. C57BL/6 and Cas9Tg mice (JAX stock #028555) were purchased from the Jackson laboratory. Both male and female mice were used throughout the study, with sex and age matched T cell donors and recipients. All mice were maintained in specific-pathogen-free facilities and used according to protocols approved by the animal care and use committees of LJI. Mice were infected via the intraperitoneal route with 2×105, 4×105 or 5×105 plaque-forming units of LCMV-Armstrong for days 6, 4 or 3 analysis, respectively. 10 μg of KLH conjugated to gp66–77 peptide of LCMV mixed with 2.5 μg of SMNP (27) in 100 μL PBS was injected at the base of tail. 1 mg BrdU in PBS was injected via intraperitoneal route 16 hours before analysis.
In vivo shRNA screen.
We included a set of 42 control shRNAmir-RV (Supplemental Table 1). 322 CRs genes expressed in mouse are targeted by 3 or 4 shRNAmir each for a total of 1230 shRNAmir-RV in the library (Supplemental Table 1). The initial screen was performed twice as previously described (25). Subsequent screens were modified as follows. Equal amounts of every shRNAmir were pooled to create 22 tubes. PLAT-E cells were transfected with 2 μg of pooled shRNAmir (1 pool/well) and 1 μg pCL-Eco plasmids. Anti-CD3/CD28 activated SMARTA CD4 T cells were transduced once with RV supernatant. 5×105 sorted cells were transferred into 20 recipient mice by intravenous injection. An aliquot of 1.1–1.5×106 sorted cells was saved for RNA isolation (Input). Mice were infected with 2×106 PFU LCMV-Armstrong 4 days after transfer.
3 days after infection, spleens were isolated and pooled. CD45.1+ SMARTA cells were pre-enriched and sorted into CXCR5+SLAMlo TFH and CXCR5−SLAM+ TH1 SMARTA cells. 1.7×105 TFH and 1.6×106 TH1 cells were sorted for the first screen. 7.2×105 TFH and 3.2×106 TH1 cells were sorted for the second screen. Total RNA was isolated using mirVana miRNA Isolation Kit (Invitrogen). RNA was quantified with High Sensitivity RNA ScreenTape kit and TapeStation (Agilent). 70 ng of RNA was used to synthesize cDNA using random oligonucleotides in triplicate reactions that were subsequently pooled.
Sequencing the shRNAmir library.
The amount of shRNAmir-derived cDNA template in each sample preparation was quantified using qPCR and a standard curve prepared from known molar amounts of gel-purified shRNAmir amplicons. To build shRNAmir amplicon libraries capturing passenger strand sequences, an amount of cDNA template in each sample resulting in equivalent amplification to previously analyzed libraries amplified from 100 ng of total genomic DNA template from transduced T cells was amplified using primers including Illumina P5 adapter (5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTCTCGAGAAGGTATATTGCT-3’) and P7 (5’-CAAGCAGAAGACGGCATACGAGATNNNNNNGTGACTGGAGTTCAGACGTGTGCTCTTCGATCTACATCTGTGGCTTCACTA-3’) adapter sequences. The miR30 (P5) and shRNAmir-loop (P7) annealing sequences are underlined and the custom barcode is indicated (Ns). Sequencing libraries were amplified in triplicate reactions using Phusion High-Fidelity DNA polymerase in 1X HF buffer for 24 PCR cycles. Replicate reactions were pooled, purified with 1.6X AMPure XP beads (Beckman Coulter), and quantified with Qubit DNA assay and Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). The individually barcoded libraries were pooled in equimolar ratios, mixed with separate base-balanced libraries to increase diversity and sequenced on the NextSeq 500 at 1.8 pM concentration with paired-end 75bp reads.
A FASTA formatted reference database comprising 97mer sequences of all analyzed shRNAmirs in the library was built. Raw sequencing reads were merged using FLASH and the merged reads were compared to the reference database using nucleotide BLAST. Only the top alignment for each read was retained and the number of times each reference sequence was aligned in each sample library was counted and reported. Normalized reads for all shRNAmir targeting the same gene were averaged to create a single gene read number and gene log2 TFH/TH1 ratios were calculated.
Retroviral vectors, transductions and cell transfers.
pMIG, Lef1, Tcf7-p45 and Bcl6 pMIG were described previously (6, 7, 19). Guide RNAs were selected by CHOP-CHOP (https://chopchop.cbu.uib.no) and cloned into the BbsI site of a modified LMPd-Ametrine vector. Transduction and transfer of CD4 T cells were performed as previously detailed (25). Transferred cells were allowed to rest in host mice for 3–4 days before infection or immunization. 2×104, 5×104 or 4×105 transduced CD4+ T cells were transferred for day 6, 4 or 3 analysis, respectively. For protein immunization, 2×105 transduced cells were transferred. For TH1 cultures, cells were cultured in the presence of 10 ng/mL IL-12 (Peprotech) and 10 μg/mL anti-IL-4 (R&D Systems).
Flow cytometry and antibodies.
Single-cell suspensions of spleen or draining inguinal lymph nodes were prepared by standard gentle mechanical disruption. Surface staining for flow cytometry was done with monoclonal antibodies against CD4 (RM4–5, 1:400), CD8 (53–6.7, 1:400), CD45.1 (A20, 1:400), B220 (RA3–6B2, 1:400) (eBiosciences); PSGL-1 (2PH1, 1:400), (from BD Biosciences); SLAM (TC15–12F12.2, 1:400), CD25 (PC61, 1:400), CD44 (IM7, 1:400) (BioLegend). CXCR5 staining was done using biotinylated anti-CXCR5 (SPRCL5, eBioscience), followed by PE-Cy7-, BV421- or APC-labeled streptavidin (BioLegend). Intranuclear staining was performed with a monoclonal antibody to Bcl6 (K112–91, BD Biosciences), TCF1 (C63D9), LEF-1 (C12A5, Cell Signaling) or Blimp-1 (5E7, BioLegend), and the Intracellular Fixation & Permeabilization Buffer Set (Invitrogen). For measurement of cytokines, cells from spleen were cultured with 10 μg/ml gp66 peptide and Brefeldin A. Intracellular staining for cytokines was performed with monoclonal antibody to IFN-γ (XMG1.2), CD40L (MR1), IL-2 (JES6–5H4, Invitrogen) and recombinant mouse IL-21 receptor Fc (R&D Systems), followed by anti-human IgG (Invitrogen), using the Fixation/Permeabilization buffer kit (BD Biosciences). BrdU staining was performed with the BrdU Flow Kit (BD Biosciences). Stained cells were analyzed using LSRII or Celesta (BD Biosciences) and FlowJo software (TreeStar). Anti-TBP (ab63766, Abcam) and anti-Mll1 (14689, Cell Signaling Technologies) were used in western blots.
RNA-Seq.
CD45.1+ gRNA+ Cas9Tg SMARTA cells were pre-enriched from spleens pooled from 4 mice per group. 5,000–150,000 CXCR5+SLAMlo TFH, CXCR5−SLAM+ TH1 SMARTA cells, or naïve SMARTA cells were sorted into Trizol LS (Invitrogen). RNA-Seq was performed as previously described (2). 0.5 ng cDNA was used to prepare a standard Nextera XT sequencing library (Nextera XT DNA library preparation kit and index kit; Illumina). Libraries were sequenced using a HiSeq2500 (Illumina) to generate 50-bp single-end reads, generating median of >17 million mapped reads per sample. Three biological replicates were generated for TFH and TH1 samples. RNA-Seq was analyzed as previously described (2) except for the following modifications. The reads were aligned with the mm10 reference genome using STAR (v 2.6.1) and the RefSeq gene annotation downloaded from the UCSC Genome Bioinformatics site. Read counts to each genomic feature were obtained with the featureCounts (v 1.6.5) using the default option along with a minimum quality cut off (Phred > 10). We considered genes differentially expressed between two groups of samples when the DESeq2 analysis resulted in an adjusted p-value of <0.05 and the difference in gene expression was 1.4-fold. TPMs were calculated. Gene Set Enrichment Analysis (GSEA) was run on gene lists with GSEA v3.0 (Broad Institute, Inc.). Gene signatures from in vitro TH2, TREG, Bcl6-bound gene list from human tonsil GC-TFH (18), Blimp-1-bound genes from activated CD8 T cells (28), or from in vitro-differentiated TH17 (29) and genes signatures from TFH, TH1 and Tcf7−/−Lef1−/− GC-TFH isolated after LCMV-Arm infection and from in vitro-differentiated Lef1+-RV TH1 (6) were previously published.
Statistical Methods.
