Abstract
Background
By the end of this decade, 70 per cent of all diagnosed pancreatic ductal adenocarcinomas will be in the elderly. Surgical resection is the only curative option. In the elderly perioperative mortality is higher, while controversy still exists as to whether aggressive treatment offers any survival benefit. This study aimed to assess the oncological benefit of pancreatoduodenectomy in octogenarians with pancreatic ductal adenocarcinoma.
Method
Retrospective multicentre case-control study of octogenarians and younger controls who underwent pancreatoduodenectomy for pancreatic ductal adenocarcinoma between 2008 and 2017. The primary endpoint was overall survival and the secondary endpoint was disease-free survival.
Results
Overall, 220 patients were included. Although the Charlson co-morbidity index was higher in octogenerians, Eastern Cooperative Oncology Group performance status, ASA and pathological parameters were comparable. Adjuvant therapy was more frequently delivered in the younger group (n = 80, 73 per cent versus n = 58, 53 per cent, P = 0.006). There was no significant difference between octogenarians and controls in overall survival (20 versus 29 months, P = 0.095) or disease-free survival (19 versus 22 months, P = 0.742). On multivariable analysis, age was not an independent predictor of either oncological outcome measured.
Conclusion
Octogenarians with pancreatic ductal adenocarcinoma of the head and uncinate process may benefit from comparable oncological outcomes to younger patients with surgical treatment. Due to the age- and disease-related frailty and co-morbidities, careful preoperative assessment and patient selection is of paramount importance.
By the end of this decade, 70 per cent of all diagnosed pancreatic ductal adenocarcinomas (PDACs) will be in the elderly. Pancreatoduodenectomy is the only curative option, however, the benefit of curative resection in the elderly is difficult to determine from the literature. Octogenarians with PDAC of the head and uncinate process may benefit from comparable oncological outcomes to younger patients with surgical treatment after careful preoperative assessment and patient selection.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) will be the second leading cause of cancer-related mortality by the end of this decade1. Surgical resection is the only curative option for patients with PDAC, however, only 15–20 per cent are eligible on diagnosis2. Oncological outcomes can be substantially improved with completion of the treatment pathway (neoadjuvant and/or adjuvant treatment and surgery)3,4. Nonetheless, 5-year survival is only 20–25 per cent due to local or metastatic disease recurrence5,6. Historically, the mortality associated with pancreatoduodenectomy (PD) was high and the concern of chronic co-morbidities and frailty amongst the elderly resulted in their exclusion from surgical treatment7. Similarly, the perception of elderly patients being poor candidates to receive adjuvant chemotherapy may prevent them being offered a chance of curative treatment pathways8.
Following the population surge after World War II, the ‘baby boomers’ have now become octogenarians9 and a combination of better general health and hence longer life expectancy has seen a rise in the age-specific trend of the incidence of PDAC10. By the end of this decade, 70 per cent of all diagnosed PDAC will be in the elderly11. The benefit of curative resection in this population is difficult to determine from the literature as it is underrepresented within clinical trials. Most studies on the safety and benefit of the elderly population undergoing PD refer to an age range of 70–75 years, but over the next 50 years the proportion of octogenarians within the population in Europe will double from 6 to 13 per cent12. The advances in surgical technique, technology, perioperative management and centralization have resulted in a substantial improvement in mortality (from 4.1 to 2.4 per cent) and failure to rescue (from 13 to 7.4 per cent) following PD13,14. Although in a previous study of this group, 90-day mortality of octogenarians after PD was double that of younger controls matched on extent of surgery for periampullary malignancies (9 versus 3 per cent), age was not an independent predictor of mortality15. Careful patient selection and assessment of co-morbidities are of paramount importance in preoperative planning for these patients. Nonetheless, the evidence in the literature is still controversial as to whether aggressive treatment offers any survival benefit for octogenarians with PDAC. The aim of this study was to assess the oncological benefit of PD for octogenarians with PDAC.
Methods
Study design
A multicentre retrospective case-control analysis of prospectively maintained databases was performed, including PD undertaken over a 10-year interval between January 2008 and December 2017. Octogenarians who underwent PD were matched with consecutively operated younger patients (control group) with a 1:1 ratio, based on extent of surgery (venous, arterial or additional organ resection). An invitation to participate in this study was sent out to all specialist pancreatic centres across the UK. Six centres agreed to participate, resulting in data from a total of seven centres included. Institutional board approval was sought and obtained by each centre separately. Data collection was carried out by each centre using a standardized proforma. The primary outcome set for this study was overall survival (OS; defined as time from diagnosis to death) and the secondary outcome disease-free survival (DFS; defined as time from surgery to diagnosis of recurrence). Reporting of results was performed in line with the STROBE statement16.
Data collection
The preoperative data collected included: demographics, ASA score, Eastern Cooperative Oncology Group (ECOG) performance status, Charlson co-morbidity index (CCI), co-morbidities, preoperative echocardiogram, pulmonary function test and or cardiopulmonary exercise testing (CPEX), preoperative biliary stenting, steroid use, preoperative haemoglobin, serum albumin, bilirubin and use of neoadjuvant chemotherapy and/or radiotherapy.
Intraoperative data included: type of PD (classic Whipple or pylorus preserving), additional organ resection or venous resection.
Postoperative data included: histological type of tumour, lymph node ratio, resection margin status (defined as R0: no tumour cells for at least 1 mm from margin, R1: tumour cells within 1 mm)17, presence of perineural and intravascular invasion, 30- and 90-day mortality, complications categorized using the Clavien–Dindo classification, 30-day re-admission, OS and DFS.
