Abstract
Introduction
Combined heart and liver transplantation (CHLT) is one of the most complex procedures of surgery that has been implemented in the last 35 years. The aim of our meta-analysis was to investigate the safety and efficacy of CHLT.
Materials
The meta-analysis was designed according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) and AMSTAR (A MeaSurement Tool to Assess systematic Reviews) recommendations. A literature search was conducted up to April 2020 using the MEDLINE,® SCOPUS,® ClinicalTrials.gov, Embase™, Cochrane Central Register of Controlled Trials and Google Scholar™ databases.
Results
Our meta-analysis included 16 studies with 860 patients. The mortality rate following CHLT was 14.1%. One and five-year survival rates were 85.3% and 71.4% while the heart and liver rejection rates were 6.1% and 9.1% respectively. The hospital stay was 25.8 days and the intensive care unit stay was 9.9 days. Pooled values were also calculated for cardiopulmonary bypass duration, units of transfused red blood cells and fresh frozen plasma, postoperative infection rate, mechanical ventilation rate and follow-up duration.
Conclusions
Despite its complexity, CHLT is a safe and effective procedure for the management of lethal diseases that lead to progressive heart and/or liver failure. Nevertheless, there must be strict adherence to the indications for surgery, and future studies should compare CHLT with isolated cardiac and hepatic transplantations.
Keywords: Combined, Heart, Liver, Transplantation, Meta-analysis
Introduction
In 1985, a six-year old girl suffering from homozygous familiar hypercholesterolaemia underwent a novel surgical procedure consisting of both cardiac and hepatic transplantation, the major indication of which was heart failure due to rapidly progressing coronary artery disease.1 Between 1988 and 2015, approximately 200 such operations took place in several high volume hospitals in the US.2 Over the last few decades, numerous clinical studies have described the combination of cardiac and hepatic transplantation as a life saving operation in cases of end-stage heart and liver failure.3
One of the most frequent indications for such a procedure is familial amyloidosis (also called transthyretin amyloidosis). This is a progressive and fatal disease characterised by extracellular accumulation of liver derived transthyretin in various organs (eg heart, bladder, intestine and soft tissues), and presents with a variety of overlapping syndromes of both familiar amyloid polyneuropathy and cardiomyopathy.4,5
Another indication worth mentioning is heart failure combined with cardiac cirrhosis. Cardiac cirrhosis is caused by congenital heart disease and dilated cardiomyopathy, which lead to hepatic dysfunction and cirrhosis as a result of chronic hepatic congestion secondary to elevated hepatic vein pressure, restricted blood flow and oxygen saturation in hepatic cells.6 In addition, excess iron in the heart and liver (resulting from hereditary haemochromatosis and frequent blood transfusions) often leads to combined heart and liver transplantation (CHLT).7 Last but not least, homozygous familial hypercholesterolemia, an autosomal dominant disorder characterised by severe low density lipoprotein receptor dysfunction and progressive atherosclerosis, is another common reason for such surgery.8,9
Nowadays, owing to its complexity, several clinical studies have attempted to investigate whether CHLT is superior to isolated transplantations. Although no statistically significant difference has been observed in terms of survival rates, CHLT seems to be more beneficial than an isolated cardiac transplantation, possibly because of the immunoprotective effect of the liver implant.10 On the other hand, no such correlation has been demonstrated between CHLT and isolated hepatic transplantation. However, only limited data are available on exact short and long-term complications and survival after these procedures.11
The aim of this study was to gather the available evidence published between 1985 and 2020 in order to investigate the potential role of CHLT as a gold standard therapeutic approach for simultaneous heart and liver failure. Compared with the last reported meta-analysis in this field,12 the present systematic review contains a larger number of patients, who were included in more recent studies in the literature.
Methods
The design of our meta-analysis adhered to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) and AMSTAR (A MeaSurement Tool to Assess systematic Reviews) recommendations.13,14 The MEDLINE®, SCOPUS®, ClinicalTrials.gov, Embase,™ Cochrane Central Register of Controlled Trials and Google Scholar™ databases were scanned up to 23 April 2020. In addition, the chances of including all available articles that met the inclusion criteria were maximised by searching the references of all full-text articles retrieved. The main search algorithm applied was: (combined[All Fields] AND (“liver”[MeSH Terms] OR “liver”[All Fields]) AND (“heart transplantation”[MeSH Terms] OR (“heart”[All Fields] AND “transplantation”[All Fields]) OR “heart transplantation”[All Fields]). The PRISMA flow diagram in Appendix 1 (available online) illustrates the article selection process.
