Abstract
Poorly differentiated gastroenteropancreatic neuroendocrine carcinomas are aggressive neoplasms of challenging clinical management. A small proportion of patients with early-stage disease may achieve long-term survival, but the majority of patients present with rapidly lethal metastatic disease. Current standard of care still follows the treatment paradigm of small cell lung cancer, a far more common G3 neuroendocrine neoplasm, although emerging molecular and clinical data increasingly question this approach. In this article, we will briefly summarize epidemiology and prognosis of gastroenteropancreatic neuroendocrine carcinomas to emphasize the very low incidence, aggressive nature, and orphan status of this tumor entity. We will also discuss the current pathological classification and its limitations, as well as recent data on their differential biological background compared with small cell lung cancer, and its potential implications for patients care. Then, we will review the standard of care of systemic therapy, basically focused on platinum-based cytotoxic chemotherapy, including some recent randomized trials providing evidence regarding efficacy of irinotecan vs etoposide platinum doublets. Finally, we will present a comprehensive overview of novel therapeutic strategies in current clinical development, including recently reported data on immunotherapy, tumor-agnostic therapies (microsatellite instability, high tumor mutational burden, NTRK and RET gene fusions, BRAF or KRAS inhibitors), and additional treatment strategies targeting other tumor vulnerabilities (ie, Notch pathway, novel targets for radioligand therapy), and provide some insights regarding unmet needs and future perspectives to improve patient's care and prognosis.
Keywords: neuroendocrine carcinomas, G3, therapy, gastroenteropancreatic
Graphical Abstract
Graphical Abstract.
Essential Points.
Poorly differentiated gastroenteropancreatic neuroendocrine carcinomas (GEP NECs) are rare tumors with limited treatment options and a dismal prognosis
Some patients with early-stage GEP NECs may achieve long-term survival with surgery or multimodality therapy, but most present or eventually develop rapidly lethal metastatic disease
Systemic chemotherapy (etoposide- vs irinotecan-platinum doublets) is the mainstay of care in the metastatic setting, though responses are generally short lasting
Standard of care shall shift from the small cell lung cancer treatment paradigm because emerging data points out major biological differences between GEP and lung NECs, and even within different GEP sites, suggesting site-specific approaches should be explored
The role of immunotherapy in GEP NECs is still a matter of debate; better understanding of the immune landscape of tumors and their interaction with the host is needed to improve patient selection and most adequate strategy
More than 20% of GEP NECs harbor potentially druggable molecular alterations (involving DNA damage repair, cell-cycle regulation, Notch or MAPK pathways, NTRK or RET gene fusions, among others) that should shift the treatment paradigm toward a more personalized, molecularly driven therapy that could eventually improve patient's survival
High-grade (G3, Ki-67 > 20%) neuroendocrine neoplasms (NENs) are rare aggressive tumors with a dismal prognosis (1–9). Only 9% are extrapulmonary, and 3% are of gastroenteropancreatic (GEP) origin. The majority are poorly differentiated small or large cell neuroendocrine carcinomas (NECs), but a small subset is well-differentiated tumors (neuroendocrine tumors [NETs]) and have a different biology and less ominous clinical course. Diagnosis and treatment are challenging because of the lack of high-quality data. Most patients are diagnosed with advanced disease, and systemic therapy is the mainstay of care. Treatment strategies are commonly adopted from those of high-grade lung NECs given their histological and clinical resemblance, with platinum/etoposide-based regimens recommended in the first-line setting (1–3). Recent evidence, however, indicates some significant differences in the molecular background of these tumors according to the anatomical site of origin, with potentially relevant therapeutic implications. Indeed, whereas TP53 and RB1 genomic alterations are almost universal in small cell lung carcinomas (SCLC), this is not the case for GEP NECs, particularly the more common large cell subtype (4, 5). Frequently mutated genes in GEP NECs include TP53, APC, KRAS, and BRAF, whereas RB1 copy number alterations are more common than mutations (<15%). MEN1, ATRX, DAXX, and SETD2 mutations may be typically identified in G3 GEP NETs, but a small proportion of well-differentiated tumors may also harbor TP53 mutations. More importantly, more than 20% of GEP NECs have potentially targetable genomic alterations (eg, DNA damage repair, BRAF/KRAS mutations, microsatellite instability)(5). Therefore, optimal therapy for GEP NECs may be site-specific and different from SCLC. But the biological background is still poorly understood, and further fundamental research is needed for progress to be made. Specific GEP NEC trials will be conducted taking into account the organ of origin, histological subtype, and proliferation index, and will incorporate comprehensive tumor molecular profiling to identify predictive biomarkers that foster personalized medicine. The aim of this manuscript is to review the state of the art of treatment strategies for patients with advanced high-grade GEP NECs, to discuss novel therapeutic approaches recently assessed in clinical trials including immunotherapy, radioligand and other targeted therapies, and to provide some insights regarding unmet needs and future perspectives to improve patient's care and prognosis.
Epidemiology and Prognosis
High-grade (G3, Ki-67 > 20%) NENs are rare aggressive tumors with a dismal prognosis. The incidence of high-grade NENs is 0.6 to 1 new cases/105 habitants/year according to the largest published US and European population-based registries (6–8). Only 9% are extrapulmonary, one-third of which are of GEP origin (9). Regarding risk factors, there are also some differences with lung primaries because GEP NECs are not related to tobacco exposure (with the only exception being esophageal NECs) and some primary sites are related to human papillomavirus (rectal and anal canal). The great majority of lung NECs have small cell morphology (95%), whereas gastrointestinal NECs are more commonly (61%) of large cell (LC) morphology, except for esophageal and anal canal primaries. Less than 20% are diagnosed with stage I-II disease, and up to 65% of patients present metastatic disease at diagnosis. Brain metastases are common for lung NECs but occur in <2% of GEP NECs. Prognosis is overall poor but is better for GEP than lung primaries and large vs small cell NECs (5-year overall survival [OS] of 32%, 15%, and 6% for LC-GEP, LC-lung, and any site small cell NECs, respectively) (10). Within the GEP tract, most common primary sites are the pancreas (19%), colon (18%), esophagus (18%), and rectum (16%). About 25% to 30% of patients undergo surgical resection with curative intent, and their OS at 5 years ranges from 20% to 40% (7, 11). Prognosis within the GEP tract also varies by primary tumor location, with small bowel and esophageal NECs having the best and worst survival rates, respectively (9, 12). A small subset of G3 NENs (∼10%) are well-differentiated tumors (G3 NETs) and have a different biology and less ominous clinical course (13).
Pathological Classification: Implications for Patient's Care
The wide anatomical distribution of NENs has led to the development of multiple, not always consistent, organ-dependent classifications of these neoplasms, giving place to complex and sometimes confusing nomenclature. The World Health Organization (WHO) has progressively evolved toward a universal classification based on tumor differentiation and grade based on proliferation rate, and the most recent 2022 WHO Classification has, for the first time, included both endocrine and neuroendocrine tumors also of nonendocrine organs (14). Briefly, based on tumor differentiation, NENs are first stratified in well-differentiated tumors (NETs) and poorly differentiated carcinomas (NECs). Then, based on proliferation index, NETs are further subdivided in grade 1 (Ki-67 < 3%), grade 2 (Ki-67 3-20%), and grade 3 (Ki-67 > 20%), whereas NECs are always grade 3. Although this could seem quite simple and straightforward, morphological criteria to discriminate NETs from NECs are not well standardized, and significant discrepancies exist even among expert pathologists, who recommend considering additional clinical and molecular data to help in the differential diagnosis of these 2 entities. A prior or coexisting NET component would suggest a G3 NET, whereas a coexisting adenocarcinoma or squamous cell carcinoma would suggest a NEC or a mixed neuroendocrine-nonneuroendocrine neoplasm (MINEN). NECs do not resemble their nonneoplastic cell counterparts and have a solid growth pattern, often with abundant geographic necrosis and severe cell polymorphism, high nuclear to cytoplasm ratio, and frequent mitoses. NECs generally have reduced or absent hormone secretion and somatostatin receptor expression (though not always) (15–17). There are 2 different morphological subtypes of NECs, small cell and large cell NECs, and increasing evidence suggests they may have different pathogenetic pathways and clinical behavior. Whereas the small cell morphology is almost universal in lung NECs (95%), large cell is the more common GEP NEC (61%), particularly in small and large bowel NECs (9). The use of a panel of immunohistochemical markers is recommended for diagnosis. Positivity for cytokeratin and at least 1 general neuroendocrine marker (2 highly advisable for LC-NECs) is required for the diagnosis of NECs. The most commonly used are synaptophysin, chromogranin A, and INSM1 because enolase and PGP9.5 are highly nonspecific and may be misleading. CD56 should be interpreted with caution and is not to be used as a single marker. Very importantly, if morphological criteria for NENs are not met, positivity of general neuroendocrine markers is not sufficient to establish the diagnosis of NECs. The WHO classification also indicates that lack of pRb (protein encoded by the RB1 gene) immunostaining or overexpression or global loss of p53 are common in NECs and may help in the differential diagnosis of LC-NECs from G3 NETs. However, recent next generation sequencing data challenge this recommendation in GEP NECs because pRb mutations are particularly much less commonly encountered than in lung primaries (5).
Adequate pathological and molecular assessment is essential for optimal patient care. Indeed, discriminating G3 NETs from NECs (particularly LC-NECs) has relevant biological, prognostic, and therapeutic implications (16, 18). NET G3 have generally molecular alterations common to low-grade NETs, including loss of DAXX or ATRX expression (pancreatic NETs) and lack of p53/pRb alterations. More often, they can secrete hormones and express somatostatin receptors (15–17) and may thus benefit from radioligand therapy (RLT), and they have better survival than NECs but respond poorly to platinum-based chemotherapy (19). Thus, management of G3 NETs tends to resemble that of high-G2 NETs and will not be discussed in this review.
