Significance
Many neuromodulatory brain regions corelease neurotransmitters along with their neuromodulators. Because the function of such corelease is largely unknown, we have examined modulation of the claustrum by the cholinergic system of the basal forebrain. Our findings show that two different claustral output neuron populations respond in opposite ways to acetylcholine (ACh) and GABA coreleased by the cholinergic system. These opposing actions toggle network computations between the two different output neuron pathways. This toggle provides a microcircuit basis for attention-related and learning-related cholinergic computations and affords a previously unappreciated degree of flexibility to the cholinergic system. Thus, our results reveal a functional logic for corelease of ACh and GABA in the claustrum and potentially in other brain areas.
Keywords: neurotransmitter corelease, cholinergic, claustrum, cell-type specificity
Abstract
The cholinergic system of the basal forebrain plays an integral part in behaviors ranging from attention to learning, partly by altering the impact of noise in neural populations. The circuit computations underlying cholinergic actions are confounded by recent findings that forebrain cholinergic neurons corelease both acetylcholine (ACh) and GABA. We have identified that corelease of ACh and GABA by cholinergic inputs to the claustrum, a structure implicated in the control of attention, has opposing effects on the electrical activity of claustrum neurons that project to cortical vs. subcortical targets. These actions differentially alter neuronal gain and dynamic range in the two types of neurons. In model networks, the differential effects of ACh and GABA toggle network efficiency and the impact of noise on population dynamics between two different projection subcircuits. Such cholinergic switching between subcircuits provides a potential logic for neurotransmitter corelease in implementing behaviorally relevant computations.
The cholinergic system of the basal forebrain (BF) is a crucial pathway that regulates multiple aspects of cognition by modulating attention, arousal, and learning (1–3). Such actions arise from the ability of the cholinergic system to impact neural dynamics at multiple scales. At the level of individual neurons, cholinergic input can alter neuronal excitability and improve the ability of neurons to discriminate between diverse inputs (4). At a population level, cholinergic input can shape the correlational structure of population dynamics and improve robustness of a circuit to noise, thereby allowing information to become more accessible to the network in the presence of noise (5–9). These actions mimic the effects of attention and learning on cortical networks across species and hence have been proposed to mediate the actions of the cholinergic system in attention and learning (10–12).
The prevailing view that the cholinergic neurons in the BF implement such computations solely by releasing acetylcholine (ACh) has been challenged by the recent discovery that nearly all forebrain cholinergic neurons corelease the inhibitory transmitter, gamma-aminobutyric acid (GABA), along with ACh (4, 13). While such corelease of ACh and GABA has been observed in multiple brain areas—including the cortex (12, 13), hippocampus (14), and entorhinal cortex (15)—the logic for this is unknown: Does corelease differ according to cellular targets, and how does it impact cholinergic computations relevant for attention?
We have addressed these questions by analyzing cholinergic modulation of the claustrum, a thin sheet of neurons between the insular cortex and the striatum. The claustrum has been implicated in attention, where it reduces the impact of distracting environmental stimuli within the cortex (16, 17). Because the claustrum also receives input from BF cholinergic neurons (18), we reasoned that the ability of the claustrum to impact attention might be mediated by these cholinergic inputs and thereby investigated the microcircuit effects of such inputs to the claustrum (16, 19, 20).
We identified a surprising logic for cholinergic corelease, namely that two different output pathways of the claustrum receive opposing modulation by ACh and GABA released from the cholinergic pathway. We propose that by acting as a switch on the input discriminability of individual neurons and network resistance to noise of the two output pathways, the opposing actions produced by transmitter corelease afford a previous unappreciated degree of flexibility to the cholinergic system and provide a microcircuit basis for attention-related and learning-related cholinergic computations.
Results
The Claustrum Receives Cell-Type-Specific Monosynaptic Cholinergic Input from the BF.
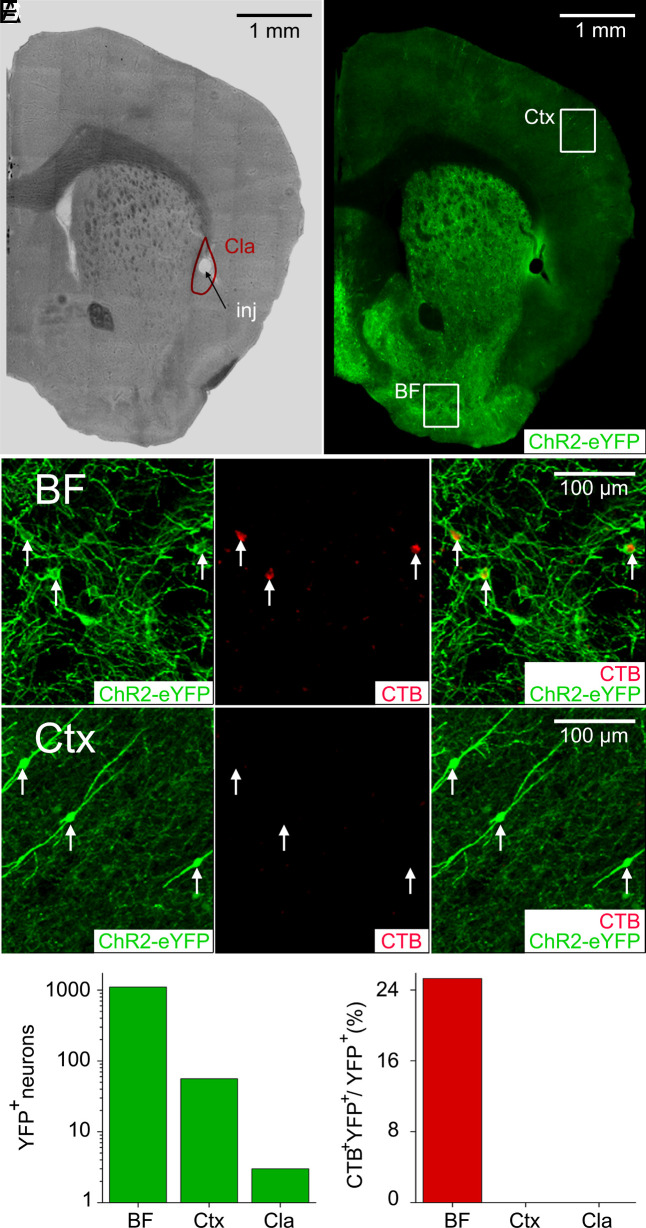
Cholinergic modulation of claustrum neurons was examined in a ChAT-Cre mouse line crossed with another line with Cre-dependent expression of channelrhodopsin fused to the yellow fluorescent protein [ChR2-YFP; (21)], thereby targeting ChR2 expression to cholinergic neurons. Because this double-transgenic strategy can also yield ChR2 expression in cholinergic VIP-expressing interneurons (VIP-INs) in the cortex (22), we examined the origin of ChR2-expressing inputs to the claustrum. For this purpose, neurons that innervate the claustrum were labeled via injection of the retrograde tracer, cholera toxin beta subunit (CTB), into the claustrum of ChR2-expressing double-transgenic mice (Fig. 1 A and B). While numerous brain regions exhibited either CTB or ChR2-YFP labeling, neurons labeled with both CTB and ChR2-YFP were found exclusively in the BF (Fig. 1 C and F). Cortical cholinergic VIP-INs expressing ChR2-YFP (Fig. 1D) were much less abundant than the ChR2-YFP expressing neurons in the BF (note logarithmic scale in Fig. 1E) and were not labeled by CTB (Fig. 1 D and F), indicating that they do not provide input to the claustrum. We identified a very small number of ChR2-YFP-labeled neurons within the claustrum itself (Fig. 1E); these rare cells also were not labeled by CTB (Fig. 1F) and were never observed in the brain slice experiments described next. We conclude that ChR2-expressing inputs to claustrum neurons originate exclusively from the BF, rather than the cortex or the claustrum.
Fig. 1.