Statistical tests were performed using Prism 8.0 (GraphPad). Significance was determined by unpaired Student’s t-test with a 95% confidence interval, by one-way ANOVA with Tukey’s multiple comparisons test or by two-way ANOVA with Sidak’s multiple comparisons test. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
RESULTS
shRNA screen for chromatin regulators of early TFH differentiation
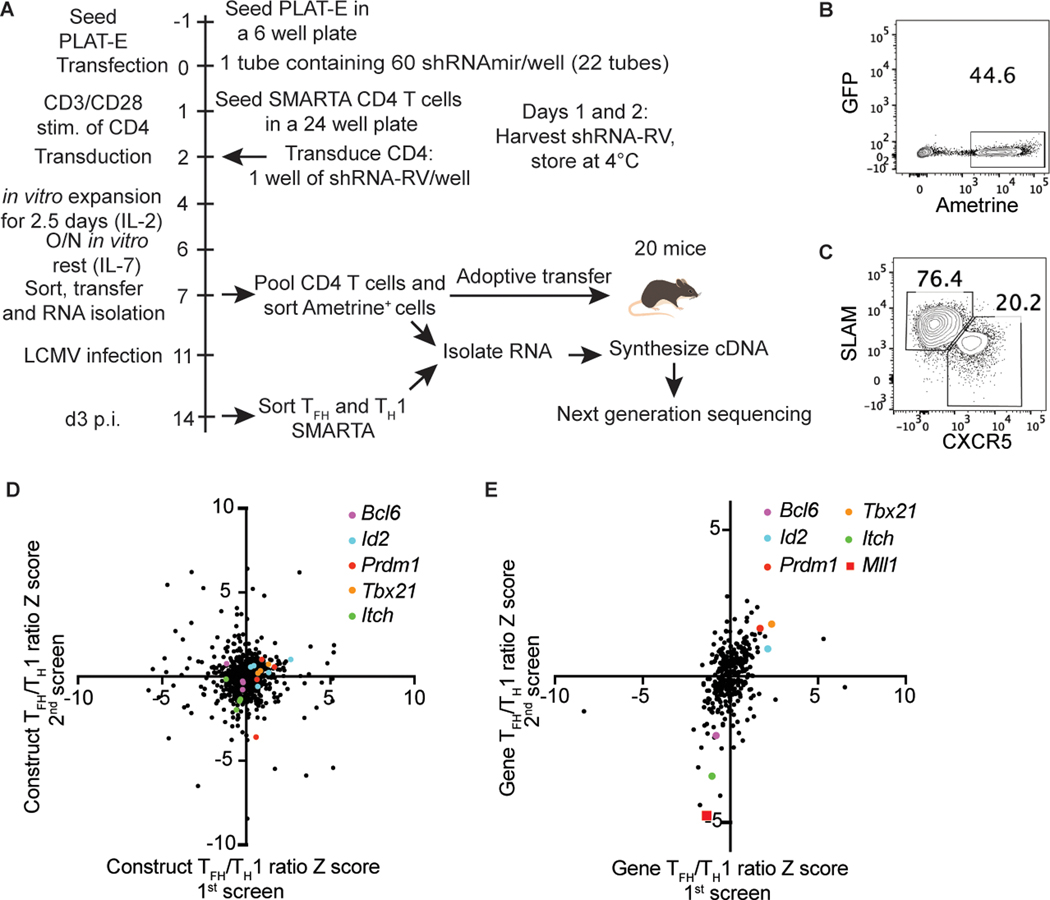
To discover novel CRs regulating TFH differentiation, we generated an shRNAmir retroviral vector (RV) library targeting all known mouse CRs, 322 genes, each targeted by 3–4 shRNAmir for a total of 1230 shRNAmir-RVs. (Fig. 1a). Briefly, LCMV gp66–77-specific TCR transgenic SMARTA CD4 T cells were transduced once with shRNAmir-RV-containing supernatant. shRNAmir-RV+ SMARTA CD4 T cells were sorted based on Ametrine marker expression and adoptively transferred into recipient C57BL/6 mice (Fig. 1b). Total RNA was isolated from an aliquot of CD4 T cells for the Input sample. Recipient mice were infected with LCMV-Arm and, three days after infection, SMARTA CD4 T cells were sorted into CXCR5+SLAMlo TFH and CXCR5−SLAM+ TH1 populations (Fig. 1c). cDNA sequencing libraries were prepared for the SMARTA Input, TFH and TH1 samples. Sequenced cDNA was aligned to a list of all shRNAmir sequences in the library to determine read counts for every shRNAmir in the library. The screen was performed twice in independent experiments.
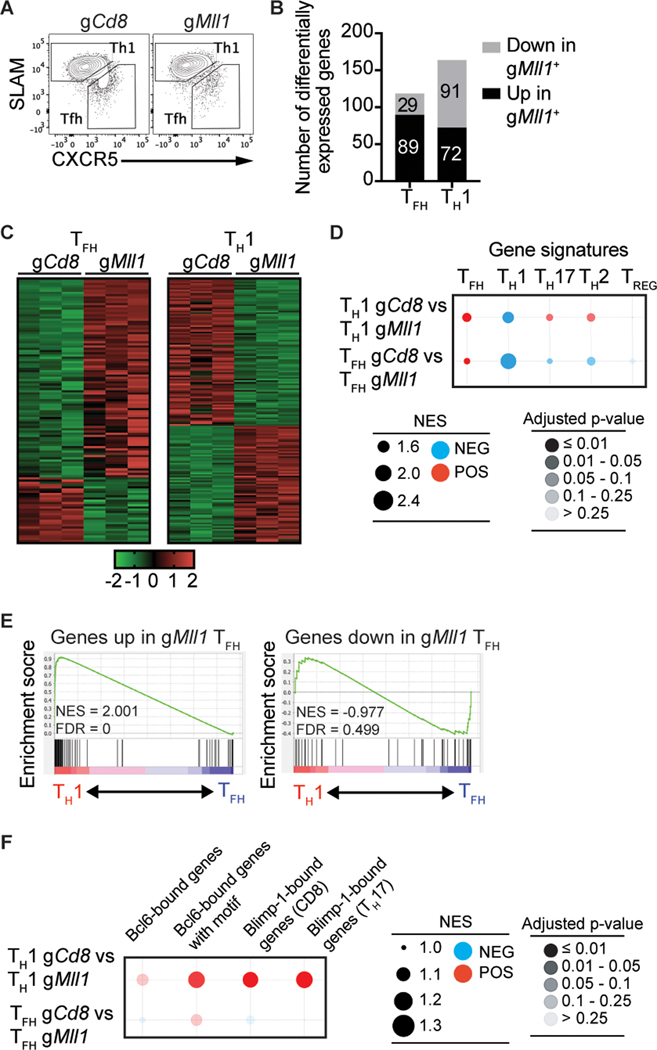
Figure 1: An in vivo RNAi screen identifies Mll1 as a novel regulator of TFH differentiation.
(A) Scheme for screening the shRNAmir library targeting CRs. (B) Transduction efficiency of SMARTA CD4 T cells infected with pooled shRNAmir-RV from the CR library. (C) Sorting strategy of shRNAmir+ SMARTA CXCR5+SLAMlo TFH and CXCR5−SLAM+ TH1 cells 3 days after LCMV infection. (D) Construct TFH/TH1 ratio Z scores for all shRNAmir in the library in two independent experiments. (E) Gene TFH/TH1 ratio Z scores for all genes in the library in two independent experiments.
We calculated Z score values of the TFH/TH1 ratio for every shRNAmir in the library. Direct comparison of Z scores per shRNAmir construct was not informative (Fig. 1d). It is possible that differences in shRNA knockdown efficacy was the source of the variability. We averaged the reads numbers for each shRNA targeting the same gene to create a single Z score for each targeted gene. We hypothesized that calculating a single Z score value for each targeted gene would reduce variability and be more informative. This analysis method showed improved reproducibility between experiments (Fig. 1e, Supplemental Table 1). We assessed the gene Z score distribution of known positive and negative regulators of TFH differentiation. Bcl6 and Itch had negative Z score values in both experiments, indicating their shRNAmir constructs were depleted from the TFH population (Fig. 1e), consistent with their known roles as positive TFH regulators (3–5, 30). Conversely, Prdm1, Tbx21 and Id2 had positive Z score values, indicative of enrichment in TFH cells (Fig. 1e), consistent with their known roles in directly inhibiting TFH differentiation or promoting TH1 cells (5, 13, 31). We then filtered the gene Z score data for novel regulators of TFH differentiation by ranking genes depleted from TFH cells. The gene coding for the histone methyltransferase Mll1 was one of the most depleted genes from TFH cells, with Z score values of −1.4 and −4.8, suggesting Mll1 is a positive regulator of TFH differentiation (Fig. 1e). Mll1 methylates lysine 4 of histone 3 (H3K4), a mark associated with active transcription, and assembles with numerous regulatory proteins such as RbBP5, Wdr5, Ash2l, Dpy30 and Menin-1 (32). Mll1 regulates the expression of TH1 and TH2 cytokines in CD4 T cells (33, 34) but a role for Mll1 in TFH was unknown. Our shRNA library included shRNA for the Ezh2 and NCoR1, two CR known to regulate Tfh differentiation (2, 23). NCoR1 had Z score values of −0.50 and −2.46 while Ezh2 had Z score values of −1.29 and −0.91 suggesting our screen can detect CRs known to regulate TFH differentiation.
