Abstract
Introduction
Despite early enthusiasm, minimally invasive cardiac surgery has had a low uptake compared with novel techniques in interventional cardiology. Steep learning curves from high-volume centres have deterred smaller units from engaging, even though low-volume centres undertake a large proportion of surgical interventions worldwide. We sought to identify the safety and experience of learning minimally invasive cardiac surgery after undertaking a structured fellowship at Blackpool Victoria Hospital, a low-volume centre.
Materials and methods
A retrospective analysis of outcomes for all consecutive minimally invasive cardiac surgery procedures performed via a right mini-thoracotomy at our institution between 2007 and 2017 was undertaken. Clinical outcomes included death, conversion to sternotomy, stroke, renal failure and other organ support. Cardiopulmonary bypass, aortic cross-clamp times and learning cumulative sum sequential probability method curves were also assessed to determine how safely the procedure was adopted.
Results
A total of 316 patients were operated on for mitral, tricuspid, atrial fibrillation, septal defects or other conditions. The mean logistic European System for Cardiac Operative Risk Evaluation score was 7.0 (± 8.5). Conversion to sternotomy occurred in 12 patients (3.8%) and in-hospital mortality was 7 (2.2%). None of the converted patients died. The learning curves showed an accelerated process of adoption, similar to reference figures from a high-volume German centre.
Discussion
It is possible for low-volume cardiac surgical centres to undertake minimally invasive surgical programmes with good outcomes and short learning curves. Despite technical complexities, with a team approach, the learning curve can be navigated safely.
Keywords: Minimally invasive surgical procedures, Mitral valve, Learning curve
Introduction
Minimally invasive mitral surgery through a right mini-thoracotomy has been evolving since it was first described nearly two decades ago.1 Experience tends to be concentrated in large referral centres with high throughput and unparalleled resource allocation. While substantial risk reduction has been possible in such super-centres, most units have much lower caseloads per annum.2
The learning curve experiences of many centres were captured prior to wider dissemination of the technologies and techniques now used. Early adoption conferred both historical advantages to these pioneers and subsequent institutional benefits to surgeons who trained within them.3,4 Unlike transcatheter aortic valve implantation, which has showed exponential growth,5 minimally invasive cardiac surgery has lower adoption rates, especially in the UK. In part, this may be due to the short learning curve involved in transcatheter aortic valve implantation, which has allowed cardiologists to increase their experience quickly.6,7 In contrast, minimally invasive cardiac surgical centres, especially those using endovascular clamping techniques, take longer to establish their programmes.8
Encouragement for units to adopt minimally invasive mitral surgery have, so far, lacked the evidence to support that this can be done safely in low-volume centres.9 We sought to identify the safety and learning curve for minimally invasive cardiac surgery in such a setting.
Materials and methods
We undertook a retrospective cohort study of all consecutive patients undergoing minimally invasive port-access cardiac surgery at our institution from 2007 to 2017. The surgeon had spent three months as a fellow at the OLV hospital in Aalst, Belgium, which had previously published a large series of minimally invasive cardiac surgery procedures with excellent results.10 The institutional review board waived the need for patient consent. Inclusion criteria were surgery employing peripheral cannulation for cardiopulmonary bypass and right mini-thoracotomy as the intended surgical approach. We included patients undergoing surgery for mitral and/or tricuspid repair or replacement, atrial septal defect closure (including patent foramen ovale), excision of cardiac tumours and atrial fibrillation surgery. There were no exclusion criteria from the study.
Clinical assessment and surgical planning
All patients had either a computed tomography aorto-femoral angiogram or fluoroscopic angiography of the iliofemoral vasculature. The ascending aorta was measured to determine a suitable landing zone for the endoaortic balloon, defined as a maximum aortic diameter of 40mm and landing zone (sinotubular junction to brachiocephalic artery) length of at least 40mm. The femoral vessels were assessed for calcification that might preclude cannulation and severe tortuosity of the vascular tree that could impede guidewire advancement. The presence of mobile (grade V) atherosclerotic plaques was considered a contraindication to peripheral bypass. The endoaortic balloon was contraindicated in patients with more than mild aortic regurgitation. Where an endovascular balloon was contraindicated but peripheral bypass was not, a transthoracic aortic clamp or fibrillating heart strategy was considered.
Anaesthetic preparation
All patients had bilateral radial (or brachial) arterial lines, an internal jugular central venous catheter and single lumen endotracheal intubation with bronchial blocker placement under video bronchoscopy for selective ventilation.