Cholinergic innervation of claustrum neurons by the basal forebrain. (A) Brightfield image showing that CTB was injected (inj) into the center of the claustrum (Cla). The claustrum boundary is outlined in red. (B) ChR2-YFP expression (green) driven by the ChAT promoter in the brain of a double-transgenic mouse. CTB labeling was examined in the cholinergic neurons of the basal forebrain (BF) or the cortex (Ctx). (C) CTB labeling of ChR2-expressing BF neurons. ChR2-YFP expression is shown in green (Left), CTB labeling of neurons is in red (Center), and the merger of ChR2-YFP and CTB images is shown at Right. Arrows indicate neurons that are positive for both CTB and ChR2-YFP. (D) Lack of CTB labeling of ChR2-expressing Ctx neurons. ChR2-YFP expression (Left), CTB labeled neurons (Center), and merger of ChR2-YFP and CTB signals (Right). Arrows indicate several cortical neurons that expressed ChR2-YFP but were not labeled by CTB. (E) Number of neurons expressing ChR2-YFP in the BF, Ctx, and claustrum. Note the logarithmic scale for the y axis. (F) Percentage of neurons in the regions shown in E that expressed ChR2-YFP and were also labeled with CTB.
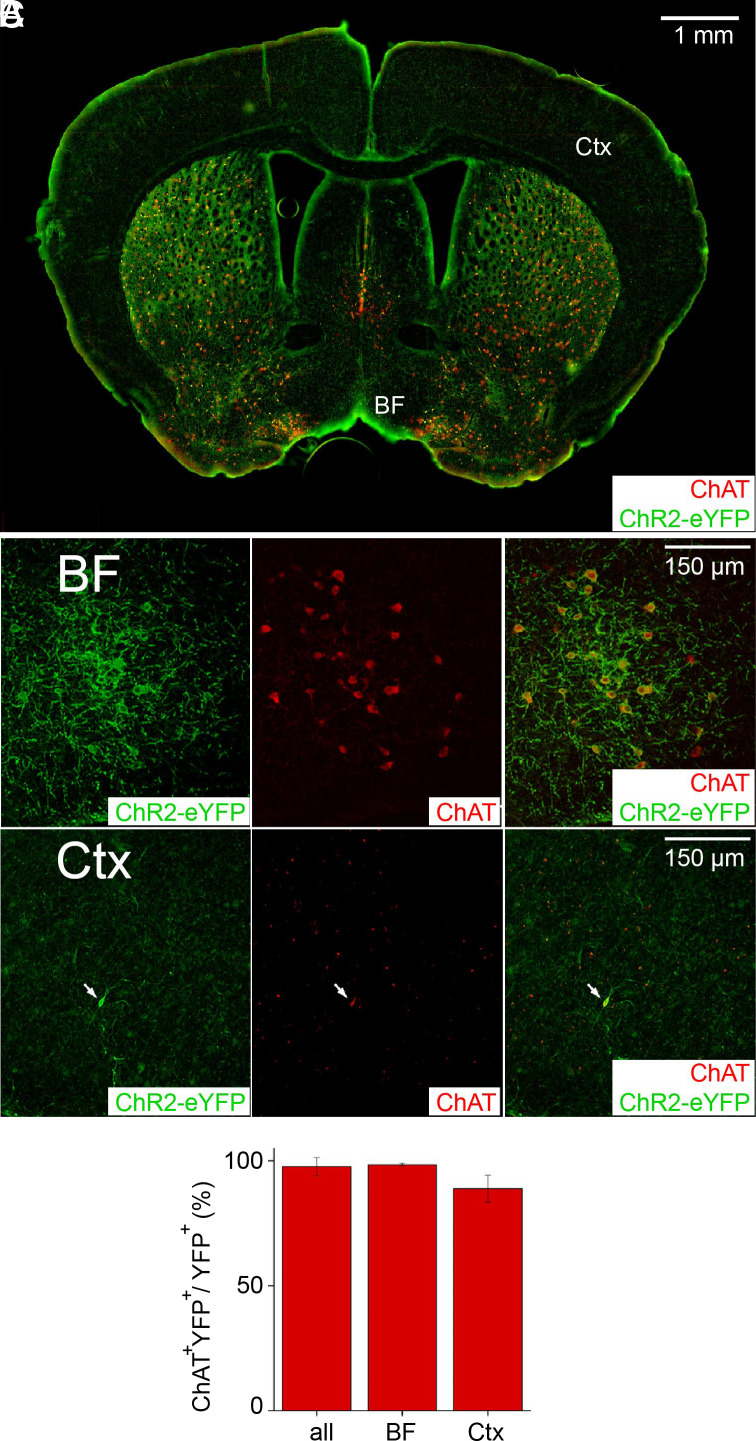
To determine whether the double-transgenic mice express ChR2-YFP exclusively in cholinergic neurons, endogenous ChAT expression was used as a label of cholinergic neurons (Fig. 2). ChAT labeling was observed in both subcortical structures (BF, striatum, and medial septum) and cortex (Fig. 2A). In general, labeling was much stronger in subcortical structures, due in part to the somata of most cholinergic neurons residing within the BF. To examine the relationship between ChAT and YFP-ChR2 expression, 2-photon imaging was performed in multiple regions of the BF and cortex (Fig. 2 B and C). In almost every case, neurons expressing YFP-ChR2 also expressed ChAT; on average, 97.7% of neurons expressing YFP-ChR2 also expressed ChAT (Fig. 2D). Among the 430 YFP+ neurons analyzed to quantify coexpression of ChAT and YFP-ChR2, most were in the BF (91.6% of eYFP+ neurons). Almost all the BF neurons expressed both YFP-ChR2 and ChAT (98.5% of 394 YFP-ChR2-expressing neurons), while a somewhat smaller fraction of YFP-ChR2-expressing cortical neurons (88.9% of 36 neurons) coexpressed ChAT (Fig. 2D). Taking together the results shown in Figs. 1 and 2, we conclude that the only ChR2-expressing neurons projecting into the claustrum of the double-transgenic mice are cholinergic neurons from the BF.
Fig. 2.
ChR2-YFP expression in cholinergic neurons. (A) Coronal brain section stained for endogenous ChAT expression (red) and ChR2-YFP expression (green) driven by ChAT-Cre. ChAT expression was high in the basal forebrain (BF), striatum, and medial septum, while cortical expression (Ctx) was relatively low. (B and C) Higher magnification, 2-photon images of endogenous ChAT (red) and ChR2-YFP (green) in cholinergic neurons of the BF (B) and cortex (arrow, C). (D) Fraction of neurons in the indicated regions that expressed ChR2-YFP and were also labeled with ChAT.
We next used whole-cell patch-clamp recordings to examine the physiological actions of this cholinergic input on claustrum neurons in brain slices (23). To isolate cholinergic responses, slices were treated with a glutamate receptor blocker (kynurenic acid, KYN; 1 mM) and a GABA receptor blocker (gabazine, GBZ; 10 µM). The claustrum consists of multiple types of projection neurons and interneurons (23). A trained classifier was applied to measurements of intrinsic electrical properties to identify neurons that project to cortical or noncortical targets, as well as to identify the three known types of local interneurons (23).