Loss of Mll1 impairs early TFH differentiation
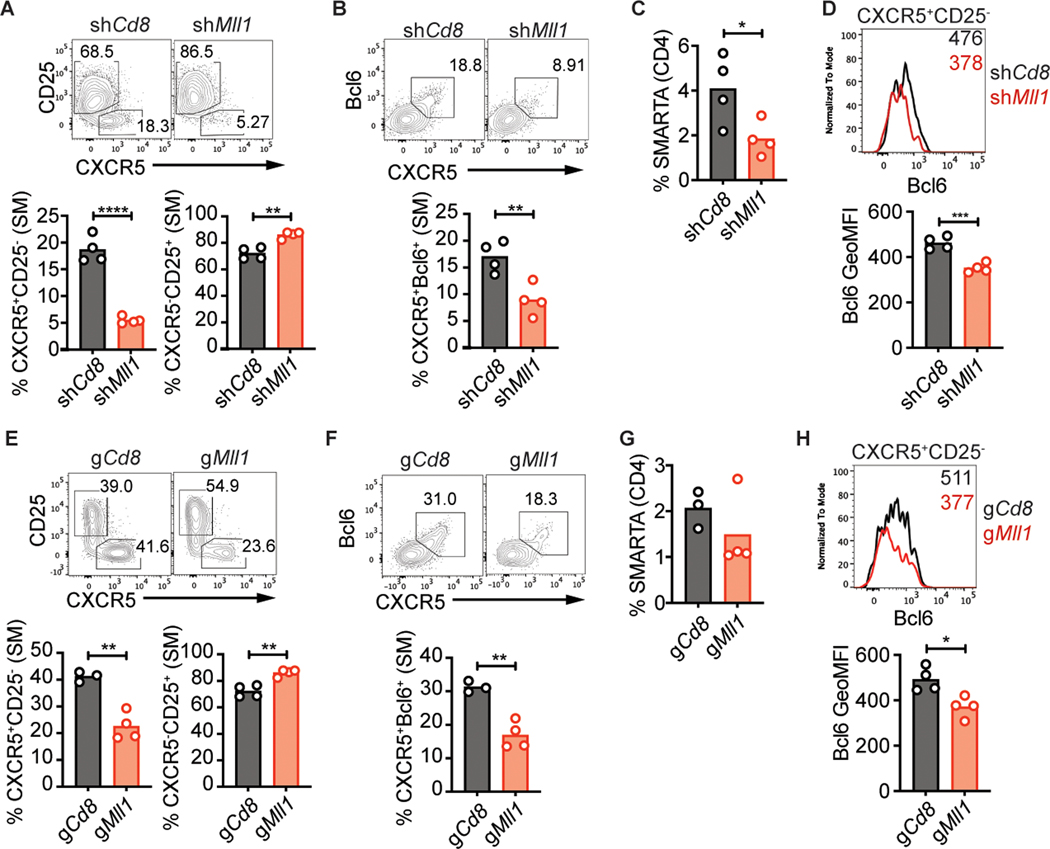
In published RNA-Seq data (2, 6), we observed that Mll1 is expressed at similar levels between naive CD4, TFH and TH1 cells (Supplemental Fig. 1a). Two shRNAmirs that efficiently inhibited Mll1 expression in vitro were tested individually for their effect on TFH differentiation in vivo (Supplemental Fig. 1b). We transduced SMARTA CD4 T cells with shMll1-RV or shCd8-RV, transferred the SMARTA CD4 T cells into C57BL/6 mice, and analyzed TFH differentiation 3 days after infection with LCMV-Arm. shMll1+ SMARTA CD4 T cells exhibited a significantly smaller early TFH cell population (CXCR5+CD25− or CXCR5+Bcl6+) when compared to shCd8+ SMARTA cells (Fig. 2a, b, Supplemental Fig. 1c, d). shRNAmirs targeting Mll1 had a small but significant impact on the accumulation of SMARTA CD4 T cells in vivo (Fig. 2c, Supplemental Fig. 1e, f). Importantly, we observed reduced expression of Bcl6 in Mll1-deficient CXCR5+CD25− early TFH cells (Fig. 2d). These results confirmed the identification of Mll1 as a novel positive regulator of TFH differentiation after LCMV infection.
Figure 2: Mll1 is required for proper differentiation of early TFH after LCMV infection.
shRNA+ SMARTA (A-D) or gRNA+ Cas9Tg SMARTA (E-H) CD4 T cells were transferred into C57BL/6 mice and analyzed 3 days after infection with LCMV-Arm. (A) CXCR5+CD25− TFH and CXCR5−CD25+ TH1 and (B) CXCR5+Bcl6+ TFH in SMARTA CD4 T cells. (C) Quantitation of SMARTA CD4 T cells. (D) Bcl6 expression in CXCR5+CD25− SMARTA CD4 T cells. Numbers in the histogram indicate geometric MFI values. (E) CXCR5+CD25− TFH and CXCR5−CD25+ TH1 and (F) CXCR5+Bcl6+ TFH in CasTg SMARTA CD4 T cells. (G) Quantitation of Cas9Tg SMARTA CD4 T cells. (H) Bcl6 expression in CXCR5+CD25− Cas9Tg SMARTA CD4 T cells. Data shown are from one experiment representative of 5 (A-D) or 3 (E-H) independent experiments with 3–4 mice per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 (unpaired two-tailed Student’s t-test).
We next sought to confirm the role of Mll1 using an independent experimental approach. Cas9Tg SMARTA CD4 T cells transduced with a control gCd8-RV or gMll1-RV (Supplemental Fig. 1g) were transferred into C57BL/6 mice and analyzed 3 days after LCMV-Arm infection. gMll1+ Cas9Tg SMARTA CD4 T cells had substantially reduced potential to differentiate into CXCR5+CD25− or CXCR5+Bcl6+ TFH cells (Fig. 2e, f, Supplemental Fig. 1h, i). Accumulation of gMll1+ Cas9Tg SMARTA CD4 T cells was normal after infection (Fig. 2g, Supplemental Fig. 1j). Bcl6 expression was also significantly reduced in gMll1+ CXCR5+CD25− cells (Fig. 2h). Thus, disruption of Mll1 expression by gRNA phenocopied shRNA-mediated knockdown of Mll1, establishing Mll1 as a novel positive regulator of TFH differentiation.
Reduced peak TFH differentiation in Mll1-deficient CD4 T cells
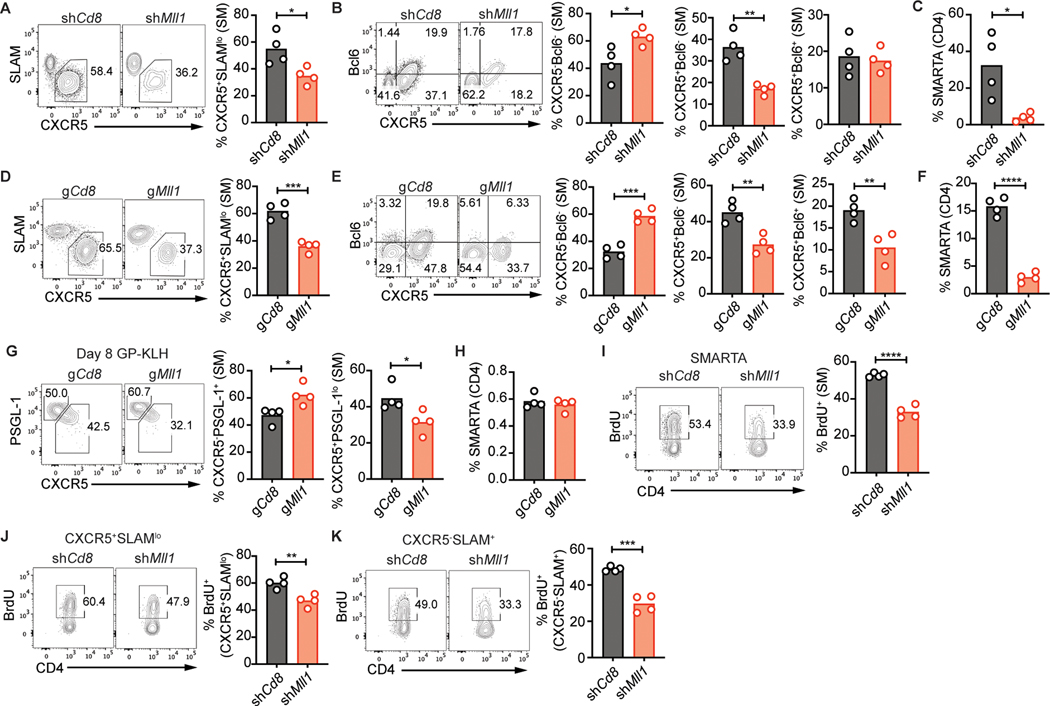
To assess if Mll1 is dispensable for later stages of TFH differentiation, we analyzed TFH differentiation of Mll1-deficient CD4 T cells 6 days after infection. Compared to control shCd8+ cells, shMll1+ SMARTA CD4 T cells showed impaired formation of TFH cells (CXCR5+SLAMlo, Fig. 3a). We next analyzed formation of GC-TFH. The percentages of CXCR5+Bcl6+ GC-TFH cells in shMll1+ SMARTA CD4 T cells were comparable to those of control shCd8+ SMARTA (Fig. 3b). Unlike what we observed at 3 days post infection, the reduction in TFH differentiation was coupled to a significant decrease in the accumulation of shMll1+ SMARTA CD4 T cells by day 6 (Fig. 3c, Supplemental Fig. 1k).
