Abstract
Background
One-step nucleic acid amplification (OSNA) assay is a proven, accurate, intraoperative method for the detection of lymph node (LN) metastases. The aim of this study was to assess if the total tumour load (TTL) as calculated by OSNA could be used to predict N2 stage disease, ie ≥4 LN containing metastases, in invasive breast cancer patients.
Methods
Between 2011 and 2019 at St Richard’s Hospital, Chichester, all macro-metastasis-positive OSNA cases for invasive breast cancer were retrospectively reviewed. The association between clinicopathological variables and ≥4 LNs containing metastases was analysed using regression analysis.
Results
In total, 134 patients with positive sentinel lymph node (SLN) on OSNA undergoing axillary node clearance were analysed, 53% of whom had no further positive LN, 25% had ≥4 lymph nodes positive. TTL was calculated as the aggregate of cytokeratin-19 mRNA copy count of all SLN tissue analysed via OSNA. TTL ≥1.1×105copies/μl and lymphovascular invasion (LVI) were both significant predictors of N2 stage disease on both univariate (TTL p=0.04, LVI p=0.005) and multivariate (TTL p=0.008, LVI p=0.039) regression analysis.
Conclusion
Our findings show that SLN TTL via intraoperative OSNA assay can predict four or more positive axillary LN involvement in invasive breast cancer. This is important in that it may be used intraoperatively by surgeons to decide on whether to proceed with a full axillary node clearance in order to stage the axilla. Further research is required to shape future guidance.
Keywords: OSNA, Breast cancer, Sentinel lymph node, Total tumour load, Axillary clearance
Introduction
Sentinel lymph node (SLN) analysis in breast cancer has been the standard staging procedure for the axilla for over a decade for patients with a clinically and radiologically negative axilla.1 A positive SLN (macro-metastasis) would warrant further axillary node clearance (ANC) to fully stage the axilla, whereas a negative result (micro-metastasis or isolated tumour cells) would warrant no further axillary surgery. Analysis of the SLN has evolved over time, moving from histological analysis including frozen section, to machine-based such as one-step nucleic acid amplification (OSNA), which via mRNA assays detects the level of cytokeratin-19 (CK19) gene expression,2 an epithelial marker associated with breast cancer not found in healthy lymph tissue. Results are typically given as negative (<250copies/μl), micrometastases (>250–<5.0×103copies/μl) or macrometastases (≥5.0×103copies/μl),3 although recent studies have suggested this figure could be raised.4 More recently, ANC has been shown to be of no oncological benefit with respect to disease-free survival or survival rate of patients with micrometastases.5 OSNA (Sysmex corporation, Kobe, Japan) has the capability to intraoperatively detect SLN macrometastases accurately in breast cancer patients, allowing further axillary surgery if appropriate in a single-stage procedure.6,7 It has been shown to be at least as accurate as frozen section while facilitating a significantly higher proportion of single-stage surgery.8 A recent 2018 meta-analysis by Shi et al confirmed this, with sensitivity and specificity for detecting macrometastases being 0.85 and 0.98, respectively.9 It is known that around 50% of women with a positive SLN will have no further lymph node (LN) metastases and therefore not benefit from ANC.10 Following results from the Z0011 study,11 an increasingly conservative approach towards the axilla has been suggested, in order to avoid complications such as lymphoedema and nerve injury, with minimal oncological benefit.