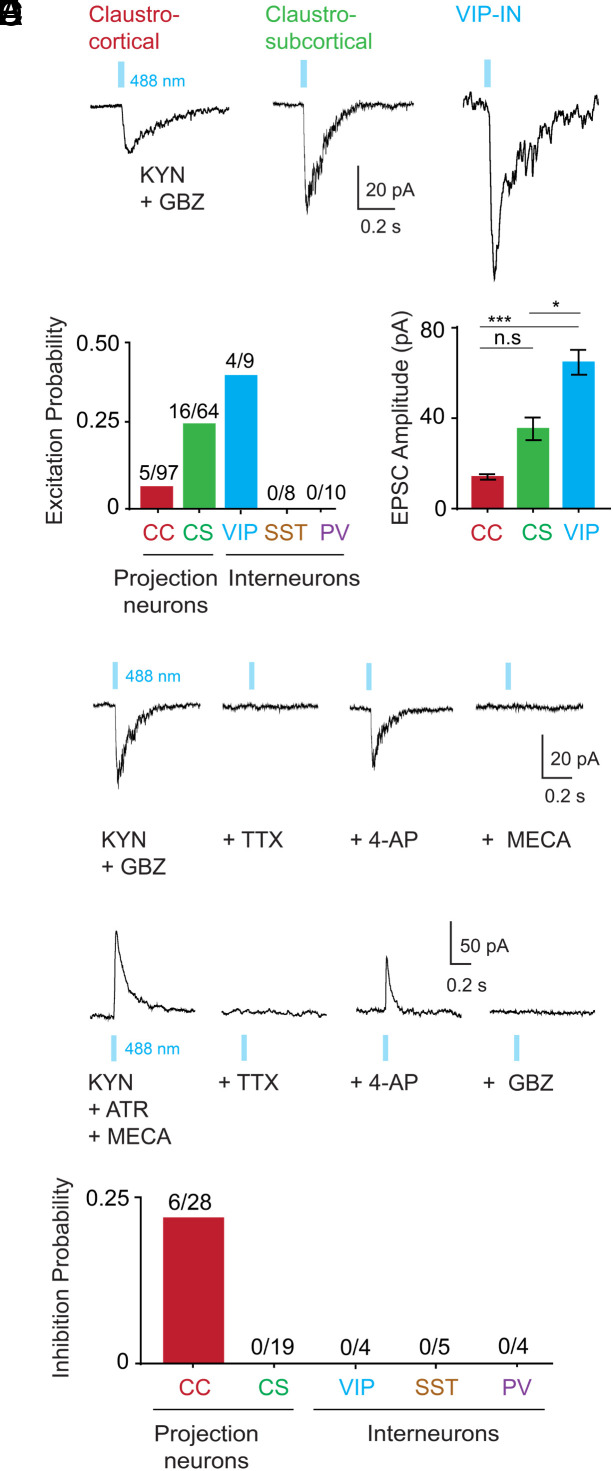
ChR2-mediated photostimulation of cholinergic input elicited responses in postsynaptic claustrum neurons (Fig. 3A). These were excitatory postsynaptic currents (EPSCs) because their reversal potential was near 0 mV (SI Appendix, Fig. S1A), much more positive than the action potential (AP) threshold of any claustrum neuron (23). Among the 5 different types of claustrum neurons examined, only 3 responded to cholinergic input: claustrocortical projection neurons (CC), claustro-subcortical projection neurons (CS), and VIP-INs (Fig. 3A). ChR2-mediated photocurrents were not observed in VIP-INs during photostimulation (5 neurons from 3 mice); this indicates that these neurons were not expressing ChR2, unlike the cortical VIP-IN shown in Fig. 1D (12). While a small fraction (5%, n = 5/97 neurons) of CC neurons received cholinergic excitatory input, five times more CS neurons (25%, n = 16/64 neurons) and even more VIP-INs (44%, n = 4/9 neurons) received such excitation (Fig. 3B). There were also target-specific differences in the amplitude of these responses, with EPSCs being largest for VIP-INs (64.7 ± 5.5 pA), smaller for CS projection neurons (35.3 ± 5 pA), and smallest for CC projection neurons (14 ± 1.2 pA; Fig. 3C). These were monosynaptic inputs because they persisted after tetrodotoxin (TTX; 1 µM) was used to block APs and 4-AP (4-aminopyridine) was applied to enhance ChR2-mediated depolarization (24–26) (Fig. 3D). These excitatory responses were mediated by nicotinic ACh receptors because they were blocked by a nicotinic receptor blocker (mecamylamine, MECA; 10 µM; Fig. 3D) and their reversal potential near 0 mV is typical of nicotinic responses (27). SST and PV interneurons never responded to cholinergic photostimulation (Fig. 1B, SST: n = 0/8, PV: n = 0/10). These results reveal cell-type-specific modulation of claustrum neurons and are consistent with reports that VIP-INs are a critical target for cholinergic modulation in other parts of the brain (28, 29).
Fig. 3.
Cell-type-specific cholinergic inputs to claustrum neurons. (A) Excitatory postsynaptic currents (EPSCs) evoked in three types of claustrum neurons by photostimulation of cholinergic axons using blue light pulses (488 nm, 50 ms duration; indicated by blue bars). Responses were measured in the presence of kynurenic acid (KYN; 1 mM) and gabazine (GBZ; 10 µM). (B) Probability of cholinergic excitation differed across claustrum cell types: claustrocortical (CC) projection neurons; claustro-subcortical (CS) projection neurons; and VIP, SST, and PV subtypes of interneurons. To facilitate comparison, all cells were held at a potential of −40 mV. (C) Amplitude of EPSCs evoked by cholinergic photostimulation varied according to cell type (*P < 0.05 and ***P < 0.005, Kruskal–Wallis test with Dunn’s post hoc test for multiple comparison). (D) A subset of claustrum neurons were tested for monosynaptic connectivity using tetrodotoxin (TTX; 1 µM) and then TTX plus 4-aminopyridine (4-AP; 500 µM). The presence of EPSCs during TTX/4-AP treatment revealed that these inputs are monosynaptic. Blockade of these responses by mecamylamine (MECA; 10 µM) indicated that they are mediated by nicotinic ACh receptors. (E) Only CC neurons received direct inhibition: monosynaptic connectivity was established by TTX and 4-AP treatment, as in D. Responses were measured in the presence of KYN, atropine (ATR; 10 µM), and MECA and were blocked by GBZ, indicating that they were mediated by GABAA receptors. (F) Probability of direct GABAergic inhibition in all cell types tested.
Because cholinergic neurons can corelease the inhibitory neurotransmitter, GABA, along with ACh (13), we asked whether claustrum neurons also receive GABAergic inhibition. For this purpose, cholinergic inputs were photostimulated while blocking excitatory responses with KYN, as well as MECA and atropine (10 µM), a blocker of muscarinic ACh receptors. Under such conditions, outward currents were observed (Fig. 3E). These were inhibitory postsynaptic currents (IPSCs) because their reversal potential of −70 mV (SI Appendix, Fig. S1B) was more negative than the AP threshold of CC neurons (−34 mV; ref. 23). These cholinergic IPSCs were monosynaptic because they persisted in the presence of TTX and 4-AP (Fig. 3E) and had a mean amplitude of 49.3 ± 6.2 pA at a membrane potential of −40 mV. These responses were mediated by GABAA receptors because they were blocked by GBZ (Fig. 3E). Remarkably, we found that only CC neurons received inhibitory inputs (Fig. 3F). Indeed, cholinergic input was much more likely to evoke inhibitory responses (21%, n = 6/28 neurons) than excitatory responses (5%) in CC neurons. These results reveal a circuit logic for cholinergic corelease of GABA: while claustral neurons projecting to subcortical structures, as well as VIP-INs, are excited via cholinergic activation of nicotinic receptors, neurons projecting to cortical structures are more likely to be inhibited by coreleased GABA.
Opposing Cholinergic Control of Gain and Dynamic Range of Claustrum Neurons.