Figure 3: Mll1 is required for proper differentiation of TFH.
shRNA+ SMARTA (A-C) or gRNA+ Cas9Tg SMARTA (D-H) CD4 T cells were transferred into C57BL/6 mice and analyzed 6 days after infection with LCMV-Arm (A-F) or 8 days after immunization with GP-KLH mixed with SMNP (G-H). (A) CXCR5+SLAMlo TFH or (B) CXCR5−Bcl6− TH1, CXCR5+Bcl6− TFH and CXCR5+Bcl6+ GC-TFH in SMARTA CD4 T cells. (C) Quantitation of SMARTA CD4 T cells. (D) CXCR5+SLAMlo TFH or (E) CXCR5−Bcl6− TH1, CXCR5+Bcl6− TFH and CXCR5+Bcl6+ GC-TFH in Cas9Tg SMARTA CD4 T cells. (F) Quantitation of Cas9Tg SMARTA CD4 T cells. (G) CXCR5−PSGL-1+ TH1 and CXCR5+PSGL-1lo TFH in Cas9Tg SMARTA CD4 T cells. (H) Quantitation of Cas9Tg SMARTA CD4 T cells. (I-K) shRNA+ SMARTA CD4 T cells were transferred into C57BL/6 mice and mice were infected with LCMV-Arm. Mice were injected with BrdU 3 days after infection and analyzed 16 hours later. (I) BrdU+ cells in SMARTA CD4 T cells. (J) CXCR5+SLAMlo TFH SMARTA CD4 T cells and (K) CXCR5−SLAM+ TH1 SMARTA CD4 T cells. Data shown are from one experiment representative of 5 (A-C), 1 (D-G) or 2 (I-K) independent experiments with 4 mice per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 (unpaired two-tailed Student’s t-test).
gRNA-mediated disruption of Mll1 also significantly reduced CXCR5+SLAMlo TFH differentiation 6 days after LCMV infection (Fig. 3d). gMll1+ Cas9Tg SMARTA CXCR5+Bcl6+ GC-TFH cells were reduced compared to gCd8+ (Fig. 3e). These results suggest that Mll1 mostly regulates TFH differentiation with less impact on GC-TFH differentiation because a reduction in CXCR5+Bcl6+ GC-TFH was observed only in gMll1+ Cas9Tg SMARTA CD4 T cells but not in shMll1+ SMARTA CD4 T cells. gMll1+ Cas9Tg SMARTA CD4 T cells also accumulated significantly less than gCd8+ Cas9Tg SMARTA CD4 T cells (Fig. 3f). These results showed that Mll1 is a positive regulator of TFH differentiation after an acute viral infection.
We next tested Mll1 disruption in the context of protein immunization. C57BL/6 mice that received gCd8+ or gMll1+ Cas9Tg SMARTA CD4 T cells were immunized subcutaneously with KLH conjugated to GP66–77 peptide from LCMV (GP-KLH) mixed with the SMNP adjuvant (saponin monophosphoryl lipid A nanoparticle). Draining lymph nodes were harvested for analysis 8 days later. Differentiation of gMll1+ Cas9Tg SMARTA CD4 T cells into CXCR5+PSGL-1lo TFH cell was reduced following immunization (Fig. 3g) while accumulation of Mll1-deficient SMARTA CD4 T cells was not compromised (Fig. 3h). These experiments demonstrate a role for Mll1 in TFH cell differentiation in different contexts.
We tested if reduced proliferation of Mll1-deficient CD4 T cells was causing their reduced accumulation after LCMV infection. Four days after infection, total shMll1+ SMARTA CD4 T cells showed significantly reduced incorporation of BrdU when compared to shCd8+ SMARTA cells (Fig. 3i). The decrease in BrdU incorporation was observed for both CXCR5+SLAMlo TFH and CXCR5−SLAM+ TH1 cells (Fig. 3j, k). These results indicate that Mll1 is required for optimal proliferation of CD4 T cells following infection and suggest that the reduced TFH differentiation of shMll1+ SMARTA CD4 T cells was not caused by a selective proliferation defect of TFH cells. These experiments demonstrated the importance for Mll1 in supporting TFH differentiation.
Mll1 is required for proper IL-21 expression
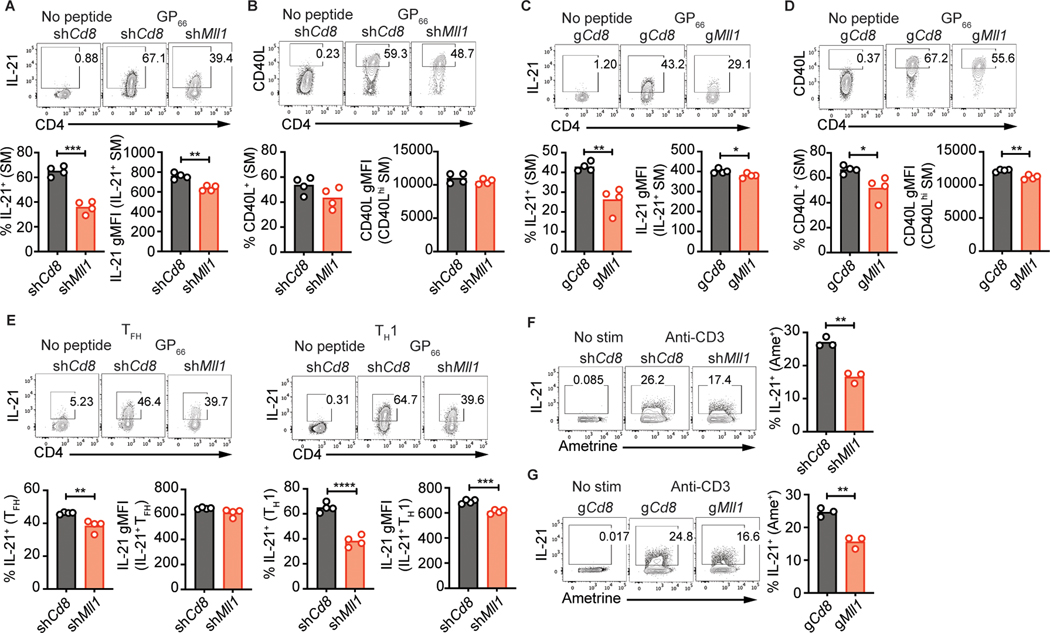
To test the functionality of Mll1-deficient TFH cells, we assessed cytokine production in shCd8+ and shMll1+ SMARTA CD4 T cells 6 days after infection. A substantial fraction of IL-21-producing cells were observed in control shCd8+ SMARTA, but IL-21 expression was greatly reduced in shMll1+ SMARTA (Fig. 4a, Supplemental Fig. 2a). Expression of CD40L or IFN-γ was not affected by loss of Mll1 (Fig. 4b, Supplemental Fig. 2b). Production of IL-2, whose gene is immediately adjacent to Il21, was mostly unaffected by Mll1-deficiency (Supplemental Fig. 2c). IL-21 expression was similarly disrupted in gMll1+ Cas9Tg SMARTA CD4 T cells (Fig. 4c). A small but significant reduction in CD40L was observed while IFN-γ production was not significantly impacted (Fig. 4d, Supplemental Fig. 2d). IFN-γ expression in TH1 cells in response to LCMV infection was also reduced after gRNA-mediated abrogation of Mll1 expression (Supplemental Fig. 2e, f) similar to what was observed in Mll1+/− CD4 T cells responding to tuberculosis antigens (34).
