Abstract
Introduction
Thyroid nodules are lesions that are radiologically distinct from the thyroid parenchyma. Cervical ultrasound diagnoses 19–67% of nodules and is crucial in identifying those that lack cytological characterisation. Approximately 25% of biopsies reveal an indeterminate cytological result (Bethesda III), in which the risk of malignancy is variable (5–15%). The clinical importance of the diagnostic strategy used for thyroid nodules results from the need to exclude malignancy. The aim of this study was to evaluate the usefulness of serum thyroid-stimulating hormone (TSH) levels as a predictor of malignancy in cytologically indeterminate thyroid nodules.
Methods
Our retrospective study included 40 patients with cytologically indeterminate thyroid nodules seen in our hospital between January 2013 and December 2017. Clinical parameters were reviewed, including age, gender, serum TSH levels, family history of thyroid carcinoma, radiation exposure and some sonographic features of the nodules. Statistical analysis was performed using SPSS. Statistical significance was defined as p<0.05.
Results
Female gender was predominant (85%) and the mean (SD) age was 53.3 (15) years. Thyroid carcinoma was confirmed in 28% of patients. Median TSH levels were higher in patients with malignant (2.73µIU/ml) compared with benign (1.56µIU/ml) nodules (p<0.05). We demonstrated an increased risk of malignancy in patients with TSH levels of 2.68µIU/ml or above (p<0.05).
Conclusion
Higher serum TSH levels are associated with an increased risk of thyroid carcinoma in cytologically indeterminate nodules. TSH can become a fundamental diagnostic tool in stratifying the risk of malignancy and assist in diagnostic and therapeutic approaches to these nodules.
Keywords: Thyroid-stimulating hormone, Indeterminate thyroid nodule, Thyroid carcinoma
Introduction
Thyroid nodules are lesions that are radiologically distinct from the remaining parenchyma and are a relatively common finding. Cervical ultrasound diagnoses 19–67% of thyroid nodules,1–3 and is crucial in identifying those that lack cytological characterisation.
The characterisation of thyroid nodules is based on the Bethesda System for Reporting Thyroid Cytopathology (BSRTC) which specifies six diagnostic categories that provide an assessment of malignancy risk and management guidelines related to each category.4,5 Approximately 25% of biopsies give an indeterminate cytological result (Bethesda III),6 which does not allow selection of the most appropriate therapeutic approach. According to the BSRTC, atypia of undetermined significance (AUS) or a follicular lesion of undetermined significance (FLUS) are classified as Bethesda III, which includes architectural or nuclear abnormalities observed in fine-needle aspiration biopsies that do not fit into any other category.7 The risk of malignancy is difficult to assess in the Bethesda III category but is suggested to be between 5% and 15%.5 However, some studies have reported malignancy rates ranging from 6% to 76% for surgically confirmed cases.8–10 This shows that the reported risk of malignancy may not be accurate as many nodules are not removed surgically. Causes of malignancy include follicular carcinoma, papillary carcinoma, Hürthle cell carcinoma, anaplastic carcinoma, medullary carcinoma, primary lymphoma, sarcoma and others. Thyroid carcinoma is rare, with an incidence of 4–6.5%. It presents clinically as a nodule (solitary or in a multinodular gland) and can be indistinguishable from benign disease.11,12 The most frequent benign thyroid nodules consist of simple or haemorrhagic cysts, multinodular goitre, chronic lymphocytic thyroiditis, subacute thyroiditis and follicular adenoma.
The clinical importance of the diagnostic strategy used for indeterminate thyroid nodules results from the need to exclude malignancy.11 The relationship between serum thyroid-stimulating hormone (TSH) levels and the risk of malignancy in thyroid nodules has been evaluated in several studies.13–17 The first prospective study to describe this association was conducted in 2006 by Boelaert et al, who proposed a serum TSH level within normal range as an independent predictor of thyroid malignancy in a euthyroid population.14 The role of TSH in follicular cell growth is a crucial factor that can guide diagnostic and therapeutic decisions when approaching cytologically indeterminate nodules and can be a noninvasive tool to determine surgical intervention in differentiated thyroid carcinoma.18
The aim of the current study was to evaluate the usefulness of serum TSH levels as a predictor of malignancy in cytologically indeterminate thyroid nodules.
