Abstract
Introduction
A recent Association of Breast Surgery summary statement on fibroadenoma management recommends excision only for cellular fibroepithelial lesions and rapidly growing lesions with a core biopsy diagnosis of fibroadenoma; persistent pain is a relative indication for excision.
Methods
This retrospective study looked at the impact this approach would have on the diagnosis of phyllodes tumours.
Results
From 2014 to 2018, there were 1,058 core biopsy diagnoses of fibroadenoma; 112 lesions were excised, of which 98 were fibroadenomas, 4 were hamartomas and 10 were phyllodes tumours. In this group, an excision diagnosis of phyllodes tumour was associated with size more than 40 mm, age more than 40 years and radiological suspicion of phyllodes tumour or carcinoma. One hundred and sixty-six excised fibroepithelial lesions with no previous core biopsy included eight phyllodes tumours; in this group, rapid growth was associated with phyllodes tumour diagnosis. Twelve of the 26 fibroepithelial lesions classified as B3 (cellular fibroepithelial lesion or phyllodes tumour) were diagnosed as phyllodes tumours on excision. Using a combination of radiological, clinical and pathological features it was possible to create an excision policy that would recommend excision of 22 of the 31 phyllodes tumours in this period. Eight of the nine ‘missed’ phyllodes tumours were benign.
Conclusion
The Association of Breast Surgery summary statement will reduce the number of fibroadenomas excised, but may also result in delayed diagnosis of some phyllodes tumours. Appropriate safety netting advice should be provided to identify rapidly growing lesions.
Keywords: Fibroadenoma, Phyllodes tumour, Excision, Needle core biopsy, Diagnosis, Radiology
Introduction
The management of fibroadenomas has evolved over recent decades. Until the 1980s, surgical excision was the standard practice. The use of fine needle aspiration cytology as part of the triple approach became more widespread around the time of the introduction of mammographic screening. Excision of a fibroadenoma was not considered necessary following a benign cytology result and clinical and radiological findings consistent with a fibroadenoma.1 In the 1990s with the introduction of needle core biopsy, a more definite nonoperative diagnosis of fibroadenoma became possible. More recently, there is evidence that a biopsy is not necessary in women aged under 25 years with clinical and ultrasound appearance consistent with a fibroadenoma because the risk of malignancy is very low in this group.2
The distinction of fibroadenoma and phyllodes tumour in surgical specimens is not always easy, so it is not surprising that this distinction in needle core biopsy can be challenging. Both are fibroepithelial lesions and there are no absolute histological criteria to distinguish these two entities. Phyllodes tumours often have areas resembling fibroadenoma and between about 10% and 40% of phyllodes tumours have a false-negative core biopsy usually with a diagnosis of fibroadenoma.3 Clinically, the distinction is important as excision of phyllodes tumour is recommended because of the risk of local recurrence and because a small proportion are malignant. In addition, identification and excision at first presentation can reduce the cosmetic impact of surgery to remove a phyllodes tumour compared with later excision. After a core biopsy diagnosis of fibroadenoma a subsequent diagnosis of phyllodes tumour is made in between 0.4% and 2% of cases.4–7
The literature provides conflicting advice on which lesions with a core biopsy or fine needle aspiration cytology diagnosis of fibroadenoma should be excised. Proposed criteria include patient age, lesion size and growing lesions.8–12
This review of our experience of excision of fibroadenomas was prompted by the recent Association of Breast Surgery summary statement which states that ‘If imaging and pathological features are concordant with the diagnosis of benign fibroadenoma the patient can be reassured and discharged’.13 Excision is recommended for cellular fibroepithelial lesions on core biopsy and rapidly growing lesions with a biopsy diagnosis of fibroadenoma, and persistent pain is a relative indication for considering excision.
Methods
A search of the pathology computer records at Nottingham University Hospitals was made for surgical specimens of phyllodes tumours diagnosed from 2014 to 2019 and compared with the results of any previous biopsies.
