Abstract
Introduction
Oncoplastic breast conserving surgery allows higher volume excision to achieve oncological safety with minimal aesthetic compromise. The primary outcome of this study was to assess the oncological safety in the setting of volume replacement oncoplastic breast conserving surgery. The secondary objective was to assess cosmetic outcome.
Methods
A systematic literature review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines to explore the oncological safety of oncoplastic breast conserving surgery, with particular focus on volume replacement. Resection margin rates, re-excision rates, conversion to mastectomy rates, local and distant disease recurrence, volume replacement techniques, cosmetic outcomes and patient-reported outcome measures were assessed.
Findings
The search criteria identified 155 articles, of which 40 met the inclusion criteria. These studies included 2,497 patients with a mean age of 47.8 years (range 38.4–59.6 years), a body mass index of 24.3kg/m2 (22.1–28.0kg/m2), with a mean follow-up of 37.1 months (6–125 months). A variety of volume replacement techniques were used, most commonly latissimus dorsi and chest wall perforator flaps. Whole mean pathological tumour size was 29.7mm (17–65mm) and mean specimen weight was 123.6g (46.5–220g). Mean re-excision rate was 7.2% and completion mastectomy rate was 2.3%. Locoregional and distant recurrence rate was 2.5% (0–8.1%) and 3.1% (0–14.6%), respectively. There were a variety of patient-reported outcome measures employed, with overall good to excellent outcomes.
Conclusions
This review demonstrates that volume replacement oncoplastic breast conserving surgery is a safe option in terms of re-excision, completion mastectomy rates, and local and distant recurrence. Available patient-related outcome measures and cosmetic assessment tend towards better outcomes compared with wide local excision and mastectomy. However, data are significantly limited, with a paucity of high-level evidence, and it is therefore necessary to be cautious regarding the strength and interpretation of data in this review. Further prospective studies are required on this subject.
Keywords: Oncological safety, Oncoplastic surgery, Breast conservation, Breast conserving surgery, Volume replacement, Resection margins, Recurrence, Survival, Aesthetic outcome, Cosmetic outcome, Cosmesis
Introduction
Breast cancer remains the most frequently diagnosed cancer among women worldwide, affecting 1.5 million women each year.1 The primary aim of breast conserving surgery is achieving oncological safety through complete tumour excision with clear margins. Breast conserving surgery with adjuvant whole breast irradiation is well established as an oncologically safe option in the management of early breast cancer. As surgical techniques have moved away from more radical approaches towards breast conservation and the use neoadjuvant therapy increased, oncoplastic surgery has evolved from breast conserving surgery, allowing for higher volume excision without compromising cosmesis and maintaining breast appearance.2,3 This avoids the need for mastectomy while still maintaining local control, achieving better cosmetic outcome and reducing complications.4
Percentage of breast tissue excised has been found to correlate with aesthetic outcome, which is still reported as poor in up to half of the patients.5,6 Oncoplastic breast conserving surgery (OBCS) has developed as a means of addressing this issue. OBCS can be classified into volume displacement or volume replacement techniques. Volume displacement involves the filling of a defect through transposition of a glandular or a dermoglandular flap of breast tissue, which often requires symmetrisation of the contralateral breast. Volume replacement, which is the main focus of this review, involves the use of autologous tissues to replace volume loss. Breast symmetrisation of the contralateral breast is an integral part of OBCS and should always be considered in either an immediate or a delayed fashion.
The term ‘volume replacement’ was first described in a full paper by Raja et al in 1997.7 Multiple oncoplastic volume replacement techniques have evolved including latissimus dorsi flap, thoracodorsal artery perforator (TDAP) flap, lateral/anterior/medial intercostal artery perforator (LICAP/AICAP/MICAP) flap, lateral thoracic artery perforator (LTAP) flap, thoracoepigastric flap, omental flap and lateral adipose tissue flap. The aim of these techniques is to fill the excised defect thus eliminating deformity and maintaining breast appearance.
The purpose of this review was to assess the best level of evidence available on the oncological safety of volume replacement OBCS. Positive margin rates, re-excision rates, conversion to mastectomy rates, effect on adjuvant therapy, local and distant disease recurrence and volume replacement techniques in the setting of breast cancer were evaluated. The second aim was to assess cosmetic outcomes and the use of patient-related outcome measures (PROMs) in this setting. Assessment of such evidence could aid both clinicians and patients in making an informed decision on OBCS.
Methods
A search was conducted through MEDLINE, EMBASE and Dynamed Plus databases to identify randomised controlled trials, cohort studies and case series of more than 10 patients who underwent volume replacement OBCS. The databases were searched from 1974 to 2020. The protocol from Hu et al was used as guidance.8 This was supplemented by a manual search of relevant references.
A comprehensive search was performed using the following keywords: ‘breast cancer’ [All Fields] AND ‘oncoplastic’ [All Fields] AND ‘partial breast reconstruction’ [All Fields] AND ‘breast conserving therapy’ [All Fields] AND ‘volume replacement’ [All Fields].
Two researchers (CLR and SB) performed the search independently and assessed results using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines to identify and filter out relevant articles.9 All searches were conducted in February 2019 and March 2020. Only full publications in English were considered, abstracts were excluded.
Inclusion criteria were: (i) patients undergoing volume replacement OBCS for breast cancer; (ii) immediate reconstruction; (iii) evaluation of oncological and/or aesthetic outcomes; (iv) peer-reviewed articles. Exclusion criteria were: (i) breast conservation for benign tumours; (ii) reconstruction following mastectomy; (iii) case reports; (iv) series with less than 10 patients; (v) less than six months of follow-up; (vi) articles not in English; (vii) incomplete papers.
Study quality was evaluated based on study design, patient numbers, selection criteria and duration of follow-up. Studies were manually filtered for the following data: author name, year of publication, country/institution, study type, number of cases, patient age, preoperative breast/bra size, tumour type, grade and stage, lymph node status, hormone receptor status, multifocality, neoadjuvant and adjuvant therapies, volume replacement technique and patient follow-up time. Oncological outcomes including re-excision rates, conversion to mastectomy, local and distant disease recurrence and survival were assessed, as well as complications, cosmetic outcomes and PROMs.
The data were extracted and stored in a standardised database by two independent investigators. Following initial blinding of the reviewers, on completion of data collection the articles selected were discussed and assessed in greater detail.
Outcomes of interest were presented in tables and text format around the primary and secondary outcomes. There was limited scope for meta-analysis owing to the small, heterogeneous studies assessed.
Findings
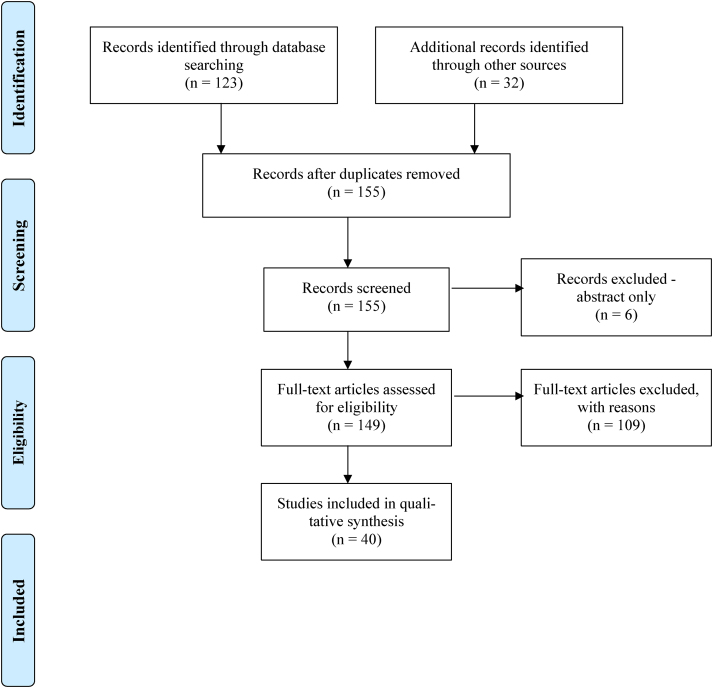
The literature search identified 155 articles, 40 of which met the inclusion criteria (Figure 1).6,10–48 The publication period for the search was from 1974 to 2020 and the studies that were included were published between 1997 and 2020. Data were extracted from the 40 relevant studies that collectively assessed 2,497 patients with a mean age of 47.8 years (range 38.4–59.6 years) and a body mass index of 24.3kg/m2 (22.1–28.0kg/m2). Study types included 26 prospective and 14 retrospective studies, all of which were observational. Follow-up time ranged from 6 to 125 months, with a mean of 37.1 months.