Methods
A retrospective study was conducted at our hospital between January 2013 and December 2017. The study participants were patients in whom a biopsy of the dominant thyroid nodule was cytologically indeterminate. The geographical area serviced by the hospital is not iodine deficient. Clinical files were consulted and several parameters were evaluated, namely age, gender, personal background (family history of thyroid carcinoma or other neoplasms and exposure to cervical radiation), ultrasound characteristics of the nodules and serum TSH levels. Presence of microcalcifications, number of thyroid nodules and histological examination of the surgical specimen were documented. All patients for whom serum TSH levels were not available, patients with known thyroid disease already undergoing levothyroxine treatment and patients in whom it was decided to continue with follow-up or repeat the third biopsy were excluded from the study. The diagnostic method used to distinguish between benign and malignant nodules was anatomopathological analysis of a surgical specimen, in accordance with World Health Organization guidelines. The study was approved by the ethics committee at our hospital, who did not require informed consent because clinical data were collected anonymously.
Statistical analysis
Statistical analysis was performed using SPSS v. 24. Statistical significance was defined as p<0.05. Categorical variables were expressed in counts or proportions and were analysed using the chi-square test and the binomial test. Continuous variables are shown as mean/median and standard deviation (SD). Receiver operator characteristic (ROC) curve analysis was used to determine a cut-off value that best predicted the risk of malignancy for cytologically indeterminate thyroid nodules.
Results
In this study, 75 patients with thyroid nodules classified as Bethesda III were evaluated (Figure 1), of whom 40 were included: 36 underwent hemithyroidectomy or total thyroidectomy and in four, their second cytological result revealed the nodule to be benign or malignant. Thirty-five patients were excluded: 28 had no available serum TSH levels and seven continued with follow-up or chose to repeat the third biopsy. There was a predominance of females (85%) and mean (SD) age was 53.3 (15) years. A family history of thyroid carcinoma or other neoplasms was found in two patients (5%) and none had experienced previous exposure to cervical radiation. Regarding ultrasound characteristics, solid nodules were identified in 32 patients (80%), irregular margins in 33 patients (83%) and hypoechogenicity in 32 (80%). Microcalcifications were detected in 12 patients (30%) and solitary nodules in 17 (43%). Differentiated thyroid carcinoma was thus confirmed in 28% of nodules. Median preoperative serum TSH level was 1.71µIU/ml (interquartile range 1.14–2.60). FLUS was observed in 24 nodules (60%) and AUS in 16 (40%).
Figure 1 .
Flow chart of patients included and excluded in the study
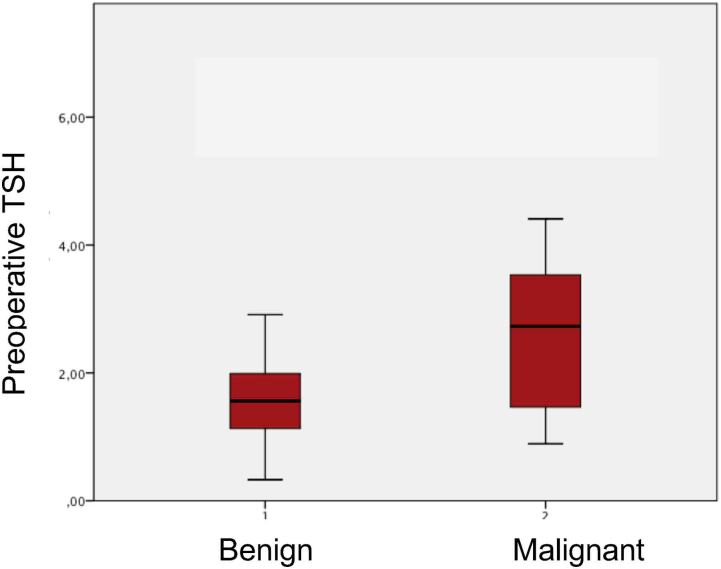
