Abstract
Background:
Chronic atrophic gastritis is a premalignant lesion with a high risk of developing into gastric cancer. Rhein is a key active ingredient of several traditional Chinese medicines with multiple pharmacological effects. Nevertheless, the role of rhein in chronic atrophic gastritis is unclear.
Methods:
Helicobacter pylori infection was used to establish chronic atrophic gastritis in a mouse model. Murine gastric mucosa treated with saline or rhein was used in experiments. Hematoxylin and eosin staining and Alcian blue-periodic acid-Schiff staining were utilized for histopathological observation of murine gastric mucosa. The levels of proinflammatory factors were detected by enzyme-linked immunosorbent assay, and oxidative stress-associated markers were detected by commercially available assay kits. Western blotting was used for measuring the levels of nuclear factor, erythroid 2-like bZIP transcription factor 2, and mitogen-activated protein kinase signaling-related proteins.
Results:
Rhein mitigated the gastric mucosal injury and suppressed inflammation and oxidative stress in H. pylori-infected chronic atrophic gastritis mouse models. Rhein inactivated mitogen-activated protein kinase and activated erythroid 2-like bZIP transcription factor 2 signaling in gastric mucosa of mice with chronic atrophic gastritis.
Conclusion:
Rhein exhibits anti-inflammatory and antioxidant effects in chronic atrophic gastritis via erythroid 2-like bZIP transcription factor 2 and mitogen-activated protein kinase signaling.
Keywords: Chronic atrophic gastritis, inflammation, MAPK signaling, Nrf2, rhein
Main Points
Rhein mitigates Helicobacter pylori-induced gastric mucosal injury.
Rhein restrains inflammation in gastric mucosa of chronic atrophic gastritis (CAG) mouse models.
Rhein inhibits oxidative stress in gastric mucosa of mice with CAG.
Rhein inactivates mitogen-activated protein kinase and activates erythroid 2-like bZIP transcription factor 2 signaling pathways.
Introduction
Chronic atrophic gastritis (CAG) is a chronic digestive disorder characterized by atrophy of gastric mucosa epithelium and glands, reduced number of glands, thinning of gastric mucosa, thickening of mucosal base layer, or accompanied by pyloric and intestinal metaplasia.1 Most cases of CAG do not exhibit obvious clinical manifestations, and patients with CAG might suffer from gastric cavity fullness, pain, indigestion, belching, and anemia.2 Chronic atrophic gastritis is a disease with multiple pathogenic factors, including Helicobacter pylori (H. pylori) infection, poor dietary habits, environmental factors, and genetic factors.3 Among these factors, H. pylori infection is the main cause of CAG and other gastric disorders.4 H. pylori is a gram-negative, spiral bacterium that colonizes the human stomach.5 In view of this, H. pylori infection was used to establish the CAG in mouse model. Chronic atrophic gastritis is a premalignant lesion that is strongly associated with an elevated risk of gastric cancer.6 Hence, finding a novel effective approach for treating CAG is of great value.
Gastritis is defined as gastric mucosal inflammation.7 Mounting evidence has validated that sustained inflammatory response of gastric mucosa is the major inducement of CAG.8,9 Inflammation is characterized by increased levels of proinflammatory cytokines like interleukin-1 beta (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α), and cyclooxygenase-2 (COX-2).10,11 Prostaglandin E2 (PGE2) is a downstream product of COX-2 and also plays a significant role in the regulation of inflammation.12 Moreover, oxidative stress is considered to be a crucial factor in the pathogenesis of CAG.13 For example, fermented kimchi can ameliorate CAG by attenuating H pylori-related oxidative stress and endoplasmic reticulum stress.14 The major indicators of oxidative stress include oxidants like malondialdehyde (MDA) and antioxidants like superoxide dismutase (SOD).15
Rhein (4,5-dihydroxyanthraquinone-2-carboxylic acid) is the major component of several traditional Chinese medicines, including Sennae folium, rhubarb, and aloe, which have been indicated to alleviate edema, inflammation, and oxidative stress in multiple disorders.16 For example, rhein was reported to exert a protective effect on uric acid nephropathy by suppressing the inflammatory response.17 Additionally, rhein mitigates β-amyloid-induced oxidative stress in Alzheimer’s disease by promoting SIRT1/PGC-1α signaling activation.18 Rhein was also reported to be a critical ingredient of San-Huang-Xie-Xin-Tang, which was used for treating gastritis.19 The above evidence suggests that rhein might exert a gastroprotective impact on gastric injury. However, whether rhein has an effect on CAG is unclear. It was reported that rhein is able to inhibit lipopolysaccharide (LPS)-induced inflammation and oxidative stress in an intestinal barrier injury rat model via nuclear factor, erythroid 2 (NFE2)-like bZIP transcription factor 2 (Nrf2, also known as NFE2L2), and mitogen-activated protein kinase (MAPK) pathways.20 Furthermore, activation of Nrf2 and MAPK signaling has been demonstrated to be involved in affecting gastric injury.21,22
This study aimed to probe the function and potential mechanism of rhein in H. pylori-infected CAG mouse models. It was hypothesized that rhein might have an effect on inflammation and oxidative stress in CAG by regulating Nrf2 and MAPK pathways. Our findings might provide a new option for treating CAG.
