Abstract
Recent work demonstrating that asymptomatic carriers of P. falciparum parasites make up a large part of the infectious reservoir highlights the need for an effective malaria vaccine. Given the historical challenges of vaccine development, multiple parasite stages have been targeted, including the sexual stages required for transmission. Using flow cytometry to efficiently screen for P. falciparum gamete/zygote surface reactivity, we identified 82 antibodies that bound live P. falciparum gametes/zygotes. Ten antibodies had significant transmission-reducing activity (TRA) in a standard membrane feeding assay and were subcloned along with 9 nonTRA antibodies as comparators. After subcloning, only eight of the monoclonals obtained have significant TRA. These eight TRA mAbs do not recognize epitopes present in any of the current recombinant transmission-blocking vaccine candidates, Pfs230D1M, Pfs48/45.6C, Pf47 D2 and rPfs25. One TRA mAb immunoprecipitates two surface antigens, Pfs47 and Pfs230, that are expressed by both gametocytes and gametes/zygotes. These two proteins have not previously been reported to associate and the recognition of both by a single TRA mAb suggests the Pfs47/Pfs230 complex is a new vaccine target. In total, Pfs230 was the dominant target antigen, with five of the eight TRA mAbs and 8 of 11 nonTRA gamete/zygote surface reactive mAbs interacting with Pfs230. Of the three remaining TRA mAbs, two recognized non-reduced, parasite-produced Pfs25 and one bound non-reduced, parasite-produced Pfs48/45. None of the TRA mAbs bound protein on an immunoblot of reduced gamete/zygote extract and two TRA mAbs were immunoblot negative, indicating none of the new TRA epitopes are linear. The identification of eight new TRA mAbs that bind epitopes not included in any of the constructs currently under advancement as transmission-blocking vaccine candidates may provide new targets worthy of further study.
Keywords: Malaria transmission, Vaccines, Monoclonal antibody screen, Plasmodium falciparum gametes, Mosquito membrane feed
Gene IDs: alpha tubulin 2 (PF3D7_0708400), endoplasmin (PF3D7_1222300), HAP2 (PF3D7_1014200), HSP90 (PF3D7_0422300), MDV1 (PF3D7_1216500), Pf77 (PF3D7_0621400), Pfg27 (PF3D7_1302100), Pfs25 (PF3D7_1031000), Pfs28 (PF3D7_1030900), Pfs47 (PF3D7_1346800), Pfs48/45 (PF3D7_1346700), Pfs230 (PF3D7_0209000)
Despite a significant reduction in malaria mortality after the introduction of insecticide-treated bed nets and artemisinin combination therapy, malaria continues to be a major health burden. The annual death rate has plateaued at roughly half a million since 2015, after its height of approximately 900,000 deaths per year before 2004. The infectious reservoir has also been found to include asymptomatic individuals who are unaware of their infection status, so they do not seek treatment and consequently provide a persistent source of Plasmodium parasites for transmission1–5. For transmission to occur, a subpopulation of intraerythrocytic parasites undergoes sexual differentiation producing mature female or male gametocytes. Once these intraerythrocytic gametocytes are taken up in a blood meal, they are stimulated to emerge from the red blood cell (RBC) as gametes that mate generating zygotes and developing into sporozoites. The sporozoites migrate to the mosquito salivary gland and during the next blood meal they are released into humans initiating an infection. The RTS,S/AS01 (RTS,S) malaria vaccine targets the Plasmodium falciparum sporozoite surface antigen, circumsporozoite protein (CSP), and was recommended by the World Health Organization for expanded use in children living in areas of moderate to high transmission areas. The vaccine reduces malaria episodes in 5–17 month-old children by approximately 40% in the first 32 months but does not confer sterilizing immunity or block transmission6,7. Combining RTS,S with a malaria transmission-blocking vaccine has the potential to enhance its impact by limiting the spread of parasites through the community and decreasing selection for vaccine-resistant strains. A transmission-blocking vaccine could also reduce the spread of drug-resistant parasites, which have been associated with higher gametocyte levels8,9.
In the 1980s, three targets of transmission-blocking monoclonal antibodies (mAbs) -- Pfs230, Pfs48/45, and Pfs25 – were identified and named based on their migration in sodium dodecyl sulfate (SDS) polyacrylamide gels10,11,12. These mAbs target the parasite in the mosquito midgut where, after emergence from RBCs as gametes, the parasite is exposed to the contents of the blood meal, including antibodies and complement13,14. Pfs230 and Pfs48/45 were found to co-immunoprecipitate15, but the structure of the complex has yet to be defined. After their sequences were identified, Pfs48/45 and Pfs25, but not Pfs230, were predicted to be attached to the plasma membrane by a glycosylphosphatidylinositol (GPI) anchor16,17, leading to the suggestion that Pfs230 was retained on the surface by its association with Pfs48/4518. Paralogs were subsequently identified for all three proteins -- Pfs29019,20, Pfs4721, and Pfs2822--and two of these, Pfs4723 and Pfs2822, were also found to induce transmission-blocking immunity. A gamete fusogen, HAP2, that was shown to be required for fertilization in the rodent malaria parasite, P. berghei 24,25, two antigens upregulated in gametocytes, Pf77and MDV126, and a mosquito midgut antigen, AnAPN127, complete the current set of transmission-blocking antigens. Except for AnAPN1, all the antigens have the potential to be exposed on the gamete/zygote surface after RBC emergence in the mosquito midgut, although this has not been formally demonstrated for Pf77 and MDV126.
Generating properly-folded recombinant proteins that induce transmission-blocking immunity in mice has been difficult14. However, clinical-grade recombinant forms of sections of Pfs2528,29, Pfs23030,31 and Pfs48/4532,33 have been successfully produced and advanced to clinical trials34, and efforts to develop clinical-grade Pfs4735 are ongoing. To date, the immunogenicity of these antigens in humans has been relatively low. Recently, Pfs230D1M conjugated to a non-toxic recombinant form of Pseudomonas aeruginosa exotoxin A (identified as EPA) was found to induce higher titers of antibodies with superior transmission-reducing activity (TRA) than Pfs25-EPA.36 The Pfs230D1M-EPA conjugate has been tested in malaria-endemic areas with different adjuvants including Alhydrogel (ClinicalTrials.gov Identifier: NCT02334462) in a phase 1 clinical trial, AS01 adjuvant in phase 2 clinical trials (ClinicalTrials.gov Identifier: NCT03917654),37 and currently with Matrix-M adjuvant in a phase 1 trial (ClinicalTrials.gov Identifier: NCT05135273).
Except for the secretory and GPI anchor signal sequences, Pfs25 vaccine candidates encode the entire protein (aa 22–193) and most of the exposed surface is included in the 3 TRA mAb binding sites (I-III) identified to date38–40. In contrast, Pfs230D1M encodes only aa 542–736, located at the N-terminus of the ~300 kDa processed form of Pfs23030 that is exposed on the gamete/zygote surface41,42. Consequently, D1M only represents 7.4% of the processed surface-exposed protein. The Pfs230D1M vaccine candidate produced in Pichia pastoris also includes glutamine in place of asparagine 585 (N585Q) to prevent potential glycosylation. Pfs230-specific TRA mAbs, have been analyzed structurally and recognize discontinuous epitopes on one side of Pfs230D1M43–45.
A large field study demonstrated 3.4% of the IgG samples purified from 647 malaria-exposed individuals’ serum had ≥90% TRA46. This naturally-acquired TRA was associated with higher IgG levels against recombinant Pfs48/45 10C (aa 159–428) and Pfs230 CMB (aa 444–730), a region similar to Pfs230D1M, as well as 43 additional recombinant antigens included on the protein microarray. Ten of the high TRA samples did not react with Pfs48/45.10C or Pfs230 CMB, but still reacted with the surface of gametes/zygotes from transgenic parasites that do not express either Pfs48/45 or Pfs230. These data suggest that there are additional antigenic targets on the gamete/zygote surface. The objective of our study was to screen for additional TRA antigens, utilizing flow cytometry to identify gamete/zygote surface-reactive mAbs. More than 588 IgG+ supernatants from hybridomas produced from splenocytes isolated from mice immunized with gamete/zygote membranes or with material immunoprecipitated using a combination of Pfs25-specific (4B747) and Pfs230-specific (1B312) TRA mAbs were screened. The gamete-positive hybridoma supernatants were assayed for TRA using a standard membrane mosquito feed assay (SMFA) and the hybridomas with TRA, as well as selected nonTRA hybridomas, were cloned, reassessed for TRA, and the target antigens identified.
Materials and methods
P. falciparum culture and parasite isolation
In vitro cultures of the NF54 strain of P. falciparum were grown in complete RPMI 1640 media (cRPMI) containing 25 mM HEPES and 100 μg/mL hypoxanthine (KD Medical, Columbia, MD), supplemented with 25 mM NaHCO3 (pH 7.3, GIBCO), 5 μg/mL gentamicin (SIGMA, St Louis, MO) and 10% human serum (Interstate Blood Bank, Memphis, TN) and incubated at 37°C in blood gas (90% N2, 5% O2, 5% CO2). Stock cultures were split every 2–3 days to maintain parasitemia below 3% and used to set up gametocyte cultures at 0.2% parasitemia. Gametocyte cultures were fed daily for the next two weeks without splitting or adding new RBCs, and growth was monitored by Giemsa-stained culture smear. Once stage II gametocytes were observed, N-acetyl glucosamine (NAG, 50 mM) was added for ≥ three days to block further asexual growth and the gametocytes were allowed to mature to stage V. Stage V gametocytes were isolated by centrifugation (1,800 xg for 10 m without breaks) on 18% Nycodenz or, to generate gametes, the stage V gametocytes were pelleted by centrifugation (1,200 xg for 3 min) and gametogenesis was stimulated by resuspension in an equal volume of room temperature serum and xanthurenic acid (10µM). Thirty minutes later, the cells were diluted four times with room temperature SA-8.3 buffer [glucose (1.67 mg/mL), NaCl (8 mg/ml), tris(hydroxymethyl) aminomethane (Tris-Cl) (8 mM, pH 8.3)]. Gametes/zygotes were isolated one or four hours post-stimulation by layering the cells onto a 6%/11%/16% Nycodenz gradient and centrifuging at room temperature at 1,800 xg for 10 m without breaks. The gametes/zygotes were isolated from the 6% - 11% interface, diluted two- to four-fold in SA-8.3 buffer, centrifuged and the pellet resuspended in 1.5 ml SA-8.3 buffer and transferred to a microcentrifuge tube. A small aliquot was removed for quantification by hemocytometer and the remaining cells were centrifuged (600 xg for 30 seconds). The supernatant was removed and the pellet was stored at −80. Preparations that were > 80% gametes/zygotes were used for the mouse immunization studies.
Immunogen Preparation
Three different preparations, 1) whole gametes, 2) gamete/zygote membranes, or 3) mAbs 1B3 and 4B7-precipitated antigen, were used to immunize CAF1 mice (Jackson Laboratories, Bar Harbor, ME). In the whole gamete/zygote preparation, the entire Nycodenz-purified gamete/zygote pellet obtained from the 6 – 11% Nycodenz interface was stored frozen for shipment to Aragen Life Sciences (Morgan Hill, CA) to be resuspended in sterile phosphate-buffered saline (PBS) and emulsified in monophosphoryl Lipid A and trehalose dicorynomycolate in 2% oil (squalene)-Tween® 80-water (MPL-TDM) (Sigma, St Louis, MO) immediately before injection into mice. Gamete/zygote membranes were prepared by resuspending the gamete/zygote pellet in 150 µl sterile TE Buffer (10 mM Tris-HCl pH 8.0 and 0.1 mM ethylenediaminetetraacetic acid (EDTA)) with protease inhibitors (PI) (2 mM phenylmethylsulfonyl fluoride, 1 µg/ml pepstatin, 2 µg/ml aprotinin, and 2 µg/ml leupeptin), then freezing and thawing them at −80°C / 4°C three times before centrifugation (1,000 xg for 5 m at 4°C) to pellet any remaining intact cells. The supernatant was harvested and centrifuged at 18,000 xg for 30 m at 4°C to isolate the membrane fraction, which was stored frozen for shipment to Aragen Life Sciences to be resuspended in PBS and MPL-TDM before immunizing mice. For transmission blocking mAb immunoprecipitation, mAbs 4B7 and 1B3 were purified from ascites fluid using Pierce Protein A resin (Thermo Fisher Scientific, Waltham, MA), eluted in citrate-phosphate buffer (0.1 M, pH 3.0), and neutralized immediately with sodium phosphate. The purified mAbs were conjugated to Invitrogen Dynabeads M-270 Epoxy using the Antibody Coupling Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. The conjugated beads were added to gamete/zygote membranes that had been solubilized on ice in NETT buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris-Cl pH 7.4, and 0.5% Triton X-100) with PIs for 20 minutes then centrifuged at 16,000 x g for 10 m at 4°C to remove any insoluble material. The gamete/zygote NETT extract-mAb-Dynabead mixture was incubated for 1 hour at room temperature and then 2 hours at 4°C before being placed in a magnet to isolate the Dynabeads and associated immunoprecipitated material. The Dynabead-mAb bound material was washed 3 times with sterile PBS and stored frozen for shipping to Aragen Life Sciences to be resuspended in PBS and MPL-TDM prior to immunizing mice.