Molecular Profiling of GEP NECs
Small cell NECs, more commonly encountered within the GEP tract in the esophagus, gall bladder, and anal canal, are generally molecularly more homogeneous and, similarly to SCLC, are often characterized by biallelic inactivation of TP53 and RB1. On the contrary, large cell NECs are molecularly more complex and heterogeneous, often resembling nonneuroendocrine tumors of similar anatomic site (ie, KRAS mutations in pancreatic NECs, BRAF mutations in right colon NECs) (4, 5, 20). In fact, up to 40% of large cell NECs may harbor a nonneuroendocrine component, suggesting a common clonal precursor. Differential diagnosis of large cell NECs from G3 NETs is often challenging, and molecular profiling may be an important aid in this context. Progression from a NET to an NEC is extremely rare because NECs generally originate de novo or from a nonneuroendocrine epithelial cancer through a phenomenon known as lineage plasticity, as has been documented in lung or prostate adenocarcinomas under selective pressure of targeted therapies (anti-EGFR tyrosine kinase inhibitors or antiandrogens) (4).
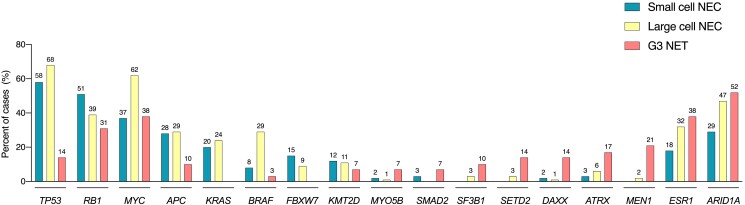
The WHO classification of digestive tumors (2019) and classification of endocrine and neuroendocrine tumors (2022) point out TP53 and RB1 mutations as main differential molecular markers to discriminate NECs from high-grade NETs (14, 21). However, recent data indicate the incidence of these mutations in GEP NECs, particularly in those of large cell morphology, is much lower than that observed in lung NECs, and they may also occur in a small proportion of G3 NETs (Fig. 1). Indeed, in 1 of the largest reported molecularly profiled series of G3 GEP NENs (N = 181), TP53 mutations were observed in 64% of NECs vs 14% of NETs, whereas RB1 mutations were rare (14% of NECs) but copy number alterations (losses or deletions) affecting RB1 were common in both NECs (34%) and NETs (31%) (5). Other frequently encountered gene copy number losses were ARID1A (35%), ATM (31%), and ESR1 (25%), and common gene amplifications were found in MYC (51%) and KDM5A (45%). Of note, gene mutations associated with the nonneuroendocrine counterparts could better discriminate NECs from NETs, including BRAF (20% vs 3%) or KRAS (22% vs 0%) mutations, particularly in certain anatomic sites (ie, BRAF mutations occur in 49% of colon NECs and 70% if located in the right colon; FBXW7 mutations were seen in 25% of rectal cancers). In fact, a 4-gene classifier including TP53, APC, KRAS, and BRAF mutations was found to yield 92% sensitivity, 61% specificity, and 83% positive predictive value for discerning large cell NECs from G3 NETs. Moreover, specificity (89%) and positive predictive value (96%) were further improved if 5 additional genes were included in a 9-gene model (TP53, APC, KRAS, BRAF, ATRX, DAXX, MEN1, MYOB5B, and SMAD2). These are interesting findings that, if validated, could be potentially implemented in clinical practice.
Figure 1.
Common genomic alterations in GEP NECs and G3 NETs. Bar plots depict the frequency of samples harboring any genomic alteration (point mutations, copy number gains or losses, amplifications or deletions) for the most frequently mutated genes in small cell NECs, large cell NECs, and G3 NETs of GEP origin (5). G3 NET, grade 3 neuroendocrine tumor; GEP: gastroenteropancreatic; NEC: neuroendocrine carcinoma.
Very interestingly, microsatellite instability (MSI) has been documented in ∼5% to 12% of GEP NECs and even in ∼3% of G3 NETs (5, 18, 22–24). As in colorectal adenocarcinoma, cooccurrence with BRAF mutations is common. Because both MSI and BRAF mutations are potentially targetable with drugs approved for tumor agnostic indications, their routine testing should be considered in patients with advance disease, at least in those of colonic origin. Overall, at least 20% of NECs may have these and other druggable molecular alterations (ie, deficient DNA damage repair) and, thus, molecular profiling is highly recommended if available in this setting.
MSI is an established predictive marker of response to immunotherapy because it is the consequence of deficient DNA mismatch repair that leads to highly mutated and immunogenic tumors. A high tumor mutational burden (TMB-H) may also occur through other mechanisms and is also associated with sensitivity to immunotherapy. Although up to 20% of G3 NENs have been reported to be TMB-H, this seems less common in those of GEP origin, with median TMB generally below 10 mutations/megabase (5, 25).
Disruption of epigenetic regulation also plays an important role in GEP NECs, with the AT-Rich Interaction Domain 1A (ARIDA1A), histone lysine methyltransferase 2 (KMT2), and histone lysine demethylase (KMD) family genes being the most frequently affected (5, 26–29). Methylation profiles seem to differ by primary tumor site, with highly methylated tumors located in the stomach, intestine, and gallbladder (30–32). Interestingly, high methylation levels are associated with MSI phenotype, as frequently methylated genes in MSI NECs include MLH1 (23). Other commonly methylated genes include MGMT and RASSF1A, particularly in foregut NECs. Methylation analysis also clearly discriminated NECs from adenocarcinomas in different primary sites, such as the pancreas or colorectum (31, 33, 34). Phylo-epigenetic and cell-type signature features derived from alpha, beta, acinar, and ductal adult cells suggest an exocrine cell of origin for pancreatic NECs, thus separating them in cell lineage from other pancreatic NENs of endocrine origin. Genes differentially methylated in colorectal NECs (co-NECs) were involved in immune cell differentiation, cytoskeleton dynamics and cell polarity, DNA damage, or apoptosis. Of note, genes typically expressed in neuroendocrine cells, such as somatostatin and other neurotransmitters, were highly methylated in colorectal adenocarcinomas. Moreover, EGFR methylation was also more commonly encountered in co-NECs than in adenocarcinomas, and EGFR methylation status and its related protein expression was directly correlated with response and acquired resistance to BRAF inhibitors in patient-derived xenograft models of co-NECs. Finally, recently published data by Kawasaki et al have also reported mutations in epigenetic modifier genes and promoter demethylation of key neuroendocrine transcription factors in GEP NEC organoids, further supporting the relevance of epigenetic alterations in the development of these neoplasms and as a potential therapeutic target that deserves to be further explored (35).
Standard of Care of GEP NECs: How Far Can We Go With Chemotherapy?
Surgery with curative intent is usually recommended for patients with locoregional disease because large retrospective series suggest it significantly improves survival regardless of primary tumor site and perioperative therapies (36). In patients with important comorbidities or in anatomical sites where surgical resection is associated with high morbidity (ie, anal canal, esophagus), radiotherapy may be the local treatment of choice, generally combined with chemotherapy (2). Based on the high risk of systemic relapse after primary tumor resection, platinum-based adjuvant chemotherapy is generally indicated following surgery in patients with localized NECs, although evidence to support this common practice is rather weak. Moreover, retrospective data suggest the potential added value of adjuvant chemotherapy may differ depending on pathological features (greater benefit in T3-T4, node positive, or positive resection margins) and primary tumor site (greater benefit reported in esophageal and colorectal primaries; no clear benefit in gastric, small bowel, or pancreatic NECs) (37–41). Consistent with this, treatment patterns generally differ by age, stage, morphology, and primary tumor location (36). Some experts/guidelines also support the use of neoadjuvant chemotherapy with or without radiotherapy and resection in selected patients with locally advanced disease, potentially enabling less aggressive surgery or avoiding major surgery in high-risk patients who experience early progression (1, 42). However, no randomized trials have properly assessed to date the role of adjuvant or neoadjuvant chemotherapy and/or radiotherapy in this setting. Nevertheless, a relevant proportion of patients with locoregional GEP NECs achieve long-term survival generally with bi- or trimodality therapy, with 5-year survival rates of 40% to 50% for patients with gastric, pancreatic, or colorectal primaries, and of 25% for those with esophageal NECs (9). Long-term cure therefore seems achievable in some patients.
In summary, surgery is generally recommended for fit patients with localized or locally advanced resectable GEP NECs, and adjuvant platinum-etoposide may be considered. Exceptions include anatomical sites where surgical resection is highly morbid, such as esophageal or anal canal NECs, in which case chemoradiation is the preferred therapeutic approach. There is less consensus regarding optimal management of resectable distal rectal NECs. Neoadjuvant chemotherapy or chemoradiotherapy may be considered before surgery, but treatment decisions should be individualized and discussed within a multidisciplinary team.