For our study population, we subdivided clinical variables into two categories: malignant and benign nodules (Table 1). Benign nodules were detected in 29 patients (73%) and malignant nodules in 11 (28%). The difference in age between the two groups was not significant; mean age was 52.1 (13) years for the benign nodule group and 56.6 (18) years for the malignant group (p>0.05). Female gender was more prevalent in both groups: 25 patients (63%) were female in the benign nodule group and nine (23%) in the malignant nodule group (p>0.05). Family history of thyroid carcinoma or other neoplasms was found in one patient (3%) with benign nodules, and one patient (9%) with malignant nodules. Analysing the ultrasound characteristics, we observed that solid nodules and microcalcifications were slightly more common in the malignant nodule group: 81% and 36%, respectively. By contrast, irregular margins, hypoechogenicity and solitary nodules predominated in the benign nodule group: 76%, 82% and 52%, respectively. These sonographic findings were compared between benign and malignant nodules and no significant statistical difference was found (p>0.05) and no independent risk factor was identified using binary regression and multivariate analysis. Serum TSH levels were higher in patients with malignant (2.73µIU/ml) compared with benign (1.56µIU/ml) nodules (p<0.05). Exploring Bethesda III category, malignant nodules subcategorised as FLUS were discovered in four patients (16%) and AUS in seven (44%). Through correlation between these findings and serum TSH levels, we found that there were no statistical differences between median TSH values when comparing malignant FLUS (1.14µIU/ml) and malignant AUS (1.28µIU/ml) (p>0.05) (Figure 2).
Table 1 .
Characteristics of clinical variables in patients with benign and malignant nodules
| Clinical Variants | Benign nodules (n=29) |
Malignant nodules (n=11) |
p-value |
|---|---|---|---|
| Age, years (SD) | 52.1 (13) | 56.6 (18) | 0.232 |
| Female:male, n (%) | 25:4 (63:10) | 9:2 (23:5) | 0.541 |
| Family history of thyroid carcinoma, n (%) | 1 (3) | 1 (9) | – |
| Exposure to cervical radiation, n (%) | 0 (0) | 0 (0) | – |
| Solid nodules, n (%) | 23 (79) | 9 (81) | 0.622 |
| Irregular margins, n (%) | 22 (76) | 8 (73) | 0.631 |
| Hypoechogenicity, n (%) | 24 (82) | 9 (81) | 0.762 |
| Microcalcifications, n (%) | 8 (28) | 4 (36) | 0.431 |
| Solitary nodule, n (%) | 15 (52) | 2 (18) | 0.079 |
| Serum TSH (median) | 1.56 (1.09–2.05) | 2.73 (1.40–3.66) | 0.025 |
| FLUS/AUS/ n (%) | 20/9 (83/56) | 7/4 (44/16) | 0.453 |
Values are shown as Mean (SD) or median (25th to 75th percentile). Microcalcifications and number of nodules were evaluated by ultrasound.
Figure 2 .
Preoperative TSH level in benign and malignant thyroid nodules
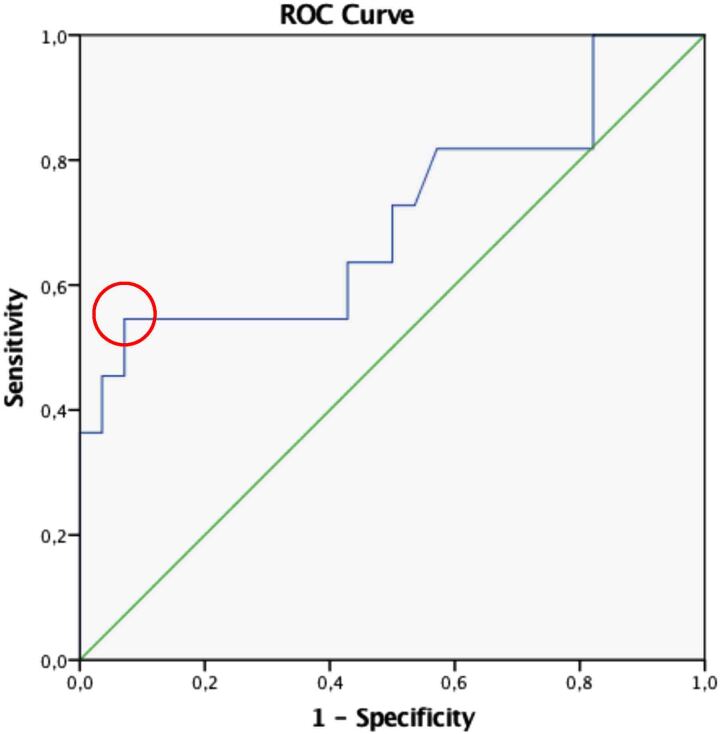