Mouse Immunization
Five CAF1 mice were immunized with one of the immunogens for a total of three times at three-week intervals (Aragen Life Sciences). Each dose was comprised of equal parts one and four-hour gametes/zygotes. The dosing schedule was the equivalent of 2 x 107 gametes/zygotes / mouse for the first injection, 1 x 107 gametes/zygotes / mouse for the second injection, and 5 x 106 gametes/zygotes / mouse for the third injection. Serum was obtained one week after the 2nd immunization (Day 29) and one week after the 3rd immunization (Day 50) and sent to Loyola University Chicago for analysis.
Mice selected for hybridoma production were re-immunized with the same immunogen, except for fusion 6 (F6); where a mouse originally immunized with mAb immunoprecipitated material was reimmunized with gamete/zygote membranes obtained from Pfs230 minus parasites, Pfs230Δ452–3135, to select for IgG that recognize other antigens. For this final boost, the immunogens were resuspended only in sterile PBS and administered to the selected mice. One week post the final immunization the spleen was harvested for hybridoma production. Following selection in hypoxanthine, aminopterin, and thymidine (HAT) media, the hybridoma supernatant was screened for IgG production and the IgG-positive samples were sent to Loyola University Chicago for further screening. Twenty hybridomas were selected for further subcloning by Aragen Life Sciences based on their TRA, gamete/zygote surface, and immunoblot reactivity profiles. Supernatants from the clones were reanalyzed and the same criteria were used to select clones for expansion and cryopreservation. The cryopreserved clones were sent to Loyola University Chicago to generate mAbs for further characterization, as well as SriSai Biopharmaceutical Solutions, Frederick, MD for long-term storage.
mAb production and purification
Clonal hybridomas were thawed and expanded in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 with penicillin (100 units/µl) streptomycin (100 µg/µl), HT supplement (1x), glutaMAX (1x) (complete DMEM/F12) and 20% heat-inactivated (HI) fetal bovine serum (FBS). When the cells were 80% confluent, they were harvested using trypsin (0.05%) for expansion in complete DMEM/F12 with 10% HI FBS or cryopreservation in complete DMEM/F12 with 10% HI FBS and 10% dimethyl sulfoxide. After the expanded culture was again >80% confluent, the medium was changed to complete DMEM/F12 with 5% HI FBS and when the cells were >90% confluent, the media was changed to serum-free complete DMEM/F12. The next day, the supernatant was harvested for IgG purification and the cells were harvested using trypsin for expansion in complete DMEM/F12 with 10% HI FB.
To isolate IgG, the serum-free hybridoma supernatant was diluted 2:1 in Pierce Protein G IgG Binding Buffer (BB) (Thermo Fisher Scientific) and applied to a protein G column (0.5–1 ml) pre-equilibrated with 5 column volumes of BB. After all the diluted supernatant had been applied, the column was washed with 5 column volumes of BB, before the IgG was eluted with Pierce Protein G IgG Elution Buffer (EB). The eluted material was collected in 0.5 column volumes fractions and neutralized with 0.05 column volumes of Tris-Cl (1M, pH 9.0). The protein concentration of the eluted fractions was determined using Bio-Rad Protein assay (Bio-Rad, Hercules, CA) and purity was assessed by size fractionation on a 4–20% SDS polyacrylamide gel.
Gamete/zygote surface reactivity
Antibody reactivity with the gamete/zygote surface was tested by resuspending freshly purified gametes/zygotes in RPMI media (2x104 cells/µl RPMI) and adding 25 µl to each well of a siliconized 96 well V-bottom plate (Sigmacote, Sigma, St Louis, MO) that contained 25 µl of 2x the final concentration of serum, hybridoma supernatant, or antibody. After incubation for 30 minutes on ice, SA-8.3 (100 µl) was added and the plate was centrifuged (300 xg) for 5 minutes in a swinging bucket rotor. The cell pellet was washed again with SA-8.3 (100 µl) before adding 50 µl of Alexa Fluor 488-labeled anti-mouse secondary antibody (1:1000 dilution), incubating in the dark for 45 minutes on ice and then repeating the wash steps with SA-8.3 plus 4% bovine serum albumin (BSA). The pellet was resuspended in SA-8.3 (75 µl) with MitoProbe DiIC1(5) (50 nM) (Thermo Fisher Scientific) and incubated in the dark at room temperature for 15 minutes before analysis on an Accuri C6 flow cytometer (BD Bioscience, Franklin Lakes, NJ). The forward and side scatter data were used to gate for single cells, the FL1 gate (Ex 488 Em 533/30) used to detect Alexa Fluor 488, and the FL4 gate (Ex 640 Em 675/25) used to detect DiIC1(5) which only stains parasites, not uninfected RBCs. The Accuri 6 Software was used for analysis.
Transmission blocking activity
An SFMA was used to assess transmission-blocking activity as previously described by Miura et al48. Mature stage V, P. falciparum strain NF54 gametocytes produced using the in vitro culture system described above were diluted to 0.15% gametocytemia with fresh uninfected RBC and then resuspended in the indicated concentration of serum or IgG (60 µl) and 100 µl of human non-heat-inactivated serum. The suspension was immediately added to a membrane feeder and Anopheles stephensi mosquitoes were allowed to feed for 20 minutes. The fed mosquitoes were maintained at 26°C and 80% humidity with access to sugar water for the next week. The midguts were then dissected and stained with mercurochrome to detect oocysts. Only midguts from mosquitoes with eggs at the time of dissection were analyzed. The percent inhibition in oocyst intensity was calculated using the formula 100x(1-mean number of oocysts in the test)/(mean number of oocysts in the control), and the model generated in the previous study was used to determine the best estimates of percent inhibition, the 95% confidence intervals and the p-value of each test sample.
Immunoassays
Immunoblots: Nycodenz-purified stage V gametocytes or gametes/zygotes isolated 1 hour post stimulation and extracted in NETT+PI or recombinant Pfs2529 (aa 22–193) produced in Pichia pastoris were resuspended to a final concentration of 1% SDS/10% glycerol/125 mM Tris-Cl (pH 6.8) with or without 5% beta-mercaptoethanol. The samples were size-fractionated on a 4–20% polyacrylamide gel and then transferred to nitrocellulose. The nitrocellulose blot was stained with Ponceau S (Sigma) to confirm protein transfer to the membrane, then blocked in PBS with 5% nonfat milk, and incubated with the indicated primary antibodies overnight. The immunoblot was then washed with PBS and probed with alkaline phosphatase-conjugated anti-mouse secondary antibodies (1:2000) (Sigma) for 1 hour before visualization with 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt (BCIP) and nitroblue tetrazolium chloride (NBT) (Invitrogen).
Enzyme-linked immunosorbent assay (ELISA): A 96 well plate was incubated overnight with 100 µl of the indicated recombinant proteins, Pfs230 regions30 or Pfs48/45.6C49, in 10 µg/ ml carbonate buffer and Pfs47 regions23 in 1 µg/ ml carbonate buffer,). The plate was then washed three times with TBS-T and blocked with ELISA Blocking Reagent (ImmunoChemistry cat # 640) overnight before the addition of the indicated mAb (1/200 dilution). The plate was incubated for 2 hours at 37°C, then washed with TBS-T three times before adding an alkaline phosphatase-labeled anti-mouse secondary antibody (0.1 µg/ml). After incubation for 1 hour, the plate was washed three times with TBS-T before p-nitrophenyl disodium phosphate solution (Sigma 104 phosphatase substrate) was added and 40 minutes later, the absorbance at 405 nm was quantified using a VersaMax ELISA plate reader.
Peptide microarray: Protein G purified mAb 1B3, 5E1, and 7E5 were shipped to PEPperPRINT GmbH, (Heidelberg, Germany) to be screened against linear 15 aa peptides with a 14 aa overlap covering the entire processed form of Pfs230 exposed on the gamete/zygote surface, aa 543–3135. The mAbs were incubated with the peptides overnight at 4°C then washed and incubated with DyLight-conjugated secondary antibodies for 45 minutes at room temperature. An LI-COR Odyssey Imaging System was used to quantify the signal and DyLight800-labeled-anti-c-Myc was used as a positive control.
Protein G purified mAb 4B7 and 2C7 were shipped to Pepscan (Lelystad, The Netherlands) for a Pfs25 linear and constrained peptide screen. The linear peptides represented the entire Pfs25 with overlapping 11, 15, or 22 aa peptides and an offset of one. Additional sets of the 11 and 15 linear peptides were synthesized with the native cysteine protected with an acetamidomethyl group with and without alanine residues inserted at positions 8 and 9. The constrained peptides included a 17 or 13 aa loop with cysteines added at the first and last positions; a β-turn mimic, which is a 22 aa peptide with cysteines added in the first and last position and a proline glycine pair at positions 11 and 12; a discontinuous mimic with every 15 aa peptide tested in combination with every other 15 aa peptide with cysteines added at positions 1, 16 and 33; and both combinatorial and linear disulfide bridge mimics. The combinatorial disulfide bridge assay screened every 11 aa cysteine-containing peptide with every other 11 aa cysteine-containing peptide with a GlyGlySerGlyGly linker between the two peptides and the linear disulfide bridge assay included every 22 aa peptide that contained a pair of cysteines. Additional cysteines were protected with an acetamidomethyl group. The peptides were incubated with the primary mAb overnight at 4°C then washed and incubated with a 1:1000 dilution of peroxidase-labeled secondary antibody for 1 hour at room temperature. After washing, peroxidase substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) and 2 μl/ml of 3 % H2O2 was added and one hour later, color development was monitored with a charge-coupled device (CCD) camera and image processing system. Herceptin and its peptide target were included as a positive control.
Mass spectrometry
Protein G-purified mAbs were conjugated to Dynabeads M-270 Epoxy using the antibody coupling kit (Thermo Fisher Scientific). The mAb coupled beads were washed and incubated with a NETT+PI extract of gametes/zygotes that were Nycodenz-purified 1 hour after stimulation. After 1 hour at room temperature and 2 hours at 4°C, the beads and immunoprecipitated material were isolated using a magnet, washed 3 times with PBS, and the immunoprecipitated material was eluted by boiling in 1% SDS for 5 minutes. The Dynabeads were removed using a magnet and the eluted material was run 1 cm into a 4–15% polyacrylamide gel. A 1x1x0.1 cm gel slice was excised, washed with 50% acetonitrile in water before freezing, and shipping to the Harvard Center for Mass Spectrometry (Cambridge, MA) for FASP protein digestion, liquid chromatography–mass spectrometry, and peptide identification.