A search was also made for core biopsy diagnoses of fibroadenoma, hamartoma and B3 cellular fibroepithelial lesion from 2014 to 2018. The results were compared with the excision diagnosis. Core biopsies with epithelial atypia were excluded. Also excised fibroepithelial lesions with no previous core biopsy or normal core biopsy were reviewed. During this period, the indications for the excision of fibroepithelial lesions were if phyllodes tumour was in the needle core biopsy histological differential diagnosis, growing lesions, lesions larger than 30mm, radiological features suggesting phyllodes tumour and patient request. The potential value of patient age, tumour size on ultrasound, tumour growth and concern expressed by the radiologist as predictors of phyllodes tumour were assessed. The percentage increase in volume per month was calculated using the largest diameters in sequential ultrasound scans and a formula similar to that of Gordon et al14:
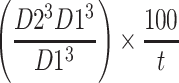 |
D2 is largest diameter at the second time point, D1 is the largest diameter at first time point, and t is the time in months between the two measurements. Cut-offs as proposed by Gordon et al were used: the 95th percentile for change in volume in women under 50 was 15.6% per month and the 90th percentile was 9% per month. Statistical analysis of the difference in frequency of these clinical and radiological features in phyllodes tumours and the group of other fibroepithelial lesions (fibroadenoma, tubular adenoma and hamartoma) was performed using chi-squared tests with Yates’ correction.
This study has been registered with Nottingham University Hospitals as a service evaluation.
Results
Between 2014 and 2019, 44 phyllodes tumours were excised (Table 1). The diagnosis was based on the surgical specimen. In 24 cases, the previous core biopsy diagnosis was cellular fibroepithelial lesion or phyllodes tumour (B3), suspicious of malignancy (B4) or malignant (B5).15 The core biopsy was normal (B1) in 1 case and benign (B2) in 12. Eleven of the B2 core biopsy diagnoses were fibroadenoma, but in five cases the features were not typical: stromal overgrowth in three, cellular stroma in one and fragmentation in one. Eight patients had no core biopsy. One tumour was classified as fibroadenoma in the first core biopsy and phyllodes tumour in the second so is included twice in Table 1.
Table 1 .
Phyllodes tumours diagnosed 2014 to 2019 with previous core biopsy diagnosis
| Previous core diagnosis | Phyllodes tumour grade | ||
|---|---|---|---|
| Benign | Borderline | Malignant | |
| Normal B1 | 1 | ||
| Fibroadenoma B2 | 8 | 3 | |
| Hamartoma B2 | 1 | ||
| Cellular fibroepithelial lesion or phyllodes tumour B3 | 12 | 6 | 2 |
| Suspicious of malignancy B4 | 2 | ||
| Malignant B5b | 2 | ||
| No core biopsy | 7 | 1 | |
| Total | 29 | 10 | 6 |
Between 2014 and 2018, 1,058 diagnoses of fibroadenoma were made on core biopsy. Only 112 lesions were excised: 98 were fibroadenomas, 4 were hamartomas and 10 were phyllodes tumours (Table 2). During this period, 12 of the 26 fibroepithelial lesions classified as B3 on core biopsy were phyllodes tumours on excision. During the same period, 166 excised fibroepithelial lesions had no previous core biopsy and 8 of these were phyllodes tumours in the excision specimen. It is not possible to say how many lesions with a clinical and radiological diagnosis of fibroadenoma with no core biopsy did not have the lesion excised. Two core biopsies with fibroadenoma containing lobular neoplasia were excluded from the analysis. In lesions with a core biopsy diagnosis of fibroadenoma the following features were associated with an excision diagnosis of phyllodes tumour: size more than 40mm, age more than 40 years, and radiological suspicion of phyllodes tumour or carcinoma (Table 3). In fibroepithelial lesions with no core biopsy or a normal core biopsy diagnosis a growth rate of more than 15% per month was associated (borderline statistical significance) with an excision diagnosis of phyllodes tumour (Table 4).
Table 2 .
