Abstract
Background
This study was conducted to assess the concentration of heavy metals (arsenic and mercury) and estimate the probability that consumption of hen egg products collected in Iran has carcinogenic or non-carcinogenic consequences.
Methods
A total of eighty-four hen eggs from 21 major brands were randomly selected from among thirty local supermarkets in two seasons (winter (January) and summer (August) 2022). Arsenic (As) and Mercury (Hg) was determined by using ICP-MS. The human health risk assessment refers to the formulation of the USEPA standard focused on Estimated Daily Intake (EDI), International Lifetime Cancer Risk (ILCR), Target Hazard Quotient (THQ), and Monte Carlo simulation (MCS) as a probabilistic method. Data analysis was carried out using the statistical software SPSS. Differences in mean concentrations of As and Hg in two seasons were tested by paired t-test.
Results
Over two seasons, the average As and Hg concentrations in hen eggs were 0.79 and 0.18 µg.kg−1, respectively. Seasonal difference in As concentration (p = 0.451) was not significant, whereas that of Hg concentration (p < 0.001) was significant. The calculated value of EDI was 0.29 µg As/day and 0.06 µg Hg/day. The EWI in the maximum scenario of as level in hen eggs was estimated to be 8.71 µg As and 1.89 µg Hg/month for Iranian adults. THQ's mean for As and Hg in adults was determined to be 0.00385 and 0.00066, respectively. In addition, ILCRs by MCS for As were 4.35E-4.
Conclusion
In total, the result indicates that there was not a significant risk of developing cancer; the calculation of THQ was still below the accepted level of 1, indicating that there was no risk while, according to most regulatory programs (ILCR > 10− 4) shows a threshold carcinogenic risk of arsenic through consuming in hen eggs. Therefore, policymakers need to be aware that it is prohibited to establish chicken farms in heavily polluted urban areas. It is essential to regularly conduct examinations to measure the presence of heavy metals in both ground waters used for agriculture and the feed provided to chickens. Additionally, it is advisable to focus on raising public awareness about the importance of maintaining a healthy diet.
Keywords: Hen eggs, Arsenic, Mercury, Risk assessment, ICP-MS, THQ, Monte Carlo simulations
Background
The perfect balance and diversity in its high-quality nutrients along with its high availability and play a key role in the daily diet of individuals worldwide, its affordable price makes the conventional hen egg a food product [1–3]. However, eggs may contain a high concentration of heavy metals that come primarily from food and water feed, mainly impacted by the environment [4]. Environmental contamination brought on by the growth of livestock and poultry production has sparked worries about food safety, particularly about potential heavy metal residues in feed additives or poultry feed and products, including eggs [5]. Heavy metals can be transferred from poultry to eggs [1]. Consequently, hens can take up heavy metals from various environmental sources and pass those chemicals on to their eggs [6]. Season, location, chicken age, nutritional behaviors, and metabolic cycle are some of the elements that influence how much the laying hens absorb heavy metals [7].
Heavy metals, including arsenic (As) and mercury (Hg), can be hazardous and not biologically necessary even in small amounts [8, 9]. Inorganic As is a naturally occurring element found in the earth's crust and is known as the "king of poisons" due to its ability to induce liver and lung cancer [2, 10]. It is widely dispersed throughout the environment in the air, water, and land [10]. As is the substance that has raised the most significant questions regarding potential harmful effects on human health because it is easily transported across food chains, and is not known to perform any crucial biological functions. It has been demonstrated that children are more sensitive than adults, and the effects are cumulative [2]. Symptoms of As toxicity include abdominal pain, nausea, and diarrhea, which may lead to severe diseases including neurological, respiratory, reproductive, hepatic, and cardiovascular, as well as various cancers [11].
Mercury is one of the most hazardous metals after lead which has no known benefits for human physiology. It is still widely employed in the industry. An adult of normal weight, weighing 70 kg, is thought to have 13 mg of mercury in his body. In humans, high Hg levels are found in the skin, nails, hair, and kidneys. It is a highly reactive molecule that produces toxic effects by binding highly to sulfhydryl and a lesser degree to hydroxyl, carboxyl, and phosphoryl groups [12]. Mercury deposited in soil may continue to be discharged into surface waters for thousands of years because soils have a long residence time for the metal. Feed ingredients, such as the usage of fish and crops tainted with Hg, may also contaminate poultry feed while it is being processed. Plants can accumulate mercury from the atmosphere [13].
Additionally, heavy metals can increase oxidative stress by generating free radicals, which harms antioxidant defense. Long-term exposure to As and Hg can have harmful consequences even in small doses. The food chain can become contaminated as a result of the poisoning of the soil, water, plants, and animals by these harmful metals. As a result, exposure to heavy metals through inhalation, ingestion, skin contact, and drinking water can be hazardous to human health [14–18].
In many countries, including Iran, is an increasing prevalence of various types of cancer, and one of the main parameters affecting cancer is the environment. Microbial, chemical, and radiation contamination can affect the majority of cancer [19]. The contamination of the environment can also be detected in the food chain. The major sources of high metal contamination in the environment include mining, industry, household trash, pesticides, and agricultural practices [20].
Knowledge of the mineral content of eggs is becoming increasingly important for many reasons that are related to their health and nutritional value of eggs including, the consequences of egg metals on its embryonic development, and the use of eggs as bio-indicators for environmental metal pollution [21]. Tehran is one of the world's most polluted cities [22]. This pollution can affect human health directly or indirectly through contamination of the food chain. Chickens play an essential role in the food chain and their contamination can have irreparable consequences. Hen eggs may become tainted with heavy metals through chicken feed and drinking water, both of which are primarily influenced by the environment. Consistently consuming heavy metals in food at hazardous levels may have negative impacts on humans by impairing a variety of biological and metabolic systems [19].
Iran, the Middle East’s largest poultry producer, produces up to 1.2 million tons of eggs annually [23]. Policymakers and risk managers, in particular, can receive comprehensive information through risk assessments [24]. It is possible to identify complicated cause-and-effect linkages and reduce risk using even modest estimates of risk variance. The environmental health risk assessment technique is so naturally cautious and strongly protective of human health [25].
There have been limited studies published about the levels of As and Hg in hen eggs. There have been limited studies published about the levels of As and Hg in hen eggs, Iran faces problems with poultry feed because of the sanctions, and hen eggs are one of the most popular foods in the food baskets of the majority of low-income households. This study was also conducted under the framework of a heavy metal monitoring plan and risk assessment in the food chain, according to the results of which policymakers can design plans for how to reduce these heavy metals and improve guidelines for protecting consumer health and increasing food safety.
So this study for the first time evaluated consumer health risk assessment of arsenic and mercury in hen eggs through Monte Carlo simulations.
