Abstract
In metabolic conditions such as obesity and diabetes, which are associated with deregulated signaling of the insulin/insulin-like growth factor system (IIGFs), inflammation plays a dominant role. In cancer, IIGFs is implicated in disease progression, particularly during obesity and diabetes; however, further mediators may act in concert with IIGFs to trigger meta-inflammation. The receptor for advanced glycation end-products (RAGE) and its ligands bridge together metabolism and inflammation in obesity, diabetes, and cancer. Herein, we summarize the main mechanisms of meta-inflammation in malignancies associated with obesity and diabetes; we provide our readers with the most recent understanding and conceptual advances on the role of RAGE at the crossroad between impaired metabolism and inflammation, toward disease aggressiveness. We inform on the potential hubs of cross-communications driven by aberrant RAGE axis and dysfunctional IIGFs in the tumor microenvironment. Furthermore, we offer a rationalized view on the opportunity to terminate meta-inflammation via targeting RAGE pathway, and on the possibility to shut its molecular connections with IIGFs, toward a better control of diabetes- and obesity-associated cancers.
Keywords: insulin/IGF system, RAGE, meta-inflammation, cancer, obesity, diabetes
Graphical Abstract
Graphical Abstract.
Essential points.
The insulin/insulin-like growth factor system (IIGFs) is implicated in cancer progression, particularly during obesity and type 2 diabetes
Metabolic imbalances occurring during obesity and type 2 diabetes are associated with the onset and perpetuation of inflammation (meta-inflammation)
The receptor for advanced glycation end-products (RAGE) is a relevant player of meta-inflammation in obesity, diabetes, and cancer
Evidence suggests cooperation between the IIGFs and the RAGE axis in cancer progression
The pharmacological manipulation of the cross-talk between IIGFs and RAGE may represent a novel promising tool to control cancer meta-inflammation, particularly in patients affected by obesity and type 2 diabetes
Impaired metabolic health may trigger inflammatory responses involved in the development and progression of several pathological conditions that strongly impact on global disability and mortality, including cardiovascular disease, diabetes, kidney and liver disorders, as well as cancer (1, 2). In fact, certain environmental, social, and lifestyle factors contribute to the establishment of metabolic dysfunctions that promote the onset of chronic inflammation toward the initiation and progression of neoplastic disease (2-4). In this context, revealing the distinguishing molecular and biological features of what is currently defined as “metabo-inflammation” or meta-inflammation may help identify novel actionable targets of molecular intervention in anticancer therapies (4).
In support of such an approach, an epidemiological link between metabolic conditions associated with nutrition overload, such as obesity and type 2 diabetes (T2DM), and the incidence and prognosis of certain types of tumors has been clearly evidenced (5-7). This epidemiological correlation is attributable to multiple causative elements, among which hormones, growth factors, oxidative stress, and dysfunctional microbioma play a major role (2). However, dysregulated adipose tissue promotes detrimental effects also by impacting on the inflammatory state of the whole body and by rearranging the composition of several immune cellular components and molecular mediators (4); as a result, a permissive milieu for tumor progression is enabled. A better understanding of how dysregulated bioenergetic pathways facilitate and maintain chronic inflammation in metabolically unhealthy patients requires thorough investigation of the multifaceted paracrine interactions occurring between the activated adipose stroma and other components of the tumor microenvironment (4, 8). On the other hand, the inflamed adipose compartment may impact on the activity and biological function of certain hormones and growth factors, thus fueling a vicious cycle that prompts the progression of certain types of tumors (9, 10).
Far from being a mere energy storage depot, adipose tissue works as an active endocrine system by secreting adipokines, cytokines, chemokines, and growth factors (11, 12). Hypertrophy and hyperplasia are 2 of the most common early cellular responses occurring in adipose tissue when energy intake chronically exceeds energy expenditure. At the upper limit of adipocyte enlargement and recruitment, if nutrition overload persists, fat will accumulate into ectopic sites, consisting of visceral depots, liver, skeletal muscle, and pancreas.
In these last 3 major glucose regulatory organs, ectopic fat elicits its detrimental action by interfering with insulin signaling and secretion, thus facilitating the establishment of insulin resistance and the onset of T2DM (11, 13). Not surprisingly, the vast majority (almost 90%) of T2DM patients are also overweight or obese (14, 15). Strikingly, the activation of inflammatory pathways also occurs as a response to elevated glucose concentrations, where glycation damage represents the immediate outcome of the upstream inflammatory cascade (16).
In these scenarios, an aberrant activation of the insulin/insulin-like growth factor system (IIGFs) drives stimulatory responses that contribute to the acquisition of malignant cancer phenotypes, particularly in those tumors associated with obesity and T2DM (17). Despite the promising results obtained in in vitro studies and preclinical animal models, targeting IIGFs has proven limited efficacy in clinical studies (18); therefore, further efforts are required to better dissect the role of IIGFs in cancer progression, in order to optimize the therapeutic options and identify predictive and prognostic biomarkers, particularly for populations of cancer patients with imbalanced metabolic health.
As early as 1992, a protein was isolated from bovine lung on the endothelial cell surface and characterized for its ability to bind to advanced glycation end-products (AGEs) (19, 20), which result from the nonenzymatic glycation of proteins, nucleic acids, and lipids chronically exposed to elevated concentrations of glucose (16). Despite being named RAGE (receptor for advanced glycation end products) for the peculiar capability to serve as a receptor for AGEs, later studies showed that RAGE has multiligand capability, with more than 25 ligands being currently identified (21).
The binding of putative ligands to RAGE induces the activation of intracellular signaling pathways that culminate in the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)–dependent transcription of inflammatory cytokines and chemokines (21). Not surprisingly, RAGE levels are generally low in physiological conditions, whereas an increase in RAGE expression and function has been detected in several diseases, including cancer (22, 23). Most notably, RAGE signaling has also been implicated in the detrimental responses to imbalanced glucose and lipid metabolism (24, 25). Based on these observations, RAGE may serve as a crosswise actor bridging together disrupted metabolism and chronic inflammation in cancer.
In this review, we first provide an overview on meta-inflammation in cancer, particularly in those malignancies associated with obesity and diabetes. Next, we describe the main advances in understanding RAGE action in cancer meta-inflammation, focusing on the signaling nodes shared by RAGE and IIGFs toward tumor progression. Furthermore, we present current translational efforts based on RAGE inhibition as a novel strategy to halt meta-inflammation in cancer, and relay the future clinical opportunities raised by most recent findings in the field. Last, we offer a rationalized view on the opportunity to terminate RAGE and IIGFs cross-talk in order to achieve a better control of neoplastic disease, particularly in those cancer types associated with obesity and diabetes.
Meta-inflammation and Cancer
Inflammation can be regarded as an ancestral defense response enacted with the aim of restraining and repairing the damage caused by external cues. However, unopposed inflammation may cause detrimental effects. For instance, an inflammatory microenvironment represents a crucial component in most malignancies; in fact, chronic inflammation is recognized as a relevant etiological factor in 20% to 25% of all cancers (26-28). The roots of inflammation in cancer are multiple and interconnected, and covering this topic goes far beyond the scope of this review. However, it is well acknowledged that chronic inflammation may result as a consequence of altered energetic pathways, particularly in those cancer patients affected by metabolic disorders (29, 30). Of note, chronic inflammation can impact on the activity of hormones and growth factors implicated in maintaining metabolic and cellular homeostasis, with a relevant tumor-promoting effect taking over in such conditions. The hypothesis of a metabolic origin of cancer inflammation deserves a detailed analysis. First of all, cancer cells are characterized by clearly identifiable alterations of main metabolic pathways, which frequently raise the levels of reactive oxygen species (ROS), thus setting up the stage for oxidative stress-induced inflammation. For instance, the preferential utilization of glycolysis rather than oxidative phosphorylation in aerobic conditions, a phenomenon known as the Warburg effect, is typically observed in cancer cells, which also exhibit higher levels of steady-state ROS compared with the noncancer counterpart (31).