Cardiopulmonary bypass and myocardial protection techniques
Cardiopulmonary bypass was established with direct femoral arterial and femoral venous cannulation using open cut-down. In patients weighing over 80kg or those requiring tricuspid surgery, a superior vena cava cannula was also inserted after induction of anaesthesia, under echocardiographic guidance with 2500iu heparin bolus cover. Arterial inflow was calculated to maintain indexed normothermic flow and supplemented with bilateral femoral arterial cannulas if required or a planned reduction in core temperature during surgery. The arterial line pressures were maintained didactically below 300mmHg. The endo-aortic clamp was placed under direct transoesophageal echocardiography guidance into the ascending aorta at the level of the pulmonary artery and, at the time of aortic occlusion, inflated with approximately n ± 5ml of saline (where n was the measured aortic diameter in millimetres). At approximately 15–20ml of inflation (two-thirds of n), 250μg/kg of adenosine was administered through the distal lumen of the intra-aortic balloon directly into the partially occluded aortic root, creating temporary cardiac standstill to allow accurate landing of the balloon. The balloon was inflated further to approximately 1ml/mm aortic diameter until a complete drop in the endoballoon root manometry was seen (indicating occlusion of the ascending aorta and isolation from the cardiopulmonary bypass pressures in the distal aorta). During inflation and cardioplegia delivery, the position of the balloon was monitored with transoesophageal echocardiography and attention to the bilateral radial arterial pressures and cerebral oxygenation to indicate any migration of the balloon. Cold crystalloid hyperkalaemic (extracellular) cardioplegia was delivered every 15–20 minutes to maintain cardiac arrest.
Surgical technique was via a utility incision at the right mini-thoracotomy at the level of the fourth intercostal space (and, latterly, periareolar incisions in women). A soft-tissue retractor was employed to protect the wound and allow instruments to be passed through uninhibited. Additional ports for a 5mm camera, initially and now a 10-mm EisteinVision® 2.0 scope (Aesculap AG, Tuttlingen, Germany) in a posterior position in the fourth space and a 5mm port for CO2 insufflation and retraction sutures in the fifth space were used. The diaphragm was retracted by means of a stay suture in the most caudal part of the pericardiotomy incision rather than through the diaphragm muscle.
Analysis
Data were analysed using R version 3.3.3 for Mac and the rcusum (Zenodo) open software package.11,12 Demographic data were analysed using Wilcoxon signed rank test for continuous variables and chi square for categorical data. Learning curves were assessed using the cumulative sum (CUSUM) sequential probability method curves described by Holzhey et al.3 Reference ranges for the expected complication rates were taken from their large series and the acceptability margin was considered as a 50% deviation from the expected rates. For in-hospital mortality, event occurrence was measured against the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) probability.13 Logarithmic regression curves for the cardiopulmonary bypass times were examined for evidence of an early vertical slope decay, indicating the end of a learning curve.14
Study endpoints were assessed as a composite measure of individual complications both including and excluding intraoperative conversion to sternotomy. The adverse outcomes measured were inpatient mortality, re-exploration (for any cause) within the same admission, stroke, acute kidney injury requiring renal replacement therapy, intra-aortic balloon pump use or myocardial infarction (defined as haemodynamic compromise associated with cardiac enzyme rise or any electrocardiogram changes).
Results
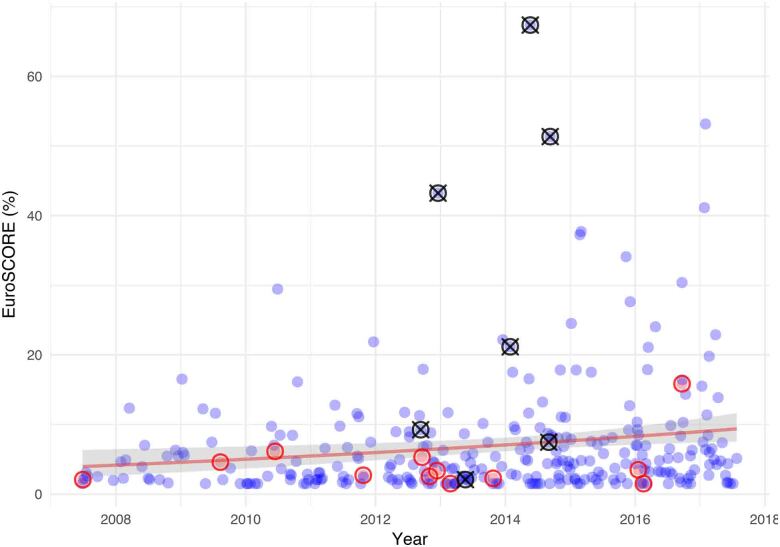
A total of 316 operations were performed during the study period. Patient characteristics are summarised in Table 1. The mean age of patients was 61 years (range 23–92 years) with a logistic EuroSCORE of 7.0 ± 8.5. Urgent operations were performed in 8.2% of patients and 2.2% of patients had poor left ventricular function (ejection fraction < 35%). There was a demonstrable rise in the preoperative risk profile of patients over time and experience (Figure 1) with over 50% of the in-hospital mortality occurring in patients with logistic EuroSCOREs over 20%.
Table 1 .
