Abstract
Building tissues from scratch to explore entirely new cell configurations could revolutionize fundamental understanding in biology. Bioprinting is an emerging technology to do this. Although typically applied to engineer tissues for therapeutic tissue repair or drug screening, there are many opportunities for bioprinting within biology, such as to explore cellular crosstalk or cellular morphogenesis. The overall goal of this Primer is to provide an overview of bioprinting with the biologist in mind, including outlining the steps in extrusion bioprinting (the most widely used and accessible technology), as well as discussing alternative bioprinting technologies and future opportunities for bioprinting in biology.
1. Introduction to bioprinting
Biological questions are traditionally investigated either with cells seeded on 2-dimensional (2D) hard surfaces (e.g., tissue culture plates, glass) or with animal models. 2D cultures can be explored with human cells but are non-physiological with regards to biophysical properties and cellular organization, whereas animal models may be challenging to implement, particularly with human relevance, and the monitoring of spatiotemporal cell behavior is difficult (Benam et al., 2015; Ingber, 2020). 3-dimensional (3D) cultures where cells are encapsulated and cultured within soft materials are seeing increased use, as exemplified by Matrigel, which has particularly improved the culture and range of in vivo-like collective cell behaviors within organoids (Hughes et al., 2010). Motivated by limitations of Matrigel such as batch variability and biochemical complexity, alternative 3D hydrogels from numerous biological and synthetic molecules have been developed and applied to the culture of cells (Aisenbrey and Murphy, 2020; Cruz-Acuña et al., 2017; Gjorevski et al., 2016). Although these approaches are advancing biology, they are still often limited to uniform structures and cultures of single cell types.
To bring further organization to the culture of cells, the field of biofabrication has developed for the creation of cellular constructs that are inspired by or mimic biological processes. These constructs incorporate living cells and extracellular or other biochemical components and are configured into desired structures, particularly for the engineering of tissue constructs for translational applications such as tissue repair and drug screening (Groll et al., 2016). Within the field of biofabrication there are a number of enabling technologies, one of which is bioprinting. Bioprinting is “the use of computer aided transfer processes for patterning and assembly of living and non-living materials with a prescribed 2D or 3D organization to produce bio-engineered structures”(Moroni et al., 2018).
The range of bioprinting technologies available to biomedical researchers is broad. The most common and accessible method is that of extrusion bioprinting, where the pressure-driven extrusion of a bioink from a printer nozzle (sometimes referred to as printer head) is used to print filaments with a user-defined design (Fig. 1) (Ozbolat and Hospodiuk, 2016). Inkjet printing falls under the umbrella of extrusion bioprinting and involves the deposition of bioink droplets through a nozzle rather than as continuous filaments (Li et al., 2020). In contrast, lithography bioprinting methods have also emerged where light is used to spatially pattern a cell-laden hydrogel resin (bioresin) into 3D constructs (Groll et al., 2016; Sun et al., 2020), and these techniques offer improved resolution when compared to extrusion bioprinting (Bertlein et al., 2017). Cell spheroid-based bioprinting technologies (often termed Bioassembly) have also emerged, where cell aggregates can be precisely assembled into cell-dense 3D constructs or structures containing organoids (Moldovan et al., 2016). The bioprinting method used depends on the biological question and requisite considerations with respect to complexity, resolution, and cellularity.
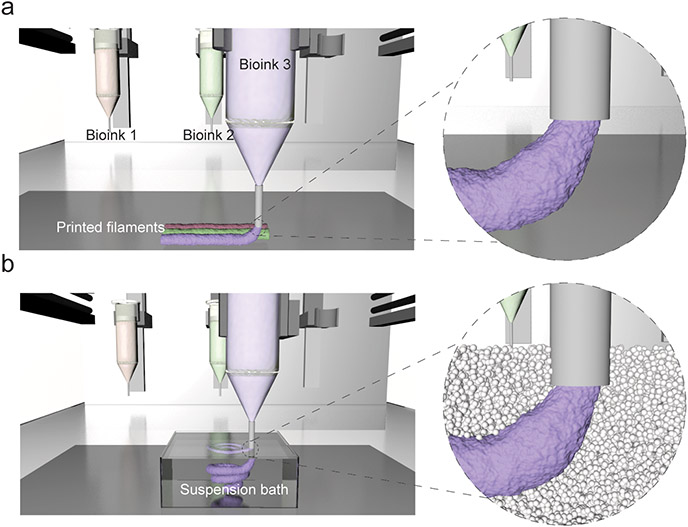
Figure 1. Extrusion bioprinting process.
In extrusion bioprinting, a bioink (formulation of cells, often with a material) is deposited from a printer nozzle either onto a surface (top) or within a suspension bath (bottom) with a user-defined pattern. There are numerous commercially available bioprinters and biomaterials for use in bioinks that are making bioprinting accessible to many users.
With respect to extrusion bioprinting, bioinks can generally be described as “a formulation of cells that is suitable to be processed by an automated biofabrication technology”(Groll et al., 2018). Common to the field is that the bioink is a hydrogel formulation containing single cell suspensions or cell aggregates. The bioink may also be combined with cell-free biomaterial inks that are structural (to help support printed construct stability) or are sacrificial (meaning that they are only present temporarily during processing) (Kang et al., 2016). Further, although bioprinting commonly involves the deposition of bioinks onto surfaces with 3D structures built through the layering of printed filaments, an emergent technique of great interest is the deposition of bioinks into suspension baths (also referred to as suspension media or support hydrogels) that provide support during the printing process (Fig. 1)(Highley et al., 2015; Hinton et al., 2015; McCormack et al., 2020). This technique enables the extrusion printing of bioinks that are otherwise challenging to process using layer-by-layer methods.
Although bioprinting has been widely explored for tissue fabrication in translational medicine (Moroni et al., 2018; Ozbolat and Hospodiuk, 2016), there is much opportunity for the application of bioprinting to address fundamental biological questions. Diverse cell-laden configurations are possible with extrusion bioprinting that span the cell-matrix, cell-soluble factor, and cell-cell interactions that drive biology. This is possible through the bioink selection and the use of multiple bioinks to create 3D constructs, where the bioinks ultimately control the local cellular microenvironment (i.e., biochemical and biophysical signals) and the placement of printed bioinks governs the macroscopic structure and length scales across cell populations. It should be noted that it is still challenging to replicate all of the structural, biochemical, and biophysical properties of tissues and simplified versions are often bioprinted.
The goal of this Primer is to provide an overview of bioprinting for the biologist, including defining the steps and components to extrusion bioprinting, reviewing literature where bioprinting has been used to address biological questions, highlighting emerging bioprinting technologies, and ending with an outlook of where bioprinting technology may be used in the future to address complex biological questions.
2. Practical steps to bioprinting
There are several steps required to implement bioprinting, which we define as (i) Plan, (ii) Print, and (iii) Process, referring to (i) the design of the overall print pattern and bioprinting components (e.g., cells, bioinks, biomaterial inks), (ii) printing the desired construct with the appropriate bioprinter, and (iii) processing the printed construct in the study, respectively (Fig. 2). This all begins by identifying the biological question of interest, which will inherently guide the other decisions during the planning and printing stages. The biological question may dictate the various cell populations that are printed or seeded onto printed constructs, the number and types of bioinks that are used, and the dimensions and geometrical features that are needed. In this section, we walk through the steps in bioprinting in a general manner, with an emphasis on the commonly used extrusion bioprinting technique. Additional information and resources, such as commercially available bioinks and bioprinters and links to user manuals for specific bioprinters, are included in Supplementary Tables 1-4. The following sections then provide numerous examples where bioprinting has been implemented in biological questions.
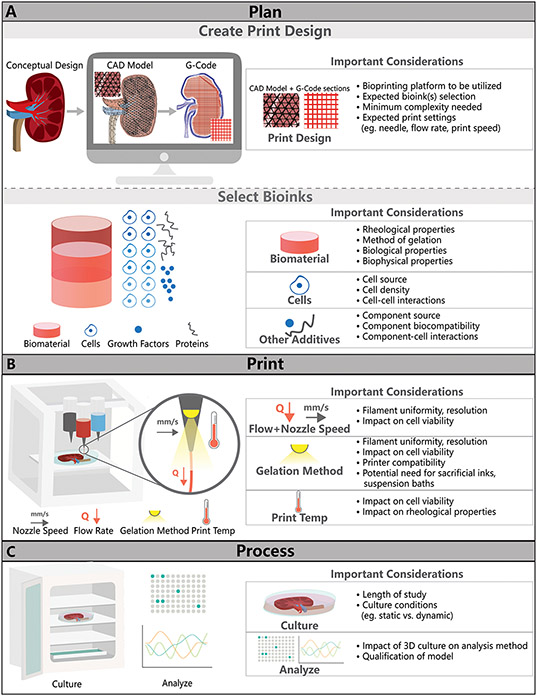
Figure 2. The bioprinting experimental workflow.
This consists of three general parts: (A) planning (e.g., creating a print design and defining the bioink and biomaterial ink to be used), (B) printing the construct, and (C) processing the printed construct over time with cell culture and identifying appropriate analytical outcomes.
