Abstract
Introduction
The Future of Surgery report from the Royal College of Surgeons of England acknowledges the important role that three-dimensional imaging will play in support of personalised surgical interventions. One component of this is preoperative planning. We investigated surgeons’ and patients’ perceptions of this evolving technology.
Materials and methods
Ethical approval was obtained. From a normal computed tomography scan, three-dimensional models of the stomach, pancreas and rectum were rendered and printed on an Ultimaker™ three-dimensional printer. Semi-structured interviews were performed with surgeons and patients to explore perceived model effectiveness and utility. Likert scales were used to grade responses (1 = strongly disagree; 10 = strongly agree) and qualitative responses recorded.
Results
A total of 26 surgeons (9 rectal, 9 oesophagogastric, 8 pancreatic) and 30 patients (median age 62 years, interquartile range, IQR, 68–72 years; 57% male) were recruited. Median surgeon scores were effectiveness for preoperative planning, 6 (IQR 3–7), authenticity, 5 (IQR 3–6), likability, 6 (IQR 4–7), promoting learning, 7 (IQR 5–8), utility, 6 (IQR 5–7) and helping patients, 7 (IQR 5–8). Median patient scores were usefulness to the surgeon, 8 (IQR 7–9), authenticity, 8 (IQR 6–8), likability, 8 (IQR 7–8), helping understanding of condition, 8 (IQR 8–9), helping understanding of surgery, 8 (IQR 7–9) and feeling uncomfortable, 1 (IQR 1–4). Median overall decisional conflict score (0 = no; 100 = high) was 22 (IQR 19–28) and decision effectiveness was 25 (IQR 19–30).
Discussion
Overall, patients and surgeons considered that three-dimensional printed models were effective and had potential utility in education and, to a lesser extent, preoperative planning. Patient decisional conflict and effectiveness scores were weighted towards certainty in decision making but had room for improvement, which three-dimensional models may help to facilitate.
Keywords: Surgical oncology, Three-dimensional printing, Gastrointestinal cancer, Education of patients, Informed consent
Introduction
The Royal College of Surgeons of England report on the Future of Surgery (2018) recognises that technology will transform surgery over the next two decades.1 ‘Imaging, virtual reality and augmented reality’ comprise one of the four core areas that is expected to have the biggest impact, the others being ‘minimally invasive surgery’, ‘big data, genomics and artificial intelligence’ and ‘specialised interventions’. It is anticipated that advancements in three-dimensional (3D) planning and printing will allow regular use for teaching, training and for the surgical forethought needed to support personalised surgical interventions.
The report acknowledges that 3D models could be commonplace in surgical clinics and multidisciplinary teams in the near future and goes on to state that within this timeframe it is likely that patients will continue to experience an increasing burden of non-communicable chronic diseases. Decision making and the consent process is therefore likely to become more complex.2 Adjuncts such as 3D models could be of increasing value for patient consultations and to help patients to make fully informed decisions.
We aimed to investigate surgeon and patient perception of the utility of this evolving technology. Within this aim, we wished specifically to explore possible disadvantages of the use of this technology and to understand surgeons’ opinions regarding efficacy given the increasing financial constraints placed upon the NHS and other care organisations.
Methods
Ethical approval was obtained from Solihull Research Ethics Committee (West Midlands; REC reference 17/WM/0318). The study was conducted in a single NHS teaching hospital in the UK. Written consent was obtained from the patients.
Creating the 3D models
A normal computed tomography (CT) image of the abdomen and pelvis performed in the portal venous phase was used for model production. Digital Imaging and Communications in Medicine (DICOM) files were downloaded from the picture archiving and communication system radiology portal. Files were uploaded into Slic3r (developed by Alessandro Ranellucci), an open-source image rendering software programme. This software transforms images from two-dimensional DICOM format into a 3D file suitable for printing. Having established a 3D reconstruction of the whole scan, excess data were removed by cropping the views until only the component of interest remained. Subsequently, the specific parts to be printed were highlighted using the organ/tissue selector, and further refined with adjustment bars within the software. Once the outline of the model was complete, the file conversion was undertaken.