Despite this general observation, aerobic glycolysis does not necessarily reflect the full spectrum of metabolism possible in cancer, with certain types of tumors being more reliant on mitochondrial respiration (31). In addition, a high degree of metabolic heterogeneity can be observed within the same tumor, as plastic adaptations of energetic pathways frequently occur in response to spatio-temporal availability of nutrients and cellular demands (32).
Therefore, in certain conditions, mitochondrial respiration can take over from glycolysis, thus contributing to the generation of oxidative stress. Notably, high but nontoxic levels of ROS promote the acquisition of malignant features also by reprogramming intracellular signaling cascades and addressing the transcriptional cellular machinery toward the acquisition of biological features of aggressiveness. For instance, ROS may directly interact with specific receptors, inducing the redox activation of protein kinases, phosphatases, and transcription factors (33), which ultimately orchestrate the cellular response to stress. The main transcription factors activated by ROS are HIF-1α (hypoxia inducible factor-1 alpha) and NF-κB, which induce the transcription of several inflammatory mediators, including CCL2/MCP-1 (C-C motif chemokine ligand 2/monocyte chemoattractant protein-1), CXCL1/GRO-α (C-X-C motif chemokine ligand 1/growth-regulated oncogene-alpha), CXCL8/IL-8 (C-X-C motif chemokine ligand 8/interleukin 8), and COX-2 (cyclooxygenase-2) together with PGE2 (prostaglandin E2) (34). In addition, within the poorly vascularized adipose tissue, the activation of NF-κB and HIF-1α is accountable for the upregulation of TNFα (tumor necrosis factor alpha), IL-1 (interleukin 1), IL-6 (interleukin 6), matrix metalloproteinases (MMP) 9 and MMP2, MCP-1, plasminogen activator inhibitor-1, macrophage migration-inhibition factor, and inducible nitric oxide synthase (35). These factors rearrange the immune compartment of the tumor microenvironment by multiple mechanisms, thus facilitating the recruitment of macrophages, neutrophils, and other immune cells potentially implicated in tumor progression (34). Along with ROS, other metabolic intermediates are known to contribute to the inflammatory milieu, including nitric oxide and lactate. In this context, the establishment of an acidic microenvironment as a consequence of high glycolytic flux and accumulation of lactate in the tumor generates a proinflammatory and IL-8–driven microenvironment, permissive to tumor progression (36). Additionally, a high-fat diet alters the composition of gut microbioma thus increasing gut vulnerability to endotoxins which contribute to the propagation of inflammation. Among the mechanisms proposed, both endotoxins and free fatty acids trigger the NF-κB-mediated release of the aforementioned cytokines and chemokines, which play a key role in maintaining and disseminating inflammation in both normal and cancer cells (37-41).
These examples nicely recapitulate some of the mechanisms through which disrupted energetic pathways may impact on tumor progression through the induction of chronic inflammation. In addition, certain inflammatory mediators reprogram the surrounding microenvironment by impacting on the bioavailability and function of hormones and growth factors, which in turn regulate tumor metabolic processes. A paradigmatic example is the inflammatory-driven insulin resistance, which contributes to the onset of T2DM and to the progression of diverse types of tumors. A large body of evidence collected from animal models and human population studies have clearly established a causative link between inflammation and insulin resistance, defined by the inability of this hormone to regulate nutrient metabolism in peripheral tissues. Therefore, insulin resistance and the consequent hyperinsulinemia derive, at least in part, from an inflammatory process initiated in the adipose tissue (42). Insulin is itself a potent mitogen and may induce the proliferation of cancer cells and enhance their migratory properties; such stimulatory effects are mainly supported by an enriched capacity to gain energy through the modulation of both glycolysis and oxidative phosphorylation (43). It should be noted that insulin resistance is enacted in response to proinflammatory signals deriving from the expanding adipose tissue; indeed, more than half of the total pathways differentially regulated in animal models of obesity compared with lean animals are inflammatory pathways (42). More specifically, TNFα, IL-1β, and interferon γ are considered major factors released by inflammatory cells recruited in the adipose tissue and involved in the impairment of insulin signaling through a receptor-mediated mechanism (44). Adding to this, hyperglycemic and hyperlipidemic stress trigger certain responses of the innate immune compartment, including the activation in macrophages of inflammasome-mediated responses, that further contribute to the recruitment of infiltrating immune cells and to the expansion of the cytokine network (38, 45). These signals synergize with TNFα in activating JUN and mitogen-activated protein kinase (MAPK)-dependent expression of SOCS (suppressor of cytokine signaling) 1 and SOCS3, which trigger the direct inhibition of insulin signaling toward insulin resistance (4).
Clearly, insulin resistance represents only the tip of an iceberg whose central core is built over time by the chronic release of inflammatory mediators.
What Can Be Learnt From Obesity and Diabetes
The role of obesity and T2DM in increasing the overall cancer risk and severity is largely recognized for breast, ovarian, endometrial, prostate, colorectal, pancreatic, hepatic, renal, esophageal, gallbladder, stomach, and thyroid cancer, together with multiple myeloma and meningioma; however, an organ-specific hierarchy of susceptibility is well documented (46-50). As molecular responses instigated in the adipose tissue trigger insulin resistance, the development of chronic inflammation also sets the stage for diverse molecular and biological features included among the hallmarks of cancer (51). Therefore, meta-inflammation represents a pivotal node in the epidemiological link between obesity, T2DM, and cancer.
In this regard, numerous clinical and experimental data have revealed that the obesity-related inflammatory signature is not solely dependent on resident adipocytes, but also on relevant components of innate immunity like macrophages, whose rate of accumulation in the adipose tissue increases parallel with the increase of body mass index. Likewise, macrophages represent the largest immune cell population in the visceral adipose tissue (42, 52-54). Moreover, the accumulation of fat is both associated with an increased infiltration and a phenotypic switch of macrophages toward a proinflammatory state (55, 56). Tumor-associated macrophages (TAMs) are actively implicated in cancer progression. They play a key role in tumor cell survival, angiogenesis, remodeling of extracellular matrix (ECM), metastasis, and resistance to both spontaneous and therapy-induced antitumor immunity (57). However, macrophages have also the potential of restraining cancer cell proliferation by promoting immune recognition, especially during the early phases of carcinogenesis (58). These variegate biological functions of TAMs are a consequence of their extreme plasticity in response to environmental stimuli (58). Historically, macrophages have been simplistically divided into activation status, referred to as “M1” and “M2,” which reflected the T cell nomenclature associated with type 1 and type 2 responses (59). However, in tumors, mixed macrophage phenotypes can coexist, as highlighted by the most recent transcriptional studies (60). Thus, the signals, the type of tissue and the stage of tumor progression orchestrate the landscape of TAMs diversity and functional programs in cancer.
Not surprisingly, the metabolic features of the tumor microenvironment strongly impact on the biological behavior of TAMs. For instance, while M1-like macrophages mainly rely on glycolysis, M2-like macrophages preferentially employ oxidative metabolism (61-64). Therefore, if glycolytic flux is inhibited the polarization toward M2-like phenotype predominates (65), whereas blocking OXPHOS metabolism substantially shifts macrophages toward an M1-like phenotype with tumor-restraining properties (62). In this context, uptake and utilization of fatty acids has been shown to trigger an OXPHOS-dependent M2-like polarization (66); likewise, both the polarization and the survival of protumoral macrophages are impaired when lipolysis is inhibited (67). Not surprisingly, in stressful and metabolically hyperactivated lung tumor microenvironments, the generation of ROS supports M2-like phenotype acquisition (68); accordingly, an antioxidant treatment prevents the accumulation of TAMs in animal models of lung cancer (68). These findings clearly suggest that manipulating oxidative metabolism in TAMs may represent an efficient tool for their education toward a tumor-suppressive phenotype, particularly in the obese setting where enhanced fuels are available for OXPHOS metabolism. The implication is that innate immune compartment may play a remarkable role in modulating meta-inflammation leading to the complications of obesity, diabetes, and neoplastic progression. For instance, the NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome is a molecular complex formed by macrophages and monocytes in response to tissue damage or infection (69). Evidence that the NLRP3 is implicated in obesity-induced inflammation and insulin resistance has been clearly established and related to caspase-1 activation and release of IL-1β (70). Notably, data obtained in animal models of obesity indicate that NLRP3 functions as an intracellular sensor of metabolic derangement associated with lipotoxic damage, enabling a complex inflammatory response that leads to insulin resistance (70). Of note, caloric restriction and exercise-mediated weight loss are associated with reduced expression of NLRP3, decreased inflammation, and improved insulin sensitivity in obese patients with T2DM (70). It should be mentioned that inflammasomes may also trigger pyroptosis, a rapid form of cell death associated with inflammation, which could be of benefit if exploited to eliminate inflammatory cancer cells. Despite the role of inflammasomes in obesity and diabetes-related inflammation being well established, these complexes may elicit both a tumor-promoting, and a tumor-restraining effect in cancer (depending on the tumor context, the type of inflammasome, and the downstream effector molecule). On the other hand, it is well recognized that high levels of glucose (71) and fatty acids (72) prompt NLRP3 activation, which might represent a hub of molecular signals of metabolic danger driving inflammation and cancer development, particularly in those types of cancer associated with nutrients overload, obesity, and T2DM.