Figure 1 .
PRISMA flowchart
A total of 19 papers included tumour type, 14 included stage and only 5 included hormone receptor status. Five papers included patients with multifocal tumours, one of which all patients included had multifocal disease. Lymph node status was reported in 15 studies.
Only four papers included preoperative breast/bra size.20,35,47,48 Tumour location was reported in 23 papers, although there was variation in how location was described. The most commonly used oncoplastic volume replacement techniques included latissimus dorsi, TDAP, ICAP and LTAP flaps in 20, 12, 11 and 8 papers, respectively. Less commonly used flaps included thoracoepigastric, omental, medial circumflex femoral artery perforator (MCFAP) and internal mammary artery perforator (IMAP) flaps.
Mean tumour size was 29.7mm (17–65mm) and mean specimen weight was 123.6g (46.5–220g). Although the majority considered an involved or positive margin less than 1–2mm, there was some variation among institutions, with some considering a positive margin as less than 5mm,41 and others less than 10mm.26 Overall, there was an 11.3% (0–29.3%) positive margin rate. The re-excision rate, however, was only 7.2% (0–26.7%) because of the variability in what was deemed a positive margin. Conversion to mastectomy was only 2.3% (0–10.3%).
There was limited information on oncological outcomes (Table 1). Thirty-one papers were included in this assessment.11,14,16–20,22–27,29–31,33–43,45–48 A total of 1,729 patients were included, with a mean follow-up of 40.8 months (6–125 months). Locoregional and distant recurrence was 2.5% (0–8.1%) and 3.1% (0–14.6%) at 43.7 months and 36.4 months, respectively.
Table 1 .
Oncological outcomes in volume replacement surgery
| Authors | Year | Institution, country | Cases (n) | Mean follow-up (months) | Pathological characteristics | Stage (%) | Neoadjuvant therapy | PMR | RE | CM | Adjuvant therapy | LRR (%) | DR (%) | OS (%) | DFS (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soumian et al40 | 2020 | University Hospitals of North Midlands NHS Trust, UK | 112 | 15 | Inv 96 (85.7%); DCIS 16 (14.3%); ER + ve 85 (88.5%) | NR | Chemo 16 (14.3%) | 15 (13.4%) | 15 (13.4%) | 1 (0.9%) | Radio 110 (98.2%), chemo 31 (27.7%), endocrine 85 (75.9%) | 0.9 | 0.9 | NR | NR | NR |
| Jae Bong et al22 | 2019 | Kyungpook National University, Korea | 40 | 25.6 | NR | NR | NR | 3 (7.5%) | NR | NR | NR | NR | NR | NR | NR | NR |
| Zhou et al48 | 2019 | Sun Yat-sen University Cancer Centre, China | 32 | 12 | IDC 29 (91%); DCIS 1 (3%); other 2 (6%) | T1 13 T2 87 |
NR | 0 (0%) | 0 (0%) | 0 (0%) | Radio 32 (100%) | 0 | 0 | NR | NR | NR |
| Schwartz et al39 | 2018 | Georgia Breast Surgery, United States | 25 | 6 | NR | NR | Chemo 7 (28%) | 3 (12%) | 3 (12%) | 0 (0%) | Radio 25 (100%), chemo 6 (24%) | NR | NR | NR | NR | NR |
| Wang et al43 | 2017 | Peking University Cancer Hospital & Institute, China | 33 | 12 | NR | NR | NR | NR | NR | NR | NR | 0 | 0 | NR | NR | NR |
| Jae Bong et al23 | 2017 | Kyungpook National University, Korea | 33 | 25.2 | IDC 31 (93.9%) DCIS 2 (6.1%) | 0 6.1 1A 33.3 2A 33.3 2B 27.3 |
NR | 3 (9.1%) | 3 (9.1%) | NR | Radio 33 (100%), chemo 21 (63.6%) | NR | NR | NR | NR | NR |
| Harman et al18 | 2017 | St Mark’s Women’s Health Centre, New Zealand | 15 | 36 | NR | NR | NR | NR | NR | NR | Radio 15 (100%) | 0 | 0 | NR | NR | 0 |
| Lee et al29 | 2017 | Kyungpook National University, Korea | 90 | 72 | NR | 0 6.7 1A 47.8 2A 25.6, 2B 16.7, 3A 3.4 |
NR | NR | NR | NR | NR | 1.1 | 4.4 | 98.9 | NR | 1.1 |
| Khan et al25 | 2017 | Edinburgh Breast Unit, UK | 35 | 36 | NR | NR | NR | 8 (22.9%) | 8 (22.9%) | NR | Radio 35 (100%) | NR | NR | NR | NR | NR |
| Mele et al31 | 2017 | Winchester Breast Unit, UK | 261 | 125 | NR | NR | NR | NR | NR | NR | Radio 188 (83.6%) | 8 | NR | NR | NR | NR |
| Van Huizum et al42 | 2017 | Netherlands Cancer Institute, Netherlands | 12 | 35.3 | IDC 7 (58.3%) DCIS 3 (25%) ILC 1 (8.3%) Phyllodes 1 (8.3%) | T1 66.7, T2 8.3 | Chemo 3 (27.3%) | 1 (8.3%) | 1 (8.3%) | 1 (8.3%) | Radio 9 (81.1%), chemo 3 (37.5%), endocrine 2 (18.2%) | NR | NR | NR | NR | NR |
| Ho et al20 | 2016 | Queen Elizabeth University Hospital, UK | 30 | 48.5 | IDC 23 (76.7%) ILC 5 (16.7%) DCIS 1 (3.3%) mixed 1 (3.3%) ER + ve 79.3% PR + ve 72.4% HER2 +ve 13.8% |
0 3.3, 1A 26.7, 1B 3.3, 2A 53.3, 3A 3.3, 3C 3.3 | Chemo 2 (6.7%) | 3 (10%) | 3 (10%) | 0% | Radio 30 (100%), chemo 14 (46.7%), Herceptin 4 (13.3%), endocrine 22 (73.3%) | 3.3 | 0 | NR | NR | NR |
| Zaha et al46 | 2015 | Nakagami Hospital, Japan | 30 | 64 | NR | T0 20, T1 33.3, T2 46.7 | NR | 1 (3.3%) | 1 (3.3%) | 0% | NR | 0 | 0 | NR | NR | NR |
| Lee et al27 | 2014 | Kyungpook National University, Korea | 72 | 40.9 | IDC 63 (87.5%) ILC 2 (2.8%) DCIS 6 (8.3%) mucinous 1 (1.4%) ER + ve 76.4%, PR + ve 68.1%, HER2 +ve 18.1%, triple –ve 12.5% |
0 8.3, 1A 41.7, 2A 25, 2B 15.3, 3A 4.2, 3B 1.4, 3C 4.2 | Chemo 4 (5.6%) | NR | 0 (0%) | NR | Radio 68 (94.4%), chemo 49 (68.1%), Herceptin 49 (68.1%), endocrine 53 (73.6%) | 2.8 | 5.6 | NR | 39.7m | 0 |
| Kijima et al26 | 2013 | Kagoshima University Graduate School of Medicine and Dental Sciences, Japan | 15 | 11.2 | T1 93.3, T2 6.7 | Chemo/radio/endocrine 0 | NR | NR | NR | Radio 5 (33.3%), chemo 6 (40%), endocrine 14 (93.3%) | 0 | 0 | NR | NR | NR | |
| Hamdi et al17 | 2013 | Brussels University Hospital, Belgium | 93 | 48 | NR | NR | NR | 2 (2.2%) | NR | NR | NR | NR | NR | NR | NR | NR |
| Rose et al38 | 2012 | Sydvestjysk Sygehus, Denmark | 15 | NR | IDC 15 (100%) | NR | NR | 4 (26.7%) | 4 (26.7%) | 0 (0%) | Radio 15 (100%) | NR | 6.7 | 93.3 | 93.3 | 6.7 |