Study selection was performed in three stages. First, duplicate publications were removed, following which the titles and abstracts were read of all electronic articles that appeared in the search in order to assess their eligibility. Second, the full text of all articles that met the inclusion criteria was downloaded, and all prospective and retrospective observational studies were selected. Study search and data tabulation was conducted by two authors on similar, predefined forms. A consensus of all authors resolved any conflicts after retrieving all available data.
The eligibility criteria were predefined and there were no data restrictions during the search procedure. All English language articles were included as well as those written in other languages using the Latin-script alphabet as long as this could be translated with the Google Translate™ service. Prospective and retrospective observational studies that demonstrated operative outcomes after CHLT were eligible for inclusion. Case reports, experimental animal studies and reviews were excluded.
Outcome measures
The primary outcome measures of our meta-analysis were survival rates after one and five years, overall mortality rate, cardiac and hepatic graft rejection rates, intensive care unit (ICU) stay and length of hospitalisation. Cardiopulmonary bypass duration, fresh frozen plasma (FFP) and red blood cell (RBC) transfusion, postoperative infection rate, mechanical ventilation requirement and follow-up duration after CHLT were secondary outcome measures.
Quality assessment of studies
The Newcastle–Ottawa scale was employed to assess the methodological quality of the included articles.15 The scale comprises eight items, divided into three groups: selection of the study cohorts, comparability of the cohorts and ascertainment of the outcome of interest. Studies were graded with stars for each item. Nine stars was the maximum possible score for each study. (A maximum of two stars can be awarded for comparability.)
Surgical technique
CHLT is a combination of the surgical procedures that are followed for the isolated transplantation of each organ separately. Most of the studies included in our meta-analysis followed the same technique and CHLT was undertaken during the same operation.
The procedure commenced with heart transplantation so as to minimise the cold ischaemia time of the cardiac graft and eliminate the possibility of heart failure during reperfusion of the hepatic graft. It was conducted using extracorporeal circulation and open chest liver transplantation followed. The liver transplantation was performed in two possible ways. Either an en bloc resection (including the retrohepatic segment of the inferior vena cava) was carried out under venovenous extracorporeal circulation or the piggyback technique was employed, using a temporary portocaval shunt, in which end-to-side or side-to-side anastomosis united both venae cavae. Two important variations that were adopted in some centres were en bloc transplantation of both heart and liver (minimising the cold ischaemia time) and maintaining extracorporeal membrane oxygenation during liver transplantation.16
Statistical analysis
MedCalc® version 18.2.1 (MedCalc Software, Mariakerke, Belgium) was employed to perform the proportional meta-analysis of dichotomous variables and OpenMeta[Analyst] (Brown University, Providence, RI, US) was used for continuous variables.17 Confidence intervals (CIs) were preassumed at 95%. Pooled proportions and 95% CIs were derived after calculation of proportions and 95% CIs for dichotomous measures while pooled means and 95% CIs were derived after calculation of means and 95% CIs for continuous measures. When standard deviations were not provided, the appropriate transformations were undertaken.18
The included studies mostly demonstrated a high degree of heterogeneity. Consequently, a random effects (DerSimonian–Laird) model using arcsine square root transformation was employed to derive pooled estimates of all variables and to back-transform the weighted means of the transformed variables as well as their CIs.19 Double arcsine (Freeman–Tukey) transformation was utilised for this single arm meta-analysis instead of logit or arcsine transformation as our review includes only a small number of studies with small numbers of patients.20 Study heterogeneity was estimated according to the I2 statistic.21 A significance level of p<0.05 and an I2 value of ≥50% indicated high heterogeneity.
Protocol registration
Our meta-analysis was registered in the Open Science Framework (www.osf.io) with the unique identifying number 10.17605/OSF.IO/5T6VQ.
Results
A total of 16 studies were included in our meta-analysis (Appendix 1 – available online). These comprised 860 patients who were listed for CHLT owing to several diseases that led to end-stage heart and liver failure.3,4,10,22–34 All studies were retrospective and one study compared the postoperative outcomes after CHLT between patients with and without congenital heart disease.32 Only 21 CHLTs were performed in Europe (Italy and Denmark)23,27 with the rest being undertaken in the US although only 2 medical centres have performed more than 10 CHLTs.24,29
Data tabulation
Appendix 2 (available online) summarises the methodology of the included studies along with each study’s indications for cardiac and hepatic transplantation. The most common source of data for the included studies was the United Network for Organ Sharing registry. Appendix 3 (available online) shows the baseline characteristics of the patients (including sex, age, body mass index, previous cardiac surgery, left ventricular ejection fraction, MELD [Model for End-stage Liver Disease] score and preoperative bilirubin and creatinine levels). Among the included patients, 514 were male and 346 were female, and 160 patients had a history of previous cardiac surgery.