2.1. Plan.
2.1.1. Print design.
The planning phase is a very important step in the bioprinting process and includes two important aspects: creating the print design and selecting bioinks (Fig. 2). Printing designs are often created through computer-aided-design (CAD) software, including with commercially-supplied or free software such as FreeCAD, Solidworks, Blender, Onshape and OpensCAD (Junk and Kuen, 2016). Users can create a novel design from scratch or modify pre-existing designs, such as from patient/tissue scans or from other users. Additionally, many commercially available bioprinters come equipped with user-friendly software and support teams to help users with CAD models. For complex prints, such as those mimicking tissue structures, there are numerous open-source resources such as the NIH 3D print exchange, which provides medical and scientifically relevant CAD models (Coakley et al., 2014). Once the CAD model is created, it can be saved and uploaded to printing software to create G-code. Most commercial bioprinters accept STL file formats of CAD files, which save 3D structures as triangular tessellations, as is depicted in Fig. 2. Open source software such as Repetier Host or software provided by bioprinting manufacturers is used to convert these STL files to G-code (Highley et al., 2015). G-code defines the printing path for deposition of the bioink and can specify which bioinks are used throughout the print, if more than one bioink is used. While STL file formats are acceptable for most bioprinting applications, methods to directly convert datasets into G-code are sometimes needed to avoid resolution loss (Bader et al., 2018).
Some important considerations when creating or choosing a print design include considering the minimum complexity needed in the print design and potential print settings such as needle used, extrusion flow rate, and print speed. The bioprinting platform is also important to consider as it can determine restrictions on bioink compatibility and achievable print resolution (e.g., ability to heat or cool ink during extrusion, minimum extrusion pressure). Key parameters in the printing process are interdependent on one another and as a result the extrusion optimization process is often iterative. For example, the needle diameter or filament flow rate chosen during the “Print” process inform the G-code design (print path, fill factor, and print speed). The needle diameter will influence the filament width and therefore the smallest geometrical features sizes possible for the printed geometry (Blaeser et al., 2016).
Most commercial platforms have excellent user manuals and training programs to guide new users through these parameters and a selected list of these commercial platforms can be found in Supplementary Tables 3 and 4. Ideally, it is best to simplify print designs as much as possible to decrease unnecessary complexity in the experimental workflow. For example, large, intricate designs such as the kidney model depicted in Fig. 2 may be possible to fabricate but will take extended time to print and will likely be difficult to culture and analyze. To address this, researchers have simplified the kidney to an appropriate in vitro model (as discussed in more detail in Section 3) to study crosstalk between renal kidney tubules and vasculature (Lin et al., 2019). Instead of creating multiple channels or trying to recapitulate complex kidney microarchitecture, the successful model focuses on a two-channel design that is easier to create and analyze but still effectively probes experimental study questions.
2.1.2. Bioink selection.
Selection of bioinks is the other major step in the planning process of the bioprinting experimental workflow. While a brief overview of this selection process is provided here, numerous publications offer excellent in-depth reviews of commercially available and state-of-the-art bioinks (Malda et al., 2013; Sun et al., 2020). The selection of the bioink is based on the printability of the ink (e.g., compatibility with the printer, print resolution) and the impact of the bioink on cell behavior. General considerations in bioink selection are described in Fig. 2 and in more detail in Table 1. There are many commercially available bioinks that can be readily combined with desired cells (outlined in Supplementary Table 1), as well as potentially useful biomaterial inks (to provide structure or that are sacrificial) and suspension baths (outlined in Supplementary Table 2).
Table 1.
Considerations in the selection of bioinks for extrusion bioprinting.
| Specification | Consideration |
|---|---|
| Rheological Properties | Rheological properties of a bioink will impact both cell response and printability. Shear-thinning hydrogels are often considered ideal for bioprinting, as these materials can flow during extrusion and may protect cells from shear stresses. Polymer concentration can be varied to control shear-thinning behaviour with higher polymer concentrations often possessing improved rheological properties (Liu et al., 2017a). Rheological additives such as gelatin or methylcellulose can be used to induce shear-thinning behaviour (Ahlfeld et al., 2020; Ouyang et al., 2020). |
| Method of Gelation | The method of gelation (e.g., photo-crosslinking, thermal) for a bioink should ideally be fast and nontoxic to cells. The gelation method will determine compatibility with select bioprinters while length of gelation will determine whether extra support, such as a suspension bath, is needed during bioprinting. |
| Biological Properties | Biological properties of a bioink will impact encapsulated cell response. Properties such as adhesion to cells and the ability to degrade in culture will be important characteristics to understand in the context of an experiment. |
| Biophysical Properties | Biophysical properties, such as the elastic modulus of a bioink, can impact cellular responses such as growth and differentiation. |
| Suspension Bath Recommended? | If a bioink does not have ideal rheological properties or if the bioink has a long gelation time, the bioink can be printed into a suspension bath or alongside a sacrificial biomaterial ink such as pluronic to offer temporary support and improve resolution. |
Printability is generally related to the rheological properties of the bioink that permits extrusion during printing and the mechanism that allows stabilization upon deposition onto a surface or within a suspension bath. Traditional bioinks are viscous solutions, which may shear-thin during printing (meaning the viscosity decreases as mechanical shear is applied during extrusion from the nozzle) and then recover after deposition and in many cases the bioink will undergo further stabilization and crosslinking (i.e., gelation), such as with light (photo-crosslinking), chemical reaction (mixing, ionic, enzymatic), or temperature change (thermal). As bioprinting technologies and methods have developed, compatibility with various biomaterial formulations has improved and techniques such as use of a suspension bath can aid in the processing of low viscosity bioinks (Fig. 1).
With regards to cellular interactions of a bioink, the bioprinting process may impact cell viability and guidance should be taken from previous reports and commercial conditions to avoid exposing to excess shear stresses during extrusion (Blaeser et al., 2016). Each bioink will present different biochemical and biophysical features to cells and the desired bioink may be related to the specific cell type and biological question. For instance, if the question relates to mechanobiology (the translation of local mechanics to biochemical signaling), a bioink where the mechanical properties of the bioink can be easily modulated should be considered. In addition, the bioink must provide a suitable microenvironment for the cell type being printed (primary, embryonic, or pluripotent derived); however, a detailed description of cell-hydrogel interactions is outside the scope of this paper and the reader is directed to published reviews (Caliari and Burdick, 2016; Tibbitt and Anseth, 2009).
When possible, the use of commercially available and off-the-shelf materials is encouraged as these products come complete with rheological testing and suggested print settings, limiting laborious troubleshooting and characterization needed from the user. Some of these available bioinks are detailed in Supplementary Table 2. If developing a custom bioink for extrusion bioprinting or other bioprinting platforms, the reader is directed to previous publications detailing the bioink development process (Gillispie et al., 2020). Other resources may also be found through manufacturers of commercial printers, who often provide useful guides for characterization of novel bioinks for specific platforms (Supplementary Table 4).
2.2. Print.
Once the planning process is complete, the user can move to printing. There are a variety of bioprinting technologies that are well defined in previous reviews (Matai et al., 2020). Of these technologies, extrusion-based systems tend to be the most versatile platforms for bioprinting. Extrusion bioprinting creates 3D constructs via the dispensing of bioink filaments through nozzles, which are controlled through pneumatic pressure or syringe pumps (Matai et al., 2020). These systems are compatible with a wide variety of bioinks and include various features (heating, cooling, light exposure) that allow processing of the aforementioned bioinks (Fig. 2). Many systems also include multiple extruders to allow the users to print with multiple bioinks in a single print. There are a variety of commercial solutions that allow access to extrusion bioprinting technology without requiring the ability or time commitment to build custom systems, some of which are outlined generally in Supplementary Table 3, with further details provided in Supplementary Table 4. Commercially available systems also come with significant support, standardization and communities of users and their costs range from entry-level to expert, based on features such as print resolution, number of print nozzles, and range of printing technology included.
2.3. Process.
The final step in the bioprinting experimental plan is to process bioprinted constructs (Fig. 2). This step involves both culture and analysis of printed constructs and is dependent on the specific biological question being asked. Important considerations include the length of the study, which may dictate the stability of the bioink and printed structure, and media formulations that are dependent on the various cell populations included. As with previous steps in the bioprinting process, users may have to revisit the planning portions of the process to adjust bioink formulation or print parameters based on results or updated protocols in the process phase. Constructs that are too large may limit nutrient transport to incorporated cells. Custom bioreactors may also be needed, such as to perfuse the channels within bioprinted structures (Lin et al., 2019). While not the focus of this article, more information on analysis of 3D cell-laden constructs and qualification of these models for industry or clinical use is detailed in previous publications (Caliari and Burdick, 2016; Ekert et al., 2020).
3. Applications of bioprinting in biology
There are numerous examples where bioprinting has tackled biological questions, particularly using extrusion bioprinting, and there are many opportunities to explore. These studies have been largely motivated by either the development or repair of tissues and have involved printing constructs with spatially patterned cell populations and/or biochemical factors. This section will provide the reader with various examples where bioprinting has been implemented in biological questions already and identify why bioprinting was useful over traditional fabrication techniques.