It should be considered that a transient, low-grade increase in the inflammasome activity of the adipose tissue macrophages is usually observed in healthy individuals in response to postprandial hyperglycemia (73). Such molecular programs of innate immunity not only ensure protection against potential microorganisms found in food, but also induce IL-1β maturation and release, which in turn potentiates glucose-induced insulin secretion. In addition, the increase of glucose uptake in macrophages further stimulates IL-1β production thus fueling a feedforward loop where both insulin and IL-1β cooperate in lowering plasma glucose levels and in facilitating its utilization (74). At odds with the transient action elicited by IL-1β on insulin secretion and activity, which is classically accompanied by the activation of diacylglycerol and protein kinase C (PKC) transduction pathways, long-term exposure to this cytokine may trigger a signaling shift toward the noncanonical NF-κB and MAPK cascades, which are accountable for the increased susceptibility of pancreatic β-cells to glucose and lipid damage typically observed in T2DM (75, 76). It should be recalled that an aberrant activation of NF-κB and MAPK transduction signaling is frequently observed in numerous types of tumors. Therefore, untamed insulin signaling, boosted upon inflammatory cascades, may contribute to the activation of key oncogenic pathways.
Collectively, these data suggest that despite inflammatory signals prime insulin secretion and metabolic homeostasis in physiological conditions, a chronic low-grade inflammation triggered by altered metabolism contributes to the pathogenesis of obesity, diabetes, and cancer. Being both a target and an effector of meta-inflammation in cancer, the complex action elicited by IIGFs requires a thorough elucidation of the potential therapeutic opportunities and manipulation of such complex hub of signaling networks.
The Role of the Insulin/IGF System
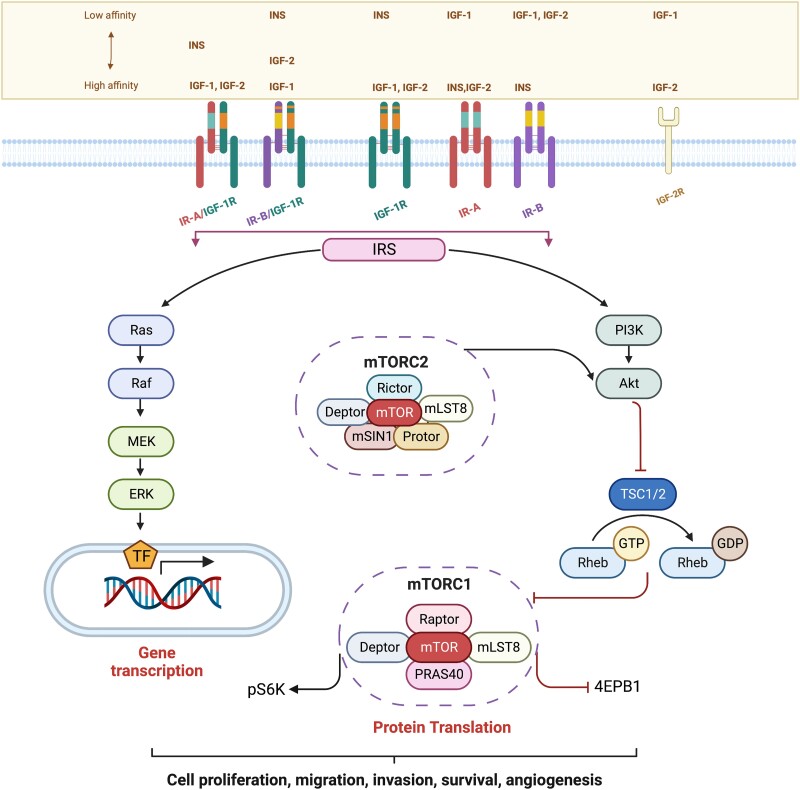
With the establishment of obesity-driven insulin resistance, hyperinsulinemia, and increased bioavailability of IGF-1, an unopposed activation of the insulin receptor (IR) and the insulin-like growth factor receptor (IGF-1R) (77), which are part of the complex IIGFs, occurs. IIGFs plays a pivotal role in maintaining energetic homeostasis in healthy tissues through the regulation of glucose, lipid, and protein metabolism; in addition, this signaling system is implicated in cell growth and differentiation (78, 79). IIGFs comprises the ligands insulin, IGF-1, IGF-2 (IGFs) and the high homologous cognate receptors (IR, IGF-1R, IR/IGF-1R hybrids, and IGF-2R) (Fig. 1), together with 6 IGF-binding proteins (IGF-BP1-6) (17, 80). IGF-IR binds to IGF-1 and IGF-2, whereas IR occurs in 2 isoforms, IR-A and IR-B, both of which can be assembled as IR/IGF-1R hybrid receptors that retain IGF-1 and IGF-2 binding ability (Fig. 1). Such pharmacological promiscuity is consistent with the elevated degree of structural homology between IGF-1R and IR. In addition, IR-A is a bona fide receptor for IGF-2 (17). Considering its crucial role in metabolic homeostasis, it is not surprising that deregulation of IIGFs may pave the way to a number of pathological conditions including obesity, diabetes and cancer (17, 81-85). In this latter context, the increased expression of both IGF-1R and IR has been broadly detected and correlated with malignancy (17, 86). Indeed, IIGFs promotes biological features of disease aggressiveness like enhanced cell proliferation, survival, migration, invasion, epithelial mesenchymal transition (EMT) transition, acquisition of stem-like features and angiogenic potential (87-93) (Fig. 1); some of these actions are mediated by IIGFs and associated signaling partners such as the collagen receptor discoidin domain receptor-1, the estrogen receptor ERα, as well as the alternate estrogen receptor, G protein–coupled estrogen receptor 1, known as GPER (94-98). Both IR and IGF-1R may prompt cell cycle progression leading to proliferative effects in cancer cells (94-98).
Figure 1.
Signaling pathways activated by IIGFs in cancer cells. Although with different affinities, ligands belonging to IIGFs induce IRS-dependent activation of several signaling cascades (RAS/RAF/MEK/ERK, PI3K/AKT/mTOR) leading to gene transcription and protein translation toward stimulatory effects in cancer cells. IGF2-R is structurally unrelated to IGF-1R and IR and is a monomeric receptor serving as scavenger for circulating IGF-2. Abbreviations: AKT, protein kinase B; ERK, extracellular signal–regulated kinase; GDP, guanosine diphosphate GTP, guanosine triphosphate; IGF-1, insulin-like growth factor-1; IGF-2, insulin-like growth factor-2; IGF-1R, insulin-like growth factor-1 receptor; IGF-2R, insulin-like growth factor 2 receptor; INS, insulin; IR-A, insulin receptor isoform A, IR-B, insulin receptor isoform B; IRS, insulin-receptor substrate; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian/mechanistic target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PRAS40, proline-rich AKT substrate of 40 kDa; p70S6k, p70S6 kinase; RAS, rat sarcoma family of proteins; RAF, rapidly accelerated fibrosarcoma protein; Rheb, Ras homolog enriched in brain; TSC, tuberous sclerosis complex; 4EBP1, Eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1. All Figures were created using Biorender.com.