However, more than 60% to 70% of patients with GEP NECs present metastatic disease at diagnosis and have a very poor prognosis, with a median OS of up to 12 months with best available therapy, and of barely 1 month in patients who only receive supportive care (12, 13, 43). Evidence to support treatment recommendations in GEP NECs is scarce, and clinical guidelines have been primarily based on the more solid data available from SCLC studies, even though there are important etiological, clinical, and molecular differences between these 2 entities, as has been discussed (4, 9). The standard of care for patients with advanced unresectable or metastatic GEP NECs is systemic chemotherapy, provided the patients have adequate performance status and organ function. Cisplatin or carboplatin in combination with etoposide are the most widely used chemotherapy regimens, although objective response rates (ORRs; 28%-48%) are generally lower than those reported for SCLC (9, 12, 43–45). Carboplatin is generally preferred over cisplatin because it has similar antitumor activity and better toxicity profile, with no nephro- nor neurotoxicity (12). Response to chemotherapy is lower in NECs with Ki-67 < 55% (15% vs 42%), although they have better survival than patients with Ki-67 ≥ 55% (14 vs 10 months) (12). Metastatic colorectal NECs have a particularly dismal prognosis, with more than 50% experiencing early disease progression with standard chemotherapy and a median survival of about 8 months. Some experts advocate the use upfront of fluoropyrimidine-based regimens with irinotecan or oxaliplatin in large cell GEP NECs, particularly if they are associated with a non-neuroendocrine component (MINEN). An ongoing randomized trial (FOLFIRINEC) is currently assessing FOLFIRINOX vs platinum-etoposide in NECs of GEP or unknown origin. Two randomized trials conducted in Asian population with GEP NECs have compared cisplatin and etoposide with cisplatin and irinotecan, demonstrating similar ORR (53%-63%), progression-free survival (PFS; 5.1-6.4 months), and OS (10.2-12.5 months) for both treatment regimens (Table 1) (46, 47). Substituting irinotecan for etoposide is therefore an alternative treatment option for these patients that is associated with less myelosuppression although a greater incidence of diarrhea. More recently, a small, randomized trial conducted in the United States compared platinum and etoposide vs CAPTEM in non–small cell GEP-NENs (including NETs and NECs), and was closed for futility, concluding CAPTEM was better tolerated but not associated with increased efficacy (Table 1) (48). No subgroup analysis has been provided yet by tumor differentiation or Ki-67 index in this trial.
Table 1.
Randomized trials of chemotherapy in GEP NECs
| Trial and author | N | Primary site | Treatment | Primary Endpoint | ORR (%) | PFS (median) | OS (median) |
|---|---|---|---|---|---|---|---|
| First-line therapy | |||||||
|
TOPIC NEC
Morizane et al |
170 | GEP (100 GI, 70 HBP) |
CDDP + etoposide CDDP + irinotecan |
OS | 54.5% 52.5% |
5.6 mo 5.1 mo |
12.5 mo 10.9 mo |
| Zhang et al | 66 | GEP | CDDP + etoposide CDDP + irinotecan |
ORR | 63.2% 61.5% |
6.4 mo 5.8 mos |
11.3 mo 10.2 mo |
| Second-line therapy | |||||||
|
NET-02
McNamara et al |
58 | GEP (40) Other EP NECs (11) UKP NECs (7) |
Nal-IRI + 5FU docetaxel |
6 mo | 10.3% 10.3% |
3 mos (31% 6-mo PFS) 2 mo (14% 6-mo PFS) |
9.0 mo 5.0 mo |
|
PRODIGE 41 (BEVANEC)
Walter et al |
126 | GEP UKP |
FOLFIRI + Beva FOLFIRI |
6 mo OS | 25.5% 18.3% |
3.7 mo 3.5 mo |
7.0 mo (52.5% 6- mo OS) 8.9 mo (58.2% 6-mo OS) |
Abbreviations: CDDP, cisplatin; EP-NEC, extrapulmonary neuroendocrine carcinoma; GEP, gastroenteropancreatic; GI, gastrointestinal; HBP, hepatobiliopancreatic; IRI, irinotecan; NEC, neuroendocrine carcinoma; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
The efficacy of chemotherapy in the refractory setting (second line and beyond) is very limited and survival is short (∼9 months with best available therapy). Although second-line regimens have not been extensively evaluated either, emerging data from recent trials consolidate infusional 5-FU and irinotecan (standard or pegylated liposomal formulation) (FOLFIRI or nal-IRI-5FU) as the treatment of choice in this setting (Table 1) (49, 50). The PRODIGE 41-BEVANEC phase 2 trial randomized 126 patients with advanced NECs of GEP or unknown origin, after failure to first-line platinum etoposide, to receive FOLFIRI (control arm) vs FOLFIRI with bevacizumab (experimental arm) (25). The trial reached its primary endpoint (6-month OS >50% in the experimental arm), but the addition of bevacizumab did not seem to improve PFS nor OS over FOLFIRI alone. A smaller phase 2 randomized, noncomparative study (NET-02) included 58 patients with extrapulmonary NECs (40 were of GEP origin) that were allocated to receive nal-IRI-5FU vs docetaxel as second-line therapy on progression to first-line platinum-based therapy (49). Liposomal irinotecan-5FU, but not docetaxel, met the primary endpoint with a 31% 6-month PFS rate that exceeded the prespecified threshold for efficacy. ORR were similar in both arms, and OS also favored the nal-IRI-5FU arm. This outcome is consistent with that reported in the BEVANEC trial for the FOLFIRI control arm, and both studies provide the most solid evidence available to date on salvage chemotherapy in NECs. Other treatment options include oxaliplatin- or temozolomide-based regimens, although evidence derives from retrospective series or tumor registries (2, 12, 43, 45).
In conclusion, carboplatin and etoposide is the preferred first-line treatment option for patients with metastatic GEP NECs. Irinotecan and cisplatin are an alternative treatment regimen that may be considered for patients with poor bone marrow reserve or who at risk of recurrent or severe infections because it has demonstrated similar efficacy and is associated with less myelosuppression. In the second-line setting, on failure of platinum-based chemotherapy, FOLFIRI is the preferred chemotherapy schedule based on best available evidence. Alternative regimens in the refractory setting include combinations of fluoropyrimidines with oxaliplatin or temozolomide, although efficacy is rather limited for any therapy in this context.
Theragnostics in NECs: Does it Play any Role?
177Lutetium-DOTATATE is currently approved for the treatment of advanced, progressive, somatostatin receptor-positive well-differentiated G1-2 GEP NETs, though emerging evidence suggests it could also be effective in some G3 NENs. Consistent data from several noncontrolled studies reported ORR in G3 NETs or low-G3 NECs (Ki-67 < 55%) of 42% to 43%, with immediate disease progression more common in lowG3-NEC than in G3 NETs (26 vs 7%), and much higher in NECs with Ki-67 > 55% (45%) (51). PFS was 19, 11, and 4 months for G3 NETs, lowG3-NECs, and highG3-NECs, and OS 44, 22, and 9 months, respectively. Therefore, RLT may be considered in G3 NETs or lowG3-NECs homogeneously expressing somatostatin receptors.
Two ongoing randomized trials are currently assessing the role of somatostatin receptor-targeted radioligand therapy in somatostatin receptor-positive, low G3 GEP NENs. The NETTER-2 trial (NCT03972488) aims to determine if Lu177-oxodotreotide in combination with standard doses of long-acting octreotide (30 mg) prolongs PFS in advanced G2-3 GEP NETs (Ki-67 10%-55%), compared with treatment with high-dose (60 mg), long-acting octreotide. The COMPOSE trial (NCT04919226) is currently recruiting patients with G2-3 GEP NETs (Ki-67 15%-55%) that are randomized to 177Lu-edotreotide vs investigator's choice of standard chemotherapy (CAPTEM or FOLFOX) as first- or second-line therapy in the advanced setting. Both trials will provide relevant information regarding safety and efficacy of peptide receptor radionuclide therapy (PRRT) relative to other treatment options in this setting and will help define the role of PRRT in the therapeutic algorithm of G2-3 GEP NENs.
Other potential theragnostic targets being explored in high-grade NENs include C-X-C motif chemokine receptor-4 and urokinase plasminogen activator receptor (uPAR) (52, 53). Overexpression of CXCR4 has been documented in SST2-negative, high-grade NENs. 68Ga-Pentixafor show reliable assessment of CXCR4 expression, thereby identifying potential candidates for CXCR4-targeted RLT with 177Lu/90Y-pentixather (52). The main pitfall of this approach is that CXCR4 is also highly expressed in hematopoietic stem cells, making myelotoxicity a dose-limiting factor for therapy.
uPAR is a cell membrane receptor part of the plasminogen activation system, which is physiologically involved in tissue reorganization events (ie, wound healing). uPAR interacts with urokinase plasminogen activator, integrins, and other proteins, and through cross-talk with tyrosine kinase receptors regulates the shift between tumor dormancy and proliferation and promotes cell motility and invasion and angiogenesis. It is overexpressed in many human cancers, including high-grade NENs and correlates with early invasion and metastasis and a poor prognosis. 68Ga-NOTA-AE105 positron emission tomography (PET) was explored in a phase 2 trial to image uPAR in 99 patients with G1-3 NENs, and uPAR-positive lesions were seen in both low-grade (57% G1, 69% G2) and high-grade NENs (75%) (53). A high uPAR expression was associated with a worse PFS and OS. uPAR PET may thus be useful for risk stratification of patients in the clinic and as a potential new target for RLT in patients with NENs.
Novel Therapeutic Strategies
Immunotherapy
The immune system plays a pivotal role in cancer prevention, development, and progression, and immune checkpoint inhibition (ICI) has changed the treatment paradigm of many cancer types over the past decade, including some high-grade NENs such as Merkel cell carcinoma (54) or small cell lung cancer (55, 56). Nevertheless, the role of immunotherapy remains controversial in G3 digestive NENs.