Data and methods
Samples
In one week in two seasons (winter (January) and summer (August) 2022), the top twenty-one egg brands in Tehran were sampled from among thirty local supermarkets in five districts (North, Sought, Center, West, and East) of Tehran. Forty-two samples were randomly selected from 21 brands (two eggs chosen from each batch content of 12 eggs) for each season. A total of 84 fresh, unbroken, and unfertilized hen eggs were selected after being visually assessed by candling. They were then cleaned with deionized water, coded, and packed in polyethylene zip bags. Refrigerated conditions were used to transport items to the laboratory for chemical analysis. Samples were quickly chilled at 4 °C before being prepared. Instead of using metal tools, chemically stable sterile flacon tube tools were utilized to prevent any chemical contamination. The analytical preparation was carried out immediately. To avoid contamination elements before use, the entire piece of equipment was cleaned with diluted HNO3 (10%) and then distilled water [26].
Samples preparation
Carefully, the egg's contents were separated from the eggshell. The next stage involved mixing and homogenizing each whole egg (yolk and white) component before pouring it into Petri dishes to be dried in an oven for 24 h at 70 °C to become a fine powder.
To digest 0.5 g of dried egg samples, 10 ml of 70% nitric acid and 30% hydrogen peroxide (v/v) were purchased from Merk (Darmstadt, Germany) and left at room temperature for one night.
The digestion took place for 4 h at 150 °C until the solution was clear. The solution was cooled to room temperature (22–23 °C), diluted with deiodine water to 50 ml, and filtered through 0.45 L acid-resistant filter paper. The solution was stored at 4 °C for later analysis. The liquefied solution was filtrated and diluted with 20% HNO3 before being analyzed by ICP-MS (ULTIMA2, 6100 DRC-e Perkin Elmer Elan). In addition, the glassware containers used for analysis were first washed with detergent and rinsed several times with tap water several times and then they have soaked overnight in 6 N HNO3 (Merk) solutions and finally rinsed with deionized water.
According to the FDA Elemental Analysis Manual, samples were assessed for total As and Hg using heat-block-assisted acid digestion and the ICP-MS technique. The blank solution was made in the same way but without an egg. For quality assurance, blanks and certified standards were analyzed after every ten samples. The samples were analyzed in triplicates. Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It is known and used for its ability to determine metals and several non-metals in liquid samples at very low concentrations (ppb = parts per billion = µg/l). It is well-recognized that this technique is the fastest and most reliable for determining the content of heavy metals in the food business [26].
The goal of a quality assurance and quality control (QA/QC) program is to monitor the quality of data from sampling in the field through the generation of the final results, to ensure that both the user and external parties are confident in the quality of the data obtained. Furthermore, a properly designed and implemented QA/QC program will also identify errors and the potential stage of the analysis. To ensure proper quality, a QA/QC program is therefore aimed at understanding the following [27, 28]:
Sampling:
At an appropriate location from five districts in Tehran and in two seasons,
Taking the sample properly,
Storing the sample not too long
The sampling equipment was plastic and stainless steel
Double-checking sample labels before starting each test
In laboratory:
Regularly calibrating equipment and machines.
Consistently and regularly documenting testing methods.
Procuring personal and lab-wide certifications.
Sterilizing equipment and preventing personal contamination.
Regularly evaluating standard procedures carried out by lab techs and interns.
Running both a triple sample and a blank sample to compare test results.
All the mentioned above have been tried to be included as (QA/QC) in the present study and also all parameters for ICP-Mass were used as shown in the Table 1.
Table 1.
Conditions of ICP-MS apparatus for determining Arsenic and Mercury in hen eggs
| Parameter | value |
|---|---|
| Radiofrequency | 1200W(40 MHz) |
| Plasma gas (Argon) flow | 16 l/min |
| Nebulizer gas (Argon) flow | 1 l/min |
| Read delay and analysis speeding | 30 s |
| Wash | 60 s |
| Wash speeding | 30 rpm |
| Dwell time | 50 ms |
| Resulting/amu10%peak | 0.7 |
| Integration time | 3.5 |
| Linear working (total element) ppb | 0.053 |
| Precision%RSD ( n = 10) | 1.3 |
| Addition/Recovery | 93–103 |
| Repetition | 3 |
| LOD (As, Hg) | 0.00033 μg kg−1 |
| LOQ (As, Hg) | 0.001 μg kg−1 |
Health risk assessment
In this study, the assessment of human health risk is used to describe the potential risk of heavy metals from consuming hen eggs obtained from Iranian commercial hen eggs. The United State Environmental Protection Agency (US EPA) proposed the method to calculate health risk needs (estimated daily intake, target hazard quotient, and carcinogenic risks) [29].
The average body weight of adult consumers, the mean amounts of these metals in eggs, and the number of eggs ingested were used to determine the Estimate daily intake (EDI) for Hg and As.
The EDI of As and Hg was calculated according to Eq. 1.
| 1 |
EDI is the estimated daily intake (µg kg −1 b.w/day); FIR, is the consumption of the daily eggs (ml/day-1); CM, is the mean level of metal (mg /mL−1); and WAB, is the average body weight (kg). According to the National Institute of Nutritional Research and Food Industry of Iran research [30], adults (18 to 50) in Iran consume 25.4 g of eggs daily. Also, the WAB (body weight) for adults is 70, according to body weight studies by the United States Environmental Protection Agency (EPA) [31, 32]. As and Hg exposure from eating eggs was calculated. In addition to EDI, estimated weekly intake (EWI, g kg -1 b.w/week) and estimated monthly intake (EMI, g kg -1 b.w/month) of As and Hg for adults were computed to compare with the PTWI and PTMI, respectively, set by JECFA (Joint FAO/WHO Expert Committee on Food Additives) [31]. The PTWI of As and Hg was established at 15 and 4(µg kg−1 BW/week) by JECFA [33].
Non-carcinogenic risk estimation
Target Hazard Quotient (THQ) estimation was performed to determine the non-carcinogenic risk among hen egg consumers [34, 35] by Eq. 2.
| 2 |
According to research, 365 days per year and 70 years were determined to be the frequency of exposure (EF) and the exposure time equivalent to the mean lifetime in Iran. As and Hg had oral reference doses (RFD) of 0.0003 and 0.0001(mg kg−1 BW/day), respectively [36]. TA (exposure duration for non-carcinogens) was 25,550 days.
Carcinogenic risk estimation
To assess the potential cancer risk of As in people who consume eggs [36], the Incremental Lifetime Cancer Risk (ILCR) was computed from Eq. 3.
| 3 |
A lifetime means the dosage of 1 mg kg-1 BW/day results in a risk known as the cancer slope factor (CSF) [19] 1.5 mg kg-1 day of CSF for As [36].
According to the U.S. Environmental Protection Agency (US-EPA), the safe limit for cancer risk is below approximately 1 chance in 1,000,000 lifetime exposure (ILCR < 10–6), the threshold risk limit (ILCR > 10–4) for a chance of cancer is above 1 in 10,000 exposure, where corrective measures are significant, and the moderate risk level (ILCR > 10–3) is above 1 in 1,000 where public health safety assessment is more critical [37, 38].