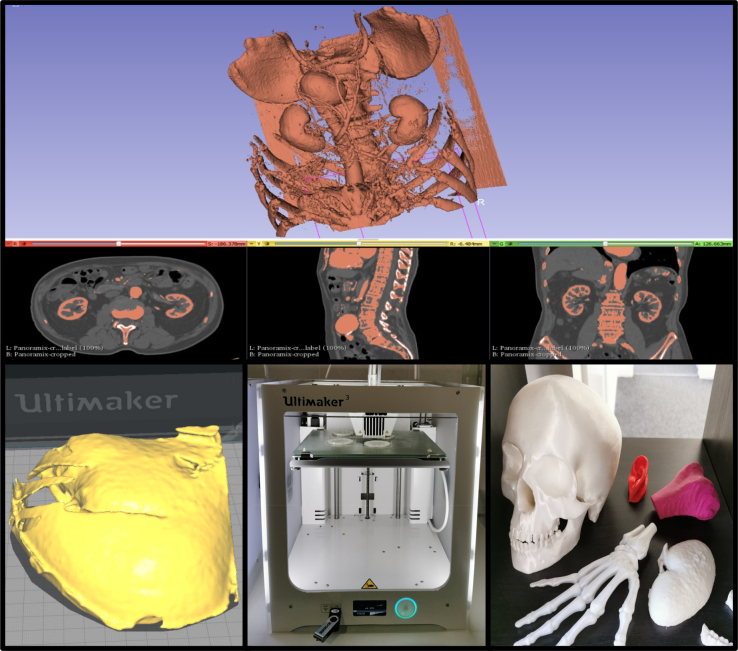
The exported file from Slic3r was imported into Meshmixer (Autodesk Inc, San Rafael, CA) to manipulate and make further refinements, including smoothing and infilling for missing data. The file was then exported in stereolithography (STL) format into Cura (Ultimaker BV, Utrecht, Netherlands), an open-source software programme designed to generate the G-code file required by the Ultimaker 3 (Ultimaker BV, Utrecht, Netherlands) 3D printer to print the model. The G-code file generates a series of coordinates in X, Y and Z directions, as well as specifying the amount of plastic to be extruded. The file is printed, with average print times of around one to three hours. After printing, any support material placed during the printing process is dissolved in water to generate the final product (Figure 1). The models were printed at scale to make them portable and usable with a size of around 20cm.
Figure 1 .
Model production. Images converted from DICOM files into three-dimensionall images (top) which are then cropped (bottom left) and rendered on a three-dimensional printer (bottom centre) to produce final models (bottom right).
Evaluating patient and surgeon opinion
A data capture tool was designed through consensus by the research team. Questions were determined to ensure coverage of the potential uses, including preoperative planning and patient/trainee education, as well as general assessment of the model including authenticity. Patients undergoing workup for oncological surgery were approached at clinics with a convenience sample taken based on the availability of the researcher. The researcher, who was independent of the clinical team, introduced themselves and the study to the participant, placed the models on a desk and allowed them to handle them, and after a few minutes of familiarisation proceeded to administer the questionnaire in person. First impression responses to these questions were recorded on a Likert scale (0 = strongly disagree; 10 = strongly agree) and supplemented by free text responses. Patients were also presented with a decisional conflict scale to evaluate their personal uncertainty about making a decision related to their surgery. A 16-question, 5-response category (strongly disagree to strongly agree) traditional decisional conflict scale was used. Subscale analysis of the decisional conflict scale was performed to assess decision effectiveness; a way of assessing whether the patient feels they are making an effective decision (ie that the decision is informed, values-based and likely to be implemented).3
Questionnaires were presented to general surgeons (consultants or senior trainees completing their subspecialty training) in different subspecialties, all of whom offer oncological resections. Data were recorded by a sole interviewer (SP) and collated and analysed by the wider research team. Descriptive statistical analyses are reported with supporting qualitative data. Decisional conflict and effectiveness scores were calculated in line with published methodology for the traditional decisional conflict scale.3 The questionnaire was reviewed after the initial five responses, following which no changes were made.
Results
A total of 26 surgeons (9 colorectal, 9 oesophagogastric, 8 pancreatic) and 30 patients (median age 62 years, interquartile range, IQR, 68–72 years; 57% male) were recruited to the study. Median (IQR) patient scores were usefulness to the surgeon 8 (7–9), authenticity 8 (6–8), likability 8 (7–8), helping understanding of the condition 8 (8–9), helping understanding of operation 8 (7–9) and feeling uncomfortable 1 (1–4). Median (IQR) overall decisional conflict score (0 = no decisional conflict; 100 = extremely high decisional conflict) was 22 (19–28) and decision effectiveness (0 = good decision; 100 = bad decision) was 25 (19–30). Median (IQR) surgeon scores (Likert scale from 1 = strongly disagree to 10 = strongly agree) were effectiveness for preoperative planning 6 (3–7), authenticity 5 (3–6), likability 6 (4–7), promoting learning 7 (5–8), utility 6 (5–7) and helping patients 7 (5–8). A summary of patient and surgeon scores can be seen in Tables 1 and 2, respectively.