Materials and Methods
H. pylori Culture
H. pylori strain ATCC43504 was obtained from American Type Culture Collection (Manassas, Va, USA) and cultured as previously described.23 The bacteria were incubated on trypticase soy (TS) agar plates supplemented with 5% sheep blood at 37°C for 3 days under microaerophilic conditions (CampyGen Atmosphere Generation Systems, Thermo Scientific, Vienna, Austria). Then, the bacteria were collected in clean TS broth, subjected to centrifugation at 3000 g for 5 minutes, and resuspended in the broth at 109 colony-forming units (CFUs)/mL. Cultures incubated for 72 hours on plates were used in the following experiments.
Animal Models and Drug Treatment
Totally 40 male C57BL/6 mice (5-week-old) were purchased from HFK Bioscience (Beijing, China). The CAG mouse model was established according to previous studies.1,23 The mice were randomly divided into 4 groups: sham + normal saline (NS), sham + rhein, CAG + NS, and CAG + rhein (n = 10 per group). To establish the CAG mouse model, mice in the CAG groups were treated with H. pylori infection combined with high-salt diet. In brief, the mice were injected intraperitoneally with pantoprazole (20 mg/kg, 3 times/week; Pacific Pharma, Korea) as proton pump inhibitor to enhance the successful colonization of H. pylori by reducing gastric acid. Afterward, gastric intubation needles were used to inoculate the stomach of the mice with 108 CFUs/mL H. pylori suspension or an equal volume (0.1 mL) of clean TS broth (4 times/week, for 24 weeks). In parallel, the mice were fed with high-salt diet containing 8% NaCl (AIN-76A; Biogenomics, Seongnam, Korea). Mice in the sham groups received the same procedure without H. pylori infection and high-salt diet. Rhein (purity ≥ 98%) was purchased from Sigma-Aldrich (St. Louis, Mo, USA), and its chemical structure was shown in Figure 1. Twenty-four weeks later, mice in the sham + rhein and the CAG + rhein groups were orally administrated with 100 mg/kg rhein (once daily, for 8 weeks)16,24 and mice in the sham + NS and the CAG + NS groups received saline treatment. All animal experiments were approved by the Animal Ethics Committee of Wuhan Myhalic Biotechnology Co., Ltd (No: 20210617088) and complied with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Figure 1.
Chemical structure of rhein.
Sample Collection
The drug was stopped after 8 weeks, and mice were fasted for 24 hours before the end of the experiment. Mice were anesthetized with sodium pentobarbital (50 mg/kg) by intraperitoneal injection. Blood samples were collected from the abdominal aorta with a syringe, injected into the blood collection tube for coagulation, and centrifuged at 3000 rpm for 15 minutes. Then, the serum was harvested and stored at –70°. Mice were then sacrificed by cervical dislocation under anesthesia, and murine gastric mucosa was collected. A portion of gastric mucosa was fixed with formalin for histological analysis and the remaining was stored at –80° for further experiments.
Hematoxylin and Eosin Staining
Murine gastric mucosa was fixed with 10% formalin and embedded in paraffin. Tissue sections of 4 µm thickness were dewaxed with xylene solution, hydrated with ethanol gradient, and subsequently stained with hematoxylin and eosin solution (Sigma-Aldrich). After being washed with 95% ethanol for 1 minute and xylene for 3 times, the slices were sealed with neutral gum and a microscope (Nikon, Tokyo, Japan) was used for histopathological observation. The inflammation score was used to show the level of inflammatory cell infiltration from 0 (none) to 3 (all mucosa). The erosion score was defined as proportion of erosive lesions from 0 (none) to 3 (all mucosa). Pathological score of hematoxylin and eosin (HE) was computed as erosion sore + inflammation score.23
Alcian Blue-Periodic Acid-Schiff) Staining
Murine gastric tissue sections were stained with Alcian blue (Sigma-Aldrich) dropwise for 10 minutes, treated with 0.5% periodic acid solution, and washed in running water. After being washed twice in distilled water, tissue sections were drip-stained with Schiff reagent (Sigma-Aldrich) for 20 minutes away from light. Then, the sections were rinsed and dried. After that, Mayer hematoxylin was used to stain the nucleus lightly for 1 minute. Eventually, the slices were rinsed, dehydrated, sealed with neutral gum, and observed under a microscope (Nikon) for analyzing mucous metaplasia.