Statistical analysis
After gathering the necessary data, data analysis was done using the SPSS software (SPSS Inc., version 16, Chicago, IL, USA). The frequency (%), mean (Standard Error), and median (minimum–maximum) for both normal and non-normal distributions were used to summarize data for categorical variables. The Kolmogorov–Smirnov test was used to determine whether the data were normal. A paired t-test was used to determine significant seasonal differences between paired egg samples. Statistics were considered significant for P-values under 0.05.
The calculation of the limit of detection, LOD (µgkg−1), was based on.the measurement results obtained with blank filters in the present study for As and Hg was 0.0003. The results were given in micrograms per kilogram of the sample's moist weight. The limit of quantification (LOQ) was estimated to be 0.001 for both metals [39].
Monte Carlo simulation (MCS) technique
There may be some uncertainties in the estimate of health risks [36, 40]. When single-point data are utilized to estimate health hazards caused by exposure to pollutants such as toxic metals, a high level of uncertainty is seen. To lessen the uncertainty in the assessment of health risks, MCS was used in our experiment as a probabilistic method [41, 42]. For the creation of risk assessment models, Oracle, Inc.'s Crystal Ball software (version 11.1.2.4, USA) was utilized. A percentile of 95% of THQ and ILCR in the cumulative probability graph is the threshold for threatened exposed populations in this study, which included 10,000 repeats [43].
Results and discussion
Eggs are a great source of protein and other vital elements. In many commercial and homemade dishes, it is also a common ingredient. Therefore, everyone must be aware of the trace-element content of eggs [44]. The number of eggs consumed rises daily. Consumption may vary depending on various variables, including socioeconomic position, age group, and urban versus rural residence. Compared to poorer people, who eat boiled or fried eggs, the higher classes consume more eggs in cakes, biscuits, and salads [44].
Heavy metals hen egg residue of As and Hg in winter and summer
The results of this study showed that the mean residue of As were 0.52 and 01.07 μg kg−1 in the winter and summer, respectively (Table 2). In total, the average concentration of As was relatively low (0.79 μg kg−1), especially when compared with the data in other countries such as Italy 7 μg kg−1 [39], France 8 μg kg−1 [45], the United Kingdom 0. 9 μg kg−1 [15], Turkey 2.96 μg kg−1 [20], Bangladesh 30 μg kg−1 [46], and Belgium 16 μg kg−1 [47]. In two investigations conducted in Iran, the mean levels of As in hen eggs were determined to be 30 μg kg−1 [48] and 8 μg kg−1 [49], respectively.
Table 2.
Comparison of As and Hg residue (µg kg−1) in hen eggs (two seasons)
| Heavy metals | Min | Max | Mean ± SE |
|---|---|---|---|
| AS | |||
| Winter | 0.10 | 1.50 | 0.52 ± 0.050 |
| Summer | 0.00 | 7.50 | 1.07 ± 0.557 |
| Hga | |||
| Winter | 0.00 | 0.50 | 0.26 ± 0.021 |
| Summer | 0.10 | 0.10 | 0.10 ± 0.00 |
aDifferences in As between winter and summer were not significant with Paired t-test (p = 0.451) but were significant in Hg (p < 0.001)
Moreover, based on the current study in Table 2, the average Hg levels were 0.26 and 0.10 μg kg−1 in winter and summer, respectively. The results showed the level of Hg in hen eggs was similar to or less than those of other countries (0.18 μg kg−1), such as China 0.1 μg kg−1 [50], France 4 μg kg−1 [45], the United Kingdom 1 μg kg−1 [15], Belgium 2 μg kg−1 [47], Turkey 0.34 μgkg−1 [20], and Denmark 2 μg kg−1 [51]. In a few studies conducted in Iran, the concentration of Hg was 70 μg kg−1 [49] and 26 μg kg−1 [4].
Overall, findings demonstrated that, compared to past Iranian assessments, As and Hg levels have decreased through these years. Differences in As concentration was not significant in the two seasons, according to the paired t-test result (p = 0.451); however, differences in Hg concentration (p < 0.001) were significant, which can be the sum of the following factors involved in the difference. The safety of poultry feed and any potential risks to human health have become significant concerns as commercial production of poultry and poultry feed has advanced on a bigger scale [52].
Challenges and threats in heavy metal residues in hen eggs
Globally, pollution and technological growth are posing enormous challenges and threats to both humans and animals as follows:
Use of insecticides and poisonous plants on crops [53]
Antibiotics used in chicken feed, both therapeutic and non-therapeutic [54]
Lead, Mercury, Arsenic, Antimony, and other heavy metals have contaminated feed through water, crops, and industrial waste [52].
However, the primary source of poultry feeding and watering is based on mineral and agricultural products and water. There is still a chance that hazardous environmental elements or food additives could contaminate chicken feed, so this must be monitored appropriately. The presence of heavy metals in the chicken feed may be caused by several components, including minerals, additions for marine feed (such as fish meal, algae), trace elements (copper sulfate, zinc oxide), Roxarson (kills parasites and improves meat color), and anti-caking chemicals. As and Hg are a significant concern due to their poisonous qualities and the absence of a necessary biological function [55, 56].To the point that European Commission has set maximum limits on heavy metals, such as lead, cadmium, and mercury, in certain foods but not for hen eggs (European Commission Regulation, EC No 18812006) [57].
Health risk assessment
In addition to assessing the total concentration of As and Hg by comparing the permissible limits, other factors, including exposure time, per capita intake, metal toxicity, and body weight, are crucial in assessing the possible health risk. Appropriate data interpretation was used to carry out exposure and non-carcinogenic and carcinogenic risk assessments for adults [19, 35].
Exposure assessment
Dietary exposure of As and Hg through consuming hen eggs was assessed by calculating EDI, EWI, and EMI in two scenarios of the overall and maximum concentrations of these metals and compared to provisional tolerable weekly intake (PTWI) and provisional tolerable monthly intake (PTMI) established joint FAO/WHO Expert Committee on Food Additives( JECFA). The results were summarized in Table 3.
Table 3.
EDI, EWI, EMI of As and Hg in adults due to consumption of hen eggs in two seasons
| As | EDIa | EWIb | EMIc |
|---|---|---|---|
| Winter | 0.19 | 1.32 | 5.66 |
| Summer | 0.39 | 2.72 | 11.65 |
| Mean | 0.29 | 2.03 | 8.71 |
| Hg | EDI | EWI | EMI |
| Winter | 0.09 | 0.66 | 2.83 |
| Summer | 0.03 | 0.25 | 1.09 |
| Mean | 0.06 | 0.42 | 1.89 |
aEDI Estimated Daily Intake, bEWI Estimated Weekly Intake,cEMI Estimated Monthly Intake
The PTWI of As and Hg was established at 15 and 4 (µg kg−1 BW/week) by JECFA
The risk values (PTWI and PTMI) depend on the amount of consumption, the pollution rate of the desired food, and the weight of the target group. The PTWI of As and Hg was established at 15 and 4( µg kg−1 BW/week) by JECFA [33].
Therefore, the weekly intake of AS and Hg (EWI) from ingested eggs (in the maximum scenario) were 2.03 and 0.25 µg/week for an adult with 70 kg body weight, respectively (EDI: 0.29 As/day and 0.06 µg Hg/day for an adult) the EWI in the maximum scenario of as level in eggs were estimated to be 2.03 As and 0.42 Hg (µg/week) for Iranian adults in Table 3.