Patient demographics (n = 316)
| Demographic | Value |
|---|---|
| Age/years, median (IQR) | 64.50 (51.00,71.00) |
| Male (%) | 193 (61.1) |
| Height/cm, median (IQR) | 171.50 (162.75,178.00) |
| Weight/kg, median (IQR) | 77.50 (65.07,89.08) |
| Body surface area/m2, median (IQR) | 1.90 (1.72,2.04) |
| Body mass index kg/m2, median (IQR) | 26.16 (22.74,29.59) |
| Canadian Cardiovascular Society grade of angina, n (%): | |
| 0 | 227 (71.8) |
| 1 | 45 (14.2) |
| 2 | 31 (9.8) |
| 3 | 11 (3.5) |
| 4 | 2 (0.6) |
| Dyspnoea status (NYHA), n (%): | |
| 1 | 57 (18.0) |
| 2 | 118 (37.3) |
| 3 | 125 (39.6) |
| 4 | 16 (5.1) |
| Previous myocardial infarction, n (%): | |
| 0 | 297 (94.0) |
| 1 | 15 (4.7) |
| ≥2 | 4 (1.3) |
| Previous percutaneous coronary intervention, n (%) | 13 (4.1) |
| Diabetes, n (%): | |
| Diet controlled | 7 (2.2) |
| Not diabetic | 296 (94.0) |
| Oral therapy | 12 (3.8) |
| Smoking history, n (%): | |
| Current smoker | 25 (7.9) |
| Ex-smoker | 135 (42.7) |
| Never smoked | 156 (49.4) |
| Hypertension, n (%) | 141 (45.3) |
| Hypercholesterolaemia, n (%) | 19 (30.6) |
| Renal disease, n (%): | |
| Acute renal failure | 4 (1.2) |
| Chronic renal failure with dialysis | 1 (0.3) |
| None | 311 (98.4) |
| Pulmonary disease (%): | |
| Asthma | 6 (1.9) |
| COPD/emphysema | 38 (12.0) |
| No | 272 (86.1) |
| Neurological disease, n (%): | |
| Cerebrovascular accident with full recovery | 9 (2.8) |
| Cerebrovascular accident with residual deficit | 6 (1.9) |
| Transient ischaemic attack | 18 (5.7) |
| No history of neurological disease | 283 (89.6) |
| Carotid bruits, n (%) | 3 (4.8) |
| Neurological dysfunction, n (%) | 5 (7.0) |
| Extracardiac arteriopathy, n (%) | 17 (5.4) |
| Sinus rhythm, n (%) | 282 (89.2) |
| Ejection fraction, n (%): | |
| Good (LVEF > 50%) | 261 (82.6) |
| Fair (LVEF 30–50%) | 48 (15.2) |
| Poor (LVEF < 30%) | 7 (2.2) |
| Cardiogenic shock, n (%) | 1 (0.3) |
| Urgent priority, n (%) | 26 (8.2) |
| EuroSCORE, median (IQR) | 5.00 (3.00, 7.00) |
| EuroSCORE (mean ± SD) | 5.58 ± 2.91 |
| Logistic EuroSCORE, median (IQR) | 4.38 (2.09, 8.13) |
| Logistic EuroSCORE (mean ± SD) | 6.98 ± 8.51 |
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association grade of dyspnoea; SD, standard deviation
Figure 1 .
EuroSCORE of operated patients over time (blue circles). Red circles indicate patients who were converted to full sternotomy. Black circles with a cross indicate patients who died. A logarithmic regression line of fit (shading shows 95% confidence interval) for all patients demonstrates a rising EuroSCORE with time.
Surgery and outcomes
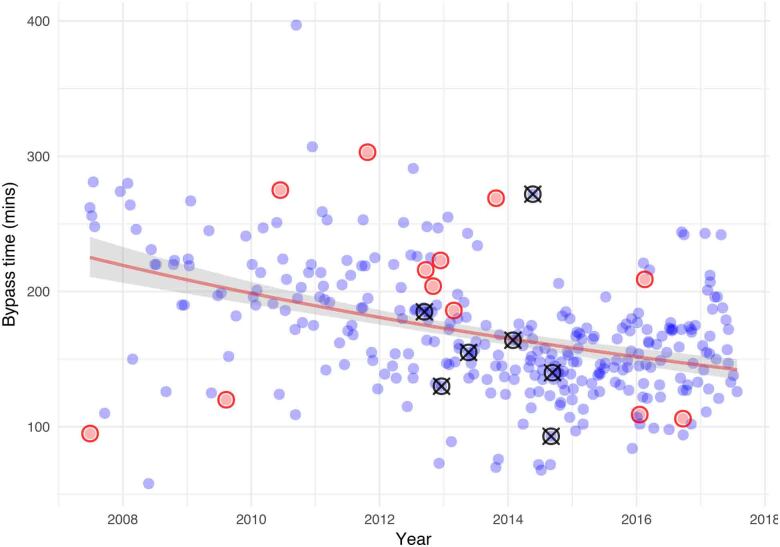
The outcomes are shown in Table 2, with distribution of operation types and subset outcomes for these outcomes in Table 3. Mean cross-clamp and bypass times were 112 ± 48 minutes and 169 ± 47 minutes, respectively. Median and interquartile ranges for cross-clamp times and bypass times were 111 minutes (range 88–133 minutes) and 163 minutes (range 139–195 minutes), respectively. Of the 316 patients, 12 (3.8%) were converted from mini-thoracotomy to sternotomy intraoperatively, distributed evenly throughout the experience. Cardiopulmonary bypass times, conversions and in-hospital mortality are shown in Figure 2. There were no in-hospital deaths for the first 100 cases, followed by 7 deaths from 2012 to 2014, and no further mortality for the final 100 cases. The period of higher mortality correlated with a significant rise in the EuroSCORE of patients accepted for minimally invasive surgery. Median blood loss following surgery was 220ml and blood transfusions were required in fewer than 1/20 patients. The majority of patients were discharged from intensive care after one night and from hospital within six days of surgery. Adequacy of repair (no more than mild mitral regurgitation) was present in all patients at the index procedure. Five patients (1.6%) required late reoperations for repair failure.