Enzyme-Linked Immunosorbent Assay
Concentrations of proinflammatory factors (TNF-α, COX-2, IL-6, and IL-1β) in gastric mucosa and PGE2 in murine serum were detected by the following mouse enzyme-linked immunosorbent assay kits: TNF-α (ab100747; Abcam, Cambridge, UK), COX-2 (SEKM-0156; Solarbio, Beijing, China), IL-6 (BMS603-2; Invitrogen, Carlsbad, Calif, USA), IL-1β (ab197742, Abcam), PGE2 (SEKM-0173, Solarbio), respectively, according to the instructions of manufacturers.
Measurement of Oxidative Stress-Related Markers
Levels of oxidative stress-associated markers were measured by commercially available assay kits. Myeloperoxidase (MPO) activity and contents of MDA and SOD were determined by MPO assay kit (A044-1-1, colorimetric method), MDA assay kit (A003-1-1, TBA method), and SOD assay kit (A001-3-2, WST-1 method) (all from Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively, following the manufacturer’s protocols.
Western Blotting
Proteins were isolated from murine gastric mucosa using Radio Immunoprecipitation Assay (RIPA) lysis buffer (Beyotime, Shanghai, China) supplemented with 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). After incubation for 30 minutes and centrifugation at 12 000 g at 4°C for 15 minutes, the supernatants were harvested, and the proteins were quantified with a bicinchoninic acid (BCA) assay kit (Beyotime). Subsequently, protein samples (30 µg) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to polyvinylidene fluoride membranes (Invitrogen), which were then blocked with 5% defatted milk, and incubated at 4°C overnight with the primary antibodies as follows: anti-Nrf2 (ab92946, 1:1000), anti-Heme oxygenase 1 (HO-1) (ab52947, 1:2000), anti-p-Jun N-terminal kinase (JNK)1/2 (ab124956, 1:1000), anti-JNK1/2 (ab179461, 1:1000), anti-p-p38 (ab195049, 1:1000), anti-p38 (ab170099, 1:1000), and anti-β-actin (ab8227, 1:1000) (all from Abcam). Afterward, the membranes were incubated with the secondary antibody (ab288151, Abcam). Eventually, protein bands were visualized with an enhanced chemiluminescence (ECL) detection kit (Cwbiotech, Beijing, China) and quantified with Image Lab 3.0 software (Bio-Rad, Hercules, Calif, USA).
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences 16.0 software (SPSS Inc.; Chicago, IL, USA). Data are presented as the mean ± standard deviation. Student’s t-test was utilized for 2 group comparisons, while analysis of variance followed by Tukey’s post hoc analysis was used for multiple group comparisons. The value of P < .05 was regarded as statistically significant.
Results
Rhein Mitigates Gastric Mucosal Injury
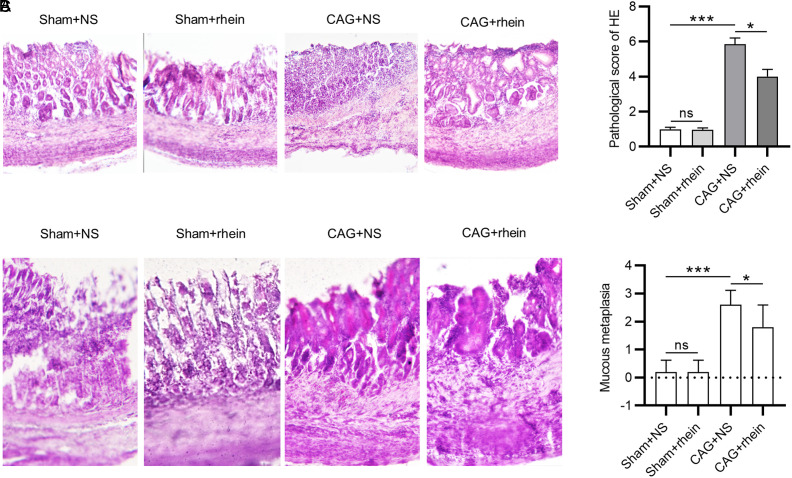
We first examined the effect of rhein on the pathology of gastric mucosa of mice with CAG. As shown by HE staining, compared with the sham group, mice in the CAG + NS group exhibited a disordered structure of gastric mucosal epithelium, a reduction in glands and sparse arrangement, accompanied by inflammatory cell infiltration (Figure 2A). Intriguingly, it was displayed that the pathological conditions of gastric mucosa were improved after rhein treatment (Figure 2A and 2B). A similar trend was observed in the results of Alcian blue-periodic acid-Schiff staining. The CAG + NS group displayed evident mucous metaplasia relative to the sham group, which was ameliorated in the rhein-treated CAG mice (Figure 2C and 2D). These suggest that rhein can mitigate H. pylori-induced gastric mucosal injury in mice with CAG.