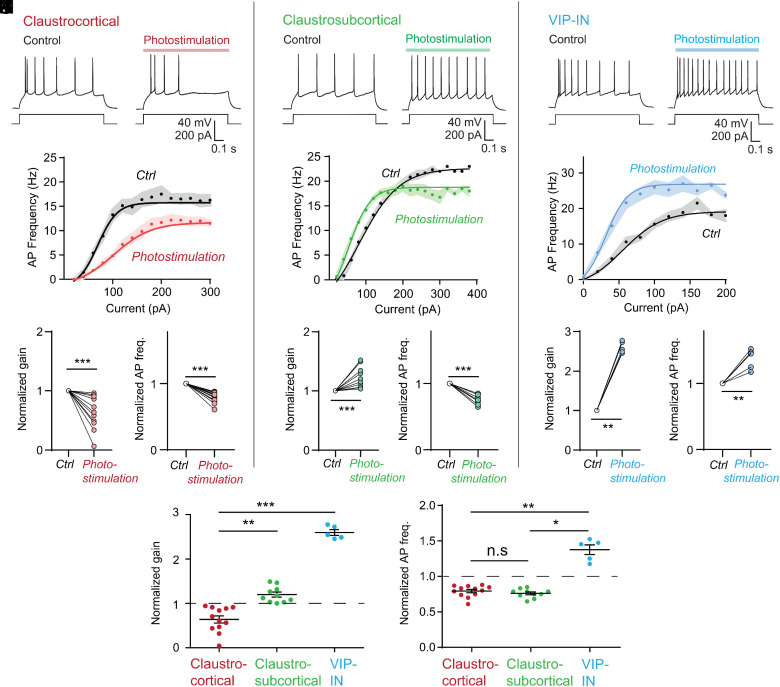
To understand the functional consequences of the dual modulation produced by corelease of ACh and GABA, we determined the effects of cholinergic input on APs evoked by depolarizing current pulses. Cholinergic input was activated by a train of blue light pulses (10 Hz; 488 nm) delivered for 1 s. In CC projection neurons, cholinergic input reduced the frequency of APs evoked by a 100 pA depolarizing current pulse (Fig. 4A; n = 12 neurons), presumably due to the inhibitory action of GABA. In contrast, this input increased AP firing in both CS projection neurons (Fig. 4B; n = 10) and VIP-INs (Fig. 4C; n = 5), consistent with the excitatory actions of ACh that we observed in these cells.
Fig. 4.
Opposing cholinergic control of neuronal gain and dynamic range in different claustrum cell types. (A–C) Cholinergic actions on claustrocortical neurons (A); claustro-subcortical neurons (B); and VIP-INs (C). Action potentials evoked by current pulses (100 pA) during control conditions (Left) and during cholinergic photostimulation (Right; 20 ms pulses, 10 Hz, 1 s duration). (D–F) Relationship between current (input) and AP frequency (output) in both control and cholinergic photostimulation conditions for CC neurons (D), CS neurons (E), and VIP-INs (F). Points indicate the means of n = 12 neurons in D, n = 11 neurons in E, and n = 5 neurons in F; shaded areas indicate 1 SEM. Smooth curves indicate fits of sigmoidal tanh functions to data (see Eq. 1 in Methods). (G, H) Quantification of cholinergic reduction of gain (G; normalized to control) and maximum action potential frequency (H) of CC neurons. (I, J) Cholinergic actions on gain (I) and maximum action potential frequency (J) of CS neurons. (K, L) Cholinergic enhancement of gain (K) and maximum action potential frequency (L) of VIP-IN. (M) Comparison of cholinergic effects on neuronal gain across cell types. (N) Comparison of cholinergic effects on neuronal dynamic range across cell types. Asterisks indicate statistical significance in the Kruskal–Wallis test with Dunn’s post hoc test for multiple comparison: *P < 0.05, **P < 0.01, and ***P < 0.005.
By varying the amplitude of the depolarizing current pulses, we could determine the relationship between current magnitude and AP frequency; this yields the neuronal input–output (IO) curve. Photostimulation of cholinergic input altered the IO curves of all three types of claustrum neurons (Fig. 4 D–F). The slope of these curves, representing neuronal gain (30), was decreased in CC neurons; this was evident both in averaged IO curves (Fig. 4D) and in the responses of individual CC neurons (Fig. 4G). Pharmacological manipulations indicated that the gain decrease of CC neurons was mediated by GABA receptors (SI Appendix, Fig. S2 A and B), consistent with the predominance of GABAergic input on these cells (Fig. 3 C and F). Conversely, cholinergic input increased the gain of both CS projection neurons (Fig. 4 E and I) and VIP-INs (Fig. 4 F and K), again consistent with the excitatory actions of ACh on these cells. These gain increases are reminiscent of the ability of cholinergic modulation to enhance gain in the visual cortex (31).
Cholinergic photostimulation also altered the maximal AP frequency, or dynamic range, for the IO curves of all three claustrum neuron populations. The dynamic range of both types of projection neurons—CC and CS cells—was reduced (Fig. 4 H and J). In contrast, cholinergic input increased the dynamic range of VIP-INs (Fig. 4I). As a result, the maximum AP frequency was achieved at higher values of input current compared to control for CC neurons following cholinergic photostimulation, but at lower values of input for CS neurons and VIP-INs (Fig. 4 D–F). Thus, the cotransmitters released by cholinergic input produce opposing, cell-type-specific modulation of the electrical properties of claustrum neurons (Fig. 4 M and N).
Opposing Cholinergic Modulation Toggles Input Discriminability of Single Neurons.
To understand the implications of these opposing modulatory effects on the ability of claustrum neurons to encode information, we turned to computational modeling. Because cholinergic input is known to alter information encoding at different scales—both in single neurons (31) and in neural populations (5)—we used simulations aimed at each scale.
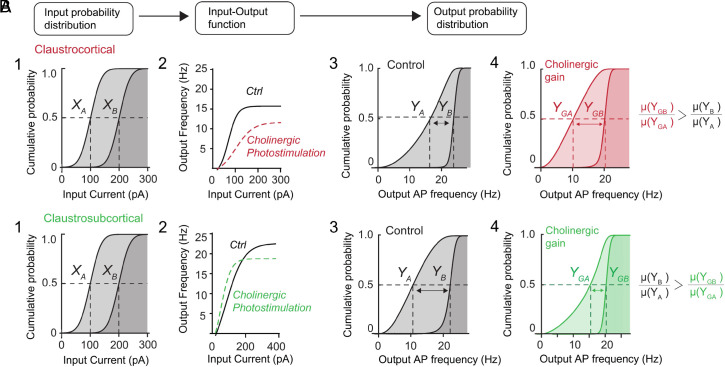
In the cortex, optogenetic activation of forebrain cholinergic input improves the signal-to-noise ratio (SNR) of individual neurons, allowing neurons to discriminate diverse inputs (4, 31). We used cell-level simulations to predict how the opposing effects on neuronal gain and dynamic range that we observed could alter this cholinergic computation. These simulations determined how responses to weak (XA) and strong (XB) synaptic inputs (Fig. 5 A1 and B1) would be transformed by the empirically measured IO functions of single CC (Fig. 5A2) and CS neurons (Fig. 5B2). To estimate the SNR, we compared the neuronal outputs produced in response to weak (YA) and strong (YB) inputs (Fig. 5 A3 and B3). Such analyses have been used to demonstrate the ability of norepinephrine to alter neuronal SNR and input discriminability (32).
Fig. 5.
Opposing cholinergic control toggles input discriminability of single neurons. (A) Paradigm used for signal–noise simulations for CC neurons: (1): Two populations of inputs (XA, XB) are transformed by the empirical IO functions of CC neurons (2) to (3) produce outputs (YA, YB). (4) Output distributions for CC neurons with cholinergic photostimulation (YGA, YGB). The impact of cholinergic input on input discriminability—the separation between distribution means (µ)—is indicated by arrows. (B) The same inputs (1) are transformed by the empirical IO functions of CS neurons (2) to produce output for native IO functions (3) and output with cholinergic photostimulation (4). Impact of cholinergic input is again indicated by arrows.
In CC neurons, for inputs centered around 100 and 200 pA (with SD of 100 pA), weak and strong inputs produced outputs with means centered around 16 Hz [µ(YA)] and 23 Hz [µ(YB)] (Fig. 5A3). The ratio of these two values yielded an SNR of 1.43. In contrast, cholinergic action increased the separation between responses to weak [µ(YGA) = 10 Hz] and strong [µ(YGB) = 21 Hz] inputs, yielding an SNR of 2.1 (Fig. 5 A4). Thus, cholinergic inputs improved the separation between responses to weak and strong input by 47% compared to control conditions, indicating that cholinergic input improves input discriminability for CC neurons. However, for CS neurons, the situation was reversed: cholinergic input reduced the separation of output distributions by 35% compared to control conditions, thereby reducing the input discriminability for CS neurons (Fig. 5 B3 and B4).
In summary, by differentially acting on neural gain and dynamic range, corelease of ACh and GABA acts as a toggle for input discriminability of single CC and CS projection neurons. This effect occurs across a wide range of input sizes for both types of projection neurons (SI Appendix, Fig. S3 A and B).
