Abstract
Introduction
The debate on the best surgical management strategy for acute malignant left-sided colonic obstruction is ongoing. Decompressing colostomy (DC) and stenting as a bridge to surgery (SBTS) are the currently proposed alternative approaches to emergency colectomy (EC). However, the results of a traditional meta-analysis were inconclusive. Therefore, a network meta-analysis (NMA) was conducted to compare the three approaches for acute left-sided colonic obstruction.
Methods
A systematic literature search of Embase, PubMed, Google Scholar and the Cochrane library was performed. A traditional meta-analysis and subsequent NMA were conducted.
Findings
A significantly greater number of primary anastomoses were performed in the DC cohort than in the EC and SBTS cohorts. The 90-day mortality rate was significantly lower in the DC cohort than in the EC and SBTS cohorts. Higher costs were associated with the SBTS cohort (by US$2,000) than with the EC cohort. The locoregional recurrence rate was higher for the SBTS cohort than for the EC cohort.
Conclusions
Evidence from the first NMA suggests there may be some clinical advantages associated with DC as an alternative approach to the EC and SBTS approaches for adequately selected patients with malignant large bowel obstruction.
Keywords: Diversion, Endoluminal stenting, Acute colectomy, Colonic obstruction, Acute resection
Introduction
Approximately 7–30% of colorectal cancers present with acute obstruction, with approximately 30% in the proximal colon and 70% the distal colon.1 The reported postoperative complications and mortality rates of emergency colonic resections are 45–50% and 15–20%, respectively.1,2 The common surgical procedure for acute left-sided colonic obstruction is Hartmann’s procedure, with a reported overall morbidity ranging from 10% to 50% and mortality from 4% to 30%. Another major drawback of the Hartmann procedure is the high permanent stoma rate. Evidence from the published literature suggests that as many as 40% of patients never have their stoma reversed and metastatic disease, anaemia and impaired renal function are independent predictors for nonreversal.3,4
Two bridge-to-surgery approaches have been proposed as alternatives to resection, initially the decompressing colostomy (DC) and subsequently endoluminal stenting. However, reports on the efficacy, efficiency and safety of stenting as a bridge to surgery (SBTS) are contradictory. A Dutch multicentre randomised trial was terminated early due to a high morbidity rate in the stent cohort (49%) compared with the emergency colectomy (EC) cohort (30%).5 A recent meta-analysis reported nonsignificant survival benefits and lower overall complication and 30-day mortality rates in patients having SBTS compared with the EC cohort. However, a major caveat of the study was the simultaneous inclusion of studies with curative and palliative intent.6
A French consensus conference recommends DC as the primary approach, followed by elective colectomy within 10 days.7 Consequently, a French retrospective study comparing initial colostomy, Hartmann’s procedure, and subtotal colectomy concluded that initial colostomy is a simple procedure and permits a planned oncological resection with decreased morbidity and mortality rates.7 Based on the existing evidence, there is an ongoing debate whether initial colostomy, emergency colectomy or stent placement for bridge to surgery is the optimal procedure for management of acute malignant left-sided colonic obstruction. Network meta-analysis (NMA) is an appropriate method to compare more than two interventions and to estimate direct and indirect evidence. Therefore, a NMA was conducted to detect the evolution of evidence over time and to detect any positive or negative impacts and turning points. The present study included only studies of treatments with potentially curative intent.
Methods
This NMA was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)8 checklist and the Cochrane Handbook for Systematic Reviews of Intervention.9
Literature search
With the use of search terms in the free text and Medical Subject Headings (‘malignant; colonic obstruction; diversion; loop colostomy; acute resection; Hartmann’s procedure; endoluminal; stent; colectomy; large bowel resection’), a systematic search of the literature published from inception until April 2020 was performed using the EMBASE, Medline (PubMed), Cochrane Library, and Google Scholar databases. A grey literature search was also performed on the ‘clinicaltrials.gov’ website. References of the retrieved articles were investigated manually for additional studies. Disagreements between authors were resolved by consensus-based discussions.
Study selection and inclusion and exclusion criteria
Studies that compared diversion, EC or endoluminal stenting for malignant left-sided colonic obstruction with potential curative intent were included in the study. Studies that included interventions with palliative intent were excluded. In cases of multiple publications by the same institution, only the most recent publication was included. Abstracts, case series, and non-English publications were excluded.
Data extraction and outcomes
Two reviewers (PG and AA) independently extracted the following summary data from the included studies: name of authors; year of publication; number of patients included in the DC, EC and SBTS cohorts; age; body mass index (BMI), American Society of Anaesthesiology score (ASA), tumour site; type of stent, primary anastomosis, permanent stoma, anastomotic leak, lymph nodes retrieved, overall complication rate, recurrence rate, length of stay, cost and 90-day mortality.