Therefore, in IGFs and insulin-rich milieu, higher cancer cell proliferative rates are usually expected, as observed in both in vitro and in vivo models (99, 100). In conditions of insulin resistance, insulin action mediated by insulin receptor substrate (IRS)-1/phosphatidylinositol 3-kinas (PI3K) protein kinase B (AKT) signaling in target tissues is compromised, whereas extracellular regulated kinase (ERK) 1/2 signaling remains unaffected (101). In these conditions, blood insulin levels increase in a compensatory effort aimed at overcoming insulin resistance (101). The subsequent hyperinsulinemia is sufficient to prompt a biological reprogramming suggestive of aberrant proliferation, as insulin induces mitogenic effects through its binding to the nonmetabolic and oncofetal isoform IR-A (17, 80, 100). In addition, hyperinsulinemia suppresses hepatic synthesis of IGFBP-1, thus increasing the free circulating levels of IGF-1 (102-105). A reduction of the secretion of pituitary growth hormone is subsequently observed due to the negative feedback elicited by free IGF-1, thus triggering a decrease in IGFBP-3, 1 and 2 (103, 104). Thereafter, the increase in free IGF-1 prompts potent mitogenic and oncogenic responses almost ubiquitously (17, 83, 85, 87). Based on these observations, dysregulations of IIGFs overall contribute to stimulatory responses typical of aberrantly proliferating cancer cells.
Among the tumor-promoting actions elicited by the IIGFs, it is not surprising that this signaling axis manipulates cell metabolism to address the high energetic demands of cancer cells. In fact, IIGFs addresses energetic fuels toward their utilization for an increased adenosine triphosphate (ATP) production (106). Furthermore, the IIGFs has also been shown to regulate the expression of inflammatory mediators in several tumor contexts through multiple mechanisms. For instance, IGF-1 induces transcriptional regulation of COX-2 in ovarian cancer cells. Moreover, in cervical, liver, and colorectal cancer cells IGF-1 promotes the activation of NF-κB and NLRP3 inflammasome signaling, which is known to trigger the maturation of IL-1β (107), thus amplifying the inflammatory and metastatic cascades. In this scenario, microenvironmental IL-1β was shown to promote the NF-κB and cyclic adenosine monophosphate response element-binding protein (CREB)–dependent activation of WNT signaling in breast cancer cells toward the formation of bone metastases (108). Extending these findings, in a primary breast cancer cell line established from an invasive ductal carcinoma, IR was involved in a cross-talk with a unique 46 kDa splicing variant of Erα (Erα46) to prompt IL-11 expression and the subsequent activation of malignant transcriptional programs and biological responses (95). In addition, the tumor-promoting and inflammatory role elicited by IR was recently identified in a murine cell model of triple negative breast cancer. More specifically, a transcriptomic interrogation of insulin-stimulated breast cancer cells followed by gene pathway enrichment analysis allowed one to establish that insulin triggers the expression of genes primarily implicated in the regulation of both metabolic pathways and immune evasions, including several cytokines and chemokines (100). These findings are consistent with several studies showing that IIGFs may be regarded as a novel orchestrator of inflammation and innate immunity. For instance, macrophages express a functional insulin signaling and develop insulin resistance in the context of systemic insulin resistance, where an M2-like phenotype, characterized by reduced secretion of proinflammatory mediators and potential immunosuppressive function, is privileged (109, 110).
Interestingly, both primary and metastatic breast cancer TAMs and cancer associated fibroblasts (CAFs) are regarded as main sources of IGF-1 and IGF-2, which in turn promote paracrine stimulatory actions in cancer cells (111). These data further corroborate that a bidirectional cross-talk between IIGFs and cellular effectors of meta-inflammation like TAMs and CAFs reprogram the tumor microenvironment toward the acquisition of malignant features.
Undoubtedly, the IIGFs is placed at the crossroad of several intertwined cancer signaling pathways that dictate the complex biological response to environmental stimuli also through the regulation of meta-inflammation. A better characterization of IIGFs action, of its molecular partners and downstream effectors may help identifying novel target of anticancer intervention, particularly in neoplastic disease associated with a dysregulated metabolic component.
This is certainly necessary to extend the currently available portfolio of therapeutic opportunities, particularly for obese and diabetic cancer patients. Despite the undoubtable role played by IIGFs and the very promising opportunities raised by targeting this signaling axis in preclinical models, expectations from implementing IIGFs-blocking strategies in the clinics have been poorly fulfilled for the great majority of cancer types (112, 113). For instance, strategies directed at blocking IGF-1R (monoclonal antibodies and inhibitors) failed in the clinical setting (114-117) for a number of reasons. First, blocking IGF-1R disrupts a negative feedback loop in the pituitary gland, resulting in a compensatory increase in growth hormone, leading to insulin resistance and increased hepatic production and serum IGF-1 levels (118, 119). Growth hormone itself contributes to oncogenic signals on 1 side (120) and facilitates the establishment of hyperinsulinemia because of the increased hepatic lipolysis and subsequent free fatty acid production (121). In this context, blocking IGF-1R may not be sufficient to contrast the supraphysiological levels of IGF-1; in addition, IGFs and insulin may activate IRs and hybrid receptors thus transmitting stimulatory signals even when IGF-1R is inhibited (17, 122). Other possible mechanisms of therapeutic resistance include the inadequate inhibition of pathways downstream of IGF-1R, the activation of alternative signaling routes in the context of receptor reciprocity and extensive cross-talks, as well as the aberrant autocrine and/or paracrine production of ligands (123, 124).
As it concerns IR, blocking this receptor would determine an undesirable diabetic-like state; therefore, pharmacological agents able to discriminate between the oncogenic A isoform and the metabolic B isoform of IR could bypass the complexity of insulin signaling, shutting down the mitogenic effects while sparing the metabolic actions mediated by IR. In light of the complicated and interconnected hub of signals mediated by IIGFs, a better dissection of the signaling partners employed by this axis to convey stimulatory messages could offer novel opportunities for pharmacological manipulation in cancer. Building knowledge in this field, sharpening our ability to predict and stratify the patients who could benefit most from anti-IIGF therapies, and dissecting the potential of combination targeted approaches will lead to greater clinical antitumor efficacy.
RAGE Signaling in Cancer Meta-inflammation
A number of studies have revealed the role of the RAGE in inflammatory, degenerative, and hyperproliferative diseases, as well as in cancer. RAGE is a 45 kDa single-spanning multiligand membrane receptor belonging to the superfamily of immunoglobulin receptors and is mainly implicated in the regulation of innate immunity and inflammation. Consistent with this role, the RAGE gene (Ager), which is highly conserved in mammalians, is located within a region on chromosome 6 that comprises the major histocompatibility complex class III. Alternate splicing of Ager results in more than 20 different splicing variants, 1 of the most clinically relevant being the endogenous secretory (es)RAGE, which lacks the transmembrane and intracellular domain, thus presenting as a circulating soluble RAGE (sRAGE) isoform (125); sRAGE may also derive from the proteolytic cleavage of the full-length RAGE membrane protein, which generates cleaved IRAGE, by enzymatic activity of MMPs (126). Despite RAGE expression being elevated during embryonic development, in the adult tissues low levels of RAGE are usually detected, consistent with the observation of promoter methylation in adult healthy subjects (127). On the other hand, RAGE expression increases in diverse pathological conditions associated with inflammation, like obesity, diabetes, and cancer. In the latter context, higher tissue levels of RAGE and lower blood levels of sRAGE have been detected and correlated with the severity of pancreatic, lung and breast cancer (128-130). An exception to this general trend is represented by multiple myeloma, where patients present higher levels of circulating sRAGE than healthy subjects (131).