Fibroepithelial tumours biopsied 2014 to 2018 with excision diagnosis
| Core diagnosis | Excision diagnosis | No excision | Total | |||
|---|---|---|---|---|---|---|
| Fibroadenomaa | Cellular fibroadenoma | Hamartoma | Phyllodes tumourb | |||
| Fibroadenoma B2 | 96c | 2 | 4 | 10d | 946 | 1,058 |
| Hamartoma B2 | 1 | 0 | 3 | 1 | 23 | 28 |
| Cellular fibroepithelial lesion or phyllodes tumour B3 | 5 | 8 | 1 | 12 | 0 | 26 |
| No core biopsy | 150 | 6 | 2 | 8 | 0 | 166 |
| Normal B1 | 4 | 0 | 0 | 0 | 0 | 4 |
aTwo tubular adenomas were included in the fibroadenoma group.
bOne tumour was called fibroadenoma in the first core biopsy and phyllodes tumour in the second.
cThree had atypical features.
dFive had atypical features.
Table 3 .
Clinical and radiological features and excision diagnosis after core biopsy diagnosis of fibroadenoma
| Radiological or clinical feature | Excision specimen diagnosis | χ2 with Yates’ correctiona | |
|---|---|---|---|
| Fibroadenoma, tubular adenoma, hamartoma | Phyllodes tumour | ||
| Size up to 30mm | 47 | 1 | χ2=3.5 |
| >30mm | 55 | 9 | p=0.06 |
| Size up to 40mm | 86 | 5 | χ2=5 |
| >40mm | 16 | 5 | p=0.03 |
| Age up to 25 years | 15 | 1 | χ2=0.0005 |
| >25 years | 80 | 9 | p=0.98 |
| Age up to 30 years | 37 | 1 | χ2=1.8 |
| >30 years | 65 | 9 | p=0.19 |
| Age up to 40 years | 69 | 2 | χ2=7 |
| >40 years | 33 | 8 | p=0.008 |
| Radiological growth | 23 | 3 | χ2=0.02 |
| No growth | 79 | 7 | p=0.89 |
| Growth >15%/month | 10 | 2 | χ2=0.2 |
| <15%/month | 92 | 8 | p=0.65 |
| Growth >10%/month | 17 | 3 | χ2=0.4 |
| <10%/month | 85 | 7 | p=0.53 |
| Radiological suspicion | 12 | 6 | χ2=12 |
| No suspicion | 90 | 4 | p=0.0004 |
aStatistical comparison of the frequency of these features in phyllodes tumours compared with other fibroepithelial lesions was made with chi squared with Yates’ correction.
Table 4 .
Clinical and radiological features and excision diagnosis in patients with no core biopsy (166 cases) or normal core biopsy diagnosis (four cases)
| Radiological or clinical feature | Excision specimen diagnosis | χ2 with Yates’ correctiona | |
|---|---|---|---|
| Fibroadenoma, tubular adenoma, hamartoma | Phyllodes tumour | ||
| Size up to 30mm | 71 | 3 | χ2=0.0002 |
| >30mm | 91 | 5 | p=0.99 |
| Size up to 40mm | 129 | 5 | χ2=0.5 |
| >40mm | 33 | 3 | p=0.47 |
| Age up to 25 years | 128 | 7 | χ2=0.02 |
| >25 years | 34 | 1 | p=0.90 |
| Radiological growth | 40 | 3 | χ2=0.2 |
| No growth | 122 | 5 | p=0.69 |
| Growth >15%/month | 15 | 3 | χ2=3.8 |
| <15%/month | 147 | 5 | p=0.05 |
| Growth >10%/month | 20 | 3 | χ2=2.3 |
| <10%/month | 142 | 5 | p=0.13 |
| Radiological suspicion | 0 | 0 | |
| No suspicion | 162 | 8 | |
aStatistical comparison of the frequency of these features in phyllodes tumours compared with other fibroepithelial lesions was made with chi squared with Yates’ correction.