Figure 4: Mll1 is required for production of IL-21.
shRNA+ SMARTA (A-B, E) or gRNA+ Cas9Tg SMARTA (C-D) CD4 T cells were transferred into C57BL/6 mice. 6 days after infection with LCMV-Arm, splenocytes were restimulated with LCMV gp66–77 peptide. Expression of IL-21 (A) and CD40L (B) in SMARTA CD4 T cells or TFH and TH1 SMARTA CD4 T cells (E). Expression of IL-21 (C) and CD40L (D) in Cas9Tg SMARTA CD4 T cells. (F) shRNA+ CD4 T cells were cultured in TH1 conditions. Expression of IL-21 in shRNA+ CD4 T cells restimulated with anti-CD3. (G) gRNA+ Cas9Tg SMARTA CD4 T cells were cultured in TH1 conditions. Expression of IL-21 in gRNA+ Cas9Tg SMARTA CD4 T cells restimulated with anti-CD3. Data shown are from one experiment representative of 5 (A-B, E), 3 (C-D) or 2 (F-G) independent experiments with 3–4 mice per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 (unpaired two-tailed Student’s t-test).
Notably, we observed reduction of IL-21 production by both TH1 and TFH cells (Fig. 4e), suggesting that Mll1 regulates IL-21 production independently of TFH differentiation. We next tested if Mll1 is required for IL-21 production in a system independent of TFH differentiation. In vitro shMll1+ TH1 cells and gMll1+ Cas9Tg TH1 cells exhibited impaired IL-21 expression (Fig. 4f, g). Impacts on IFN-γ in vitro were variable with shMll1+ TH1 producing normal levels of IFN-γ while IFN-γ production was reduced in gMll1+ TH1 (Supplemental Fig. 2g, h). Altogether, these results demonstrate that IL-21 production by CD4 T cells depends on Mll1.
Mll1-deficient TFH cells have a dysregulated TH1 genetic program
To gain further understanding of the function of Mll1, we performed a transcriptomics analysis of Mll1-deficient TFH and TH1 cells (Fig. 5a). Mll1-deficient TFH cells had expression changes of a select number of genes in comparison to control TFH cells (89 upregulated and 29 downregulated genes, |FC| > 1.4, padj < 0.05, Fig. 5b, c). In TH1 cells, loss of Mll1 resulted in the downregulation of 91 and the upregulation of 72 genes (Fig. 5b, c). We next asked how different gene expression signatures associated with CD4 T cell subsets were affected by loss of MLL1 expression. We performed gene set enrichment analysis of TFH, TH1, TH17, TH2 and Treg gene signatures against control and Mll1-deficient TH1 and TFH populations. In these analyses, a negative normalized enrichment score (NES) denotes enrichment of the indicated gene signature in the gMll1+ populations while a positive NES indicates enrichment of the specified gene signature in gCd8+ cells. TFH-associated gene expression was depleted from Mll1-deficient TFH cells (Fig. 5d). Conversely, a TH1 signature was enriched in Mll1-deficient TFH cells (Fig. 5d). Enrichment of a TH1 gene signature was also observed in Mll1-deficient TH1 cells (Fig. 5d). Genetic signatures associated with TH2, TH17 and Treg cells were slightly enriched in Mll1-deficient TFH cells (Fig. 5d). In a separate analysis, we observed that wild-type TH1 cells were enriched for genes upregulated in Mll1-deficient cells (Fig. 5e). It was possible that Mll1 controls TFH differentiation by acting in concert with Bcl6 or Blimp-1. We tested the enrichment of Bcl6-bound and Blimp-1 genes in control and Mll1-deficient TH1 and TFH populations but found no strong evidence of Mll1 involvement in the regulation of Bcl6-bound genes (Fig. 5f). Expression of Blimp-1-bound genes was decreased in Mll1-deficient TH1 cells suggesting dysregulation of Blimp-1 activity in the absence of MLL1 (Fig. 5f). Together, these GSEA analyses suggest that in the absence of Mll1, TFH cells gain expression of genes associated with the TH1 program.
Figure 5: Transcriptomics analysis of Mll1-deficient TFH.
(A) gRNA+ CasTg SMARTA CD4 T cells were transferred into C57BL/6 mice and sorted into CXCR5+CD25− TFH and CXCR5−CD25+ TH1 3 days after infection with LCMV-Arm. (B) Quantitation of differentially expressed genes in gCd8+ and gMll1+ TFH and TH1. The numbers of DEGs are indicated in the columns. (C) Heatmaps of differentially expressed genes in gCd8 and gMll1 TFH and TH1. (D, F) Bubblegum plots of GSEA analyses of genes differentially expressed between gCd8+ and gMll1+ TFH or TH1 against the indicated gene signatures. (E) GSEA of genes down- or up-regulated in gMll1+ TFH compared to genes differentially expressed in gCd8+ TFH and TH1.
Dysregulated expression of LEF-1 and TCF-1 in Mll1-deficient TFH
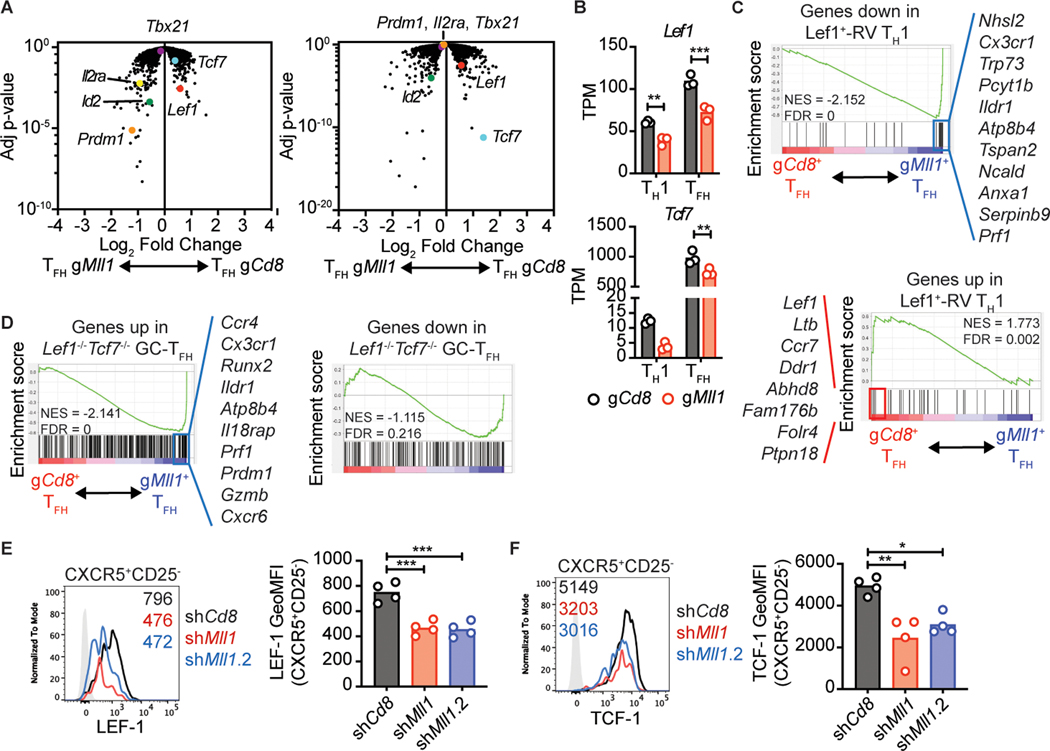
We searched a curated list of known TFH regulators for genes with dysregulated expression in Mll1-deficient TFH cells. Mll1-deficient TFH expressed significantly less Lef1 and Tcf7, (Fig. 6a, b). We also observed that Prdm1, the gene encoding Blimp-1 had higher expression in Mll1-deficient TFH in comparison to control TFH (Fig. 6a). Id2 and Il2ra, two genes associated with suppression of TFH fate (13, 35, 36), were also upregulated in Mll1-deficient TFH cells (Fig. 6a). Interestingly, Lef1 also showed reduced expression in Mll1-deficient TH1 cells (Fig. 6a, b).
Figure 6: Mll1 regulates expression of LEF-1 and TCF-1 in TFH cells.