Statistical methods
Chi squared with exact statistics and ANOVA were used as appropriate to compare variables and outcomes between the two groups, with statistical significance set at P < 0.050. Survival analysis (OS and DFS) was performed using the Kaplan–Meier method and the log-rank test for comparing differences between survival curves. Univariable and multivariable time to event analyses were performed using the Cox proportional hazard model for OS and DFS. Variables were subjected to a univariable analysis first and those with P < 0.20 were introduced into a multivariable model. Hazard ratios (HR) and associated 95 per cent confidence intervals were calculated. A two-tailed P value <0.050 was considered statistically significant. All statistical analyses were performed using the software package SPSS Statistics for Windows® (version 23.0; SPSS Inc., Chicago, IL, USA).
Results
Cohort characteristics
A total of 220 patients comprising 110 octogenerian and 110 non-octogenerian patients underwent PD (Table 1). The octogenarian cohort was matched on complexity of resection with consecutive patients from a younger cohort where 54 (24.5 per cent) of patients underwent vein resections. Although CCI was higher in octogenerians, ECOG performance status and ASA were comparable. Neoadjuvant therapy was not commonly performed in either cohort, but adjuvant therapy was delivered more commonly in the younger cohort (n = 80 (72.7 per cent) versus n = 58 (52.7 per cent), P = 0.006). There was no difference in the tumour stage, lymph node ratio or resection margin status between the groups. There was no significant difference in the recurrence pattern between the groups.
Table 1.
Comparison of demographic, treatment and pathology characteristics between octogenarians and controls
| Demographics | Total n = 220 |
Controls n = 110 |
Octogenarians n = 110 |
P |
|---|---|---|---|---|
| Age (years), median (range) | 79 (36–88) | 69 (36–79) | 81 (80–86) | <0.001* |
| Sex | 0.135 | |||
| Male | 123 (55.9) | 67 (60.9) | 56 (50.9) | |
| Female | 97 (44.1) | 43 (39.1) | 54 (49.1) | |
| Charlson co-morbidity index | <0.001* | |||
| 1–2 | 6 (2.7) | 6 (5.5) | 0 | |
| 3–4 | 34 (15.5) | 34 (30.9) | 0 | |
| >5 | 167 (75.9) | 62 (56.4) | 105 (95.5) | |
| ECOG performance status | 0.756 | |||
| 0–1 | 197 (89.5) | 98 (89.1) | 99 (90) | |
| 2–4 | 9 (4.1) | 4 (3.6) | 5 (4.5) | |
| ASA | 0.635 | |||
| I–II | 149 (67.7) | 77 (70) | 72 (65.5) | |
| III–IV | 38 (17.3) | 18 (16.4) | 20 (18.2) | |
| Treatment | ||||
| Neoadjuvant therapy | ||||
| Chemotherapy | 5 (2.3) | 3 (2.7) | 2 (1.8) | 0.651 |
| Radiotherapy | 3 (1.4) | 2 (1.8) | 1 (0.9) | 0.561 |
| Operation | 0.786 | |||
| Whipples | 122 (55.5) | 60 (54.5) | 62 (56.4) | |
| PPPD | 98 (44.5) | 50 (45.5) | 48 (43.6) | |
| Vein resection | 54 (24.5) | 28 (25.5) | 26 (23.6) | 0.754 |
| Adjuvant chemotherapy | 138 (62.7) | 80 (72.7) | 58 (52.7) | 0.006* |
| Pathology | ||||
| T stage | 0.271 | |||
| pT1 | 8 (3.6) | 3 (2.7) | 5 (4.6) | |
| pT2 | 10 (4.5) | 7 (6.4) | 3 (2.7) | |
| pT3 | 197 (89.6) | 99 (90) | 98 (89.1) | |
| pT4 | 5 (2.3) | 1 (0.9) | 4 (3.6) | |
| Resection margin | 0.499 | |||
| R0 | 101 (45.9) | 48 (43.6) | 53 (48.2) | |
| R1 | 119 (54.1) | 62 (56.4) | 57 (51.8) | |
| Lymph node ratio, median (range) | 0.15 (0–1) | 0.19 (0–1) | 0.14 (0–0.75) | 0.132 |
| Perineural invasion | 187 (85) | 93 (84.5) | 94 (85.5) | 0.278 |
| Perivascular invasion | 171 (77.7) | 84 (76.4) | 87 (79.1) | 0.183 |
| Outcomes | ||||
| 30-day mortality | 5 (2.3) | 2 (1.8) | 3 (2.7) | 0.636 |
| 90-day mortality | 13 (5.9) | 4 (3.6) | 9 (8.2) | 0.143 |
Values are n (%) unless otherwise stated. *P values are significant. ECOG, Eastern Cooperative Oncology Group; PPPD, pylorus-preserving pancreatoduodenectomy.
Survival analysis
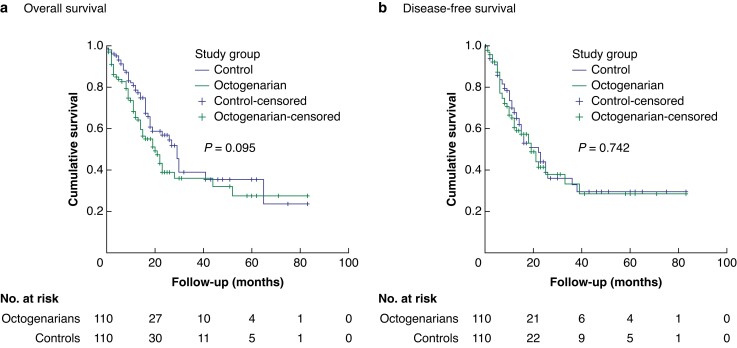
There was no significant difference in OS (octogenarians median: 20 months, range: 14–26 months versus controls median: 29 months, range: 24–34 months; P = 0.095) or DFS (octogenarians median: 19 months, range: 13–24 months versus controls median: 22 months, range: 15–29 months; P = 0.742) between octogenarians and controls (Fig. 1).