It has been suggested in multiple previous studies that OSNA may hold the key to providing both an oncologically safe yet surgically conservative approach to staging the axilla. The exact number of macrometastatic positive lymph nodes in the axillary basin is of the utmost clinical relevance with regard to staging of the disease, and subsequent adjuvant oncological treatment. No nodes involved (negative SLN) is classified as N0 disease, 1–3 nodes positive is N1 disease and ≥4 LNs positive is N2 disease.12 Additional adjuvant therapy is advised for N2 disease13 as well as further irradiation of the preserved breast and supra-and-subclavian nodes.14
Nomograms have been proposed as tools for clinicians in predicting the likelihood of N2 disease and identifying those most likely to benefit from ANC.15–17 Factors identified as significant include pathological tumour size and histology, presence of lymphovascular invasion, extranodal extension and size of the largest sentinel node metastasis.18–20 In reality their application is limited owing to the fact that these are postoperative findings. Newer nomograms have looked to utilise intraoperative factors to guide surgical management for a single-stage procedure.21–23 These include OSNA findings, most notably the highest copy count in the SLN,24 or more recently total tumour load (TTL),25–27 an aggregate of the copy count of a single or multiple SLN(s). TTL was shown by Cuffolo et al in 2018 as the best predictive tool available from OSNA data as compared with average or highest copy number.28 Peg et al in 2017 followed up 950 women with a median follow-up of 5.1 years. They found a TTL of >2.5×104copies/μl was able to differentiate a low and high-risk group for both disease-free survival (HR 1.07; p=0.0014) and overall survival (HR 1.08, p=0.0032).29 Recent studies have attempted to identify the cut-off value for TTL that could predict ≥4 lymph node involvement. Kubota et al found a cut-off 5.4×104copies/μl was correlated with ≥4 LN metastases (odds ratio=2.95, 95% confidence interval (CI): 1.17–7.97, p=0.022).30
The aim of our study is to analyse our local data to see if a cut-off value for TTL could be identified as a satisfactory predictor of ≥4 positive lymph nodes, and therefore identify which patients would most benefit from intraoperative completion ANC.
Methods
Inclusion criteria for this study were women with invasive breast cancer clinically and radiologically assessed as TNM stage T1–3, N0, M0 disease undergoing surgery with SLN biopsy via OSNA analysis. Preoperative nodal status was determined by ultrasonography performed by a breast radiologist. Exclusion criteria included women undergoing neoadjuvant chemotherapy and endocrine therapy, primary tumour histology showing no evidence of invasive disease, preoperative identification of metastases in the lymph nodes and those undergoing axillary node sampling or primary clearance instead of sentinel node biopsy. In total, 938 patients met the inclusion criteria between November 2011 and October 2019 at St Richard’s Hospital, Chichester, UK, identified by clinical coding of OSNA analysis and cross-referenced with finalised pathology reports. Of the 938 patients, 134 positive OSNA results with subsequent ANC were performed, and these patients were selected for further analysis.
Data acquisition
SLN biopsy was performed using dual technique. Day-1 of surgery patients were injected with radioisotope tracer (technetium-99) into the subdermal plane of the areola border. Lymphoscintigraphy was performed two hours after injection, with images and a report provided to the surgeon outlining presence of primary and secondary nodes. On the day of surgery following general anaesthesia, 2ml of patent blue V dye diluted with 2–3ml of 0.9% sodium chloride was injected into the subdermal space of the areola at the 3, 6 and 9 o’clock position. Background radiation at the injection site was recorded. SLN was identified intraoperatively using hand-held gamma probe and presence of blue dye. Following extraction of the SLN, residual cavity radiation via gamma probe was assessed and if less than 10% of injection site, SLN was deemed as successfully removed and sent for immediate OSNA analysis intraoperatively. The result of the OSNA analysis was given via telephone directly to the surgeon as: negative (<250copies/μl), micrometastases (+: >250–<5.0×103copies/μl) or positive macrometastases (++: ≥5.0×103copies/μl). ANC was performed for a positive (++) OSNA result. No further axillary surgery was performed for negative or micrometastases. TTL was defined as the total number of CK19 copies in all positive SLN(s). Further axillary lymph nodes harvested during clearance underwent histopathological analysis postoperatively using haematoxylin and eosin staining and reported by qualified histopathologists. Final tumour histology, total tumour size (mm), nuclear grade (1–3), lymphovascular invasion (LVI), hormone receptor status (ER/PR/HER-2) and total lymph nodes (and status) harvested from ANC were all obtained in the final postoperative histology report.
Statistical analysis
Statistical analysis was performed using MedCalc software version 19.1.7 (Ostend, Belgium). Both univariate and multivariate analyses using logistic regression modelling were performed to assess the association of the variables with the presence of four or more positive lymph nodes. p<0.05 indicates statistical significance. A receiver operator characteristic (ROC) curve was created to assess predictive accuracy of TTL, with measurement of the area under the curve (AUC) for comparison to literature.