Table 2 .
Postoperative outcomes (n = 316)
| Outcome | Value | |
|---|---|---|
| (n) | (%) | |
| Reoperation: | ||
| No reoperation required | 308 | 97.5 |
| Reoperation for bleeding or tamponade | 6 | 1.9 |
| Reoperation for valve problems including SAM | 2 | 0.6 |
| New postoperative stroke: | ||
| Permanent | 3 | 1.0 |
| Transient | 5 | 1.6 |
| New haemofiltration | 3 | 0.9 |
| Discharge destination: | ||
| Home | 296 | 93.7 |
| Convalescence (non-acute hospital) | 6 | 1.9 |
| Other acute hospital | 7 | 2.2 |
| Patient deceased | 7 | 2.2 |
| Duration of ventilation: | ||
| <12 hours | 290 | 91.8 |
| <24 hours | 16 | 5.1 |
| >24 hours | 10 | 3.2 |
| Intensive care stay/days, median [IQR] | 1.00 | [1.00, 1.00] |
| Donor blood use | 14 | 4.4 |
| Blood loss first 12 hours, median [IQR] | 220 | [160, 340] |
| Inotropes use | 91 | 28.8 |
| Arrhythmias: | ||
| None | 257 | 81.3 |
| Permanent pacemaker | 5 | 1.5 |
| Myocardial infarction | 0 | 0.0 |
| Pulmonary complications: | ||
| None | 287 | 90.8 |
| Tracheostomy and long term wean | 4 | 1.2 |
| GI complications: | ||
| None | 313 | 99.1 |
| Other | 2 | 0.6 |
| Upper GI bleed | 1 | 0.3 |
| Acute kidney injury (%) | 9 | 2.8 |
| Postoperative stay/days, median [IQR] | 6.00 | [5.00, 8.00] |
| Multisystem organ failure | 3 | 0.9 |
| Cause of death: | ||
| Cardiac | 1 | 0.3 |
| Neurological | 1 | 0.3 |
| Respiratory | 3 | 1.0 |
| Septicaemia | 2 | 0.6 |
| Patient status: | ||
| Alive | 309 | 97.8 |
| Died in hospital | 7 | 2.2 |
GI, gastrointestinal; IQR, interquartile range; SAM, systolic anterior motion
Table 3 .
Distribution of operation performed in 316 patientsa
| Operation | Patients (n) | Age | Male Gender) | EuroSCORE | Postoperative stay in days | Inpatient mortality |
|---|---|---|---|---|---|---|
| MVP alone | 128 | 63.00 [51.00, 70.00] | 99 (77.3) | 4.00 [2.00, 6.00] | 6.00 [4.00, 7.00] | 1 (0.8) |
| MVR alone | 41 | 61.00 [50.00, 71.00] | 16 (39.0) | 6.00 [4.00, 7.00] | 7.00 [6.00, 10.00] | 1 (2.4) |
| MV + other | 77 | 68.00 [61.00, 74.00] | 49 (63.6) | 6.00 [4.00, 7.00] | 7.00 [6.00, 9.00] | 1 (1.3) |
| TVR alone | 7 | 71.00 [56.50, 75.50] | 3 (42.9) | 7.00 [6.00, 7.00] | 10.00 [7.50, 15.50] | 1 (14.3) |
| Otherb | 32 | 54.50 [42.25, 65.00] | 11 (34.4) | 5.00 [3.00, 5.00] | 5.00 [4.00, 7.00] | 0 (0.0) |
| Redo | 31 | 68.00 [61.00, 75.00] | 15 (48.4) | 9.50 [8.00, 12.00] | 8.00 [6.00, 12.00] | 3 (9.7) |
MV, mitral valve surgery; MVP, mitral valve repair; MVR, mitral valve replacement; TVR, tricuspid valve surgery
a Data shown as n (%) for categorical variable and median [interquartile range] for continuous variables
b Atrial septal defect, patent foramen ovale closure or atrial fibrillation ablation surgery
Figure 2 .
Cardiopulmonary bypass times of operated patients over time (blue circles). Red circles indicate patients who were converted to full sternotomy. Black circles with a cross indicate patients who died. A logarithmic regression line of fit (shading shows 95% confidence interval) for all patients demonstrates a reduction in bypass times with increasing experience.