Results
Immunization and hybridoma cloning strategy
Given the complex structure of many gamete/zygote surface antigens, we screened intact parasites using flow cytometry to expand the repertoire of P. falciparum transmission-blocking mAbs. Five mice were immunized with one of three different preparations of purified P. falciparum gametes/zygotes isolated one and four hours after stimulating gametogenesis in vitro: 1) whole gametes, 2) membranes isolated after 3 freeze thaw cycles, and 3) proteins immunoprecipitated from gamete/zygote Triton X-100 extracts using TRA mAb 4B7 specific for Pfs2547 and TRA mAb 1B3 specific for Pfs23012. MAb 1B3 was selected because it immunoprecipitates both Pfs230 and Pfs48/45 and therefore would focus the response to this complex. All sera collected 1 week post-immunization, at days 29 and 50, reacted with live intact gametes/zygotes using flow cytometry and fluorescence microscopy. The reactivity of the sera was further tested for TRA activity using an SMFA (Table 1). At a 1:20 dilution, all serum samples significantly reduced transmission and sera from mice immunized with gamete/zygote membranes (Mem) or mAb-precipitated antigen (Ippt) completely blocked transmission. Furthermore, when the sera were diluted 1:100, the sera from the Mem and Ippt immunized mice reduced transmission by more than 96%.
Table 1:
Standard membrane feed using serum collected 1 week after the 3rd injection (Day 50)
| Day 50 | 1:20 dilution | |||||||
|---|---|---|---|---|---|---|---|---|
| Mouse | Mosquito Inf/dissect | Oocyst | % Inhib | |||||
| Ave | Range | |||||||
| α-GAMa ms1 | 2/20 | 0.1 | (0–1) | 99.5 | ||||
| α-GAM ms2 | 1/20 | 0.05 | (0–1) | 99.7 | ||||
| α-GAM ms3 | 2/20 | 0.1 | (0–1) | 99.5 | 1:100 dilution | |||
| α-GAM ms4 | 1/20 | 0.15 | (0–3) | 99.2 | Mosquito Inf/dissect | Oocyst | % Inhib | |
| α-GAM ms5 | 3/20 | 0.15 | (0–1) | 99.2 | Ave | Range | ||
| α-mem ms1 | 1/20 | 0.05 | (0–1) | 99.7 | 5/20 | 0.25 | 0–2 | 99.3 |
| α-mem ms2 | 0/20 | 0 | 0 | 100.0 | 2/20 | 0.20 | 0–3 | 99.5 |
| α-mem ms3 | 0/20 | 0 | 0 | 100.0 | 12/20 | 1.50 | 0–11 | 96.0 |
| α-mem ms4 | 0/20 | 0 | 0 | 100.0 | 6/20 | 0.30 | 0–1 | 99.2 |
| α-mem ms5 | 0/20 | 0 | 0 | 100.0 | 11/20 | 1.40 | 0–6 | 96.3 |
| α-ippt ms1 | 0/20 | 0 | 0 | 100.0 | 2/20 | 0.10 | 0–1 | 99.7 |
| α-ippt ms2 | 0/20 | 0 | 0 | 100.0 | 5/20 | 0.50 | 0–3 | 98.7 |
| α-ippt ms3 | 0/20 | 0 | 0 | 100.0 | 4/20 | 0.35 | 0–3 | 99.1 |
| α-ippt ms4 | 0/20 | 0 | 0 | 100.0 | 1/20 | 0.05 | 0–1 | 99.9 |
| α-ippt ms5 | 0/20 | 0 | 0 | 100.0 | 7/20 | 0.70 | 0–5 | 98.1 |
| Prebleed | 19/20 | 18.4 | (0–52) | 0 | 18/20 | 37.60 | 0–94 | 0.0 |
| 20/20 | 37.60 | 6–112 | 0.0 | |||||
| mAb 4B7 | 16/20 | 3 | (0–8) | 86.1 | 9/20 | 2.05 | 0–9 | 95.3 |
| 9/20 | 1.05 | 0–5 | 97.1 | |||||
| Human serum | 20/20 | 21.65 | (1–54) | 0 | 20/20 | 44.30 | 1–81 | 0.0 |
α-GAM indicates mice immunized with whole gametes/zygotes, α-mem indicates mice immunized with gamete/zygote membranes, and α-ippt indicates mice immunized with mAb immunoprecipitate. Mice were immunized 3 times with decreasing concentrations of gametes/zygotes. 2 x 107 gametes/zygotes / mouse or the equivalent amount of membranes or immunoprecipitated material was used for the first injection, 1 x 107 gametes/zygotes / mouse for the second injection, and 5 x 106 gametes/zygotes / mouse for the third injection. The immunogen was emulsified in monophosphoryl Lipid A and trehalose dicorynomycolate in 2% oil (squalene)-Tween® 80-water (MPL-TDM) (Sigma, St Louis, MO) immediately before injection into mice.
Six of the fifteen vaccinated mice, 3 Mem and 3 Ippt, were selected for hybridoma formation after an additional antigen boost just before splenic harvest and fusion. 82 of 588 hybridoma supernatants (n=588) were positive for gamete/zygote surface reactivity and selected for SMFA testing, along with 3 negative supernatants as controls. Ten of the 82 hybridoma supernatants had greater than 45% TRA and were further sub-cloned. An additional 9 hybridoma supernatants with <45% TRA that recognized the gamete/zygote surface, as well as a negative control hybridoma with supernatant that did not recognize the gamete/zygote surface, were also subcloned. Clones from 8 different parental hybridomas produced IgG with significant TRA at ≤ 375 µg/ml (TRA mAbs) (Fig 1, Tables 2 and S1). IgG produced by the remaining clones recognized the gamete/zygote surface but did not have significant TRA.
Figure 1.
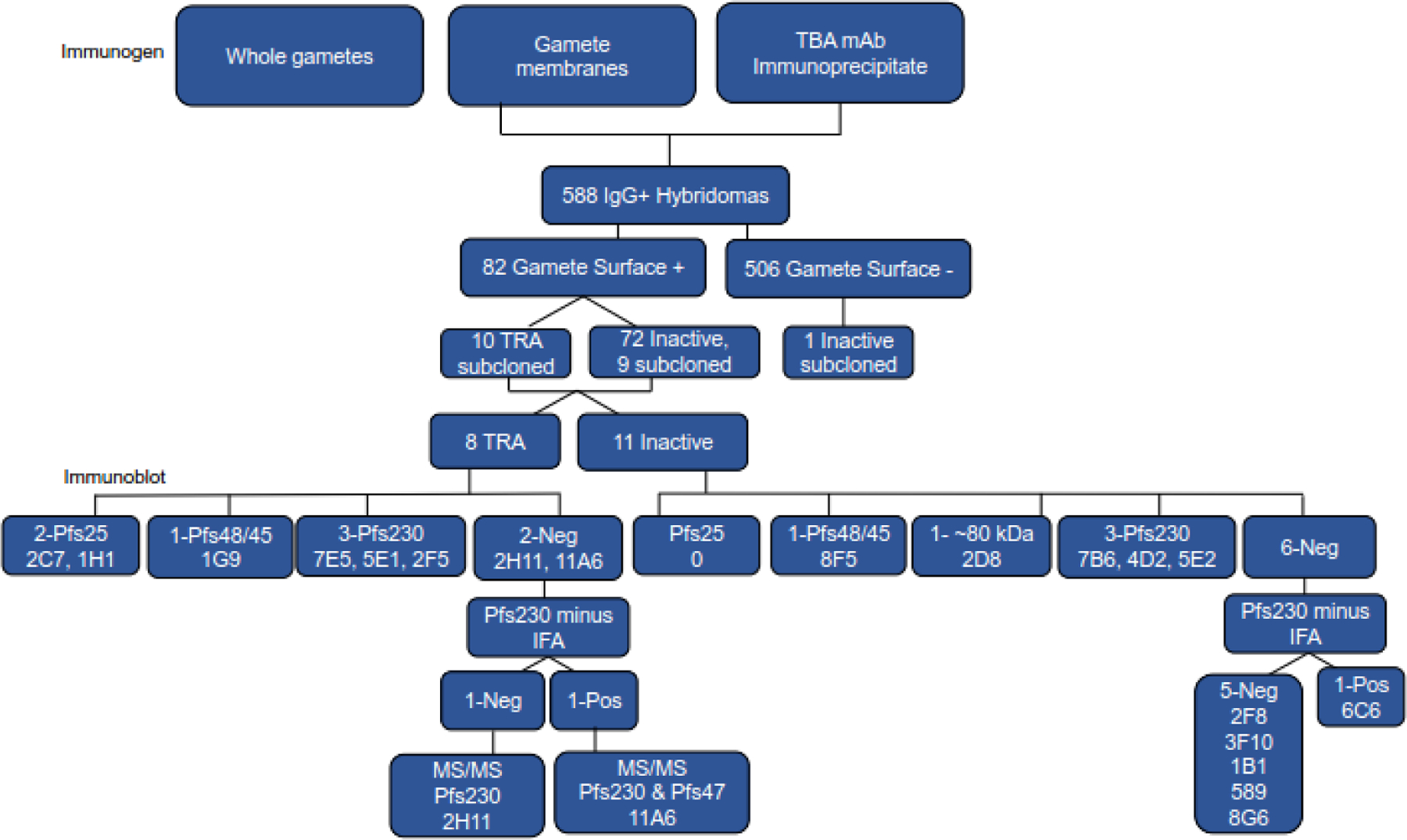
Monoclonal (mAb) selection schematic. Mice were immunized with whole gametes/zygotes, gamete/zygote membranes, or material immunoprecipitated with Pfs230-specific mAb 1B3 or Pfs25-specific mAb 4B7. The latter two immunogens induced more potent transmission-reducing activity (TRA) and splenocytes from 3 mice in each of these groups were used to generate hybridomas. The IgG+ hybridoma supernatants were screened for gamete/zygote surface reactivity using flow cytometry and the surface reactive IgGs were tested for TRA using a standard membrane feed assay. All the TRA and selected nonTRA hybridomas were cloned and rescreened for gamete/zygote surface reactivity, TRA and antigen reactivity. Antigen reactivity was assessed by immunoblot, surface reactivity with gametes/zygotes that do not express Pfs230 (Pfs230 minus IFA), and mass spectroscopy (MS/MS).
Table 2:
Standard membrane feeding assay using monoclonals purified from serum-free hybridoma media using Protein G.