3.1. Bioprinted models to study tissue development and repair.
3.1.1. Biochemical gradients.
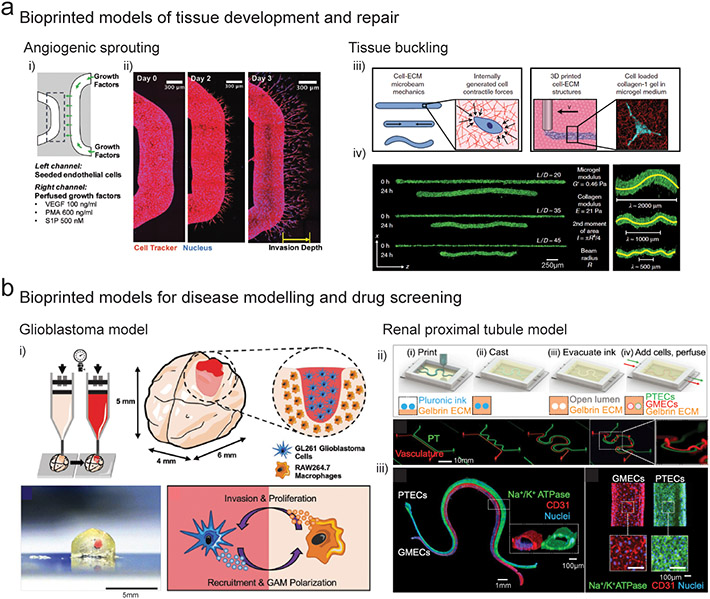
Biochemical gradients provide spatiotemporal cues to direct cell differentiation in developing tissues (Rogers and Schier, 2011). It is challenging to recapitulate the spatiotemporal complexity of developmental cascades where multiple signaling centers transiently arise to direct differentiation and morphogenesis; however, bioprinting is a promising technology for such applications as the spatially patterning of multiple biochemical species in 3D hydrogels is possible that are then subject to diffusive mass transfer. To study vascular angiogenesis in response to soluble factors, printing into suspension baths was used to create vascular channels inside cell degradable hydrogels and a second channel was used to present a gradient of a cocktail of growth factors (Fig. 3a) (Song et al., 2018). Interestingly, when endothelial cells sprouted from the channel towards the biochemical gradient, increased sprouting was observed at curved locations. This study highlights how bioprinting technology is useful to probe how biochemical signaling is interpreted in complex geometric contexts to influence biological processes such as collective cell migration. Further, such geometrically complex channels (e.g., introducing curvature, creating interconnected channel networks) would be challenging with other more traditional methods (e.g., sacrificial molding). Combinatorial arrays of morphagen gradients are also possible (Miller et al., 2009), where traditional techniques are limited to single gradients.
Figure 3. Bioprinted models of tissue development and disease.
(a) i) Angiogenic sprouting - Schematic of 3D printed microchannels within cell degradable hydrogels where the left channel is seeded with endothelial cells and the right channel is perfused with angiogenic factors, and ii) endothelial cell sprouting from the vascular channel towards the VEGF gradient over 3 days of culture. (Song et al., 2018) iii) Tissue buckling - Schematic demonstrating embedded 3D printing of collagen filaments containing fibroblasts into a granular yield stress media (i.e., suspension bath), and fibroblast contraction of the collagen matrix inside the printed filament, and iv) contraction and buckling of the collagen filament over 24 hours of culture as a function of the filament aspect ratio (length/diameter). (Morley et al., 2019) (b) i) Glioblastoma model – Schematic and image of extrusion bioprinting of a mini-brain model containing compartmentalized regions of glioblastoma cells and macrophages to study the role of macrophages in glioblastoma progression. (Heinrich et al., 2019) ii) Renal proximal tubule model – Schematic demonstrating 3D printing of a sacrificial pluronic ink to generate convoluted perfusable channels inside a gelatin/fibrin matrix, and iv) seeding of the microchannels with proximal tubule epithelial cells (PTECS) and glomerular microvascular endothelial cells (GMECs) to generate parallel vascular and renal epithelial channels to study solute renal reabsorption. (Lin et al., 2019)
3.1.2. Biophysical morphogenesis.
During development as tissues grow and expand, internal and interfacial pressures and tensions are generated which can lead to mechanical instabilities such as folding and buckling (Nelson, 2016). These shape-morphing events contribute to tissue patterning through mechanotransduction-mediated cell specification and remodeling of the ECM (Mammoto and Ingber, 2010). Reconstructing biophysical models of tissue morphogenesis using traditional in vitro culture methods is challenging; however, cell laden bioinks offer a promising approach where cell-generated forces and ECM mechanics can be spatially controlled. Morley et al. used printing within a suspension bath to extrude a fibroblast laden collagen bioink into a granular support slurry, and then measured the time dependent changes in filament geometry as a result of cell generated traction on the collagen (Morley et al., 2019). By varying the filament length and diameter, and also the mechanics of the bioink and the suspension bath, a range of mechanical deformations including buckling, axial contraction, failure, and total static stability were observed (Fig. 3b). This platform holds tremendous potential for studying biophysical morphogenesis in 3D across multiple cell types, and the design flexibility afforded by bioprinting technology allows investigation into how geometrical features arise during morphogenesis. This approach provides advantages of the freedom of design and control over geometrical features when compared to traditional methods of molded hydrogels (e.g., collagen, fibrin).
3.1.3. Paracrine signaling and co-culture.
During tissue development, cells communicate using paracrine signals via several highly conserved receptors and pathways. Bioprinting technologies offer a promising platform to study paracrine signaling in vitro as multiple cell populations can be compartmentalized in 3D matrices with biomimetic patterning, which can be challenging to achieve using traditional cell culture methods. In the context of liver development, hepatocytes and endothelial cells have been printed into lobule-like geometries with biomimetic heterocellular localization, resulting in enhanced maturation compared to co-cultures that lacked geometric structure (Kang et al., 2018). Bioprinting allows control over distinct cell populations to investigate paracrine signaling (Jeon et al., 2020), which is challenging or not possible with traditional methods (e.g., Transwell inserts, sequential micromolding).
3.2. Bioprinted tissues for disease modeling
3.2.1. Cancer disease models.
Ex vivo cancer models are aiding in the design of personalized drug treatment regimes and to understand the basic biology that underlies disease. However, recreating the complexity of the cancer environment in vitro - including stroma and immune interactions, angiogenesis, and ECM remodeling - is challenging with traditional culture methods. In particular, the resistance of cancer cells to chemotherapy drugs is well known to be modulated by interactions with surrounding stromal and immune cells and simplified 2D cell cultures do not capture this complexity (Pauli et al., 2017). To develop a model of the glioblastoma microenvironment, extrusion bioprinting of decellularized ECM inks was used to create compartmentalized regions of glioblastoma cells and endothelial cells, which better mimicked the tumor-stroma interactions when compared to mixed co-cultures and reproduced clinically observed patient-specific resistance to treatment (Yi et al., 2019). In another study, a mini-brain model with compartmentalized regions of glioblastoma cells and macrophages was developed through extrusion bioprinting using a GelMA bioink (Fig. 3c) (Heinrich et al., 2019). Glioblastoma cells were observed to actively recruit macrophages into the tumor region and polarized them into a glioblastoma-macrophage phenotype, which demonstrated correlations with clinically generated transcriptome data. Future studies will likely build on these techniques to integrate additional vascular, immune, and stromal components to provide predictive tissue models to dissect the multifactorial complexity of the cancer microenvironment.
3.2.2. Tubular disease models.
3D bioprinting approaches using sacrificial inks offer an elegant approach to generate perfusable microchannels inside 3D hydrogels (Highley et al., 2015). While these approaches have been predominantly focused on engineering tissues for implantation, there is a significant opportunity to develop vascular and epithelial disease models. For example, sacrificial embedded printing has been used to engineer tissue models to study reabsorption of solutes and crosstalk between renal kidney tubules and vasculature, which are in tight juxtaposition along non-linear paths, a difficult construction problem that warranted bioprinting innovation (Lin et al., 2019). Using a sacrificial pluronic ink, two parallel microchannels were printed inside a fibrin matrix and epithelial and vascular monolayers were generated by seeding one channel with proximal tubule epithelial cells and a second channel with vascular endothelial cells (Fig. 3d). Flow through the channels was controlled using a closed-loop perfusion system to study renal reabsorption of glucose from the epithelial channels into the vascular channels, and the model was able to recapitulate endothelial cell dysfunction and enhanced reabsorption in hyperglycemic disease conditions.
3.2.3. Fibrosis disease models.
Following tissue injury in the heart, liver, and lung, adverse pathological remodeling can lead to the development of non-functional fibrotic tissue that disrupts surrounding healthy tissue and leads to eventual organ failure. Engineered models of tissue fibrosis could offer a significant opportunity to study disease progression or tissue repair; however, it is challenging to reconstruct the heterogenous cellular and extracellular patterning that arises following scarring using traditional culture methods. To develop a model of liver fibrosis, extrusion bioprinting was used to create structured layers of hepatocytes, activated stellate cells, and endothelial cells (Lee et al., 2020). The model exhibited characteristics of fibrotic remodeling including collagen accumulation, cell apoptosis, and reduced liver function that was attributed to the presence of the stellate cell population, and it was possible to attenuate fibrosis using drugs targeting stellate cell activation.