In 2010, the JECFA stated that daily ingestion of As through food consumption has minimal effect on overall exposure due to its long half-life. Therefore, tolerable and dietary ingestion of As for assessing the long-term and short-term health risks should be determined over 1 month or several months, respectively. Considering the maximum level of As and Hg in hen eggs, the EMI of adults was 8.71 and 1.89(µg/month), respectively.
In the present study, the exposure of As and Hg through consuming eggs (EWI and EMI) was lower than the risk values suggested, which indicates low risk for consumers [58]. It should be highlighted that this study only refers to the consumption of hen eggs, which may only contribute minimally to Iranian consumers' overall exposure to As and Hg.
Non-carcinogenic risk
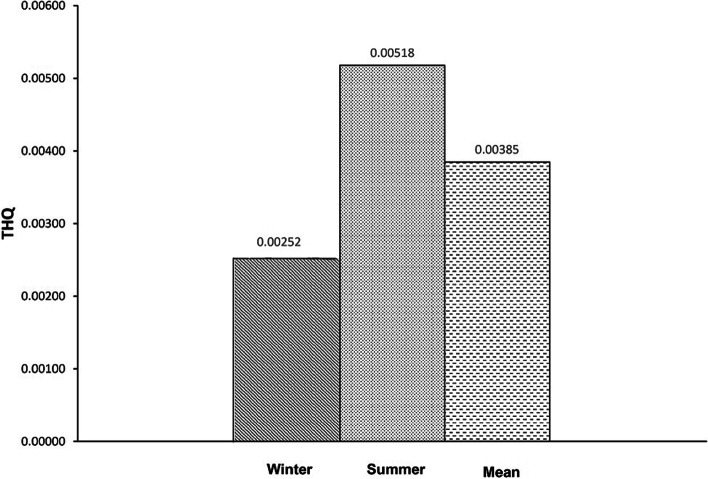
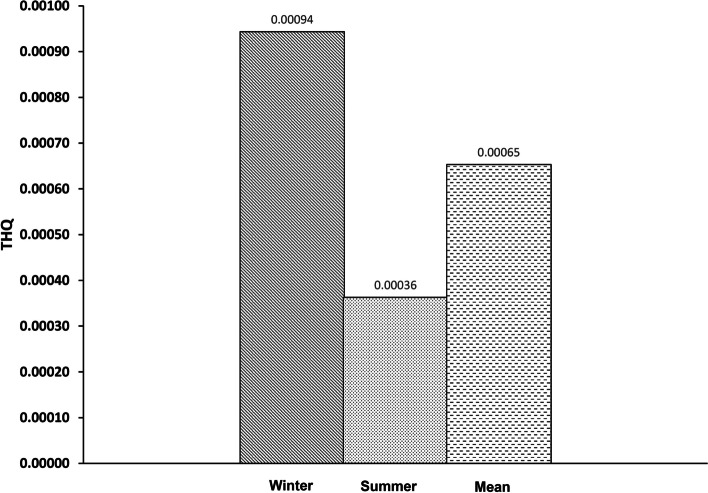
The non-carcinogenic risk of As and Hg in consumers was calculated by determining the THQ (Target Hazard Quotient) value. The results of the ICP-MS analysis were utilized to calculate the adult population's overall and maximum levels of THQ for As and Hg’, as shown in Figs. 1 and 2. This value has been acknowledged as an appropriate variable for assessing the dangers associated with eating hazardous metals through contaminated food.
Fig. 1.
Target hazard quotient (THQ) of As in two seasons (winter, summer, and mean) through hen eggs consumption
Fig. 2.
Target hazard quotient (THQ) of Hg in two seasons (winter, summer, and mean) through hen eggs consumption
The THQ is described as the ratio of a contaminant's observed amount to the drug's reference oral dosage (RFD) [59]. Negative consequences may happen when a metal’s THQ value is more than 1, but they are less probable to occur when it is lower than 1 [60].
Based on the total amount of hen eggs consumed daily by Iranian adults (25.4 g/day) [30], the THQ’s mean of As and Hg for adults was calculated to be 0.00385 (winter 0.00252, summer 0.00518) and 0.00065 (winter 0.00094, summer 0.00036), respectively.
Several authors in other countries [61], including Bangladesh (0.260) (-), Belgium (3.539) (0.065), China (0.195) (-), Egypt (0.069) (-), Germany (3.006) (0.161), India (0.270) (-), Italy (0.072) (-), Malaysia (2.228) (-), USA (2.270) (8.511), and South Korea(-) (0.116) have reported adult As and Hg THQ values from eating hen eggs, respectively.
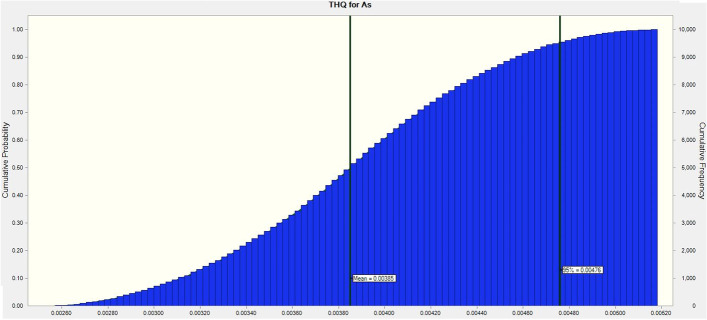
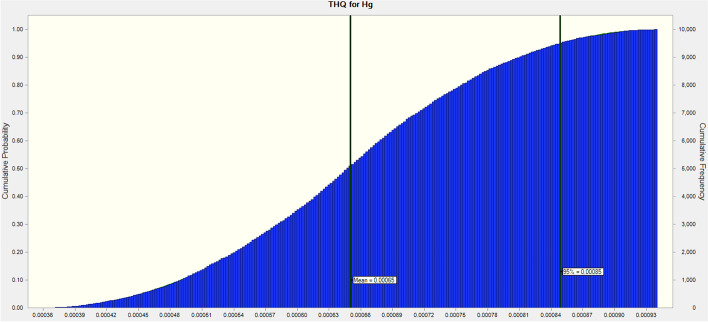
Because the THQ values of As and Hg for adult consumers in this research indicate values below one, the outcomes of this investigation demonstrate that eating eggs did not pose any risks to the health of Iranian consumers. In addition, the MCS revealed that the estimated THQ values for As and Hg for adults at the 95% percentile were 0.00385 and 0.00066, respectively, indicating that Iranian consumers are not possibly at risk for health issues as a result of consuming eggs (Figs. 3 and 4). It's essential to keep in mind that there are other ways to be exposed to these dangerous metals, including through water, skin contact, inhaling dust, and eating other foods. In this regard, a study conducted recently in Iran revealed that various heavy metal concentrations in tea samples were higher than the levels allowed by Iran National Standard and WHO [17]. A study conducted in Iran in 2022, proved that using depilatory products on the skin would not increase the risk of cancer or other serious illnesses, but continued usage might be harmful because of the excessive accumulation of these heavy metals [16]. As the most widely used tobacco in the world, cigarettes are also well-known. The assessment of the levels of heavy metals in smoked and non-smoked cigarettes in Iran revealed that heavy metals in cigarette butts can have both potentially harmful and beneficial consequences for the health of smokers who are subjected to inhalation [18].