Fig. 1.
Kaplan–Meier curves a Overall survival and b disease-free survival.
Risk analysis
Overall survival
Table 2 shows the results of the univariable Cox regression analysis for OS. Multivariable analysis identified history of angina/percutaneous coronary intervention/coronary surgery (OR = 3.149; c.i. = 1.351–7.341; P = 0.008), preoperative albumin levels (OR = 0.592; c.i. = 0.383–0.914; P = 0.018) and lymph node ratio (OR = 10.048; c.i. = 3.388–29.801; P < 0.001) as independent predictors of OS. Of note, age was not significant.
Table 2.
Univariable Cox regression analysis for overall survival
| Overall survival | ||||
|---|---|---|---|---|
| Parameters | Univariable analysis: whole cohort | Univariable analysis: octogenarians subgroup | ||
| OR (95% c.i.) | P | OR (95% c.i.) | P | |
| Preoperative | ||||
| Age Indicator: control; < 80 years |
1.410 (0.935–2.125) | 0.101 | — | — |
| Sex Indicator: female |
1.001 (0.663–1.511) | 0.996 | 0.928 (0.529–1.628) | 0.793 |
| ASA score Indicator: class 1 |
1.326 (0.905–1.945) | 0.148 | 0.993 (0.556–1.774) | 0.981 |
| ECOG performance status Indicator: grade 1 |
1.126 (0.779–1.629) | 0.527 | 1.040 (0.616–1.756) | 0.884 |
| ECOG groups Indicator: grades 0–1 |
1.172 (0.428–3.211) | 0.757 | 1.392 (0.335–5.773) | 0.649 |
| Diabetes Indicator: no |
1.709 (1.118–2.612) | 0.013* | 2.054 (1.154–3.654) | 0.014* |
| COPD Indicator: no |
0.502 (0.158–1.594) | 0.242 | 0.507 (0.070–3.688) | 0.502 |
| Congestive heart failure Indicator: no |
2.776 (0.864–8.914) | 0.086 | 1.862 (0.244–14.208) | 0.549 |
| Myocardial infarction Indicator: no |
1.746 (0.837–3.641) | 0.138 | 1.402 (0.497–3.955) | 0.523 |
| Prior PCI/previous coronary surgery/angina Indicator: no |
1.918 (0.918–4.005) | 0.083 | 1.330 (0.318–5.571) | 0.696 |
| Hypertension Indicator: no |
1.042 (0.667–1.629) | 0.856 | 1.036 (0.572–1.878) | 0.907 |
| Impaired sensorium Indicator: no |
7.052 (0.955–52.057) | 0.055 | NA | NA |
| Dementia Indicator: no |
4.778 (0.654–34.885) | 0.123 | NA | NA |
| Peripheral vascular disease Indicator: no |
1.680 (0.410–6.889) | 0.471 | 0.048 (0–98692.140) | 0.683 |
| TIA / CVA Indicator: no |
2.592 (1.186–5.665) | 0.017* | 1.683 (0.600–4.723) | 0.323 |
| Neurological deficit Indicator: no |
17.073 (2.204–132.244) | 0.007* | NA | NA |
| Connective tissue disease Indicator: no |
0.047 (0–32.697) | 0.361 | 0.046 (0–34.921) | 0.363 |
| Peptic ulcer disease Indicator: no |
0.292 (0.040–2.116) | 0.223 | 6.804 (0.890–52.025) | 0.065 |
| Liver disease Indicator: no |
0.047 (0–8.606) | 0.250 | 0.046 (0–53.310) | 0.393 |
| Hypercoagulability Indicator: no |
2.307 (0.724–7.354) | 0.158 | 1.985 (0.612–6.439) | 0.254 |
| Chronic kidney disease Indicator: stage 1 |
0.996 (0.927–1.069) | 0.902 | 0.988 (0.908–1.075) | 0.780 |
| Biliary stent Indicator: no |
0.813 (0.526–1.256) | 0.350 | 0.485 (0.270–0.872) | 0.016* |
| Steroid use prior to operation Indicator: no |
1.841 (1.011–3.353) | 0.046* | 4.070 (2.020–8.197) | <0.001* |
| Preoperative haemoglobin | 0.952 (0.846–1.071) | 0.414 | 0.852 (0.698–1.042) | 0.118 |
| Preoperative bilirubin | 1.001 (0.999–1.003) | 0.183 | 1.001 (0.999–1.003) | 0.163 |
| Preoperative albumin | 0.668 (0.502–0.890) | 0.006* | 0.482 (0.323–0.718) | <0.001* |
| Neoadjuvant chemotherapy Indicator: no |
1.259 (0.309–5.126) | 0.748 | 1.287 (0.176–9.398) | 0.803 |
| Neoadjuvant radiotherapy Indicator: no |
0.048 (0–29.994) | 0.355 | 0.049 (0–6709.6999) | 0.616 |
| Intraoperative | ||||
| Classical or PPPD Indicator: classical |
0.819 (0.542–1.239) | 0.344 | 0.635 (0.357–1.129) | 0.122 |
| Venous resection Indicator: no |
1.113 (0.687–1.803) | 0.664 | 1.083 (0.563–2.082) | 0.811 |
| Additional organ resection Indicator: no |
0.610 (0.224–1.666) | 0.335 | 0.266 (0.036–1.948) | 0.192 |
| Histopathological | ||||
| pT Indicator: pT1 |
1.408 (0.792–2.502) | 0.244 | 1.452 (0.692–3.048) | 0.324 |
| Lymph node ratio | 8.654 (4.038–18.549) | <0.001* | 16.928 (4.299–66.656) | <0.001* |
| Resection margin Indicator: R0 |
1.790 (1.161–2.762) | 0.008* | 2.291 (1.259–4.171) | 0.007* |
| Perineural invasion Indicator: no |
3.055 (1.239–7.533) | 0.015* | 2.881 (0.698–11.892) | 0.143 |
| Intravascular invasion Indicator: no |
1.960 (1.087–3.532) | 0.025* | 3.353 (1.040–10.808) | 0.043* |
| Postoperative | ||||
| Postoperative complications Indicator: no |