Opposing Cholinergic Modulation Toggles Claustrum Network Efficiency.
Cholinergic input has been reported to shape the encoding of information in neuronal networks (5). If a population of neurons receiving an input also experiences noise (due to random fluctuations, such as spontaneous activity) that is also highly correlated, it will be more difficult to decode the original signal. Thus, a strong relationship between neuronal signal correlations and noise correlations is harmful because it reduces discrimination of the signal from the noise. In the cortex, cholinergic input (as well as attention) weakens this relationship (5, 10, 32), thereby enabling a network to be less influenced by noise and thereby improving the efficiency of encoding information (5, 33, 34).
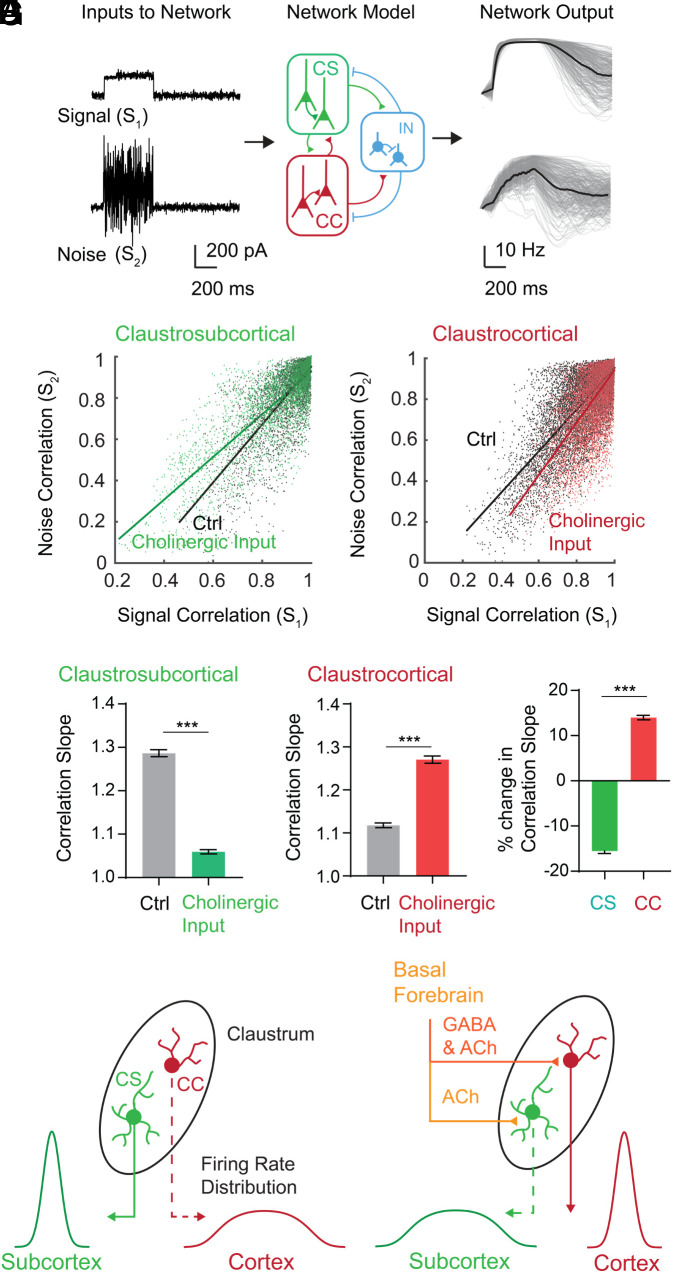
We therefore predicted the impact of cholinergic corelease on network correlation structure in the claustrum. We employed a recurrent circuit model based on an inhibition-stabilized network (35). This model contained 300 neurons, including excitatory CC and CS projection neurons and the three types of inhibitory interneurons, with the IO function of each neuron defined by experimental measurements (Methods and SI Appendix, Fig. S5).
To understand how cholinergic input effects the impact of noise in the claustrum network, we first gauged the ability of this network to distinguish signals with different amounts of additive noise. For this purpose, the network was driven by two types of stimuli. One input had a Gaussian amplitude distribution and an SD of 1/10th of the mean, called signal (S1); a second was a noisier version of S1, called noise (S2), with a Poisson amplitude distribution and an SD equal to its mean (Fig. 5 A, Left). We examined the impact of cholinergic input on network output by assessing the correlations between neurons in response to the signal, S1 (called signal correlation), and in response to the noisy input, S2 (called noise correlation).
Remarkably, in response to input signals above 140 pA, changes in the gain of CS neurons associated with cholinergic photostimulation decreased the slope (ΔSlope = −22%) of the relationship between signal correlation and noise correlation (Fig. 6 B and D and SI Appendix, Fig. S3C). Thus, cholinergic input to CS neurons weakens the relationship between signal and noise correlations, as both predicted by theory and observed in vivo (5, 34). Because this leads to better signal discrimination, the reduction in correlation slope is associated with greater robustness to noise and thus improves encoding capacity and population coding fidelity in networks (5, 32, 34). In contrast, for CC neurons, the slope increased (ΔSlope = +16%) for input sizes above 140 pA (Fig. 6 C and E and SI Appendix, Fig. S3D). This strengthening of the relationship between signal and noise correlations will reduce population coding fidelity because similarly tuned neurons will tend to have higher noise correlations and thus more susceptibility to noise.
Fig. 6.
Opposing cholinergic control toggles network efficiency of model circuits. (A) Paradigm used for modeling: a signal, S1 [Gaussian distributed; mean (µ) = 200 pA, SD (δ) = 20 pA] and noise, S2 (Poisson distributed; µ = 100 pA, δ = 100 pA) were presented to an inhibition-stabilized claustrum network model. (Right) Outputs of individual neurons (gray) and population average (black). (B) Relationship between signal and noise correlations for CS neurons. Lines indicate regression fits: control (Ctrl) slope = 1.28; cholinergic input slope = 1.05. (C) Relationship between signal and noise correlations for CC neurons. Ctrl slope = 1.12; cholinergic input slope = 1.27. (D) Quantification of correlation slopes following 100 repeated simulations, as in B, for CS neurons in control and cholinergic input conditions (***P < 0.005). (E) Quantification of correlation slopes following 100 repeated simulations, as in C, for CC neurons in control and cholinergic input conditions (***P < 0.005). (F) Percentage change in correlation slopes (cholinergic input – control) for 100 repeated simulations of model for CS and CC neurons (***P < 0.005). (G) Diagram summarizing our findings. Cell-type-specific cholinergic modulation in the claustrum leads to a toggle of signal-to-noise ratio (as indicated by distribution of neuronal firing rates) and encoding efficiency between cortical and subcortical projections.
Our claustrum network model predicts that cholinergic input will toggle encoding efficiency and coding fidelity between CC and CS projection neurons (Fig. 6F). The direction of this toggle depends on input size: for signal inputs smaller than 100 pA, the toggle will go in the opposite direction, with efficiency increasing for CC neurons while decreasing for CS neurons (SI Appendix, Fig. S4 C and D). Hence, our model indicates that the opposing cholinergic effects on gain and dynamic range of the two projection neuron types, caused by corelease of ACh and GABA, will toggle network computations between these neurons in a manner that depends on input size, increasing encoding efficiency for one neuronal population while reducing it for the other (Fig. 6G).
This model illustrates the ability of cholinergic input to toggle encoding efficiency in the presence of stimuli with differing noise; this is highly relevant for the claustrum, which receives inputs from a multitude of cortical and subcortical regions (16, 20). However, we also considered a situation where noise arises from variations in neural responses to repeated presentations of the same stimulus, as has been used in vivo in the visual system (5). For this purpose, we provided neurons in the network with a time-varying input that was repeated 30 times (SI Appendix, Fig. S4A). As in the visual system studies (5), we then constructed a signal covariance matrix, by using the mean response of each neural population in the network across signal presentations, and a noise covariance matrix using the mean-subtracted response. This alternative approach also evinced a switch in encoding efficiency (SI Appendix, Fig. S4B) that was qualitatively identical to that shown in Fig. 5: for small inputs (e.g., 50 pA), the slope of the signal correlation–noise correlation plot increased for CS neurons, while that of CC neurons decreased. For larger inputs (e.g., 400 pA), the switch occurred in the reverse direction (SI Appendix, Fig. S4B). Therefore, we conclude that the input-dependent cholinergic switch in the claustrum circuit occurs for two different forms of signal and noise input.