4. Advanced bioprinting technologies
Although extrusion bioprinting is a common and accessible bioprinting technology, there are other related technologies that may be of use in the pursuit of biological questions. This section highlights examples where these advanced bioprinting technologies have been implemented, and the advantages and disadvantages of these techniques over extrusion technologies (Table 2).
Table 2.
Comparison of different bioprinting technologies.
| Bioprinting technology |
Advantages | Disadvantages |
|---|---|---|
| Extrusion bioprinting |
|
|
| Lithography bioprinting |
|
|
| Spheroid bioprinting/ bioassembly |
|
|
4.1. Lithography bioprinting.
Lithography is achieved by concentrating light into a 2D plane to locally solidify a hydrogel resin, and then a robotic stage vertical translates the crosslinked layer to allow sequential layer-by-layer crosslinking into a 3D solid. Several variations of lithography exist depending on how light is delivered – stereolithography technologies (SLA) utilize a scanning laser beam, whereas digital light processing technologies (DLP) utilize a digital mirror device to rapidly mask light into 2D patterns (Lim et al., 2020). Lithography technologies can create physical features in 10-100μm range which is a significant advantage compared to extrusion technology which has a minimum resolution of ~100μm (Bertlein et al., 2017; Lim et al., 2018). Further, the development of biocompatible photo-crosslinking chemistries has enabled the design of hydrogel resins that can support high cell-viability (i.e. bioresins) (Lim et al., 2020).
Deciding between extrusion or lithography bioprinting depends on the cell/tissue model being developed. For example, extrusion bioprinters are relatively cheap compared to lithography systems. In addition, extrusion bioprinting is compatible with a wide variety of bioinks, whereas lithography bioprinting is only compatible with photo-crosslinkable bioinks/bioresins. It can also be challenging to fabricate heterogenous constructs using lithography methods as the bioresin cannot be easily switched out during fabrication, limiting applicability toward co-culture models, although it should be noted that newer technologies have recently been developed to address this limitation (Miri et al., 2018). As lithography technologies become more widespread and commercially available, they are increasingly being used to engineer tissue and organ models; however, a detailed description of the steps to lithographic techniques and their components is outside the scope of this article.
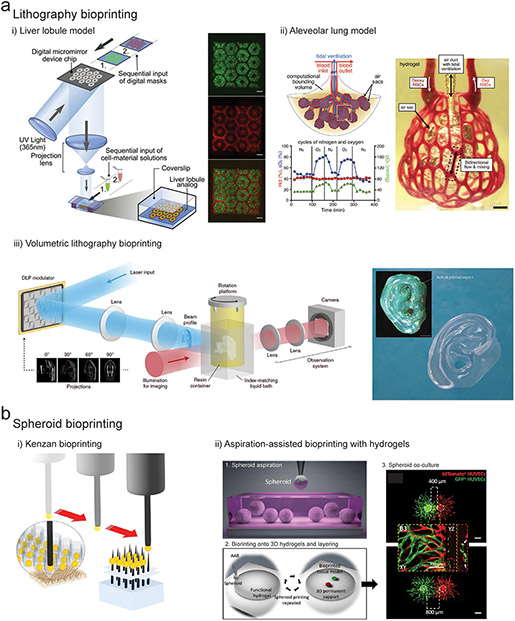
There are numerous interesting examples where lithography techniques have been used to fabricate cell-laden structures or structures that are subsequently seeded with cells for biological questions. For example, to recreate the microarchitectural complexity of a liver lobule, Ma et al. used DLP lithography to pattern iPSC-hepatocytes, endothelial cells, and adipose-derived stem cells in a biomimetic hexagonal microarchitecture using a gelatin bioresin (Fig. 4a i) (Ma et al., 2016). The bioprinted tri-culture model enhanced hepatocyte functionality compared to 2D and 3D controls, with increases in liver specific gene expression, albumin secretion, and drug metabolizing enzymes.
Figure 4. Advanced bioprinting technologies.
(a) i) DLP lithography bioprinting process where light is spatially projected onto a cell laden bioresin using a digital micromirror device to create a liver lobule construct (green regions contain iPSC-hepatocytes, and red regions contain endothelial cells & adipose-derived stem cells. Scalebars 500μm. (Ma et al., 2016) ii) DLP bioprinting of an alveolar lung model containing a central mechanically ventilated air sac surrounded by vascular channels perfused with red blood cells, and demonstration of gaseous exchange through measurement of reoxygenation of the red blood cell population (green line) following oxygenation of the air sac (blue line). Scalebars 1mm. (Grigoryan et al., 2019) iii) Experimental setup for volumetric bioprinting including laser input followed by DLP projection modulation of light onto a rotating platform containing the bioresin. Image of bioprinted human ear model created using a cell laden GelMA bioresin, total printing time 22.7 seconds. Scalebar 2mm. (Bernal et al., 2019; Loterie et al., 2020) (b) i) Kenzan bioprinting method where cell spheroids are aspirated and then skewered onto metal needles for fusion into 3D constructs. (Moldovan et al., 2016) ii) Aspiration-assisted bioprinting of spheroids (labelled red and green) onto fibrin hydrogels at different separation distances to study paracrine signaling and angiogenic sprouting. Scalebar 400μm. (Ayan et al., 2020)
Lithography bioprinting can also be used to create microchannels inside 3D hydrogels, to create hierarchical interconnected networks (e.g. capillary beds). Grigoryan et al. utilized lithography bioprinting to engineer two entangled open-channel networks within a synthetic hydrogel (Grigoryan et al., 2019). The first network was perfused with deoxygenated red blood cells (RBCs) and the second network was perfused with humidified gaseous oxygen, resulting in reoxygenation of the RBCs during flow. To further demonstrate the power of this technology, the authors bioprinted a vascularized alveolar lung model containing ventilated air sacs surrounded by perfused vascular beds to study oxygenation of RBCs in response to mechanical ventilation in the air sacs (Fig. 4a ii).
Due to the layer-by-layer nature of SLA and DLP lithography technologies, particularly long processing times are required to create large volumes which is a disadvantage of this technology. To address this fundamental limitation, volumetric lithographic technologies have recently been developed where light energy is delivered to a material volume from a set of 2D image projections delivered simultaneously from multiple angles (Fig. 4a iii) (Kelly et al., 2019; Loterie et al., 2020). The additive light dose exposure from multiple angles results in a 3D energy dose that rapidly solidifies a resin volume. In an important study, Bernal et al. demonstrated that this technology could be adopted for bioprinting purposes, enabling rapid fabrication of anatomically shaped and human-scale cell-laden constructs (Bernal et al., 2019).
4.2. Spheroid bioprinting.
The printing of high cell density constructs is an important consideration, as cells rarely exist in isolation and coordinated cellular collective processes mediated by cell-cell contact underlie developmental morphogenesis (Hall and Miyake, 1995). In addition, many disease states such as fibrosis or cancer are challenging to faithfully recapitulate when single cells are dispersed throughout gels. Cells self-organize into miniaturized spheroid or organoid structures in vitro, and for years biologists have been using these systems to study human development and disease in vitro (Fatehullah et al., 2016). In particular, organoid models can display emergent levels of physiological structure and function due to their high cell-density and capacity to support developmental like cell sorting and differentiation (Rossi et al., 2018). However, there is limited control over the self-organization processes, and organoids possess non-polarized structurally immature microarchitectures compared to native organs (Laurent et al., 2017). This has led to the development of hybrid bioprinting technologies capable of processing self-organized tissues (often cell spheroids) into 3D constructs to scale and direct self-organization.
Early work in this area demonstrated that pre-formed spheroids can fuse through liquid-like coalescence to minimize adhesive-free energy (Fleming et al., 2010). To scale this into a bioprinting process, multiple spheroids were fused into tissue strands, followed by automated extrusion of the strands into larger 3D constructs (Norotte et al., 2009). The kenzan method has also been developed where cell spheroids are skewered onto supporting metallic needles for fusion into 3D constructs, followed by removal of the fused tissue from the needle supports (Fig. 4b i) (Moldovan et al., 2016). This system is commercially available and has been used to fabricate a range of different tissue models (Moldovan et al., 2016). More recently, hydrogel based spheroid bioprinting technologies have been developed where spheroids are printed into 3D constructs through sequential layering of an uncrosslinked hydrogel precursor and spheroids, followed by crosslinking of the hydrogel layer (Fig. 4b ii) (Ayan et al., 2020). These systems avoid mechanical disruption of spheroids, enabling precise positioning in 3D with improved control over geometry and heterogeneity (Ayan et al., 2020). To study how far paracrine signals can travel in the ECM, Ayan et al. used aspiration-assisted bioprinting to print endothelial cell spheroids in a fibrin hydrogel at varying degrees of separation (400, 800, & 1200μm) (Ayan et al., 2020). Limited interactions were observed at high separation; however, at closer proximity enhanced EC sprouting and capillary network formation were observed.