1.125 (0.746–1.696) | 0.575 | 0.933 (0.529–1.644) | 0.810 |
| Clavien–Dindo classification based on higher category recorded Indicator: grade I |
0.989 (0.832–1.175) | 0.897 | 0.926 (0.720–1.191) | 0.551 |
| Adjuvant chemotherapy Indicator: yes |
1.763 (1.120–2.775) | 0.014* | 2.052 (1.115–3.775) | 0.021* |
*P values are significant. ECOG, Eastern Cooperative Oncology Group; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack; CVA, cerebrovascular accident; PPP, pylorus preserving pancreatoduodenectomy; NA, not applicable.
In an effort to identify predictors of mortality that can be used to council octogenarian patients, separate multivariable analyses were performed within that subgroup for all parameters and for preoperative parameters only as shown in Table S1. Amongst preoperative parameters history of peptic ulcer disease (OR = 18.502; c.i. = 2.205–155.284; P = 0.007), preoperative use of steroids (OR = 2.440; c.i. = 1.105–5.389; P = 0.027) and preoperative albumin levels (OR = 0.528; c.i. = 0.330–0.844; P = 0.008) were independent predictors of OS. When the postoperative parameters were also included in the model, preoperative haemoglobin levels (OR = 0.653; c.i. = 0.475–0.898; P = 0.009), lymph node ratio (OR = 16.300; c.i. = 3.039–87.419; P = 0.001), type of resection (OR = 0.234; c.i. = 0.100–0.546; P = 0.001) and adjuvant chemotherapy (OR = 2.227; c.i. = 1.004–4.939; P = 0.049) were also significant predictors.
Disease-free survival
Table 3 shows the results of the univariable Cox regression analysis for DFS. Multivariable analysis identified history of angina/percutaneous coronary intervention (PCI)/coronary surgery (OR = 2.394; c.i. = 1.173–4.884; P = 0.016), preoperative albumin levels (OR = 0.625; c.i. = 0.446–0.876; P = 0.006), lymph node ratio (OR = 6.383; c.i. = 2.713–15.020; P < 0.001) and perineural invasion (OR = 7.022; c.i. = 1.684–29.281; P = 0.007) as independent predictors of DFS. Of note, age was not significant.
Table 3.
Univariable Cox regression analysis for disease-free survival
| Disease-free survival | ||||
|---|---|---|---|---|
| Parameters | Univariable analysis: whole cohort | Univariable analysis: octogenarians subgroup | ||
| OR (95% c.i.) | P | OR (95% c.i.) | P | |
| Preoperative | ||||
| Age Indicator: control; < 80 years |
1.070 (0.710–1.612) | 0.746 | — | — |
| Sex Indicator: female |
0.931 (0.618–1.404) | 0.735 | 1.202 (0.660–2.191) | 0.548 |
| ASA score Indicator: class 1 |
1.044 (0.720–1.515) | 0.819 | 0.638 (0.347–1.172) | 0.147 |
| ECOG performance status Indicator: grade 1 |
1.086 (0.744–1.586) | 0.668 | 1.160 (0.647–2.078) | 0.618 |
| ECOG groups Indicator: grades 0–1 |
2.217 (0.895–5.491) | 0.085 | 14.351 (3.707–55.552) | <0.001* |
| Diabetes Indicator: no |
1.496 (0.957–2.337) | 0.077 | 1.637 (0.838–3.199) | 0.149 |
| COPD Indicator: no |
0.953 (0.414–2.191) | 0.909 | 0.578 (0.079–4.232) | 0.590 |
| Congestive heart failure Indicator: no |
2.267 (0.710–7.234) | 0.167 | 3.056 (0.384–24.307) | 0.291 |
| Myocardial infarction Indicator: no |
1.539 (0.707–3.349) | 0.277 | 0.960 (0.295–3.125) | 0.946 |
| Prior PCI / previous coronary surgery / angina Indicator: no |
2.346 (1.167–4.717) | 0.017* | 2.071 (0.632–6.787) | 0.229 |
| Hypertension Indicator: no |
1.357 (0.892–2.065) | 0.154 | 1.218 (0.662–2.239) | 0.526 |
| Impaired sensorium Indicator: no |
2.955 (0.408–21.415) | 0.284 | NA | NA |
| Dementia Indicator: no |
19.769 (2.504–156.043) | 0.005* | NA | NA |
| Peripheral vascular disease Indicator: no |
1.485 (0.469–4.708) | 0.501 | 1.724 (0.407–7.291) | 0.459 |
| TIA / CVA Indicator: no |
1.965 (0.853–4.527) | 0.113 | 2.067 (0.731–5.843) | 0.171 |
| Neurological deficit Indicator: no |
0.049 (0–6E + 014) | 0.873 | NA | NA |
| Connective tissue disease Indicator: no |
0.550 (0.076–3.975) | 0.553 | 0.727 (0.098–5.371) | 0.755 |
| Peptic ulcer disease Indicator: no |
0.295 (0.041–2.119) | 0.225 | 0.049 (0–8E + 011) | 0.846 |
| Liver disease Indicator: no |
0.639 (0.157–2.598) | 0.531 | 0.046 (0–43.892) | 0.379 |
| Hypercoagulability Indicator: no |
2.287 (0.835–6.266) | 0.108 | 1.808 (0.556–5.876) | 0.325 |
| Chronic kidney disease Indicator: stage 1 |
1.000 (0.975–1.025) | 0.982 | 0.999 (0.975–1.025) | 0.961 |
| Biliary stent Indicator: no |
1.099 (0.718–1.684) | 0.663 | 0.608 (0.330–1.123) | 0.112 |
| Steroid use prior to operation Indicator: no |