Appendix 4 (available online) lists the perioperative outcomes for each study (operative time, cardiopulmonary bypass duration, RBC and FFP transfusion, ICU stay, postoperative infection and mechanical ventilation requirement) as well as the immunosuppressive regimens that were employed for graft maintenance. Appendix 5 (available online) summarises each study’s postoperative outcomes (hospital stay, mortality, one and five-year survival, follow-up duration, and heart and liver rejection rates). Finally, Table 1 presents the pooled perioperative (cardiopulmonary bypass duration, RBC and FFP transfusion, ICU stay, postoperative infection, mechanical ventilation requirement, hospital stay and mortality) and long-term (1 and 5-year survival rates, follow-up duration, heart and liver rejection rates) outcomes after CHLT.
Table 1 .
The pooled perioperative and long-term outcomes after combined heart and liver transplantation
| Pooled value | 95% CI | Number of studies | Heterogeneity (I 2 ) | p-value | |
|---|---|---|---|---|---|
| Operative outcomes | |||||
| Cardiopulmonary bypass duration (min) | 183.5 | 142.9–224.2 | 6 | 93.4% | <0.1 |
| RBC transfusion (units) | 17.9 | 7.7–28.1 | 4 | 98.4% | <0.1 |
| FFP transfusion (units) | 14.3 | 6.1–22.6 | 3 | 89.1% | <0.1 |
| ICU stay (days) | 9.9 | 7.5–12.2 | 6 | 46.9% | <0.1 |
| Postoperative infection | 20.8% | 6.8–40.1% | 8 | 90.2% | <0.0001 |
| Mechanical ventilation | 66.5% | 28.1–95.1% | 8 | 98.7% | <0.0001 |
| Survival outcomes | |||||
| Hospital stay (days) | 25.8 | 21.2–30.4 | 7 | 32.2% | 0.2 |
| Mortality rate | 14.1% | 8.3–21.1% | 13 | 78.6% | <0.0001 |
| 1-year survival rate | 85.3% | 80.3–89.6% | 11 | 65.7% | 0.0012 |
| 5-year survival rate | 71.4% | 57.9%–83.1% | 12 | 94.2% | <0.0001 |
| Follow-up duration (months) | 56.8 | 31.5–82.1 | 16 | 100% | <0.1 |
| Heart rejection rate | 6.1% | 1.6–13.1% | 12 | 78.2% | <0.0001 |
| Liver rejection rate | 9.1% | 3.1–18.1% | 12 | 81.6% | <0.0001 |
CI = confidence interval; FFP = fresh frozen plasma; ICU = intensive care unit; RBC = red blood cell
Transplantation indications and immunosuppression
The patients included in our meta-analysis underwent CHLT owing to several diseases that progressively led to end-stage heart and/or liver failure. The most common indications for heart transplantation were restrictive cardiomyopathy (11.8%), familial amyloidosis (11.1%), congenital heart disease (9.4%) and idiopathic cardiomyopathy (9.4%) whereas the most common indications for hepatic transplantation were cirrhosis of cardiac, alcoholic or cryptogenic aetiology (14.6%), familial amyloidosis (9.5%), hepatitis C virus associated cirrhosis (4.5%) and haemochromatosis (2.2%). The most common immunosuppressive agents for graft maintenance following CHLT were steroids, tacrolimus, cyclosporine, azathioprine and mycophenolate mofetil, which were included in the immunosuppressive regimens of almost all of the studies in our analysis.