| Hernanz et al19 | 2011 | University of Cantabria, Spain | 41 | 58 | IDC 27 (65.9%) ILC 10 (24.4%) Phyllodes/apocrine/solid/mixed each 1 (2.4%), ER + ve 46.3% PR + ve 53.7% HER2 +ve 26.8% triple –ve 31.7% |
NR | NR | 12 < 2mm (29.3%), 0 involved | 0 (0%) | NR | Radio 38 (100%) | 2.4 | 14.6 | NR | NR | 7.3 |
| El-Marakby et al14 | 2011 | National Cancer Institute, Cairo, Egypt | 50 | 33 | IDC 82%, ILC 10% |
NR | NR | 0 (0%) | 0 (0%) | 0 (0%) | Radio 92%, chemo 86%, endocrine 74% | 4 | 2 | NR | NR | NR |
| Zaha et al47 | 2010 | Nakagami Hospital, Japan | 24 | 35 | NR | Tis 25, T1 8, T2 50, T3 17 | NR | 1 (4.2%) | NR | NR | Radio 54% | 0 | 0 | NR | NR | NR |
| Almasad et al11 | 2008 | Jordan University Hospital, Amman, Jordan | 25 | 48 | IDC 21 (84%) medullary 3 (12%) tubulolobular 1 (4%) | NR | Chemo 1 (4%) | NR | NR | NR | Radio 25 (100%), chemo 24 (96%) | 8 | 4 | NR | NR | 4 |
| Navin et al35 | 2007 | Bassetlaw Hospital, UK | 51 | 33 | IDC 40 (78.5%) ILC 3 (5.9%) Mixed inv 4 (7.9%) DCIS 2 (3.9%), medullary/mucinous each 1 (1.9%) |
NR | NR | 2 (3.9%) | 0 (0%) | 4 (7.8%) | Radio 47 (92.2%) | 0 | 0 | 100 | 100 | 0 |
| Naguib et al34 | 2006 | National Cancer Institute Cairo, Egypt | 29 | 19 | NR | NR | Chemo 7 (24.1%) | NR | NR | NR | Radio 29 (100%) | 0 | 6.9 | NR | NR | NR |
| Munhoz et al33 | 2006 | University of Sao Paulo, Brazil | 34 | 23 | NR | NR | NR | NR | NR | NR | Radio 34 (100%), chemo 12 (35.3%) | 0 | NR | NR | NR | NR |
| Takeda et al41 | 2005 | Tohoku University School of Medicine, Japan | 266 | 72 | NR | NR | NR | NR | NR | NR | Radio 165 (62%) | 5.6 | NR | NR | NR | NR |
| Woerdeman et al44 | 2004 | Netherlands Cancer Institute, Netherlands | 20 | 60 | NR | NR | Radio 20 (100%) | 5 (25%) | NR | NR | Chemo 5 (25%), endocrine 8 (40%) | 5 | NR | NR | NR | 35 |
| Losken et al30 | 2004 | Emory University School of Medicine, United States | 39 | 44.4 | IDC 30 (77%), ILC 4 (10.3%), DCIS 2 (5.1%), Phyllodes 2 (5.1%), ADH 1 (2.5%), ER/PR + ve 64%, ER/PR −ve 20% | NR | Chemo 5 (13%), radio 1 (2.6%) | 9 (23.1%) | 4 (10.3%) | 4 (10.3%) | Radio 33 (91.7%) | 5.1 | 7.7 | 94.9 | 84.6 | 5.1 |
| Gendy et al16 | 2003 | Royal Hampshire County Hospital, UK | 49 | 53 | IDC 37 (75.5%), ILC 2 (4.1%), mixed 2 (4.1%), DCIS 4 (8.1%), special type 4 (8.1%) | NR | NR | 0 (0%) | 0 (0%) | 0 (0%) | Radio 37 (75.5%) | 4.1 | NR | NR | NR | NR |
| Kat et al24 | 1999 | Stoke Mandeville Hospital NHS Trust, UK | 30 | NR | IDC 27 (90%), ILC 2 (6.7%), tubular 1 (3.3%) | NR | NR | 0 (0%) | 0 (0%) | 0 (0%) | Radio 30 (100%) | NR | NR | NR | NR | NR |
| Rainsbury et al37 | 1998 | Royal Hampshire County Hospital, UK | 62 | 43 | NR | NR | NR | 8 (12.9%) | 4 (6.5%) | 0 (0%) | Radio 32 (54.8%) | 8.1 | NR | NR | NR | NR |
| Ohuchi et al36 | 1997 | Tohoku University School of Medicine, Japan | 66 | 48 | IDC 66 (100%) | NR | NR | NR | NR | NR | NR | 0 | NR | NR | NR | 0 |
ADH, atypical ductal hyperplasia; CM, completion mastectomy; DCIS, ductal carcinoma in situ; DFS, disease-free survival; DR, distant recurrence; ER, estrogen receptor; IDC, invasive ductal carcinoma; ILC, infiltrating ductal carcinoma; Inv, invasive; LRR, locoregional recurrence; NR, not recorded; OS, overall survival; PMR, positive margin rate; PR, progesterone receptor; RE, re-excision rate; +ve, positive; –ve, negative
Overall survival and disease-free survival were 96.8% (93.3–100%) and 92.6% (84.6–100%) at 49.8 months and 39.0 months respectively, with a mortality rate of 5.9% (0–35.0%) at a mean follow-up of 48.9 months. The mortality was skewed due to low numbers of papers reporting these data and one outlying paper, which had a mortality rate of 35% at a mean follow-up of 60 months.44 In this paper, all patients were given preoperative radiotherapy and had a positive margin rate of 25%. Discounting this paper, the mean mortality is 2.7% (0–7.3%) at 47.5 months.
Several of the older studies were involved with adjuvant radiotherapy trials at the same time as patient recruitment to the volume replacement studies.37,44 There were also institutions that did not employ routine adjuvant radiotherapy,26,27,41 hence there is a considerable discrepancy in its use, ranging from 33.3% to 100%, with more recent studies tending towards 100%.11,18–20,23,25,33,34,38,39,48
Overall complications ranged from 0% to 65.7%, with a mean of 21.1%. Complications described were divided into minor (I–II) and major (III–IV) as per the Clavien–Dindo classification: 17.1% (0–52%) and 5.6% (0–13.7%), respectively. The majority of these complications were seroma formation (particularly donor site), fat necrosis, haematoma and wound infection. These results demonstrate that most volume replacement surgery complications can be managed conservatively. There was little information on complications leading to delay in adjuvant therapy, with only four studies mentioning this; three leading to no delay and one study quoting 16% of patients experiencing delay secondary to complications.39 Patient-reported and cosmetic outcomes (Table 2) lacked standardisation; however, among patients the Breast-Q,13,23,25 with satisfaction expressed on a scale of 1–1016 or poor to excellent,10–12,22 and the Modified Michigan cosmetic and overall outcomes6,45 were most commonly used. In terms of surgeon reported cosmetic outcome, panel assessments were most frequently used.12,18,19,22,46,47 Overall, results tended towards good to excellent outcomes as reported by the patients and surgeons.