Upon ligand binding, RAGE triggers the activation of intracellular signaling cascades that culminate on the recruitment of transcription factors like NF-κB, activator protein-1 and signal transducer and activator of transcription 3 (STAT3), and the subsequent modulation of the cellular transcriptional machinery. The engagement of RAGE signaling in cancer cells executes a large and heterogeneous range of biological responses identifying several cancer hallmarks, like cell proliferation, migration, invasion, survival, angiogenesis, altered metabolism, inflammation, and immune evasion (23). RAGE was first identified as the receptor for AGEs which result from the nonenzymatic glycation of proteins, lipids, and DNA occurring during chronic glucose exposure. However, it is now largely recognized that RAGE propagates transduction signals initiated also by non-AGE molecules (21). In fact, RAGE ligands include a broad repertoire of endogenous and exogenous molecules like β2 integrin/Mac-1, amyloid β-peptide, β-sheet fibrils, collagen I/IV, bacterial lipopolysaccharide, CpG DNA, high-mobility group box 1 (HMGB-1), and proteins of the S100 family, to name a few. Upon ligand binding, RAGE engages diverse intracellular adaptor proteins, such as diaphanous-1/mDia1, ERK1/2, PKC, PKB, c-Jun N-terminal kinase, TIRAP, dedicator of cytokinesis 7 (DOCK7), DOCK7, and Rac-1/Cdc42, which in turn promote transcription factor-dependent gene changes and biological responses (21). In addition, a PKC-ζ (protein kinase c type zeta)-dependent phosphorylation of RAGE at Ser391 has been demonstrated upon binding of ligands (132); however, the molecular and functional consequences of this post-translational modification have not been established yet. On the other hand, it is clear that the multimeric form of RAGE, rather than the monomeric, is mainly implicated in ligand binding and signal transduction, as the multimeric receptor complexes recruit aggregates of ligands, which are usually found at site of inflammation.
RAGE is classified within the pattern recognition receptors (PRRs), a group of proteins that recognize and respond to pathogen-associated molecular pattern molecules and danger-associated molecular pattern molecules (DAMPs) (133). While pathogen-associated molecular pattern molecules boost PRR-mediated innate immunity in response to pathogens (134), DAMPs are typically released by damaged and/or stressed cells undergoing necrosis and nontolerogenic apoptosis, in order to eliminate the cellular debris through the recruitment of immune cells and mediators (134). Nonetheless, an unopposed activation of PRR signaling may facilitate the establishment of a chronic inflammatory environment, ultimately leading to inflammation-dependent damage progression (135, 136). With a similar mechanism, RAGE action elicits detrimental effects in obesity and T2DM, where the initial trigger for RAGE activation is represented by DAMPs released by enlarged dying adipocytes, and by hyperglycemic damage (135). Similarly, in cancer RAGE propagates DAMP- and glycoxidative-dependent cell damage through multiple and interdependent mechanisms, which reprogram the tumor microenvironment for the establishment of chronic inflammatory niches that drive disease progression (136, 137).
Consensus has been broadly reached on the role of RAGE in mediating inflammatory responses that prompt cancer development and progression, also for cancers not directly associated with obesity and diabetes (22). Current knowledge is suggestive of an involvement of RAGE in driving the evolution from a transient inflammatory reaction into a persistently microenvironmental response that supports disease development and progression. For instance, early evidence has shown that RAGE deficiency, in animal models of chemically induced skin carcinogenesis, impairs tumor initiation due to the instigation of an inflammation-resistant phenotype (22). The inability to mount an inflammatory response in RAGE−/− animals exposed to the skin carcinogens TPA/DMBA (12-O-tetradecanoylphorbol-13-acetate/dimethylbenz[a]anthracene) is supported by the dramatically reduced expression of inflammatory mediators such as COX-2, S100A8, S100A9, and macrophage inflammatory proteins compared with wild-type animals (22). Interestingly, RAGE expression on immune cells, but not keratinocytes, is required for chemical-induced dermal immune infiltration, toward inflammation-dependent tumorigenesis (22). These observations are consistent with other studies showing that RAGE prompts NF-κB-mediated inflammation, leading to colitis-associated carcinogenesis (138). In this model, RAGE appears to be dispensable for the instigation of the initial acute inflammation, but it is required for the transition toward chronic inflammatory conditions that anticipate colorectal cancer development (138). Notably, in both studies RAGE deficiency is accompanied by a reduced accumulation of Gr1 + CD11b + myeloid precursor cells, suggesting immune escape responses consistent with the recruitment of myeloid-derived suppressor cells (MDSCs) and resulting in T cell tolerance (22, 138). Evidence that RAGE deficiency is characterized by the lack of inflammatory RAGE ligands, as observed in the abovementioned study by Gebhardt and collaborators (22), suggests that RAGE may regulate the expression of its own binding activators, thus contributing to establish and maintain an inflammatory environment that facilitates neoplastic development and progression. Extending these observations, several ligands of RAGE prompt the recruitment of transcription factors like NF-κB at binding sites located within RAGE promoter, thus fostering RAGE expression which further propagates the initial inflammatory response (139-141). This codependency enables a cycle of prolonged activation of the intracellular transcriptional machinery toward chronic inflammatory reprogramming after the initial damage (142). It should be mentioned that RAGE-dependent activation of sustained NF-κB signaling is partly due to the de novo synthesis of RelA (p65), which contributes to establishing an increasingly growing reservoir of active NF-κB pool, thus overcoming the physiological mechanisms of negative feedback control (143). Therefore, a globally accepted working model for RAGE/ligands axis in inflammation and cancer specifies that the accumulation of RAGE ligands at sites of inflammation drives the formation of receptor oligomers, which are further stabilized by interaction with ligands in the extracellular tumor milieu (Fig. 2); this step is required for receptor activation toward the instigation of inflammatory responses that are chronically propagated also through the upregulation of RAGE and RAGE ligands themselves (21, 142). These observations are in accordance with evidence showing that RAGE is generally expressed at very low levels in physiological conditions (with the exception of epithelial lung alveolar cells) (144, 145), whereas its levels raise in pathological conditions associated with inflammation (like obesity, diabetes and cancer). In these pathological contexts, the increase of RAGE levels is supportive of disease progression (21). On the other hand, RAGE elicits a tumor-suppressive role in those tissues where the receptor is highly expressed at physiological levels; likewise, the loss/reduction of RAGE expression in these contexts is associated with neoplastic progression and poor prognostic outcomes (146, 147). In this intricate scenario, the relative prevalence of certain isoforms of RAGE compared with others may favor distinct molecular and biological responses in the tumor microenvironment. For instance, Downs and collaborators performed a proteomic analysis of lung adenocarcinoma cells and found that dominant-negative (DN)-RAGE, which differs from full length RAGE for lacking the intracellular signaling tail, prompts the transition from a proinflammatory to a prometastatic phenotype (148).
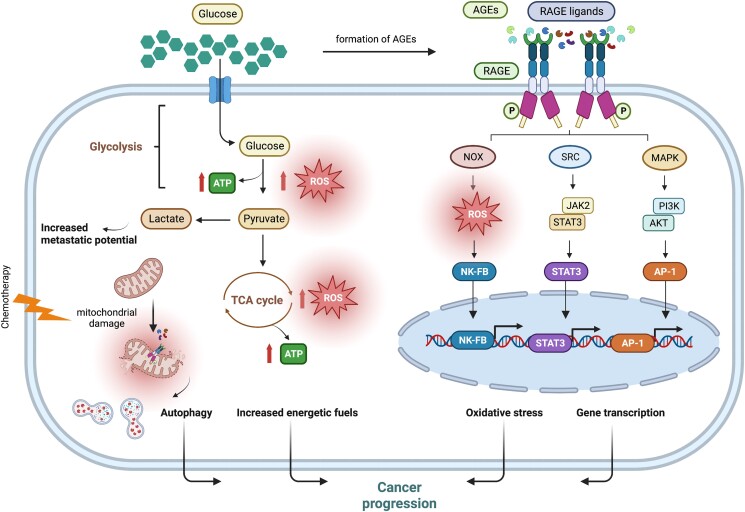
Figure 2.