| Immuno-blot | Sample name | IgG conc [µg/ml] | % Uninfect | Oocyst | % Inhibition | ||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Ave | Estimate | 95% CI Lo | 95% CI Hi | p-valu e | ||||
| Human serum | 0 | 0 | 16–90 | 56.1 | |||||
| Pfs25 | 4B7 | 93.8 | 5 | 0–19 | 5.4 | 90.5 | 72.1 | 96.8 | 0.001 |
| Neg Ctrl | F2 03G2 1A12 | 375.0 | 5 | 0–123 | 43.8 | ||||
| Pfs230 | F3 09F4 5E1 | 375.0 | 100 | 0 | 0.0 | 100.0 | 99.7 | 100.0 | 0.001 |
| F5 6D11 7E5 | 375.0 | 80 | 0–2 | 0.3 | 99.4 | 97.4 | 100.0 | 0.001 | |
| F5 05A6 2F5 | 90.0 | 25 | 0–33 | 8.0 | 81.7 | 52.1 | 93.8 | 0.003 | |
| Pfs48/45 | F2 14E5 1G9 | 375.0 | 10 | 0–29 | 5.6 | 87.3 | 63.8 | 95.7 | 0.001 |
| Pfs25 | F5 04F5 2C7 | 375.0 | 55 | 0–5 | 1.0 | 97.7 | 92.7 | 99.4 | 0.00 1 |
| F3 09B9 1H1 | 375.0 | 60 | 0–7 | 1.6 | 96.5 | 89.9 | 98.9 | 0.001 | |
| Neg | F6 30C5 11A6 | 375.0 | 60 | 0–6 | 0.9 | 97.9 | 93.9 | 99.4 | 0.001 |
| F3 14A1 2H11 | 375.0 | 30 | 0–46 | 9.3 | 78.9 | 36.7 | 93.1 | 0.006 | |
| Human serum | 0 | 0 | 18–98 | 58.4 | |||||
| Pfs25 | 4B7 | 93.8 | 25 | 0 – 9 | 2.9 | 95.0 | 89.0 | 97.9 | 0.001 |
| Neg Ctrl | F2 03G2 1A12 | 375.0 | 0 | 9 – 70 | 36.4 | ||||
| Pfs230 | F3 09F4 5E1 | 375.0 | 80 | 0 – 1 | 0.2 | 99.5 | 98.8 | 99.8 | 0.001 |
| “ | 93.8 | 80 | 0 – 1 | 0.2 | 99.5 | 98.5 | 99.9 | 0.001 | |
| F5 6D11 7E5 | 375.0 | 90 | 0–2 | 0.2 | 99.5 | 98.0 | 100.0 | 0.001 | |
| “ | 93.8 | 70 | 0–4 | 0.6 | 98.4 | 95.2 | 100.0 | 0.001 | |
| F5 05A6 2F5 | 75.0 | 0 | 6–72 | 31.1 | 14.6 | −138.5 | 71.4 | 0.746 | |
| Pfs48/45 | F2 14E5 1G9 | 375.0 | 5 | 0 – 46 | 16.4 | 54.9 | 0.1 | 79.5 | 0.050 |
| “ | 93.8 | 5 | 0 – 35 | 11.3 | 69.0 | 30.6 | 86.2 | 0.011 | |
| Pfs25 | F5 04F5 2C7 | 375.0 | 70 | 0 – 3 | 0.6 | 98.5 | 96.4 | 99.4 | 0.001 |
| “ | 93.8 | 30 | 0 – 16 | 3.9 | 89.4 | 75.3 | 95.3 | 0.001 | |
| F3 09B9 1H1 | 375.0 | 35 | 0 – 7 | 1.3 | 96.4 | 92.1 | 98.5 | 0.001 | |
| “ | 93.8 | 5 | 0 – 28 | 9.4 | 74.2 | 34.7 | 90.7 | 0.003 | |
| Neg | F6 30C5 11A6 | 375.0 | 30 | 0 – 5 | 1.9 | 94.9 | 88.4 | 98.0 | 0.001 |
| “ | 93.8 | 5 | 0–34 | 13.3 | 63.6 | 16.4 | 84.0 | 0.017 | |
| F3 14A1 2H11 | 375.0 | 0 | 2–47 | 22.3 | 38.6 | −36.2 | 73.4 | 0.235 | |
| “ | 93.8 | 5 | 0–39 | 18.1 | 50.3 | −10.1 | 79.2 | 0.090 | |
Monoclonal target identification
The targets of the monoclonals were initially evaluated using immunoblots of reduced and non-reduced gamete/zygote extract. Three TRA mAbs and three nonTRA mAbs (Fig S1) have the same binding pattern as antibodies against recombinant Pfs230 suggesting they target Pfs230. Two mAb, one TRA and one nonTRA, have patterns similar to anti-recombinant Pfs48/45 antibodies (Fig 2B, Fig S1) and two TRA mAb have patterns similar to Pfs25-specific mAb 4B7 (Fig 2C) consistent with Pfs48/45 or Pfs25 being their targets. The remaining two TRA mAb, (Fig 2B) and six nonTRA mAb (Fig S1), were negative by immunoblot, while one nonTRA mAb reacted with a ~85 kDa band that has not been previously reported as a gamete/zygote surface antigen. None of the TRA mAbs recognized proteins on an immunoblot of reduced gamete/zygote extract (Fig 2A-D) in accordance with prior observations of TRA mAbs11,50. However, in contrast to prior work50, one of the anti-Pfs230 TRA mAbs (5E1) is IgG1, as is the anti-Pfs48/45 TRA mAb (1G9). The rest of the TRA mAbs are IgG2a, except for 2H11 which is IgG2b (Table 3).
Figure 2.
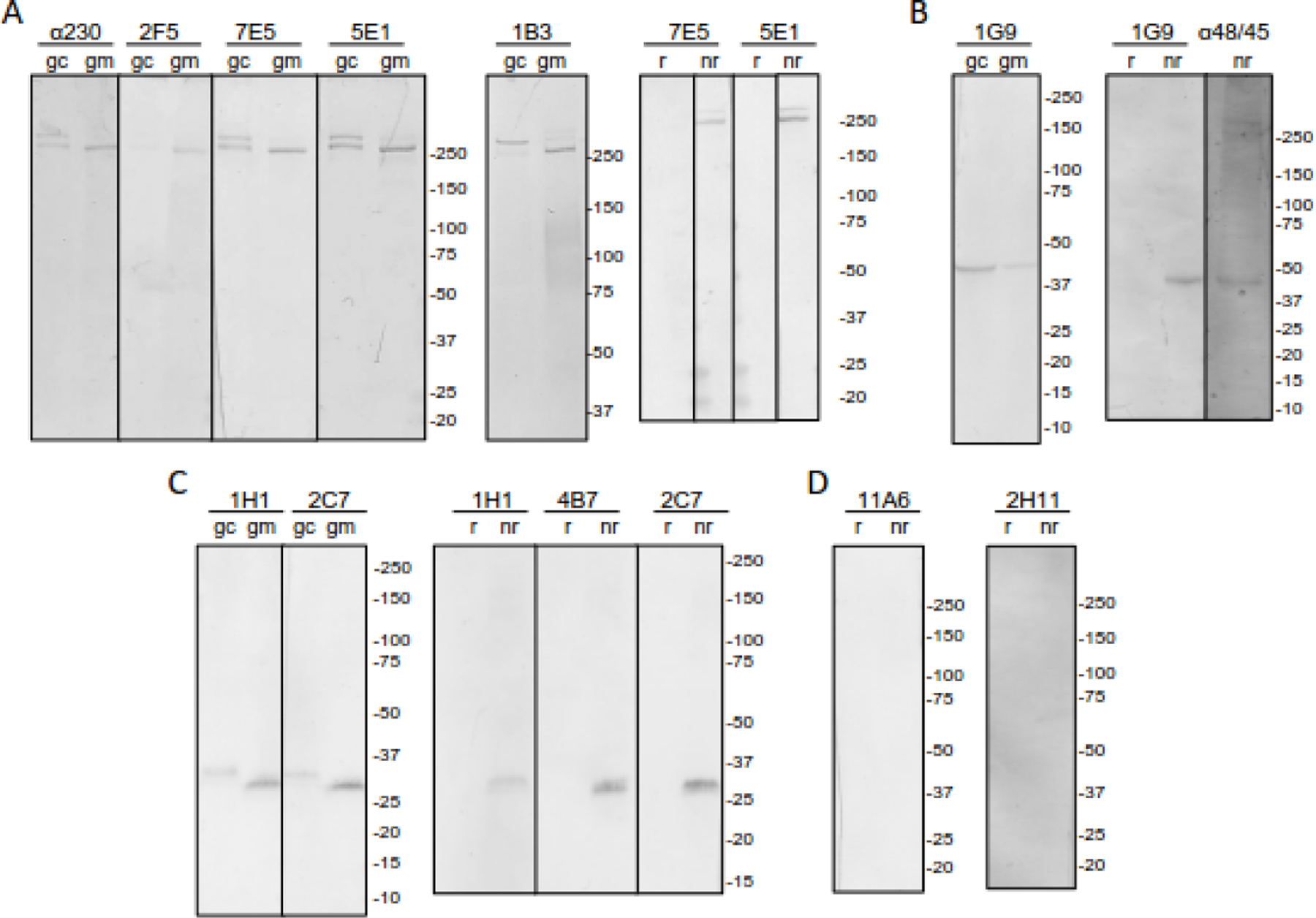
Monoclonal (mAb) sexual stage parasite reactivity. Immunoblots of purified gametocyte (gc) or gamete/zygote (gm) Triton X-100 extract without β-mercaptoethanol or gamete/zygote Triton-X-100 extract with (r) and without (nr) β-mercaptoethanol were probed with the indicated mAb or as positive controls, anti-recombinant MBP.Pfs230.C (α230) (A) or MBP.Pfs48/45 (α48/45) serum (B). Previously reported mAb specific for Pfs230 (1B3) (A) or Pfs25 (4B7) (C) are also included as controls. Immunoblots are grouped according to the mAb pattern: A) Pfs230-like, B) Pfs48/45-like, C) Pfs25-like, and D) No reactivity. 5-Bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro blue tetrazolium (NBT) were used to visualize the bands.
Table 3:
Monoclonal antibody characterization.
| Isotype | Gamete Immunoblot | Gamete surface reactivityb | Immuno-precipitation | |||||
|---|---|---|---|---|---|---|---|---|
| TRAa | ID | Full name | Heavy & Light Chain | Non-reduce d | Reduced | WT | 230 minus | |
| 1 | 2C7 | F5 04F5.2C7 | IgG2a, Kappa | 25 | neg | pos | pos | |
| 2 | 1H1 | F3 09B9.1H1 | IgG2a, Kappa | 25 | neg | pos | pos | |
| 3 | 1G9 | F2 14E5.1G9 | IgG1, Kappa | 48 | neg | pos | pos | |
| 4 | 7E5 | F5 06D11.7E5 | IgG2a, Kappa | 230 | neg | pos | neg | |
| 5 | 5E1 | F3 09F4.5E1 | IgG1, Kappa | 230 | neg | pos | neg | |
| 6 | 2F5 | F3 05A6.2F5 | IgG2a, Kappa | 230 | neg | pos | neg | |
| 7 | 2H11 | F3 14A1.2H11 | IgG2b, Kappa | neg | neg | pos | neg | 230 |
| 8 | 11A6 | F6 30C5.11A6 | IgG2a, Kappa | neg | neg | pos | pos | 230 & 47 |
| nonTRA | ||||||||
| 1 | 8F5 | F6 13C10.8F5 | IgG2a, Kappa | 48 | neg | pos | pos | |
| 2 | 2D8 | F4 05H5.2D8 | IgG2a, Kappa | 80–100 | neg | pos | ND | HSP90+ |
| 3 | 4D2 | F3 14D10.4D2 | IgG1, Kappa | 230 | neg | pos | neg | |
| 4 | 5E2 | F3 08F1.5E2 | IgG1, Kappa | 230 | neg | pos | neg | |
| 5 | 7B6 | F3 08D6.7B6 | IgG2a, Kappa | 230 | >230 | pos | neg | |
| 6 | 5B9 | F3 05H1.5B9 | IgG2a, Kappa | neg | neg | pos | neg | |
| 7 | 8G6 | F3 04C6.8G6 | IgG2b, Kappa | neg | neg | pos | neg | |
| 8 | 3F10 | F5 09G9.3F10 | IgG1, Kappa | neg | neg | pos | neg | |
| 9 | 2F8 | F3 06D6.2F8 | IgG1, Kappa | neg | neg | pos | neg | |
| 10 | 1B1 | F4 11D9.1B1 | IgG1, Kappa | neg | neg | pos | neg | |
| 11 | 6C6 | F2 18F12.6C6 | IgG2a, Kappa | neg | neg | pos | pos | |
| Control | ||||||||
| 12 | 1A12 | F2 03G2.1A12 | IgG1, Kappa | neg | neg | neg | ND | |
Characteristics of the 8 transmission-reducing (TRA), 11 non-transmission-reducing (nonTRA), and 1 control monoclonal are listed.
Wild-type (WT) and Pfs230Δ452–3135 gametes/zygotes (230 minus) were used to detect surface reactivity.
To identify the target of the two immunoblot-negative TRA mAbs and identify the ~85 kDa band recognized by the nonTRA mAb, immunoprecipitation followed by mass spectroscopy (MS/MS) was performed (Table S2). One immunoblot-negative TRA mAb (2H11) was found to immunoprecipitate Pfs230 from a gamete/zygote Triton X-100 extract while the other TRA mAb (11A6) immunoprecipitated both Pfs230 and Pfs47. The nonTRA mAb that recognized a ~85 kDa protein immunoprecipitated several heat shock and cytoskeleton proteins, as well as Pfg27, an abundant, internal gametocyte-specific protein51. Most of the peptides identified, except HSP90, endoplasmin with 42.3% identity to HSP90, and alpha tubulin 2, were also found in low abundance in the other pull-downs suggesting that these were nonspecific interactions. All three genes are expressed in stage V gametocytes52 and the molecular weights of HSP90 (86.1 kDa) and endoplasmin (95 kDa) are consistent with mAb 2D8’s immunoblot profile, but none are predicted to be expressed on the gamete/zygote surface. More detailed analysis is needed to clearly define the target antigen of nonTRA mAb 2D8.