Table 2 .
Patient reported outcomes and cosmetic outcomes
| Authors | Year | Institution/Country | VR Technique (%) | Overall complications (%) | Complications CD I-II (%) | Complications CD III-IV (%) | PROMs | Surgeon-reported outcomes |
|---|---|---|---|---|---|---|---|---|
| Soumian et al40 | 2020 | University Hospitals of North Midlands NHS Trust/UK | LICAP (± LTAP) 75, LTAP 2.7, AICAP 12.5, MICAP 9.8 | 7.1 | NR | 2.7 | NR | NR |
| Gwak et al15 | 2020 | The Catholic University of Korea, Korea | Diced ADM 100 | 20.5 | 12 | 8.5 | Cosmetic satisfaction 9.7/10, overall satisfaction 9.5/10 | Cosmetic satisfaction 9.7/10, overall satisfaction 9.4/10 |
| Abdelrahman et al10 | 2019 | Benha University, Egypt | LD 50, TDAP 50 | 40.5 | NR | NR | Patient satisfaction: excellent 26.2%, good 52.4%, fair 11.9%, poor 9.5% | NR |
| Jae Bong et al22 | 2019 | Kyungpook National University, Korea | LICAP 100 | 22.5 | NR | NR | Cosmetic satisfaction: excellent 57.5%, good 37.5%, fair 7.5% | Panel assessment: excellent 47.5%, good 42.5%, fair 10.0% |
| Zhou et al48 | 2019 | Sun Yat-sen University Cancer Centre, China | LD miniflap 100 | 9 | NR | NR | DASH 10.6/30, cosmetic satisfaction > 90% very or extremely | NR |
| Schwartz et al39 | 2018 | Georgia Breast Surgery, USA | LICAP 100 | 40 | 40 | 0 | NR | NR |
| Chand et al13 | 2017 | Royal Hampshire County Hospital, UK | LD miniflap 100 | NR | NR | NR | BREAST-Q: overall experience excellent or very good 78%, preferable to mastectomy 88%, would undergo again 73% | NR |
| Wang et al43 | 2017 | Peking University Cancer Hospital and Institute, China | TDAP 100 | 6.1 | NR | NR | NR | NR |
| Amin et al12 | 2017 | National Cancer Institute, Egypt | TDAP 100 | 20 | NR | 2.5 | Cosmetic satisfaction: excellent 10.0%, good 70.0%, fair 15.0%, poor 5.0% | Panel assessment: excellent 5.0%, good 57.5%, fair 30.0%, poor 7.5% |
| Jae Bong et al23 | 2017 | Kyungpook National University, Korea | LICAP 57.6, TDAP 42.2 | 48.5 | NR | 12.1 | Modified BREAST-Q: excellent 45.5%, good 39.4%, fair 15.2% | NR |
| Harman et al18 | 2017 | St Mark’s Women’s Health Centre, NZ | Biozorb (polylactic acid and titanium clips) 100 | 6.7 | 6.7 | NR | NR | Panel assessment: 8% palpable at 12m, all excellent at 36 months |
| Lee et al29 | 2017 | Kyungpook National University, Korea | ICAP/LTAP, TDAP, LD – numbers NR | NR | NR | NR | NR | NR |
| Khan et al25 | 2017 | Edinburgh Breast Unit, UK | Lipofilling 100 | NR | NR | NR | BREAST-Q – VR better than standard BCS in all domains | NR |
| Mele et al31 | 2017 | Winchester Breast Unit, UK | LD miniflap 100 | NR | NR | NR | NR | NR |
| Van Huizum et al42 | 2017 | Netherlands Cancer Institute, The Netherlands | IMAP 100 | 0 | 0 | 0 | Breast cosmesis 7.9/10, nipple cosmesis 9.3/10 | NR |
| Ho et al20 | 2016 | Queen Elizabeth University Hospital, UK | TE 43.3, LICAP 16.7, matrix rotation 26.7, TDAP 6.7, LTAP 3.3, crescent 3.3 | 26.7 | 20 | 6.7 | NR | NR |
| Zaha et al46 | 2015 | Nakagami Hospital, Japan | Omental 100 | 10 | 6.7 | 3.3 | BCCT.core: excellent 43.3%; good 43.3%, fair 13.3%, poor 0% | Panel assessment: excellent 66.7%, good 20.0%, fair 3.3%, poor 10.0% |
| Lee et al27 | 2014 | Kyungpook National University, Korea | LD 63.4, ICAP 13.4, LTAP 10.2, TDAP 9.3, TE 3.7 | 14.1 | NR | NR | Cosmetic satisfaction: 82.3% satisfied |
NR |
| Park et al6 | 2014 | Daegu Fatima Hospital, Korea | ICAP 50, LD 21.4, TE 14.3, TDAP 7.1 | 21.4 | NR | NR | Modified Michigan: cosmetic satisfaction 86.0%, overall satisfaction 79.0% | Panel assessment 1–5: 4.16/5 |
| Lee et al28 | 2014 | Kyungpook National University, Korea | ICAP 34.7, LTAP 18.1, ELD 18.1, TDAP 15.3, LD 6.9, TE 4.2, inf chest wall 2.8 | 18.1 | 18.1 | NR | NR | NR |
| Morishima et al32 | 2014 | Osaka Rosai Hospital, Japan | Lateral tissue flap 100 | NR | NR | NR | NR | Sawai’s criteria: Bp 8.87, Bq 6.67 |
| Izumi et al21 | 2013 | Osaka General Medical Center, Japan | MCFAP 100 | 20 | NR | NR | NR | Photographic assessment: excellent 53.3%, very good 26.7%, good 13.3%, fair 6.7% |
| Kijima et al26 | 2013 | Kagoshima University Graduate School of Medicine and Dental Sciences, Japan | TDAP 100 | NR | NR | NR | NR | Sawai's criteria: excellent 36.3%, good 63.6% |
| Hamdi et al17 | 2013 | Brussels University Hospital, Belgium | LD/TDAP/LICAP/SAAP – numbers NR | 14.0 | NR | 3.2 | NR | NR |
| Yang et al45 | 2012 | Kyungpook National University, Korea | LD 50.5, ICAP 23.4, TDAP 11.2, LTAP 8.4, TE 6.5 | 13.5 | NR | NR | Modified Michigan: cosmetic satisfaction 76.9%, overall satisfaction 81.7% | NR |
| Rose et al38 | 2012 | Sydvestjysk Sygehus, Denmark | LTAP 100 | 6.7 | 0 | 6.7 | NR | NR |
| Hernanz et al19 | 2011 | University of Cantabria, Spain | LD 100 | NR | NR | NR | NR | Panel assessment: excellent 6.8%, good 51.7%, fair 37.9% BCCT.core: excellent 13.0%, good 52.2%, fair 24.8% |
| El-Marakby et al14 | 2011 | National Cancer Institute, Egypt | LD 90, LD miniflap 10 | 18 | NR | 12 | NR | NR |
| Zaha et al47 | 2010 | Nakagami Hospital, Japan | Omental 100 | 12.5 | 8.3 | 4.2 | NR | Panel assessment: excellent 67%, good 26%, fair 4%, poor 0%, BRA 0.5 |
| Almasad et al11 | 2008 | Jordan University Hospital, Amman | Lateral tissue flap 100 | NR | NR | NR | Cosmetic satisfaction: very good 56.0%, good 28.0%, fair 16.0% | NR |
| Navin et al35 | 2007 | Bassetlaw Hospital, Worksop, UK | LD miniflap 100 | 2.0 | NR | NR | Overall satisfaction 86% | NR |
| Naguib et al34 | 2006 | National Cancer Institute, Egypt | LD 100 | 65.7 | 52.0 | 13.7 | Cosmetic satisfaction: overall satisfaction 69%, asymmetry 17.2%, skin colour discrepancy 6.9%, NAC discrepancy 6.9% | NR |
| Munhoz et al33 | 2006 | University of Sao Paulo School of Medicine, Brazil | LTAP 100 | 52.9 | 23.5 | 8.8 | Cosmetic satisfaction very good–satisfactory: breast shape 96.8%, NAC 97.9%, breast symmetry 95.9%, overall cosmesis 97.0%, regret surgery 0% | Panel assessment: Overall cosmetic result very good-satisfactory 97.0% |
| Takeda et al41 | 2005 | Tohoku University School of Medicine, Japan | Lateral tissue flap 100 | NR | NR | NR | NR | NR |
| Woerdeman et al44 | 2004 | Netherlands Cancer Institute, The Netherlands | LD 100 | NR | NR | NR | Cosmetic satisfaction: 2.8/3 | Panel assessment of cosmesis: 2.6/3 |
| Losken et al30 | 2004 | Emory University School of Medicine, Atlanta, USA | LD 100 | 31.0 | 12.8 | 2.6 | NR | NR |
| Gendy et al16 | 2003 | Breast Unit, Royal Hampshire County Hospital, UK | LD miniflap 100 | 18.4 | 6.4 | 12.0 | Cosmetic outcome 83.5%, freedom of dress 89.0%, altered skin sensation 10%, altered NAC sensation 2% | Photographic panel assessment: 3.8, BRA: 2.2 |