Figure 2.
Rhein mitigates gastric mucosal injury. (A-B). HE staining for evaluating the histopathological changes of murine gastric mucosa in each group. (C-D). AB-PAS staining for assessing the changes in mucous metaplasia of murine gastric mucosa. *P < .05, ***P < .001. HE, hematoxylin and eosin; AB-PAS, Alcian blue-periodic acid-Schiff.
Rhein Restrains the Inflammation in Murine Gastric Mucosa
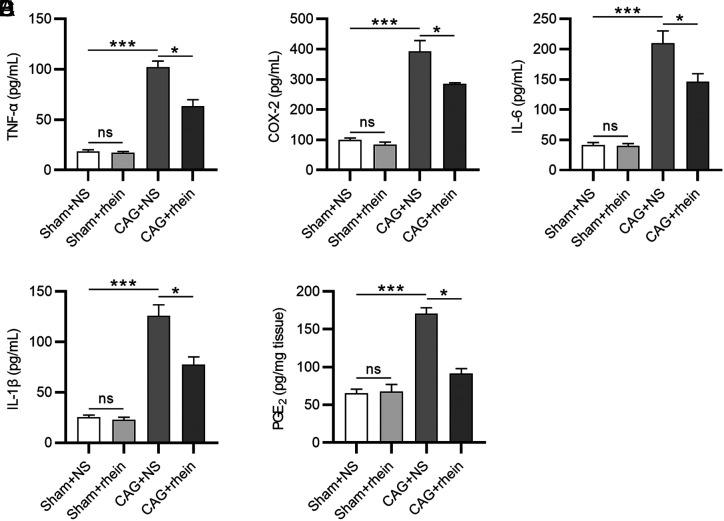
Next, we tested whether rhein influenced the inflammatory response in CAG. Enzyme-linked immunosorbent assay was used to assess the levels of proinflammatory cytokines. As revealed by the results, high levels of proinflammatory cytokines (TNF-α, COX-2, IL-6, and IL-1β) were shown in the CAG + NS group, while rhein treatment led to a decrease in the cytokine levels (Figure 3A-D). Then, we evaluated the production of serum PGE2, a downstream product of COX-2. In comparison to the sham group, the level of serum PGE2 was evidently higher in the CAG + NS group and rhein treatment was shown to reduce its level (Figure 3E), indicating that rhein greatly suppresses the inflammation in gastric mucosa infected by H. pylori.
Figure 3.
Rhein restrains the inflammatory response in murine gastric mucosa. (A-E). ELISA for assessing concentrations of proinflammatory cytokines (TNF-α, COX-2, IL-6, and IL-1β) in murine gastric mucosa and PGE2 in murine serum. *P < .05, ***P < .001. ELISA, enzyme-linked immunosorbent assay; IL, interleukin; TNF- α, tumor necrosis factor-alpha; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2.
Rhein Inhibits Oxidative Stress in the Gastric Mucosa
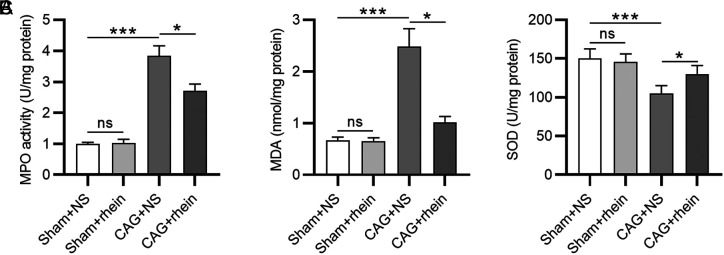
We subsequently explored the function of rhein in regulating oxidative stress in CAG. Myeloperoxidase is a peroxidase enzyme that catalyzes the release of cytotoxic hypochlorite and other chlorinated species, resulting in potential oxidative stress.25 As a result, MPO activity was evaluated by colorimetric method, which showed that MPO activity was remarkedly increased in CAG group compared to that in the sham group (Figure 4A). However, the increased MPO activity was reduced by treatment of rhein (Figure 4A). After that, we assessed the levels of oxidant MDA and antioxidant SOD. Notably, the gastric mucosa of mice with CAG showed elevated production of MDA and decreased production of SOD compared with those of the normal mice (Figure 4B and 4C). The altered levels of MDA and SOD were both reversed after rhein treatment, suggesting that rhein can mitigate H. pylori-induced oxidative stress in mice with CAG.
Figure 4.