Learning curves
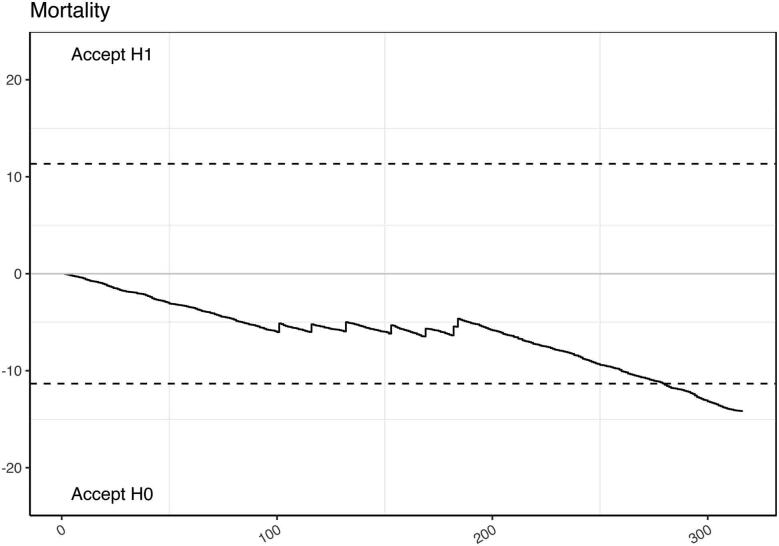
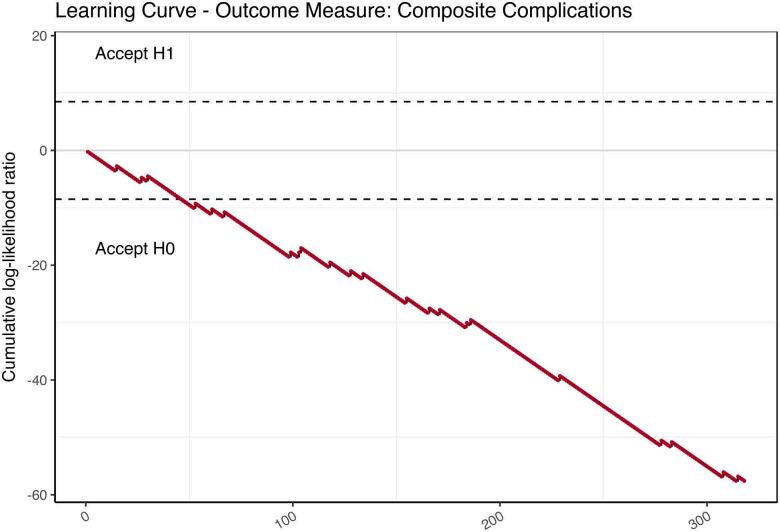
The learning curve for mortality, benchmarked against the logistic EuroSCORE is demonstrated in Figure 3 and shows a cluster of mortality in the middle portion of the experience. The CUSUM curves with respect to conversion to sternotomy, myocardial infarction, cerebrovascular accident, renal replacement therapy and reoperations all showed event occurrences well within the expected limits. Learning curves against composite outcome measures also showed institutional outperformance compared with the reference ranges (Figure 4).
Figure 3 .
Cumulative sum (CUSUM) probability curve using mortality as outcome measure. The upper limit (H1) indicates unacceptable learning performance. The lower bound (H0) indicates acceptable learning performance.
Figure 4 .
Cumulative sum (CUSUM) probability curve using a composite of complications (mortality, bleeding, stroke, renal replacement, reoperation and myocardial infarction) as the outcome measure. The upper limit (H1) indicates unacceptable learning performance. The lower bound (H0) indicates acceptable learning performance.
Operative times
The logarithmic regression curve of cardiopulmonary bypass times against increasing experience demonstrated no marked early acceleration in learning (Figure 2). There was, however, a general trend towards reduced operative time with increasing experience. Notably, the patients who were converted in this series did not suffer any adverse outcomes leading to in-hospital death. Additionally, there was no correlation between cardiopulmonary bypass times and either conversion or death.
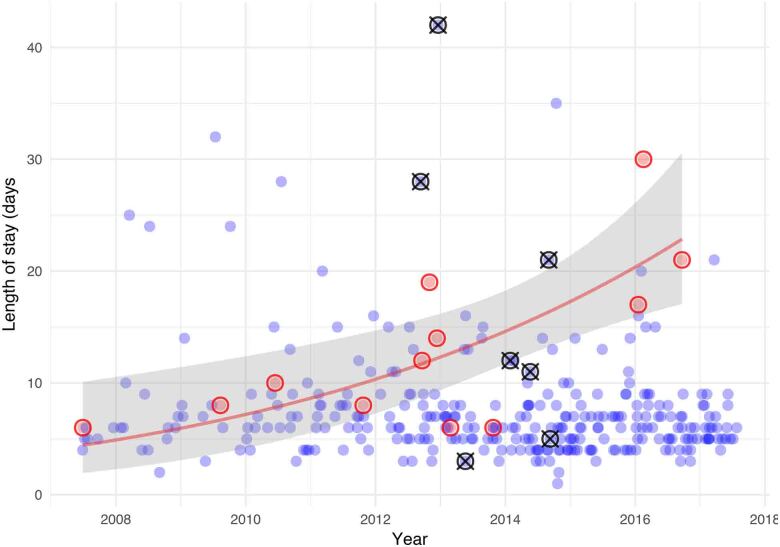
Hospital length of stay
The length of in-hospital stay following surgery showed a decreasing trend over the period of the study. Patients who were converted early in the experience did not have prolonged hospital stays, but over the whole study period there was a trend towards prolonged inpatient admissions in patients who were converted (Figure 5). There was a higher proportion of mortality in patients who were hospitalised for over two weeks.
Figure 5 .
Length of postoperative hospital stay of operated patients over time (blue circles). Red circles indicate patients who were converted to full sternotomy. Black circles with a cross indicate patients who died. A logarithmic regression line of fit (shading shows 95% confidence interval) for converted patients only shows increasing length of hospital stay for later conversions.