Statistical analysis
Cochrane’s criteria were used to assess the methodological quality of the included Randomised Control Trials (RCTs). Two authors (PG and AA) independently assessed the risk of selection bias, attrition bias, detection bias, performance bias and reporting bias. Consequently, they are categorised as unclear, high or low. The included nonrandomised studies were evaluated for risk of bias using the newly developed tool, Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I).10 Bias was assessed by seven domains: confounding, selection of participants into the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of reported results. An important feature of ROBINS-I is the use of signalling questions to detect the risk of bias and facilitate assessment in seven bias domains.
First, an updated meta-analysis was conducted to compare two of the approaches to left-sided colonic malignant obstruction. A NMA was conducted to compare DC versus EC versus SBTS. Statistical analysis was performed using Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK) and General Mixed Treatments Comparisons (GeMTC) software. Heterogeneity was assessed using the I2 test, and cut off values of 25%, 50% and 75% were considered low, moderate and high heterogeneity, respectively.11 For studies that did not report the means and variances of the two groups, these values were estimated from the median, range and sample size using the technique described by Hozo et al where possible.12
A NMA was conducted using hierarchical random-effects models.13 A fixed-effects model was also used to estimate whether any discrepancy could be detected between the results of the two models. Quantitative data synthesis of the connected network of the studies was performed using the software package WinBUGS (version 1.4.3, MRC Biostatistics Unit, Cambridge, UK).14 For each model, 200,000 simulations were generated for the two sets of initial values, and the first 5,000 were discarded as the burn-in period. The point-estimate was defined as the median of the posterior distribution based on 200,000 simulations; the corresponding 95% credible intervals (CrIs) were obtained using the 2.5th and 97.5th percentiles of the posterior distribution, which can be interpreted in a similar way as 95% confidence intervals (CI).14 Inconsistency and heterogeneity of the direct and indirect evidence for the three approaches for management of acute left-sided malignant colonic obstruction were estimated. In all analyses, the point estimate was considered significant if p<0.05.
Sensitivity analysis
Analyses of both primary and secondary outcomes were calculated using the random- and fixed-effects models to assess the impact of heterogeneity on the robustness of the conclusions. After the traditional meta-analysis, a NMA was conducted and the results were compared to detect any discrepancies between them.
Results
Search strategy and study characteristics
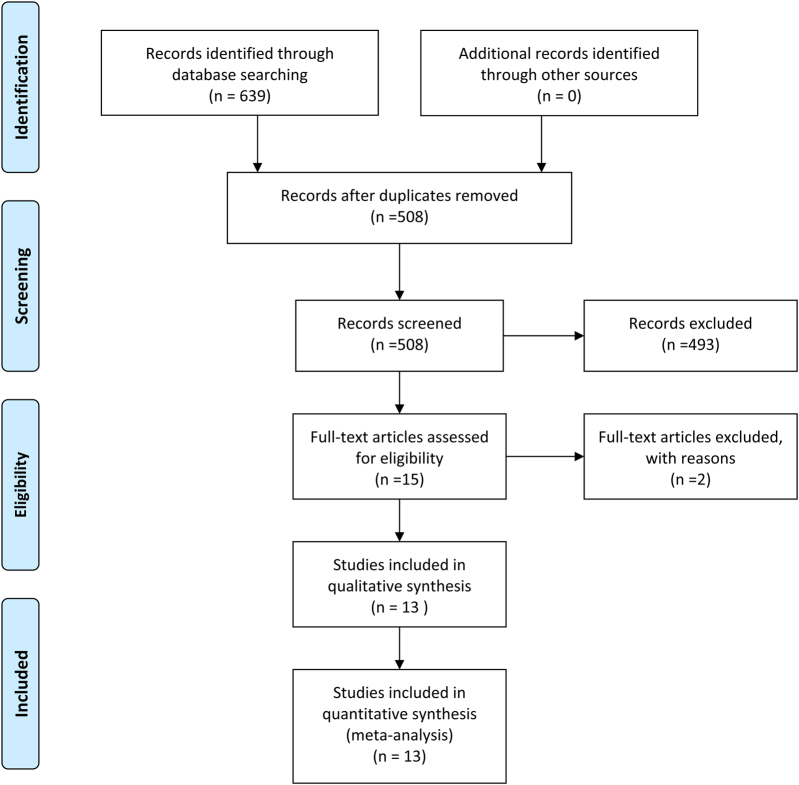
Search results returned a total of 639 manuscripts and, after exclusion of 131 duplicates (Figure 1), 508 abstracts were screened. Of these, 493 were excluded as they were not relevant to the aims of the study, leaving 15 full text articles for assessment. Of these, two were excluded due to comparing nonmalignant obstruction, resulting in a total of 13 studies included for NMA. These 13 studies5,15–26 included a total of 3,739 patients, of whom 16.3% (n=608/3,739), 67.2% (n=2,515/3,739) and 16.5% (n=617/3,739) underwent DC, EC and SBTS for acute malignant left-sided colonic obstruction (Tables 1 and 2). Eight studies5,19–25 including 246 (49.60%) and 250 (51.40%) patients compared SBTS and EC, respectively. Two studies compared DC versus EC,15,17 two studies compared DC versus EC with SBTS and one study compared DC16,26 with SBTS.18
Figure 1 .