Fig. 3.
THQ distribution for As through Monte Carlo simulation
Fig. 4.
THQ distribution for Hg through Monte Carlo simulation
This would indicate a severe risk to the health of the exposed population.
Carcinogenic risk
Although a variety of characteristics, such as age, race, and gender, may contribute to the development of cancer, several studies have shown that exposure to environmental pollutants, such as toxic elements, increases the risk of cancer [62]. The ILCR value was applied to the current investigation's calculations to determine the carcinogenic risk for adult egg consumers. ILCR was only calculated for arsenic since the cancer slope factor (CSF) for Mercury's risk of oral cancer hasn't yet been determined.
There might be considerable ambiguity when estimating health risks [40]. When single-point data are utilized to evaluate health risks related to exposure to pollutants such as toxic metals, a high level of uncertainty is seen. As a consequence, MCS was used in our study as a probabilistic strategy to lower the uncertainties in the assessment of health risks.
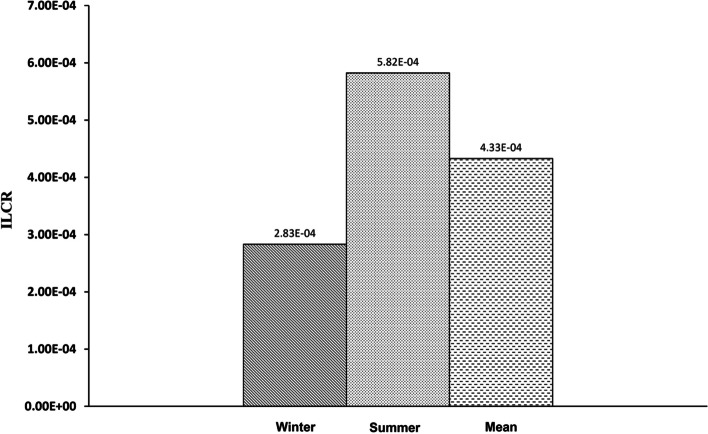
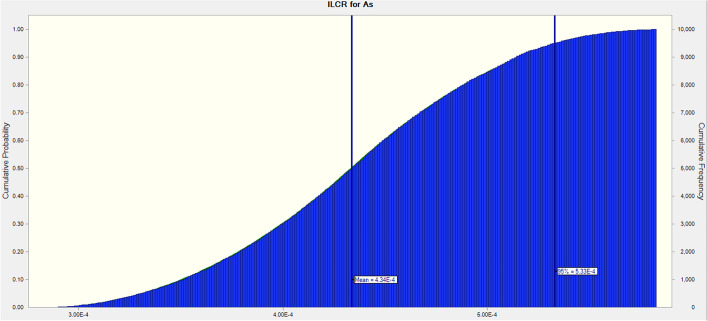
It was calculated that the mean ILCR for As in adults was 4.33E-04 (winter: 2.83E-04, summer: 5.82E-04), which indicated the threshold risk limit (ILCR > 10–4) for a chance of cancer is above 1 in 10,000 exposure (Figs. 5 and 6).
Fig. 5.
Incremental lifetime cancer risk (ILCR) of As in adults through hen eggs consumption in two seasons (winter, summer, and mean)
Fig. 6.
ILCR distribution for As through Monte Carlo simulation
These contaminants enter hen eggs through contaminated food, unclean water sources, and additional factors like age, species, and lying cycles. Other elements influencing the quality of hen eggs include supplemental nourishment and insecticides used to control pests [63]. According to most studies, contaminated feed consumed orally by hens can more frequently lead to the contamination of eggs [64].
Interestingly, our data described the amount of As and Hg in hen eggs as lower than in the majority of studies in other countries. The intake of staple foods such as rice raises major health problems due to the level of As and Hg in Iranian food. Further risk-based monitoring studies should be recommended to reduce exposure to As and Hg from other food sources. Human exposure to arsenic is a complex issue because it is closely related to the exposed population's environmental pollution, occupation, lifestyle, and dietary patterns. While drinking water has traditionally been considered a principal contributor to consumer exposure to arsenic, recent studies have shown that food represents an even more significant source of exposure to arsenic [65, 66]. Recently a study by Mohammad Pour, et al. (2023) showed by Monte Carlo simulation that water intake rate and mercury concentration were the most critical parameters in the hazard index for children and adults in Shiraz, Iran [67]. Environmental toxins like heavy metals can impact the safety and quality of hen eggs. Pesticides like phosphate fertilizers and contaminated air are the main sources of heavy metals in soil and crops. Heavy metals are absorbed from plant roots, moved to leaves, and stored in tissues depending on the kind and variety of plants, the type of sound water supply, the duration of irrigation, metal ionization, and transfer parameters [68]. Such as a study in Shiraz (Iran) (2023),heavy metals residue in fruit were As:7.5, Hg:4.38 µg.kg−1 [69].
Most research has shown that the concentration of As in groundwater in various geographic regions was beyond the limits set by WHO and the National Standards of Iran, another issue with heavy metals in groundwater resources [70]. The findings of an investigation conducted in Tehran, Iran, in 2015 indicated that the amount of heavy metals in the hen eggs collected was less than the permitted levels and therefore regarded as safe. The continual monitoring of these contaminants in the food chain was, however, strongly recommended by policymakers due to the significance of food contamination for public health [48, 49, 71].
Chemical contamination rates are greatly influenced by the distances that should be kept between drinking water supplies and industrial, mining, and agricultural activities. Farmers can reduce heavy metal pollution in crops, which results in the food that is hen eggs, by using fertilizer and implementing surveillance systems for agricultural areas. The national regulations for these contamination issues in hen eggs' food products have not established legally binding limitations.
Additionally, Particular attention has been made to exposure to children under the age of five because there are no reliable standard limits for As and Hg in hen eggs. In response to the requests of the food safety authorities, it is recommended that the international adopt this restriction. The presence of heavy metals in chicken feed, water, and meat should be assessed individually in future investigations.
Limitation
Our samples might not represent all the hen egg samples in Iran. In addition, this study only looked at the heavy metals in hen eggs and not necessarily other food consumption. Thorough consideration of arsenic speciation among foods is necessary for reliable evaluations of exposure to inorganic arsenic in the food supply. It is possible to overestimate exposures that suggest a considerably higher risk than is present if arsenic exposure is expressed in terms of total arsenic and results are compared to the RFD for inorganic arsenic.