| Kat et al24 | 1999 | Stoke Mandeville Hospital NHS Trust, UK | LD 100 | 33.3 | 33.3 | 0 | NR | NR |
| Rainsbury et al37 | 1998 | Royal Hampshire County Hospital, UK | LD miniflap 100 | 11.3 | NR | NR | Physical and psychological assessment – more freedom of dress and less worried about residual cancer compared with BCS | BRA 10% < 2.0 |
| Ohuchi et al36 | 1997 | Tohoku University School of Medicine, Japan | Lateral tissue flap 84.4, LD 15.2 | NR | NR | 1.6 | NR | NR |
ADM, acellular dermal matrix; AICAP, anterior intercostal artery perforator; BCS, breast conserving surgery; Bp, partial breast resection; Bq, breast quadrantectomy; BRA, breast retraction assessment; DASH, disabilities of arm, shoulder and hand; ELD = extended latissimus dorsi; IMAP, internal mammary artery perforator; LICAP, lateral intercostal artery perforator; LD, latissimus dorsi; LTAP, lateral thoracic artery perforator; MCFAP, medial circumflex femoral artery perforator; MICAP, medial intercostal artery perforator; NAC, nipple–areolar complex; NR, not recorded; PROMs, patient-reported outcome measures; TDAP, thoracodorsal artery perforator; TE, thoracoepigastric; VR, volume replacement.
Discussion
As with any emerging technique, oncological safety is of prime concern. OBCS has the advantage of allowing for higher volume surgical resection, thereby reducing positive resection margins and subsequent re-excision and mastectomy rates. This may have the added benefit of preventing delays in adjuvant therapy due to lower rates of positive margins encountered and obviating further surgery.49 OBCS can also improve cosmesis and patient satisfaction as compared with mastectomy and standard breast conserving surgery.
Oncological safety: resection margins and re-excision
Re-excision rates are of the upmost importance in allowing for accurate assessment of tumour size, margin status and local recurrence, as well as minimising impact on cosmetic outcome.
A 1mm margin is generally accepted as clear for both ductal carcinoma in situ and invasive breast cancer, and does not warrant re-excision according to the Association of Breast Surgeons guidelines.50 National Institute for Health and Care Excellence guidelines recommend margins of 2mm or more in breast cancer resection specimens. However, margins of greater than 1mm are not associated with lower recurrence rates. Positive surgical margins occur in 20–40% of all conventional breast conserving surgery, with 20% undergoing re-excision.51,52
Previous studies have clearly demonstrated a reduction in re-excision rates in the setting of OBCS as compared with standard breast conserving surgery or wide local excision.53,54 Despite this, some critics are concerned that tissue rearrangement may inhibit the ability to perform an accurate re-excision.
De La Cruz et al carried out the largest comprehensive literature review assessing oncological safety in OBCS. They evaluated over 6,000 patients and found re-excision rates of 6.0%.49 Crown et al assessed a total of 812 patients undergoing either OBCS or wide local excision. Of these, 18% underwent re-excision in the OBCS cohort, as compared with 32% in the standard wide local excision group (p < 0.0001).54 These findings are supported by a similar study by Chakravorty et al, who reported significantly lower rates of re-excision in the OBCS group (2.7%) as compared with standard breast conserving surgery (13.4%; p < 0.001).53 This study supports these findings, demonstrating a re-excision rate of 7.2% in the setting of volume replacement OBCS, but it also highlights that the management of positive margins is not standardised.
Oncological safety: conversion to mastectomy rates
Several papers have demonstrated a reduction in mastectomy rates with the introduction of OBCS. Crown et al demonstrated that, in the OBCS cohort, 15% required completion mastectomy, as compared with 34% in the wide local excision cohort (p < 0.001), despite the average tumour size in the OBCS group being larger.16 In this study, the whole mean tumour size was 29.7mm, with a completion mastectomy rate of only 2.3%.
Oncological safety: adjuvant therapy
Radiotherapy is advocated in breast conserving surgery, whether conventional or oncoplastic. Current UK guidelines recommend that adjuvant therapy be commenced within 31 days of completion of surgery wherever clinically possible. Delaying radiotherapy beyond eight weeks has been demonstrated to have a detrimental effect on local recurrence.5 Yoon et al also highlighted that boost after whole breast radiotherapy has been demonstrated to reduce local recurrence. This finding is of particular importance in this setting, as young women are at greater risk of local recurrence and more likely to undergo OBCS.55 There was very little information in this review about the impact of complications in the setting of volume replacement and its impact on adjuvant therapy.
Oncological safety: local and distant disease recurrence rates
Evidence relating to local and distant recurrence rates after OBCS have shown similarities to mastectomy, together with striking similarities in histopathological results.56 Previously, this has been demonstrated by Mansell et al in consecutive patients treated with breast conserving surgery, mastectomy with or without reconstruction and OBCS.57 Histological variables including tumour size, grade, nodal and hormonal status were found to be more adverse in OBCS compared with breast conserving surgery and similar to mastectomy. Five-year local recurrence was found to be similar in all three groups, while distant recurrence was higher after mastectomy and OBCS, most probably related to tumour biology. This is supported by both Losken and Chand, who found that therapeutic mammoplasty and volume replacement with mini-latissimus dorsi flaps, respectively, had no effect on local recurrence.13,58 However, Chakravorty et al demonstrated similar local recurrence rates of 2.7% and 2.2.%, but distant recurrence rates of 1.3% and 7.5% for OBCS and breast conserving surgery, respectively,53 but this study had a median follow-up of only 28 months.53
Yoon et al assessed 10 papers in their systematic review, where local recurrence ranged from 0% to 10% at a mean follow-up of 40 months.55 This systematic review once again supported these findings, with a locoregional and distant recurrence rate of 2.5% and 3.1% and a mean follow-up of 43.7 months and 36.4 months, respectively.