Diagram of search strategy
Table 1 .
Study characteristics and outcomes in RCTs
| Author | Year | Country | Number of patients n | Mean Age | Mean BMI | ASA>III | Tumour site SBTS n (%) | Tumour site EC n (%) | Type of stent |
|---|---|---|---|---|---|---|---|---|---|
| Arezzo | 2016 | Italy | SBTS: 56 | SBTS: 72±11.75 | SBTS: 24.8±5.17 | SBTS:17 | Sigmoid: 8 | Sigmoid: 12 | NR |
| EC: 59 | EC: 71±12.5 | EC: 24.5±4.25 | EC: 20 | Descending: 43 | Descending: 34 | ||||
| Splenic flexure: 5 | Splenic flexure: 13 | ||||||||
| Tung | 2012 | China (Hong Kong) | SBTS: 24 | SBTS: 64.5±7.25 | SBTS: 23.8±2.42 | NR | NR | NR | Wallstent |
| EC: 24 | EC: 68.5±14.8 | EC: 24±3.22 | |||||||
| Ghazal | 2012 | Egypt | SBTS: 30 | SBTS: 52±7.75 | NR | NR | Rectosigmoid: 12 | Rectosigmoid: 10 | NR |
| EC: 30 | EC: 51±7.75 | Sigmoid: 14 | Sigmoid: 17 | ||||||
| Descending: 4 | Descending: 3 | ||||||||
| Alcántara | 2011 | Spain | SBTS: 15 | SBTS: 71.9±8.96 | NR | SBTS: 10 | Rectosigmoid: 0 | Rectosigmoid: 3 | Wallstent |
| EC: 13 | EC: 71.15±9 | EC: 12 | Sigmoid: 11 | Sigmoid: 4 | |||||
| Descending: 1 | Descending: 2 | ||||||||
| Splenic flexure: 2 | Splenic flexure: 4 | ||||||||
| Ho | 2011 | Singapore | SBTS: 20 | SBTS: 68±8.5 | NR | NR | Rectosigmoid: 5 | Rectosigmoid: 3 | Wallflex |
| EC: 19 | EC: 65±8.75 | Sigmoid: 10 | Sigmoid: 8 | ||||||
| Descending: 3 | Descending: 6 | ||||||||
| Splenic flexure: 2 | Splenic flexure: 2 | ||||||||
| van Hooft | 2011 | Netherlands | SBTS: 47 | SBTS: 70.4±11.9 | NR | SBTS: 6 | NR | NR | Wallstent, Wallflex |
| EC: 51 | EC: 71.4±9.7 | EC: 6 | |||||||
| Pirlet | 2010 | France | SBTS: 30 | SBTS: 70.4±10.3 | SBTS: 24.2±7.6 | NR | Rectosigmoid: 8 | Rectosigmoid: 7 | Nitimol |
| EC: 30 | EC: 74.7±11.3 | EC: 23.3±4.2 | Sigmoid: 15 | Sigmoid: 18 | |||||
| Descending: 6 | Descending: 2 | ||||||||
| Splenic flexure: 0 | Splenic flexure: 3 | ||||||||
| Cheung | 2009 | China (Hong Kong) | SBTS: 24 | SBTS: 64.5±7.25 | SBTS: 23.8±2.42 | NR | NR | NR | Wallstent |
| EC: 24 | EC: 68.5±14.75 | EC: 24±3.22 | |||||||
| Pooled estimates | SBTS: 246 | MD=−0.48 | MD=−0.04 | OR=0.78 | Rectosigmoid: 25 (17%) | Rectosigmoid: 23 (15%) | |||
| EC: 250 | (−2.3, 1.3) | (−1.06, 0.99) | (0.42, 1.4) | Sigmoid: 58 (39%) | Sigmoid: 59 (39%) | ||||
| Total: 496 | p=0.60 | p=0.94 | p=0.42 | Descending: 57 (38%) | Descending: 47 (31%) | ||||
| Splenic flexure: 9 (6%) | Splenic flexure: 22 (15%) | ||||||||
| Total: n=149 | Total: n=151 |
OR = odds ratio; MD = mean difference; CI = confidence intervals; SBTS = stenting bridge to surgery; EC = emergency colectomy; NR = not reported; ASA = American society of Anesthesiologists; RCT randomised controlled trial; BMI = body mass index
Table 2 .