1.030 (0.473–2.245) | 0.940 | 2.722 (1.128–6.569) | 0.026* |
| Preoperative haemoglobin | 0.911 (0.799–1.038) | 0.161 | 0.938 (0.765–1.151) | 0.540 |
| Preoperative bilirubin | 1.001 (0.999–1.002) | 0.568 | 1.000 (0.998–1.003) | 0.736 |
| Preoperative albumin | 0.708 (0.536–0.936) | 0.015* | 0.632 (0.410–0.972) | 0.037* |
| Neoadjuvant chemotherapy Indicator: no |
1.039 (0.256–4.228) | 0.957 | 1.275 (0.174–9.336) | 0.811 |
| Neoadjuvant radiotherapy Indicator: no |
0.457 (0.064–3.287) | 0.437 | 0.048 (0–7921.938) | 0.621 |
| Intraoperative | ||||
| Classical or PPPD Indicator: classical |
0.687 (0.453–1.042) | 0.078 | 0.807 (0.441–1.475) | 0.486 |
| Venous resection Indicator: no |
1.057 (0.652–1.713) | 0.823 | 0.741 (0.341–1.607) | 0.447 |
| Additional organ resection Indicator: no |
0.656 (0.241–1.787) | 0.410 | 0.913 (0.220–3.798) | 0.901 |
| Histopathological | ||||
| pT Indicator: pT1 |
1.431 (0.829–2.473) | 0.199 | 1.130 (0.575–2.221) | 0.722 |
| Lymph node ratio | 7.881 (3.725–16.674) | <0.001* | 20.937 (4.727–92.741) | <0.001* |
| Resection margin Indicator: R0 |
1.733 (1.129–2.660) | 0.012* | 0.807 (0.441–1.475) | 0.486 |
| Perineural invasion Indicator: no |
4.202 (1.541–11.455) | 0.005* | 5.200 (0.714–37.868) | 0.104 |
| Intravascular invasion Indicator: no |
2.599 (1.381–4.890) | 0.003* | 2.334 (0.912–5.973) | 0.077 |
| Postoperative | ||||
| Postoperative complications Indicator: no |
1.288 (0.853–1.944) | 0.228 | 1.201 (0.657–2.196) | 0.552 |
| Clavien–Dindo classification based on higher category recorded Indicator: grade I |
1.143 (0.964–1.355) | 0.124 | 1.162 (0.902–1.497) | 0.245 |
| Adjuvant chemotherapy Indicator: yes |
1.090 (0.672–1.767) | 0.727 | 0.791 (0.407–1.540) | 0.491 |
*P values are significant. ECOG, Eastern Cooperative Oncology Group; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack; CVA, cerebrovascular accident; PPP, pylorus preserving pancreatoduodenectomy; NA, not applicable.
In an effort to identify predictors of mortality that can be used to council octogenarian patients, separate multivariable analyses were performed within that subgroup for all parameters and for preoperative parameters only. Amongst preoperative parameters low ECOG (OR = 15.053; c.i. = 3.552–63.794; P < 0.001) and preoperative albumin levels (OR = 0.588; c.i. = 0.381–0.907; P = 0.016) were independent predictors of DFS. When the postoperative parameters were also included in the model, lymph node ratio (OR = 10.704; c.i. = 2.148–53.338; P = 0.004) was also a significant predictor as shown in Table S2.
Discussion
The elderly comprises the fastest expanding portion of Western society and this shift in the population demographics is depicted in the patients being diagnosed with and assessed for treatment for PDAC. Cancer outcomes have taken large strides as a result of centralization and high-volume units bringing together multidisciplinary expertise and advances in perioperative and oncological management18,19. These overall improvements in time-dependent mortality have also been observed in the elderly, where risk of mortality before and after 2000 has almost halved20. Nonetheless, the elderly population appears to be disadvantaged due to selection bias for aggressive oncological treatment pathways. The reason behind this is the concern over the fitness of this subset of patients and their ability to withstand the effects of systemic treatment and the stress of surgical resection. Furthermore, even if this is achieved, doubt still rests in any oncological benefit that may be produced in respect to the patients’ life expectancy in the 9th decade of their life. The elderly are at a higher risk of mortality from both non-PDAC cancers and non-cancer-related causes21,22. Therefore, the propriety of curative treatment needs to take the patient's remnant life expectancy, as well as quality of life, into consideration23. Discerning any survival benefit to octogenarians treated for PDAC from the current literature is difficult. This is due to the small proportion of the elderly patients being included within studies or where the age cut-off excludes a significant proportion of elderly patients24,25. A vast majority of the literature uses age cut-offs much lower than 80 years to define the ‘elderly’ and yet the median age of patients presenting with PDAC is 72 years, where only 7 per cent are under 50 years26–28.