Rhein inhibits oxidative stress in the gastric mucosa. (A-C). Measurement of MPO, MDA, and SOD levels in murine gastric mucosa. *P < .05, ***P < .001. MPO, myeloperoxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
Rhein Activates Erythroid 2-Like bZIP Transcription Factor 2 and Inactivates Mitogen-Activated Protein Kinase Signaling Pathways
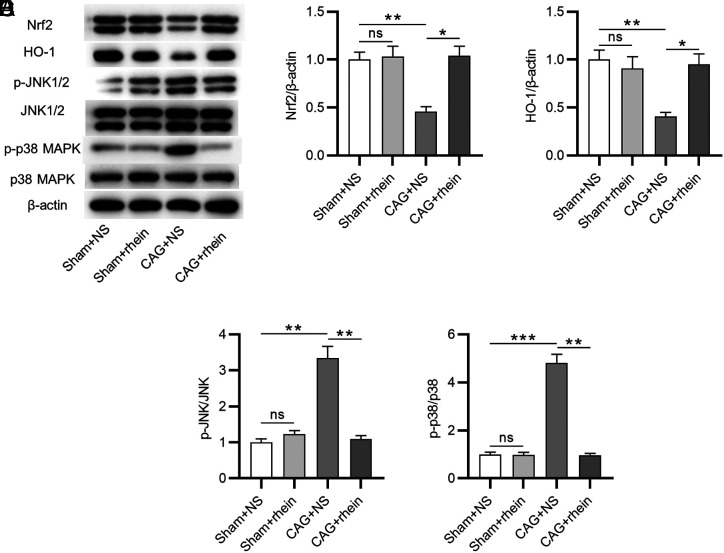
To reveal the potential mechanism of rhein underlying CAG, we examined whether rhein had an effect on Nrf2 and MAPK pathways. Western blotting was utilized to evaluate the levels of Nrf2 and MAPK signaling-associated proteins (Figure 5A). As displayed by the results, Nrf2 and HO-1 protein levels were markedly reduced in CAG + NS group (Figure 5B and 5C). However, the effects were shown to be partially reversed after rhein treatment (Figure 5B and 5C), suggesting that rhein activates Nrf2 signaling in CAG. Next, we analyzed the protein levels of MAPK family members JNK and p38. Compared with the sham group, the ratios of phosphorylated (p)-JNK1/2 to total JNK1/2 and p-p38 to total p38 in the CAG + NS group were all increased; however, the increased levels were markedly reduced after treatment of rhein (Figure 5D and 5E). This indicates that pretreatment of rhein in murine gastric mucosa can inactivate MAPK signaling.
Figure 5.
Rhein inactivates MAPK signaling and activates Nrf2 signaling. (A-E). Western blotting for evaluating the levels of Nrf2 and MAPK signaling-associated proteins in murine gastric mucosa. *P < .05, **P < .01, ***P < .001. MAPK, mitogen-activated protein kinase; Nrf2, erythroid 2-like bZIP transcription factor 2.
Discussion
Chronic atrophic gastritis is a precancerous lesion that has a high risk of developing into gastric cancer.26 H. pylori infection is regarded as the major inducement of CAG.27 Previous studies have demonstrated that H. pylori infection induces oxidative stress and inflammation in gastric mucosa, consequently resulting in adverse gastric mucosal injury.14,28 Thus, we established H. pylori-infected mouse models of CAG in this study. Rhein, a natural polymer with high safety, has proven anti-inflammatory, anti-edema, and antibacterial capabilities.29 Owing to its wide properties, rhein has been used in multiple diseases. For example, rhein attenuates LPS-induced injury by inhibiting the inflammatory response in intestinal epithelial cells.30 Importantly, rhein was reported to suppress ethanol-induced inflammation and oxidative stress in gastric ulcer, exhibiting a gastroprotective effect.13 In the present study, rhein was shown to improve the disordered structure and mucous metaplasia of gastric mucosa in CAG mice induced by H. pylori, confirming that rhein exerts a protective role in CAG.
Inflammation is featured with enhanced production of proinflammatory factors including TNF-α, COX-2, IL-6, and IL-1β.31 Additionally, COX-2 participates in the conversion of arachidonic acid into PGH2, a precursor of PGE2 which further promotes the inflammatory response in tissues.32 In accordance with previous evidence, it was found in this study that rhein treatment reversed the elevated secretion of proinflammatory factors in gastric mucosa of mice with CAG, indicating that rhein has an anti-inflammatory effect on H. pylori-induced inflammation in mice with CAG. Furthermore, MPO has been indicated to mediate oxidative stress and function as a biomarker of neutrophil infiltration.33 Neutrophil infiltration plays a pivotal role in the development of oxidative stress-induced inflammatory response.34 Overproduction of MPO and its mediated oxidative stress can contribute to inflammation and lead to severe tissue damage.25 In this study, MPO activity in gastric mucosa of mice with CAG was significantly high but was decreased upon rhein treatment. An imbalance between the production of oxidants and antioxidants results in oxidative stress, which exacerbates the condition of CAG.35 In the current study, rhein-treated CAG mice displayed a decreased level of the oxidant MDA and an increased level of the antioxidant SOD compared to those of NS-treated mice. Collectively, the above results demonstrate that rhein can alleviate the oxidative stress in gastric mucosa of mice with CAG.