Discussion
We found that two output pathways of the claustrum receive opposing cholinergic modulation: 1) A subcortically projecting neuron population (CS cells) receives monosynaptic cholinergic excitation mediated by nicotinic receptors, while 2) a cortically projecting neuron population (CC cells) receives monosynaptic inhibition mediated by GABAA receptors. These opposite postsynaptic actions translate into opposing changes in the gain and dynamic range of the two types of projection neurons. Incorporating this motif of opposing cholinergic control into model circuits revealed that cholinergic corelease serves as a toggle, improving the input discriminability of neurons in one population while reducing it for the other, thereby switching the ability of network output to efficiently encode signals and ignore noise.
Cholinergic modulation has been investigated in diverse experimental paradigms and model systems, with several studies suggesting that the cholinergic system contributes to attention and learning by improving access to information via alteration of the SNR of neural populations (2, 5). These studies predict that attention and learning, via cholinergic input, induce specific changes in the cortical network, rather than changing neuronal gain uniformly (6). Our results connect these studies and provide a microcircuit basis for this nonuniformity of cholinergic control of SNR and encoding capacity, based on opposing control of gain and dynamic range of specific cell types.
Previous investigations of cholinergic transmitter corelease have revealed that this phenomenon is widespread, from superficial layers of the cortex (13) to the entorhinal cortex (15), and even in the hippocampus, where corelease is essential for the suppression of hippocampal sharp-wave ripples (14). By investigating the effects of corelease on neuronal electrical properties, we have uncovered a circuit logic for cholinergic corelease in the claustrum that toggles information between subpopulations, from a cortically projecting to a subcortically projecting population in the case of the claustrum (Fig. 6G). This toggle was also observed to depend on input size, providing flexibility to the cholinergic system to either improve or reduce access to information during attention depending on context; such a phenomenon has been observed in the cortex (10).
Opposing cholinergic control of projection neurons might also explain the ability of the claustrum to inhibit the cortex and coordinate the generation of slow waves (20). Recent theoretical work suggests that cholinergic control of inhibitory gain enables the transition of slow wave generation in the entorhinal cortex (36). Our observation of gain and dynamic range control of VIP-INs by cholinergic input in the claustrum suggests a similar mechanism: cholinergic corelease of ACh and GABA could introduce a state change responsible for the generation of slow waves, with a low cholinergic tone switching the discriminability of input toward the cortically projecting claustral population.
Materials and Methods
Animals.
All animal experiments were performed according to the Guidelines of the Institutional Animal Care and Use Committee of Nanyang Technological University, Singapore (Protocol number: 151075). Thirty-five adult ChAT-Cre x floxed ChR2-YFP (B6;129S6-Chattm2(cre)Lowl/J; # 006410) mice of both sexes were used to study cholinergic input to claustrum cells. The mean age of mice used in our experiments was postnatal day 65 ± 0.6.
For the anatomical experiments shown in Fig. 1, an additional 6 male mice were used; their mean age was postnatal day 198.5 ± 2.1.
Tracer Injection.
Mice were anesthetized with isoflurane and placed in a stereotaxic frame (Harvard Apparatus). An incision was made along the scalp to access the bregma and the injection site. A craniotomy was performed at −1.18 mm anterior and 2.55 mm lateral from the bregma. Pulled glass pipettes were back-filled with mineral oil and 0.5% w/v Alexa Fluor 555–conjugated cholera toxin subunit B (CTB; Invitrogen). The glass pipettes were lowered 2.7 mm below the brain surface, and 30 to 40 nL of CTB solution was pressure injected at 10 nL/s for 1 s and then at a rate of 2 nL/s for the remaining volume. Mineral oil was also injected (5 nL) to visualize the injection site post hoc. To minimize leakage during withdrawal, the pipette was held in place for 5 min before being slowly retracted from the brain. Out of 6 injected mice, CTB was successfully injected into the claustrum in 2, while the remaining 4 were not considered further.
Immunohistochemistry.
Mice were anesthetized and transcardially perfused 10 d postsurgery with 25 mL ice-cold phosphate-buffered saline (PBS), followed by 25 mL 4% paraformaldehyde and PBS. Brains were dissected and kept in 30% sucrose in PBS for 2 d. The brains were mounted in mounting medium (tissue-tek O.C.T, Sakura) and sectioned at 60 µm on a microtome (AO instruments). Brain slices were washed in PBS with 0.25% Triton X-100 (PBSTx) and incubated with primary antibodies against CTB (rabbit-anti-CTB subunit, 1:500, Abcam: ab34992, RRID: AB_726859) and YFP (chicken-anti-GFP, 1:1,000, Abcam: ab13970, RRID: AB_300798) in PBSTx for 48 h at 4 °C. Brain slices were washed with PBSTx and incubated with fluorophore-conjugated secondary antibodies against chicken (1:500, Alexa Fluor 488, Thermo Fisher: A-11039, RRID: AB_142924) and rabbit (1:500, Alexa Fluor 555, Thermo Fisher: A32732, RRID: AB_2633281) for 24 h at 4 °C. The brain sections were rinsed in PBSTx and mounted in antifade reagent (polyvinyl alcohol mounting medium with DABCO, Merck).
To check for the endogenous expression of ChAT, the protocol was adjusted using TBS with 0.5% Triton X-100 (TBSTx) instead of PBS, and brain slices were incubated with 1:300 goat-anti-ChAt antibody (1:300, Merck: AB144P, RRID:AB_2079751) and YFP (chicken-anti-GFP, 1:1,000, Abcam: ab13970, RRID: AB_300798) in TBSTx for 72 h at 4 °C. Brain slices were washed with TBSTx and incubated with fluorophore-conjugated secondary antibodies against chicken (1:400, FITC, Abcam: AB63507; RRID: AB_1139472) and goat (1:400, Alexa Fluor 568, Thermo Fisher: A-11057, RRID: AB_2534104) for 24 h at 4 °C. The brain sections were rinsed in TBSTx and mounted in antifade reagent (Polyvinyl alcohol mounting medium with DABCO, Merck).
Image Acquisition and Processing.
To access the quality of the injection side, overview images were acquired on a Zeiss Axio Scan Z1 slide scanner. For the quantification of CTB and/or YFP labeled neurons, a total of 33 brain sections were imaged (19 BF sections and 14 cortical sections, including the claustrum, endopiriform nucleus, insula, and primary and secondary somatosensory and motor cortices) across their entire depth. Imaging was performed on an Olympus FV-1000 confocal microscope equipped with a 10× objective (0.3 NA). Two continuous discharge lasers (488 nm and 559 nm) were used for excitation, and fluorescence emission for the two channels was acquired sequentially. Emitted light was split with a dichroic mirror with a separation wavelength of 560 nm. Fluorescence signals were bandpass filtered at 505 to 540 nm for YFP and 575 to 675 nm for CTB. For the ChAT-YFP immunostaining, overview images were taken with an Axio Scan.Z1 system (Carl Zeiss) and a 20× objective (0.8 NA) in sequential scan mode. For the eYFP-FITC signal, an LED with an excitation wavelength of 450 to 488 nm was used, while the emitted signals were bandpass filtered between 504 and 546 nm. For the ChAT-568 signal, the sample was illuminated at 577 to 604 nm, while emitted signals were sampled between 615 and 756 nm. Two-photon images were acquired with a Fluoview FVMPE-RS system (Olympus) and a 25× objective (1.05 NA). For the YFP-FITC signal, an excitation wavelength of 950 nm was used, and emitted signals were sampled between 495 and 540 nm. For the ChAT-Alexa 568 signal, an excitation wavelength of 1,100 nm was used, and emitted signals were sampled after passing through a dichroic mirror (SDM570) in a range from 575 to 645 nm.