The elderly are frequently affected by reduced physiological reserve from chronic co-morbidities29–31. Compounded by PDAC-induced malnutrition, this may result in frailty syndrome and thus a reduced capacity to withstand major stress such as undergoing a PD32. Evidence suggests that in well selected octogenarians, perioperative complications15,21–23 and 30-day and index admission mortality are comparable with younger controls15. Ninety-day mortality though was higher (9 per cent versus 3 per cent) and co-morbidities such as previous cerebrovascular event or history of dementia were identified as independent predictors, pointing to a general decline with possible failure to reverse this in the community after hospital discharge. On the contrary, age was not an independent predictor of mortality in any multivariable model15. Therefore, meticulous assessment and patient selection is of paramount importance when surgical treatment is considered33. Additional specialized tests, such as pulmonary function tests, cardiopulmonary exercise testing and echocardiography33,34, use of frailty scoring systems35 and assessment of sarcopenia36,37 should all be carefully considered in the preoperative assessment, accepting their limitations.
With regards to oncological outcomes, OS has been reported as shorter in elderly patients undergoing pancreatic resections, however, the treatment selection bias with regards to receiving standard therapies, such as venous resection and adjuvant chemotherapy, was also highlighted20. On the other hand, in subgroup analysis of cohorts that receive adjuvant treatment, no difference in survival was reported38. A study of nonagenarians showed that an OS of 20.4 months could be achieved with multimodal therapy, however, 70 per cent of the study group did not receive multimodal therapy39. In this study, OS was defined as the time from the date of diagnosis to death rather than from the date of surgery to death. The rationale behind this choice stands in the inclusion of patients who received preoperative chemotherapy, as well as taking into account the differences in the time between diagnosis and surgery or first treatment among the patients. Any disease progression or stability during this time is also a measure of disease biology that primarily affects OS. There was no significant difference in OS or DFS between octogenarians and controls. The recorded OS in the octogenarian subgroup of 20 months is consistent with the range of 15–30 months in the published literature40,41. Similarly, there is also no demonstrable difference in DFS between the elderly and younger groups where use of adjuvant therapy has been shown to be an independent prognostic variable41,42. On risk analysis, history of cardiac co-morbidities and low preoperative albumin were identified as independent predictors of both OS and DFS. High lymph node ratio and perineural were also identified as predictors of oncological outcomes (OS and DFS respectively). The significance of low preoperative albumin levels persisted after analysis of only preoperative variables. Low albumin levels may indicate a status of relative malnutrition which would predispose the patient to prolonged hospitalization and recovery43 and in turn reduce the opportunity to be offered any adjuvant treatment. Similarly, cardiac history may prevent or limit the use of systemic treatment due to the described chemotherapy-related cardiotoxicities44. The fact that elderly patients are in general less likely to receive any systemic treatment has been documented by various studies45 (possibly due to the concerns regarding tolerability of multiagent regimens), even though evidence supports their safety and efficacy resulting in comparable survival to younger cohorts8,46,47. Elderly patients are more likely to receive reduced doses of adjuvant chemotherapy and only in the presence of lymph-node-positive disease40, a treatment selection bias which is not present in younger patients22.
Limitations of this study include its retrospective nature and the consequent inability to also assess parameters that have not been captured such as frailty and quality of life, type of systemic treatment and follow-up. Details of cause of death after hospital discharge were lacking, hence the inability for any non-cancer-related mortality to be assessed. Nonetheless, this is a multicentre study over a 10-year interval on a large cohort of patients that are underrepresented in the published surgical literature for the management of PDAC. Matching for the extent of surgery was also utilized to account for any possible intraoperative selection bias for utilizing a more radical surgical technique.
In summary, octogenarians with PDAC of the head and uncinate process benefit from comparable oncological outcomes to younger patients with surgical treatment after careful preoperative assessment and patient selection, which should be the focus of future studies.
Supplementary Material
Contributor Information
Rupaly Pande, HPB and Liver Transplant Unit, Queen Elizabeth Hospital, Birmingham, UK.
Joseph A Attard, HPB and Liver Transplant Unit, Queen Elizabeth Hospital, Birmingham, UK.
Bilal Al-Sarireh, Department of Surgery, Morriston Hospital, Swansea, UK.
Ricky Harminder Bhogal, HPB Unit, Royal Marsden Hospital, London, UK.
Alexia Farrugia, Department of Surgery, University Hospitals Coventry and Warwickshire NHS trust, Coventry, UK.
Giuseppe Fusai, HPB and Liver Transplant Unit, Royal Free Hospital, London, UK.
Simon Harper, HPB Unit, Cambridge University Hospital, Cambridge, UK.
Camila Hidalgo-Salinas, HPB and Liver Transplant Unit, Royal Free Hospital, London, UK.
Asif Jah, HPB Unit, Cambridge University Hospital, Cambridge, UK.
Gabriele Marangoni, Department of Surgery, University Hospitals Coventry and Warwickshire NHS trust, Coventry, UK.
Matthew Mortimer, Department of Surgery, Morriston Hospital, Swansea, UK.
Michail Pizanias, HPB Unit, King’s College Hospital, London, UK.