Discussion
Minimally invasive surgery on the mitral valve using femoral cardiopulmonary bypass and an endovascular aortic occlusion device was first described in humans in 1996.1 The early outcomes for this procedure were poor, with a mortality of 9.8% and aortic dissections in 3.9% of patients.15 Seventeen years later, the same group described their learning curves for minimally invasive mitral surgery. The overall mortality had fallen to 2.3% and conversions were 1.7% compared with 11.7% in the initial series.3
Anecdotally, much of the concern over adopting minimally invasive strategies for the mitral valve and associated surgeries has been directed at three areas of uncertainty: management of the clamped aorta and aortic root in a closed chest; the effects of retrograde perfusion in atheromatous aortae; and the risks of substantially increased cardiopulmonary bypass and ischaemic times resulting from the technical difficulties of reduced exposure and access.
The options for aortic cross-clamping include either transthoracic external clamping or intravascular occlusion. The former is cheaper, familiar to surgeons experienced in open surgery but requires closed-chest cannulation of the aortic root and blind closure of the distal end of the clamp near the pulmonary artery and left atrial appendage, which can be iatrogenically perforated. Even in experienced centres, the dissection required around the great vessels precludes use of the transthoracic clamp in cardiac reoperation. Conversely, endoaortic occlusion is more expensive and requires vigilance by the perfusionist and anaesthetist to prevent balloon migration or subtotal occlusion. Vanerman et al reported a dissection rate of 2.7%.16 Initially, these events were thought to be related to the use of the intra-aortic occlusion devices, but they are now understood to be the result of high arterial line pressures in the cardiopulmonary bypass circuit. Subsequent series of patients undergoing minimally invasive surgery with endovascular occlusion systems have shown that the technique can be used safely.14 In our series, avoidance of line pressures greater than 300mmHg has been didactically enforced and we have seen no iatrogenic dissections.
One group described a 17.4% endoaortic clamp dysfunction rate which is likely to have been user error rather than device failure.17 Another group described a 35% rate of technical issues with endoaortic clamping.18 Within five years of the technology being introduced, however, centres were performing the technique with no conversions or dissections.19 Endoaortic balloon clamping is associated with fewer cerebral microemboli and lower rates of intimal, medial and adventitial damage compared with mechanical cross-clamp.20–22 A recent meta-analysis of endovascular versus transthoracic aortic clamping found no difference in cerebrovascular events or all-cause mortality but identified a higher rate of iatrogenic dissection (0.93% vs 0.13%).23 Excluding early series where perfusion pressures were run much higher, there are no increased risks compared with transthoracic clamping.
Concerns that retrograde perfusion, regardless of cross-clamping, increase incidence of stroke have also been unfounded.24 Developments in the technology such as decreased resistance despite a smaller catheter size with higher flow rates at lower pressures should also ameliorate some of the initial issues with femoral cannulation.8 By performing routine preoperative computed tomography of the vascular tree, together with transoesophageal echocardiography of the descending aorta prior to use of femoral cannulation, we exclude patients with highly mobile atheroma. Our findings suggest that in patients with lower grades of atherosclerotic disease, even in the presence of substantial but fixed plaques, stroke and distal organ ischaemia are rare.
We have also demonstrated that prolonged cardiopulmonary bypass times and aortic cross-clamp times are not associated with increased risks of mortality, morbidity or conversion. Although our default strategy is for single-femoral arterial inflow and femoral-only venous drainage, we use a second superior vena caval venous cannula to assist drainage in patients over 80kg and cannulate a second femoral artery (or drop systemic temperature) if perfusion pressures approach 300mmHg or adequate flows cannot be maintained for the patient’s calculated cardiac index. Careful preoperative planning in conjunction with the anaesthetists and perfusionists is required to select appropriate cannulas and cannulation strategies for each patient and we believe that this can reduce complications associated with malperfusion.
Importantly, and contrary to the findings of other groups, we did not find that conversion was associated with higher morbidity or mortality.25 Consequently, we disagree with the assertion that conversion should be avoided at all costs.26 It is our opinion that conversion is a strategy change that should be considered early in the operation to avoid clinical complications. Reversion to sternotomy, which remains the standard of care for mitral valve surgery worldwide, should not be considered a failure of treatment. Recognising that high levels of conversions could be a surrogate marker of inexperience in minimally invasive procedures, however, we performed a sensitivity analysis and found the composite outcome learning curve remained within limits. It is interesting to note that conversions early in our experience did not experience prolonged hospital stays. With increasing experience, however, a trend emerged for longer hospital stays in patients converted to sternotomy (Figure 5). While the numbers are small, this may represent a change in philosophy or reflect a tendency to convert more complex cases with increasing experience.
While operative costs are higher, minimally invasive mitral surgery reduces total hospital costs compared with sternotomy as a result of shorter stays and reduced complications.27,28 Much of the information comes from observational studies, however, and a thorough prospective randomised controlled trial of minimally invasive versus sternotomy approach for mitral valve surgery is only now being undertaken in the UK.29 Nonetheless, there is growing demand for less invasive methods in cardiac surgery and, as patients increasingly request such methods, expertise will have to proliferate. It is likely that this expectation will include patients requiring redo surgery, those with high body mass index and other risk factors which have traditionally been considered contraindications.