Single-agent PD-1 or PD-L1 blockade (ie, pembrolizumab, spartalizumab, avelumab) has shown to be mostly ineffective (ORR 3%-7%, PFS 1.8-4 months, OS 5.1-7 months) in molecularly unselected, heavily pretreated patients with advanced GEP NECs (57–59) (Table 2). The only exception was toripalimab, which reported an ORR of 20% in G2high-G3 NENs (primarily GEP NECs), with a median duration of response of 15.1 months (60). Interestingly, ORR was significantly higher in patients with PD-L1 expression ≥10% or TMB-H than PD-L1 < 10% (50.0% vs 10.7%, P = .019) and TMB-low patients (75.0% vs 16.1%, P = .03), and 3 of the 8 (37.5%) responders harbored ARID1A mutations. ARID1A gene product is a member of the switching/sucrose nonfermentable complex involved in chromatin remodeling and interacts with the DNA mismatch repair system. ARID1A alterations have been associated with MSI and TMB-H in several cancer types and have been also correlated with improved outcomes after immune checkpoint blockade (61). PD-L1 expression and TMB has been positively correlated to tumor grade and are associated with a poorer survival in NENs (62).
Table 2.
Clinical trials assessing immunotherapy in GEP NECs
| Trial and author | N | Primary site | Treatment | Primary endpoint | ORR (%) | PFS (median) | OS (median) |
|---|---|---|---|---|---|---|---|
| First-line therapy | |||||||
|
NICE NEC trial
Riesco-Martinez et al |
38 | GEP or UKP G3 NENs |
Nivolumab + CBDCA + etoposide |
12-mo OS | 54.1% | 5.7 mo (43.2% 6-mo PFS, 17.5% 12-mo PFS) |
13.9 mo (54% 12-mo OS, 44% 18-mo OS) |
| Second-line therapy and beyond | |||||||
|
NIPINEC trial
Walter et al |
185 | 93 lung LC-NEC 92 GEP NECs |
Nivolumab vs nivolumab + ipilimumab |
ORR 8 wk | Nivo vs Nivo-Ipi: Lung: 7.3% vs 18.2% GEP: 7.1% vs 11.6% |
1.8 vs 1.9 mo | 7.2 vs 5.8 mo |
|
DUNE trial
Capdevila et al |
33 | C4: G3 GEP NENs | Durvalumab + tremelimumab | 9-moOS | 9.1% | 2.4 mo | 5.9 mo (9-mo OS 36%) |
|
CA209-538
Basket trial Klein et al |
29 | NENs any site (13 G3, 10 GEP, 4 GEP NECs) |
Nivolumab + ipilimumab | DCR | 24% (G3 31%) (DCR 72%) |
4.8 mo | 14.8 mo |
|
DART SWOG 1609
Basket trial Patel et al |
32 | NENs any site (18 G3, 15 GEP, 8 GEP NECs) |
Nivolumab + ipilimumab | ORR | 25% (G3 44%) |
4 mo (G3 6-mo PFS 44%) |
11 mo |
|
Mulvey et al
Chan et al |
14 (A) 22 (B) |
EP-NECs (6 + 16 GEP) |
Part A: Pembro Part B: Pembro + CT (IRI or PAC) |
ORR | A: 7% B: 5% |
A: 1.9 mo B: 2.0 mo |
A: NR B: 4.9 mo |
| Vijayvergia et al | 29 | G3 EP-NENs (19 NECs, 24 GEP) |
Pembrolizumab | ORR | 3.4% | 2.2 mo | 5.1 mo |
| Yao et al | 116 | 21 GEP-NECs | Spartalizumab | ORR | 4.8% | 1.8 mo | 6.8 mo |
| Lu et al | 40 | G2-3 NENs (Ki67 > 10%) (32 GEP, 32 NEC) |
Toripalimab | ORR | All patients 20% GI NEN 13% Pan NEN 22% Other primaries 38% PD-L1 > 1 50% TMB-H 75% |
2.5 mo | 7.8 mo |
|
AVENEC trial
Fottner et al |
29 | G3 NENs (21 GEP, 19 NEC) |
Avelumab | DCR | 6.9% (DCR 27.6%) |
4.0 months | 7.0 mo (NEC 4.7 mo, G3 NET NR) |
Abbreviations: CBDCA, carboplatin; CDDP, cisplatin; CT, chemotherapy; C4, cohort 4; DCR, disease control rate; EP-NEC, extrapulmonary neuroendocrine carcinoma; GEP, gastroenteropancreatic; LC-NEC, large-cell neuroendocrine carcinoma; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm; NET, neuroendocrine tumors; ORR, objective response rate; NR, not reported or not reached; OS, overall survival; Pembro, pembrolizumab; PFS, progression-free survival; TMB-H, high tumor mutational burden; UKP, unknown primary.
Dual blockade using anti–PD-1 and anti–CTLA-4 monoclonal antibodies (MAbs) has demonstrated increased efficacy compared with single-agent ICI in several tumor types such as melanoma, renal cell carcinoma, MSI colorectal cancer, and certain subsets of non-SCLC, among others. The combination of nivolumab (anti-PD1 Mab) and ipilimumab (anti-CTLA4 MAb) showed promising activity in small cohorts of patients with high-grade NENs enrolled in 2 basket trials conducted in patients with rare cancers, DART SWOG 1609 and CA209-538 (63, 64). The DART trial reported an ORR of 44% in 18 patients with high-grade nonpancreatic NECs (including lung primaries and 8 GEP NECs), with a 6-month PFS rate of 44% (63). The CA209-538 documented an ORR of 31% in an additional cohort of 13 patients with high-grade NENs (4 GEP NECs) (64).
However, more recent data from the DUNE trial suggested only modest activity for durvalumab and tremelimumab in a cohort of 33 patients with G3 GEP NENs, with an ORR of 9.1% and a 9-month OS rate of 36.1%, although it surpassed the preestablished OS futility threshold (primary endpoint) (65). Of note, 10 (30.3%) patients were alive 12 months after treatment initiation. Outcomes in this cohort did not substantially differ by tumor differentiation (NET vs NEC) and Ki-67 index, and PD-L1 combined score did not correlate with treatment efficacy.
Consistent with results of the DUNE trial, the NIPINEC study (GCO-001) also showed in a larger patient population modest activity of dual blockade with nivolumab and ipilimumab in GEP NECs (66). This was a randomized noncomparative trial that included 185 patients with advanced, refractory large-cell pulmonary (N = 93) or GEP NECs (N = 92) on progression to 1 or 2 previous systemic treatment lines including platinum-based chemotherapy. These patients were randomly allocated to receive nivolumab or nivolumab plus ipilimumab. In this trial, combination of nivolumab and ipilimumab, but not nivolumab as single agent, reached its primary endpoint of ORR at 8 weeks >10%. Dual ICI induced higher ORR in lung large-cell NECs (18.2%) than in GEP NECs (11.6%). Global median PFS (1.9 months) and OS (5.8 months) were not encouraging, although further follow-up is needed to identify whether a proportion of patients achieve long-term benefit from this treatment strategy.
In summary, overall efficacy of dual immune checkpoint blockade seems lower in digestive than in lung NECs, although a small subset of patients may be long-term survivors. Thus, further efforts will be pursued to identify who these patients may be. Unfortunately, MSI was not assessed in any of these trials. The lack of treatment control and short follow-up of these studies further limits proper assessment of the real impact of this therapeutic approach on patient's long-term outcomes. Despite these modest results, however, and taking into account the limited efficacy of chemotherapy in this refractory setting, the National Comprehensive Cancer Network guidelines indicate that dual immune checkpoint blockade can be considered in NECs when the disease progresses following chemotherapy.
On the other hand, emerging evidence suggests that conventional chemotherapy induces an immunogenic cell death that can prime antitumor immunity within an immunosuppressive microenvironment and may be synergistic with immune checkpoint inhibition (67). This has been demonstrated in chemotherapy-näive patients with advanced SCLC because the addition of durvalumab or atezolizumab to first-line platinum-based chemotherapy improves survival and is now the standard of care for these patients (55, 56). In this context, the NICE NEC trial assessed this strategy in advanced untreated G3 NENs of GEP or unknown origin. The combination of nivolumab with carboplatin and etoposide induced an ORR of 54% and sustained response beyond 12 months in 6 of 38 patients (16%) with a median OS of 13.9 months (68). On the contrary, limited efficacy was reported with the combination of ICI (pembrolizumab) and chemotherapy (investigator's choice of either irinotecan or paclitaxel) in refractory extrapulmonary NECs (patients progressed to 1 to 3 prior lines of therapy) (69). Ongoing translational studies may help identify predictive or prognostic biomarkers to improve selection of patients most likely to benefit from this treatment strategy, and randomized trials would be required to properly assess the role of adding ICI to chemotherapy in this setting.
Finally, preclinical evidence suggests the genomic instability induced by drugs that target cell-cycle or DNA damage repair (DDR) regulators such as PARP, CHK1, or CDKs, may boost sensitivity to ICI, and these combinations are currently being explored in clinical trials in NECs. Other alternative immunotherapeutic approaches include bispecific antibodies or bispecific T-cell engagers that concurrently target CD3 in lymphocytes and specific tumor targets such as somatostatin receptors or DLL3, to redirect cytotoxic T cells against cancer cells (70). These therapies show promising activity in preclinical models and are currently in early stages of clinical development in NENs.