Andreas Prachialias, HPB Unit, King’s College Hospital, London, UK.
Keith J Roberts, HPB and Liver Transplant Unit, Queen Elizabeth Hospital, Birmingham, UK.
Chloe Sew Hee, HPB Unit, Cambridge University Hospital, Cambridge, UK.
Fiammetta Soggiu, HPB and Liver Transplant Unit, Royal Free Hospital, London, UK.
Parthi Srinivasan, HPB Unit, King’s College Hospital, London, UK.
Nikolaos A Chatzizacharias, HPB and Liver Transplant Unit, Queen Elizabeth Hospital, Birmingham, UK.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data sets generated during and/or analysed during the present study are available from the corresponding author on reasonable request.
Author contributions
Rupaly Pande (Data curation, Formal analysis, Writing—original draft), Joseph Attard (Data curation, Formal analysis, Writing—review & editing), Bilal Al-Sarireh (Data curation, Writing—review & editing), Ricky Bhogal (Data curation, Writing—review & editing), Alexia Farrugia (Data curation, Writing—review & editing), Giuseppe Fusai (Data curation, Writing—review & editing), Simon Harper (Data curation, Writing—review & editing), Camila Hidalgo Salinas (Data curation, Writing—review & editing), Asif Jah (Data curation, Writing—review & editing), Gabriele Marangoni (Data curation, Writing—review & editing), Matthew Mortimer (Data curation, Writing—review & editing), Michael Pizanias (Data curation, Writing—review & editing), Andreas Prachalias (Data curation, Writing—review & editing), Keith Roberts (Data curation, Writing—review & editing), Chloe Sew Hee (Data curation, Writing—review & editing), Fiammetta Soggiu (Data curation, Writing—review & editing), Parthi Srinivasan (Data curation, Writing—review & editing) and Nikolaos Chatzizacharias (Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing—review & editing).
References
- 1. Amin S, Lucas AL, Frucht H. Evidence for treatment and survival disparities by age in pancreatic adenocarcinoma: a population-based analysis. Pancreas 2013;42:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gong J, Tuli R, Shinde A, Hendifar AE. Meta-analyses of treatment standards for pancreatic cancer. Mol Clin Oncol 2016;4:315–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujii T, Yamada S, Murotani K, Kanda M, Sugimoto H, Nakao Aet al. Inverse probability of treatment weighting analysis of upfront surgery versus neoadjuvant chemoradiotherapy followed by surgery for pancreatic adenocarcinoma with arterial abutment. Medicine (Baltimore) 2015;94:e1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JLet al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–2406 [DOI] [PubMed] [Google Scholar]
- 5. Kalisvaart M, Broadhurst D, Marcon F, Pande R, Schlegel A, Sutcliffe Ret al. Recurrence patterns of pancreatic cancer after pancreatoduodenectomy: systematic review and a single-centre retrospective study. HPB (Oxford) 2020;22:1240–1249 [DOI] [PubMed] [Google Scholar]
- 6. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski Ket al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–1481 [DOI] [PubMed] [Google Scholar]
- 7. Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman Jet al. 1423 Pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 2006;10:1199–1210 [DOI] [PubMed] [Google Scholar]
- 8. Kenig J, Richter P. Pancreatoduodenectomy due to cancer in the older population. Nowotwory J Oncol 2021;71:321–327 [Google Scholar]
- 9. Matsui Y, Hirooka S, Yamaki S, Kotsuka M, Kosaka H, Yamamoto Tet al. Assessment of clinical outcome of cholecystectomy according to age in preparation for the “silver tsunami”. Am J Surg 2019;218:567–570 [DOI] [PubMed] [Google Scholar]
- 10. Wu W, He X, Yang L, Wang Q, Bian X, Ye Jet al. Rising trends in pancreatic cancer incidence and mortality in 2000–2014. Clin Epidemiol 2018;10:789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–2765 [DOI] [PubMed] [Google Scholar]
- 12. Eurostat . https://ec.europa.eu/eurostat/databrowser/view/proj_19ndbi/default/table?lang=en (accessed August 2022)
- 13. Hulzebos EHJ, van Meeteren NLU. Making the elderly fit for surgery. Br J Surg 2016;103:463. [DOI] [PubMed] [Google Scholar]
- 14. Suurmeijer JA, Henry AC, Bonsing BA, Bosscha K, van Dam RM, van Eijck CHet al. Outcome of pancreatic surgery during the first six years of a mandatory audit within the Dutch pancreatic cancer group. Ann Surg 2022; DOI: 10.1097/SLA.0000000000005628[Epub ahead of print] [DOI] [PubMed]
- 15. Attard JA, Al-Sarireh B, Bhogal RH, Farrugia A, Fusai G, Harper Set al. Short-term outcomes after pancreatoduodenectomy in octogenarians: multicentre case-control study. Br J Surg 2021;109:89–95 [DOI] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499 [DOI] [PubMed] [Google Scholar]
- 17. Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 2009;11:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balzano G, Guarneri G, Pecorelli N, Paiella S, Rancoita PMV, Bassi Cet al. Modelling centralization of pancreatic surgery in a nationwide analysis. Br J Surg 2020;107:1510–1519 [DOI] [PubMed] [Google Scholar]
- 19. Latenstein AEJ, Mackay TM, van der Geest LGM, van Eijck CHJ, de Meijer VE, Stommel MWJet al. Effect of centralization and regionalization of pancreatic surgery on resection rates and survival. Br J Surg 2021;108:826–833 [DOI] [PubMed] [Google Scholar]