The effect of using multiple factors to guide excision was assessed in the groups of patients from 2014 to 2018 with either a core biopsy diagnosis of a fibroepithelial lesion, or no core biopsy of lesion that was later found on excision to be a fibroepithelial lesion.
1. If a core diagnosis of cellular fibroepithelial lesion or phyllodes tumour (B3, B4 or B5) was used, 13 phyllodes tumours and 14 fibroadenomas would be excised.
2. Adding core biopsies with a diagnosis of fibroadenoma with cellular stroma, fragmentation or overgrowth would identify a further 3 phyllodes tumours (16 in total) and 4 fibroadenomas (18 in total).
3. Adding radiological suspicion of phyllodes tumour or carcinoma would identify an additional 2 phyllodes tumours (18 in total) tumours and 12 fibroadenomas (30 in total).
4. Adding tumours greater than 40mm would identify 5 phyllodes tumours (23 in total) tumours and 49 fibroadenomas (79 in total).
If instead of (4), percentage change in volume per month greater than 15% was used, 4 phyllodes tumours (22 in total) and 8 fibroadenomas (38 in total) would be identified; using the 10% cut-off no further phyllodes tumours and 8 fibroadenomas would be identified. These strategies would fail to identify all 31 phyllodes tumours. Using the last approach, the missed phyllodes tumours are eight benign and one borderline.
Discussion
During the study period from 2014 to 2018, 31 phyllodes tumours were diagnosed. In 13 cases the core biopsy included phyllodes tumour in the histological differential diagnosis. Ten phyllodes tumours had a core biopsy diagnosis of fibroadenoma. In the group with a core biopsy diagnosis of fibroadenoma, lesion size above 40mm, age above 40 years and radiological features suggestive of phyllodes tumour or carcinoma were associated with an excision diagnosis of phyllodes tumour. Eight phyllodes tumours had no core biopsy; in this group, rapid growth was associated with phyllodes tumour. No combination of clinical, radiological and pathological features could enable the nonoperative diagnosis of all phyllodes tumours.
A concern with a conservative approach to the management of fibroadenomas has been that the diagnosis may not be accurate. However, the risk of missing malignancy is very low. Neville et al studied 3,438 lesions with a core biopsy diagnosis of fibroadenoma.10 Two hundred and ninety lesions were excised and one had a 2mm focus of invasive carcinoma separate from the fibroadenoma. The majority of carcinomas in fibroadenomas are in situ, particularly lobular carcinoma in situ.16–18 In the present series, there were two core biopsies with fibroadenoma containing lobular neoplasia; on excision, one had fibroadenoma containing atypical lobular hyperplasia and the other had fibroadenoma containing lobular carcinoma in situ. No carcinoma was diagnosed after a core biopsy diagnosis of fibroadenoma during the study period.
The risk of missing a phyllodes tumour is higher than the risk of missing a carcinoma. On clinical examination, fibroadenomas are rubbery or firm, mobile, non-tender masses with a smooth outline. Of clinically diagnosed fibroadenomas, the diagnosis is confirmed on excision in about two-thirds of cases; the majority of the remainder are other benign diagnoses including occasional phyllodes tumours, but a few per cent are malignant (usually carcinomas).19 There is a greater degree of certainty of diagnosis with ultrasound. However, there is considerable overlap between the imaging appearance of fibroadenomas and phyllodes tumours. On mammography, both appear as well-defined, oval, lobulated or rounded masses. Coarse, benign ‘pop-corn’ calcifications are common in fibroadenomas but rare in phyllodes tumours.20 Fibroadenomas and phyllodes tumours both frequently exhibit the features of a benign mass on ultrasound, appearing as a well-circumscribed mass with an oval or gently lobulated contour, often with a thin echogenic pseudocapsule.21 The diagnosis of a phyllodes tumour is more likely if elongated cystic spaces or clefts or internal acoustic heterogeneity are observed within a well-defined solid mass, but these features are only present in a minority of cases.20,22,23 Phyllodes tumours with a size greater than 3cm are more likely to be associated with malignancy.20 Other than size, there are no reliable imaging features to differentiate a benign from a malignant phyllodes tumour.20 We found that radiological suspicion of phyllodes tumour or carcinoma was a useful feature: 6 of 18 such lesions were phyllodes tumour on excision.