Although less widely used than extrusion bioprinting, spheroid bioprinting technologies hold significant promise for developing organ and tissue models. As an example, several varieties of brain organoids have been developed to mimic different regions of the brain (Di Lullo and Kriegstein, 2017), and simple fusion between two organoid phenotypes has been used to study regional interactions in vitro (Birey et al., 2017). Spheroid bioprinting methods could provide a powerful method to direct fusion into more biomimetic organotypic assemblies. Finally, there has been increased interest in engineering vascularized organoids to enhance oxygen and nutrient delivery in core regions, and also to model vascular interactions during development and disease (Cakir et al., 2019). To facilitate scaling-up of organoids into vascularized 3D tissues, thousands of aggregates have been jammed together in supporting molds to create self-healing granular tissue matrices that can support sacrificial embedded 3D printing of perfusable vascular channels (Skylar-Scott et al., 2019b). Embryoid bodies, cerebral organoids, and cardiac spheroids were compatible with the process, and the inclusion of vascular channels enhanced cell viability within core regions of the tissues. It should be noted that spheroid bioprinting has some limitations of relatively long processing times and limitations in the complexity of printed structures; however, there are many benefits related to the high cell densities that mimic tissue-like features.
5. Outlook for bioprinting in biology
Bioprinting has great potential for the exploration of biological questions where traditional techniques are insufficient to build-in desired complexity and organization, and the technology is in its infancy with regards to its potential in biological research. Below we have outlined several biological contexts where the technology may be particularly useful.
Biochemical signaling at a distance.
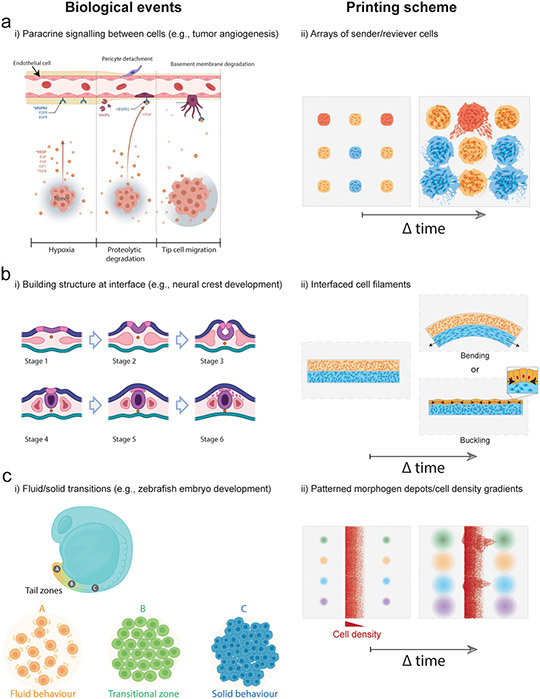
The term “morphogen” was coined by the computer scientist Alan Turing to describe factors that form a spatially non-uniform distribution spanning multiple cell-lengths to instruct different cell fates at distinct levels (Green and Sharpe, 2015). Understanding morphogen gradients in vivo is complex due to a limited ability to change spatial features of developing tissues (Hiscock and Megason, 2015). Bioprinting could play a key role here by juxtaposing engineered or primary tissue-derived morphogen “sender” cells with morphogen “receiver” cells, perhaps in periodic arrays, extending emerging efforts in 2D to a 3D context (Li et al., 2018). Questions such as tissue size and composition, diffusion distance, and diffusion/absorption rates are ideally suited to a combination of cell engineering and bioprinting methods, which can control these variables quantitatively (Ozbolat and Hospodiuk, 2016; Song et al., 2018). For example, extrusion bioprinting could be used to create arrays of cell depots enabling combinational screening of paracrine signaling between distinct cell populations such as interactions between vascular and tumor cells (Fig. 5a). Parameters such as the depot spacing could be varied across multiple cell types followed by mapping out of functional (proliferation, migration, ECM secretion) and phenotypic variables (protein/gene expression) through live imaging (Fig. 5a). Cells could also be engineered for signaling or biochemical secretion triggered by light or small-molecules to study how specific morphogens influence paracrine interactions in a highly controlled manner.
Figure 5. Bioprinting approaches to biological questions in tissue development and homeostasis.
(a) To study interactions between endothelial and tumor cells in a highly controlled manner, spatial combinatorial patterning of engineered “sender” and “receiver” cell arrays could test hypotheses around diffusible biochemical signaling and their influence on cell phenotype and function (e.g., protein/gene expression, migration, proliferation). This could be achieved by patterning cell depots of distinct compositions at prescribed spacing within ECM hydrogels and then monitoring cell behaviour during culture. (b) To study biophysical morphogenesis in neural crest development strains at bioprinted tissue interfaces could be generated through internally generated cell-cell or cell-ECM forces to create dynamic changes in tissue shape. Bioprinting could be used to interface two filaments where differences in cell behaviour (e.g., contractility, proliferation) within filaments drive bending or buckling behaviours. (c) Gradients in growth factor concentrations could stimulate formation of fluid-like and solid-like domains to guide dynamic remodeling of bioprinted tissues, and to study microenvironmental conditions that drive tissue fluidity (i.e., interplay between cell-cell interactions, cell-ECM interactions, and morphogen presentation), such as during head-to-tail elongation in the zebrafish embryo. Models could be produced by patterning morphogen depots adjacent to filaments containing cell density gradients, allowing combinatorial screening of cell migration behaviour across conditions.
Building structure at Interfaces.
Biological structure is often built at interfaces between cell populations with different properties and eventual fates. Examples across developmental time include induction and spatial segregation of the three germ layers during gastrulation (endoderm, mesoderm, ectoderm), and self-organization of distinct early embryonic structures such as the neural tube and somites (early body segments). Engineering efforts to control interfacial structure formation will be crucial to forming new in vitro tissues for disease modeling and to explore biological questions. Indeed, cells show a range of dynamics at these interfaces that are open to engineering. These dynamics include cell sorting on the basis of cell-cell adhesion or cell-ECM adhesion properties (Cerchiari et al., 2015), establishment of polarity -- distinguishing apical (up) and basal (down) directionality to cells (Andrew and Ewald, 2010; Nissen et al., 2018), as well as in-plane directionality (“planar cell polarity”) (Butler and Wallingford, 2017), and collective migration (Cetera et al., 2018). The positioning of interfaces and the behavior of cells at interfaces can also be refined by cell-cell or cell-ECM repulsion/avoidance cues such as Eph-Ephrin and Versican signaling (Scheideler et al., 2020; Szabó et al., 2016). In total, these collective cell decisions then create a “blueprint” for future events that sculpt tissues. For example, planar cell polarity appears to have an important role in driving epithelial tubule elongation and sculpting craniofacial cartilage (Kaucka et al., 2017). This is achieved by setting the direction of oriented cell divisions and cell “intercalation”, in which groups of cells adjust their geometric relationship to each other in such a way as to elongate in one direction while contracting along a radial or orthogonal direction (Kaucka et al., 2017). Further, mechanical tension within tissues feeds back into planar cell polarity and oriented cell division (Aw et al., 2016).
This reveals an opportunity to explore the effect of patterned tension fields on cell collective behaviors within bioprinted objects over time. Indeed, bioprinting has a distinctive role to play here, because placing cells at synthetic interfaces in 3D would begin to create a biochemical-to-morphological map that could be exploited to study the development of tissue interfaces such as the neural crest (Fig 5 b). 3D bioprinting could be used to interface two filaments within supportive ECM hydrogels and several parameters could be varied such as the cell type (epithelial, mesenchymal), the ECM type (collagen/laminin rich), the filament ECM mechanics (stiffness, viscoelasticity), and the initial filament geometry (straight, curved) (Fig. 5b). Such approaches could be used to study how local differences in collective cell behaviour can generate internal tensions and forces that drive morphological changes in the interface development such as local bending and buckling (Fig. 5b).
Shape change.
Coordinated shape change in cell sheets and tubules through intercalation and oriented cell division is complemented by a range of other shape-change phenomena at interfaces. In principle, a shape-change will accompany any local or global mechanical strain (change in length) parallel to an interface that is not relieved by viscoelastic dissipation (Clément et al., 2017). Developing tissues employ several strategies for inducing strain at interfaces, including changes in apical dimension of cell sheets (“apical constriction”) (Martin et al., 2009); and differential growth, contractility, or mechanical constraint in one tissue layer at the interface relative to the other (Hughes et al., 2018; Spurlin et al., 2019). Other shape changes can also be achieved by appropriate spatial patterning of fluid-like and solid-like (jammed) cell domains (Fig. 5 c) (Mongera et al., 2018), where fluidity of a domain is associated with lower cell-cell adhesion, higher cell motility, and/or lower cell density (Ihermann-Hella et al., 2014; Sadati et al., 2013). Bioprinting methods could aid in establishing the relationship between fluid-like states and geometric, mechanical, and biochemical features of the tissue microenvironment. Bioprinting could be used to create tissues with cell density gradients to determine if cell density alone is sufficient to trigger formation of a tip-stalk phenotype, and if not, which additional microenvironmental features need to be specified. For example, engineered tissue with intrinsic mechanical stress profiles that occur at high aspect-ratio features due to cell-matrix traction could be patterned within biochemical gradients thought to reinforce “tip” cell states (such as GDNF in the developing kidney) (Gjorevski et al., 2015; Menshykau et al., 2019). This could be achieved by patterning depots of morphogens adjacent to cell density gradients for combinatorial screening of cell migration across a wide range of microenvironmental conditions (Fig. 5c). Such experiments could establish fundamental understanding for designing bioprinted tissues that undergo, for example, programmed branching morphogenesis processes in vitro. They would also lend quantitative understanding to shape-change phenomena in new embryo-like organoids (van den Brink et al., 2020).