RAGE-dependent regulation of metabolic pathways toward cancer progression. In cancer cells, persistently elevated glucose levels enhance glycolytic flux, which prompts the generation of ROS, directly implicated in the activation of RAGE. Hyperglycemic environments also promote the formation of AGEs, which bind to and activate RAGE. RAGE ligands released from dying cancer cells as a consequence of chemotherapy damage activate RAGE signaling toward cancer progression. Activation of mitochondrial RAGE enhances ATP production and fosters the generation of ROS, setting the stage for mitochondrial fission and autophagy. Abbreviations: AGEs, advanced glycation end-products; AKT, protein kinase B; AP-1, activator protein 1; ATP, adenosine triphosphate; ERK, extracellular signal–regulated kinase; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; NOX, nicotinamide adenine dinucleotide phosphate oxidase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphatidylinositol 3-kinase; RAC1, ras-related C3 botulinum toxin substrate 1; RAGE, receptor for advanced glycation end-products; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; TCA cycle, tricarboxylic acid cycle.
To address unanswered questions and resolve the complexity of RAGE signaling in inflammation and cancer, omics tools could be employed. In this context, a transcriptomic profiling of gastric cancer tissue vs noncancer tissue coupled with gene set enrichment analysis identified the AGE/RAGE pathway as 1 of the most strongly enriched pathways together with neutrophil activation- and T cell activation-signaling (149), suggestive of RAGE-dependent regulation of inflammatory responses. A critical point emerging from these observations is that more comprehensive interrogating tools could help deciphering the multiple aspects of RAGE pathway, regulatory networks and signaling companion, possibly uncovering tissue-specific biological responses and molecular targets. Such an approach will alleviate the difficulties encountered when trying to translate promising preclinical data into clinics, thereby improving the feasibility of anti-RAGE therapies particularly in inflammation-associated tumors.
RAGE Signaling in Tumor Metabolism
Cancer cells
An altered energetic metabolism strongly contributes to the progression of cancer, which counts metabolic aberration as 1 of its typical hallmarks. Tumor cells mainly rely on glycolysis for energy supply during both aerobic conditions and hypoxia, when intratumoral delivery of oxygen is dramatically reduced. Such metabolic dependency induces an increased uptake of glucose and turnover of metabolic intermediates, thus facilitating the formation of the glycoxidative adducts (AGEs) (16). Also, metabolic imbalances associated with hyperglycemia contribute to the raise of AGE levels (16). Endogenous AGEs are generated from nonenzymatic glycation of proteins, lipids and nucleic acids, a reaction that occurs when the amino group of these molecules interacts with the carbonyl group of reducing sugars abundantly and chronically present in the micromilieu, forming an unstable but reversible chemical product named the Schiff base (16). If high sugar levels persist, these compounds evolve into more stable structures named Amadori products, which go through permanent chemical modifications, ultimately becoming AGEs (16). The pool of body AGEs also derives from dietary intake of exogenous AGEs present in thermally processed and in protein/lipid-rich foods. Whichever their origin, AGEs trigger diverse oncogenic pathways by binding to RAGE (16) (Fig. 2). In addition, increased AGE levels instigate a feedforward loop of stimulatory responses by upregulating RAGE expression (150, 151), thus contributing to maintain a chronic inflammatory environment instructed upon an initial metabolic trigger.
For example, the AGE named Nε-carboxymethyllysine (CML) induces RAGE-dependent activation of tumorigenic pathways and growth effects in human pancreatic adenocarcinoma cell lines; likewise, CML administration in pancreatic adenocarcinoma–prone mice promotes RAGE accumulation and facilitates the progression of neoplasia toward invasive pancreatic cancer (152).
Further supporting these findings, Liao et al found that high glucose triggers RAGE and nicotinamide adenine dinucleotide phosphate oxidase (NOX) expression, which promote the acquisition of malignant features in lung cancer (153), whose incidence and severity are increased among diabetic patients (154, 155). In turn, RAGE mediates the activation of the HIF-1α/VEGF (vascular endothelial growth factor)-dependent pathway, thus suggesting that RAGE may be implicated in modulating angiogenic responses in dysfunctional metabolic environments associated with prediabetes and diabetes (153).
Recently, a comparative tissue analysis of gastric mucosa has demonstrated that the expression of both RAGE and its ligand HMGB-1 is increased in cancer patients with and without diabetes, compared with noncancer individuals (156); furthermore, RAGE and HMGB-1 expression is associated with worse prognostic parameters in patients affected by gastric cancer and diabetes simultaneously (156).
In addition, AGE–RAGE signaling has been shown to boost the expression and function of carbohydrate responsive element binding protein, a key transcription factor implicated in glycolytic and anabolic activity, leading to increased cancer cell proliferation (157); thus, the elevated AGE levels observed in diabetic and obese patients may prompt tumorigenic effects by boosting RAGE transduction pathway (157). Of note, RAGE may act as a relevant effector of metabolic-driven chemoresistance, which represents an important clinical issue also in cancers associated with obesity and diabetes (158). In this regard, Huang et al demonstrated that the release of the RAGE ligand HMGB-1 from dying cancer cells treated with chemotherapeutic agents induces RAGE-dependent activation of ERK1/2 and the subsequent phosphorylation of the dynamin-related protein 1 (Drp1) (159), a factor implicated in mitochondrial fission; the subsequent activation of autophagic programs prompts the regrowth of surviving cancer cells, thus conferring chemoresistance (159). Likewise, in multiple myeloma cells, HMGB-1 was involved in metabolic-driven chemotherapy resistance, whereas its repression prompted chemotherapy sensitivity through the induction of apoptosis and the inhibition of autophagic response (160); however, in this study the authors did not assess if the effects of HMGB-1 were specifically mediated by RAGE (160). Extending these findings, RAGE, localized at mitochondria, and its ligand HMGB-1 were shown to promote tumor growth in vitro and in vivo through the regulation of mitochondrial complex I activity, which increased ATP production (161). Interestingly, AGE/RAGE signaling was implicated in pancreatic tumorigenesis through the induction of the metabolic process of autophagy, which triggered IL-6 release and activation of mitochondrial STAT3 pathway, toward increased ATP formation and cell proliferation (162).
A role for Cancer Stem Cells
Cancer stem cells (CSCs) are a rare population of cancer cells implicated in tumor initiation, as well as in malignant recurrence, metastasis formation, and therapeutic resistance. RAGE has been shown to maintain stemness properties and tumorigenicity of CSCs in diverse types of tumors, an ability shared by more than 1 RAGE ligand (163-165). CSCs propagation may be facilitated during hypoxic conditions. The molecular and biochemical mechanisms underlying this response are complex and multifactorial, however an increased mitochondrial biogenesis appears to be implicated in CSCs maintenance in low oxygen environment (166). In this context, the RAGE ligand S100A4, which is upregulated in hypoxic conditions (167), mediates metabolic reprogramming toward an OXPHOS dependency through the upregulation of the mitochondrial complex I protein NDUFS2 (168); however, the involvement of RAGE in this action has not been investigated (168). S100A4 is considered itself a stemness marker, and is associated with enhanced self-renewal and tumorigenic properties of CSCs (163). Extending these findings, a low oxygen tension was shown to induce the expression of RAGE, which cooperated with oncogenic KRAS signaling, largely implicated in stemness (169), to prompt RAGE-dependent pancreatic tumor growth (170). Adding to this, an integrative genomic analysis of breast CSCs identified 1q21.3 amplification as a relevant chromosomal aberration associated with enhanced S100A7, S100A8, and S100A9 production, increased tumor sphere formation in breast cancer cells and in patient-derived samples, and higher tumor recurrence (171). Likewise, S100A9 was the top upregulated gene in transcriptomic analysis of radioresistant brain metastasis derived from melanoma, lung, and breast cancer (172). In this context, S100A9/RAGE contributed to establish a gene signature reminiscing the acquisition of stem-like features and therapeutic resistance (172).
Tumor microenvironment
the contribution of the tumor microenvironment to cancer development and progression is well acknowledged. CAFs, and adipocytes represent main cellular components that, together with noncellular factors (ECM, hypoxia, environmental pH) orchestrate a permissive milieu for neoplastic growth and expansion, particularly in obesity-related diseases. RAGE signaling pathway features many aspects of the tumor microenvironment, enabling cancer-conducive biological responses.