One of the concerns among surgeons has been the number of procedures required to get over the learning curve, in particular when using non-traditional technologies such as an endoaortic occlusion device. Recent series suggest a learning curve of approximately 75 cases.3 This volume of cases would be limited to very few centres, however, and would deter many centres from adopting the technology. Our experience began with less than 20 cases/year for several years (1.8% of our annual cardiac surgery activity), typifying our experience as a low-volume mitral centre. The surgeon involved had undertaken a structured fellowship in a high-volume centre and had access to mentors who helped him to navigate the learning curve. A team involving anaesthesia, perfusion and dedicated surgical nursing staff is also fundamental to the success of such a programme.30 Our team included a single surgeon, two anaesthetists, one perfusionist (eventually expanding to three) and two scrub nurses. No cases were performed without a team member from each core discipline. Investment in setting up a team approach and using a fellowship in an existing minimally invasive centre were crucial factors in achieving these results and may have ameliorated the expected differences between high- and low-volume centres. There is a political push to create large centres in the UK, but this is against patient preference, as most heart valve disease is seen in the elderly population who are less likely to travel further afield to access heart surgery.
All our mortalities occurred in the middle third of our experience, when patients with substantially higher EuroSCOREs had also started to be accepted for minimally invasive surgery. This probably represented a degree of confidence in the minimally invasive technique that encouraged much higher risk patients to be adopted on compassionate grounds, similar to the approach adopted early in transcatheter aortic valve replacement. Close scrutiny of minimally invasive cardiac surgery does not encourage this approach in the long-term, however, and more focused patient selection, even with higher EuroSCORES, seems to have kept our series event-free following that experience.
We conclude that the endoaortic occlusion technique for minimally invasive cardiac surgery can be safely adopted in centres performing low volumes in the initial learning curve, where a coherent and consistent team has been established. Using the device as a routine strategy ensures that when more difficult combined or redo procedures are undertaken, the endoaortic clamp does not add an additional layer of confounding complexity.
Limitations
Small iterative changes in surgical technique, technology or postoperative medical management may have been overlooked in the methodology. The complete, heterogenous experience of a single centre may limit the wider applicability of this study.
Conclusions
Minimally invasive mitral, tricuspid and atrial septal defect surgery can be performed with safe and effective learning curves even in centres with low initial volumes. Our technique allows progression to more complex cases later in the learning curve with minimal impact and no obvious learning curve. We credit the good results to a structured fellowship in a large volume centre prior to embarking on the programme.
Acknowledgements
We thank Cathy Malpas for her assistance in collecting data for the study and Dr Hugo Vanerman for his mentorship both during the fellowship and after.
References
- 1.Falk V, Walther T, Diegeler Aet al. Echocardiographic monitoring of minimally invasive mitral valve surgery using an endoaortic clamp. J Heart Valve Dis 1996; 5: 630–637. [PubMed] [Google Scholar]
- 2.Gammie JS, O’Brien SM, Griffith BPet al. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation 2007; 115: 881–887. 10.1161/CIRCULATIONAHA.106.634436 [DOI] [PubMed] [Google Scholar]
- 3.Holzhey DM, Seeburger J, Misfeld Met al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume centre. Circulation 2013; 128: 483–491. 10.1161/CIRCULATIONAHA.112.001402 [DOI] [PubMed] [Google Scholar]
- 4.Murzi M, Miceli A, Cerillo AGet al. Training surgeons in minimally invasive mitral valve repair: a single institution experience. Ann Thorac Surg 2014; 98: 884–9. 10.1016/j.athoracsur.2014.05.040 [DOI] [PubMed] [Google Scholar]
- 5.Ludman PF, Moat N, de Belder MAet al. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6-year follow-up: a report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation 2015; 131: 1181–90. 10.1161/CIRCULATIONAHA.114.013947 [DOI] [PubMed] [Google Scholar]
- 6.Kempfert J, Rastan A, Holzhey Det al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124: S124–129. 10.1161/CIRCULATIONAHA.110.013425 [DOI] [PubMed] [Google Scholar]
- 7.Alli OO, Booker JD, Lennon RJet al. Transcatheter aortic valve implantation: assessing the learning curve. JACC Cardiovasc Interv 2012; 5: 72–79. 10.1016/j.jcin.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 8.Marullo AGM, Irace FG, Vitulli Pet al. Recent developments in minimally invasive cardiac surgery: evolution or revolution? Biomed Res Int 2015; 2015: 483025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czesla M, Götte JM, Doll N. How to establish video assisted, minimally invasive mitral valve surgery. Heart 2012; 98: 1172–1178. 10.1136/heartjnl-2011-300348 [DOI] [PubMed] [Google Scholar]
- 10.Casselman FP, Van Slycke S, Wellens Fet al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 2003; 108(Suppl 1): 1148–1154. 10.1161/01.cir.0000087391.49121.ce [DOI] [PubMed] [Google Scholar]
- 11.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org (cited December 2020). [Google Scholar]
- 12.Meyer A, Kempfert J, Falk V. rcusum v1.0.1 https://sandbox.zenodo.org/record/51159#.X8u4vWT7TKI (cited December 2020).