(A) Volcano plots of gene expression in gCd8+ and gMll1+ TFH or TH1 plotted against adjusted p-value. Genes of interest are indicated. (B) TPM values for Tcf7 and Lef1 in the indicated populations. (C) GSEA of genes down- or up-regulated in Lef-RV+ TH1 compared to genes differentially expressed in gCd8+ TFH and gMll1+ TFH. (D) GSEA analysis of genes down- or up-regulated in Lef1−/−Tcf7−/− GC-TFH compared to genes differentially expressed in gCd8+ TFH and gMll1+ TFH. Selected genes that form the core enrichment signatures are listed. (E-F) shRNA+ SMARTA CD4 T cells were transferred into C57BL/6 mice and analyzed 3 days after infection with LCMV-Arm. (E) LEF-1 or (F) TCF-1 expression in CXCR5+CD25− SMARTA CD4 T cells. Numbers in the histograms indicate geometric MFI values. Data shown are from one experiment representative of 3 (E-F) independent experiments with 4 mice per group. (B) **p ≤ 0.01, ***p ≤ 0.001 (two-way ANOVA with Sidak’s multiple comparison test), (E-F) *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 (one way ANOVA with Tukey’s multiple comparisons test).
GSEA analysis revealed that genes upregulated following ectopic expression of Lef1 in CD4 T cells are depleted from Mll1-deficient TFH cells (Fig. 6c). Conversely, genes suppressed when Lef1 is overexpressed are over-represented in TFH lacking Mll1 (Fig. 6c). In a separate GSEA analysis, Mll1-deficient TFH cells were enriched for genes upregulated in Lef1−/−Tcf7−/− GC-TFH (Fig. 6d). These findings indicated that LEF-1- and TCF-1-regulated genes were dysregulated, implicating roles for LEF-1 and TCF-1 downstream of Mll1. We measured the expression of LEF-1 and TCF-1 in Mll1-deficient TFH cells by flow cytometry. Disruption of Mll1 reduced expression of both LEF-1 and TCF-1 in early TFH cells (CXCR5+CD25−, Fig. 6e, f, Supplemental Fig. 3a–c) These experiments demonstrate that in the absence of Mll1, LEF-1 and TCF-1 expression is impaired.
We sought to confirm the increased Prdm1 and Il2ra mRNA level in Mll1-deficient TFH by analyzing Blimp-1 and CD25 expression by flow cytometry. We first validated the staining of Blimp-1 by showing that CXCR5−CD25+ early TH1 cells express more Blimp-1 than CXCR5+CD25− early TFH cells (Supplemental Fig. 3d). We observed sporadic increases in Blimp-1 expression in Mll1-deficient CXCR5+Bcl6+ TFH 3 days after LCMV infection (Supplemental Fig. 3e). Similarly, CD25 expression was not reproducibly increased in Mll1-deficient TFH (Supplemental Fig. 3f).
TCF-1 partially rescues TFH differentiation of Mll1-deficient CD4 T cells
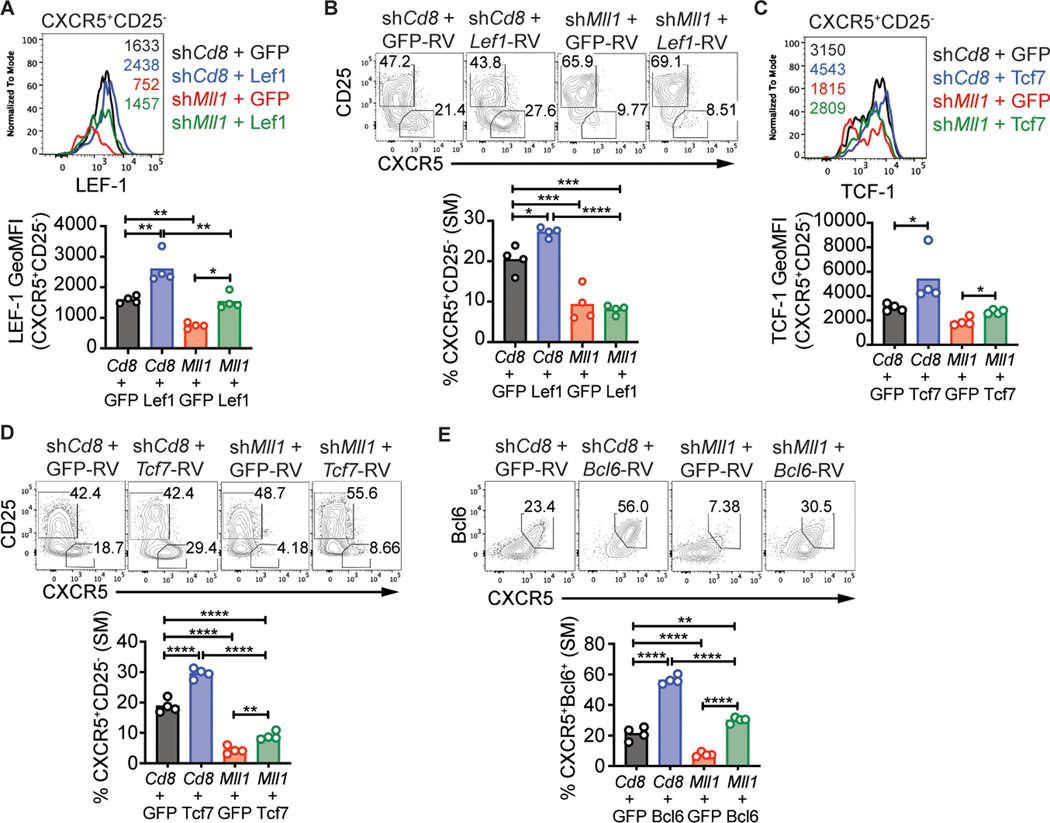
We hypothesized that ectopic expression of Bcl6, TCF-1 or LEF-1 in Mll1-deficient CD4 T cells may rescue TFH differentiation, as the expression of all three is decreased in the absence of Mll1. To test this hypothesis, we transduced shCd8+ and shMll1+ SMARTA CD4 T cells with RV-Lef1, RV-Tcf7-p45 (full-length isoform of TCF-1), RV-Bcl6 or control RV expressing GFP alone. RV+shRNAmir+ SMARTA CD4 T cells were transferred into C57BL/6 recipient mice and TFH differentiation was analyzed 3 days after LCMV-Arm infection. The expression of LEF-1 in shMll1+Lef1-RV+ TFH cells was equivalent to that of shCd8+GFP+ TFH cells (Fig. 7a). As expected, forced expression of LEF-1 resulted in increased TFH differentiation of shCd8+ SMARTA CD4 T cells (Fig. 7b). However, ectopic expression of wild-type levels of LEF-1 in shMll1+ SMARTA CD4 T cells did not rescue TFH differentiation (Fig. 7b). The levels of LEF-1 may not have quantitatively compensated for the reduced levels of TCF-1 (Supplemental Fig. 3g), as Tcf7 is more highly expressed than Lef1 at the mRNA level in mouse CD4 T cells (Fig. 6b), supporting a speculation that TCF1 protein is more highly expressed than LEF1.
Figure 7: TCF-1 partially rescues TFH differentiation of Mll1-deficient CD4 T cells.
SMARTA CD4 T cells transduced with the indicated RV were transferred into C57BL/6 mice and analyzed 3 days after LCMV-Arm infection. (A) LEF-1 or (C) TCF-1 expression in CXCR5+CD25− SMARTA CD4 T cells. Numbers in the histogram indicate geometric MFI values. (B,D) CXCR5+CD25− TFH or (E) CXCR5+Bcl6+ TFH in SMARTA CD4 T cells. Data shown are from one experiment representative of 2–3 independent experiments with 4 mice per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.001 (one way ANOVA with Tukey’s multiple comparisons test).
Expression of Tcf7-p45 in Mll1-deficient CD4 T cells resulted in TCF-1 expression equivalent to control shCd8+GFP+ cells (Fig. 7c). TCF-1 partially rescued the differentiation of early TFH differentiation by Mll1-deficient SMARTA CD4 T cells (Fig. 7d), including minimal changes in expression of Bcl6 and LEF-1 (Supplemental Fig. 3h). Overall, the results indicate Mll1 regulates multiple aspects of TFH differentiation, which can only be partially restored by TCF-1 expression.
If Mll1 primarily functions upstream of Bcl6 in TFH differentiation, ectopic expression of Bcl6 may bypass the loss of Mll1 and support TFH differentiation. TFH differentiation of shMll1+ SMARTA CD4 T cells was rescued by ectopic expression of Bcl6 (Fig. 7e) but did not increase LEF-1 and TCF-1 expression in TFH cells (Supplemental Fig. 3i). Overall, these data indicate that Mll1 regulates TFH differentiation by regulating expression of a network of transcription factors that includes, but is most likely not limited to, Bcl6, LEF-1 and TCF-1.