Other immunotherapeutic strategy in development, which may render immunologically “cold” tumors more sensitive to immune checkpoint blockade, are oncolytic viruses. Their antitumor activity is based on the ability to selectively replicate in tumor cells causing cell lysis, to amplify the initial therapeutic dose through replication in vivo, and to induce innate and adaptive antitumor immunity. A variety of native and, more commonly, genetically modified viruses have been successfully developed as oncolytic agents, such as T-Vec (talimogene laherparepvec) for melanoma or oncorine (H101) for head and neck tumors. Oncolytic viruses explored in NET preclinical models with some preliminary evidence of efficacy include GLV-1 h68, a genetically engineered vaccinia virus; HSV T-01, a third-generation herpes simplex; or Ad5fkFWKT (CgA-E1A-miR122), a modified adenovirus with somatostatin motifs for selective infection of neuroendocrine tumor cells, and some early clinical trials in NEN patients are currently under way (71–73). Oncolytic virus may also be engineered to express different transgenic payloads, such as extracellular matrix-degrading enzymes, pro-inflammatory cytokines, antibodies (ie, ICI), and specific antigens that can expand their therapeutic potential. Still, many challenges posed by tumor heterogeneity, physical barriers, antiviral immunity, and the immunosuppressive tumor microenvironment need to be overcome to improve antitumor efficacy of oncolytic virotherapy.
Tumor Agnostic Therapy
Deficient mismatch repair and high mutational burden
Immune checkpoint inhibition has proven to be capable of eliciting a T cell–mediated cytotoxic effect that induces profound and durable tumor responses in certain subsets of patients with cancer. To identify this subset of tumors, several molecular determinants have been explored in specific tumor types with somewhat discordant results, including, among others, PD-L1 expression, RNA expression signatures, mutational burden, and patterns of lymphocytic infiltrates. But probably the most robust pan-tumor predictive biomarker to ICI is DNA mismatch repair deficiency (dMMR) or its phenotypic consequence, MSI. Mismatch repair deficiency results in an inability to recognize and repair spontaneous mutations during DNA replication and, thus, dMMR/MSI tumors harbor thousands of mutations (TMB > 50 mutations/megabase), which are highly immunogenic. Indeed, their typically prominent tumor lymphocytic infiltrates is consistent with an antitumor immune response. However, their active immune microenvironment is counterbalanced by immune inhibitory signals (PD-1, PDL1, CTLA-4, LAG-3, IDO) that lead to tumor immune scape. In this context, ICI can unlock a potent tumor-antigen specific immunological response leading to significant clinical benefit.
In 2017, pembrolizumab was granted accelerated approval by the US Food and Drug Administration (FDA) for patients with advanced dMMR/MSI solid tumors that have progressed from prior treatment and who have no satisfactory alternative treatment options. This was the first tumor agnostic indication ever approved in cancer. The approval was based on data from 149 patients with 15 types of MSI/dMMR solid tumors (90 patients had colorectal cancer, 59 had other tumor types) enrolled across 5 multicohort single-arm clinical trials (74). The global ORR was 39.6%, and responses were profound and durable, with median PFS and OS that had not been reached. Several years later (2021-2022), the European Medicines Agency also approved pembrolizumab for a more restricted spectrum of MSI/dMMR tumors, including colorectal, endometrial, gastric, small intestine, or biliary cancer on progression to at least 1 prior therapy. No specific data have been reported, however, in patients with dMMR/MSI NENs, which occurs in 3% to 12% of high-grade GEP NENs. MSI NECs are more commonly encountered in those of gastric or colorectal origin and are enriched in BRAF mutations and CpG island methylator phenotype.
In 2020, the FDA granted pembrolizumab its second tumor agnostic indication for the treatment of advanced TMB-H solid tumors (≥10 mutations/megabase) that have progressed to prior therapy and with no satisfactory alternative treatment options, and also approved the FoundationOneCDx assay (Foundation Medicine, Inc.) as a companion diagnostic. Approval was based on the results of a planned retrospective analysis of 10 cohorts of patients included in the KEYNOTE-158 trial (NCT02628067) (75). A total of 105 patients (13%) had TMB-H tumors, including 5 of 87 (5.7%) patients with neuroendocrine tumors (tumor site, differentiation, and grade not specified). The global ORR was 29%, 28% among the 81 patients with non-MSI TMB-H tumors. Two of the 5 (40%) patients with TMB-H neuroendocrine tumors achieved an ORR. The median duration of response was not reached, with 50% of patients having response durations ≥24 months. Although TMB-H has been reported in up to 20% of G3 NENs, the precise incidence in GEP NECs is uncertain and seems less common than in other anatomic locations such as head and neck or lung NECs (5, 25).
Based on these data, pembrolizumab may be considered for the treatment of patients with advanced dMMR/MSI and/or TMB-high NECs that have progressed following prior treatment and have no alternative treatment options.
Neurotrophic tyrosine receptor kinase and RET gene fusions
Other molecular alterations that may be encountered in NECs and are efficiently targeted by approved tumor-agnostic drugs (larotrectinib, entrectinib) include neurotrophic tyrosine receptor kinase (NTRK) fusions (76, 77). NTRK gene fusions, resulting in chimeric TRK proteins, are oncogenic drivers in a diverse range of solid tumor types. Entrectinib is a potent, central nervous system-active TRK inhibitor approved for the treatment of patients with advanced solid tumors harboring an NTRK gene fusion. Approval was based on results of 3 single-arm clinical trials (ALKA, STARTRK-1 [NCT02097810] and STARTRK-2 [NCT02568267]) that enrolled 54 patients with NTRK-positive tumors (78). The ORR was 57% and in 45%, the patient response duration was 12 months or longer. The most common cancers included in these trials were sarcoma, non-SCLC, mammary analogue secretory carcinoma, breast, thyroid, and colorectal. Among the 5 patients with NENs enrolled in these trials, the ORR was 40% (79). However, relevant patients’ features of these NENs (tumor differentiation, grade, or primary site) have not been reported. More recently, a phase 1 trial with taletrectinib (AB-106/DS-6051b), a tyrosine kinase inhibitor with high affinity for ROS1 and NTRK1/2/3, was conducted in patients with neuroendocrine tumors, tumor-induced pain, or tumors harboring ROS1/NTRK rearrangements (80). Patients with NETs were eligible for this trial based on preclinical evidence suggesting TrkB (NTRK2) signaling pathway could be involved in the tumorigenesis, proliferation, and invasive nature of NETs. However, the ORR among the 12 included patients with molecularly unselected NENs was modest, with 1 patient (8.3%) with a heavily pretreated small bowel NEC achieving a confirmed partial response (PR), and a median PFS of 10.2 months (80). These results would only justify the use of NTRK inhibitors in NEN patients with NTRK rearrangements, although the incidence of NTRK fusions may be somewhat higher than in many other common malignancies. In fact, comprehensive genomic profiling identified NTRK fusions in 6 of 2417 NENs (0.3%), including patients with pancreatic (N = 2), uterine (N = 1), lung (N = 1), and unknown (N = 2) primary tumor sites. Three of them involved translocations of NTRK1 with unique fusion partners (GPATCH4, PIP5K1A, CCDC19). However, detailed information of the full study cohort regarding tumor differentiation, grade, and primary site was not provided.
RET inhibitors have also demonstrated efficacy in patients with a variety of RET fusion–positive solid tumors. RET is a proto-oncogene involved in many essential physiological and developmental functions, including spermatogonial stem cell maintenance, renal morphogenesis, and neural and neuroendocrine tissue development. Aberrant activation of RET through gain-of-function mutations is associated with the hereditary predisposition syndrome multiple endocrine neoplasia 2, which increases the risk of developing medullary thyroid cancer, pheochromocytoma, and parathyroid hyperplasia or adenoma. RET rearrangements occur in 10% to 20% of sporadic papillary thyroid cancer and in 1% to 3% of patients with non-SCLC, and are far less common in other tumor types (81). Pralsetinib is a selective RET inhibitor that potently inhibits RET kinases, including RET fusion proteins. The phase 1-2 ARROW trial enrolled 29 patients with 12 different RET fusion–positive solid tumor types, excluding thyroid and non-SCLC (82). ORR was 57%, and responses were observed regardless of tumor type or RET fusion partner, including 2 of 3 patients (67%) with RET-fusion positive neuroendocrine cancer. Median duration of response was 12 months, and median PFS and OS were 7 and 14 months, respectively. These data indicate RET could also be a tissue-agnostic target, although the incidence of RET fusions in NENs is uncertain and, to date, RET inhibitors are only approved for the treatment of lung and thyroid tumors harboring RET rearrangements.
MAPK signalling pathway
As in adenocarcinomas of similar anatomical location, aberrant activation of mitogen-activated protein kinase signaling pathway is common in GEP NECs. KRAS and BRAF mutations are frequently encountered in digestive NECs (24% and 29% of large cell NECs, respectively), particularly in those of pancreatic or colorectal origin (5). Of note, up to 70% of right colon NECs harbor BRAF V600E mutations. BRAF inhibition, alone or in combination with MEK or EGFR inhibition, has demonstrated antitumor activity in preclinical models and patients (case reports) (83–85). A recent study in patient's samples and patient-derived xenografts showed a distinctive methylome profile of co-NECs compared with colorectal adenocarcinomas, resulting in EGFR expression silencing (34). Thus, authors suggest BRAF-mutated co-NECs could potentially benefit from single-agent BRAF inhibition and support the addition of EGFR inhibition in the setting of acquired resistance. It should be pointed out, however, that these tumors often harbor a nonneuroendocrine component, and the combination of encorafenib and cetuximab is approved for the treatment of colorectal cancer (histology not specified). Moreover, in June 2022, the FDA approved the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) for the treatment of adult and pediatric patients with advanced solid tumors with the BRAF V600E mutation. The efficacy of this combination was evaluated in 131 adult and 36 pediatric patients included in several open-label basket trials (BRF117019 [NCT02034110], NCI-MATCH [NCT02465060], CTMT212X2101 [NCT02124772]), and supported by results in COMBI-d, COMBI-v, and BRF113928 (86). The studies enrolled patients with 24 tumor types, including 2 patients with gastrointestinal MINEN and 2 patients with colon neuroendocrine carcinomas. The ORR was 41% in adults and 25% in pediatric patients. Duration of response was ≥24 months for 44% of patients. No ORR was documented in the few NEN patients included in the NCI-MATCH trial. Overall, these data support further specific clinical assessment in co-NECs of these therapeutic strategies.