Primary outcome measures
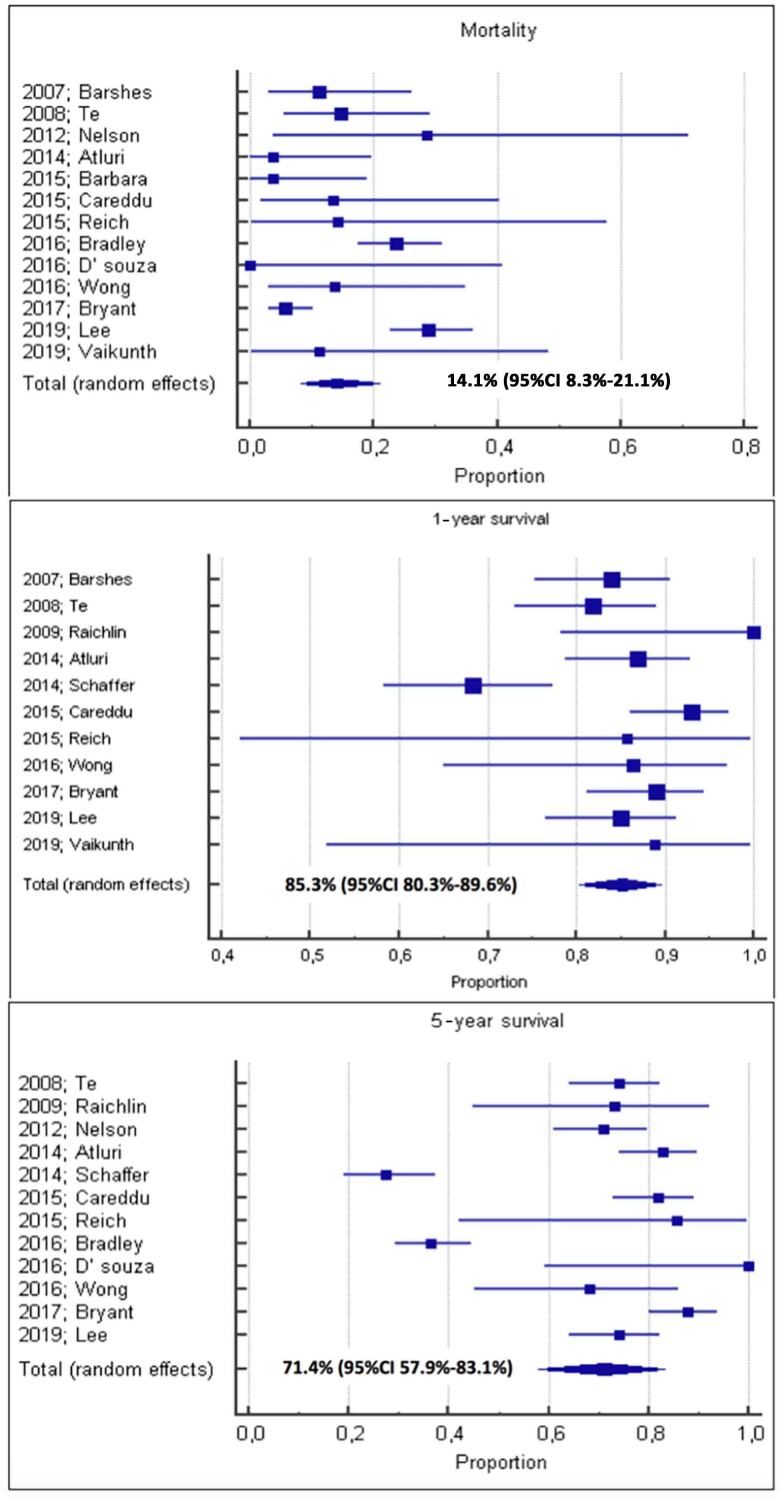
Thirteen of the sixteen studies provided data on mortality; the pooled mortality rate after CHLT was 14.1% (95% CI: 8.3–21.1%). Meta-analysis of 11 studies revealed the pooled 1-year survival rate to be 85.3% (95% CI: 80.3–89.6%). The pooled 5-year survival rate was 71.4% (95% CI: 57.9–83.1%), based on results from 12 studies. Forest plots for these data are provided in Figure 1.
Figure 1 .
Forest plots showing a pooled mortality rate of 14.1%, and pooled 1-year and 5-year survival rates of 85.3% and 71.4% respectively
Meta-analysis of 12 studies gave a pooled cardiac graft rejection rate of 6.1% (95% CI: 1.6–13.1%) and a pooled hepatic graft rejection rate of 9.1% (95% CI: 3.1–18.1%). Appendix 7 (available online) shows the forest plots for graft rejection while Appendix 8 (available online) illustrates the data for length of hospital stay and ICU stay. Seven studies demonstrated that the pooled hospital stay after CHLT was 25.8 days (95% CI: 21.2–30.4 days). Six studies provided ICU data, with a pooled ICU stay of 9.9 days (95% CI: 7.5–12.2 days).
Secondary outcome measures
Meta-analysis of six studies revealed that the pooled cardiopulmonary bypass duration was 183.5 minutes (95% CI: 142.9–224.2 minutes). The pooled mean number of RBC units that were required to be transfused during CHLT was 17.9 (95% CI: 7.7–28.1) whereas for FFP, this was 14.3 (95% CI: 6.1–22.6). Four studies were meta-analysed for RBC data while three studies provided information on FFP.