- 20. Tan E, Song J, Lam S, D’Souza M, Crawford M, Sandroussi C. Postoperative outcomes in elderly patients undergoing pancreatic resection for pancreatic adenocarcinoma: a systematic review and meta-analysis. Int J Surg 2019;72:59–68 [DOI] [PubMed] [Google Scholar]
- 21. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 2010;58:783–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burgdorf SK, Storkholm JH, Chen IM, Hansen CP. Postoperative and long-term survival in relation to life-expectancy after pancreatic surgery in elderly patients (cohort study). Ann Med Surg (Lond) 2021;69:102724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Søreide K, Wijnhoven BPL. Surgery for an ageing population. Br J Surg 2016;103:e7–e9 [DOI] [PubMed] [Google Scholar]
- 24. von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore Met al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol 2012;30:2036–2038 [DOI] [PubMed] [Google Scholar]
- 26. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Yet al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825 [DOI] [PubMed] [Google Scholar]
- 27. Khalaf N, El-Serag HB, Abrams HR, Thrift AP. Burden of pancreatic cancer: from epidemiology to practice. Clin Gastroenterol Hepatol 2021;19:876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699–708 [DOI] [PubMed] [Google Scholar]
- 29. Sperti C, Moletta L, Pozza G. Pancreatic resection in very elderly patients: a critical analysis of existing evidence. World J Gastrointest Oncol 2017;9:30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wagner D, Büttner S, Kim Y, Gani F, Xu L, Margonis GAet al. Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. Br J Surg 2016;103:e83–e92 [DOI] [PubMed] [Google Scholar]
- 31. Audisio RA. Tailoring surgery to elderly patients with cancer. Br J Surg 2016;103:e10–e11 [DOI] [PubMed] [Google Scholar]
- 32. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moran J, Wilson F, Guinan E, McCormick P, Hussey J, Moriarty J. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth 2016;116:177–191 [DOI] [PubMed] [Google Scholar]
- 34. Shim CY. Preoperative cardiac evaluation with transthoracic echocardiography before non-cardiac surgery. Korean J Anesthesiol 2017;70:390–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katz M, Silverstein N, Coll P, Sullivan G, Mortensen EM, Sachs Aet al. Surgical care of the geriatric patient. Curr Probl Surg 2019;56:260–329 [DOI] [PubMed] [Google Scholar]
- 36. Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck Met al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 2012;23:2166–2172 [DOI] [PubMed] [Google Scholar]
- 37. Kallogjeri D, Piccirillo JF, Spitznagel EL, Steyerberg EW. Comparison of scoring methods for ACE-27: simpler is better. J Geriatr Oncol 2012;3:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin SH, Park Y, Hwang DW, Song KB, Lee JH, Kwon Jet al. Prognostic value of adjuvant chemotherapy following pancreaticoduodenectomy in elderly patients with pancreatic cancer. Anticancer Res 2019;39:1005–1012 [DOI] [PubMed] [Google Scholar]
- 39. Meltzer RS, Kooby DA, Switchenko JM, Datta J, Carpizo DR, Maithel SKet al. Does major pancreatic surgery have utility in nonagenarians with pancreas cancer? Ann Surg Oncol 2021;28:2265–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Geest LGM, Besselink MGH, Busch ORC, de Hingh IHJT, van Eijck CHJ, Dejong CHCet al. Elderly patients strongly benefit from centralization of pancreatic cancer surgery: a population-based study. Ann Surg Oncol 2016;23:2002–2009 [DOI] [PubMed] [Google Scholar]
- 41. Turrini O, Paye F, Bachellier P, Sauvanet A, Sa Cunha A, le Treut YPet al. Pancreatectomy for adenocarcinoma in elderly patients: postoperative outcomes and long-term results: a study of the French Surgical Association. Eur J Surg Oncol 2013;39:171–178 [DOI] [PubMed] [Google Scholar]
- 42. Frakes JM, Strom T, Springett GM, Hoffe SE, Balducci L, Hodul Pet al. Resected pancreatic cancer outcomes in the elderly. J Geriatr Oncol 2015;6:127–132 [DOI] [PubMed] [Google Scholar]
- 43. Chang EH, Sugiyama G, Smith MC, Nealon WH, Gross DJ, Apterbach Get al. Obesity and surgical complications of pancreaticoduodenectomy: an observation study utilizing ACS NSQIP. Am J Surg 2020;220:135–139 [DOI] [PubMed] [Google Scholar]
- 44. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CMet al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;387:1011–1024 [DOI] [PubMed] [Google Scholar]
- 45. Daiku K, Ikezawa K, Morishima T, Kai Y, Takada R, Yamai Tet al. Chemotherapy effectiveness and age-group analysis of older adult patients with metastatic pancreatic cancer: a Japanese cancer registry cohort study. J Geriatr Oncol 2022;13:1208–1215 [DOI] [PubMed] [Google Scholar]
- 46. Oba A, Wu YHA, Lieu CH, Meguid C, Colborn KL, Beaty Let al. Outcome of neoadjuvant treatment for pancreatic cancer in elderly patients: comparative, observational cohort study. Br J Surg 2021;108:976–982 [DOI] [PubMed] [Google Scholar]
- 47. Baldini C, Escande A, Bouché O, El Hajbi F, Volet J, Bourgeois Vet al. Safety and efficacy of FOLFIRINOX in elderly patients with metastatic or locally advanced pancreatic adenocarcinoma: a retrospective analysis. Pancreatology 2017;17:146–149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analysed during the present study are available from the corresponding author on reasonable request.