Image analysis and processing were done with Fiji/ImageJ software (37). To analyze the YFP signal, the maximum intensity projections of the YFP signal across the entire z-stack were used. To analyze the CTB signal and optimize the signal/noise ratio, SD projections of the CTB signal were generated, spatially filtered with a median filter (1 pixel radius), and subtracted against the average background signal within a 20-pixel radius. To visualize and detect CTB-positive cell bodies, a detection threshold was set based on the mean intensity value plus 2 SDs from all CTB-positive pixels within the brain slide. The resulting CTB signals were overlaid with the YFP signal, and CTB+ and/or YFP+ neurons were quantified manually using the cell count plug-in of ImageJ. For the analysis of ChAT and FITC signals, FITC signals were smoothened using a Gaussian blur filter (sigma = 0.5), while the ChAT signals were first processed using the following routine: a) calculation of the local mean signal (radius = 1 px), b) background subtraction (rolling radius = 100 px, sliding stack), and c) calculation of the local median signal (radius = 2 px). The mean and SD of the ChAT-Alexa568 signal was calculated and visualized with an upper detection threshold of mean+2 SD. If an YFP-expressing neuron overlapped with the processed ChAT signal, a neuron was counted ChAT positive.
Brain Slice Recording.
Acute brain slices were prepared according to the procedures described in ref. 23. In brief, mice were deeply anesthetized with isoflurane and euthanized via decapitation. The brains were isolated and transferred into ice-cold sucrose solution containing the following: 250 mM sucrose, 26 mM NaHCO3, 10 mM glucose, 4 mM MgCl2, 3 mM myoinositol, 2.5 mM KCl, 2 mM sodium pyruvate, 1.25 mM NaH2PO4, 0.5 mm ascorbic acid, 0.1 mM CaCl2, and 1 mM KYN, with an osmolality of 350 to 360 mOsm and a pH of 7.4. Coronal brain slices (250 µm) were cut with a Leica VT 1000S vibratome. Slices were kept for 0.5 h at 34 °C in artificial CSF (ACSF) containing the following: 126 mM NaCl, 24 mM NaHCO3, 1 mM NaH2PO4, 2.5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose, and 0.4 mM ascorbic acid, 300 to 310 mOsm, pH 7.4, and gassed with a 95% O2/5% CO2 mixture before transfer to ACSF at room temperature for recordings.
Whole-cell patch-clamp recordings were performed using borosilicate glass pipettes (5 to 9 MΩ) filled with internal solution containing the following: 130 mM K-gluconate, 10 mM KOH, 2.5 mM MgCl2, 10 mM HEPES, 4 mM Na2ATP, 0.4 mM Na3GTP, 5 mM EGTA, 5 mM, Na2 phosphocreatinin, and 0.2% neurobiotin (290 to 295 mOsm, pH 7.4). Recordings were performed at 24 °C with a MultiClamp 700B amplifier (Molecular Devices) and a Digidata 1440 interface (Molecular Devices). Signals were acquired at 50 kHz and filtered at 10 kHz. Access resistance (Ra) was measured, and only cells with Ra < 30 MΩ were used for further analysis. Cell-type identity was determined using an automated classifier using electrical properties described in ref. 23. Fourteen electrophysiological properties were extracted using software made available by the authors at https://github.com/adityanairneuro/claustrum. A trained classifier was used to distinguish between the two subtypes of claustral projection neurons and three subtypes of claustral interneurons.
For optogenetic photoactivation of cholinergic terminals, slices were illuminated by a 130-W mercury lamp (Olympus) passed through an EYFP filter set and a 25× water-immersion objective. For voltage-clamp experiments, 50 ms light pulses were delivered while holding neurons at −40 mV. For current-clamp experiments, blue light pulses (10 Hz, 20 ms duration) were delivered for 1 s. We choose this stimulation protocol to mimic the average firing rates of BF cholinergic neurons, which range from 7 to 14 Hz during wakefulness and rapid eye movement sleep (38, 39).
Analysis of Neuronal Gain.
In current-clamp experiments, we constructed IO curves for each neuron by injecting depolarizing current pulses in the range 0 to 400 pA in 20 pA steps and measuring output firing frequency. Empirically determined input current–output frequency curves were fit with sigmoidal tanh functions of the form:
| [1] |
where is the maximum observed firing frequency, is the baseline firing frequency, is the input current, and is the gain or slope of the function at baseline and thus represents the IO sensitivity of the neuron k.
Analysis of SNR with Simulations Using Empirical IO Functions.
Given that cholinergic input can alter gain, we analyzed whether these empirically observed differences in IO curves of CC and CS neurons might be sufficient for these projections to process input differently. We modeled this possibility by considering how two input probability distribution functions (PDF: XA, XB) would be transformed by IO functions of neurons with and without cholinergic photostimulation (Fig. 3A). To quantify the amount of separation between output PDFs, we compared the change in the ratio of their means with and without cholinergic gain control as this reflects the SNR of the two signals XA and XB (40). Formally:
| [2] |
where YGB and YGA are output PDFs in the presence of cholinergic gain control, whereas YB and YA are output PDFs in its absence. indicates the mean of distribution Y.
We verified that the SNR results we observed generalized for a range of input (PDF: XA, XB) means and SDs by systematically varying either the mean of XA, XB (SI Appendix, Fig. S2 A1 and B1) or the SD of XA, XB (SI Appendix, Fig. S2 A2 and B2).
Analysis of Signal and Noise Correlations in Model CLA-Like Networks.
To understand the role of cholinergic gain control in CLA-like networks, we constructed a recurrently connected network model using stability-optimized circuits (SOCs), a class of networks where inhibition stabilizes the network to create a non-chaotic network with transient dynamics. Below we briefly describe this model.
We first generate synaptic weight matrices W with N = 300 neurons (with excitatory and inhibitory neurons in the ratio 9:1 as empirically determined (23) as detailed in Hennequin et al. (35).
We begin with set of sparse weights with nonzero elements set to for excitatory neurons and for inhibitory neurons, where with connection probability P being 0.03 for excitatory neurons and 0.4 for interneurons as empirically determined (25). We construct W with an approximately circular spectrum (i.e., set of eigenvalues) of radius ρ = 10 and inhibition/excitation ratio γ = 3 in line with Hennequin et al. (35) (SI Appendix, Fig. S3 B, Left).
Following construction of W, we never change the excitatory weight, but refine the inhibitory connections to minimize the “spectral abscissa” of W, which is the largest real part among the eigenvalues of W (SI Appendix, Fig. S3A). This optimization is performed according to Stroud et al. (37), and the resulting matrix, referred to as a SOC is nonchaotic (41) (SI Appendix, Fig. S3 B, Right).
The use of SOCs is an approximation used due to the lack of precise cell-type-specific connectivity for the claustrum. SOCs have been used to study the effect of gain modulation in motor cortex circuits (41). Since we were interested in gain control of the claustrum network, we used SOCs to obtain a nonchaotic network with claustrum-like connectivity for projection neuron to projection neuron and interneuron to interneuron connections and empirically determined IO curves for each cell type.
Our model is governed by a differential equation which controls neuronal activity (Eq. 3) using the gain function (Eq. 1) and the synaptic connectivity matrix W.
| [3] |
We integrate Eq. 1 using the ODE45 function in Matlab using default parameters.
The initial condition was chosen among the “most observable” modes which elicit the strongest transient dynamics according to Hennequin et al. (35).
To gauge the ability of the network to distinguish different types of input with differing variance, we delivered time varying inputs as shown in Fig. 3C. We delivered two sets of inputs, a signal (S1) which consists of Gaussian distributed input where the SD of input is 1/10th of its mean. In a different set of trials, we provided a second noisy input (S2) to the network which was Poisson distributed with SD equal to the mean of the signal. Signal correlation–noise correlation graphs are obtained by plotting the Pearson’s correlations coefficient between every pair of excitatory neurons during the presentation of the Gaussian signal vs. during the presentation of Poisson noise. The slope of the signal correlation–noise correlation graph is obtained using least-squares regression. To ensure that the results we observed were robust for a range of input sizes, we systematically varied the mean of either the Gaussian signal or Poisson noise and examined the slope of the signal–noise correlation plot (SI Appendix, Fig. S2 C and D).