Results
In total, 134 patients were identified as having macrometastasis positive (++) OSNA results intraoperatively during the study period and subsequently underwent ANC for analysis (Table 1); 102 patients had one OSNA positive SLN, 32 patients had two OSNA positive SLN removed. The median number of lymph nodes removed during clearance was 11 (range 2–39). Of the 134 positive OSNA patients, 34 had ≥4 positive lymph nodes (25.4%) (Table 1).
Table 1 .
Patient characteristics (n=134)
| Characteristics | No. (%) |
|---|---|
| Age, years (median, range) | 59 (33–89) |
| <55 | 51 (38.1) |
| ≥55 | 83 (61.9) |
| Operation type | |
| Lumpectomy | 57 (42.5) |
| Mastectomy | 77 (57.5) |
| Histological type | |
| Ductal | 103 (76.9) |
| Lobular | 28 (20.9) |
| Other | 3 (2.2) |
| Total tumour size (median, range) | 26 (9–140) |
| Nuclear grade | |
| 1 | 11 (8.2) |
| 2 | 80 (59.7) |
| 3 | 43 (32.1) |
| Oestrogen receptor status | |
| Negative | 12 (9.0) |
| Positive | 122 (91.0) |
| Unknown | 0 (0) |
| Her2 receptor status | |
| Negative | 114 (85.1) |
| Positive | 13 (9.7) |
| Unknown | 7 (5.2) |
| Lymphovascular invasion | |
| Negative | 90 (67.2) |
| Positive | 44 (32.8) |
| Unknown | 0 (0) |
The correlation between clinicopathological variables and N2 disease (≥4 lymph nodes positive) was analysed via univariate analysis (Table 2). Of the variables analysed, lymphovascular invasion (p=0.005) and TTL ≥1.1×105copies/μl (p=0.004) were significantly correlated with ≥4 positive lymph nodes. Both of these variables were again found to be significantly correlated on multivariate analysis (Table 3); lymphovascular invasion (OR=3.20, 95% CI 1.05–6.38 p=0.039) and TTL ≥1.1×105copies/μl (OR 3.37, 95% CI 1.37–8.27, p=0.008).
Table 2 .
Univariate model of variables correlated with N2 disease (≥4 lymph nodes positive)
| Variable | Lymph node metastases, no. (%) | p-value | |
|---|---|---|---|
| <4 (n=100) | ≥4 (n=34) | ||
| Age (years) | 0.659 | ||
| <55 | 39 (76.5) | 12 (23.5) | |
| ≥55 | 61 (73.5) | 22 (26.5) | |
| Operation type | 0.115 | ||
| Lumpectomy | 48 (81.2) | 11 (19.8) | |
| Mastectomy | 52 (69.3) | 23 (30.6) | |
| Tumour size (mm) | 0.052 | ||
| ≤20 | 38 (86.4) | 6 (13.6) | |
| >20 | 68 (70.8) | 28 (29.2) | |
| Nuclear grade | 0.418 | ||
| <2 | 66 (72.5) | 25 (27.5) | |
| 3 | 34 (79.1) | 9 (20.9) | |
| Oestrogen receptor status | 0.175 | ||
| Negative | 9 (0.60) | 6 (0.40) | |
| Positive | 91 (76.5) | 28 (23.5) | |
| Her-2 receptor status | 0.087 | ||
| Negative | 86 (75.4) | 28 (24.5) | |
| Positive | 10 (76.9) | 3 (23.1) | |
| Lymphovascular invasion | 0.005 | ||
| Negative | 74 (82.2) | 16 (17.8) | |
| Positive | 26 (59.1) | 18 (40.9) | |
| Total tumour load (copies/μl) | 0.004 | ||
| <1.1 × 105 | 56 (86.2) | 9 (13.8) | |
| ≥1.1 × 105 | 44 (63.8) | 25 (36.2) | |
The data in bold represent the significant findings.
Table 3 .