The pooled postoperative infection rate was 20.8% (95% CI: 6.8–40.1%), based on meta-analysis of eight studies. Analysis of eight studies also gave a pooled mechanical ventilation rate of 66.5% (95% CI: 28.1–95.1%). Finally, all 16 studies provided information on the follow-up period. The pooled follow-up duration was 56.8 months (95% CI: 31.5–82.1 months).
Quality assessment of studies
The methodological quality of the included studies was evaluated using the Newcastle–Ottawa scale.15 Fourteen of the studies received seven stars while the remaining two studies received six stars (Appendix 6 – available online).
Discussion
Our meta-analysis has shown the perioperative and long-term outcomes after CHLT indicated for several diseases that led to progressive end-stage heart and/or liver failure. Restrictive cardiomyopathy, familial amyloidosis, congenital heart disease and idiopathic cardiomyopathy were the most common indications for heart transplantation whereas cardiac or alcoholic cirrhosis, familial amyloidosis, hepatitis C virus associated cirrhosis and haemochromatosis were the most common indications for liver transplantation.
CHLT appears to be one of the most complicated surgical procedures as it requires prolonged cardiopulmonary bypass circulation, large amounts of transfused RBCs and FFP, and prolonged ICU stay and hospitalisation. Nevertheless, postoperative morbidity indices such as infection rate and mechanical ventilation requirements remain low. Overall mortality, however, is moderate, with satisfactory one and three-year survival. Despite its surgical complexity, the safety of CHLT has been correlated both with the immunosuppressive agents (steroids, tacrolimus, cyclosporine, azathioprine and mycophenolate mofetil), which keep graft rejection rates low, and with the prolonged follow-up period, which provides intensive medical support for patients with potentially life threatening diseases.
Our meta-analysis of 16 studies (with 860 patients) has demonstrated a mortality rate of 14.1% (95% CI: 8.3–21.1%). This is similar to the mortality rates after isolated heart transplantation (7.4%) and isolated liver transplantation (10%).35,36 The most common causes of perioperative death were infections and cardiovascular disease.32 Survival after CHLT was 85.3% (95% CI: 80.3–89.6%) at one year and 71.4% (95% CI: 57.9–83.1%) at five years. The one-year survival rate following isolated cardiac transplantation has been reported as 85–90%,37 which is comparable with that for CHLT. The survival rate after isolated hepatic transplantation has been calculated as approximately 90% at one year and 80% at five years,38 which is also similar to our findings for CHLT.
Safety aside, CHLT provides an effective therapeutic option for improvement of both heart and liver failure simultaneously with a cardiac graft rejection rate of 6.1% (95% CI: 1.6–13.1%) and a hepatic graft rejection rate of 9.1% (95% CI: 3.1–18.1%). The effectiveness of this complex procedure has been reinforced by work from Cannon et al, who observed that hepatic graft survival at one, five and ten years was similar between CHLT patients and those undergoing isolated heart transplantation (83.4%, 72.8% and 71.0% vs 79.4%, 71.0% and 65.1% respectively; p=0.894).11 In addition, they demonstrated that cardiac allograft survival at one, five and ten years was also similar for CHLT and isolated cardiac transplantation (83.5%, 73.2% and 71.5% vs 82.6%, 71.9% and 63.2% respectively; p=0.341).
Nevertheless, 5-year graft survival rates after isolated hepatic transplantation have been shown to decrease if the recipients present certain risk factors (ventilator support, age >60 years, haemodialysis, diabetes or serum creatinine ≥1.5 mg/dl) from 77.2% to approximately 50%, depending on the number of risk factors and the presence of liver failure.39 Moreover, diabetes has been associated with increased risk for graft failure and patient mortality after CHLT (hazard ratio [HR]: 2.28, 95% CI: 1.05–4.92, p=0.036), and the post-2006 transplant era has been linked to significantly improved graft survival as well as overall survival (HR: 0.45, 95% CI: 0.24–0.87, p=0.017).40
The pathophysiological relationship between the heart and liver has been the basis for combined transplantation of these two organs when their function is impaired. However, the heart also has a close functional relationship with the lungs. The survival rates after combined transplantation of these two organs are 71% at three months, 63% at one year, 44% at five years and 32% at ten years, with a reported operative mortality rate of 16.8%;41 these are significantly worse than our findings for CHLT. Heart and liver transplantation is performed using a chest-abdominal approach, as is lung–liver transplantation but this procedure has a one-year survival rate of 69% and a five-year survival rate of 49%,42 which are also worse than our pooled data for CHLT. Consequently, heart and liver transplantation seems to be the safest among combined organ transplantations.