CAFs
Within the tumor microenvironment, CAFs provide with mechanical support to the growing mass through the release of ECM proteins; in addition, CAFs actively release numerous signaling molecules implicated in the regulation of tumor metabolism, neoangiogenesis, cell migration, invasion, inflammation, and immune evasion. Recently, the accumulation of AGEs in the ECM was shown to promote collagen glycation, leading to RAGE-dependent regulation of mechano-transduction signaling and acquisition of CAF-like phenotype in surrounding stromal fibroblasts (173). Furthermore, the accumulation of nutrition-associated glycoxidative damage induced an AGE/RAGE–dependent regulatory program of prostate CAF activation, toward increased cancer cell migration and tumor growth in vivo (174). Additional evidence that the reciprocal interaction between cancer cells and CAFs may be mediated by RAGE-dependent metabolic processes comes from the observation that HMGB-1, released by breast cancer cells, prompts fibroblasts activation and induces a RAGE-mediated metabolic shift toward aerobic glycolysis; in turn, the accumulation of lactate enhances the metastatic potential of breast cancer cells (175) in a feed-forward stimulatory loop.
Extending these findings, data collected from both in vitro and in vivo studies showed that the secretion of HMGB-1 from autophagic CAFs augments the metastatic propensity of lung cancer (176), reinforcing the idea that metabolic processes like autophagy may foster tumor-promoting responses through RAGE activation. On the other hand, we should mention that certain RAGE ligands, including HMGB-1, may signal also through receptors other than RAGE. For instance, despite the fact that maintenance of luminal breast cancer cells reportedly relies on the paracrine release of HMGB-1 from autophagic CAFs, the receptor implicated in this oncogenic response is the Toll-like receptor (TLR) 4 rather than RAGE (177). Thus, RAGE may cooperate with TLRs in triggering stimulatory responses which should be investigated in inflammation-related disease and cancer.
Adipocytes
The RAGE system regulates key functional aspect of adipocyte biology, which contributes to energy storage and to the maintenance of lipid homeostasis. First, RAGE and ligands accumulate in obese murine and human adipose tissues (178); adding to this, global knockout as well as adipocyte-specific deletion of Ager confers protection from obesity and insulin resistance in mice fed a high-fat diet (25, 179). RAGE also confers higher metabolic recovery after fasting or after a cold challenge through an increased thermogenic gene program and an intensified mitochondrial activity (178). Likewise, RAGE overexpression induces adipocyte hypertrophy accompanied by decreased glucose transporter 4 and adiponectin expression together with enhanced insulin resistance (180). These effects appear to rely not only on RAGE, but also on its ligands HMGB-1 and S100B, thus confirming the role of RAGE axis in obesity-driven metabolic imbalances toward an insulin resistant phenotype (180). Interestingly, HMGB-1 secreted by adipocytes triggers RAGE-mediated expression of IL-6, a major mediator of tissue inflammation in diverse pathological conditions including cancer (181).
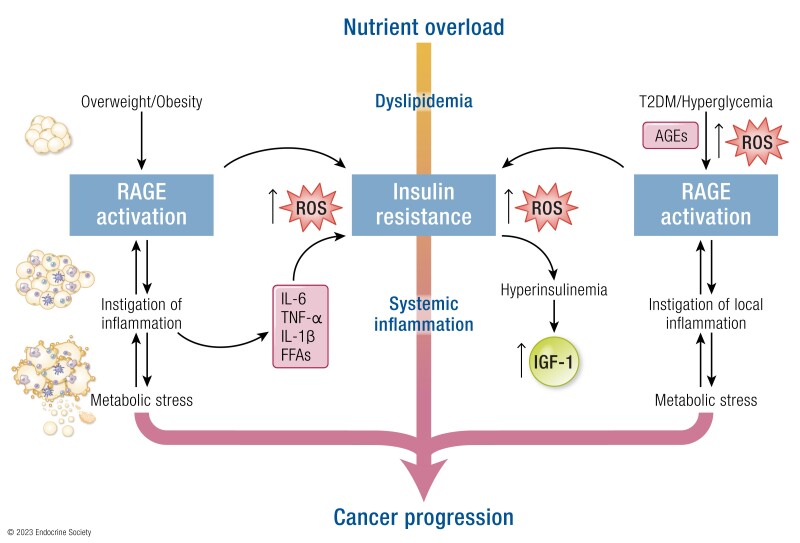
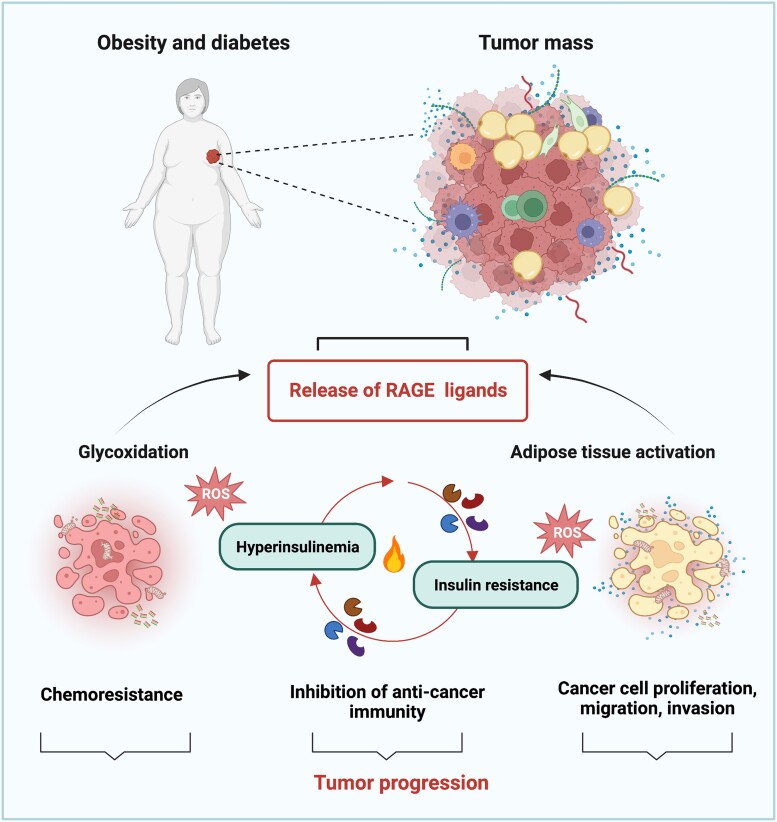
Further characterizing the role of the RAGE pathway in adipocyte-dependent microenvironmental responses of tumor progression, Sakurai and collaborators demonstrated that conditioned medium from adipose-derived stromal cells induce breast cancer cell proliferation and migration through the activation of the S100A7/RAGE axis (182). It should be mentioned that the RAGE pathway seems to be implicated in the acquisition of detrimental features in the adipose tissue adjacent the tumor mass, toward the establishment of tumor inflammation (Fig. 3). In this regard, it was recently shown that the AGE–RAGE pathway is among the most strongly enriched pathways driving the transition to a cancer-associated–like phenotype in adipose-derived mesenchymal stem/stromal cells exposed to triple negative breast cancer secretome (183). In addition, a thorough investigation of the molecular, biological and functional features of tumor-associated adipose tissue from 3 different tumor models established loss of adipocyte specification, necrosis and lipids release, together with robust expression of HMGB-1 and infiltration of lipid-containing and foam cell-resembling macrophages (184). Thus, the RAGE pathway might contribute to the instigation of an inflammatory tumor microenvironment which propagates detrimental stimuli initiated in the aberrant adipose tissue (Fig. 3). Collectively, these observations suggest that RAGE and its ligands may play a crucial role in obesity-driven inflammation and impaired insulin sensitivity which put together diabetes, obesity and cancer (Fig. 3).
Figure 3.
RAGE action in obese- and diabetic-related cancers. RAGE ligands may be released by the tumor mass in response to stressful conditions, such as glycoxidative stress and adipose tissue activation. Once released in the tumor milieu, RAGE ligands facilitate the establishment of insulin resistance and hyperinsulinemia. In addition, RAGE ligands prompt stimulatory effects like cell proliferation, migration, invasion, immuno-evasion and chemoresistance. Abbreviation: ROS, reactive oxygen species.