6. Summary.
This review provides an overview of bioprinting as a field, describing the steps to implement the commonly used extrusion bioprinting technology, and reviewing examples where this technology has been used to address biological questions. In some instances, the information provided here can act as a guide for the bioprinting of simple structures, whereas in other cases it may be useful to develop collaborations with the appropriate engineers or directly with bioprinting companies to help accelerate the adoption of bioprinting by biologists.
Efforts in the use of bioprinting in biology will only grow as bioprinting technology advances – from new bioinks developed to mimic the dynamic nature of biology to new bioprinters and bioprinting approaches that match the complexity of biology. To further increase widespread adoption, engineers are continuing to streamline bioprinting technologies to improve automation in order to limit the experience required by the operator. For example, the development of automated “mid-print” feedback mechanisms between the machine and the bioprinted object could fully automate the bioprinting process (Sun et al., 2020). Bioprinters are also being developed with microfluidic extrusion printheads that enable fast and smooth switching across different bioink reservoirs during the print process making it easier to recapitulate the biological complexity of native tissue and organs (Liu et al., 2017b). Lastly, extrusion printers with parallelized nozzles have also emerged to offer significantly increased throughput (Skylar-Scott et al., 2019a).
One particular area that will likely see advances with bioprinting is that of morphogenesis, which involves complex cell, biochemical, and biophysical dynamics that sculpt the shape and composition of living organisms and their constituent organs and that can be recapitulated in some form with bioprinted constructs, including merging with the rapidly expanding area of organoid engineering. Thus, the future of bioprinting provides great potential across wide-ranging biological questions.
Supplementary Material
Acknowledgements
The authors acknowledge support through the American Heart Association (postdoctoral fellowship to A.C.D.) and the National Science Foundation (GRFP award to M.E.P., STC Program (CMMI: 15-48571)).
References
- Ahlfeld T, Doberenz F, Kilian D, Vater C, Korn P, Lauer G, Lode A, and Gelinsky M (2018). Bioprinting of mineralized constructs utilizing multichannel plotting of a self-setting calcium phosphate cement and a cell-laden bioink. Biofabrication 10, 045002. [DOI] [PubMed] [Google Scholar]
- Ahlfeld T, Guduric V, Duin S, Akkineni AR, Schütz K, Kilian D, Emmermacher J, Cubo-Mateo N, Dani S, Witzleben M.v., et al. (2020). Methylcellulose – a versatile printing material that enables biofabrication of tissue equivalents with high shape fidelity. Biomaterials science 8, 2102–2110. [DOI] [PubMed] [Google Scholar]
- Aisenbrey EA, and Murphy WL (2020). Synthetic alternatives to Matrigel. Nature Reviews Materials 5, 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DJ, and Ewald AJ (2010). Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol 341, 34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw WY, Heck BW, Joyce B, and Devenport D (2016). Transient Tissue-Scale Deformation Coordinates Alignment of Planar Cell Polarity Junctions in the Mammalian Skin. Curr Biol 26, 2090–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayan B, Heo DN, Zhang Z, Dey M, Povilianskas A, Drapaca C, and Ozbolat IT (2020). Aspiration-assisted bioprinting for precise positioning of biologics. Science Advances 6, eaaw5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C, Kolb D, Weaver JC, Sharma S, Hosny A, Costa J, and Oxman N (2018). Making data matter: Voxel printing for the digital fabrication of data across scales and domains. Science Advances 4, eaas8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang K-J, Karalis K, Kim HJ, MacQueen L, Mahmoodian R, et al. (2015). Engineered In Vitro Disease Models. Annual Review of Pathology: Mechanisms of Disease 10, 195–262. [DOI] [PubMed] [Google Scholar]
- Bernal PN, Delrot P, Loterie D, Li Y, Malda J, Moser C, and Levato R (2019). Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Advanced Materials 31, 1904209. [DOI] [PubMed] [Google Scholar]
- Bertlein S, Brown G, Lim KS, Jungst T, Boeck T, Blunk T, Tessmar J, Hooper GJ, Woodfield TBF, and Groll J (2017). Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Advanced Materials 29, 1703404. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee T, Zehnder SM, Rowe KG, Jain S, Nixon RM, Sawyer WG, and Angelini TE (2015). Writing in the granular gel medium. Science Advances 1, e1500655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaeser A, Duarte Campos DF, Puster U, Richtering W, Stevens MM, and Fischer H (2016). Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv Healthc Mater 5, 326–333. [DOI] [PubMed] [Google Scholar]
- Butler MT, and Wallingford JB (2017). Planar cell polarity in development and disease. Nat Rev Mol Cell Biol 18, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang Y-J, Chapeton K, Patterson B, Yuan Y, He C-S, et al. (2019). Engineering of human brain organoids with a functional vascular-like system. Nature Methods 16, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, and Burdick JA (2016). A practical guide to hydrogels for cell culture. Nat Methods 13, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerchiari AE, Garbe JC, Jee NY, Todhunter ME, Broaders KE, Peehl DM, Desai TA, LaBarge MA, Thomson M, and Gartner ZJ (2015). A strategy for tissue self-organization that is robust to cellular heterogeneity and plasticity. Proc Natl Acad Sci U S A 112, 2287–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetera M, Leybova L, Joyce B, and Devenport D (2018). Counter-rotational cell flows drive morphological and cell fate asymmetries in mammalian hair follicles. Nat Cell Biol 20, 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansoria P, Narayanan LK, Schuchard K, and Shirwaiker R (2019). Ultrasound-assisted biofabrication and bioprinting of preferentially aligned three-dimensional cellular constructs. Biofabrication 11, 035015. [DOI] [PubMed] [Google Scholar]
- Chansoria P, and Shirwaiker R (2019). Characterizing the Process Physics of Ultrasound-Assisted Bioprinting. Sci Rep 9, 13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément R, Dehapiot B, Collinet C, Lecuit T, and Lenne P-F (2017). Viscoelastic Dissipation Stabilizes Cell Shape Changes during Tissue Morphogenesis. Curr Biol 27, 3132–3142.e3134. [DOI] [PubMed] [Google Scholar]
- Coakley MF, Hurt DE, Weber N, Mtingwa M, Fincher EC, Alekseyev V, Chen DT, Yun A, Gizaw M, Swan J, et al. (2014). The NIH 3D Print Exchange: A Public Resource for Bioscientific and Biomedical 3D Prints. 3D Print Addit Manuf 1, 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, et al. (2017). Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol 19, 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly AC, and Kelly DJ (2019). Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials 197, 194–206. [DOI] [PubMed] [Google Scholar]
- Di Lullo E, and Kriegstein AR (2017). The use of brain organoids to investigate neural development and disease. Nature Reviews Neuroscience 18, 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, and Chang R (2018). Printability Study of Bioprinted Tubular Structures Using Liquid Hydrogel Precursors in a Support Bath. Applied Sciences 8, 403. [Google Scholar]
- Duin S, Schutz K, Ahlfeld T, Lehmann S, Lode A, Ludwig B, and Gelinsky M (2019). 3D Bioprinting of Functional Islets of Langerhans in an Alginate/Methylcellulose Hydrogel Blend. Adv Healthc Mater 8, e1801631. [DOI] [PubMed] [Google Scholar]
- Ekert JE, Deakyne J, Pribul-Allen P, Terry R, Schofield C, Jeong CG, Storey J, Mohamet L, Francis J, Naidoo A, et al. (2020). Recommended Guidelines for Developing, Qualifying, and Implementing Complex In Vitro Models (CIVMs) for Drug Discovery. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 2472555220923332. [DOI] [PubMed] [Google Scholar]
- Fatehullah A, Tan SH, and Barker N (2016). Organoids as an in vitro model of human development and disease. Nat Cell Biol 18, 246–254. [DOI] [PubMed] [Google Scholar]
- Fleming PA, Argraves WS, Gentile C, Neagu A, Forgacs G, and Drake CJ (2010). Fusion of uniluminal vascular spheroids: A model for assembly of blood vessels. Developmental Dynamics 239, 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillispie G, Prim P, Copus J, Fisher J, Mikos AG, Yoo JJ, Atala A, and Lee SJ (2020). Assessment methodologies for extrusion-based bioink printability. Biofabrication 12, 022003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Piotrowski AS, Varner VD, and Nelson CM (2015). Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Sci Rep 5, 11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, and Lutolf MP (2016). Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564. [DOI] [PubMed] [Google Scholar]
- Green JBA, and Sharpe J (2015). Positional information and reaction-diffusion: two big ideas in developmental biology combine. Development 142, 1203–1211. [DOI] [PubMed] [Google Scholar]
- Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, Greenfield PT, Calafat NJ, Gounley JP, Ta AH, et al. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll J, Boland T, Blunk T, Burdick JA, Cho DW, Dalton PD, Derby B, Forgacs G, Li Q, Mironov VA, et al. (2016). Biofabrication: reappraising the definition of an evolving field. Biofabrication 8, 013001. [DOI] [PubMed] [Google Scholar]
- Groll J, Burdick JA, Cho DW, Derby B, Gelinsky M, Heilshorn SC, Jüngst T, Malda J, Mironov VA, Nakayama K, et al. (2018). A definition of bioinks and their distinction from biomaterial inks. Biofabrication 11, 013001. [DOI] [PubMed] [Google Scholar]
- Guo T, Holzberg TR, Lim CG, Gao F, Gargava A, Trachtenberg JE, Mikos AG, and Fisher JP (2017). 3D printing PLGA: a quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication 9, 024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK, and Miyake T (1995). Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol 39, 881–893. [PubMed] [Google Scholar]
- Heinrich MA, Bansal R, Lammers T, Zhang YS, Michel Schiffelers R, and Prakash J (2019). 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Advanced Materials 31, 1806590. [DOI] [PubMed] [Google Scholar]
- Highley CB, Rodell CB, and Burdick JA (2015). Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater 27, 5075–5079. [DOI] [PubMed] [Google Scholar]
- Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, and Feinberg AW (2015). Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Advances 1, e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock TW, and Megason SG (2015). Mathematically guided approaches to distinguish models of periodic patterning. Development 142, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, and Lewis JA (2016). Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci Rep 6, 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospodiuk M, Dey M, Sosnoski D, and Ozbolat IT (2017). The bioink: A comprehensive review on bioprintable materials. Biotechnology Advances 35, 217–239. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Miyazaki H, Coyle MC, Zhang J, Laurie MT, Chu D, Vavrušová Z, Schneider RA, Klein OD, and Gartner ZJ (2018). Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Dev Cell 44, 165–178.e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CS, Postovit LM, and Lajoie GA (2010). Matrigel: A complex protein mixture required for optimal growth of cell culture. PROTEOMICS 10, 1886–1890. [DOI] [PubMed] [Google Scholar]
- Ihermann-Hella A, Lume M, Miinalainen IJ, Pirttiniemi A, Gui Y, Peränen J, Charron J, Saarma M, Costantini F, and Kuure S (2014). Mitogen-activated protein kinase (MAPK) pathway regulates branching by remodeling epithelial cell adhesion. PLoS Genet 10, e1004193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE (2020). Is it Time for Reviewer 3 to Request Human Organ Chip Experiments Instead of Animal Validation Studies? Advanced Science n/a, 2002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus AE, Rutz AL, Jordan SW, Kannan A, Mitchell SM, Yun C, Koube KD, Yoo SC, Whiteley HE, Richter CP, et al. (2016). Hyperelastic "bone": A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci Transl Med 8, 358ra127. [DOI] [PubMed] [Google Scholar]
- Jakus AE, Secor EB, Rutz AL, Jordan SW, Hersam MC, and Shah RN (2015). Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 9, 4636–4648. [DOI] [PubMed] [Google Scholar]
- Jeon O, Lee YB, Jeong H, Lee SJ, Wells D, and Alsberg E (2019). Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries. Mater Horiz 6, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Heo J-H, Kim MK, Jeong W, and Kang H-W (2020). High-Precision 3D Bio-Dot Printing to Improve Paracrine Interaction between Multiple Types of Cell Spheroids. Advanced Functional Materials n/a, 2005324. [Google Scholar]
- Jiang T, Munguia-Lopez JG, Gu K, Bavoux MM, Flores-Torres S, Kort-Mascort J, Grant J, Vijayakumar S, De Leon-Rodriguez A, Ehrlicher AJ, et al. (2019). Engineering bioprintable alginate/gelatin composite hydrogels with tunable mechanical and cell adhesive properties to modulate tumor spheroid growth kinetics. Biofabrication 12, 015024. [DOI] [PubMed] [Google Scholar]
- Junk S, and Kuen C (2016). Review of Open Source and Freeware CAD Systems for Use with 3D-Printing. Procedia CIRP 50, 430–435. [Google Scholar]
- Kang D, Ahn G, Kim D, Kang H-W, Yun S, Yun W-S, Shim J-H, and Jin S (2018). Pre-set extrusion bioprinting for multiscale heterogeneous tissue structure fabrication. Biofabrication 10, 035008. [DOI] [PubMed] [Google Scholar]
- Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, and Atala A (2016). A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnology 34, 312–319. [DOI] [PubMed] [Google Scholar]
- Kaucka M, Zikmund T, Tesarova M, Gyllborg D, Hellander A, Jaros J, Kaiser J, Petersen J, Szarowska B, Newton PT, et al. (2017). Oriented clonal cell dynamics enables accurate growth and shaping of vertebrate cartilage. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BE, Bhattacharya I, Heidari H, Shusteff M, Spadaccini CM, and Taylor HK (2019). Volumetric additive manufacturing via tomographic reconstruction. Science 363, 1075. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Chi BH, Yoo JJ, Ju YM, Whang YM, and Chang IH (2019). Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer. PLoS One 14, e0223689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JM, Kang J, Shin S-H, Jegal J, Cha HG, Choy S, Hakkarainen M, Park J, Oh DX, and Hwang SY (2020). Biobased thermoplastic elastomer with seamless 3D-Printability and superior mechanical properties empowered by in-situ polymerization in the presence of nanocellulose. Composites Science and Technology 185, 107885. [Google Scholar]
- Krishnamoorthy S, Zhang Z, and Xu C (2019). Biofabrication of three-dimensional cellular structures based on gelatin methacrylate-alginate interpenetrating network hydrogel. J Biomater Appl 33, 1105–1117. [DOI] [PubMed] [Google Scholar]
- Kuo C-Y, Eranki A, Placone JK, Rhodes KR, Aranda-Espinoza H, Fernandes R, Fisher JP, and Kim PCW (2016). Development of a 3D Printed, Bioengineered Placenta Model to Evaluate the Role of Trophoblast Migration in Preeclampsia. ACS Biomaterials Science & Engineering 2, 1817–1826. [DOI] [PubMed] [Google Scholar]
- Kutlehria S, Dinh TC, Bagde A, Patel N, Gebeyehu A, and Singh M (2020). High-throughput 3D bioprinting of corneal stromal equivalents. J Biomed Mater Res B Appl Biomater 108, 2981–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent J, Blin G, Chatelain F, Vanneaux V, Fuchs A, Larghero J, and Thery M (2017). Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nat Biomed Eng 1, 939–956. [DOI] [PubMed] [Google Scholar]
- Lee A, Hudson AR, Shiwarski DJ, Tashman JW, Hinton TJ, Yerneni S, Bliley JM, Campbell PG, and Feinberg AW (2019). 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482. [DOI] [PubMed] [Google Scholar]
- Lee H, Han W, Kim H, Ha DH, Jang J, Kim BS, and Cho DW (2017). Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 18, 1229–1237. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim J, Choi Y, and Cho D-W (2020). Application of Gelatin Bioinks and Cell-Printing Technology to Enhance Cell Delivery Capability for 3D Liver Fibrosis-on-a-Chip Development. ACS Biomaterials Science & Engineering 6, 2469–2477. [DOI] [PubMed] [Google Scholar]
- Lewicki J, Bergman J, Kerins C, and Hermanson O (2019). Optimization of 3D bioprinting of human neuroblastoma cells using sodium alginate hydrogel. Bioprinting 16, e00053. [Google Scholar]
- Li P, Markson JS, Wang S, Chen S, Vachharajani V, and Elowitz MB (2018). Morphogen gradient reconstitution reveals Hedgehog pathway design principles. Science 360, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu B, Pei B, Chen J, Zhou D, Peng J, Zhang X, Jia W, and Xu T (2020). Inkjet Bioprinting of Biomaterials. Chemical Reviews 120, 10793–10833. [DOI] [PubMed] [Google Scholar]
- Lim KS, Galarraga JH, Cui X, Lindberg GCJ, Burdick JA, and Woodfield TBF (2020). Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chemical Reviews. [DOI] [PubMed] [Google Scholar]
- Lim KS, Levato R, Costa PF, Castilho MD, Alcala-Orozco CR, van Dorenmalen KMA, Melchels FPW, Gawlitta D, Hooper GJ, Malda J, et al. (2018). Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication 10, 034101. [DOI] [PubMed] [Google Scholar]
- Lin NYC, Homan KA, Robinson SS, Kolesky DB, Duarte N, Moisan A, and Lewis JA (2019). Renal reabsorption in 3D vascularized proximal tubule models. Proceedings of the National Academy of Sciences 116, 5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, Liu X, Guan X, Zhong Z, Jin X, Khademhosseini A, et al. (2017a). Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Advanced healthcare materials 6, 10.1002/adhm.201601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, Alvarez MM, Yang J, Li Y-C, Trujillo-de Santiago G, et al. (2017b). Rapid Continuous Multimaterial Extrusion Bioprinting. Advanced Materials 29, 1604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loterie D, Delrot P, and Moser C (2020). High-resolution tomographic volumetric additive manufacturing. Nature Communications 11, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, Liu J, Wang P, Lai CSE, Zanella F, et al. (2016). Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proceedings of the National Academy of Sciences 113, 2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJA, Groll J, and Hutmacher DW (2013). 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Advanced Materials 25, 5011–5028. [DOI] [PubMed] [Google Scholar]