Oncological safety: survival
There is little data on overall and disease-free survival in the setting of volume replacement surgery specifically. However, in reference to OBCS, five-year disease-free survival has been found to be 91.7%, overall survival 93.8% and cancer-specific survival 96.1% in previous studies.59 This is comparable with the findings in this review, with overall and disease-free survival of 96.8% (93.3–100%) and 92.6% (84.6–100%) at 49.8 months and 39.0 months, respectively. However, it is important to highlight that the limited studies reporting these data were mostly in the context of latissimus dorsi reconstruction.
Aesthetic outcome
Breast appearance after surgery can have a significant psychological impact on patients. Despite this, there is no consensus on how best to evaluate breast cosmesis. There are multiple factors that have the potential to affect cosmesis. These include tumour location, adjuvant therapy and patient factors; however, volume of tissue excised compared with breast volume is the single most important factor influencing cosmetic outcome.
Estimated percentage of breast volume excised has been shown to have a significant effect on patient satisfaction. By estimating volume through mammograms, subjective cosmetic assessment tools can be used to measure patient satisfaction. In relation to breast conserving surgery, studies have demonstrated less than 10% estimated percentage of breast volume excised correlates with a majority of patients being satisfied (83.5%), as compared with over 10% where volume is significantly reduced (37%).60 In terms of location in the setting of conventional breast conserving surgery, Pukancsik et al demonstrated maximum breast volumes that were resectable without resulting in unacceptable aesthetic and functional outcomes or decreased quality of life. Percentage volumes were 18–19% in the upper outer quadrant (p < 0.0001), 14–15% in the lower outer quadrant (p < 0.0001), 8–9% in the upper inner quadrant (p < 0.0001) and 9–10% in the lower inner quadrant (p < 0.0001).61
In the setting of OBCS, patient satisfaction remains high with volume excision of less than 20%.62 Once 20% of breast volume or more is excised, there is a significant risk of deformity. However, tumours located in the upper inner quadrant and lower pole have been found more commonly to lead to breast deformity, even when the volume excised is less than 20%.63
Current methods for evaluating cosmetic outcome vary widely from clinical assessment to photographic and geometric tools. Scoring methods most commonly involve subjective evaluation through patient self-evaluation and panel evaluation.64 Studies have demonstrated that results between panel evaluation and observers, regardless of breast surgery experience, have similar results, indicating that a reliable cosmetic outcome score can be achieved. Objective evaluation is becoming increasingly widespread due to the increased efficiency this offers. This can be carried out through breast cancer conservation treatment – cosmetic results (BCCT.core) software. Studies have demonstrated BCCT.core software can provide valid cosmetic outcome scores when compared with panel evaluation. Not only does this allow for rapid and accurate assessment, it can also facilitate comparison among units.51 A patient-centred cosmetic scoring method that is reliable and reproducible still requires development to aid decision making.
Patient-reported outcome measures
There is no standardised PROM currently used routinely in the setting of OBCS, although several tools are in existence. The BREAST-Q is a validated questionnaire-based tool using a Likert scale, which assesses physical, psychological and sexual wellbeing, cosmetic appearance and overall satisfaction.
Chand et al used the BREAST-Q questionnaire to assess breast appearance, physical, emotional and sexual wellbeing in patients who underwent either therapeutic mammoplasty or mini-latissimus dorsi flap with those who underwent mastectomy and immediate autologous reconstruction. Overall satisfaction was high in both groups, with 82% reporting ‘excellent/very good’ (mammoplasty 88%; mini-latissimus dorsi 78%), with therapeutic mammoplasty patients being significantly more satisfied with breast shape (p < 0.05), size (p < 0.05) and natural feel (p < 0.01) as compared with the mini-latissimus dorsi group, although they demonstrated similar scores for physical and emotional wellbeing; 89% felt that OBCS was better than mastectomy. Mean outcome scores for breast appearance, physical and emotional wellbeing persisted beyond 15 years.13
Kelsall et al used the validated Hopwood body image scale scores of psychosocial function and PROMs for breast appearance and return to function analysis comparing case-matched OBCS with mastectomy and immediate reconstruction. They found that overall body image scale score (p = 0.002), self-rated breast appearance, return to work and function (all p < 0.001) significantly favoured OBCS. This difference was most marked in women with larger breasts.65
Limitations
There are several difficulties interpreting the literature on volume replacement OBCS. Several papers were excluded purely on difficulty in separating volume displacement from replacement. The majority of publications that are included are small, single-centre observational studies with heterogeneous patient groups and short-term follow-up. The data gathered are hugely variable and tend towards focusing on oncological outcomes, cosmetic outcomes or complications. Few studies cover all these parameters.
There are a few studies included that were conducted prior to the standardised treatment of radiotherapy following breast conserving surgery or OBCS and, as such, some patients may have had this preoperatively or omitted all together, influencing rates of local recurrence and skewing data as a result.
There is variability in what is considered a positive margin, depending on when and where the study was conducted. Eastern papers have a tendency to manage involvement of less than 10mm with radiotherapy (rather than re-excision less than 1mm) and greater than 20mm margin without.
There is paucity of data on recurrence and survival, with most of the limited data available concerning latissimus dorsi reconstruction.
In terms of function and cosmesis, there are no standardised PROMs in the setting of OBCS. There is also no standardised panel assessment. With this type of assessment comes observer expectancy bias.
Conclusion
This systematic review demonstrates that volume replacement surgery is safe compared with breast conserving surgery and mastectomy. It also demonstrates that cosmetic outcomes are generally favourable, with improved outcomes when compared with breast conserving surgery and mastectomy. However, there currently remains a lack of high-level evidence supporting the oncological safety of volume replacement OBCS.66 The interpretation and strength of data within this review must be regarded with care due to limited numbers of studies on this subject, the large variation in patient numbers within papers and the heterogeneity of data reported. There is a need for prospective multicentre studies directly comparing standard wide local excision/lumpectomy with OBCS in the setting of volume replacement.
The type of volume replacement OBCS employed is based on a range of clinical, oncological and patient factors. As OBCS continues to become more popular, achieving a balance between oncological and breast form must be sought.
Acknowledgements
The authors would like to thank the Breast Team at Gartnavel General Hospital, Glasgow. The authors would also like to thank Kirsty Coltart, Beatson Librarian, for her assistance with the literature search.
References
- 1.World Health Organization. Preventing cancer. http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en (cited March 2021).