In a second paradigm, we provided the model with biologically realistic inputs based on experiments performed in Minces et al. (5), where noise is defined as the trial–trial variance in neural dynamics after repeated presentations of the same stimulus. To this end, we provided the model with a time-varying Gaussian signal 30 times to the same network. We then construct a signal covariance matrix using the mean response of each population in the network across signal presentations and a noise covariance matrix using the mean-subtracted response as performed in Minces et al. (5).
Code used for analysis and reproduction of IO curves and the recurrent claustrum network is available (43).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank K.L.L Wong, Z. Chia, G. X. Ham, L. Mark, and G. Silberberg for insightful discussions and comments on our paper and K. Chung, S. Kay, R. Tan, P. Teo, Y. C. Teo, and M. Yeow for technical assistance. Supported by the Singapore Ministry of Education under its Singapore Ministry of Education Academic Research Fund Tier 3 (MOE2017-T3-1-002). M.G. was supported by an LKCMedicine LEARN grant (021912-00001), and A.N is supported by the National Science Scholarship awarded by the Agency of Science, Technology and Research, Singapore.
Author contributions
A.N., G.J.A., and M.G. designed research; A.N., Y.Y.T., and M.G. performed research; A.N., Y.Y.T., and M.G. analyzed data; and A.N., G.J.A., and M.G. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Software: Code used for analysis and reproduction of IOcurves and the recurrent claustrum network data have been deposited in Github (42, 43). All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Hangya B., Ranade S. P., Lorenc M., Kepecs A., Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162, 1155–1168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz T. W., Duncan J., Normalization and the cholinergic microcircuit: A unified basis for attention. Trends Cogn. Sci. 22, 422–437 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Thiele A., Bellgrove M. A., Neuromodulation of attention. Neuron 97, 769–785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colangelo C., Shichkova P., Keller D., Markram H., Ramaswamy S., Cellular, synaptic and network effects of acetylcholine in the neocortex. Front. Neural Circuits 13, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minces V., Pinto L., Dan Y., Chiba A. A., Cholinergic shaping of neural correlations. Proc. Natl. Acad. Sci. U.S.A. 114, 5725–5730 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kempen J., Panzeri S., Thiele A., Cholinergic control of information coding. Trends Neurosci. 40, 522–524 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Záborszky L., et al. , Specific basal forebrain–cortical cholinergic circuits coordinate cognitive operations. J. Neurosci. 38, 9446–9458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinke W., et al. , Cholinergic modulation of response properties and orientation tuning of neurons in primary visual cortex of anaesthetized marmoset monkeys. Eur. J. Neurosci. 24, 314–328 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgkvist A., Lieberman O. J., Sulzer D., Synaptic plasticity may underlie l -DOPA induced dyskinesia. Curr. Opin. Neurobiol. 48, 71–78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruff D. A., Cohen M. R., Attention can either increase or decrease spike count correlations in visual cortex. Nat. Neurosci. 17, 1591–1597 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeanne J. M., Sharpee T. O., Gentner T. Q., Associative learning enhances population coding by inverting interneuronal correlation patterns. Neuron 78, 352–363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen M. R., Maunsell J. H. R., Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci. 12, 1594–1600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders A., Granger A. J., Sabatini B. L., Corelease of acetylcholine and GABA from cholinergic forebrain neurons. Elife 4, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takács V. T., et al. , Co-transmission of acetylcholine and GABA regulates hippocampal states. Nat. Commun. 9, 2848 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desikan S., Koser D. E., Neitz A., Monyer H., Target selectivity of septal cholinergic neurons in the medial and lateral entorhinal cortex. Proc. Natl. Acad. Sci. U.S.A. 115, E2644–E2652 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atlan G., et al. , The claustrum supports resilience to distraction. Curr. Biol. 28, 2752–2762.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White M. G., et al. , Anterior cingulate cortex input to the claustrum is required for top-down action control. Cell Rep. 22, 84–95 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong K. L. L., Nair A., Augustine G. J., Changing the cortical conductor’s tempo: Neuromodulation of the claustrum. Front. Neural Circuits 15, 658228 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zingg B., Dong H. W., Tao H. W., Zhang L. I., Input–output organization of the mouse claustrum. J. Comp. Neurol. 526, 2428–2443 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narikiyo K., et al. , The claustrum coordinates cortical slow-wave activity. Nat. Neurosci. 23, 741–753 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Zhao S., et al. , Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods 8, 745–752 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granger A. J., et al. , Cortical ChAT+ neurons co-transmit acetylcholine and GABA in a target- and brain-region-specific manner. Elife 9, e57749 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graf M., Nair A., Wong K. L. L., Tang Y., Augustine G. J., Identification of mouse claustral neuron types based on their intrinsic electrical properties. eneuro 7, ENEURO.0216-20.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petreanu L., Mao T., Sternson S. M., Svoboda K., The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J., Matney C. J., Roth R. H., Brown S. P., Synaptic organization of the neuronal circuits of the claustrum. J. Neurosci. 36, 773–784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chia Z., Augustine G. J., Silberberg G., Synaptic connectivity between the cortex and claustrum is organized into functional modules. Curr. Biol. 30, 2777–2790.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Hammond C., “The ionotropic nicotinic acetylcholine receptors” in Cellular and Molecular Neurophysiology (Elsevier, 2015), pp. 173–197. [Google Scholar]
- 28.Poorthuis R. B., Enke L., Letzkus J. J., Cholinergic circuit modulation through differential recruitment of neocortical interneuron types during behaviour. J. Physiol. 592, 4155–4164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista-Brito R., et al. , Developmental dysfunction of vip interneurons impairs cortical circuits. Neuron 95, 884–895.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silver R. A., Neuronal arithmetic. Nat. Rev. Neurosci. 11, 474–489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto L., et al. , Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 16, 1857–1863 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sompolinsky H., Yoon H., Kang K., Shamir M., Population coding in neuronal systems with correlated noise. Phys. Rev. E 64, 051904 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Meir I., Katz Y., Lampl I., Membrane potential correlates of network decorrelation and improved snr by cholinergic activation in the somatosensory cortex. J. Neurosci. 38, 10692–10708 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y., et al. , Perceptual learning reduces interneuronal correlations in macaque visual cortex. Neuron 71, 750–761 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennequin G., Vogels T. P., Gerstner W., Optimal control of transient dynamics in balanced networks supports generation of complex movements. Neuron 82, 1394–1406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nghiem T.-A.E., et al. , Cholinergic switch between two different types of slow waves in cerebral cortex. Cereb. Cortex 30, 3451–3466 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Schindelin J., et al. , Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassani O. K., Lee M. G., Henny P., Jones B. E., Discharge profiles of identified gabaergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J. Neurosci. 29, 11828–11840 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee M. G., Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J. Neurosci. 25, 4365–4369 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Servan-Schreiber D., Printz H., Cohen J., A network model of catecholamine effects: Gain, signal-to-noise ratio, and behavior. Science 249, 892–895 (1990). [DOI] [PubMed] [Google Scholar]
- 41.Stroud J. P., Porter M. A., Hennequin G., Vogels T. P., Motor primitives in space and time via targeted gain modulation in cortical networks. Nat. Neurosci. 21, 1774–1783 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nair A., Graf M., Augustine G.J., Automated electrophysiology based classification of claustrum neurons. Github. https://github.com/adityanairneuro/claustrum. Deposited 31 October 2020.
- 43.Nair A., Teo Y. Y., Graf M., Augustine G. J., Investigating the effects of opposing cholinergic gain control in a network model of the claustrum. Github. https://github.com/adityanairneuro/cholinergic. Deposited 27 January 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Software: Code used for analysis and reproduction of IOcurves and the recurrent claustrum network data have been deposited in Github (42, 43). All study data are included in the article and/or SI Appendix.