For additional confirmation that Pfs230 was the target of the two immunoblot-negative TRA mAb, as well as the other mAb that bound a Pfs230-like band on an immunoblot their ability to recognize the surface of Pfs230 minus gametes, was tested. For these experiments, a Pfs230 disruptant parasite line was used that only expresses the N-terminal region of Pfs230. (Pfs230Δ452–3135)53. This N-terminal region of Pfs230 is released during RBC emergence in the mosquito midgut generating Pfs230 minus gametes. As predicted all three Pfs230-associated TRA mAbs, reacted with the surface of wild-type gametes, but not Pfs230Δ452–3135 gametes, (Fig 3). The TRA mAb that immunoprecipitated Pfs230 also reacted with wild-type gametes, but not Pfs230Δ452–3135 gametes/zygotes consistent with Pfs230 being the target antigen. In contrast, the TRA mAb that immunoprecipitated both Pfs230 and Pfs47, reacted with both wild type and Pfs230Δ452–3135 gametes/zygotes suggesting Pfs230 was not required for binding and that gamete/zygote surface reactivity was due to the presence of Pfs47.
Figure 3.
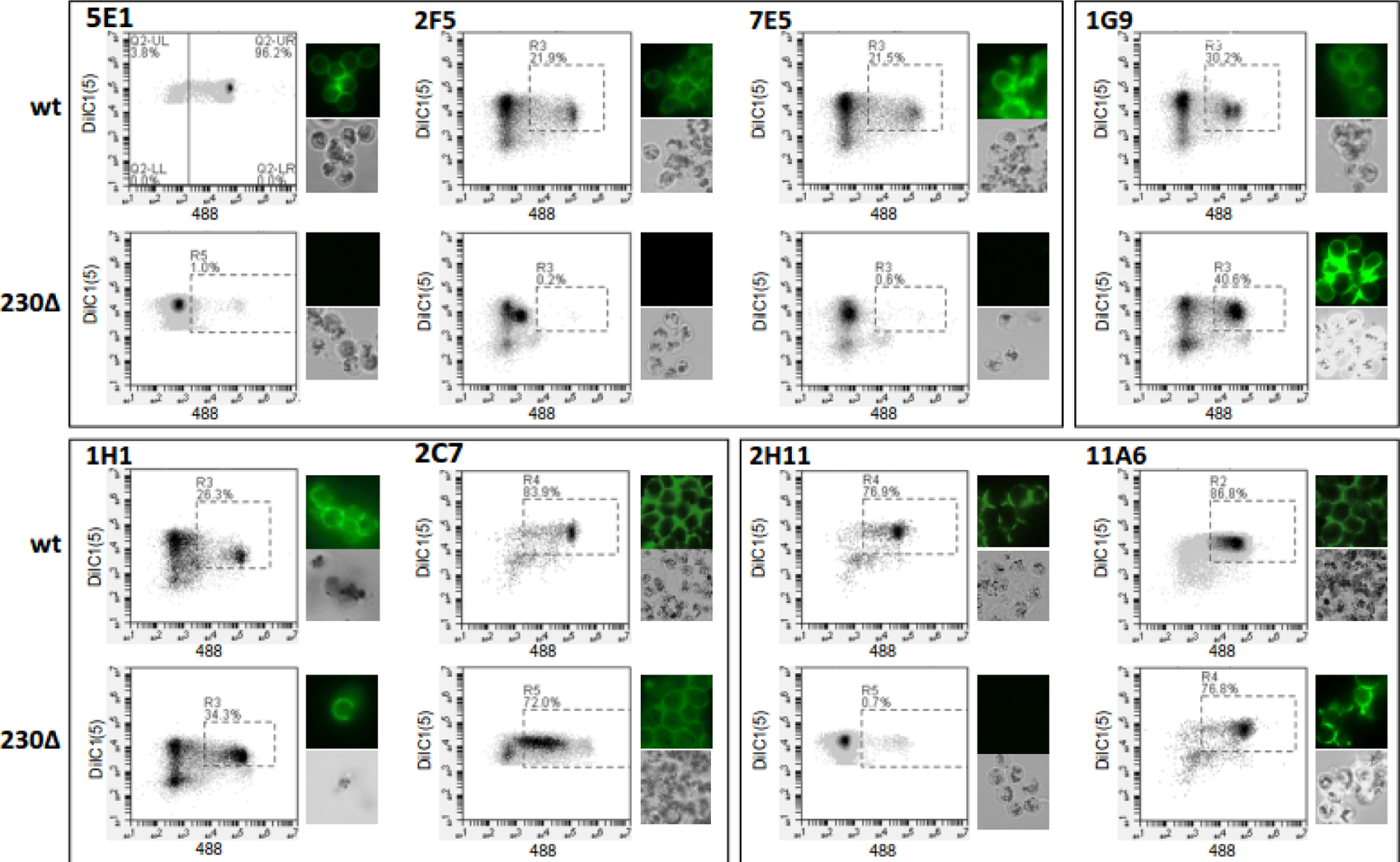
Monoclonal (mAb) gamete/zygote surface reactivity. Purified wild-type strain NF54 (wt) or Pfs230Δ452–3135 (Pfs230Δ) gametes/zygotes were incubated with the indicated mAb, washed, and then stained with Alexa Fluor 488-labeled anti-mouse IgG and membrane potential dye DiIC1 (5). Fluorescence was monitored using flow cytometry and fluorescence microscopy. For each antibody and gamete/zygote population, the DiIC1(5) and Alexa Fluor 488 fluorescence of each cell is plotted and representative fluorescent and bright field images are shown.
Given the high prevalence of mAbs targeting Pfs230, the other six nonTRA, immunoblot negative, but surface-reactive mAbs were also tested for reactivity to Pfs230Δ452–3135 gametes. Five of these six nonTRA mAb reacted with wild type, but not Pfs230Δ452–3135 gametes/zygotes consistent with targeting Pfs230 or a Pfs230-associated protein (Fig S2). As a control, the 4 mAb that reacted with Pfs25 or Pfs48/45 by immunoblot were also tested and found to bind both wild-type and Pfs230Δ452–3135 gametes/zygotes as expected (Fig 3 and S2). In total, 5 TRA and 8 nonTRA mAb were found to target Pfs230 or a Pfs230-associated antigen suggesting Pfs230 is the immunodominant gamete/zygote surface antigen when gamete/zygote membranes were used as an immunogen. In contrast, immunizing with immunoprecipitated material induced all the mAbs that recognized targets other than Pfs230 and Pfs25 (Fig 4). Of the five mAbs generated in response to immunoprecipitated material two reacted with a Pfs48/45-like band, one reacted with a ~85 kDa protein, while two were immunoblot negative, including the TRA mAb that immunoprecipitated both Pfs230 and Pfs47 and maintained an affinity for Pfs230Δ452–3135 gametes.
Figure 4.
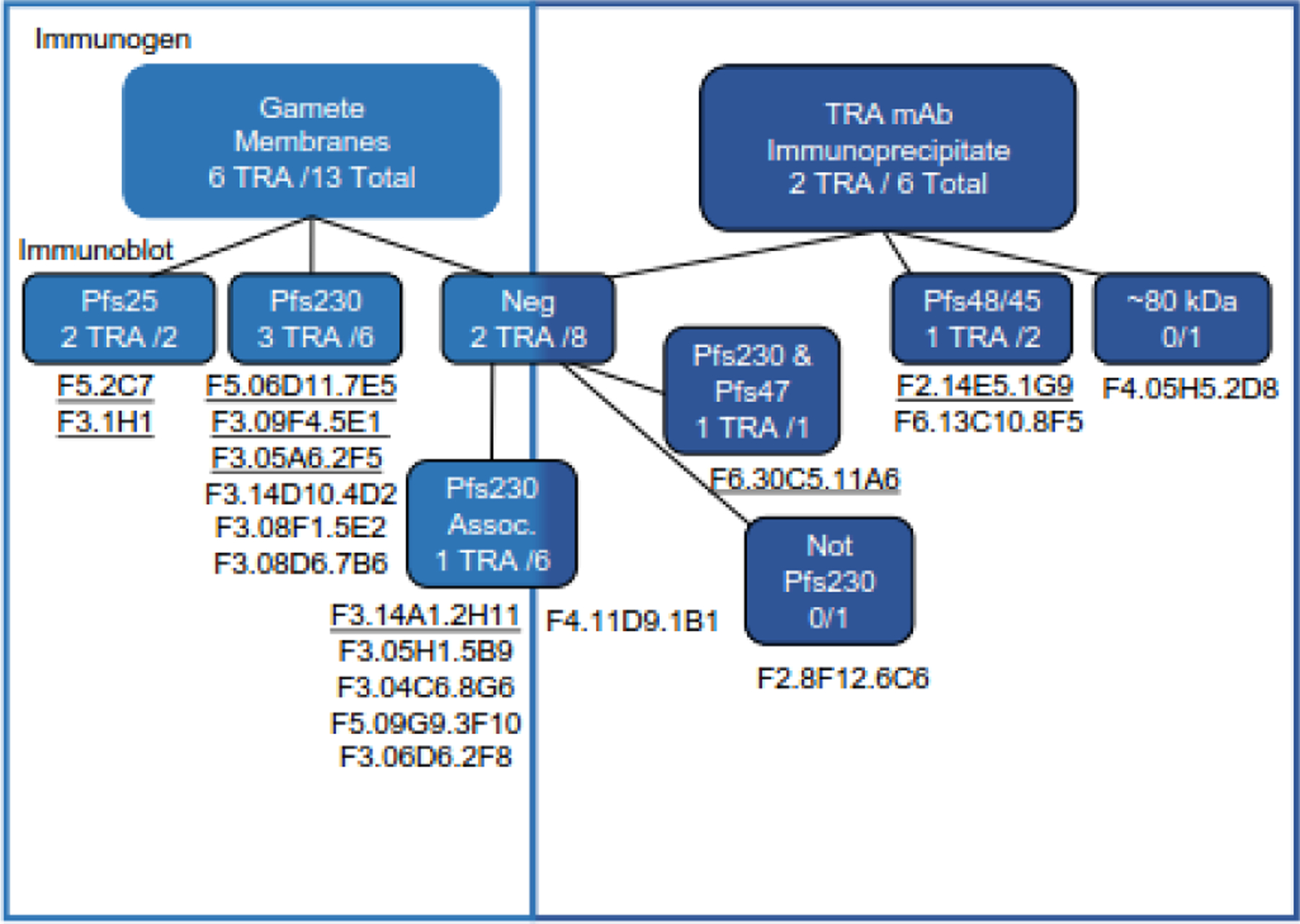
Comparison of the profiles of monoclonals (mAbs) generated by each of the immunogens. mAbs that were derived from mice immunized with gamete/zygote membranes are in the light blue boxes and those from mice immunized with material immunoprecipitated by TRA mAbs are in the dark blue boxes. Within each box mAbs have been divided first based on their immunoblot reactivity and TRA mAbs are underlined. The immunoblot-negative mAbs are further separated based on their lack of reactivity with Pfs230Δ357–3135 gametes/zygotes (Pfs230 Assoc). The two immunoblot-negative mAbs that still reacted with Pfs230Δ357–3135 gametes/zygotes, 11A6 and 6C6, were then separated based on TRA and mass spectrometry data.
Reactivity with recombinant vaccine candidates
To determine whether the TRA mAbs reacted with new transmission-blocking epitopes, they were tested by ELISA or immunoblot for reactivity with a series of recombinant proteins that included the domains currently being tested as transmission-blocking vaccine candidates, Pfs230D1M30 (Fig 5), Pfs4723(Fig 6), Pf48/45.6C1, and Pfs25 (Fig 7). Except for one Pfs25 mAb, that bound to reduced, but not non-reduced, recombinant Pfs25, none of the TRA mAbs tested bound to the recombinant proteins included in the ELISAs or immunoblots, suggesting they bind to novel epitopes that could be further targeted as vaccine candidates. Peptide mapping of the TRA mAbs that reacted with either a Pfs230 or Pfs25-sized band confirmed that the epitope was not recapitulated by linear peptides. The small size of Pfs25 also allowed extensive screening of conformationally constrained peptides, which tested every possible combination of 15mer peptides. In this assay, the well-characterized anti-Pfs25 mAb 4B7, which reacts only with non-reduced Pfs25 from a gamete/zygote extract and reduced recombinant Pfs25 on an immunoblot, reacted with both linear and constrained peptides containing aa 122–130 (Fig S3), consistent with previous work40. In marked contrast, the new Pfs25-specific TRA mAbs did not react with any of the constrained or linear peptides, consistent with these TRA mAb targeting a distinct epitope other than site 1 on Pfs25.