- 2.Bertozzi N, Pesce M, Santi PLet al. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci 2017; 21: 2572–2585. [PubMed] [Google Scholar]
- 3.Morrow ES, Stallard S, Doughty Jet al. Oncoplastic breast conservation occupies a niche between standard breast conservation and mastectomy: a population-based prospective audit in Scotland. Eur J Surg 2019; 45: 1806–1811. 10.1016/j.ejso.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 4.Masannat YA, Agrawal A, Maraqa Let al. Multifocal and multicentric breast cancer, is it time to think again? Ann R Coll Surg Engl 2020; 102: 62–66. 10.1308/rcsann.2019.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell EJ, Romics L. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer 2017; 9: 521–530. 10.2147/BCTT.S113742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HC, Kim HY, Kim MCet al. Partial breast reconstruction using various oncoplastic techniques for centrally located breast cancer. Arch Plast Surg 2014; 41: 520–528. 10.5999/aps.2014.41.5.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raja MA, Straker VF, Rainsbury RM. Extending the role of breast-conserving surgery by immediate volume replacement. Br J Surg 1997; 84: 101–105. 10.1046/j.1365-2168.1997.02477.x [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Rainsbury RM, Segaran Aet al. Objective assessment of clinical, oncological and cosmetic outcomes following volume replacement in patients undergoing oncoplastic breast-conserving surgery:protocol for a systematic review. BMJ Open 2018; 8: e020859. 10.1136/bmjopen-2017-020859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff Jet al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: 2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abderahman EM, Nawar AM, Balbaa MAet al. Oncoplastic volume replacement for breast cancer: latissimus dorsi flap versus thoracodorsal artery perforator flap. Plast Reconstr Surg Glob Open 2019; 7: e2476. 10.1097/GOX.0000000000002476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almasad JK, Salah B. Breast reconstruction by local flaps after conserving surgery for breast cancer: An added asset to oncoplastic techniques. Breast J 2008; 14: 340–344. 10.1111/j.1524-4741.2008.00595.x [DOI] [PubMed] [Google Scholar]
- 12.Amin A, Rifaat M, Farahat Aet al. The role of thoracodorsal artery perforator flap in oncoplastic breast surgery. J Egypt Natl Canc Inst 2019; 29: 83–87. 10.1016/j.jnci.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 13.Chand ND, Browne V, Paramanathan Net al. Patient-reported outcomes are better after oncoplastic breast conservation than after mastectomy and autologous reconstruction. PRS Global Open 2017; 5: 1419. 10.1097/GOX.0000000000001419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Marakby HH, Kotb MH. Oncoplastic volume replacement with latissimus dorsi myocutaneous flap in patients with large ptotic breasts. Is it feasible? J Egypt Natl Canc Inst 2011; 23: 163–169. 10.1016/j.jnci.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 15.Gwak H, Jeon YW, Lim STet al. Volume replacement with diced acellular dermal matrix in oncoplastic breast-conserving surgery: a prospective single-center experience. World J Surg Oncol 2020; 18: 60. 10.1186/s12957-020-01835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendy R, Able JA, Rainsbury RMet al. Impact of skin-sparing mastectomy with immediate reconstruction and breast-sparing reconstruction with miniflaps on the outcomes of oncoplastic breast surgery. Br J Surg 2003; 90: 433–439. 10.1002/bjs.4060 [DOI] [PubMed] [Google Scholar]
- 17.Hamdi M. Oncoplastic and reconstructive surgery of the breast. Breast 2013; 22: S100–S105. 10.1016/j.breast.2013.07.019 [DOI] [PubMed] [Google Scholar]
- 18.Harman J, Govender S, Simpson Jet al. A new method for partial breast reconstruction: 3-year New Zealand experience. Plast Reconstr Surg 2019; 143: 49–52. 10.1097/PRS.0000000000005079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernanz F, Sánchez S, Cerdeira MPet al. Long-term results of breast conservation and immediate volume replacement with myocutaneous latissimus dorsi flap. World J Surg Oncol 2011; 9: 159. 10.1186/1477-7819-9-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho W, Stallard S, Doughty Jet al. Oncological outcomes and complications after volume replacement oncoplastic breast conservations: the Glasgow experience. Breast Cancer (Auckl ) 2016; 10: 223–228. 10.4137/BCBCR.S41017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izumi K, Fujikawa M, Tashima Het al. Immediate reconstruction using free medial circumflex femoral artery perforator flaps after breast-conserving surgery. J Plast Reconstr Aesthet Surg 2013; 66: 1528–1533. 10.1016/j.bjps.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Kim JB, Eom JR, Lee JWet al. Utility of two surgical techniques using a lateral intercostal artery perforator flap after breast-conserving surgery: a single-centre retrospective study. Plast Reconstr Surg 2019; 143: 477–487. 10.1097/PRS.0000000000005374 [DOI] [PubMed] [Google Scholar]
- 23.Kim JB, Kim DK, Lee JWet al. The usefulness of pedicle perforator flap in partial breast reconstruction after breast conserving surgery in Korean women. Arch Plast Surg 2017; 45: 29–36. 10.5999/aps.2017.01200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kat C, Darcy CM, O’Donoghue JMet al. The use of the latissimus dorsi musculocutaneous flap for immediate correction of the deformity resulting from breast conservation surgery. Br J Plast Surg 1999; 52: 99–103. 10.1054/bjps.1997.3035 [DOI] [PubMed] [Google Scholar]
- 25.Khan L, Raine CR, Dixon JM. Immediate lipofilling in breast conserving surgery. Eur J Surg Oncol 2017; 43: 1402–1408. 10.1016/j.ejso.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 26.Kijima Y, Yoshinaka H, Hirata Met al. Oncoplastic surgery combining partial mastectomy and immediate volume replacement using a thoracodorsal adipofascial cutaneous flap with a crescent-shaped dermis. Surg Today 2014; 44: 2098–2105. 10.1007/s00595-013-0812-1 [DOI] [PubMed] [Google Scholar]
- 27.Lee JW, Kim MC, Park HYet al. Oncoplastic volume replacement techniques according to the excised volume and tumor location in small- to moderate-sized breasts. Gland Surg 2014; 3: 14–21. 10.3978/j.issn.2227-684X.2014.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JW, Jung JH, Kim WWet al. Oncologic outcomes of volume replacement technique after partial mastectomy for breast cancer: a single center analysis. Surg Oncol 2015; 24: 35–40. 10.1016/j.suronc.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, Jung JH, Kim WWet al. Comparison of 5-year oncological outcomes of breast cancer based on surgery type. A N Z J Surg 2018; 88: E395–E399. 10.1111/ans.14017 [DOI] [PubMed] [Google Scholar]
- 30.Losken A, Schaefer TG, Carlson GWet al. Immediate endoscopic latissimus dorsi flap: risk or benefit in reconstructing partial mastectomy defects. Ann Plast Surg 2004; 53: 1–5. 10.1097/01.sap.0000106425.18380.28 [DOI] [PubMed] [Google Scholar]
- 31.Mele S, Wright D, Paramanathan Net al. Long-term effect of oncoplastic breast-conserving surgery using latissimus dorsi miniflaps on mammographic surveillance and the detection of local recurrence. J Plast Reconstr Aesthet Surg 2017; 70: 1203–1209. 10.1016/j.bjps.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 32.Morishima H, Matsunami N, Hasegawa Jet al. Cosmetic evaluation following volume replacement using lateral tissue flap in breast-conserving surgery. Breast Cancer (Auckl ) 2014; 21: 28–32. 10.1007/s12282-012-0355-0 [DOI] [PubMed] [Google Scholar]
- 33.Munhoz AM, Montag E, Arruda EGet al. Critical analysis of reduction mammaplasty techniques in combination with conservative breast surgery for early breast cancer treatment. Plast Reconstr Surg 2006; 117: 1091–1107. 10.1097/01.prs.0000202121.84583.0d [DOI] [PubMed] [Google Scholar]