Although traditionally considered “undruggable,” recent advances in targeting KRAS in a variety of solid tumors are encouraging, particularly KRAS G12C mutations, although no specific data have been reported in NECs (87). Referral of patients with KRAS-mutant tumors for enrollment in clinical trials is highly recommended.
Other Potentially Targetable Tumor Vulnerabilities
Notch signaling plays a suppressor function in NENs. Delta-like ligand 3 (DLL3), unlike other ligands, negatively regulate Notch signaling and is upregulated in aggressive NENs including GEP NECs, whereas its expression is almost absent in well-differentiated NETs or normal cells (70). This makes DLL3 a suitable target for directed therapies, including antibody-drug conjugates, adoptive cell therapy or radioligand therapy. rovalpituzumab tesirine (Rova-T), a DLL3-targeted antibody-drug conjugate consisting of a humanized anti-DLL3 IgG1 antibody linked to a pyrrolobenzodiazepine dimer toxin, reported promising antitumor activity in phase 1-2 trials conducted in SCLC, particularly in DLL3 high-expressing tumors. However, phase 3 trials assessing Rova-T as a first-line maintenance therapy following first-line platinum-based chemotherapy (MERU trial, NCT03033511) or comparing Rova-T to topotecan as a second-line therapy (TAHOE trial, NCT03061812) in patients with advanced SCLC failed to demonstrate a survival benefit or were even detrimental (88, 89). Moreover, Rova-T was associated with significant toxicity attributed to the pyrrolobenzodiazepine payload, including pleural and pericardial effusions, peripheral edema, pneumonitis, and thrombocytopenia, altogether leading to discontinuation of its clinical development. Rova-T was tested in 28 patients with GEP NECs (68% DLL3 positive) included in a phase 1-2 basket trial reported an ORR of 18% (50% in DLL3-high tumors), with a median PFS of 3.8 months and OS of 8.1 months (90).
DLL3-targeted agents include also bispecific T-cell engagers (AMG 119, BI 764 532) and chimeric antigen receptors that activate and redirect CD3-positive T lymphocytes to DLL3-expressing tumors (AMG 757), to induce a cytotoxic immune effect. Early results are promising in patients with SCLC, and these agents are now starting to be tested in other high-grade NENs. DLL3 is also currently being explored as a target for radioligand PET imaging (Zr-89-DFO-SC16.56) and therapy (linked to actinium or lutetium) in preclinical models of SCLC (70).
Other tumor vulnerabilities that may be therapeutically exploited include cell-cycle and DDR, epigenetic regulators or MYC family dysregulations (4). Germline mutations in DDR genes have been documented in up to 20% of extrapulmonary NECs and are associated with increased sensitivity to DNA-damaging drugs such as platinum agents. Tumor cells with impaired TP53 and/or RB1 function are particularly vulnerable to cell-cycle checkpoint inhibitors (ie, Aurora kinases or cyclin-dependent kinase inhibitors) or drugs targeting the DDR pathway (ie, CHK1 or PARP inhibitors), particularly in the context of DNA-damaging therapy. Different combinations of these agents are currently being explored in patients with NECs. MYC is amplified in up to 50% of GEP NECs and drugs targeting MYCN synthetic lethal partners (ie, EZH2, Aurora kinases) have shown promising activity in preclinical models and are currently in early stages of clinical development.
Conclusions and Future Perspectives
GEP NECs are a challenging, highly aggressive type of neoplasms that commonly present widespread disease at diagnosis and have a very poor prognosis. Although increasing evidence suggests relevant molecular differences relative to their lung counterparts, SCLC-type platinum-based chemotherapy remains the standard of care. Nevertheless, a lower rate of TP53 and RB1 alterations have been documented in GEP NECs and, consistently, they seem to be less responsive to platinum agents. Novel therapeutic approaches including immunotherapy (immune-check point inhibition and others), tumor agnostic therapies targeting oncogenic drivers (RET or NTRK fusions, BRAF or KRAS mutations), or strategies targeting other tumor vulnerabilities (eg, DNA damage repair, cell-cycle dysregulations, MYC amplification, Notch pathway) may benefit some patients but comprehensive tumor molecular profiling is not widely available in standard practice. Further research is needed to consolidate these strategies and better define suitable candidates that really benefit from these therapies.
Adequate preclinical models shall be developed to support fundamental research, as an improved understanding of the underlying biology of GEP NECs and its interaction with the host is critical for progress to be made. Moreover, quality of evidence to support clinical decisions in daily practice is rather poor, and specific GEP NEC trials should be conducted considering the organ of origin, histological subtype, proliferation index, and molecular background. Clinical trials should thus incorporate comprehensive tumor molecular profiling to identify predictive biomarkers and new actionable targets to improve efficacy in the era of personalized medicine. A higher enrichment of properly characterized NEN cohorts of patients in biomarker-driven basket trials should be pursued to generate more solid evidence to support tumor agnostic therapies in these patients. To this aim, international collaboration is highly encouraged as it is essential for progress to be made.
Acknowledgments
We thank Daniel Rolin-Ferrer for his editing and administrative support to submit this manuscript.
Abbreviations
- co-NEC
colorectal neuroendocrine carcinoma
- DDR
DNA damage repair
- dMMR
DNA mismatch repair deficiency
- FDA
US Food and Drug Administration
- GEP
gastroenteropancreatic
- ICI
immune checkpoint inhibition
- LC
large cell
- MAb
monoclonal antibody
- MINEN
mixed neuroendocrine-nonneuroendocrine neoplasm
- MSI
microsatellite instability
- NEC
neuroendocrine carcinoma
- NEN
neuroendocrine neoplasm
- NET
neuroendocrine tumor
- NTRK
neurotrophic tyrosine receptor kinase
- ORR
objective response rate
- OS
overall survival
- PET
positron emission tomography
- PFS
progression-free survival
- RLT
radioligand therapy
- SCLC
small cell lung cancer
- TMB-H
high tumor mutational burden
- uPAR
urokinase plasminogen activator receptor
- WHO
World Health Organization
Contributor Information
Rocio Garcia-Carbonero, Oncology Department, Hospital Universitario 12 de Octubre, 28041 Madrid, Spain; Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain; Lung Cancer Clinical Research Unit, Hospital 12 de Octubre-Centro Nacional de Investigaciones Oncológicas (CNIO), 28029 Madrid, Spain; Department of Medicine, Faculty of Medicine, Universidad Complutense de Madrid (UCM), 28040 Madrid, Spain.
Beatriz Anton-Pascual, Oncology Department, Hospital Universitario 12 de Octubre, 28041 Madrid, Spain; Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain.
Andrea Modrego, Oncology Department, Hospital Universitario 12 de Octubre, 28041 Madrid, Spain; Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain.
Maria del Carmen Riesco-Martinez, Oncology Department, Hospital Universitario 12 de Octubre, 28041 Madrid, Spain; Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain; Lung Cancer Clinical Research Unit, Hospital 12 de Octubre-Centro Nacional de Investigaciones Oncológicas (CNIO), 28029 Madrid, Spain.
Alberto Lens-Pardo, Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain; Lung Cancer Clinical Research Unit, Hospital 12 de Octubre-Centro Nacional de Investigaciones Oncológicas (CNIO), 28029 Madrid, Spain.
Carlos Carretero-Puche, Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain; Lung Cancer Clinical Research Unit, Hospital 12 de Octubre-Centro Nacional de Investigaciones Oncológicas (CNIO), 28029 Madrid, Spain.
Beatriz Rubio-Cuesta, Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain; Lung Cancer Clinical Research Unit, Hospital 12 de Octubre-Centro Nacional de Investigaciones Oncológicas (CNIO), 28029 Madrid, Spain.
Beatriz Soldevilla, Centro de Oncologia Experimental, Grupo de Investigación en Tumores Gastrointestinales y Neuroendocrinos, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), 28041 Madrid, Spain; Lung Cancer Clinical Research Unit, Hospital 12 de Octubre-Centro Nacional de Investigaciones Oncológicas (CNIO), 28029 Madrid, Spain; Department of Genetics, Physiology and Microbiology, Faculty of Biology, Universidad Complutense de Madrid (UCM), 28040 Madrid, Spain.
Funding
M.R. is partially funded by the AECC (CLSEN19003RIES). A.L.P. is funded by the Instituto de Salud Carlos III (PFIS; FI20/00131). C.C.P. was partially funded by CAM (PEJD-2016-PRE/BMD-2666). B.R.C. was partially funded by CAM (PEJD-2017-PRE/BMD-4981). B.S. is partially funded by the AECC (POSTDO46SOLD, Spain).
Disclosures
R.G.C. has provided scientific advice and/or received honoraria or funding for continuous medical education from AAA, Advanz Pharma, Amgen, Bayer, BMS, Boerhringer, Esteve, Hutchmed, Ipsen, Merck, Midatech Pharma, MSD, Novartis, PharmaMar, Pierre Fabre, Roche, and Servier, and has received research support from Pfizer, BMS, and MSD. B.A.P. received honoraria for medical advisory from AAA, Advanz. Merck, Servier, and Novartis. A.M. received support for medical education from IPSEN, Merck, Servier, leo-pharma, and AstraZeneca. M.C.R.M. received honoraria or funding for consultant of advisory role from Bayer, AAA, Servier, and Roche, for speaking from Servier, Roche, Merk, and Bayer, and has received research support from MSD and BMS.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed for this review article.