Table 1 .
Patient questionnaire responses categorised into positive, negative and indifferent, and median (IQR) Likert scale responses (1 = strongly disagree to 10 = strongly agree).
| Question | Positive, n (%) | Negative, n (%) | Indifferent, n (%) | No answer, n (%) | Median scores, n (IQR) |
|---|---|---|---|---|---|
| This would be useful to my surgeon in planning my operation | 23 (77) | 4 (13) | 3 (10) | 0 (0) | 8 (7–9) |
| The models are authentic | 9 (30) | 7 (23) | 13 (43) | 1 (3) | 8 (6–8) |
| I like the models | 23 (77) | 4 (13) | 3 (10) | 0 (0) | 8 (7–8) |
| The models will help me understand my condition | 25 (83) | 2 (7) | 1 (3) | 2 (7) | 8 (8–9) |
| The models will help me understand my operation | 19 (63) | 3 (10) | 4 (13) | 4 (13) | 8 (7–9) |
| The models make me feel squeamish or uncomfortable | 22 (73) | 4 (13) | 4 (13) | 0 (0) | 1 (1–4) |
| Totals | 121 (67) | 24 (13) | 28 (16) | 7 (4) |
IQR, interquartile range
Table 2 .
Surgeon questionnaire responses categorised into positive, negative and indifferent, and median (IQR) Likert scale responses (1 = strongly disagree to 10 = strongly agree).
| Question | Positive | Negative | Indifferent | No answer | Median scores (IQR) |
|---|---|---|---|---|---|
| This could be useful in preoperative planning | 13 (50) | 11 (42) | 1 (4) | 1 (4) | 6 (3–7) |
| The models are authentic | 4 (15) | 14 (54) | 6 (23) | 2 (8) | 5 (3–6) |
| I like the models | 8 (31) | 5 (19) | 7 (27) | 6 (23) | 6 (4–7) |
| The models could promote learning | 14 (54) | 4 (15) | 7 (27) | 1 (4) | 7 (5–8) |
| The models have potential clinical utility in my specialty | 11 (42) | 9 (35) | 4 (15) | 2 (8) | 6 (5–7) |
| I would like to use these models in my practice | 12 (46) | 9 (35) | 3 (12) | 2 (8) | 6 (3–8) |
| The models will help patients understand their condition and surgery | 16 (62) | 4 (15) | 4 (15) | 2 (8) | 7 (5–8) |
| If made available (estimated cost £10) would you make use of them in your practice | 14 (54) | 10 (38) | 2 (8) | 0 (0) | N/A |
| Totals | 92 (44) | 66 (32) | 34 (16) | 16 (8) |
IQR, interquartile range; N/A, not available
Patient responses
Twenty-three (77%) patients considered that the models would be useful to surgeons in planning an operation. Free text responses indicated that the majority of patients thought that it would help the surgeon to visualise the disease/problem and decide on the surgical approach: ‘the surgeon will see where it has spread to so that he can plan the best operation’ [P26]; ‘it could help them work out if the cancer can be taken out keyhole or not’ [P5]. Four (13%) patients thought that the utility of the models was limited for this purpose: ‘I’m not sure what it will offer in addition to my scan results’ [P15].
Nine (30%) patients thought that the models were authentic. Thirteen (43%) patients were equivocal and seven (23%) felt that they were not authentic. Four of these patients’ comments were regarding the colour and surface texture: ‘I’m not so sure, why the yellow colour and rough edges?’ [P2]. Overall, 23 (77%) patients liked the models, with the most positive comments related to the use of models for patient explanations: ‘They are helpful to me for understanding what is going on’ [P8]; ‘they’re a clever way to get across information’ [P14]. Three (10%) patients reported equivocal answers and four (13%) did not like the models.