Conclusion
Overall, findings demonstrated that, compared to past Iranian assessments, As and Hg levels have decreased through these years. Hen egg consumption by Iranian consumers did not pose a non-carcinogenic risk, based on THQ values of these hazardous metals below one. Moreover, the incremental lifetime cancer risk (ILCR) of As was estimated to be 4.33E-04, indicating that consumers in Iran are at the threshold carcinogenic risk of As through consuming hen eggs (ILCR > 10–4). However, exposure to As and Hg through food consumption, water consumption, skin contact, and inhalation can be harmful to human health. It is advised that As and Hg levels be frequently checked in hen eggs and other foods in Iran. In general, consumer consumption rates are connected with exposure to heavy metals through food chains, which may have a cancer-causing effect on people over time. Particular attention has been made to exposure to children under the age of five because there are no reliable standard limits for As and Hg in hen eggs. In response to the requests of the food safety authorities, it is recommended that the international adopt this restriction.
Therefore, policymakers need to be aware that it is prohibited to establish chicken farms in heavily polluted urban areas. It is essential to regularly conduct examinations to measure the presence of heavy metals in both groundwater used for agriculture and the feed provided to chickens. Additionally, it is advisable to focus on raising public awareness about the importance of maintaining a healthy diet.
Acknowledgements
The authors thank the Research Council of Nutrition and Food Industry Research Institute, School of Nutrition and Food Industry, Shahid Beheshti University of Medical Sciences, as well as the staff of Saba Analytical Laboratory (HMS) for performing the measurement tests.
Authors’ contributions
The study's design, supervision, data collection, experimental measuring, statistical analysis, and paper drafting were all carried out by AA, HH, and FE. NR helped with the study design, data collection, and manuscript drafting, while FMN helped with the conceptualization, methodology, and data interpretation. The authors have strictly followed all ethical standards, which include plagiarism, informed consent, misconduct, data fabrication and falsification, duplicate publishing, submission, redundancy, etc. The paper has been read and approved by all authors.
Funding
This study was supported fully by the National Nutrition and Food Technology Research Institute (NNFTRI), Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No: 30109).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The National Nutrition and Food Technology Research Institute, Faculty of Nutrition Science and Food Technology, Shahid Beheshti University of Medical Sciences approved the project. (ethical code No. SBMU.nnftri.Rec.1400.074). Human samples were not used by any of the authors in this article. Additionally, none of the authors' investigations that included animals are included.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giri S, Singh AK. Heavy metals in eggs and chicken and the associated human health risk assessment in the mining areas of Singhbhum copper belt, India. Arch Environ Occup Health. 2019;74(4):161–170. doi: 10.1080/19338244.2017.1407284. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen N, Ahmed M, Islam M, Habibullah-Al-Mamun M, Tukun AB, Islam S, MA Rahim AT. Health risk assessment of trace elements via dietary intake of ‘non-piscine protein source’foodstuffs (meat, milk and egg) in Bangladesh. Environ Sci Pollut Res. 2016;23(8):7794–806. [DOI] [PubMed]
- 3.Whiting IM, Pirgozliev V, Kljak K, Orczewska-Dudek S, Mansbridge SC, Rose SP, Atanasov AG. Feeding dihydroquercetin in wheat-based diets to laying hens: impact on egg production and quality of fresh and stored eggs. Br Poult Sci. 2022;63(6):735–741. doi: 10.1080/00071668.2022.2090229. [DOI] [PubMed] [Google Scholar]
- 4.Farahani S, Eshghi N, Abbasi A, Karimi F, Shiri Malekabad E, Rezaei M. Determination of heavy metals in albumen of hen eggs from the Markazi Province (Iran) using ICP-OES technique. Toxin reviews. 2015;34(2):96–100. doi: 10.3109/15569543.2015.1040166. [DOI] [Google Scholar]
- 5.Hu Y, Cheng H, Tao S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ Int. 2017;107:111–130. doi: 10.1016/j.envint.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Aendo P, Netvichian R, Viriyarampa S, Songserm T, Tulayakul P. Comparison of zinc, lead, cadmium, cobalt, manganese, iron, chromium and copper in duck eggs from three duck farm systems in Central and Western, Thailand. Ecotoxicol Environ Safety. 2018;161:691–698. doi: 10.1016/j.ecoenv.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 7.Hashemi M, Sadeghi A, Saghi M, Aminzare M, Raeisi M, Rezayi M, Sany SBT. Health risk assessment for human exposure to trace metals and arsenic via consumption of hen egg collected from largest poultry industry in Iran. Biol Trace Elem Res. 2019;188(2):485–493. doi: 10.1007/s12011-018-1437-4. [DOI] [PubMed] [Google Scholar]
- 8.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Mol Clin Environment Toxicol Exp Suppl. 2012;101:133–64. [DOI] [PMC free article] [PubMed]
- 9.Tassew Belete AH, Rao VM. Determination of concentrations of selected heavy metals in cow’s milk: Borena Zone, Ethiopia. J Health Sci. 2014;4(5):105. [Google Scholar]
- 10.Annar S. The characteristics, toxicity and effects of heavy metals arsenic, mercury and cadmium: a review. 2022.
- 11.Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J. 2003;79(933):391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol. 2003;18(3):149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 13.Alam MM, Haque MM. Presence of antibacterial substances, nitrofuran metabolites and other chemicals in farmed pangasius and tilapia in Bangladesh: probabilistic health risk assessment. Toxicol Rep. 2021;8:248–257. doi: 10.1016/j.toxrep.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ysart G, Miller P, Croasdale M, Crews H, Robb P, Baxter M, De L'Argy C, Harrison N. 1997 UK Total Diet Study dietary exposures to aluminium, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin and zinc. Food Addit Contam. 2000;17(9):775–786. doi: 10.1080/026520300415327. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadzadeh M, Mirzaei N, Mostafaii G, Atoof F, Miranzadeh MB, Dehghani R. Determination of potentially toxic metals in depilatory products in the Iranian markets: human health risk assessment. Environ Sci Pollut Res. 2022;29:13756–65. [DOI] [PubMed]
- 17.SeyyediBidgoli N, Mostafaii GR, Akbari H, Mohammadzadeh M, Hesami Arani M, Miranzadeh MB. Determination of the concentration of heavy metals in infused teas and their assessment of potential health risk in Kashan Iran. Int J Environment Analytical Chem. 2022;102(19):7673–7683. doi: 10.1080/03067319.2020.1836174. [DOI] [Google Scholar]
- 18.Ghaderi A, Mohammadzadeh M, Banafshe HR, Mousavi SGA, Mirzaei N, Parmoozeh Z, Mostafaei G, Rasouli-Azad M, Ghalerashidi HM, Fouladi-Fard R. The carcinogenic and non-carcinogenic risk assessment of heavy metals from the butts of smoked and non-smoked cigarettes. Hum Ecol Risk Assess Int J. 2023;29(1):187–201. doi: 10.1080/10807039.2022.2150598. [DOI] [Google Scholar]
- 19.Fakhri Y, Mohseni-Bandpei A, Conti GO, Ferrante M, Cristaldi A, Jeihooni AK, Dehkordi MK, Alinejad A, Rasoulzadeh H, Mohseni SM. Systematic review and health risk assessment of arsenic and lead in the fished shrimps from the Persian gulf. Food Chem Toxicol. 2018;113:278–286. doi: 10.1016/j.fct.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Aliu H, Dizman S, Sinani A, Hodolli G. Comparative study of heavy metal concentration in eggs originating from industrial poultry farms and free-range Hens in Kosovo. J Food Qual. 2021, 2021.