References
- 1. Shah MH, Goldner WS, Benson AB, et al. . Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2021;19(7):839‐868. [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Carbonero R, Sorbye H, Baudin E, et al. . Vienna Consensus conference participants. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103(2):186‐194. [DOI] [PubMed] [Google Scholar]
- 3. Sorbye H, Baudin E, Borbath I, et al. . Unmet needs in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Neuroendocrinology. 2019;108(1):54‐62. [DOI] [PubMed] [Google Scholar]
- 4. Frizziero M, Kilgour E, Simpson KL, et al. . Expanding therapeutic opportunities for extrapulmonary neuroendocrine carcinoma. Clin Cancer Res. 2022;28(10):1999‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venizelos A, Elvebakken H, Perren A, et al. . The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2021;29(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dasari A, Shen C, Halperin D, et al. . Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alese OB, Jiang R, Shaib W, et al. . High-Grade gastrointestinal neuroendocrine carcinoma management and outcomes: A national cancer database study. Oncologist. 2019;24(7):911‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Zwan JM, Siesling S, van Velthuysen L, Links T, Walenkamp A, Tesselaar M. Extra-pulmonary neuroendocrine carcinomas: a population-based study in The Netherlands. Neuroendocrinology. 2018;107(1):50‐59. [DOI] [PubMed] [Google Scholar]
- 9. Dasari A, Mehta K, Byers L, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: a SEER database analysis of 162983 cases. Cancer. 2018;124(4):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Korse CM, Taal BG, van Velthuysen M-LF, Visser O. Incidence and survival of neuroendocrine tumours in The Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer Oxf Engl. 2013;49(8):1975‐1983. [DOI] [PubMed] [Google Scholar]
- 11. Pommergaard H-C, Nielsen K, Sorbye H, et al. . Surgery of the primary tumour in 201 patients with high-grade gastroenteropancreatic neuroendocrine and mixed neuroendocrine-non-neuroendocrine neoplasms. J Neuroendocrinol. 2021;33(5):e12967. [DOI] [PubMed] [Google Scholar]
- 12. Sorbye H, Welin S, Langer SW, et al. . Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(1):152‐160. [DOI] [PubMed] [Google Scholar]
- 13. Nuñez-Valdovinos B, Carmona-Bayonas A, Jimenez-Fonseca P, et al. . Neuroendocrine tumor heterogeneity adds uncertainty to the world health organization 2010 classification: real-world data from the Spanish Tumor registry (R-GETNE). Oncologist. 2018;23(4):422‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rindi G, Mete O, Uccella S, et al. . Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022; 33(1):115‐154. [DOI] [PubMed] [Google Scholar]
- 15. Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P. Gastroenteropancreatic well-differentiated grade 3 neuroendocrine tumors: review and position statement. Oncologist. 2016;21(10):1191‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heetfeld M, Chougnet CN, Olsen IH, et al. . Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22(4):657‐664. [DOI] [PubMed] [Google Scholar]
- 17. Sorbye H, Baudin E, Perren A. The problem of high-grade gastroenteropancreatic neuroendocrine neoplasms: well-differentiated neuroendocrine tumors, neuroendocrine carcinomas, and beyond. Endocrinol Metab Clin North Am. 2018;47(3):683‐698. [DOI] [PubMed] [Google Scholar]
- 18. Milione M, Maisonneuve P, Spada F, et al. . The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2017;104(1):85‐93. [DOI] [PubMed] [Google Scholar]
- 19. Lacombe C, De Rycke O, Couvelard A, et al. . Biomarkers of response to etoposide-platinum chemotherapy in patients with grade 3 neuroendocrine neoplasms. Cancers (Basel). 2021;13(4):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Liu M, Zhang Y, Guo Y, Chen M-H, Chen J. Genetic characteristics of colorectal neuroendocrine carcinoma: more similar to colorectal adenocarcinoma. Clin Colorectal Cancer. 2020;20(2):177‐185.e13. [DOI] [PubMed] [Google Scholar]
- 21. Nagtegaal ID, Odze RD, Klimstra D, et al. . The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Puccini A, Poorman K, Salem ME, et al. . Comprehensive genomic profiling of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(22):5943‐5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahnane N, Furlan D, Monti M, et al. . Microsatellite unstable gastrointestinal neuroendocrine carcinomas: a new clinicopathologic entity. Endocr Relat Cancer. 2015;22(1):35‐45. [DOI] [PubMed] [Google Scholar]
- 24. Girardi DM, Silva ACB, Rêgo JFM, Coudry RA, Riechelmann RP. Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: a systematic review. Cancer Treat Rev. 2017; 56:28‐35. [DOI] [PubMed] [Google Scholar]
- 25. Sun TY, Zhao L, Van Hummelen P, et al. . Exploratory genomic analysis of high grade neuroendocrine neoplasms across diverse primary sites. Endocr Relat Cancer. 2022;29(12):665‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Busico A, Maisonneuve P, Prinzi N, et al. . Gastroenteropancreatic high-grade neuroendocrine neoplasms: histology and molecular analysis, two sides of the same coin. Neuroendocrinology. 2020;110(7-8):616‐629. [DOI] [PubMed] [Google Scholar]
- 27. Shamir ER, Devine WP, Pekmezci M, et al. . Identification of high-risk human papillomavirus and Rb/E2F pathway genomic alterations in mutually exclusive subsets of colorectal neuroendocrine carcinoma. Mod Pathol Off J U S Can Acad Pathol Inc. 2019;32(2):290‐305. [DOI] [PubMed] [Google Scholar]
- 28. Konukiewitz B, Jesinghaus M, Steiger K, et al. . Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol. 2018;77:70‐79. [DOI] [PubMed] [Google Scholar]
- 29. Li R, Yang Z, Shao F, et al. . Multi-omics profiling of primary small cell carcinoma of the esophagus reveals RB1 disruption and additional molecular subtypes. Nat Commun. 2021;12(1):3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pizzi S, Azzoni C, Bottarelli L, et al. . RASSF1A promoter methylation and 3p21.3 loss of heterozygosity are features of foregut, but not midgut and hindgut, malignant endocrine tumours. J Pathol. 2005;206(4):409‐416. [DOI] [PubMed] [Google Scholar]
- 31. La Rosa S, Marando A, Furlan D, Sahnane N, Capella C. Colorectal poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas: insights into the diagnostic immunophenotype, assessment of methylation profile, and search for prognostic markers. Am J Surg Pathol. 2012;36(4):601‐611. [DOI] [PubMed] [Google Scholar]
- 32. Furlan D, Sahnane N, Mazzoni M, et al. . Diagnostic utility of MS-MLPA in DNA methylation profiling of adenocarcinomas and neuroendocrine carcinomas of the colon–rectum. Virchows Arch. 2013;462(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 33. Simon T, Riemer P, Jarosch A, et al. . DNA methylation reveals distinct cells of origin for pancreatic neuroendocrine carcinomas and pancreatic neuroendocrine tumors. Genome Med. 2022;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Capdevila J, Arqués O, Hernández Mora JR, et al. . Epigenetic EGFR gene repression confers sensitivity to therapeutic BRAFV600E blockade in colon neuroendocrine carcinomas. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(4):902‐909. [DOI] [PubMed] [Google Scholar]
- 35. Kawasaki K, Toshimitsu K, Matano M, et al. . An organoid biobank of neuroendocrine neoplasms enables genotype-phenotype mapping. Cell. 2020;183(5):1420‐1435.e21. [DOI] [PubMed] [Google Scholar]
- 36. Dasari A, Shen C, Devabhaktuni A, Nighot R, Sorbye H. Survival according to primary tumor location, stage, and treatment patterns in locoregional gastroenteropancreatic high-grade neuroendocrine carcinomas. Oncologist. 2022;27(4):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitz R, Mao R, Moris D, Strickler JH, Blazer DG. Impact of postoperative chemotherapy on the survival of patients with high-grade gastroenteropancreatic neuroendocrine carcinoma. Ann Surg Oncol. 2021;28(1):114‐120. [DOI] [PubMed] [Google Scholar]
- 38. Mao R, Li K, Cai J-Q. et al. et al. Adjuvant chemotherapy versus observation following resection for patients with nonmetastatic poorly differentiated colorectal neuroendocrine carcinomas. Ann Surg. 2021;274(2):e126‐e133. [DOI] [PubMed] [Google Scholar]
- 39. Casas F, Ferrer F, Farrús B, Casals J, Biete A. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80(8):1366‐1372. [PubMed] [Google Scholar]
- 40. Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(13):2730‐2739. [DOI] [PubMed] [Google Scholar]
- 41. Shen C, Chen H, Chen H, et al. . Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016;16(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sørbye H, Westre B, Horn A. Curative surgery after neoadjuvant chemotherapy in metastatic poorly differentiated neuroendocrine carcinoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2007;33(10):1209‐1210. [DOI] [PubMed] [Google Scholar]
- 43. Yamaguchi T, Machida N, Morizane C, et al. . Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105(9):1176‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227‐232. [DOI] [PubMed] [Google Scholar]
- 45. Espinosa-Olarte P, La Salvia A, Riesco-Martinez MC, Anton-Pascual B, Garcia-Carbonero R. Chemotherapy in NEN: still has a role? Rev Endocr Metab Disord. 2021;22(3):595‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morizane C, Machida N, Honma Y, et al. . Effectiveness of etoposide and cisplatin vs irinotecan and cisplatin therapy for patients with advanced neuroendocrine carcinoma of the digestive system: the TOPIC-NEC phase 3 randomized clinical trial. JAMA Oncol. 2022;8(10):1447‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang P, Li J, Li J, et al. . Etoposide and cisplatin versus irinotecan and cisplatin as the first-line therapy for patients with advanced, poorly differentiated gastroenteropancreatic neuroendocrine carcinoma: a randomized phase 2 study. Cancer. 2020;126(S9):2086‐2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Randomized phase II study of platinum and etoposide (EP) versus temozolomide and capecitabine (CAPTEM) in patients (pts) with advanced G3 non-small cell gastroenteropancreatic neuroendocrine neoplasms (GEPNENs): ECOG-ACRIN EA2142. Journal of Clinical Oncology. Available at: https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.4020. Accessed November 21, 2022.