Multivariate model of variables correlated with N2 disease (≥4 lymph nodes positive)
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Lymphovascular invasion | 2.59 | 1.05–6.38 | 0.039 |
| Negative | |||
| Positive | |||
| Total tumour load (copies/μl) | 3.37 | 1.37–8.27 | 0.008 |
| <1.1 × 105 | |||
| ≥1.1 × 105 |
The association between TTL and ≥4 positive lymph nodes was evaluated using a ROC curve analysis (Figure 1). Median TTL was 1.1×105copies/μl (5.2×103–1.4×107copies/μl). The AUC analysis of the ROC curve was 0.678, p<0.001 with an optimal TTL cut-off 1.1×105copies/μl (Table 3). Nine (13.8%) patients with TTL <1.1×105copies/μl had ≥4 positive lymph nodes compared with 25 (36.2%) patients with TTL ≥1.1×105copies/μl (Table 2). At optimal TTL cut-off of 1.1×105copies/μl the sensitivity and specificity were 73% and 59%, respectively.
Figure 1 .
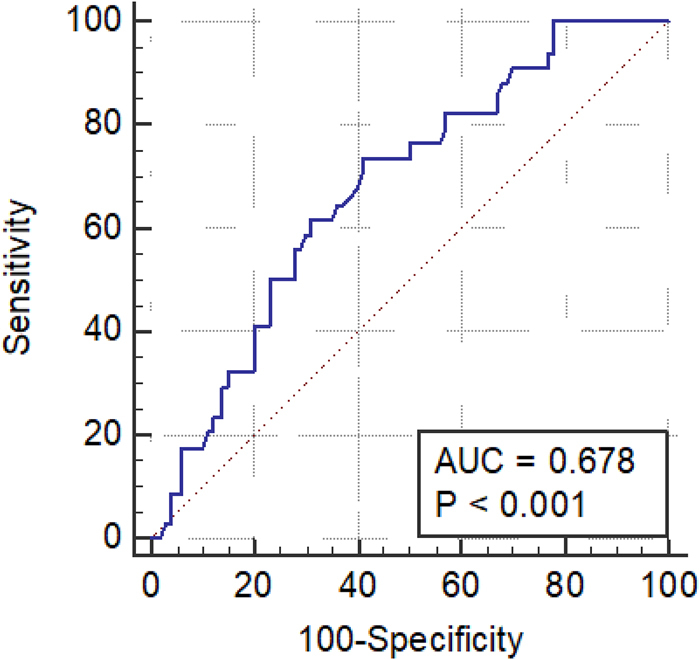
Receiver operator characteristic curve (AUC) of the total tumour load
Conclusion
The aim of this study was to assess if, as previously reported in the literature, TTL (copies/μl) from OSNA assay could accurately predict ≥4 lymph nodes containing metastatic disease from an invasive breast cancer, in our local population. If so, what was the numerical cut-off for TTL that would lead to a significant likelihood of ≥4 positive lymph nodes and therefore stage N2 disease requiring further treatment? This study forms part of a body of evidence to support the use of OSNA TTL to guide surgical management of the axilla intraoperatively. A TTL below a pre-determined cut-off value could potentially be used to support a conservative approach to the axilla avoiding the morbidity associated with a full ANC. OSNA has been the focus of intense research over the last decade, and this is not surprising given its function as an accurate predictor of LN metastases in invasive breast cancer. Moreover, the ability to provide a swift (25 minutes) intraoperative result allows surgeons to make real-time decisions regarding further axillary surgery. A recent 2018 meta-analysis by Shi et al analysed 19 recent studies concluding ‘the pooled sensitivity, specificity and AUC for detecting overall metastasis were 0.90, 0.96 and 0.98, suggesting OSNA could be used to diagnose true-positive patients with SLN metastases as well as rule out false negative results’.9
A meta-analysis performed by Van la Parra et al identified predictive factors for non-SLN involvement in patients with a positive sentinel node.20 They identified clinicopathological variables most predictive of non-sentinel lymph node metastases as SLN metastases >2mm in size, ≥1 positive SLN, ≤1 negative SLN, extracapsular extension in SLN, tumour size >2cm, ratio of positive SLN >50% and lymphovascular invasion of the primary tumour. These factors formed the basis of predictive nomograms to help guide surgical management of the axilla. With the uptake of intraoperative SLN analysis via OSNA assay, new research into predicting non-SLN involvement occurred. Initial research by Ohi et al in 2012 from a series of 130 node-positive patients undergoing ANC reported that whole- node CK19 mRNA copy count number was the strongest independent predictor of ≥4 lymph nodes containing metastatic disease (p=0.014), with lymphovascular invasion (p=0.019) and tumour size >2cm (p=0.024) also significant.24 Cuffolo et al later showed TTL across all SLNs to be of higher predictive value than the highest count from an individual SLN.28 Peg et al in 2013 first reported on the ability of TTL to independently predict non-SLN metastases.25 They analysed 697 patients across multiple centres with clinically node negative, T1–3 stage