DISCUSSION
Here, we identified Mll1 as a critical regulator of TFH cells. Our work shows that Mll1 is required for optimal TFH cell differentiation and production of IL-21. We demonstrate that absence of Mll1 results in impaired expression of Bcl6, LEF-1 and TCF-1, each of which are important regulators of TFH differentiation. Our findings add Mll1 to the CRs that regulate TFH differentiation (19–24). The results here demonstrate that Mll1 is required for TFH differentiation. Mll1 is also involved in the expression of TH1 and TH2 cytokines (33, 34). Thus, Mll1 has roles in multiple CD4 T cell subsets similar to other CRs and transcription factors. The histone methyltransferase EZH2 is required for both TFH and Treg differentiation (21, 22, 37, 38). Similarly, the transcription factors Maf, IRF4 and Batf are all required for both TH17 and TFH differentiation (9–12, 39, 40).
Accumulation of Mll1-deficient CD4 T cells was mildly impaired 3 days after LCMV infection but was more dramatically impaired 3 days later. Loss of Mll1 expression resulted in decreased TFH population at both times analyzed after LCMV infection. It is possible that Mll1 plays a more prominent role in the early stages of TFH differentiation, at a time when the impact of Mll1-deficiency on CD4 T cell accumulation is marginal. Mll1 impact on CD4 T cell accumulation may become more prominent at later times as evidenced by decreased BrdU labelling observed 4 days after LCMV infection and the substantial loss in Mll1-deficient CD4 T cells 2 days later. Six days after LCMV infection, the decrease in TFH cell frequencies may be secondary to the decreased accumulation of Mll1-deficient CD4 T cells. Nevertheless, in a protein immunization system, TFH cells were decreased 8 days after immunization even when accumulation of CD4 T cells was not impaired in the absence of Mll1.
Our study provides a mechanistic explanation for the defective TFH differentiation in the absence of Mll1. First and foremost, expression of the critical TFH regulator Bcl6 is reduced in Mll1-deficient CD4 T cells. A small reduction in Bcl6 expression is sufficient to negatively impact TFH differentiation, as seen in CD4 T cells lacking a single Bcl6 allele (13). The reduced expression of Bcl6 in the absence of Mll1 most likely impairs suppression of alternative cell fates. Partial loss of Bcl6 is probably not the only cause for defective TFH differentiation in the absence of Mll1. LEF-1 and TCF-1 have largely redundant functions to each other in CD4 T cells, and reduced expression of both is likely a key contributing factor to the observed phenotype of Mll1-deficient CD4 T cells. Ectopic expression of LEF-1 did not rescue the defective TFH in Mll1-deficient CD4 T cells. It is plausible that forced expression of LEF-1 was not elevated enough to compensate for both LEF-1 and TCF-1. TCF-1 is expressed at substantially higher levels than LEF-1 and may explain why ectopic expression of TCF-1 partially compensated for Mll1 deficiency. We suggest that Mll1 controls a network of genes that includes Bcl6, LEF-1 and TCF-1 to promote TFH differentiation. We did not observe any effect of Mll1 on the expression of Bcl6- or Blimp-1-bound genes, suggesting Mll1 does not influence their transcriptional activities. Mll1 may still act on a small subset of such genes and this influence is not detected in our bioinformatics analysis. It is also possible that Mll1 is required for the function of other transcription factors controlling formation of TFH cells.
In addition to driving TFH differentiation, Mll1 promotes production of IL-21. Loss of Mll1 does not impact expression of Maf (data not shown), a transcription factor essential for IL-21 production (11, 12). Deletion of Mll1 could limit H3K4 methylation at the Il21 locus reducing its transcription leading to impaired production of IL-21. Mll1 is required for normal hematopoiesis (41, 42), but this function does not require the histone methyltransferase domain of Mll1 (43). It is conceivable that the enzymatic activity of Mll1 is not involved in promoting IL-21 production and TFH differentiation.
Chromatin modification is a complex process, and absence of a single CR is often compensated by other enzymes. Reduction in TFH differentiation of Mll1-deficient CD4 T cells may be partial due to compensatory mechanisms by other Mll1 family members (Mll2, Mll3 or Mll5), all of which form complexes with Wdr5, Ash2l and RbBp5 (32). Expression of Mll family members and genes coding for components of the Mll1 complex (Wdr5, Ash2l, Dpy30, RbBp5, Menin-1) was normal in the absence of Mll1 (data not shown). The phenotype of Mll1-deficient CD4 T cells is similar to the incomplete loss of TFH cells when other CRs are deleted (21–24). Further studies are required to obtain a detailed understanding the role of Mll1 in the control of epigenetic modifications of enhancers and promoters and how this impacts general chromatin accessibility. The roles of members of the Mll1 complex in regulating Mll1 function in the context of TFH differentiation should also be studied.
Disruption of the interaction between Mll1 and the core complex protein Wdr5 with a small molecule drug inhibits the growth of Mll1 leukemic cells (44). Similarly, a small-molecule inhibitor blocking the interaction between Mll1 and Menin-1 reduces tumor burden in mouse (45). Targeting Mll1 to inhibit TFH differentiation or function could be a promising therapeutic approach for autoimmune disorders characterized by elevated TFH activity. Alternatively, targeting the Mll1 complex to enhance its activity may be used in the design of better vaccines.
Supplementary Material
KEY POINTS.
MLL1 is a positive regulator of TFH differentiation
MLL1 regulates expression of LEF-1, TCF-1 and Bcl6
ACKNOWLEDGEMENTS
We thank members of the Crotty lab for critical reading of the manuscript, D. Hinz, M. Haynes, S. Sehic, S. Ellis and C. Dillingham of the LJI Flow Cytometry core for cell sorting, K. Fung and J. Greenbaum of the LJI Bioinformatics Core and G. Seumois, H. Simon and A. Wang of the LJI Sequencing core.
The work of S.H. was partially supported by the Achievement Rewards for College Scientists (ARCS) Foundation of San Diego. This work was funded by grants from the USA National Institutes of Health (NIH), including National Institute of Allergy and Infectious Diseases (NIAID) U19 AI109976 and NIAID R01 AI72543, NIH S10 RR027366 (LJI), and internal La Jolla Institute institutional funds to SC.
Footnotes
COMPETING INTERESTS
S.B. is a current employee of VIR Biotechnology and may possess shares of VIR Biotechnology.
S.B. designed and performed the experiments, analyzed data and wrote the manuscript; S.H. performed the experiments and analyzed data; C.E.F. and J.C. generated reagents; A.G., H.D., P.P.K. and N.A.B. performed and analyzed the next generation sequencing of the shRNA screen; M.E.P. supervised sequencing analysis; S.C. designed the study, supervised the work and wrote the manuscript.