- 21.Abdulkhaliq A, Swaileh K, Hussein RM, Matani M: Levels of metals (Cd, Pb, Cu and Fe) in cow’s milk, dairy products and hen’s eggs from the West Bank, Palestine. 2012.
- 22.Goharipour H, Firoozabadi SS. Assessing the impacts of changes in the currency exchange rate on air pollution in tehran: a sectoral review. Eur J Bus Manag Res. 2022;7(3):12–19. doi: 10.24018/ejbmr.2022.7.3.1411. [DOI] [Google Scholar]
- 23.Shariatmadari F. Poultry production and the industry in Iran. Worlds Poult Sci J. 2000;56(1):55–65. doi: 10.1079/WPS20000006. [DOI] [Google Scholar]
- 24.Tavakoly Sany SB, Hashim R, Rezayi M, Rahman MA, Razavizadeh BBM, Abouzari-lotf E, Karlen DJ. Integrated ecological risk assessment of dioxin compounds. Environ Sci Pollut Res. 2015;22(15):11193–11208. doi: 10.1007/s11356-015-4511-x. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, Wang L, Ma L, Yang Z. Speciation analysis of six arsenic species in marketed shellfish: extraction optimization and health risk assessment. Food Chem. 2018;244:311–316. doi: 10.1016/j.foodchem.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 26.Meermann B, Nischwitz V. ICP-MS for the analysis at the nanoscale–a tutorial review. J Anal At Spectrom. 2018;33(9):1432–1468. doi: 10.1039/C8JA00037A. [DOI] [Google Scholar]
- 27.Piercey SJ. Modern analytical facilities 2: a review of Quality Assurance and Quality Control (QA/QC) procedures for lithogeochemical data. Geosci Can. 2014;41(1):75–88. doi: 10.12789/geocanj.2014.41.035. [DOI] [Google Scholar]
- 28.Welna M, Szymczycha-Madeja A, Pohl P. Quality of the trace element analysis: sample preparation steps. Wide Spectry Qual Control. 2011;1:53–70.
- 29.Moya J, Phillips L, Schuda L, Clickner R, Birch R, Adjei N, Blood P, et al. Exposure Factors Handbook, 2011 Edition. US Environmental Protection Agency, vol 52. Washington: EPA/600/R-090; 2011. p. 133–164.
- 30.NNFTRI. Food consumption pattern and nutritional status in Iran (2020–2022). Tehran (Iran): National Nutrition and Food Technology Research Institute/Ministry of Health and Medical Education of Iran. In.; 2022.
- 31.US EPA (United States Environmental Protection Agency). Exposure Factors Handbook: 2011 Edition. Office of Research and Development, United States Environmental Protection Agency. Washington, DC: EPA/600/R-09/052F; 2011a. Available at http://www.epa.gov/ncea/efh/report.html.
- 32.Phillips LJ, Moya J. Exposure factors resources: contrasting EPA’s Exposure Factors Handbook with international sources. J Eposure Sci Environ Epidemiol. 2014;24(3):233. doi: 10.1038/jes.2013.17. [DOI] [PubMed] [Google Scholar]
- 33.FAO/WHO: Food and Agriculture Organization/World Health Organization, Summary of evaluations performed by the joint FAO/WHO expert committee on food additives (JECFA 1956-2003), (First Through Sixty First Meetings). Food and Agriculture Organization of the United Nations and the World Health Organization, ILSI Press International Life Sciences Institute. 2004.
- 34.EPA. Risk assessment guidance for Superfund, vol. I Human health evaluation manual (Part A) EPA/540/1–89/002. 2004.
- 35.Rahmani J, Fakhri Y, Shahsavani A, Bahmani Z, Urbina MA, Chirumbolo S, Keramati H, Moradi B, Bay A, Bjørklund G. A systematic review and meta-analysis of metal concentrations in canned tuna fish in Iran and human health risk assessment. Food Chem Toxicol. 2018;118:753–765. doi: 10.1016/j.fct.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Jara EA, Winter CK. Dietary exposure to total and inorganic arsenic in the United States, 2006–2008. Int J Food Contaminat. 2014;1(1):1–12. doi: 10.1186/s40550-014-0003-x. [DOI] [Google Scholar]
- 37.EPA. Use of Monte Carlo Simulation in Risk Assessments - EPA. 511, https://www.epa.gov/risk/use-monte-carlo-simulation-risk-assessments. 2016.
- 38.Epa U. Guidelines for carcinogen risk assessment. Washington, DC 2005.
- 39.Esposito M, Cavallo S, Chiaravalle E, Miedico O, Pellicanò R, Rosato G, Sarnelli P, Baldi L. Trace elements in free-range hen eggs in the Campania region (Italy) analyzed by inductively coupled plasma mass spectrometry (ICP-MS) Environ Monit Assess. 2016;188(6):1–9. doi: 10.1007/s10661-016-5316-1. [DOI] [PubMed] [Google Scholar]
- 40.Chen M-J, Hsu H-T, Lin C-L, Ju W-Y. A statistical regression model for the estimation of acrylamide concentrations in French fries for excess lifetime cancer risk assessment. Food Chem Toxicol. 2012;50(10):3867–3876. doi: 10.1016/j.fct.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Keramati H, Ghorbani R, Fakhri Y, Khaneghah AM, Conti GO, Ferrante M, Ghaderpoori M, Taghavi M, Baninameh Z, Bay A. Radon 222 in drinking water resources of Iran: a systematic review, meta-analysis and probabilistic risk assessment (Monte Carlo simulation) Food Chem Toxicol. 2018;115:460–469. doi: 10.1016/j.fct.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 42.Ru Q-M, Feng Q, He J-Z. Risk assessment of heavy metals in honey consumed in Zhejiang province, southeastern China. Food Chem Toxicol. 2013;53:256–262. doi: 10.1016/j.fct.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Qu C-S, Ma Z-W, Yang J, Liu Y, Bi J, Huang L. Human exposure pathways of heavy metals in a lead-zinc mining area, Jiangsu Province, China. PLoS One. 2012;7(11):e46793. doi: 10.1371/journal.pone.0046793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Freitas R, Ramos Nacano L, Lemos Batista B, Barbosa F., Jr Toxic and essential elements in conventional and home-produced eggs by ICP-MS analysis. Food Additives and Contaminants: Part B. 2013;6(1):30–35. doi: 10.1080/19393210.2012.721095. [DOI] [PubMed] [Google Scholar]
- 45.Leblanc J-C, Guérin T, Noël L, Calamassi-Tran G, Volatier J-L, Verger P. Dietary exposure estimates of 18 elements from the 1st French total diet study. Food Addit Contam. 2005;22(7):624–641. doi: 10.1080/02652030500135367. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed MK, Shaheen N, Islam MS, Habibullah-Al-Mamun M, Islam S, Islam MM, Kundu GK, Bhattacharjee L. A comprehensive assessment of arsenic in commonly consumed foodstuffs to evaluate the potential health risk in Bangladesh. Sci Total Environ. 2016;544:125–133. doi: 10.1016/j.scitotenv.2015.11.133. [DOI] [PubMed] [Google Scholar]
- 47.Van Overmeire I, Pussemier L, Hanot V, De Temmerman L, Hoenig M, Goeyens L. Chemical contamination of free-range eggs from Belgium. Food Addit Contam. 2006;23(11):1109–1122. doi: 10.1080/02652030600699320. [DOI] [PubMed] [Google Scholar]
- 48.Abbasi Kia S, Jahed Khaniki G, Shariatifar N, Nazmara S. Akbar Zadeh A. contamination of chicken eggs supplied in Tehran by heavy metals and calculation of their daily intake. J Health Field. 2017;2(4):44–51.