Study characteristics and outcome in the studies comparing DC, EC and SBTS
| Author | Year | Country | Number of patients n | Mean Age | Mean BMI | ASA>III | Tumour site DC n (%) | Tumour site EC n (%) | Tumour site SBTS n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chéreau | 2013 | France | DC: 61 | 70.3±15.1 | NR | DC: 39 | Rectosigmoid: 22 | Rectosigmoid: 4 | NA | ||
| EC: 11 | 69.3±15 | EC: 6 | Sigmoid: 21 | Sigmoid: 5 | |||||||
| Descending: 9 | Descending: 2 | ||||||||||
| Splenic flexure: 9 | Splenic flexure: 0 | ||||||||||
| Krstic | 2014 | Serbia | DC: 58 | 65.7±15 | NR | DC: 3 | All rectosigmoid | All rectosigmoid | NA | ||
| EC: 63 | 67±11 | EC: 3 | |||||||||
| Östämö | 2016 | Sweden | DC: 23 | 67±12 | NR | DC: 7 | Rectosigmoid: 1 | Rectosigmoid: 0 | Rectosigmoid: 1 | ||
| EC: 57 | 74±12 | EC: 24 | Sigmoid: 10 | Sigmoid: 39 | Sigmoid: 15 | ||||||
| SBTS: 20 | 71±10 | Stent: 7 | Descending: 7 | Descending: 7 | Descending: 2 | ||||||
| Splenic flexure: 5 | Splenic flexure: 11 | Splenic flexure: 2 | |||||||||
| Veld | 2019 | Netherlands | DC: 345 | 68±4.3 | 25.2±4 | DC: 6 | Sigmoid: 233 | Sigmoid: 1,364 | Sigmoid: 169 | ||
| EC: 2,013 | 71±4.3 | 25.6±7 | EC: 109 | Descending: 58 | Descending: 375 | Descending: 45 | |||||
| SBTS: 229 | 72±4 | 26±5 | Stent: 57 | Splenic flexure: 54 | Splenic flexure: 274 | Splenic flexure: 15 | |||||
| Veld | 2020 | Netherlands | DC: 121 | 67±12 | 24.6±3.6 | DC: 27 | Sigmoid: 62 | NA | Sigmoid: 60 | ||
| SBTS: 121 | 71±11 | 26.3±6.5 | SBTS: 22 | Descending: 21 | Descending: 20 | ||||||
| Splenic flexure: 36 | Splenic flexure: 2 | ||||||||||
| Pooled estimates | DC: 608 | Rectosigmoid: 81 (13) | Rectosigmoid: 67 (3) | Rectosigmoid: 1 | |||||||
| EC: 2,265 | Sigmoid: 326 (54) | Sigmoid: 1,408 (66) | Sigmoid: 245 (74) | ||||||||
| SBTS: 370 | Descending: 95 (16) | Descending: 384 (18) | Descending: 67( 20) | ||||||||
| Total n: 3,243 | Splenic flexure: 104 (17) | Splenic flexure: 285 (13) | Splenic flexure: 19 (6) | ||||||||
| Total: 606 | Total: 2,144 | Total: 332 | |||||||||
NA = nonapplicable; DC = decompressing colostomy; EC = emergency colectomy; NR = nonreported; Fl = flexure; SBTS = stenting bridge to surgery; ASA = American Society of Anesthesiologists
Pairwise meta-analysis
The results demonstrated that a significantly older cohort of patients (by at least 3 years) were included in the EC (71.3 years, 95% CI 69.5–73.0) and SBTS (71.6 years, 95% CI 69.8–73.4) groups compared with the DC group (68.0 years, 95% CI 67.5–68.5). A significantly larger proportion of patients underwent primary anastomosis in the SBTS cohort (77%, n=170/222, Table 3) compared with the EC cohort (61%, n=137/226). The rate of permanent stoma was lower in the SBTS cohort (24%, n=47/192) compared with the EC group (35%, n=69/196) as was the rate of overall complications (SBTS: 38% versus 55% in the EC group. However, there was a significantly higher locoregional recurrence rate in the SBTS cohort (26%, n=35/135) compared with the EC cohort (16%, n=23/145), (Table 3).
Table 3 .