The findings of our meta-analysis indicate that despite its complexity, CHLT presents similar postoperative outcomes to isolated procedures and in some series, these outcomes are better than for isolated procedures. This potential benefit offered by the combined procedure is based on the possible minimisation of alloantigen exposure, which may diminish immune activation and acute rejection of the simultaneously transplanted organ.43
On the one hand, the liver is considered tolerogenic as it is quickly accepted in animal transplantation models but it also maintains a central role in immune surveillance as it provides intrinsic mechanisms that prevent immune activation to several antigens while clearing circulating pathogens.44 As a result, when the liver is included in multiorgan transplantations, decreased rates of allograft rejection among recipients have been reported.45 The mechanisms that lead to decreased levels of donor specific antibodies have not yet been clarified but production of soluble major histocompatibility complex class I antigens or absorption of alloreactive immune complexes by the liver’s large surface has been proposed.46
Study strengths and weaknesses
To our knowledge, apart from a meta-analysis from 2020 that included 6 studies and 99 patients,12 the present meta-analysis is the first in the international literature that has systematically included all observational studies that describe operative outcomes following CHLT, based on an extensive search of a wide range of databases. No restrictions were applied during the literature search, thereby maximising the chances of including all available articles related to the topic. Furthermore, our meta-analysis has been registered in the international Open Science Framework database, giving every researcher the opportunity to assess its methodological quality and statistical adequacy.
However, the systematic nature of the present study required all papers that met the inclusion criteria to be incorporated in the meta-analysis and considerable heterogeneity was therefore observed between the included studies in terms of indications for transplantation and graft waiting times. Unfortunately, it was not possible to investigate the effect of these parameters on our primary outcome measures. In addition, given the small number of patients involved, our findings should be interpreted with caution. Moreover, control groups were not present among the included studies, and so a comparative analysis between CHLT and isolated procedures was not feasible. Finally, only one study compared CHLT between patients with and without congenital heart disease.
Implications for clinical practice and future research
CHLT seems to be a relatively safe procedure with proven clinical effectiveness compared with isolated heart and liver transplantations. Nevertheless, it remains a highly complicated and technically challenging surgical procedure with complex postoperative patient management by highly skilled and experienced multidisciplinary teams. Consequently, transplant surgeons should possess the required technical skills to perform such procedures, together with advanced immunology and critical care knowledge to handle the postoperative course of transplant recipients as well as possible complications. Indications for CHLT should be strictly adhered to for all patients. Such procedures also require well organised transplant centres that can support recipients for a prolonged period after the operation.
However, a comparison between CHLT and isolated procedures could be extremely useful; for this reason, future trials should focus on this area. In addition, the immunological basis of the protective role of the liver in multiorgan transplantation should be investigated by means of molecular and experimental studies. In that way, the immunological secrets of graft maintenance can be revealed and the clinical outcomes of transplantations can be improved.
Conclusions
CHLT seems to be the final solution for several end-stage heart and liver diseases such as restrictive cardiomyopathy, familial amyloidosis and cirrhosis secondary to various causes. Despite its high level of complexity, morbidity and mortality rates remain relatively low, while survival rates are encouraging. It therefore appears to be a safe procedure when it is performed in specified transplant centres by expert surgeons. Nevertheless, there must be strict adherence to the indications for surgery, along with multidisciplinary consultation on whether to perform CHLT or isolated heart and liver transplantations.