There is a histological continuum between cellular fibroadenoma and benign phyllodes tumour. The histological distinction can be difficult in surgical specimens so it is not surprising that the diagnosis can be challenging in core biopsy. Definite core biopsy diagnosis of phyllodes tumours is sometimes possible, particularly of borderline and malignant tumours. Several histological features are of consistent value in the literature for the diagnosis of phyllodes tumour on core biopsy: stromal cellularity more than a fibroadenoma, stromal mitoses (particularly more than three per ten high-power fields), stromal atypia, stromal overgrowth (×10 field of stroma without glands) and fragmentation.3,4,24,25 There are no absolute histological criteria to separate phyllodes tumour and fibroadenoma and all of the five features listed above can be seen occasionally in fibroadenomas. A key feature is stromal cellularity, but this is subjective. The features are less developed in benign phyllodes tumour and often the term ‘cellular fibroepithelial lesion’ has to be used because a definite diagnosis is not possible and B3 categorisation is used. Surgical excision is recommended for lesions with a definite or suspected diagnosis of phyllodes tumour.26 Only 24 of 44 phyllodes tumours had a B3, B4 or B5 diagnosis (Table 1). The remainder largely had either a core biopsy diagnosis of fibroadenoma or no core biopsy. Nine biopsies were reported as fibroadenoma and classified as benign (B2) but had atypical features: cellular stroma, fragmentation or stromal overgrowth. Five of these were phyllodes tumours on excision. This suggests that the presence of cellular stroma, fragmentation or stromal overgrowth alone should be sufficient for categorisation as cellular fibroepithelial lesion (B3) to avoid missing phyllodes tumours.
Relying only on core biopsy features or suspicious radiological appearance fails to identify all phyllodes tumours. Some phyllodes tumours have typical histological features of fibroadenoma on core biopsy or are present in young women who do not undergo core biopsy sampling at assessment.
Tumour size has been proposed as a criterion for excision. Consistent with the literature, we found that phyllodes tumours were more likely in larger lesions.9,11 In the group with a core biopsy diagnosis of fibroadenoma, 5 of 21 lesions larger than 40mm were phyllodes tumours. Size was not of value in the group with no core biopsy.
Patient age has also been proposed as a criterion for excision. In agreement with previous studies, we found that phyllodes tumours tended to arise in older women, but fibroadenomas are more common at all ages.9 Forty-one women over 40 years old had a core biopsy diagnosis of fibroadenoma and eight of these were phyllodes tumours in the surgical specimen. Age is not of value in the group with no core biopsy because most of these women are young. One difficulty with using either tumour size or patient age to guide excision is that a large of number of fibroadenomas would also be excised in addition to the much smaller number of phyllodes tumours.
The Association of Breast Surgery summary statement13 recommends excision of rapidly growing lesions with a biopsy diagnosis of fibroadenoma, but no definition of rapidly growing is given. Studies of growing lesions with a core biopsy diagnosis of fibroadenoma found phyllodes tumour in 2 of 65 cases in one study8 and none of 40 in another.12 We found no difference in the proportion of fibroadenomas and phyllodes tumours with radiological evidence of growth. We also assessed rapid growth with the percentage increase in volume per month using cut-offs as proposed by Gordon et al14: the 95th percentile for change in volume per month of 15.6% and the 90th percentile of 9% based on a series of fibroadenomas. Neither of these cut-offs was of value in patients with a core biopsy diagnosis of fibroadenoma, but growth of more than 15% per month was more common in phyllodes tumours (of borderline statistical significance) in patients with no core biopsy.