Emerging evidence has indicated that inflammation and oxidative stress in gastric injury are mediated by various signaling pathways.36,37 A previous study showed that rhein plays anti-inflammatory and antioxidant roles in intestinal injury via Nrf2 and MAPK pathways.20 Erythroid 2-like bZIP transcription factor 2 is known as transcription factor that exerts a significant effect on cellular defense against H. pylori infection-induced injury including oxidative stress.23 Upon oxidative stress, Nrf2 can bind to antioxidant response element and regulate the expression of antioxidant genes like HO-1.20 Moreover, previous studies have demonstrated that the Nrf2/HO-1 signaling pathway-related oxidative stress is involved in inflammation and apoptosis of cells.38,39 Here, we found that H. pylori induced the downregulation of Nrf2 and HO-1 proteins in murine gastric mucosa. Nevertheless, treatment of rhein effectively restrained the above effects caused by H. pylori. Furthermore, the impact of rhein on MAPK signaling pathway was also tested. Anti-p-Jun N-terminal kinase and p38 MAPK are key members of the MAPK family which have been indicated to modulate diverse cellular activities, including inflammatory response.40 Results from this study revealed that rhein reversed H. pylori infection-induced phosphorylation of JNK and p38 in gastric mucosal tissues of mice with CAG. Hence, the above results indicated that rhein can inactivate MAPK signaling and activate Nrf2 signaling in murine gastric mucosa.
However, there are some limitations to this study. The study included a limited number of animals. Future studies with larger sample size and prospective trials are needed. Additionally, more signaling pathways that might be mediated by rhein in CAG need to be further investigated in future studies.
In conclusion, we explored the function and potential mechanism of rhein in regulating inflammation and oxidative stress in a CAG mouse model. The results reveal that rhein can mitigate gastric mucosal injury and suppress inflammation and oxidative stress in murine gastric mucosa by activating Nrf2 and inactivating MAPK signaling pathways. Our findings might provide a new option for CAG therapy. Translational medicine research or clinical trials upon rhein are needed in the future.
Footnotes
Ethics Committee Approval: The study was approved by the Animal Ethics Committee of Wuhan Myhalic Biotechnology Co., Ltd (No: 20210617088).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.L., L.S.; Design – S.L., L.S., Z.h.S; Supervision – J.y.Y, Y.L., S.Z, N.W.; Resources – S.L., L.S., Z.h.S; Materials – S.L., L.S., Z.h.S; Data Collection and/or Processing – S.L., L.S., Z.h.S; Analysis and/or Interpretation – S.L., L.S., Z.h.S; Literature Search – S.L., L.S., Z.h.S; Writing Manuscript – S.L., L.S., Z.h.S; Critical Review – S.L., L.S., Z.h.S.
Acknowledgments: The authors would like to thank all participants who contribute to this work.
Declaration of Interests: The authors have no conflicts of interest to declare.
Funding: The work was supported by the Medical Research Project of Wuhan Health Commission (No. WZ21C21).