disease undergoing intraoperative SLN analysis via OSNA with a positive result: ‘The multivariate logistic regression analysis showed that (log) TTL is an independent predictor of metastatic non-SLNs, after adjusting for the tumour size, LVI, HER-2 status and the total number of affected SLNs’. They reported an optimal TTL cut-off of 1.5×104copies/μl exhibited a sensitivity and specificity of 76.7 and 55.2, respectively, at predicting non-SLN involvement, with an AUC of 0.709 comparable with available predictive scores at that time that notably relied on postoperative factors.15,16 The same unit in 2014 published an intraoperative nomogram factoring in TTL validated with an external cohort; the nomogram was accurate with an AUC=0.678.31 Later research published by Shimazu et al created a comparable intraoperative nomogram incorporating TTL with an AUC of 0.70.26 Peg et al in 2017 reported follow-up data of 950 patients, identifying TTL cut-off of 2.5×104copies/μl as a differentiator between high and low risk in terms of disease-free survival, local recurrence and overall survival.29
In direct comparison with this study, Kubota et al looked at whether TTL was an independent predictor of having ≥4 lymph nodes containing metastatic disease.30 With an identical patient cohort size (134), through multivariate analysis they found only TTL to be a significant independent predictor of cut-off for ≥4 positive lymph nodes, and identified TTL of 5.4×104copies/μl as a cut-off with a AUC of 0.70, and sensitivity and specificity of 74% and 59%, respectively. The results exhibited in this study are in agreement with those, in addition to finding lymphovascular invasion to be a significant, independent predictor in keeping with the larger body of research available to date. Differences in the most significant TTL cut-off between our data sets is explained by the relatively low numbers of cases for analysis as well as clinicopathological differences between the two cohorts.
The limitations of this study are apparent; the data set available was limited to 134 patients across a nine-year period. This is in keeping with the size of our institution as a medium-sized UK district general hospital. Our patient cohort in Western Sussex is higher in socioeconomic class as compared with the UK average and as such, exhibits a higher uptake in screening, and earlier presentation via symptomatic pathways. This leads to cancers detected earlier and as such, lower burden of N2 stage disease for analysis. To conclude, presence of lymphovascular invasion and TTL ≥1.1×105copies/μl was significantly correlated with having ≥4 positive lymph nodes. This is in keeping with available literature, and adds to the growing evidence that TTL may be safely used as an intraoperative adjunct to surgical management of the axilla. Further, multicentre research in this area is essential to improve its function and advance OSNA’s potential as an intraoperative axillary staging tool.
Acknowledgements
We would like to thank members of the pathology department at St Richard’s Hospital Chichester involved in assisting our data collection for this study. Also, thanks to Sally Reynolds and Mark Sumner, clinical librarians at Western Sussex NHS Foundation trust, for their assistance in the literature review.
Availability of supporting data
Supporting data were obtained via literature review using MEDLINE and EMBASE database via Open Athens.
Author contributions
Study design: Wendy Sotheran, Ross Kenny.
Data collection: Leila Gould, Ross Kenny.
Data analysis and interpretation: Ross Kenny.
Paper write-up and editing: Ross Kenny, Grace Wong, Olubunmi Odofin, Richard Bowyer, Wendy Sotheran.