RNA-seq data are deposited to the Gene Expression Omnibus (GEO) under the GSE226341 accession number (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE226341)
REFERENCES
- 1.Crotty S. 2019. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50: 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi J, Diao H, Faliti CE, Truong J, Rossi M, Bélanger S, Yu B, Goldrath AW, Pipkin ME, and Crotty S. 2020. Bcl-6 is the nexus transcription factor of T follicular helper cells (TFH) via repressor-of-repressor circuits. Nat Immunol 21: 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li Q-J, Parish CR, Mackay CR, and Vinuesa CG 2009. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31: 457–468. [DOI] [PubMed] [Google Scholar]
- 4.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Y-H, and Dong C. 2009. Bcl6 mediates the development of T follicular helper cells. Science 325: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, and Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325: 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue H-H, and Crotty S. 2015. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol 16: 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, He R, Hao Y, Wang H, Zhao T, Fan Z, Qin A, Ye J, Zhou X, Ye L, and Wu Y. 2015. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat Immunol 16: 991–999. [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Shin HM, Moseman EA, Ji Y, Huang B, Harly C, Sen JM, Berg LJ, Gattinoni L, McGavern DB, and Schwartzberg PL 2015. TCF1 Is Required for the T Follicular Helper Cell Response to Viral Infection. Cell Rep 12: 2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, Kim CH, and Taparowsky EJ 2010. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J Exp Med 207: 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL, and Murphy KM 2011. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat Immunol 12: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho I-C, Sharpe AH, and Kuchroo VK 2009. Costimulatory molecule ICOS plays a critical role in the development of TH-17 and follicular T-helper cells by regulating c-Maf expression and IL-21 production. Nat Immunol 10: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andris F, Denanglaire S, Anciaux M, Hercor M, Hussein H, and Leo O. 2017. The Transcription Factor c-Maf Promotes the Differentiation of Follicular Helper T Cells. Frontiers in Immunology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw LA, Bélanger S, Omilusik KD, Cho S, Scott-Browne JP, Nance JP, Goulding J, Lasorella A, Lu L-F, Crotty S, and Goldrath AW 2016. Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nat Immunol 17: 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, Nakatsukasa H, Neelapu SS, Chen W, Clevers H, Tian Q, Qi H, Wei L, and Dong C. 2014. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature 507: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J-Y, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, Jenkins MK, Rosenfeld MG, Hogquist KA, and Jameson SC 2015. The transcription factor KLF2 restrains CD4+ T follicular helper cell differentiation. Immunity 42: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vahedi G, Kanno Y, Furumoto Y, Jiang K, Parker SCJ, Erdos MR, Davis SR, Roychoudhuri R, Restifo NP, Gadina M, Tang Z, Ruan Y, Collins FS, Sartorelli V, and O’Shea JJ 2015. Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature 520: 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein JS, Lezon-Geyda K, Maksimova Y, Craft S, Zhang Y, Su M, Schulz VP, Craft J, and Gallagher PG 2014. Global transcriptome analysis and enhancer landscape of human primary T follicular helper and T effector lymphocytes. Blood 124: 3719–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, and Crotty S. 2015. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med 212: 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nance JP, Bélanger S, Johnston RJ, Hu JK, Takemori T, and Crotty S. 2015. Bcl6 middle domain repressor function is required for T follicular helper cell differentiation and utilizes the corepressor MTA3. Proc Natl Acad Sci U S A 112: 13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JA, Tubo NJ, Gearhart MD, Bardwell VJ, and Jenkins MK 2015. Cutting edge: Bcl6-interacting corepressor contributes to germinal center T follicular helper cell formation and B cell helper function. J Immunol 194: 5604–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Zeng Z, Xing S, Gullicksrud JA, Shan Q, Choi J, Badovinac VP, Crotty S, Peng W, and Xue H-H 2018. Ezh2 programs TFH differentiation by integrating phosphorylation-dependent activation of Bcl6 and polycomb-dependent repression of p19Arf. Nat Commun 9: 5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Cao G, Wu J, Wang X, Pan Z, Gao J, Tian Q, Xu L, Li Z, Hao Y, Huang Q, Wang P, Xiao M, Xie L, Tang S, Liu Z, Hu L, Tang J, He R, Wang L, Zhou X, Wu Y, Chen M, Sun B, Zhu B, Huang J, and Ye L. 2020. The histone methyltransferase EZH2 primes the early differentiation of follicular helper T cells during acute viral infection. Cell Mol Immunol 17: 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long X, Zhang L, Zhang Y, Min M, Lin B, Chen J, Ma X, Zhai S, Cai Z, Liu Y, Lu Y, Che N, Tan W, Qin J, and Wang X. 2020. Histone methyltransferase Nsd2 is required for follicular helper T cell differentiation. J Exp Med 217: e20190832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook KD, Shpargel KB, Starmer J, Whitfield-Larry F, Conley B, Allard DE, Rager JE, Fry RC, Davenport ML, Magnuson T, Whitmire JK, and Su MA 2015. T Follicular Helper Cell-Dependent Clearance of a Persistent Virus Infection Requires T Cell Expression of the Histone Demethylase UTX. Immunity 43: 703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Bélanger S, Frederick MA, Li B, Johnston RJ, Xiao N, Liu Y-C, Sharma S, Peters B, Rao A, Crotty S, and Pipkin ME 2014. In vivo RNA interference screens identify regulators of antiviral CD4+ and CD8+ T cell differentiation. Immunity 41: 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oxenius A, Zinkernagel RM, and Hengartner H. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9: 449–457. [DOI] [PubMed] [Google Scholar]
- 27.Silva M, Kato Y, Melo MB, Phung I, Freeman BL, Li Z, Roh K, Van Wijnbergen JW, Watkins H, Enemuo CA, Hartwell BL, Chang JYH, Xiao S, Rodrigues KA, Cirelli KM, Li N, Haupt S, Aung A, Cossette B, Abraham W, Kataria S, Bastidas R, Bhiman J, Linde C, Bloom NI, Groschel B, Georgeson E, Phelps N, Thomas A, Bals J, Carnathan DG, Lingwood D, Burton DR, Alter G, Padera TP, Belcher AM, Schief WR, Silvestri G, Ruprecht RM, Crotty S, and Irvine DJ 2021. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. Sci Immunol 6: eabf1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, Braun A, Wynne-Jones E, Behr FM, Stark R, Pellicci DG, Godfrey DI, Belz GT, Pellegrini M, Gebhardt T, Busslinger M, Shi W, Carbone FR, van Lier RAW, Kallies A, and van Gisbergen KPJM 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352: 459–463. [DOI] [PubMed] [Google Scholar]
- 29.Jain R, Chen Y, Kanno Y, Joyce-Shaikh B, Vahedi G, Hirahara K, Blumenschein WM, Sukumar S, Haines CJ, Sadekova S, McClanahan TK, McGeachy MJ, O’Shea JJ, and Cua DJ 2016. Interleukin-23-Induced Transcription Factor Blimp-1 Promotes Pathogenicity of T Helper 17 Cells. Immunity 44: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao N, Eto D, Elly C, Peng G, Crotty S, and Liu Y-C 2014. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol 15: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, and Glimcher LH 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669. [DOI] [PubMed] [Google Scholar]
- 32.Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, and Roeder RG 2006. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol 13: 713–719. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita M, Hirahara K, Shinnakasu R, Hosokawa H, Norikane S, Kimura MY, Hasegawa A, and Nakayama T. 2006. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity 24: 611–622. [DOI] [PubMed] [Google Scholar]
- 34.Schaller M, Ito T, Allen RM, Kroetz D, Kittan N, Ptaschinski C, Cavassani K, Carson WF, Godessart N, Grembecka J, Cierpicki T, Dou Y, and Kunkel SL 2015. Epigenetic regulation of IL-12-dependent T cell proliferation. J Leukoc Biol 98: 601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston RJ, Choi YS, Diamond JA, Yang JA, and Crotty S. 2012. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med 209: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballesteros-Tato A, León B, Graf BA, Moquin A, Adams PS, Lund FE, and Randall TD 2012. Interleukin-2 Inhibits Germinal Center Formation by Limiting T Follicular Helper Cell Differentiation. Immunity 36: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, and Bluestone JA 2015. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity 42: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X-P, Jiang K, Hirahara K, Vahedi G, Afzali B, Sciume G, Bonelli M, Sun H-W, Jankovic D, Kanno Y, Sartorelli V, O’Shea JJ, and Laurence A. 2015. EZH2 is crucial for both differentiation of regulatory T cells and T effector cell expansion. Sci Rep 5: 10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkhurst CN, Muratet M, Newberry KM, Meadows S, Greenfield A, Yang Y, Jain P, Kirigin FK, Birchmeier C, Wagner EF, Murphy KM, Myers RM, Bonneau R, and Littman DR 2012. A validated regulatory network for Th17 cell specification. Cell 151: 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamoorthy V, Kannanganat S, Maienschein-Cline M, Cook SL, Chen J, Bahroos N, Sievert E, Corse E, Chong A, and Sciammas R. 2017. The IRF4 Gene Regulatory Module Functions as a Read-Write Integrator to Dynamically Coordinate T Helper Cell Fate. Immunity 47: 481–497.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jude CD, Climer L, Xu D, Artinger E, Fisher JK, and Ernst P. 2007. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell 1: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMahon KA, Hiew SY-L, Hadjur S, Veiga-Fernandes H, Menzel U, Price AJ, Kioussis D, Williams O, and Brady HJM 2007. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell 1: 338–345. [DOI] [PubMed] [Google Scholar]
- 43.Mishra BP, Zaffuto KM, Artinger EL, Org T, Mikkola HKA, Cheng C, Djabali M, and Ernst P. 2014. The histone methyltransferase activity of MLL1 is dispensable for hematopoiesis and leukemogenesis. Cell Rep 7: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao F, Townsend EC, Karatas H, Xu J, Li L, Lee S, Liu L, Chen Y, Ouillette P, Zhu J, Hess JL, Atadja P, Lei M, Qin Z, Malek S, Wang S, and Dou Y. 2014. Targeting MLL1 H3 K4 methyltransferase activity in MLL leukemia. Mol Cell 53: 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borkin D, He S, Miao H, Kempinska K, Pollock J, Chase J, Purohit T, Malik B, Zhao T, Wang J, Wen B, Zong H, Jones M, Danet-Desnoyers G, Guzman ML, Talpaz M, Bixby DL, Sun D, Hess JL, Muntean AG, Maillard I, Cierpicki T, and Grembecka J. 2015. Pharmacologic inhibition of the menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 27: 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.