None of the individual features discussed above identifies all phyllodes tumours. A combination of B3, B4 or B5 core biopsy diagnosis, B2 diagnosis of fibroadenoma with atypical features, radiological suspicion of phyllodes tumour or carcinoma and percentage increase in volume per month greater than 15% identified 22 of 31 phyllodes tumours. Almost all the missed phyllodes tumours were benign. It is possible that using these features in an independent series would identify a lower proportion of phyllodes tumours. We have already reduced the proportion of fibroadenomas that are being excised in Nottingham consistent with the Association of Breast Surgery summary statement (the current series is from the period before we introduced this change). This study shows that these guidelines are likely to result in the later diagnosis of some phyllodes tumours. We plan to expand our criteria for core biopsy diagnosis of ‘cellular fibroepithelial lesion’ to include more minor changes, as described above, to try to reduce these late diagnoses. Some large lesions will be excised because of patient choice and these are more likely to be phyllodes tumours. Rapid growth defined as percentage increase in volume per month greater than 15% may be useful to identify some phyllodes tumours.
Conclusions
In conclusion, the Association of Breast Surgery summary statement will greatly reduce the number of fibroadenomas that are excised. However, it is also likely to result in later diagnosis of some phyllodes tumours and we suggest this should be acknowledged in future guidelines. Guidelines should acknowledge the difficulty of nonoperative diagnosis of phyllodes tumours including false-negative core biopsies. Categorising core biopsies with a fibroepithelial lesion with isolated cellular stroma, fragmentation or stromal overgrowth as B3 would result in identification of more phyllodes tumours with few additional fibroadenomas excised (three and four, respectively, in this study). We recommend that patients with a nonoperative diagnosis of fibroadenoma should be advised to return if the lesion grows rapidly. Use of the threshold of percentage change in volume per month greater than 15% is supported by this study: it identified four phyllodes tumours and eight fibroadenomas.
References
- 1.Carty NJ, Carter C, Rubin Cet al. Management of fibroadenoma of the breast. Ann R Coll Surg Engl 1995; 77: 127–130. [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GE, Burrows P. Ultrasound diagnosis of fibroadenoma - is biopsy always necessary? Clin Radiol 2008; 63: 511–515; discussion, 516–517. [DOI] [PubMed] [Google Scholar]
- 3.Lee AHS. Fibroepithelial lesions. In: Shousha S. (ed.) Breast Pathology: Problematic Lesions. Switzerland: Springer; 2017. [Google Scholar]
- 4.Lee AHS, Hodi Z, Ellis IOet al. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology 2007; 51: 336–344. [DOI] [PubMed] [Google Scholar]
- 5.Abdulcadir D, Nori J, Meattini Iet al. Phyllodes tumours of the breast diagnosed as B3 category on image-guided 14-gauge core biopsy: analysis of 51 cases from a single institution and review of the literature. Eur J Surg Oncol 2014; 40: 859–864. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs TW, Chen Y-Y, Guinee DGet al. Phyllodes tumor (PT) subsequent to a diagnosis of fibroadenoma (FA) on breast core needle biopsy (CNB): frequency and characteristics. The 103rd Annual Meeting of the United States and Canadian Academy of Pathology. March 2014; San Diego CA, USA. Mod Pathol 2014;27:56A. [Google Scholar]