- 49.Salar-Amoli J, Ali-Esfahani T. Determination of hazardous substances in food basket eggs in Tehran, Iran: A preliminary study. In: Veterinary Research Forum: 2015: Faculty of Veterinary Medicine, Urmia University, Urmia, Iran; 2015: 155. [PMC free article] [PubMed]
- 50.Zheng N, Wang Q, Zhang X, Zheng D, Zhang Z, Zhang S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city. China Sci Total Environment. 2007;387(1–3):96–104. doi: 10.1016/j.scitotenv.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 51.Larsen EH, Andersen NL, Møller A, Petersen A, Mortensen GK, Petersen J. Monitoring the content and intake of trace elements from food in Denmark. Food Addit Contam. 2002;19(1):33–46. doi: 10.1080/02652030110087447. [DOI] [PubMed] [Google Scholar]
- 52.Suleman S, Qureshi JA, Rasheed M, Farooq W, Yasmin F. Poultry feed contamination and its potential hazards on human health. Biomed Lett. 2022;8(1):70–81. doi: 10.47262/BL/8.1.20210901. [DOI] [Google Scholar]
- 53.Trampel DW, Imerman PM, Carson TL, Kinker JA, Ensley SM. Lead contamination of chicken eggs and tissues from a small farm flock. J Vet Diagn Invest. 2003;15(5):418–422. doi: 10.1177/104063870301500503. [DOI] [PubMed] [Google Scholar]
- 54.Gupta PK. Epidemiology of animal poisonings in Asia. Veterinary Toxicology. Academic Press. 2018. p. 57–69.
- 55.Gump BB, Hruska B, Parsons PJ, Palmer CD, MacKenzie JA, Bendinskas K, Brann L. Dietary contributions to increased background lead, mercury, and cadmium in 9–11 Year old children: accounting for racial differences. Environ Res. 2020;185:109308. doi: 10.1016/j.envres.2020.109308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López-Alonso M. Animal feed contamination by toxic metals. In: Animal feed contamination. Wood head publishing. 2012. p. 183–204.
- 57.European Union. Commission regulation (EC) no. 1881/ 2006 setting maximum levels for certain contaminants in foodstuffs. J Eur Union. 2006; 364, 5–24.
- 58.JECFA. Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants. 73 Report, 2010, Geneva, Switzerland; WHO technical report series, n°. 960, (237 pp.). 2011. Available from http://apps.who.int/iris/bitstream/handle/10665/44515/WHO_TRS_960_eng.pdf. Accessed 4 July 2015.
- 59.Meshref AM, Moselhy WA, Hassan NE-HY. Heavy metals and trace elements levels in milk and milk products. J Food Measure Character. 2014;8(4):381–8.
- 60.Dadar M, Adel M, Nasrollahzadeh Saravi H, Fakhri Y. Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev. 2017;36(3):222–227. [Google Scholar]
- 61.Atamaleki A, Sadani M, Raoofi A, Miri A, Bajestani SG, Fakhri Y, Heidarinejad Z, Khaneghah AM. The concentration of potentially toxic elements (PTEs) in eggs: a global systematic review, meta-analysis and probabilistic health risk assessment. Trends Food Sci Technol. 2020;95:1–9. doi: 10.1016/j.tifs.2019.11.003. [DOI] [Google Scholar]
- 62.Antwi SO, Eckert EC, Sabaque CV, Leof ER, Hawthorne KM, Bamlet WR, Chaffee KG, Oberg AL, Petersen GM. Exposure to environmental chemicals and heavy metals, and risk of pancreatic cancer. Cancer Causes Control. 2015;26(11):1583–1591. doi: 10.1007/s10552-015-0652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waegeneers N, Hoenig M, Goeyens L, De Temmerman L. Trace elements in home-produced eggs in Belgium: levels and spatiotemporal distribution. Sci Total Environ. 2009;407(15):4397–4402. doi: 10.1016/j.scitotenv.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 64.McEvoy J. Contamination of animal feedingstuffs as a cause of residues in food: a review of regulatory aspects, incidence and control. Anal Chim Acta. 2002;473(1–2):3–26. doi: 10.1016/S0003-2670(02)00751-1. [DOI] [Google Scholar]
- 65.Georgopoulos PG, Wang S-W, Yang Y-C, Xue J, Zartarian VG, Mccurdy T, Özkaynak H. Biologically based modeling of multimedia, multipathway, multiroute population exposures to arsenic. J Eposure Sci Environ Epidemiol. 2008;18(5):462–476. doi: 10.1038/sj.jes.7500637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xue J, Zartarian V, Wang S-W, Liu SV, Georgopoulos P. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003–2004 NHANES data. Environ Health Perspect. 2010;118(3):345–350. doi: 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohammadpour A. Emadi Z, Samaei MR, Ravindra K, Hosseini SM, Amin M, Samiei M, Mohammadi L, Khaksefidi R, Zarei AA. The concentration of potentially toxic elements (PTEs) in drinking water from Shiraz, Iran: a health risk assessment of samples. Environ Sci Pollut Res. 2023;30(9):23295–311. [DOI] [PMC free article] [PubMed]
- 68.Armah FA, Quansah R, Luginaah I. A systematic review of heavy metals of anthropogenic origin in environmental media and biota in the context of gold mining in Ghana. International scholarly research notices 2014, 2014. [DOI] [PMC free article] [PubMed]
- 69.Mohammadpour A, Emadi Z, Keshtkar M, Mohammadi L, Motamed-Jahromi M, Samaei MR, Allah Zarei A, Berizi E, Khaneghah AM. Assessment of potentially toxic elements (PTEs) in fruits from Iranian market (Shiraz): A health risk assessment study. J Food Compos Anal. 2022;114: 104826.
- 70.Dehghani M, Abbasnejad A. Cadmium, Arsenic, Lead and nitrate pollution in the groundwater of Anar Plain. 2011.
- 71.Sobhanardakani S. Human health risk assessment of Cd, Cu, Pb and Zn through consumption of raw and pasteurized cow's milk. PLoS Negl Trop Dis. 2018;47(8):1172–1180. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.