The vast majority of patients considered that the model would help them to understand their condition (25; 83%) and the operation (19; 63%): ‘It allows me to visualise the problem’ [P1]; ‘I can see what bits needs to be taken out’ [P11]. Negative responses regarding patient understanding of their condition and operation were offered by two (7%) and three (10%) patients respectively: ‘the models could cause more confusion’ [P2]; ‘It’s hard to understand when the model is just one part of the bowel. It’d be useful to see everything in my tummy’ [P3]. The models made four (13%) patients feel uncomfortable: ‘The model makes everything feel a bit too close to home’ [P6]. The remaining 22 (73%) didn’t experience this issue: ‘Not in the slightest’ [P10].
Surgeon responses
A similar number of surgeons thought that the models would be beneficial for preoperative planning (13; 50%) as those who thought it would be of no benefit (11; 42%). Positive comments were mainly related to the possible use of models for complex cases to determine resectability and to assess relationships to other anatomical structures: ‘for extensive invasive tumour or aberrant anatomy’ [S5], and ‘it has the potential for demonstrating tumour invasion into surrounding structures’ [S12]. Criticism of the accuracy and quality of the models were a trend in the negative comments with nine surgeons either mentioning the accuracy or lack of benefit over cross-sectional imaging: ‘these models are not of a high enough render in order to plan an operation’ [S17]; ‘it’s unlikely to offer any additional benefit over a high quality CT scan’ [S8]. Only four (15%) surgeons thought that the models were sufficiently authentic, with 14 (54%) respondents expressing concerns about authenticity: ‘This is the biggest weakness of the models’ [S16]; ‘there’s a lot of artefact on the models’ [S23].
Overall, eight (31%) surgeons liked the models: ‘This represents the future of high-fidelity surgical models’ [S9]. Five (19%) surgeons had the opposing opinion: ‘I would rather not show this to the patient as it would mislead them’ [S2]. Eight (27%) provided equivocal answers and six (23%) declined to answer. Fourteen (54%) surgeon respondents offered positive comments regarding the use of the models to promote learning especially for ‘visual and tactile learners’ [S18]. Four (15%) surgeons did not perceive this benefit. Positive comments were predominantly related to teaching of healthcare professionals: ‘they could be of use for teaching of physician associates, nurses and medical students’ [S19].
Eleven (42%) surgeons considered that the models would have potential clinical utility in their specialty. Nine (35%) surgeons could not see any benefits for clinical use, with four referencing the quality of the model as the main barrier: ‘Not at present as the quality and accuracy is too poor’ [S8]. If made available, 12 (46%) surgeons would use the models in their own practice. Eight of these responses specifically recognised the potential for use in patient explanations: ‘I would like to use the models for educating patients’ [S18] and the models ‘would be useful pre-op for patient discussions’ [S1]. Nine (35%) surgeons reported that they would not use the models in their present form: ‘due to inaccuracies I would not use the model in my practice’ [S130], and ‘not with this model’ [S20].
The majority of surgeons (16; 62%) could see the models having a positive impact on their patients’ understanding of their condition and surgery: ‘perhaps the most useful aspect’ [S5]. Four (15%) surgeons thought that the models could actually cause patient confusion and worsen understanding: ‘if I don’t understand the models how on earth could a patient?’ [S21]. Other potential utilities suggested by surgeons included radiation planning and facilitating multidisciplinary team discussions: ‘Could be used as part of the multidisciplinary team to improve communication between different specialties’ [S9].
Discussion
With the evolution of 3D imaging and modelling techniques comes the opportunity to incorporate these technological advances into important aspects of surgical practice. The median (IQR) decisional conflict score for patient decisions suggested a low level of conflict. The decisional conflict effectiveness subscale suggested a ‘good’ decision. The IQR for both these decision scores, however, cross the cut-off of 25, suggesting scope for improvement. Decisional conflict and effectiveness can be lowered by fully informing patients about the benefits and risks of any intended procedure, as well as presenting alternative options. Our data suggest that the majority of patients thought that the use of a 3D model would improve their understanding of their condition and operation, and therefore harnessing the technology in this manner may improve decisional conflict and effectiveness.
Operative planning including determination of resectability is a critical task, and it is not anticipated that this could be completely determined in the preoperative setting with a high-fidelity model. However, as highlighted in surgeon responses, a 3D model could be used as an adjunct in cases of complex surgery with respect to difficult margins and aberrant anatomy. Patient responses to this question should not be overlooked, as their confidence in a detailed preoperative planning process is likely to have an impact on their preoperative psychological status.4 Increasing technology brings greater access to information, and this may influence the relationship between the patient and the surgical team. How we present this information is important, as there is a risk of causing unnecessary stress and anxiety. We consider that this risk could be largely mitigated by asking the patient whether they would like to see a model of their organ(s).