- 7.Van Osdol AD, Landercasper J, Andersen JJet al. Determining whether excision of all fibroepithelial lesions of the breast is needed to exclude phyllodes tumor: upgrade rate of fibroepithelial lesions of the breast to phyllodes tumor. JAMA Surg 2014; 149: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 8.Sanders LM, Sara R. The growing fibroadenoma. Acta Radiol Open 2015; 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubbard JL, Cagle K, Davis JWet al. Criteria for excision of suspected fibroadenomas of the breast. Am J Surg 2015; 209: 297–301. [DOI] [PubMed] [Google Scholar]
- 10.Neville G, Neill CO, Murphy Ret al. Is excision biopsy of fibroadenomas based solely on size criteria warranted? Breast J 2018; 24: 981–985. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Mercado CL, Cangiarella JFet al. Frequency and outcomes of biopsy-proven fibroadenomas recommended for surgical excision. Clin Imaging 2018; 50: 31–36. [DOI] [PubMed] [Google Scholar]
- 12.Dialani V, Chansakul T, Lai KCet al. Enlarging biopsy-proven fibroadenoma: Is surgical excision necessary? Clin Imaging 2019; 57: 35–39. [DOI] [PubMed] [Google Scholar]
- 13.Cox K and the Clinical Practice & Standards Committee of the Association Breast Surgery. Association of Breast Surgery summary statement: management of fibroadenomas. https://associationofbreastsurgery.org.uk/media/64950/abs-summary-statement-fibroadenomas-v1.pdf
- 14.Gordon PB, Gagnon FA, Lanzkowsky L. Solid breast masses diagnosed as fibroadenoma at fine-needle aspiration biopsy: acceptable rates of growth at long-term follow-up. Radiology 2003; 229: 233–238. [DOI] [PubMed] [Google Scholar]
- 15.Non-operative Diagnosis Subgroup of the National Coordinating Committee for Breast Screening Pathology. Guidelines for non-operative diagnostic procedures and reporting in breast cancer screening. The Royal College of Pathologists; 2016. [Google Scholar]
- 16.Buzanowski-Konakry K, Harrison EG, Payne WS. Lobular carcinoma arising in fibroadenoma of the breast. Cancer 1975; 35: 450–456. [DOI] [PubMed] [Google Scholar]
- 17.Diaz NM, Palmer JO, McDivitt RW. Carcinoma arising within fibroadenomas of the breast. A clinicopathologic study of 105 patients. Am J Clin Pathol 1991; 95: 614–622. [DOI] [PubMed] [Google Scholar]
- 18.Kuijper A, Mommers EC, van der Wall Eet al. Histopathology of fibroadenoma of the breast. Am J Clin Pathol 2001; 115: 736–742. [DOI] [PubMed] [Google Scholar]
- 19.Walters TK, Zuckerman J, Nisbet-Smith Aet al. Fine needle aspiration biopsy in the diagnosis and management of fibroadenoma of the breast. Br J Surg 1990; 77: 1215–1217. [DOI] [PubMed] [Google Scholar]
- 20.Liberman L, Bonaccio E, Hamele-Bena Det al. Benign and malignant phyllodes tumours: mammographic and sonographic findings. Radiology 1996; 198: 121–124. [DOI] [PubMed] [Google Scholar]
- 21.Stavros AT, Thickman D, Rapp CLet al. Solid breast nodules: Use of sonography to distinguish between benign and malignant lesion. Radiology 1995; 196: 123–134. [DOI] [PubMed] [Google Scholar]
- 22.Cole-Beuglet C, Sopriano R, Kurtz ABet al. Ultrasound, mammography and histopathology of cystosarcoma phyllodes. Radiology 1983; 146: 481–486. [DOI] [PubMed] [Google Scholar]
- 23.Chao TC, Lo YF, Chen SC, Chen MF. Sonographic features of phyllodes tumors of the breast. Ultrasound Obstet Gynecol 2002; 20: 64–71. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs TW, Chen YY, Guinee DGet al. Fibroepithelial lesions with cellular stroma on breast core needle biopsy: are there predictors of outcome on surgical excision? Am J Clin Pathol 2005; 124: 342–354. [DOI] [PubMed] [Google Scholar]
- 25.Jara-Lazaro AR, Akhilesh M, Thike AAet al. Predictors of phyllodes tumours on core biopsy specimens of fibroepithelial neoplasms. Histopathology 2010; 57: 220–232. [DOI] [PubMed] [Google Scholar]
- 26.Pinder SE, Shaaban A, Deb Ret al. NHS breast screening multidisciplinary working group guidelines for the diagnosis and management of lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin Radiol 2018; 73: 682–692. [DOI] [PubMed] [Google Scholar]