To investigate level variation in serum TSH as a predictor of malignancy in thyroid nodules of indeterminate significance, we subdivided the sample into quartiles according to the percentile distribution of TSH levels in this study (<25th percentile, TSH≤1.14; 25th to 50th percentiles, TSH 1.15–1.70; 51st to 75th percentiles, TSH 1.71–2.59; and >75th percentile, TSH≥2.60). As shown in Figure 3, the frequency of malignancy varies between the first and fourth quartile TSH levels (p<0.05). In the first quartile, the percentage of malignant nodules was 20%, in the second quartile it was 20%, in the third quartile it was 10% and finally, in the fourth quartile, 60% of nodules were malignant (odds ratio (OR) 6.2; confidence intervals (CI) 0.5–11.6). We confirmed that the percentage of malignant nodules in the last quartile was three times higher than in the first quartile, highlighting the correlation between elevated serum TSH levels and the malignancy of these nodules (Figure 3). To identify a cut-off value for TSH levels that combines the best sensitivity and specificity, and so best predicts the risk of malignancy, we conducted an analysis using a ROC curve (Figure 4). Approaching serum TSH as a categorical variable, a cut-off of 2.68 was discovered, which allowed us to demonstrate an increased risk of malignancy in patients with TSH levels of 2.68µIU/ml or higher (p<0.05).
Figure 3 .
Frequencies of benign and malignant nodules in accordance with TSH quartiles
Figure 4 .
ROC curve. The red circle shows the cut-off value.
Discussion
Although previous studies point to a higher risk in males,16,19 in our study, this factor did not significantly affect the risk of malignancy. This may be due to the small number of male patients. Similarly, there were no statistically significant differences in terms of age between patients with benign pathology and those with malignant disease (p>0.05). Corroborating previous authors, nodules with solid components were found mainly in the malignant nodule group (81%), as were microcalcifications (36%).20,21 Contrary to the literature, irregular margins, hypoechogenicity and a solitary nodule were evidenced mostly in patients with benign nodules (76%, 82% and 52%, respectively).21–23
Regarding serum TSH levels, there is a clear demonstration that patients with malignant thyroid nodules have higher TSH levels (2.73µIU/ml) than patients with benign nodules (1.56µIU/ml) and the difference was statistically significant (p<0.05).
Thyroid carcinoma is the most common endocrine malignancy and its prevalence continues to increase. Analytical measurement of serum TSH, a highly sensitive determinant of thyroid dysfunction, is recommended as the initial biochemical test for patients presenting with thyroid nodules.16 Authors revised that TSH has a trophic effect, mediated by TSH receptors, located in the cell membrane of differentiated carcinomas. Therefore, TSH promotes carcinogenesis in thyroid tissue and could explain the increased risk of malignancy with high serum concentrations of TSH.24,25 In recent studies, it has been shown that serum TSH levels, even those within the normal range, are associated with thyroid carcinoma diagnosis in patients with thyroid nodules.26 In this study, we confirmed an association between serum TSH levels and risk of malignancy for cytologically indeterminate thyroid nodules.