Figure 5.
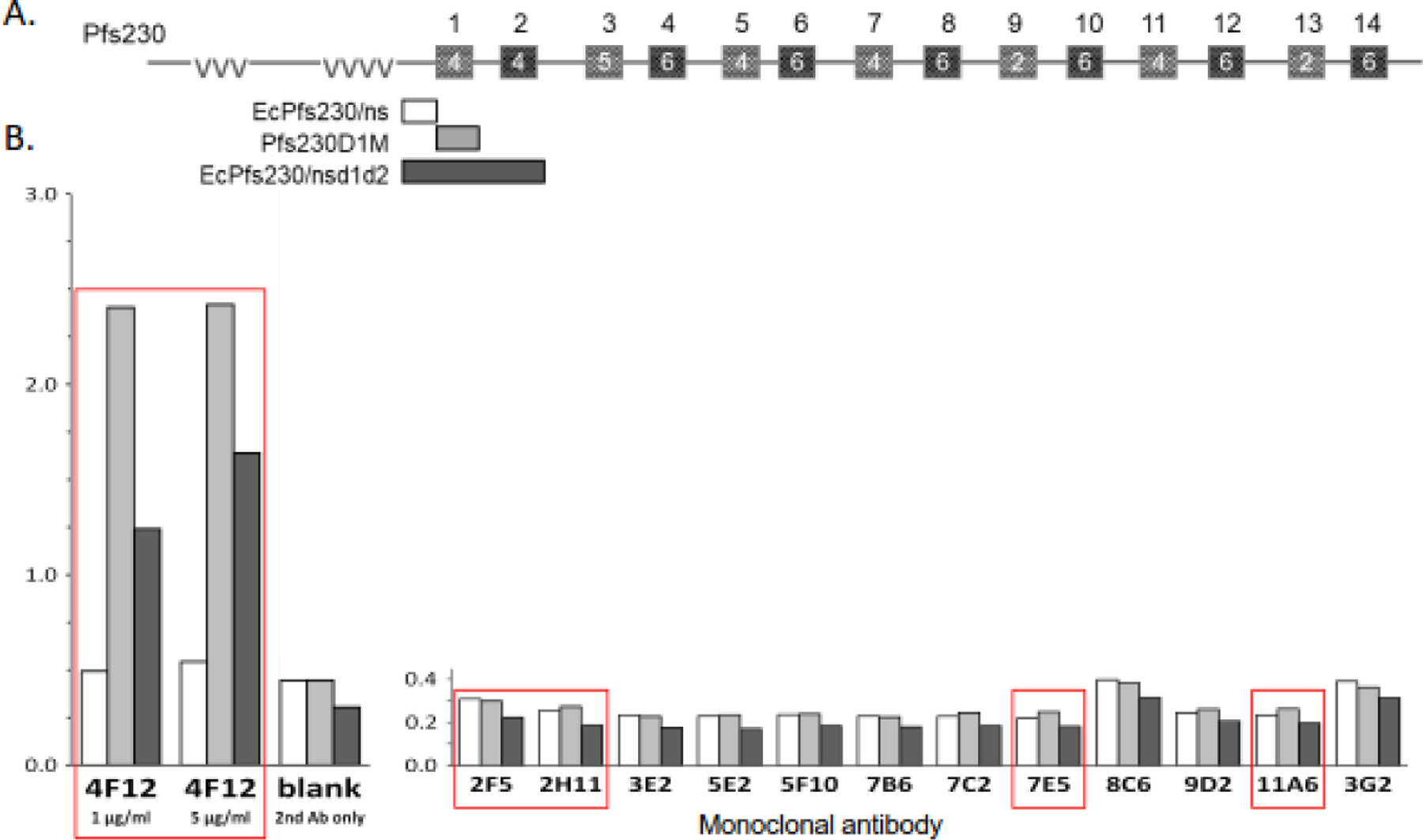
Monoclonals (mAbs) do not react with recombinant Pfs230. A) Schematic of the structure of Pfs230. Glutamate-rich repeats are indicated by vvv and 6-cys domains are depicted by boxes numbered 1 to 14 with the actual number of cysteines in each domain indicated in the box. The Pfs230 regions included in the recombinant proteins tested for reactivity are shown, the non-structured domain, Glu444-Val592 without (EcPfs230ns, □) and with domains 1 and 2, Glu444-Asn915 (EcPfs230/nsd1d2, ■) produced in E. coli (Ec) and domain 1, Ser542-Gly736 (Pfs230D1M, ■) produced in Pichia pastoris. (B) The 3 recombinant proteins were tested for reactivity with the indicated mAb using ELISA. Red boxes indicate mAbs with transmission-reducing activity, including the positive control 4F12. Alkaline phosphatase-labeled anti-mouse secondary antibody and p-nitrophenyl disodium phosphate substrate were used for detection.
Figure 6.
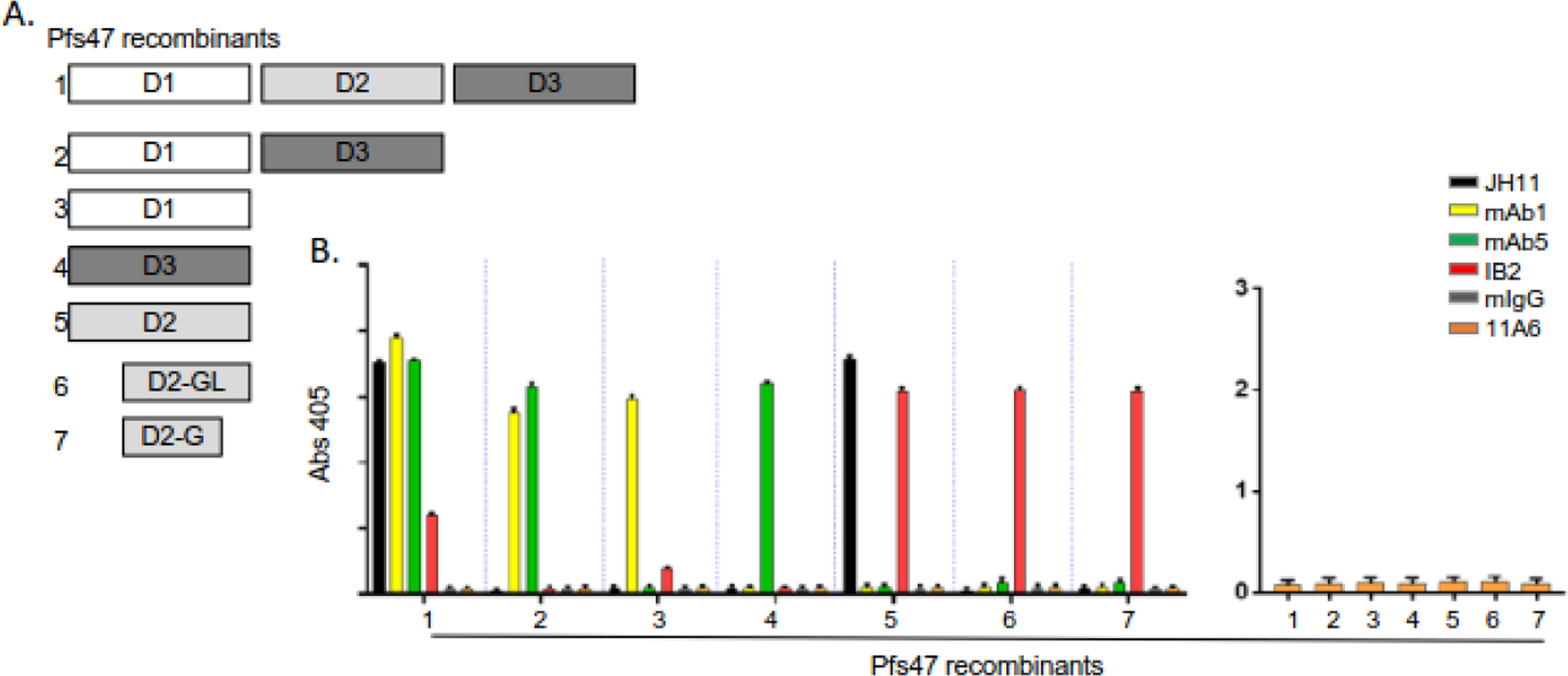
Monoclonal (mAb) F6.30C5.11A6 does not react with recombinant Pfs47. A) Schematic of the three Pfs47 6-cys domains included in the 7 E. coli-produced recombinant proteins tested for antibody recognition using ELISA. B) The reactivity of the 6 antibodies, JH11 (▪), mAb1 (▪), mAb5 (▪), IB2 (▪), mIgG (▪), 11A6 (▪) against the indicated recombinant Pfs47 proteins (1–7) is plotted. Alkaline phosphatase-labeled anti-mouse secondary antibody and p-nitrophenyl disodium phosphate substrate were used for detection.
Figure 7.
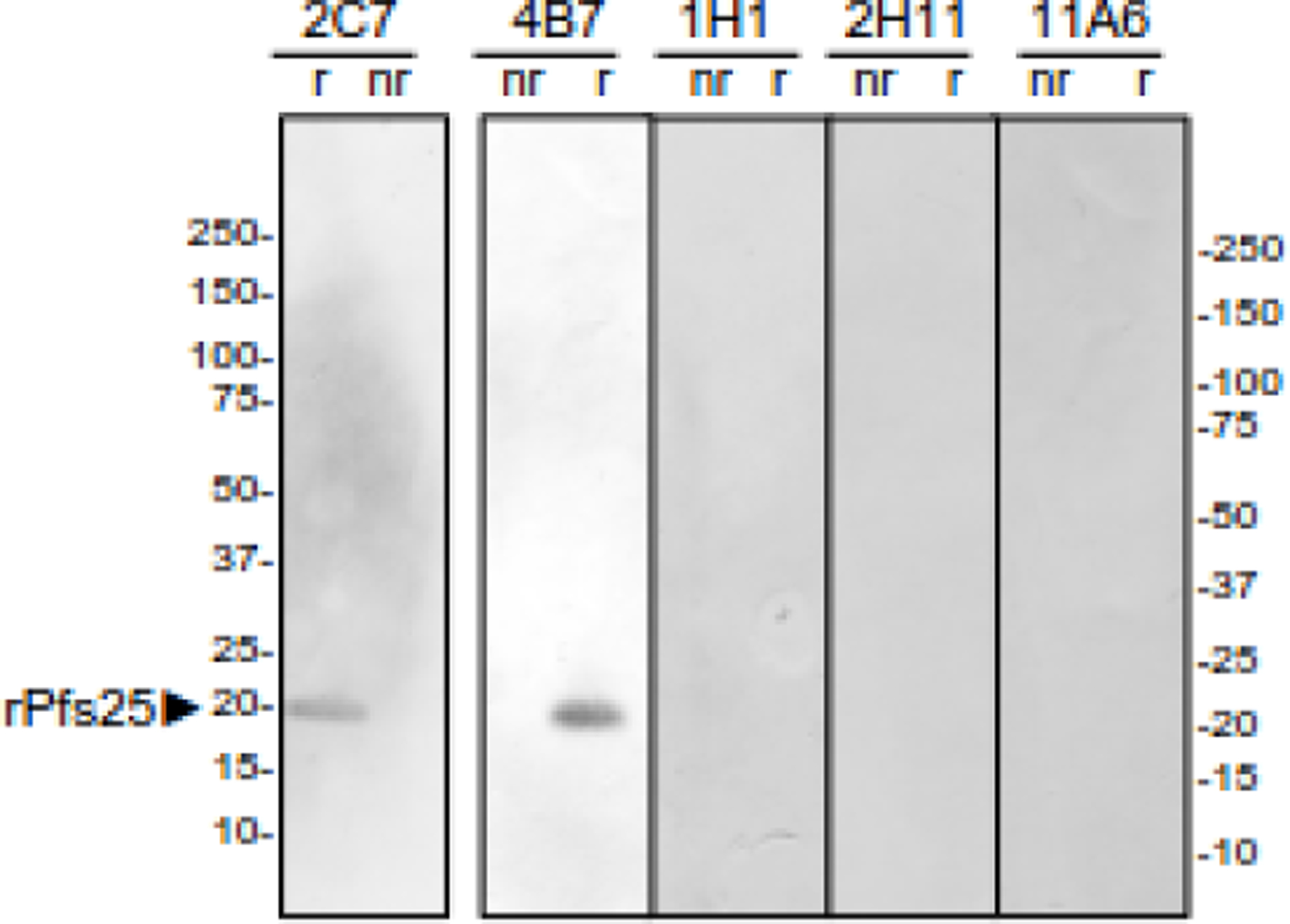
Recombinant Pfs25 Reactivity. An immunoblot of purified recombinant Pfs25 (rPfs25, aa 22–193) produced in Pichia pastoris pretreated with (r) or without (nr) 5% β-mercaptoethanol was probed with the indicated monoclonal.