References
- 1.Lyman GH, Giuliano AE, Somerfield MRet al. American society of clinical oncology: American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005; 23: 7703–7720. 10.1200/JCO.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 2.Pegolo E, Puppin C, Gerometta Aet al. One-step nucleic acid amplification (OSNA) for intraoperative evaluation of sentinel lymph node status in breast cancer: a comparative study between CK19 protein expression and CK19 mRNA level in primary tumors and lymph node metastasis. Virchows Arch 2013; 463: 7–15. 10.1007/s00428-013-1440-2 [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto M, Nakabayashi K, Yoshidome Ket al. One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res 2007; 13: 4807–4816. 10.1158/1078-0432.CCR-06-2512 [DOI] [PubMed] [Google Scholar]
- 4.Deambrogio C, Castellano I, Paganotta Aet al. A new clinical cut-off of cytokeratin 19 mRNA copy number in sentinel lymph node better identifies patients eligible for axillary lymph node dissection in breast cancer. J Clin Pathol 2014; 67: 702–706. 10.1136/jclinpath-2014-202384 [DOI] [PubMed] [Google Scholar]
- 5.Galimberti V, Cole BF, Zurrida Set al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): A phase 3 randomised controlled trial. Lancet Oncol 2013; 14: 297–305. 10.1016/S1470-2045(13)70035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Frère-Belda M, Bats A, Gillaizeau Fet al. Diagnostic performance of one-step nucleic acid amplification for intraoperative sentinel node metastasis detection in breast cancer patients. Int J Cancer 2012; 130: 2377–2386. 10.1002/ijc.26291 [DOI] [PubMed] [Google Scholar]
- 7.Tamaki Y. One-step nucleic acid amplification (OSNA): where do we go with it? Int J Clin Oncol 2017; 22: 3–10. 10.1007/s10147-016-1030-9 [DOI] [PubMed] [Google Scholar]
- 8.Espinosa-Bravo M, Navarro-Cecilia J, Boyero Met al. Intraoperative assessment of sentinel lymph node by one-step nucleic acid amplification in breast cancer patients after neoadjuvant treatment reduces the need for a second surgery for axillary lymph node dissection. The Breast 2017; 31: 40–45. 10.1016/j.breast.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 9.Shi F, Liang Z, Zhang Qet al. The performance of one-step nucleic acid amplification assay for intraoperative detection of sentinel lymph node macrometastasis in breast cancer: An updated meta-analysis. The Breast 2018; 39: 39e45. 10.1016/j.breast.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 10.Callans LS, Orel SG, Keeney GLet al. Sentinel lymph node biopsy with metastasis: Can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol 1999; 17: 1720–1726. 10.1200/JCO.1999.17.6.1720 [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AE, Hunt KK, Ballman KVet al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. J Am Med Assoc 2011; 305: 569–575. 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardaso F, Kyriakides S, Ohno Set al. Early breast cancer: ESMO clinical practice guidelines 2019. Ann Oncol 2019; 30: 1194–1220. 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 13.Goldhirsch A, Wood WC, Coates ASet al. Panel members: strategies for subtypes - dealing with the diversity of breast cancer: highlights of the St. gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 2011; 22: 1736–1747. 10.1093/annonc/mdr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 2007; 82: 247–253. 10.1016/j.radonc.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Van Zee KJ, Manasseh DM, Bevilacqua JLet al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 2003; 10: 1140–1151. 10.1245/ASO.2003.03.015 [DOI] [PubMed] [Google Scholar]
- 16.Katz A, Smith BL, Golshan Met al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol 2008; 26: 2093–2098. 10.1200/JCO.2007.11.9479 [DOI] [PubMed] [Google Scholar]
- 17.Chagpar AB, Scoggins CR, Martin RCet al. Predicting patients at low probability of requiring postmastectomy radiation therapy. Ann Surg Oncol 2007; 14: 670–677. 10.1245/s10434-006-9107-8 [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita T, Shino S, Takayama Set al. A combination of histology and CK19 mRNA transcripts in sentinel lymph nodes for axillary lymph node staging and prognostication in patients with breast cancer. J Clin Oncol 2016; 34: 1055–1055. 10.1200/JCO.2016.34.15_suppl.1055 [DOI] [Google Scholar]