As an established follicular cell growth factor, TSH plays a central role in the development of thyroid carcinomas. We reinforce this aspect through subdivision of the analytical parameter into quartiles and the difference in the percentage of malignant nodules in the first quartile compared with the fourth quartile, which proves to be more significant. As we demonstrated, in the fourth quartile, where serum TSH is 2.60µIU/ml or higher, the percentage of malignant nodules is three times higher (60%) than in the first quartile (20%) and the prevalence of malignancy was higher in patients with serum TSH of 2.68µIU/ml or more, as previously indicated in ROC curve analysis. These observations show a statistically significant difference. Previous authors investigated an optimal cut-off value that intersects the highest sensitivity and specificity and can predict the risk of malignancy for thyroid nodules that underwent surgical intervention.15,27 However, to date, these studies have not yet achieved any consensus for a specific value. In these studies, the cut-off values were within normal ranges. In several previous studies, the authors tried to find characteristics that could help predict the risk of malignancy of cytologically indeterminate thyroid nodules to facilitate their therapeutic schedule. The great majority of the literature regarding the difference between AUS/FLUS category relates to ultrasound and cytological features21,28,29
In this study, we investigated how serum TSH levels can predict the risk of malignancy and be a simple, accessible factor that can assist the clinical and/or surgical approach in these nodules. This is the main highlight of our analysis, which is the first study to regard TSH value as a predictor for malignancy in cytologically indeterminate thyroid nodules. Management of these nodules is challenging and our findings suggest that higher serum TSH levels are associated with an increased risk of malignancy, which can be an encouraging future approach to therapeutic decision-making.
This study has some limitations. First, it is a retrospective analysis, single-institution and small study which may result in sampling bias. Second, there is a lack of all thyroid nodule ultrasound characteristics, which may have been considered as independent risk factors for nodule malignancy and thus enrich our assessment. Third, to calculate a precise risk of malignancy in the Bethesda III category, all thyroid nodules should be removed and subjected to anatomopathological examination to achieve a final diagnosis. Finally, in the recent literature, it has been shown that a molecular mutation, in particular BRAFV600E mutation, is a promising marker for gauging malignancy risk, especially in indeterminate cases, and makes diagnosis and decision-making more accurate.30 However, this information is not routinely available and could not be investigated in our study.
Conclusion
From the retrospective analysis in this study we can conclude that higher serum TSH levels are associated with an increased risk of thyroid carcinoma in patients with cytologically indeterminate nodules. Because the analytical parameters are simple and accessible, serum TSH levels may complement other clinical methods, such as fine-needle aspiration cytology, in predicting risk of malignancy in patients with cytologically indeterminate nodules. Therefore, TSH can become a fundamental diagnostic tool in risk stratifying malignancy and aid in selecting a therapeutic approach for nodules whose cytology reveals an indeterminate significance.
However, the role of TSH level and its association with malignant pathology remain controversial. Individualisation and obtaining a risk profile for these patients based on genetic and molecular analysis can also improve clinical guidance and decision-making. Further multicentre, prospective studies are required to establish and define the importance of this analytical method as an indicator of malignancy for a thyroid nodule clinical approach.
References
- 1.Hegedüs L. The thyroid nodule. N Engl J Med 2004; 351: 1764–1771. [DOI] [PubMed] [Google Scholar]
- 2.Guth S, Theune U, Aberle Jet al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 2009; 39: 699–706. [DOI] [PubMed] [Google Scholar]
- 3.Burman KD, Wartofsky L. Thyroid nodules. N Engl J Med 2015; 373: 2347–2356. [DOI] [PubMed] [Google Scholar]
- 4.Crippa S, Mazzucchelli L, Cibas ESet al. The Bethesda system for reporting thyroid fine-needle aspiration specimens. Am J Clin Pathol 2010; 134: 343–345. [DOI] [PubMed] [Google Scholar]
- 5.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid 2009; 19: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 6.Bongiovanni M, Spitale A, Faquin WCet al. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol 2012; 56: 333–339. [DOI] [PubMed] [Google Scholar]