Discussion
A flow cytometry assay was developed to efficiently screen for antibody binding to the surface of live P. falciparum gametes/zygotes and then used to identify eight new TRA mAbs. None of TRA mAb reacted with current recombinant transmission-blocking vaccine candidates, suggesting the existence of additional target TRA epitopes exposed on the gamete/zygote surface. One TRA mAb (11A6) immunoprecipitated both Pfs47 and Pfs230 suggesting the two proteins are associated in a complex that has not previously been reported. Unlike Pfs230, Pfs47 is only expressed on female parasites and is predicted to be associated with the plasma membrane via a GPI anchor. Prior to this work, Pfs230 had only been reported to interact with Pfs48/45, a paralog of Pfs47, that also has a GPI anchor signal, but is expressed by both males and females. One possibility is that Pfs230 interacts with both Pfs48/45 and Pfs47 in female gametes. Recent work has demonstrated low levels of anti-Pfs230 polyclonal antibody reactivity with some Pfs48/45 disruptant parasites44 consistent with a possible association between Pfs230 and Pfs47. Further support for the hypothesis that an association exists between Pfs230 and Pf47 as well as between Pfs230 and Pf48/45 is that all three Pfs47 or Pfs48/45-binding mAbs were obtained from splenocytes from mice immunized with material immunoprecipitated by established Pfs25- and Pfs230-specific mAbs, 4B747 and 1B312, while all the mAbs isolated after immunization with gamete/zygote membranes interacted with Pfs230 or Pfs25. This data suggests that Triton X-100 solubilization followed by immunoprecipitation with 4B7 and/or 1B3 enriches, and may increase, the exposure of Pfs48/45 and Pfs47. Pfs48/45, Pfs47, and Pfs230 are members of a family of P. falciparum proteins containing multiple 6 cysteine (6-cys) domains54 and there is precedence for one GPI anchored 6-cys protein serving as a membrane anchor for another GPI anchorless family member55.
The structures of the 6-cys domains included in recombinant Pfs230 (D1M)43,44 and Pfs48/45 (6C)56,57 vaccine candidates have been reported, but their orientation on the parasite surface and interaction with each other is unknown. However, given the C-terminal GPI anchor in Pfs48/45, it is likely that the C-terminal 3rd 6-cys domain encoded by Pfs48/45 6C is membrane proximal and the N-terminal domain is more distal. The structure of full-length Pfs230 is more difficult to predict. In contrast to the other 6-cys family members that encode 1 to 3 domains, Pfs230 and its paralog, Pfs230p, contain 7 and 6 pairs of A- and B-type 6-cys domains for a total of 14 and 12 predicted domains, respectively. Polyclonal TRA serum generated against recombinant Pfs230 encoding the 1st and 2nd Pfs230 6-cys domains recognize the surface of live male and female gametes58, as does one TRA mAb that binds Pfs230D1M. However, another anti-Pfs230D1M TRA mAb only binds live male gametes and two other anti-Pfs230D1M nonTRA mAbs react only with fixed, not live, gametes44. Recombinant Pfs230 proteins produced to date that include the remaining 12 6-cys domains have only induced nonTRA antibodies 58,59 that only react with denatured Pfs23058. This data suggests that the structure of the recombinant 6-cys domains used as immunogens did not recapitulate the native structure of Pfs230 on the gamete/zygote surface. One recent model of full-length Pfs230 generated using the alpha fold algorithm predicts that the first two and last two 6-cys domains are in close proximity and provides leads for future recombinant protein designs to test for discontinuous epitopes60. Additional structural work using parasite-produced protein and TRA mAbs is needed to define the functional surface-exposed epitopes.
The predominance of Pfs230-associated mAbs in this screen suggests Pfs230 is the immunodominant membrane antigen 1 to 4 hours post-stimulating gametogenesis. Thirteen of the 19 gamete/zygote surface mAbs interact with Pfs230. Neither the 5 new Pfs230-related TRA mAbs identified here nor the original Pfs230-specific TRA mAb 1B312 binds to recombinant Pfs230D1M supporting the existence of additional transmission-blocking epitopes that could be targeted as vaccine candidates. Similarly, the other 3 TRA mAbs that reacted with Pfs48/45 or Pfs25 did not react with Pfs48/45.6C or non-reduced recombinant Pfs25, respectively. It is possible that Pfs48/45 mAb 1G9 may react with Pfs48/45.10C, which has been shown to contain another transmission-blocking epitope56, but this was not formally tested. None of the Pfs25-specific mAbs bound non-reduced recombinant Pfs25. However, mAb 2C7, but not mAb 1H1, recognized reduced recombinant Pfs25. This pattern of reactivity has been previously reported for mAb 4B747; however, unlike 4B7, mAb 2C7 did not react with any linear or constrained peptides in a peptide scan. Together the data suggest the TRA mAbs identified here, which were selected based on intact gamete/zygote surface reactivity, interact with epitopes on parasite-produced proteins that are either not present or not correctly folded in the current recombinant vaccine candidates and therefore represent new target epitopes. All four TRA mAb targets, Pfs230, Pfs48/45, Pfs47, and Pfs25 contain multiple domains with multiple disulfide bonds that may not be reproduced correctly using the heterologous expression systems tried to date. Further evaluation of insect or mammalian systems to express larger regions of Pfs230 or to co-express Pfs230 and Pfs48/45 and/or Pfs47 may be needed to recreate these new TRA epitopes.
No TRA mAbs were detected that bound PF77, MDV1, HAP2, or other gametocyte/gamete antigens26,46,61. It is possible that these antigens are not expressed one to four hours post-invasion. Both PF77 and MDV1 are expressed in gametocytes before RBC emergence26 and HAP2 is likely to be involved in fertilization62, which happens between 10–30 minutes after stimulation. Therefore, these antigens could be shed from the surface within an hour. The targets for two nonTRA, gamete-surface reactive mAbs remain unknown and could represent new surface exposed gamete/zygote antigens. Both are likely to bind discontinuous epitopes since one mAb, 6C6, does not react with any bands on a gamete/zygote immunoblot, while the other mAb, 2D8, reacts with multiple bands from 80–100 kDa on a non-reduced, but not a reduced, gamete/zygote immunoblot. Mass spectroscopic analysis of the gamete/zygote proteins immunoprecipitated by mAb 2D8 identified peptides corresponding to HSP90, endoplasmin and alpha tubulin 2 as the only abundant peptides not also identified in other gamete/zygote immunoprecipitations. Endoplasmin and HSP90 are homologs that share 42.3% identity and have molecular weights of 95 and 86.1 kDa, respectively, which are consistent with the bands recognized by mAb 2D8 on the gamete/zygote immunoblot. Therefore, it is possible that the mAb recognizes both proteins. Endoplasmin is predicted to be located in the endoplasmic reticulum, while HSP90 lacks a signal peptide consistent with a cytoplasmic location. Their locations in gametocytes and gametes/zygotes have not been investigated. In contrast, alpha tubulin 2 is 49.7 kDa and known to be a component of the axonemal structure of microgametocytes63, not the gamete/zygote surface, raising questions about whether its presence was due to cytoskeletal contamination often found in immunoprecipitations. Although it is intriguing to hypothesize that the presence of protein chaperones on the gametocyte/gamete/zygote surface facilitates the transition from the 37°C host to the <30°C mosquito, further analysis is needed to identify the target of mAb 2D8.
In summary, we have identified 8 new TRA mAbs that bind discontinuous epitopes exposed on the P. falciparum gamete/zygote surface that are not currently being targeted for vaccine development. Five of the TRA mAbs bind to Pfs230, consistent with it being an immunodominant transmission-blocking target. One of these five mAbs immunoprecipitated both Pfs230 and Pfs47, suggesting for the first time that Pfs230 may associate with Pfs47 as well as Pfs48/45. The remaining TRA mAbs recognized established TRA antigens Pfs25 and Pfs48/45. Together the results suggest these 4 proteins are the major surface-exposed antigens on gametes/zygotes one to four hours after emergence and are prime targets to block development in the mosquito midgut.
Supplementary Material
Supplemental Figure Legends
Figure S1 Monoclonal (mAb) sexual stage parasite reactivity. Immunoblots of purified gamete/zygote (gm) Triton X-100 extract with (r) and without (nr) β-mercaptoethanol (A&B) and purified gametocyte (gc) Triton X-100 extract without β-mercaptoethanol (B) were probed with the indicated mAb and the bands visualized with 5-Bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro blue tetrazolium (NBT).
Figure S2 Gamete/zygote surface reactivity of immunoblot negative monoclonals (mAbs). Purified wild-type strain NF54 (wt) or Pfs230Δ452–3135 (Pfs230Δ) gametes/zygotes were incubated with the indicated mAb, washed, and then stained with Alexa Fluor 488-labeled anti-mouse IgG (488) and membrane potential dye DiIC1(5). Fluorescence was monitored using flow cytometry and fluorescence microscopy. For each antibody and gamete/zygote population, the DiIC1(5) and Alex Fluor 488 fluorescence of each cell is plotted and representative fluorescent and bright field images are shown. Pfs230-specific mAb 1B3 and Pfs25-specific mAb 4B7 are included as controls.
Figure S3 Peptides bound by monoclonal (mAb) 4B7. The fluorescence intensity of mAb 4B7 binding to two sets of peptides corresponding to Pfs25. One set was linear fifteen aa peptides with an offset of one residue (orange) and the other set was looped by adding cysteine residues at both ends of the one aa offset 15 aa Pfs25 peptides to allow disulfide binding (cyan).
Acknowledgments
We thank Dr. A. Birkett and Ms. K. Mertes for critical reading of the manuscript.
Funding:
This research was supported by PATH Agreement # GAT.0888-30-0155844-COL (HJF Award 65252, KCW) and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (PED, CB-M, Z01AI000947; CL)
Abbreviations
- 6-cys
6 cysteine domains
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate)
- BB
Binding Buffer
- BCIP
5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt
- BSA
bovine serum albumin
- CCD
charge-coupled device
- cRPMI
complete RPMI 1640 media
- CSP
circumsporozoite protein
- Conc
concentration
- EB
Elution Buffer
- Ec
Escherichia coli
- EDTA
ethylenediaminetetraacetic acid
- ELISA
Enzyme-linked immunosorbent assay
- EPA
Pseudomonas aeruginosa exotoxin A
- F6
fusion 6
- FBS
fetal bovine serum
- Gc
gametocyte
- Gm
gamete
- GPI
glycosylphosphatidylinositol
- HAT
hypoxanthine, aminopterin, and thymidine media
- HI
heat-inactivated
- Inf
infected
- Ippt
mAb-precipitated antigen
- mAbs
monoclonal antibodies
- Mem
gamete/zygote membranes
- MPL-TDM
monophosphoryl Lipid A and trehalose dicorynomycolate in 2% oil (squalene)-Tween® 80-water
- MS/MS
mass spectroscopy
- NAG
N-acetyl glucosamine
- NBT
nitroblue tetrazolium chloride
- nr
without β-mercaptoethanol
- PBS
phosphate buffered saline
- PI
protease inhibitors
- r
with β-mercaptoethanol
- RBC
red blood cell
- RTS,S
RTS,S/AS01 malaria vaccine targets
- SDS
sodium dodecyl sulfate
- SMFA
standard membrane mosquito feed assay
- TBS-T
Tris-buffered saline and 0.1% Tween 20
- TRA
transmission reducing activity
- TRA
mAbs IgG with significant TRA at ≤ 375 µg/ml
- Tris-Cl
tris(hydroxymethyl) aminomethane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers
The opinions and assertions expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. or the Department of Defense. The authors do not have a financial interest in any commercial product, service, or organization providing financial support for this research.