The question we posed regarding model authenticity universally produced the most negative responses, with both patients and surgeons expressing concerns. As cross-sectional imaging continues to improve, so too will their 3D derivatives. Combined with improvements in materials and the manufacturing process, we are likely to see an increase in accuracy of 3D models over coming years. The challenge may be how best to embrace these developments while remaining cost efficient. The Future of Surgery report highlights how surgeons must be careful not to introduce potential inequalities through development of new technologies.1 In the case of 3D printing, slow adoption may be related to financial constraints and limited production availability. It would be beneficial to investigate further by evaluating how a centralised manufacturing process could keep cost reasonable and provide consistent, timely and high-quality production. Currently, each model takes approximately one to three hours to prepare and one to three hours to print, depending on complexity. The main financial pressure is the initial outlay for the 3D printer (information available commercially), as the cost of producing each model is limited to the cost of running the machine and the plastic, estimated at approximately £5 (€5.5, US$ 6.5) per model.
Education is clearly an area where 3D models could make a positive impact. Comments related to education were mainly aimed at teaching medical students and physician associates, but it is not clear how using case-specific models would be of significant benefit at this level. We consider that education would be most beneficial when delivered at specialty training level, with the main benefit arising from discussion of preoperative planning and strategy in cases being undertaken by the trainee and as high-quality models for trainees to practice procedures.
The use of 3D printing is becoming more commonplace, and so too are reports of its use within the literature.5 A number of small studies have attempted to evaluate the advantages and disadvantages of 3D models in surgery, although at the present time these are predominantly limited to maxillofacial and orthopaedic surgery.6 Tissue classification for rendering is often based on Hounsfield units, and bone is at the extreme end of the scale, making it easier to discern radiologically and therefore clearer in the derived 3D models. As adjacent abdominal viscera often have comparable Hounsfield units it is understandable that tissue interfaces may not be cleanly resolved, resulting in a reduction in the fidelity of the model. Furthermore, variation in the tissue density of tumours and local tissue reaction may again cause difficulty at tissue interfaces when generating 3D models for use within general surgical specialties.
The use of 3D printing for preoperative planning has already been reported. Maddox et al produced seven patient-specific 3D models of kidneys with suspected malignancies.7 Partial nephrectomies were undertaken on models prior to the live procedure and cases were compared with the institution’s prospectively maintained robotic partial nephrectomy database. Although the patients who had been ‘pre-operated’ with 3D models had fewer positive margins, shorter hospital stay and fewer postoperative complications, none of these findings was statistically significant.
A number of studies have shown a benefit in the use of 3D models for patient and family education.4,8,9 Bernhard et al conducted a prospective pilot study of seven patients who were due to undergo partial nephrectomy.9 Results showed an improvement in understanding of physiology (16.7%, p = 0.018), anatomy (50%, p = 0.026), tumour characteristics (39.3%, p = 0.068) and the planned surgical procedure (44.6%, p = 0.026) when 3D models were used as an adjunct to patient education. A similar study was conducted where generic Mohs micrographic surgery models were used with similar results.4 In comparing these studies to our own, we acknowledge the limitation that we have no control group for decisional conflict score comparison. Common patient perceived advantages mentioned in other studies include improved understanding of their condition and surgery, with very few patients describing negative psychological impact;10 our study is reflective of these findings.
Positive attitudes towards the use of 3D models for education and training have been expressed in several studies, mostly using Likert scales for evaluation. Two separate small studies looked at the use of 3D models for training of otolaryngologists and rhinologists with strong agreement that 3D models are beneficial to training/education.11,12 In contrast to our results, both these studies report positive responses regarding the fidelity of the model. Similarly positive results have also been reported in a small studies.13–15 In view of these previous studies, and indeed the data from our own, it is clear that education and training is an area of 3D model use which should be targeted for implementation.
If 3D models are to become commonplace in surgical planning, training and education, there are limitations that will need to be addressed. Manufacturing capacity would need to be evaluated and addressed prior to producing models for patient cases. Capacity is intricately related to manufacturing timeframe and we must be mindful not to introduce unnecessary delay in already tight treatment pathways in surgical oncology. With concerns regarding financial implications of future developments described in the Future of Surgery report,1 it is imperative that any development is cost effective. This will need to be balanced with ensuring models are accurate and fit for purpose, which, as our study has shown, is an area that currently requires further refinement prior to widespread adoption in abdominopelvic cancer surgery.