- 19.Jimbo K, Kinoshita T, Ogura Tet al. Prediction score model for non-sentinel and four or more nodal metastases using a combined method of one-step nucleic acid amplification and histology in sentinel node-positive breast cancer patients. Eur J Surg Oncol 2020; 46: 516e521. 10.1016/j.ejso.2019.10.040 [DOI] [PubMed] [Google Scholar]
- 20.Van la Parra RF, Peer PG, Ernst MF. et al. Metaanalysis of predictive factors for non-sentinel lymph node metastases in breast cancer patients with a positive SLN. Eur J Surg Oncol 2011; 37: 290–299. 10.1016/j.ejso.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 21.Meretoja TJ, Audisio RA, Heikkilä PSet al. International multicenter tool to predict the risk of four or more tumor-positive axillary lymph nodes in breast cancer patients with sentinel node macrometastases. Breast Cancer Res Treat 2013; 138: 817–827. 10.1007/s10549-013-2468-3 [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Zhang Y, Wu Set al. Intraoperative prediction Of Non-sentinel lymph node metastasis based On The molecular assay In breast cancer patients. Cancer Manag Res 2019; 11: 9715–9723. 10.2147/CMAR.S226733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O-charoanrat P, Sa-nguanraksa D, Kulprom A. Nomogram to predict non-sentinel lymph node status of breast cancer using total tumor load determined by one-step nucleic acid amplification (OSNA). Breast Cancer (Auckl) 2019; 26: 471–477. 10.1007/s12282-019-00945-8 [DOI] [PubMed] [Google Scholar]
- 24.Ohi Y, Umekita Y, Sagara Yet al. Whole sentinel lymph node analysis by a molecular assay predicts axillary node status in breast cancer. Br J Cancer 2012; 107: 1239–1243. 10.1038/bjc.2012.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peg V, Espinosa-Bravo M, Vieites Bet al. Intraoperative molecular analysis of total tumor load in sentinel lymph node: A new predictor of axillary status in early breast cancer patients. Breast Cancer Res Treat 2013; 139: 87–93. 10.1007/s10549-013-2524-z [DOI] [PubMed] [Google Scholar]
- 26.Shimazu K, Sato N, Ogiya Aet al. Intraoperative nomograms, based on one-step nucleic acid amplification, for prediction of Non-sentinel node metastasis and four or more axillary node metastases in breast cancer patients with sentinel node metastasis. Ann Surg Oncol 2018; 25: 2603–2611. 10.1245/s10434-018-6633-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fung V, Kohlhardt S, Vergant Pet al. Intraoperative prediction of the two axillary lymph node macrometastases threshold in patients with breast cancer using a one-step nucleic acid cytokeratin-19 amplification assay. Mol Clin Oncol 2017; 7: 755–762. 10.3892/mco.2017.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuffolo G, Gahir-Atwal HK, Smith B. One-step nucleic acid amplification CK19 copy number for sentinel node biopsy in breast cancer: identification of new cutoffs to predict nonsentinel axillary node involvement [published online ahead of print, 2020 Jul 17]. Breast J 2020; 26: 2002–2005. 10.1111/tbj.13977 [DOI] [PubMed] [Google Scholar]
- 29.Peg V, Sansano I, Vieites Bet al. Role of total tumour load of sentinel lymph node on survival in early breast cancer patients. Breast 2017; 33: 8–13. 10.1016/j.breast.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 30.Kubota M, Komoike Y, Hamada Met al. One-step nucleic acid amplification assay for intraoperative prediction of advanced axillary lymph node metastases in breast cancer patients with sentinel lymph node metastasis. Mol Clin Oncol 2016; 4: 173–178. 10.3892/mco.2015.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubio IT, Espinosa-Bravo M, Rodrigo Met al. Nomogram including the total tumoral load in the sentinel nodes assessed by one-step nucleic acid amplification as a new factor for predicting nonsentinel lymph node metastasis in breast cancer patients. Breast Cancer Res Treat 2014; 147: 371–380. 10.1007/s10549-014-3108-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supporting data were obtained via literature review using MEDLINE and EMBASE database via Open Athens.