- 7.VanderLaan PA, Krane JF, Cibas ES. The frequency of ‘atypia of undetermined significance' interpretations for thyroid fine-needle aspirations is negatively correlated with histologically proven malignant outcomes. Acta Cytol 2011; 55: 512–517. [DOI] [PubMed] [Google Scholar]
- 8.Kholová I, Ludvíková M. Thyroid atypia of undetermined significance or follicular lesion of undetermined significance: an indispensable Bethesda 2010 diagnostic category or waste garbage? Acta Cytol 2014; 58: 319–329. [DOI] [PubMed] [Google Scholar]
- 9.Carr R, Ustun B, Chhieng Det al. Radiologic and clinical predictors of malignancy in the follicular lesion of undetermined significance of the thyroid. Endocr Pathol 2013; 24: 62–68. [DOI] [PubMed] [Google Scholar]
- 10.Turkyilmaz S, Ulusahin ME, Celebi Bet al. Thyroid nodules classified as atypia or follicular lesions of undetermined significance deserve further research: analysis of 305 surgically confirmed nodules. Cytopathology 2017; 28: 391–399. [DOI] [PubMed] [Google Scholar]
- 11.Haugen B, Alexander EK, Bible KCet al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid Am Thyroid Assoc 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen QT, Lee EJ, Huang MGet al. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits 2015; 8: 30. [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HK, Yoon JH, Kim SJet al. Higher TSH level is a risk factor for differentiated thyroid cancer. Clin Endocrinol (Oxf) 2013; 78: 472–477. [DOI] [PubMed] [Google Scholar]
- 14.Boelaert K, Horacek J, Holder RLet al. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab 2006; 91: 4295–4301. [DOI] [PubMed] [Google Scholar]
- 15.Golbert L, de Cristo AP, Faccin CSet al. Serum TSH levels as a predictor of malignancy in thyroid nodules: A prospective study. PLoS ONE 2017; 12: e0188123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polyzos SA, Κita M, Efstathiadou Zet al. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J Cancer Res Clin Oncol 2008; 134: 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haymart MR, Repplinger DJ, Leverson GEet al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 2008; 93: 809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod DS, Watters KF, Carpenter ADet al. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab 2012; 97: 2682–2692. [DOI] [PubMed] [Google Scholar]
- 19.Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid 1998; 8: 377–383. [DOI] [PubMed] [Google Scholar]
- 20.Molina-Vega M, Rodríguez-Pérez CA, Álvarez-Mancha AIet al. Clinical and ultrasound thyroid nodule characteristics and their association with cytological and histopathological outcomes: A retrospective multicenter study in high-resolution thyroid nodule clinics. J Clin Med 2019; 8: 2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Pan D, Wu Yet al. Ultrasound characteristics of thyroid nodules facilitate interpretation of the malignant risk of Bethesda system III/IV thyroid nodules and inform therapeutic schedule. Diagn Cytopathol 2019; 47: 881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frates MC, Benson CB, Doubilet PMet al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab 2006; 91: 3411–3417. [DOI] [PubMed] [Google Scholar]
- 23.Chan BK, Desser TS, McDougall IRet al. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med 2003; 22: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 24.Rivas M, Santisteban P. TSH-activated signaling pathways in thyroid tumorigenesis. Mol Cell Endocrinol 2003; 213: 31–45. [DOI] [PubMed] [Google Scholar]
- 25.Chang T, Kuo SH, Liaw KYet al. Cell kinetics, DNA content and TSH receptor-adenylate cyclase system in differentiated thyroid cancer. Clin Endocrinol (Oxf) 1988; 29: 477–484. [DOI] [PubMed] [Google Scholar]
- 26.Duccini K, de Souza MV, Delfim Ret al. High serum thyrotropin concentrations within the reference range: A predictor of malignancy in nodular thyroid disease. Med Princ Pract 2018; 27: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin J, Machekano R, McHenry CR. The utility of preoperative serum thyroid-stimulating hormone level for predicting malignant nodular thyroid disease. Am J Surg 2010; 199: 294–298. [DOI] [PubMed] [Google Scholar]
- 28.Gao L-Y, Wang Y, Jiang YXet al. Ultrasound is helpful to differentiate Bethesda class III thyroid nodules: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2017; 96: e6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Han K, Kim EKet al. Risk stratification of thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) cytology using ultrasonography patterns defined by the 2015 Ata guidelines. Ann Otol, Rhinol Laryngol 2017; 126: 625–633. [DOI] [PubMed] [Google Scholar]
- 30.Choi SH, Baek JH, Lee JHet al. Evaluation of the clinical usefulness of BRAFV600E mutation analysis of core-needle biopsy specimens in thyroid nodules with previous atypia of undetermined significance or follicular lesions of undetermined significance results. Thyroid 2015; 25: 897–903. [DOI] [PubMed] [Google Scholar]