References
- 1. Jiang JY, Liu DJ, Liu MX. The protective effect of NF-κB signaling pathway inhibitor PDTC on mice with chronic atrophic gastritis. Scand J Gastroenterol. 2021;56(10):1131 1139. ( 10.1080/00365521.2021.1953130) [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Xia R, Zhang B, Li C. Chronic atrophic gastritis: a review. J Environ Pathol Toxicol Oncol. 2018;37(3):241 259. ( 10.1615/JEnvironPatholToxicolOncol.2018026839) [DOI] [PubMed] [Google Scholar]
- 3. Ji Q, Yang Y, Song X, Han X, Wang W. Banxia Xiexin Decoction in the treatment of chronic atrophic gastritis: a protocol for systematic review and meta-analysis. Medicine. 2020;99(42):e22110. ( 10.1097/MD.0000000000022110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bravo D, Hoare A, Soto C, Valenzuela MA, Quest AF. Helicobacter pylori in human health and disease: mechanisms for local gastric and systemic effects. World J Gastroenterol. 2018;24(28):3071 3089. ( 10.3748/wjg.v24.i28.3071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ge Z, Taylor DE. Contributions of genome sequencing to understanding the biology of Helicobacter pylori. Annu Rev Microbiol. 1999;53:353 387. ( 10.1146/annurev.micro.53.1.353) [DOI] [PubMed] [Google Scholar]
- 6. Zhao X, Wu M, Zhang D.et al. The relationship of interpersonal sensitivity and depression among patients with chronic atrophic gastritis: the mediating role of coping styles. J Clin Nurs. 2018;27(5-6):e984 e991. ( 10.1111/jocn.14114) [DOI] [PubMed] [Google Scholar]
- 7. Rugge M, Pennelli G, Pilozzi E.et al. Gastritis: the histology report. Dig Liver Dis. 2011;43(suppl 4):S373 S384. ( 10.1016/S1590-8658(11)60593-8) [DOI] [PubMed] [Google Scholar]
- 8. Bockerstett KA, Lewis SA, Noto CN.et al. Single-cell transcriptional analyses identify lineage-specific epithelial responses to inflammation and metaplastic development in the gastric corpus. Gastroenterology. 2020;159(6):2116 2129.e4. ( 10.1053/j.gastro.2020.08.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vannella L, Lahner E, Annibale B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: a critical reappraisal. World J Gastroenterol. 2012;18(12):1279 1285. ( 10.3748/wjg.v18.i12.1279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dogan R, Sjostrand AP, Yenıgun A, Karatas E, Kocyigit A, Ozturan O. Influence of Ginkgo biloba extract (EGb 761) on expression of IL-1 beta, IL-6, TNF-alfa, HSP-70, HSF-1 and COX-2 after noise exposure in the rat cochlea. Auris Nasus Larynx. 2018;45(4):680 685. ( 10.1016/j.anl.2017.09.015) [DOI] [PubMed] [Google Scholar]
- 11. Tseng HC, Lin CC, Wang CY, Yang CC, Hsiao LD, Yang CM. Lysophosphatidylcholine induces cyclooxygenase-2-dependent IL-6 expression in human cardiac fibroblasts. Cell Mol Life Sci. 2018;75(24):4599 4617. ( 10.1007/s00018-018-2916-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107(4):391 397. ( 10.1111/cas.12901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang C, Gao F, Gan S.et al. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food Chem Toxicol. 2019;131:110539. ( 10.1016/j.fct.2019.05.047) [DOI] [PubMed] [Google Scholar]
- 14. Park JM, Han YM, Oh JY.et al. Fermented kimchi rejuvenated precancerous atrophic gastritis via mitigating Helicobacter pylori-associated endoplasmic reticulum and oxidative stress. J Clin Biochem Nutr. 2021;69(2):158 170. ( 10.3164/jcbn.20-180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan Y, Zhao X, Yu J.et al. Lead-induced oxidative damage in rats/mice: A meta-analysis. J Trace Elem Med Biol. 2020;58:126443. ( 10.1016/j.jtemb.2019.126443) [DOI] [PubMed] [Google Scholar]
- 16. Wu J, Wei Z, Cheng P.et al. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10(23):10665 10679. ( 10.7150/thno.43528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu J, Yang Z, Wu H, Wang D. Rhein attenuates renal inflammatory injury of uric acid nephropathy via lincRNA-Cox2/miR-150-5p/STAT1 axis. Int Immunopharmacol. 2020;85:106620. ( 10.1016/j.intimp.2020.106620) [DOI] [PubMed] [Google Scholar]
- 18. Yin Z, Geng X, Zhang Z, Wang Y, Gao X. Rhein relieves oxidative stress in an Aβ(1-42) oligomer-burdened neuron model by activating the SIRT1/PGC-1α-Regulated mitochondrial biogenesis. Front Pharmacol. 2021;12:746711. ( 10.3389/fphar.2021.746711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Hu H, Chen F, Lan K, Wang A. Reduced system exposures of total rhein and baicalin after combinatory oral administration of rhein, baicalin and berberine to beagle dogs and rats. J Ethnopharmacol. 2013;145(2):442 449. ( 10.1016/j.jep.2012.11.008) [DOI] [PubMed] [Google Scholar]
- 20. Zhuang S, Zhong J, Bian Y.et al. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs. Life Sci. 2019;216:168 175. ( 10.1016/j.lfs.2018.11.048) [DOI] [PubMed] [Google Scholar]