References
- 1.Starzl TE, Bilheimer DW, Bahnson HTet al. Heart–liver transplantation in a patient with familial hypercholesterolaemia. Lancet 1984; 1: 1382–1383. 10.1016/S0140-6736(84)91876-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal EW, Mumtaz K, Hayes Det al. Combined heart–liver transplantation: indications, outcomes and current experience. Transplant Rev 2016; 30: 261–268. 10.1016/j.trre.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichlin E, Daly RC, Rosen CBet al. Combined heart and liver transplantation: a single-center experience. Transplantation 2009; 88: 219–225. 10.1097/TP.0b013e3181ac60db [DOI] [PubMed] [Google Scholar]
- 4.Te HS, Anderson AS, Millis JMet al. Current state of combined heart–liver transplantation in the United States. J Heart Lung Transplant 2008; 27: 753–759. 10.1016/j.healun.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Naharro A, Treibel TA, Abdel-Gadir Aet al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol 2017; 70: 466–477. 10.1016/j.jacc.2017.05.053 [DOI] [PubMed] [Google Scholar]
- 6.Hadi H El, Di Vincenzo A, Vettor R, Rossato M. Relationship between heart disease and liver disease: a two-way street. Cells 2020; 9: 567. 10.3390/cells9030567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol 2010; 56: 1001–1012. 10.1016/j.jacc.2010.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Santamaria M, Migliazza L, Gamez Met al. Liver transplantation in patients with homozygotic familial hypercholesterolemia previously treated by end-to-side portocaval shunt and ileal bypass. J Pediatr Surg 2000; 35: 630–633. 10.1053/jpsu.2000.0350630 [DOI] [PubMed] [Google Scholar]
- 9.Ishigaki Y, Kawagishi N, Hasegawa Yet al. Liver transplantation for homozygous familial hypercholesterolemia. J Atheroscler Thromb 2019; 26: 121–127. 10.5551/jat.RV17029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topilsky Y, Raichlin E, Hasin Tet al. Combined heart and liver transplant attenuates cardiac allograft vasculopathy compared with isolated heart transplantation. Transplantation 2013; 95: 859–865. 10.1097/TP.0b013e31827eef7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon RM, Hughes MG, Jones CMet al. A review of the United States experience with combined heart–liver transplantation. Transpl Int 2012; 25: 1223–1228. 10.1111/j.1432-2277.2012.01551.x [DOI] [PubMed] [Google Scholar]
- 12.Rizvi SS, Challapalli J, Maynes EJet al. Indications and outcomes of combined heart–liver transplant: a systematic review and met-analysis. Transplant Rev 2020; 34: 100517. 10.1016/j.trre.2019.100517 [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff Jet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea BJ, Reeves BC, Wells Get al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 16.Lebray P, Varnous S. Combined heart and liver transplantation: state of knowledge and outlooks. Clin Res Hepatol Gastroenterol 2019; 43: 123–130. 10.1016/j.clinre.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 17.Wallace BC, Dahabreh IJ, Trikalinos TAet al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49(5). 10.18637/jss.v049.i05 [DOI] [Google Scholar]
- 18.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–114. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 20.Afreixo V, Cruz S, Freitas A, Hernandez MA. Meta-analysis of a very low proportion through adjusted Wald confidence intervals. Open Access Biostat Bioinform 2019; 2(4). 10.31031/OABB.2019.02.000545 [DOI] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barshes NR, Udell IW, Joyce DLet al. A pooled analysis of posttransplant survival following combined heart–liver transplantation. Transplantation 2007; 83: 95–98. 10.1097/01.tp.0000243731.29657.87 [DOI] [PubMed] [Google Scholar]
- 23.Nelson LM, Penninga L, Sander Ket al. Long-term outcome in patients treated with combined heart and liver transplantation for familial amyloidotic cardiomyopathy. Clin Transplant 2013; 27: 203–209. 10.1111/ctr.12053 [DOI] [PubMed] [Google Scholar]
- 24.Atluri P, Gaffey A, Howard Jet al. Combined heart and liver transplantation can be safely performed with excellent short- and long-term results. Ann Thorac Surg 2014; 98: 858–862. 10.1016/j.athoracsur.2014.04.100 [DOI] [PubMed] [Google Scholar]
- 25.Schaffer JM, Chiu P, Singh SKet al. Combined heart–liver transplantation in the MELD era: do waitlisted patients require exception status? Am J Transplant 2014; 14: 647–659. 10.1111/ajt.12595 [DOI] [PubMed] [Google Scholar]