- 34.Naguib S. Expanding the role of breast conservation surgery by immediate volume replacement with the latissimus dorsi flap. J Egypt Natl Canc Inst 2006; 18: 216–226. [PubMed] [Google Scholar]
- 35.Navin C, Agrawal A, Kolar KMet al. The use of latissimus dorsi miniflap for reconstruction following breast-conserving surgery: experience of a small breast unit in a district hospital. World J Surg 2007; 31: 46–50. 10.1007/s00268-006-0396-7 [DOI] [PubMed] [Google Scholar]
- 36.Ohuchi N, Harada Y, Ishida Tet al. Breast-Coserving surgery for primary breast cancer: immediate volume replacement using lateral tissue flap. Breast Cancer (Auckl ) 1997; 4: 135–141. 10.1007/BF02967067 [DOI] [PubMed] [Google Scholar]
- 37.Rainsbury RM, Paramanathan N. Recent progress with breast-conserving volume replacement using latissimus dorsi miniflaps in UK patients. Breast Cancer (Auckl ) 1998; 5: 139–147. 10.1007/BF02966686 [DOI] [PubMed] [Google Scholar]
- 38.Rose M, Svensson H. Tunnelled lateral fasciocutaneous thoracodorsal flap with a skin island in breast reconstruction in oncoplastic breast surgery. J Plast Surg Hand Surg 2012; 46: 404–409. 10.3109/2000656X.2012.722095 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz JD. New approach to oncoplastic breast conservation: combining autologous volume replacement and the wise-pattern mammaplasty. Plast Reconstr Surg Glob Open 2018; 6: e1987. 10.1097/GOX.0000000000001987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soumian S, Chandarana M, Marla Set al. Chest wall perforator flaps for partial breast reconstruction: surgical outcomes from a multicentre study. Arch Plast Surg 2020; 47: 153–159. 10.5999/aps.2019.01186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda M, Ishida T, Ohnuki Ket al. Breast conserving surgery with primary volume replacement using a lateral tissue flap. Breast Cancer 2005; 12: 16–20. 10.2325/jbcs.12.16 [DOI] [PubMed] [Google Scholar]
- 42.Van Huizum MA, Hage JJ, Oldenburg HAet al. Internal mammary artery perforator flap for immediate volume replacement following wide local excision of breast cancer. Arch Plast Surg 2017; 44: 502–508. 10.5999/aps.2016.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, He YJ, Li JFet al. Breast-conserving surgery with immediate partial breast reconstruction using pedicled thoracodorsal artery perforator flap: a clinical analysis of 33 patients. Zhonghua Wai Ke Za Zhi 2017; 55: 120–125. 10.3760/cma.j.issn.0529-5815.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 44.Woerdeman L, Hage JJ, Thio EAet al. Breast-conserving therapy in patients with a relatively large (T2 or T3) breast cancer: long-term local control and cosmetic outcome of a feasibility study. Plast Reconstr Surg 2004; 113: 1607–1616. 10.1097/01.PRS.0000117191.10766.95 [DOI] [PubMed] [Google Scholar]
- 45.Yang JD, Kim MC, Lee JWet al. Usefulness of oncoplastic volume replacement techniques after breast conserving surgery in small to moderate-sized breasts. Arch Plast Surg 2012; 39: 489–496. 10.5999/aps.2012.39.5.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaha H. Oncoplastic volume replacement technique for the upper inner quadrant using the omental flap. Gland Surg 2015; 4: 263–269. 10.3978/j.issn.2227-684X.2015.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaha H, Sunagawa H, Kawakami Ket al. Partial breast reconstruction for an inferomedial breast carcinoma using an omental flap. World J Surg 2010; 34: 1782–1787. 10.1007/s00268-010-0535-z [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Wang Y, Cai Ret al. Pedicled descending branch latissimus dorsi mini-flap in repairing partial mastectomy defect: shoulder functional and esthetic outcomes. J Surg Oncol 2019; 120: 518–526. 10.1002/jso.25524 [DOI] [PubMed] [Google Scholar]
- 49.De La Cruz L, Blankenship SA, Chatterjee Aet al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann Surg Oncol 2016; 23: 3247–2358. 10.1245/s10434-016-5313-1 [DOI] [PubMed] [Google Scholar]
- 50.Association of Breast Surgery. Guidance Platform. https://associationofbreastsurgery.org.uk/professionals/clinical/guidance-platform (cited March 2021).
- 51.Haloua M, Krekel NM, Jacobs GJet al. Cosmetic outcome assessment following breast-conserving therapy: a comparison between BCCT.core software and panel evaluation. Int J Breast Cancer 2014: 7: 716860. 10.3978/j.issn.2227-684X.2015.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeevan R, Cromwell DA, Trivella Met al. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ 2012; 345: e4505. 10.1136/bmj.e4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakravorty A, Shrestha AK, Sanmugalingam Net al. How safe is oncoplastic breast conservation? comparative analysis with standard breast conserving surgery. Eur J Surg Oncol 2012; 38: 395–398. 10.1016/j.ejso.2012.02.186 [DOI] [PubMed] [Google Scholar]
- 54.Crown A, Wechter DG, Grumley JW. Oncoplastic breast-conserving surgery reduces mastectomy and postoperative re-excision rates. Ann Surg Oncol 2015; 22: 3363–3368. 10.1245/s10434-015-4738-2 [DOI] [PubMed] [Google Scholar]
- 55.Yoon JJ, Green WR, Kim Set al. Oncoplastic breast surgery in the setting of breast-conserving therapy: a systematic review. Adv Radiat Oncol 2016; 1: 205–215. 10.1016/j.adro.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansell J, Weiler-Mithoff E, Martin Jet al. How to compare the oncological safety of oncoplastic breast conseravation surgery – to wide local excision or mastectomy? Breast 2015; 24: 497–501. 10.1016/j.breast.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 57.Mansell J, Weiler-Mithoff E, Stallard Set al. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast 2017; 32: 179–185. 10.1016/j.breast.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 58.Losken A, Hamdi M. Partial breast reconstruction: current perspectives. Plast Reconstr Surg 2009; 124: 722–736. 10.1097/PRS.0b013e3181b179d2 [DOI] [PubMed] [Google Scholar]
- 59.Romics L, Macaskill EJ, Fernandez Tet al. A population-based audit of surgical practice and outcomes of oncoplastic breast conversations in Scotland: an analysis of 589 patients. Eur J Surg Oncol 2018; 44: 939–944. 10.1016/j.ejso.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 60.Cochrane RA, Valasiadou P, Wilson ARet al. Cosmesis and satisfaction after breast-conserving surgery correlates with percentage of breast volume excised. Br J Surg 2003; 90: 1505–1509. 10.1002/bjs.4344 [DOI] [PubMed] [Google Scholar]
- 61.Pukancsik D, Kelemen P, Újhelyi Met al. Objective decision making between conventional and oncoplastic breast-conserving surgery or mastectomy: an aesthetic and functional prospective cohort study. Eur J Surg Oncol 2017; 43: 303–310. 10.1016/j.ejso.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 62.Chan S, Chueng PS, Lam SH. Cosmetic outcome and percentage of breast volume excision in oncoplastic breast conserving surgery. World J Surg 2010; 34: 1447–1452. 10.1007/s00268-009-0278-x [DOI] [PubMed] [Google Scholar]
- 63.Noguchi M, Yokoi-Noguchi M, Ohno Yet al. Oncoplastic breast conserving surgery: volume replacement vs. volume displacement. Eur J Surg 2016; 42: 926–934. 10.1016/j.ejso.2016.02.248 [DOI] [PubMed] [Google Scholar]
- 64.Potter S, Harcourt D, Cawthorn S, et al. Assessment of cosmesis after breast reconstruction surgery: A systematic review. Ann Oncoplast Surg 2011; 18: 813–823. 10.1245/s10434-010-1368-6 [DOI] [PubMed] [Google Scholar]
- 65.Kelsall JE, McCulley SJ, Brock L, et al. Comparing oncoplastic breast conserving surgery with mastectomy and immediate breast reconstruction: case-matched patient reported outcomes. J Plast Reconstr Aesthet Surg 2017; 70: 1377–1385. 10.1016/j.bjps.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 66.Haloua M, Krekel NM, Winters HAet al. A systematic review of oncoplastic breast-conserving surgery current weaknesses and future prospects. Ann Surg 2013; 257: 609–620. 10.1097/SLA.0b013e3182888782 [DOI] [PubMed] [Google Scholar]