Conflict of Interest
The authors have no conflicts of interest.
References
- 1.Ayanful-Torgby R et al. Persistent Plasmodium falciparum infections enhance transmission-reducing immunity development. Sci Rep 11, 21380 (2021). 10.1038/s41598-021-00973-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andagalu B et al. Malaria Transmission Dynamics in High Transmission Setting of Western Kenya and the Inadequate Treatment Response to Artemether-lumefantrine in an Asymptomatic Population. Clin Infect Dis (2022). 10.1093/cid/ciac527 [DOI] [PMC free article] [PubMed]
- 3.Ouedraogo AL et al. Dynamics of the Human Infectious Reservoir for Malaria Determined by Mosquito Feeding Assays and Ultrasensitive Malaria Diagnosis in Burkina Faso. J Infect Dis 213, 90–99 (2016). 10.1093/infdis/jiv370 [DOI] [PubMed] [Google Scholar]
- 4.Goncalves BP et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8, 1133 (2017). 10.1038/s41467-017-01270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rek J et al. Asymptomatic school-aged children are important drivers of malaria transmission in a high endemicity setting in Uganda. J Infect Dis (2022). 10.1093/infdis/jiac169 [DOI] [PMC free article] [PubMed]
- 6.Zavala F RTS,S: the first malaria vaccine. J Clin Invest 132 (2022). 10.1172/JCI156588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rts SCTP Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 (2015). 10.1016/S0140-6736(15)60721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley EA et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371, 411–423 (2014). 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes KI et al. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J Infect Dis 197, 1605–1613 (2008). 10.1086/587645 [DOI] [PubMed] [Google Scholar]
- 10.Rener J, Graves PM, Carter R, Williams JL & Burkot TR Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med 158, 976–981 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen AN et al. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med 162, 1460–1476 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quakyi IA et al. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol 139, 4213–4217 (1987). [PubMed] [Google Scholar]
- 13.Miller LH, Duffy PE & Culleton R Transmission-Blocking Vaccines: From Conceptualization to Realization. Am J Trop Med Hyg (2022). 10.4269/ajtmh.22-0023 [DOI] [PubMed]
- 14.Acquah FK, Adjah J, Williamson KC & Amoah LE Transmission-Blocking Vaccines: Old Friends and New Prospects. Infect Immun 87 (2019). 10.1128/IAI.00775-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar N Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol 9, 321–335 (1987). [DOI] [PubMed] [Google Scholar]
- 16.Kaslow DC et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333, 74–76 (1988). [DOI] [PubMed] [Google Scholar]
- 17.Kocken CH et al. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol 61, 59–68 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Eksi S et al. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Molecular Microbiology 61, 991–998 (2006). https://doi.org:MMI5284 [pii] 10.1111/j.1365-2958.2006.05284.x [DOI] [PubMed] [Google Scholar]
- 19.Eksi S & Williamson KC Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PfB0400w. Molecular and Biochemical Parasitology 122, 127–130 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Gardner MJ et al. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science 282, 1126–1132 (1998). [DOI] [PubMed] [Google Scholar]
- 21.van Schaijk BC et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Molecular & Biochemical Parasitology 149, 216–222 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Duffy PE & Kaslow DC A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun 65, 1109–1113 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canepa GE et al. Antibody targeting of a specific region of Pfs47 blocks Plasmodium falciparum malaria transmission. NPJ Vaccines 3, 26 (2018). 10.1038/s41541-018-0065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura K et al. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun 81, 4377–4382 (2013). 10.1128/IAI.01056-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22, 1051–1068 (2008). 10.1101/gad.1656508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tripathi AK et al. Plasmodium falciparum Pf77 and male development gene 1 as vaccine antigens that induce potent transmission-reducing antibodies. Sci Transl Med 13 (2021). 10.1126/scitranslmed.abg2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinglasan RR et al. Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proceedings of the National Academy of Sciences of the United States of America 104, 13461–13466 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaslow DC Efforts to Develop Pfs25 Vaccines. Am J Trop Med Hyg (2022). 10.4269/ajtmh.21-1326 [DOI] [PubMed]
- 29.Zou L, Miles AP, Wang J & Stowers AW Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine 21, 1650–1657 (2003). 10.1016/s0264-410x(02)00701-6 [DOI] [PubMed] [Google Scholar]
- 30.MacDonald NJ et al. Structural and Immunological Characterization of Recombinant 6-Cysteine Domains of the Plasmodium falciparum Sexual Stage Protein Pfs230. J Biol Chem 291, 19913–19922 (2016). 10.1074/jbc.M116.732305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy PE The Virtues and Vices of Pfs230: From Vaccine Concept to Vaccine Candidate. Am J Trop Med Hyg (2022). 10.4269/ajtmh.21-1337 [DOI] [PubMed]
- 32.Singh SK et al. A Reproducible and Scalable Process for Manufacturing a Pfs48/45 Based Plasmodium falciparum Transmission-Blocking Vaccine. Front Immunol 11, 606266 (2020). 10.3389/fimmu.2020.606266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauerwein RW, Plieskatt J & Theisen M 40 Years of Pfs48/45 Research as a Transmission-Blocking Vaccine Target of Plasmodium falciparum Malaria. Am J Trop Med Hyg (2022). 10.4269/ajtmh.21-1320 [DOI] [PubMed]
- 34.Duffy PE Transmission-Blocking Vaccines: Harnessing Herd Immunity for Malaria Elimination. Expert Rev Vaccines 20, 185–198 (2021). 10.1080/14760584.2021.1878028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molina-Cruz A & Barillas-Mury C Pfs47 as a Malaria Transmission-Blocking Vaccine Target. Am J Trop Med Hyg (2022). 10.4269/ajtmh.21-1325 [DOI] [PubMed]
- 36.Healy SA et al. Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J Clin Invest 131 (2021). 10.1172/JCI146221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy PE & Patrick Gorres J Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines 5, 48 (2020). 10.1038/s41541-020-0196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLeod B et al. Potent antibody lineage against malaria transmission elicited by human vaccination with Pfs25. Nat Commun 10, 4328 (2019). 10.1038/s41467-019-11980-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scally SW et al. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nat Commun 8, 1568 (2017). 10.1038/s41467-017-01924-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stura EA et al. Crystallization of an intact monoclonal antibody (4B7) against Plasmodium falciparum malaria with peptides from the Pfs25 protein antigen. Acta Crystallogr D Biol Crystallogr 50, 556–562 (1994). 10.1107/S0907444994001782 [DOI] [PubMed] [Google Scholar]
- 41.Williamson KC, Fujioka H, Aikawa M & Kaslow DC Stage-specific processing of Pfs230, a Plasmodium falciparum transmission-blocking vaccine candidate. Mol Biochem Parasitol 78, 161–169 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Vincent AA, Fanning S, Caira FC & Williamson KC Immunogenicity of malaria transmission-blocking vaccine candidate, y230.CA14 following crosslinking in the presence of tetanus toroid. Parasite Immunology 21, 573–581 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Coelho CH et al. A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes. Nat Commun 12, 1750 (2021). 10.1038/s41467-021-21955-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh K et al. Structure and function of a malaria transmission blocking vaccine targeting Pfs230 and Pfs230-Pfs48/45 proteins. Commun Biol 3, 395 (2020). 10.1038/s42003-020-01123-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang WK et al. A human antibody epitope map of Pfs230D1 derived from analysis of individuals vaccinated with a malaria transmission-blocking vaccine. Immunity 56, 433–443 e435 (2023). 10.1016/j.immuni.2023.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone WJR et al. Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity. Nat Commun 9, 558 (2018). 10.1038/s41467-017-02646-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barr PJ et al. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med 174, 1203–1208 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miura K et al. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PLoS One 8, e57909 (2013). 10.1371/journal.pone.0057909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acquah FK et al. Antibody responses to two new Lactococcus lactis-produced recombinant Pfs48/45 and Pfs230 proteins increase with age in malaria patients living in the Central Region of Ghana. Malar J 16, 306 (2017). 10.1186/s12936-017-1955-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Read D et al. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol 16, 511–519 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Wizel B & Kumar N Identification of a continuous and cross-reacting epitope for Plasmodium falciparum transmission-blocking immunity. Proc Natl Acad Sci U S A 88, 9533–9537 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Barragan MJ et al. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics 12, 587 (2011). 10.1186/1471-2164-12-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eksi S et al. Targeting and sequestration of truncated Pfs230 in an intraerythrocytic compartment during Plasmodium falciparum gametocytogenesis. Molecular Microbiology 44, 1507–1516 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Arredondo SA & Kappe SHI The s48/45 six-cysteine proteins: mediators of interaction throughout the Plasmodium life cycle. Int J Parasitol 47, 409–423 (2017). 10.1016/j.ijpara.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tonkin ML et al. Structural and biochemical characterization of Plasmodium falciparum 12 (Pf12) reveals a unique interdomain organization and the potential for an antiparallel arrangement with Pf41. J Biol Chem 288, 12805–12817 (2013). 10.1074/jbc.M113.455667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lennartz F et al. Structural basis for recognition of the malaria vaccine candidate Pfs48/45 by a transmission blocking antibody. Nat Commun 9, 3822 (2018). 10.1038/s41467-018-06340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kundu P et al. Structural delineation of potent transmission-blocking epitope I on malaria antigen Pfs48/45. Nat Commun 9, 4458 (2018). 10.1038/s41467-018-06742-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williamson KC, Keister DB, Muratova O & Kaslow DC Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasitol 75, 33–42 (1995). [DOI] [PubMed] [Google Scholar]
- 59.Tachibana M et al. Identification of domains within Pfs230 that elicit transmission blocking antibody responses. Vaccine 37, 1799–1806 (2019). 10.1016/j.vaccine.2019.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lyons FMT, Gabriela M, Tham WH & Dietrich MH Plasmodium 6-Cysteine Proteins: Functional Diversity, Transmission-Blocking Antibodies and Structural Scaffolds. Front Cell Infect Microbiol 12, 945924 (2022). 10.3389/fcimb.2022.945924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muthui MK et al. Characterization of Naturally Acquired Immunity to a Panel of Antigens Expressed in Mature P. falciparum Gametocytes. Front Cell Infect Microbiol 11, 774537 (2021). 10.3389/fcimb.2021.774537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blagborough AM & Sinden RE Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine 27, 5187–5194 (2009). 10.1016/j.vaccine.2009.06.069 [DOI] [PubMed] [Google Scholar]
- 63.Rawlings DJ et al. Alpha-tubulin II is a male-specific protein in Plasmodium falciparum. Mol Biochem Parasitol 56, 239–250 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legends
Figure S1 Monoclonal (mAb) sexual stage parasite reactivity. Immunoblots of purified gamete/zygote (gm) Triton X-100 extract with (r) and without (nr) β-mercaptoethanol (A&B) and purified gametocyte (gc) Triton X-100 extract without β-mercaptoethanol (B) were probed with the indicated mAb and the bands visualized with 5-Bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro blue tetrazolium (NBT).
Figure S2 Gamete/zygote surface reactivity of immunoblot negative monoclonals (mAbs). Purified wild-type strain NF54 (wt) or Pfs230Δ452–3135 (Pfs230Δ) gametes/zygotes were incubated with the indicated mAb, washed, and then stained with Alexa Fluor 488-labeled anti-mouse IgG (488) and membrane potential dye DiIC1(5). Fluorescence was monitored using flow cytometry and fluorescence microscopy. For each antibody and gamete/zygote population, the DiIC1(5) and Alex Fluor 488 fluorescence of each cell is plotted and representative fluorescent and bright field images are shown. Pfs230-specific mAb 1B3 and Pfs25-specific mAb 4B7 are included as controls.
Figure S3 Peptides bound by monoclonal (mAb) 4B7. The fluorescence intensity of mAb 4B7 binding to two sets of peptides corresponding to Pfs25. One set was linear fifteen aa peptides with an offset of one residue (orange) and the other set was looped by adding cysteine residues at both ends of the one aa offset 15 aa Pfs25 peptides to allow disulfide binding (cyan).