- 49. NET-02: a multicenter, randomized, phase II trial of liposomal irinotecan (nal-IRI) and 5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line therapy in patients (pts) with progressive poorly differentiated extra-pulmonary neuroendocrine carcinoma (PD-EP-NEC). Available at: https://ascopubs.org/doi/abs/10.1200/JCO.2022.40.16_suppl.4005. Accessed November 21, 2022.
- 50. Walter TA, Lievre A, Coriat R, et al. . LBA46 bevacizumab (B) plus FOLFIRI after failure of platinum-etoposide in patients (pts) with advanced neuroendocrine carcinoma (NEC): the PRODIGE 41-BEVANEC randomized phase II study. Ann Oncol. 2022;33:S1412. [Google Scholar]
- 51. Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT In high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer. 2020;27(3):R67‐R77. [DOI] [PubMed] [Google Scholar]
- 52. Refardt J, Hofland J, Kwadwo A, et al. . Theranostics in neuroendocrine tumors: an overview of current approaches and future challenges. Rev Endocr Metab Disord. 2021;22(3):581‐594. [DOI] [PubMed] [Google Scholar]
- 53. Carlsen EA, Loft M, Loft A, et al. . Prospective phase II trial of prognostication by 68Ga-NOTA-AE105 uPAR PET in patients with neuroendocrine neoplasms: implications for uPAR-targeted therapy. J Nucl MedOff Publ Soc Nucl Med. 2022;63(9):1371‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gauci M-L, Aristei C, Becker JC, et al. . European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline—update 2022. Eur J Cancer Oxf Engl. 2022;171:203‐231. [DOI] [PubMed] [Google Scholar]
- 55. Paz-Ares L, Dvorkin M, Chen Y, et al. . Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet Lond Engl. 2019;394(10212):1929‐1939. [DOI] [PubMed] [Google Scholar]
- 56. Horn L, Mansfield AS, Szczęsna A, et al. . IMpower133 study group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220‐2229. [DOI] [PubMed] [Google Scholar]
- 57. Vijayvergia N, Dasari A, Deng M, et al. . Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;122(9):1309‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yao JC, Strosberg J, Fazio N, et al. . Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer. 2021:ERC-20-0382.R1. [DOI] [PubMed] [Google Scholar]
- 59. Fottner C, Apostolidis L, Ferrata M, et al. . A phase II, open label, multicenter trial of avelumab in patients with advanced, metastatic high-grade neuroendocrine carcinomas NEC G3 (WHO 2010) progressive after first-line chemotherapy (AVENEC). J Clin Oncol. 2019;37(15_suppl):4103‐4103. [Google Scholar]
- 60. Lu M, Zhang P, Zhang Y, et al. . Efficacy, safety, and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms: a multiple-center phase Ib trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(10):2337‐2345. [DOI] [PubMed] [Google Scholar]
- 61. Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer. 2020;8(1):e000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis. 2017;8(8):e3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patel SP, Othus M, Chae YK, et al. . A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(10):2290‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Klein O, Kee D, Markman B, et al. . Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(17):4454‐4459. [DOI] [PubMed] [Google Scholar]
- 65. Capdevila J, Teule A, López C, et al. . 1157O A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: the DUNE trial (GETNE 1601). Ann Oncol. 2020;31:S770‐S771. [Google Scholar]
- 66. Girard N, Mazieres J, Otto J, et al. . Nivolumab (nivo) ± ipilimumab (IPI) in pre-treated patients with advanced, refractory pulmonary or gastroenteropancreatic poorly differentiated neuroendocrine tumors (NECs) (GCO-001 NIPINEC). Ann Oncol. 2021;32 (suppl_5):1283‐1346. [Google Scholar]
- 67. Wang Y-J, Fletcher R, Yu J, Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis. 2018;5(3):194‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Riesco-Martinez M, Capdevila-Castillon J, Alonso V, et al. . Final overall survival results from the NICE-NEC trial (GETNE-T1913): a phase II study of nivolumab and platinum-doublet chemotherapy (CT) in untreated advanced G3 neuroendocrine neoplasms (NENs) of gastroenteropancreatic (GEP) or unknown (UK) origin. Ann Oncol. 2022;33(suppl_7):S225‐S226. [Google Scholar]
- 69. Chan JA, Raj NP, Aggarwal RR, et al. . Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: results of part B (pembrolizumab + chemotherapy). J Clin Oncol. 2021;39(15_suppl):4148‐4148. [Google Scholar]
- 70. Ranallo N, Bocchini M, Menis J, et al. . Delta-like ligand 3 (DLL3): an attractive actionable target in tumors with neuroendocrine origin. Expert Rev Anticancer Ther. 2022;22(6):597‐603. [DOI] [PubMed] [Google Scholar]
- 71. Leja J, Yu D, Nilsson B, et al. . Oncolytic adenovirus modified with somatostatin motifs for selective infection of neuroendocrine tumor cells. Gene Ther. 2011;18(11):1052‐1062. [DOI] [PubMed] [Google Scholar]
- 72. Kloker LD, Berchtold S, Smirnow I, et al. . Oncolytic vaccinia virus GLV-1h68 exhibits profound antitumoral activities in cell lines originating from neuroendocrine neoplasms. BMC Cancer. 2020;20(1):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Matsushima H, Kaibori M, Hatta M, et al. . Efficacy of a third-generation oncolytic herpes simplex virus in neuroendocrine tumor xenograft models. Oncotarget. 2019;10(67):7132‐7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Le DT, Durham JN, Smith KN, et al. . Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marabelle A, Fakih M, Lopez J, et al. . Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353‐1365. [DOI] [PubMed] [Google Scholar]
- 76. Osborne JK, Larsen JE, Gonzales JX, et al. . Neurod1 regulation of migration accompanies the differential sensitivity of neuroendocrine carcinomas to TrkB inhibition. Oncogenesis. 2013;2(8):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sigal DS, Bhangoo MS, Hermel JA, et al. . Comprehensive genomic profiling identifies novel NTRK fusions in neuroendocrine tumors. Oncotarget. 2018;9(88):35809‐35812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bazhenova L, Lokker A, Snider J, et al. . TRK fusion cancer: patient characteristics and survival analysis in the real-world setting. Target Oncol. 2021;16(3):389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bazhenova L, Liu S, Lin J, et al. . Efficacy and safety of entrectinib in patients with locally advanced/metastatic NTRK fusion-positive (NTRK-FP) solid. Ann Oncol. 2021;32:S583‐S620. [Google Scholar]
- 80. Papadopoulos KP, Borazanci E, Shaw AT, et al. . Phase I first-in-human study of taletrectinib (DS-6051b/AB-106), a ROS1/TRK inhibitor, in patients with advanced Solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(18):4785‐4794. [DOI] [PubMed] [Google Scholar]
- 81. Li AY, McCusker MG, Russo A, et al. . RET fusions in solid tumors. Cancer Treat Rev. 2019;81:101911. [DOI] [PubMed] [Google Scholar]
- 82. Subbiah V, Cassier PA, Siena S, et al. . Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022;28(8):1640‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Klempner SJ, Gershenhorn B, Tran P, et al. . BRAFV600E mutations in high-grade colorectal neuroendocrine tumors may predict responsiveness to BRAF-MEK combination therapy. Cancer Discov. 2016;6(6):594‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Burkart J, Owen D, Shah MH, et al. . Targeting BRAF mutations in high-grade neuroendocrine carcinoma of the colon. J Natl Compr Cancer Netw JNCCN. 2018;16(9):1035‐1040. [DOI] [PubMed] [Google Scholar]
- 85. Dizdar L, Werner TA, Drusenheimer JC, et al. . BRAFV600E mutation: a promising target in colorectal neuroendocrine carcinoma. Int J Cancer. 2019;144(6):1379‐1390. [DOI] [PubMed] [Google Scholar]
- 86. Salama AKS, Li S, Macrae ER, et al. . Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: results of the NCI-MATCH trial subprotocol H. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(33):3895‐3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang L, Guo Z, Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021;6(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Blackhall F, Jao K, Greillier L, et al. . Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2021;16(9):1547‐1558. [DOI] [PubMed] [Google Scholar]
- 89. Johnson ML, Zvirbule Z, Laktionov K, et al. . Rovalpituzumab tesirine as a maintenance therapy after first-line platinum-based chemotherapy in patients with extensive-stage-SCLC: results from the phase 3 MERU study. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2021;16(9):1570‐1581. [DOI] [PubMed] [Google Scholar]
- 90. Mansfield AS, Hong DS, Hann CL, et al. . A phase I/II study of rovalpituzumab tesirine in delta-like 3-expressing advanced solid tumors. NPJ precis. Oncol. 2021;5(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed for this review article.