Comparison between SBTS and EC in terms of patient demography and outcomes
| Outcome of interest | Number of studies reported in | Total patients (n) | % (n) | Measure of effect size | Estimated effect (95% CI) | p | I2 (%) |
|---|---|---|---|---|---|---|---|
| Age | 8 | 496 | – | MD | −0.48 (−2.29–1.33) | 0.600 | 0 |
| BMI | 4 | 184 | – | MD | −0.04 (−1.06–0.99) | 0.940 | 0 |
| ASA | 3 | 241 | SBTS: 28% (33/118) | OR | 0.78 (0.42–1.44) | 0.420 | 0 |
| EC: 31% (38/123) | |||||||
| Primary anastomosis | 7 | 448 | SBTS: 77% (170/222) | OR | 2.54 (1.58–4.08) | 0.001 | 0 |
| EC: 61% (137/226) | |||||||
| Permanent stoma | 6 | 388 | SBTS: 24% (47/192) | OR | 0.56 (0.35–0.91) | 0.020 | 5 |
| EC: 35% (69/196) | |||||||
| Overall complications rate | 7 | 448 | SBTS: 38% (84/222) | OR | 0.51 (0.35–0.75) | 0.005 | 74 |
| EC: 55% (124/226) | |||||||
| Anastomotic leak | 7 | 448 | SBTS: 5% (11/222) | OR | 0.93 (0.43–1.99) | 0.840 | 25 |
| EC: 5% (12/226) | |||||||
| Lymph Nodes Retrieval | 3 | 124 | 124 | MD | −6.48 (−13.69–0.72) | 0.080 | 0 |
| Length of stay | 3 | 115 | 115 | MD | 1.09 (−2.04–4.21) | 0.500 | 0 |
| Locoregional Recurrences | 4 | 280 | SBTS: 26% (35/135) | OR | 1.98 (1.08–3.63) | 0.030 | 33 |
| EC: 16% (23/145) | |||||||
| Mortality | 5 | 340 | SBTS: 10% (16/168) | OR | 0.98 (0.48–1.98) | 0.960 | 0 |
| EC: 10% (17/172) | |||||||
| Cost | 2 | 67 | – | MD | $2,002 (587–3417) | 0.006 | 5 |
BMI = body mass index, ASA = American Society of Anesthesiology, SBTS = stenting bridge to surgery; EC = emergency colectomy; OR = odds ratio; MD = mean difference
To analyse the financial cost involved in each procedure, the Euro currency was converted to the US dollar on 23 April 2020, with an exchange rate of 1€ equal to $1.08. Analysis demonstrated that SBTS was over $2,000 more expensive per patient compared with EC. There were no differences in age, BMI, ASA, rate of anastomotic leak, lymph node retrieval, length of hospital stay and mortality rates between the SBTS and EC cohorts (Table 3).
Network meta-analysis
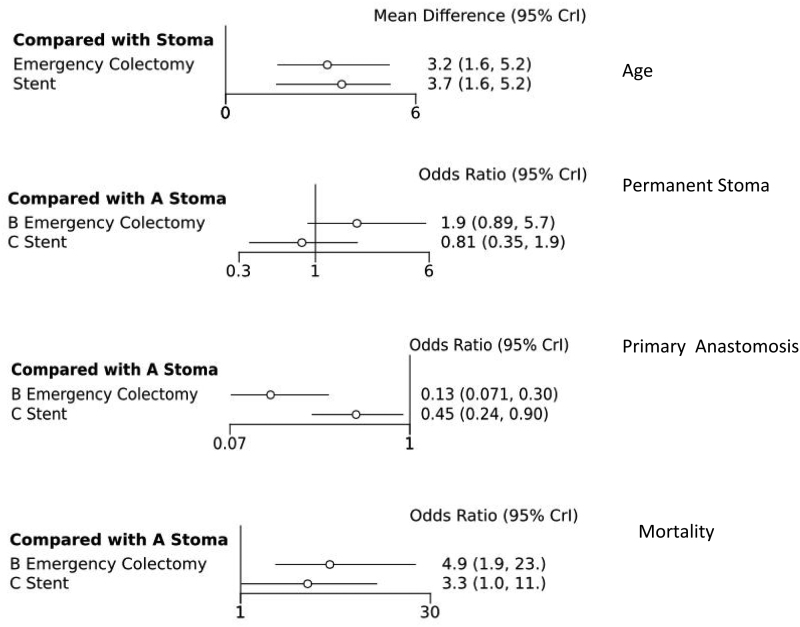
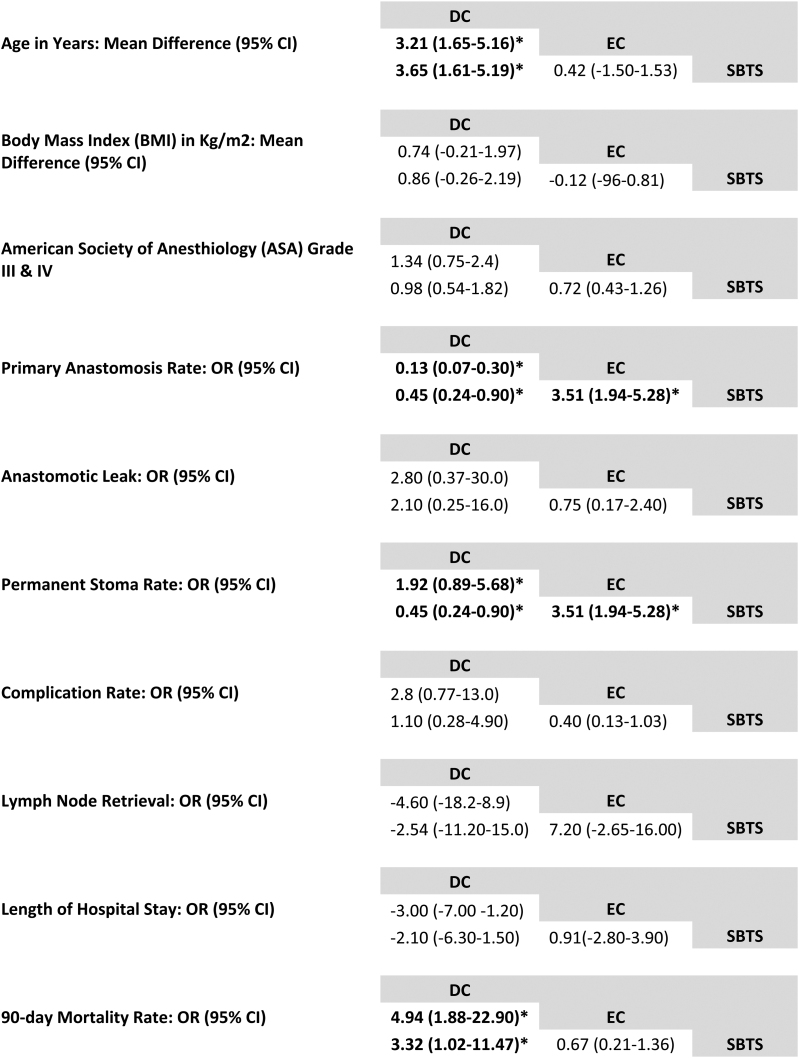
In the analyses, DC was associated with a higher rate of subsequent primary anastomosis compared with EC (odds ratio (OR) 0.13, 95% 0.07–0.030) and compared with SBTS (OR 0.45, 95% CI 0.24–0.90). SBTS patients were more likely to have an anastomosis (OR 3.51, 95% CI 1.94–5.28) compared with the EC group.