- Maloney E, Clark C, Sivakumar H, Yoo K, Aleman J, Rajan SAP, Forsythe S, Mazzocchi A, Laxton AW, Tatter SB, et al. (2020). Immersion Bioprinting of Tumor Organoids in Multi-Well Plates for Increasing Chemotherapy Screening Throughput. Micromachines (Basel) 11, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, and Ingber DE (2010). Mechanical control of tissue and organ development. Development 137, 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, and Wieschaus EF (2009). Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matai I, Kaur G, Seyedsalehi A, McClinton A, and Laurencin CT (2020). Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226, 119536. [DOI] [PubMed] [Google Scholar]
- Mazzocchi A, Devarasetty M, Huntwork R, Soker S, and Skardal A (2018). Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 11, 015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A, Highley CB, Leslie NR, and Melchels FPW (2020). 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol 38, 584–593. [DOI] [PubMed] [Google Scholar]
- Menshykau D, Michos O, Lang C, Conrad L, McMahon AP, and Iber D (2019). Image-based modeling of kidney branching morphogenesis reveals GDNF-RET based Turing-type mechanism and pattern-modulating WNT11 feedback. Nat Commun 10, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ED, Phillippi JA, Fisher GW, Campbell PG, Walker LM, and Weiss LE (2009). Inkjet Printing of Growth Factor Concentration Gradients and Combinatorial Arrays Immobilized on Biologically-Relevant Substrates. Combinatorial Chemistry & High Throughput Screening 12, 604–618. [DOI] [PubMed] [Google Scholar]
- Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz-Esparza GU, Khoshakhlagh P, Manbachi A, Dokmeci MR, Chen S, et al. (2018). Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Advanced Materials 30, 1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan NI, Hibino N, and Nakayama K (2016). Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Engineering Part B: Reviews 23, 237–244. [DOI] [PubMed] [Google Scholar]
- Mongera A, Rowghanian P, Gustafson HJ, Shelton E, Kealhofer DA, Carn EK, Serwane F, Lucio AA, Giammona J, and Campàs O (2018). A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 561, 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley CD, Ellison ST, Bhattacharjee T, O'Bryan CS, Zhang Y, Smith KF, Kabb CP, Sebastian M, Moore GL, Schulze KD, et al. (2019). Quantitative characterization of 3D bioprinted structural elements under cell generated forces. Nat Commun 10, 3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, and Yoo JJ (2018). Biofabrication strategies for 3D in vitro models and regenerative medicine. Nature Reviews Materials 3, 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM (2016). On Buckling Morphogenesis. J Biomech Eng 138, 021005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SB, Rønhild S, Trusina A, and Sneppen K (2018). Theoretical tool bridging cell polarities with development of robust morphologies. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norotte C, Marga FS, Niklason LE, and Forgacs G (2009). Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30, 5910–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang L, Armstrong JPK, Lin Y, Wojciechowski JP, Lee-Reeves C, Hachim D, Zhou K, Burdick JA, and Stevens MM (2020). Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Science Advances 6, eabc5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbolat IT, and Hospodiuk M (2016). Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76, 321–343. [DOI] [PubMed] [Google Scholar]
- Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, et al. (2017). Personalized In Vitro Cancer Models to Guide Precision Medicine. Cancer Discovery 7, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi TH, Tytgat L, Dubruel P, Duda GN, Van Vlierberghe S, and Geissler S (2019). Extrusion Printed Scaffolds with Varying Pore Size As Modulators of MSC Angiogenic Paracrine Effects. ACS Biomaterials Science & Engineering 5, 5348–5358. [DOI] [PubMed] [Google Scholar]
- Rimann M, Bono E, Annaheim H, Bleisch M, and Graf-Hausner U (2016). Standardized 3D Bioprinting of Soft Tissue Models with Human Primary Cells. J Lab Autom 21, 496–509. [DOI] [PubMed] [Google Scholar]
- Rogers KW, and Schier AF (2011). Morphogen Gradients: From Generation to Interpretation. Annual Review of Cell and Developmental Biology 27, 377–407. [DOI] [PubMed] [Google Scholar]
- Rossi G, Manfrin A, and Lutolf MP (2018). Progress and potential in organoid research. Nature Reviews Genetics 19, 671–687. [DOI] [PubMed] [Google Scholar]
- Sadati M, Taheri Qazvini N, Krishnan R, Park CY, and Fredberg JJ (2013). Collective migration and cell jamming. Differentiation 86, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheideler OJ, Yang C, Kozminsky M, Mosher KI, Falcón-Banchs R, Ciminelli EC, Bremer AW, Chern SA, Schaffer DV, and Sohn LL (2020). Recapitulating complex biological signaling environments using a multiplexed, DNA-patterning approach. Science Advances 6, eaay5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior JJ, Cooke ME, Grover LM, and Smith AM (2019). Fabrication of Complex Hydrogel Structures Using Suspended Layer Additive Manufacturing (SLAM). Advanced Functional Materials 29, 1904845. [Google Scholar]
- Skylar-Scott MA, Mueller J, Visser CW, and Lewis JA (2019a). Voxelated soft matter via multimaterial multinozzle 3D printing. Nature 575, 330–335. [DOI] [PubMed] [Google Scholar]
- Skylar-Scott MA, Uzel SGM, Nam LL, Ahrens JH, Truby RL, Damaraju S, and Lewis JA (2019b). Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Science Advances 5, eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KH, Highley CB, Rouff A, and Burdick JA (2018). Complex 3D-Printed Microchannels within Cell-Degradable Hydrogels. Advanced Functional Materials 28, 1801331. [Google Scholar]
- Sooppan R, Paulsen SJ, Han J, Ta AH, Dinh P, Gaffey AC, Venkataraman C, Trubelja A, Hung G, Miller JS, et al. (2016). In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks. Tissue Eng Part C Methods 22, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlin JW, Siedlik MJ, Nerger BA, Pang M-F, Jayaraman S, Zhang R, and Nelson CM (2019). Mesenchymal proteases and tissue fluidity remodel the extracellular matrix during airway epithelial branching in the embryonic avian lung. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Starly B, Daly AC, Burdick JA, Groll J, Skeldon G, Shu W, Sakai Y, Shinohara M, Nishikawa M, et al. (2020). The bioprinting roadmap. Biofabrication 12, 022002. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Hamid Q, Sun W, and Clyne AM (2019). Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication 11, 025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó A, Melchionda M, Nastasi G, Woods ML, Campo S, Perris R, and Mayor R (2016). In vivo confinement promotes collective migration of neural crest cells. J Cell Biol 213, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitt MW, and Anseth KS (2009). Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and bioengineering 103, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topfer E, Pasotti A, Telopoulou A, Italiani P, Boraschi D, Ewart MA, and Wilde C (2019). Bovine colon organoids: From 3D bioprinting to cryopreserved multi-well screening platforms. Toxicol In Vitro 61, 104606. [DOI] [PubMed] [Google Scholar]
- van den Brink SC, Alemany A, van Batenburg V, Moris N, Blotenburg M, Vivié J, Baillie-Johnson P, Nichols J, Sonnen KF, Martinez Arias A, et al. (2020). Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi W, Kuss M, Mirza S, Qi D, Krasnoslobodtsev A, Zeng J, Band H, Band V, and Duan B (2018). 3D Bioprinting of Breast Cancer Models for Drug Resistance Study. ACS Biomaterials Science & Engineering 4, 4401–4411. [DOI] [PubMed] [Google Scholar]
- Yi H-G, Jeong YH, Kim Y, Choi Y-J, Moon HE, Park SH, Kang KS, Bae M, Jang J, Youn H, et al. (2019). A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nature Biomedical Engineering 3, 509–519. [DOI] [PubMed] [Google Scholar]
- Zhang B, Pei X, Song P, Sun H, Li H, Fan Y, Jiang Q, Zhou C, and Zhang X (2018). Porous bioceramics produced by inkjet 3D printing: Effect of printing ink formulation on the ceramic macro and micro porous architectures control. Composites Part B: Engineering 155, 112–121. [Google Scholar]
- Zhang R, Tao Y, Xu Q, Liu N, Chen P, Zhou Y, and Bai Z (2020). Rheological and ion-conductive properties of injectable and self-healing hydrogels based on xanthan gum and silk fibroin. Int J Biol Macromol 144, 473–482. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Ng WL, An J, Chua CK, and Tan LP (2019). Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS One 14, e0216776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.