- 21. Song MY, Lee DY, Kim EH. Anti-inflammatory and anti-oxidative effect of Korean propolis on Helicobacter pylori-induced gastric damage in vitro . J Microbiol. 2020;58(10):878 885. ( 10.1007/s12275-020-0277-z) [DOI] [PubMed] [Google Scholar]
- 22. Chen K, Zhang L, Qu Z.et al. Uncovering the mechanisms and molecular targets of Weibing formula 1 against gastritis: coupling network pharmacology with GEO database. BioMed Res Int. 2021;2021:5533946. ( 10.1155/2021/5533946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park JM, Han YM, Park YJ, Hahm KB. Dietary intake of walnut prevented Helicobacter pylori-associated gastric cancer through rejuvenation of chronic atrophic gastritis. J Clin Biochem Nutr. 2021;68(1):37 50. ( 10.3164/jcbn.20-103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Fan S, Hu N.et al. Rhein Reduces Fat Weight in db/db Mouse and Prevents Diet-Induced Obesity in C57BL/6 Mouse through the Inhibition of PPARγ Signaling. PPAR Res. 2012;2012:374936. ( 10.1155/2012/374936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shao C, Yuan J, Liu Y.et al. Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc Natl Acad Sci U S A. 2020;117(19):10155 10164. ( 10.1073/pnas.1917946117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crafa P, Russo M, Miraglia C.et al. From Sidney to OLGA: an overview of atrophic gastritis. Acta Biomed. 2018;89(8-S):93 99. ( 10.23750/abm.v89i8-S.7946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lahner E, Carabotti M, Annibale B. Treatment of Helicobacter pylori infection in atrophic gastritis. World J Gastroenterol. 2018;24(22):2373 2380. ( 10.3748/wjg.v24.i22.2373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang W, Lu H, Graham DY. An update on Helicobacter pylori as the cause of gastric cancer. Gastrointest Tumors. 2014;1(3):155 165. ( 10.1159/000365310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang R, Wang D, Li H.et al. Preparation and characterization of Bletilla striata polysaccharide/polylactic acid composite. Molecules. 2019;24(11):2104. ( 10.3390/molecules24112104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo L, Liu Y, Cai X.et al. Bletilla striata polysaccharides ameliorates lipopolysaccharide-induced injury in intestinal epithelial cells. Saudi J Gastroenterol. 2019;25(5):302 308. ( 10.4103/sjg.SJG_520_18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang L, Zhou R, Tong Y.et al. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol Dis. 2020;140:104814. ( 10.1016/j.nbd.2020.104814) [DOI] [PubMed] [Google Scholar]
- 32. Liu F, Zhang X, Li Y.et al. Anti-inflammatory effects of a Mytilus coruscus α-d-glucan (MP-A) in activated macrophage cells via TLR4/NF-κB/MAPK pathway inhibition. Mar Drugs. 2017;15(9):294. ( 10.3390/md15090294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monteiro CES, Sousa JAO, Lima LM.et al. LASSBio-596 protects gastric mucosa against the development of ethanol-induced gastric lesions in mice. Eur J Pharmacol. 2019;863:172662. ( 10.1016/j.ejphar.2019.172662) [DOI] [PubMed] [Google Scholar]
- 34. Han B, Li S, Lv Y.et al. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019;10(9):5555 5565. ( 10.1039/c9fo01152h) [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Jin Z, Qin X, Zheng Q. Urinary metabolomics research for Huangqi Jianzhong Tang against chronic atrophic gastritis rats based on (1). J Pharm Pharmacol. 2020;72(5):748 760. ( 10.1111/jphp.13242) [DOI] [PubMed] [Google Scholar]
- 36. El Badawy SA, Ogaly HA, Abd-Elsalam RM, Azouz AA. Benzyl isothiocyanates modulate inflammation, oxidative stress, and apoptosis via Nrf2/HO-1 and NF-κB signaling pathways on indomethacin-induced gastric injury in rats. Food Funct. 2021;12(13):6001 6013. ( 10.1039/d1fo00645b) [DOI] [PubMed] [Google Scholar]
- 37. Raish M, Ahmad A, Ansari MA.et al. Momordica charantia polysaccharides ameliorate oxidative stress, inflammation, and apoptosis in ethanol-induced gastritis in mucosa through NF-kB signaling pathway inhibition. Int J Biol Macromol. 2018;111:193 199. ( 10.1016/j.ijbiomac.2018.01.008) [DOI] [PubMed] [Google Scholar]
- 38. Yang X, Fang Y, Hou J.et al. The heart as a target for deltamethrin toxicity: inhibition of Nrf2/HO-1 pathway induces oxidative stress and results in inflammation and apoptosis. Chemosphere. 2022;300:134479. ( 10.1016/j.chemosphere.2022.134479) [DOI] [PubMed] [Google Scholar]
- 39. Li S, Wu P, Han B.et al. Deltamethrin induces apoptosis in cerebrum neurons of quail via promoting endoplasmic reticulum stress and mitochondrial dysfunction. Environ Toxicol. 2022;37(8):2033 2043. ( 10.1002/tox.23548) [DOI] [PubMed] [Google Scholar]
- 40. Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother. 2020;122:109772. ( 10.1016/j.biopha.2019.109772) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a