Permanent stoma rates were 55% lower in the SBTS group when compared with DC (OR 0.45, 95% CI 0.24–0.90) but stoma rates were much higher in the EC group compared with SBTS (OR 3.51, 95% CI 1.94–5.28). The SBTS mortality rate was the lowest of all three treatment modalities and EC had the highest mortality (OR 4.94, 95% CI 1.88–22.90) (Figure 2). Rate of anastomotic leak, overall complications, lymph node retrieval and length of hospital stay were similar across all three modalities (Figure 3).
Figure 2 .
Forest plots of NMA age, permanent stoma, primary resection anastomosis, mortality. CrI = credible interval.
Figure 3 .
NMA league table comparing all three treatment modalities in terms of outcomes. CI = confidence interval; DC = decompressing colostomy; EC = emergency colectomy; NMA = network meta-analysis; OR = odds ratio; SBTS = stenting as a bridge to surgery.
Sensitivity and assessing bias
The overall quality of the nonrandomised studies was assessed using the ROBINS-I tool, demonstrating that they ranged from low to moderate in quality. Increased risk of bias was detected in the domains of confounding and selection of patients (Supplementary Table 1). The overall quality of the included RCTs ranged from low to moderate and only the study by van Hooft et al scored low risk of bias in all domains of assessment. None of the remaining studies blinded assessors and personnel, raising the concern that performance and detection bias might have influenced the results (Supplementary Table 2).
Discussion
This is the first NMA to demonstrate that the primary anastomosis rate was significantly higher in DC- than in EC- and SBTS-cohorts. The 90-day mortality rate was also significantly lower in the DC cohort than in the EC and SBTS cohorts. In addition, significantly more locoregional recurrences occurred in the SBTS cohort compared with the EC cohort. Due to lack of meta-analysable data for the DC cohort, further analysis of the outcome of locoregional recurrences with NMA was not feasible.
Of note, the cost analysis demonstrated that SBTS was significantly more expensive (by $2,002) when compared with EC. Other key findings were that a significantly younger patient group (by 3 years) was included in the DC cohort compared with the EC and SBTS cohorts. However, nonsignificant differences were detected between the EC and SBTS cohorts. Taking into account that DC comprised 16.3% of the total sample and the EC and SBTS cohorts were 67.2% and 16.5%, respectively, we can conclude that heterogeneity and an underpowered sample might have influenced the results.
It has previously been reported that the DC approach results in more primary anastomoses and fewer permanent stomas than the EC approach.27 The evidence of the present NMA demonstrates that significantly more primary anastomoses were performed in the DC cohort compared with the EC and SBTS cohorts. Pairwise and NMA demonstrated significantly fewer permanent stomas in the SBTS than in the EC cohort. The NMA demonstrated nonsignificant differences between the DC cohort and EC and SBTS cohorts. This discrepancy between the two methods could have been influenced by the larger sample size of the NMA compared with the traditional meta-analysis.
Considering that both surgeons and patients are reluctant to choose the colostomy first approach because of the perception of a lower quality of life related to colostomy,28,29 the evidence of this study demonstrating more primary anastomoses and nonsignificant differences in permanent stomas between the three approaches will help surgeons and patients to make more objective decisions. Regarding oncological adequacy, the DC cohort demonstrated promising results in terms of excellent subsequent lymph node retrieval. Although not statistically significant, the DC group had a higher nodal yield than either of the other two cohorts and had the added advantage of a significantly lower mortality rate compared with the EC and SBTS cohorts (Table 3).