Conclusion
Both patients and surgeons anticipate benefits with the use of 3D printed models for education and, to a lesser extent, preoperative planning. Patient decisional conflict scores suggest room for improvement in the decision making and consent process. Positive responses from surgeons and patients regarding the use of 3D models to promote patient understanding suggest they have potential to improve the consent process. The majority of concerns raised in our study relate to the authenticity of 3D models of abdominal viscera, although this is something which is expected to improve with technological advancements. To truly assess the impact of this technology, future studies should ideally compare the outcomes of 3D models compared with patients and surgeons undergoing standard verbal and written consent.
Acknowledgements
The authors wish to acknowledge the assistance provided by Jack Broughton and 3DGBIRE Ltd with the production of the 3D models and images used for this study.
References
- 1.Royal College of Surgeons of England. Future of Surgery. London: RCS; 2018. https://futureofsurgery.rcseng.ac.uk (cited December 2020). [Google Scholar]
- 2.Campbell M. Montgomery v Lanarkshire health board. Common Law World Rev 2015; 44: 222–228. 10.1177/1473779515592118 [DOI] [Google Scholar]
- 3.O’Connor AM. User manual: decisional conflict scale. Ottawa: Ottawa Hospital Research Institute; 2010. www.ohri.ca/decisionaid (cited December 2020). [Google Scholar]
- 4.Biro M, Kim I, Huynh Aet al. The use of 3D-printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol 2019; 81: 1339–1345. 10.1016/j.jaad.2019.05.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers MK, Lee BR, Silberstein J. Three-dimensional printing of surgical anatomy. Curr Opin Urol 2016; 26: 283–288. 10.1097/MOU.0000000000000274 [DOI] [PubMed] [Google Scholar]
- 6.Martelli N, Serrano C, van den Brink Het al. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery 2016; 159: 1485–1500. 10.1016/j.surg.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 7.Maddox MM, Feibus A, Liu Jet al. 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study. J Robot Surg 2018; 12: 27–33. 10.1007/s11701-017-0680-6 [DOI] [PubMed] [Google Scholar]
- 8.Ozturk AM, Sirinturk S, Kucuk Let al. Multidisciplinary assessment of planning and resection of complex bone tumor using patient-specific 3D model. Indian J Surg Oncol 2019; 10: 115–124. 10.1007/s13193-018-0852-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhard JC, Isotani S, Matsugasumi Tet al. Personalized 3D printed model of kidney and tumor anatomy: a useful tool for patient education. World J Urol 2016; 34: 337–345. 10.1007/s00345-015-1632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou PY, Hallac RR, Shih Eet al. 3D-printed models of cleft lip and palate for surgical training and patient education. Cleft Palate Craniofac J 2018; 55: 323–327. 10.1177/1055665617738998 [DOI] [PubMed] [Google Scholar]
- 11.Belt TH, Nijmeijer H, Grim Det al. Patient specific actual size 3D printed models for patient education in glioma treatment: first experiences. World Neurosurg 2018; 117: e99–e105. 10.1016/j.wneu.2018.05.190 [DOI] [PubMed] [Google Scholar]
- 12.Alrasheed AS, Nguyen LH, Mongeau Let al. Development and validation of a 3D-printed model of the ostiomeatal complex and frontal sinus for endoscopic sinus surgery training. Int Forum Allergy Rhinol 2017; 7: 837–841. 10.1002/alr.21960 [DOI] [PubMed] [Google Scholar]
- 13.Chang DR, Lin RP, Bowe Set al. Fabrication and validation of a low-cost, medium-fidelity silicone injection molded endoscopic sinus surgery simulation model. Laryngoscope 2017; 127: 781–786. 10.1002/lary.26370 [DOI] [PubMed] [Google Scholar]
- 14.Ryan JR, Almefty KK, Nakaji P, Frakes DH. Cerebral aneurysm clipping surgery simulation using patient-specific 3D printing and silicone casting. World Neurosurg 2016; 88: 175–181. 10.1016/j.wneu.2015.12.102 [DOI] [PubMed] [Google Scholar]
- 15.Hsieh TY, Cervenka B, Dedhia Ret al. Assessment of a patient-specific, 3-dimensionally printed endoscopic sinus and skull base surgical model. JAMA Otolaryngol Head Neck Surg 2018; 144: 574–579. 10.1001/jamaoto.2018.0473 [DOI] [PMC free article] [PubMed] [Google Scholar]