- 13.Nashef SAM, Roques F, Michel Pet al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999; 16: 9–13. 10.1016/S1010-7940(99)00134-7 [DOI] [PubMed] [Google Scholar]
- 14.De Praetere H, Verbrugghe P, Rega Fet al. Starting minimally invasive valve surgery using endoclamp technology: safety and results of a starting surgeon. Interact CardioVasc Thorac Surg 2015; 20: 351–358. 10.1093/icvts/ivu394 [DOI] [PubMed] [Google Scholar]
- 15.Mohr FW, Falk V, Diegeler Aet al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998; 115: 567–576. 10.1016/S0022-5223(98)70320-4 [DOI] [PubMed] [Google Scholar]
- 16.Vanermen H, Wellens F, De Geest Ret al. Video-assisted Port-Access mitral valve surgery: from debut to routine surgery. Will trocar-Port-Access cardiac surgery ultimately lead to robotic cardiac surgery? Semin Thorac Cardiovasc Surg 1999; 11: 223–234. 10.1016/S1043-0679(99)70063-8 [DOI] [PubMed] [Google Scholar]
- 17.Aybek T, Dogan S, Wimmer-Greinecker Get al. The micro-mitral operation comparing the Port-Access technique and the transthoracic clamp technique. J Card Surg 2000; 15: 76–81. 10.1111/j.1540-8191.2000.tb00446.x [DOI] [PubMed] [Google Scholar]
- 18.Dogan S, Dzemali O, Wimmer-Greinecker Get al. Minimally invasive versus conventional aortic valve replacement: a prospective randomized trial. J Heart Valve Dis 2003; 12: 76–80. [PubMed] [Google Scholar]
- 19.Greco E, Zaballos JM, Alvarez Let al. Video-assisted mitral surgery through a micro-access: a safe and reliable reality in the current era. J Heart Valve Dis 2008; 17: 48–53. [PubMed] [Google Scholar]
- 20.Maselli D, Pizio R, Borelli Get al. Endovascular balloon versus transthoracic aortic clamping for minimally invasive mitral valve surgery: impact on cerebral microemboli. Interact Cardiovasc Thorac Surg 2006; 5: 183–186. 10.1510/icvts.2005.123372 [DOI] [PubMed] [Google Scholar]
- 21.Loforte A, Luzi G, Montalto Aet al. Video-assisted minimally invasive mitral valve surgery: external aortic clamp versus endoclamp techniques. Innovations (Phila ) 2010; 5: 413–418. 10.1177/155698451000500606 [DOI] [PubMed] [Google Scholar]
- 22.Ozalp B, Canbaz S, Huseyinova Get al. Histopathological comparison of vascular wall damage created by external cross-clamp and endoluminal balloon occlusion techniques. J Cardiovasc Surg (Torino ) 2009; 50: 545–553. [PubMed] [Google Scholar]
- 23.Kowalewski M, Malvindi PG, Suwalski Pet al. Clinical safety and effectiveness of endoaortic as compared with transthoracic clamp for small thoracotomy mitral valve surgery: meta-analysis of observational studies. Ann Thorac Surg 2017; 103: 676–686. 10.1016/j.athoracsur.2016.08.072 [DOI] [PubMed] [Google Scholar]
- 24.Ad N, Holmes SD, Shuman DJet al. Minimally invasive mitral valve surgery without aortic cross-clamping and with femoral cannulation is not associated with increased risk of stroke compared with traditional mitral valve surgery: a propensity score-matched analysis. Eur J Cardiothorac Surg 2015; 48: 868–872. 10.1093/ejcts/ezv017 [DOI] [PubMed] [Google Scholar]
- 25.Vollroth M, Seeburger J, Garbade Jet al. Minimally invasive mitral valve surgery is a very safe procedure with very low rates of conversion to full sternotomy. Eur J Cardiothorac Surg 2012; 42: e13–6. 10.1093/ejcts/ezs195 [DOI] [PubMed] [Google Scholar]
- 26.Vollroth M, Seeburger J, Garbade Jet al. Conversion rate and contraindications for minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013; 2: 853–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santana O, Larrauri-Reyes M, Zamora Cet al. Is a minimally invasive approach for mitral valve surgery more cost-effective than median sternotomy? Interact CardioVasc Thorac Surg 2016; 22: 97–100. 10.1093/icvts/ivv269 [DOI] [PubMed] [Google Scholar]
- 28.Verbrugghe P, De Praetere H, Meuris Bet al. Cost analysis of minimally invasive compared with conventional mitral valve surgery. Acta Cardiol 2016; 71: 527–535. 10.1080/AC.71.5.3167495 [DOI] [PubMed] [Google Scholar]
- 29.Newcastle Clinical Trials Unit. Minimally invasive thoracoscopically-guided right minithoracotomy versus conventional sternotomy for mitral valve repair. ISRCTN1393. 0454. ISRCTN Registry 2016. 10.1186/ISRCTN13930454 (cited December 2020). [DOI]
- 30.Pisano GP, Bohmer RMJ, Edmondson AC. Organizational differences in rates of learning: evidence from the adoption of minimally invasive cardiac surgery. Manag Sci 2001; 47: 752–768. 10.1287/mnsc.47.6.752.9811 [DOI] [Google Scholar]