Tumour manipulation, breach of the tumour during passing of a guidewire, and continuous compression of the tumour wall with a stent are risk factors that may predispose to tumour spillage and perforation.30,31 Of note, a high-quality multicentre RCT reported a worrying rate of stent-related perforation (9%); worse still, when surgical specimens were examined to look for silent colonic perforations due to the prosthesis, this percentage increased to 20%.5 More recently, it has been reported that the three-year locoregional recurrence rate due to stent-related perforations is approximately 18%.19 In the present study, significantly higher locoregional recurrences occurred in the SBTS cohort (26%) than in the EC cohort (16%). This finding could be due to tumour spillage during colonoscopic manoeuvres or to silent colonic perforation by the stent. However, the above result should be interpreted with caution because the included RCTs are older than the study by Amelung, and learning curve bias might have influenced the results.32
As well as the above factors, there are also cost implications to consider in choosing between the different treatment modalities. The present study demonstrates, for the first time, that the cost of the SBTS approach was significantly more expensive by approximately $2,002 per case when compared with the EC cohort. Due to a lack of data, we did not perform cost analysis for the DC cohort, and there were not enough data to conduct an analysis of the long-term survival benefits. Whether this increased cost of stents offsets the potentially higher cost of treating complications of patients undergoing EC is unclear and is not a question that can be answered from the current data available in the literature.
The results of the present study should be interpreted in the context of its limitations. Ultimately, as with any pooling of data, the final results are only as good as the quality of the data input. Given that the majority of the studies were non-RCTs, there is potential for selection bias of patients into the different treatment cohorts (DC, EC, SBTS). Only the van Hooft study scored a low risk of bias in all domains and none of the remaining studies blinded participants, personnel or outcome assessors.5 Therefore, performance and detection bias might have influenced the results. The study by Arezzo and colleagues declared explicitly that they detected a discrepancy between the complication rates used to estimate the total sample and the observed complication rate during the performance.25 Therefore, this finding is suggestive of an underpowered sample, which might have influenced the results. Risk of bias analysis using the ROBINS-I tool demonstrated that only the studies by Veld scored low risk in six of the seven domains,16,26 raising the possibility of confounding and selection bias. Of note, the outcomes of the stenting might have been influenced by operator skills. In addition, ECs usually are performed by an on-call team that might not have expertise in colorectal surgery. Therefore, learning curve and performance bias might have influenced the results.
Conclusions
Between the two bridge-to-surgery approaches, DC demonstrated significantly more primary anastomoses and a significantly lower mortality rate than the SBTS approach. Moreover, SBTS was significantly more expensive by $2,002 per case, and significantly more locoregional recurrences occurred in SBTS than in the EC cohort. While not commonly performed at present, an initial DC remains a potential consideration in patients presenting with acute, left-sided, malignant large bowel obstruction. However, well designed multicentre studies would eliminate concerns regarding patient selection bias and provide better data on the outcomes of each modality.
Conflict of interest
All named authors hereby declare that they have no conflicts of interest to disclose.
References
- 1.Lee YM, Law WL, Chu KW, Poon RT. Emergency surgery for obstructing colorectal cancers: a comparison between right-sided and left-sided lesions. J Am Coll Surg 2001; 192: 719–725. 10.1016/S1072-7515(01)00833-X [DOI] [PubMed] [Google Scholar]
- 2.Cuffy M, Abir F, Audisio RA, Longo WE. Colorectal cancer presenting as surgical emergencies. Surg Oncol; 2004; 13: 149–157. 10.1016/j.suronc.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 3.Meyer F, Marusch F, Koch Aet al. Emergency operation in carcinomas of the left colon: value of Hartmann’s procedure. Tech Coloproctol 2004; 8(Suppl 1): s226–s229. 10.1007/s10151-004-0164-3 [DOI] [PubMed] [Google Scholar]
- 4.van Ommeren-Olijve SJ, Burbach JPM, Furnée EJB, Dutch Snapshot Research Group. Risk factors for non-closure of an intended temporary defunctioning stoma after emergency resection of left-sided obstructive colon cancer. Int J Colorectal Dis 2020; 35: 1087–1093. 10.1007/s00384-020-03559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hooft JE, Bemelman WA, Oldenburg Bet al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 2011; 12: 344–352. 10.1016/S1470-2045(11)70035-3 [DOI] [PubMed] [Google Scholar]