- 26.Barbara DW, Rehfeldt KH, Heimbach JKet al. The perioperative management of patients undergoing combined heart–liver transplantation. Transplantation 2015; 99: 139–144. 10.1097/TP.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 27.Careddu L, Zanfi C, Pantaleo Aet al. Combined heart–liver transplantation: a single-center experience. Transpl Int 2015; 28: 828–834. 10.1111/tri.12549 [DOI] [PubMed] [Google Scholar]
- 28.Reich HJ, Awad M, Ruzza Aet al. Combined heart and liver transplantation: the Cedars-Sinai experience. Transplant Proc 2015; 47: 2722–2726. 10.1016/j.transproceed.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 29.Wong TW, Gandhi MJ, Daly RCet al. Liver allograft provides immunoprotection for the cardiac allograft in combined heart–liver transplantation. Am J Transplant 2016; 16: 3522–3531. 10.1111/ajt.13870 [DOI] [PubMed] [Google Scholar]
- 30.Bradley EA, Pinyoluksana KO, Moore-Clingenpeel Met al. Isolated heart transplant and combined heart–liver transplant in adult congenital heart disease patients: insights from the United Network of Organ Sharing. Int J Cardiol 2017; 228: 790–795. 10.1016/j.ijcard.2016.11.121 [DOI] [PubMed] [Google Scholar]
- 31.D’Souza BA, Fuller S, Gleason LPet al. Single-center outcomes of combined heart and liver transplantation in the failing Fontan. Clin Transplant 2017; 31: e12892. 10.1111/ctr.12892 [DOI] [PubMed] [Google Scholar]
- 32.Bryant R, Rizwan R, Zafar Fet al. Contemporary outcomes of combined heart–liver transplant in patients with congenital heart disease. Transplantation 2018; 102: e67–e73. 10.1097/TP.0000000000001978 [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Matsuoka L, Cao Set al. Identifying predictors of outcomes in combined heart and liver transplantation. Transplant Proc 2019; 51: 2002–2008. 10.1016/j.transproceed.2019.04.038 [DOI] [PubMed] [Google Scholar]
- 34.Vaikunth SS, Concepcion W, Daugherty Tet al. Short-term outcomes of en bloc combined heart and liver transplantation in the failing Fontan. Clin Transplant 2019; 33: e13540. 10.1111/ctr.13540 [DOI] [PubMed] [Google Scholar]
- 35.Fynn-Thompson F. Heart transplantation in adults with congenital heart disease. Methodist DeBakey Cardiovasc J 2019; 15: 145–148. 10.1016/j.acvd.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baganate F, Beal EW, Tumin Det al. Early mortality after liver transplantation: defining the course and the cause. Surgery 2018; 164: 694–704. 10.1016/j.surg.2018.04.039 [DOI] [PubMed] [Google Scholar]
- 37.Bhagra SK, Pettit S, Parameshwar J. Cardiac transplantation: indications, eligibility and current outcomes. Heart 2019; 105: 252–260. 10.1136/heartjnl-2018-313103 [DOI] [PubMed] [Google Scholar]
- 38.Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med 2018; 16: 113. 10.1186/s12916-018-1110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asrani SK, Saracino G, O’Leary JGet al. Recipient characteristics and morbidity and mortality after liver transplantation. J Hepatol 2018; 69: 43–50. 10.1016/j.jhep.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Matsuoka L, Cao Set al. Identifying predictors of outcomes in combined heart and liver transplantation. Transplant Proc 2019; 51: 2002–2008. 10.1016/j.transproceed.2019.04.038 [DOI] [PubMed] [Google Scholar]
- 41.Idrees JJ, Pettersson GB. State of the art of combined heart–lung transplantation for advanced cardiac and pulmonary dysfunction. Curr Cardiol Rep 2016; 18: 36. 10.1007/s11886-016-0713-1 [DOI] [PubMed] [Google Scholar]
- 42.Grannas G, Neipp M, Hoeper MMet al. Indications for and outcomes after combined lung and liver transplantation: a single-center experience on 13 consecutive cases. Transplantation 2008; 85: 524–531. 10.1097/TP.0b013e3181636f3f [DOI] [PubMed] [Google Scholar]
- 43.Pinderski LJ, Kirklin JK, McGiffin Det al. Multi-organ transplantation: is there a protective effect against acute and chronic rejection? J Heart Lung Transplant 2005; 24: 1828–1833. 10.1016/j.healun.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 44.Roland CR, Mangino MJ, Duffy BF, Flye MW. Lymphocyte suppression by Kupffer cells prevents portal venous tolerance induction: a study of macrophage function after intravenous gadolinium. Transplantation 1993; 55: 1151–1158. 10.1097/00007890-199305000-00041 [DOI] [PubMed] [Google Scholar]
- 45.Rana A, Robles S, Russo MJet al. The combined organ effect: protection against rejection? Ann Surg 2008; 248: 871–879. 10.1097/SLA.0b013e31817fc2b8 [DOI] [PubMed] [Google Scholar]
- 46.Geissler EK, Korzun WJ, Graeb C. Secreted donor-MHC class I antigen prolongs liver allograft survival and inhibits recipient anti-donor cytotoxic T lymphocyte responses. Transplantation 1997; 64: 782–786. 10.1097/00007890-199709150-00024 [DOI] [PubMed] [Google Scholar]