- 6.Spannenburg L, Sanchez Gonzalez M, Brooks Aet al. Surgical outcomes of colonic stents as a bridge to surgery versus emergency surgery for malignant colorectal obstruction: A systematic review and meta-analysis of high quality prospective and randomised controlled trials. Eur J Surg Oncol 2020; published online May 7. 10.1016/j.ejso.2020.04.052 [DOI] [PubMed] [Google Scholar]
- 7.Seitz JF, Faivre J. The French consensus conference on cancer of the colon: simple and precise recommendations. Bull Cancer 1998; 85: 293–294. [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DGet al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, Ltd, 2008. 10.1002/9780470712184 [DOI] [Google Scholar]
- 10.Sterne JA, Hernán MA, Reeves BCet al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23: 3105–3124. 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 14.Ades AE, Sculpher M, Sutton Aet al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006; 24: 1–19. 10.2165/00019053-200624010-00001 [DOI] [PubMed] [Google Scholar]
- 15.Chéreau N, Lefevre JH, Lefrancois Met al. Management of malignant left colonic obstruction: is an initial temporary colostomy followed by surgical resection a better option? Colorectal Dis 2013; 15: e646–e653. 10.1111/codi.12335 [DOI] [PubMed] [Google Scholar]
- 16.Veld JV, Amelung FJ, Borstlap WAAet al. Changes in management of left-sided obstructive colon cancer: national practice and guideline implementation. J Natl Compr Canc Netw 2019; 17: 1512–1520. 10.6004/jnccn.2019.7326 [DOI] [PubMed] [Google Scholar]
- 17.Krstic S, Resanovic V, Alempijevic Tet al. Hartmann’s procedure vs loop colostomy in the treatment of obstructive rectosigmoid cancer. World J Emerg Surg 2014; 9: 52. 10.1186/1749-7922-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Öistämö E, Hjern F, Blomqvist Let al. Emergency management with resection versus proximal stoma or stent treatment and planned resection in malignant left-sided colon obstruction. World J Surg Oncol 2016; 14: 232. 10.1186/s12957-016-0994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung HYS, Chung CC, Tsang WWCet al. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg 2009; 144: 1127–1132. 10.1001/archsurg.2009.216 [DOI] [PubMed] [Google Scholar]
- 20.Ho K-S, Quah H-M, Lim J-Fet al. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: a prospective randomized trial. Int J Colorectal Dis 2012; 27: 355–362. 10.1007/s00384-011-1331-4 [DOI] [PubMed] [Google Scholar]
- 21.Pirlet IA, Slim K, Kwiatkowski Fet al. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 2011; 25: 1814–1821. 10.1007/s00464-010-1471-6 [DOI] [PubMed] [Google Scholar]
- 22.Alcántara M, Serra-Aracil X, Falcó Jet al. Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 2011; 35: 1904–1910. 10.1007/s00268-011-1139-y [DOI] [PubMed] [Google Scholar]
- 23.Ghazal A-HA, El-Shazly WG, Bessa SSet al. Colonic endolumenal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg 2013; 17: 1123–1129. 10.1007/s11605-013-2152-2 [DOI] [PubMed] [Google Scholar]
- 24.Tung KLM, Cheung HYS, Ng LWCet al. Endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: long-term follow-up of a randomized trial. Asian J Endosc Surg 2013; 6: 78–81. 10.1111/ases.12030 [DOI] [PubMed] [Google Scholar]
- 25.Arezzo A, Balague C, Targarona Eet al. Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc 2017; 31: 3297–3305. 10.1007/s00464-016-5362-3 [DOI] [PubMed] [Google Scholar]
- 26.Veld JV, Amelung FJ, Borstlap WAAet al. Comparison of decompressing stoma vs stent as a bridge to surgery for left-sided obstructive colon cancer. JAMA Surg 2020; published online Jan 8. 10.1001/jamasurg.2019.5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amelung FJ, Mulder CLJ, Broeders IAMJet al. Efficacy of loop colostomy construction for acute left-sided colonic obstructions: a cohort analysis. Int J Colorectal Dis 2017; 32: 383–390. 10.1007/s00384-016-2695-2 [DOI] [PubMed] [Google Scholar]
- 28.Sprangers MA, Taal BG, Aaronson NK, te Velde A. Quality of life in colorectal cancer. stoma vs. nonstoma patients. Dis Colon Rectum 1995; 38: 361–369. 10.1007/BF02054222 [DOI] [PubMed] [Google Scholar]
- 29.Elfeki H, Thyø A, Nepogodiev Det al. Patient and healthcare professional perceptions of colostomy-related problems and their impact on quality of life following rectal cancer surgery. BJS Open 2018; 2: 336–344. 10.1002/bjs5.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabbagh C, Browet F, Diouf Met al. Is stenting as ‘a bridge to surgery’ an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg 2013; 258: 107–115. 10.1097/SLA.0b013e31827e30ce [DOI] [PubMed] [Google Scholar]
- 31.Sloothaak DAM, van den Berg MW, Dijkgraaf MGWet al. Oncological outcome of malignant colonic obstruction in the Dutch stent-In 2 trial. Br J Surg 2014; 101: 1751–1757. 10.1002/bjs.9645 [DOI] [PubMed] [Google Scholar]
- 32.Amelung FJ, Borstlap WAA, Consten ECJet al. Propensity score-matched analysis of oncological outcome between stent as bridge to surgery and emergency resection in patients with malignant left-sided colonic obstruction. Br J Surg 2019; 106: 1075–1086. 10.1002/bjs.11172 [DOI] [PubMed] [Google Scholar]