RAGE Signaling in Tumor Inflammation and Immune Evasion
In healthy tissues, RAGE acts as a relevant arm of innate immunity for protection against infective agents through the instigation of an inflammatory response aimed at eliminating the pathogen. In cancer, RAGE promotes a peculiar type of sterile inflammation (not associated with infective pathogens), which is a chronic, low-grade response, aimed at eliminating cancer cells. However, a long-term and unopposed activation of inflammatory programs ends up in fostering tumor progression. In this scenario, RAGE and its ligands appear to play a critical role (Fig. 4), as indicated by mounting clinical and experimental evidence. For instance, Chen et al employed RAGE knockout animal models of invasive and noninvasive gliomas and found that the genetic depletion of Ager increases the survival rates of mice; this effect was not due to reduction growth rate, but rather to inhibition of the inflammatory cytokine network (185). In support of these findings, RAGE signaling was shown to promote a dysfunctional inflammatory microenvironment characterized by a high degree of immune cell infiltration in diverse types of tumors (186-188). Further dissecting the role of RAGE in tumor inflammation, this receptor appears to be equally expressed in both M1- and M2-like macrophages, despite the opposite role played by these phenotypes in tumor progression (189). In TAMs, RAGE/HMGB-1 signaling has been shown to empower cancer cells with invasive and angiogenic abilities (190). Similarly, in hypoxic metastatic melanoma, HMGB-1 released by cancer cells drives the release of IL-10 from TAMs and prompts their accumulation through RAGE (191), thus suggesting that the HMGB-1/RAGE pathway may represent an evading strategy used by the cancer immune compartment in low oxygen conditions. On the other hand, HMGB-1/RAGE signaling may reduce the motility of TAMs in low oxygen environments, thus indicating that additional efforts need to be undertaken to uncover the potential of HMGB-1/RAGE axis, particularly in stressful conditions associated with ROS generation (192). In this context, Rojas et al observed that RAGE expression persists during macrophages polarization from the proinflammatory M1 to the anti-inflammatory M2 phenotype. They propose that in M2-polarized macrophages, RAGE signaling circumvents the typical activation of NF-κB by recruiting NF-κB negative regulators like SOCS1 and the Src homology-2 domain-containing inositol 5-phosphatase 1 (SHIP-1), allowing for the facilitation of protumor effects of M2 macrophages and leading to enhanced invasion and angiogenesis (190). These data indicate that, despite bypassing classical NF-κB inflammatory signals, RAGE engages M2-polarized macrophages through alternate signaling pathways to foster aggressive biological responses in surrounding tumor cells (189).
Figure 4.
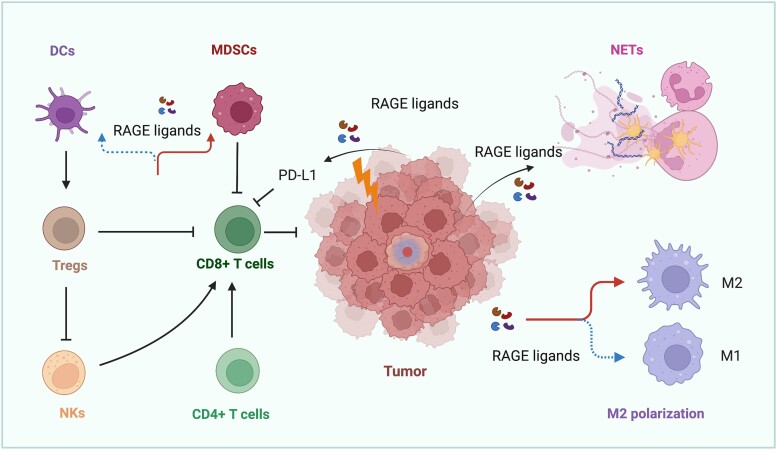
RAGE-dependent immune evasion toward cancer progression. RAGE activation by ligands induces differentiation of M2 macrophages and their accumulation, leading to the suppression of anticancer immunity due to multiple mechanisms (ie, activation of TREG cells, inhibition of NK and CD8+ T cells). Likewise, RAGE ligands released by cancer cells exposed to radiotherapy and chemotherapy stress induce the expression of the immune checkpoint PD-L1, thereby hindering CD8+ T cell–dependent cytotoxicity. Similarly, RAGE ligands inhibit DCs and mobilize MDSCs to halt immune recognition. Parallel, RAGE ligands induce the formation of NETs, which facilitate tumor progression. Abbreviations: DCs, dendritic cells; M2, M2-like macrophages; MDSCs, myeloid derived suppressor cells; NETs, neutrophil extracellular traps; NKs, natural killer cells; PD-L1, programmed death-ligand 1; RAGE, receptor for advanced glycation end products; TREGs, T regulatory cells.
As an effector of innate immunity, RAGE elicits tumor-promoting actions also through the regulation of biological responses triggered by activation of the NLRP3 inflammasomes, as well as by neutrophils and natural killer cells. For instance, the RAGE hetero-ligand S100A8/S100A9 induces ROS-dependent activation of the NLRP3 inflammasome (193), which plays a remarkable role in the progression of certain tumors like glioma and mesothelioma (194). Additionally, the increase in NLRP3 levels and the subsequent build-up of HMGB-1 appear to contribute to the establishment of chemotherapy resistance in malignant mesothelioma (195). Conversely, the HMGB-1/RAGE–NFκB–NLRP3 inflammasome pathway, engaged in glioblastoma cells treated with the anticancer agent temozolomide, contributes to drug chemosensitivity through the re-education of macrophages toward a tumor suppressive phenotype (196).
These controversial data reflect the opposite roles played by the NLRP3 inflammasome, possibly through RAGE, in cancer progression, corroborating that this molecular component of innate immunity elicits a regulatory role based on the tumor type, stage and effector involved.
RAGE signaling has been shown to promote the formation of neutrophil extracellular traps (NETs), complex macromolecular web-like structures composed of DNA chromatin extruded by activated neutrophils and decorated with histone proteins (197) (Fig. 4). First identified for their ability to entrap and get rid of pathogens, NETs have also been shown to sequester circulating cancer cells thus eliciting antitumor effects; nonetheless, several tumor-promoting actions of NETs have been described, including their ability to serve as an adhesion substrate in the metastatic niche for the formation of secondary tumors, to promote ECM remodeling and vascular permeability, as to foster EMT (197). In this context, RAGE contribution to NET-osis toward tumor growth and progression has been shown in gliomas, colorectal and pancreatic cancer, where RAGE signaling triggered autophagy-dependent NETs formation (198-200). Conversely, Sionov et al showed that neutrophils recognize and target cancer cells through RAGE, thus eliciting cytotoxic activity in a contact-dependent manner. Cathepsin G expressed on neutrophils and RAGE expressed on tumor cells represent the main molecular effectors of this anticancer effect (201). These observations are in accordance with the evidence that S100A8-A9/RAGE axis activates natural killer cells and halts tumor growth in vivo, an effect that is prevented by obliterating RAGE signaling (202).
These contrasting findings shed light on the need to deepen our knowledge on the complex mechanisms determining the fate of inflammatory RAGE signaling toward a tumor-promoting (immune-evasion) or tumor-restraining (immune-recognition) effect. However, it is clear that the RAGE axis not only regulates innate immunity, but also serves as a bridging molecule in the connection between innate and adaptive responses of the immune system in cancer. In this regard, the RAGE ligand HMGB-1 was shown to suppress dendritic cells and thus interfere with host anticancer response toward metastatic evolution in murine models of colorectal cancer (203); likewise, in colorectal cancer patients an increased expression of tumoral HMGB-1 was associated with reduced dendritic cells in primary tumors of metastasis-positive patients (203). Neoplastic keratinocytes of the genital tract were shown to secrete HMGB1, which attracted plasmacytoid dendritic cells at the tumor site, thereby inducing a RAGE-mediated phenotypic change reminiscent of tolerogenic response; these data indicate that HMGB-1 released from epithelial cancer cells coopts RAGE signaling in adjacent plasmacytoid dendritic cells to evade tumor immunity (204). Furthermore, the HMGB-1/RAGE pathway induced upon stressful conditions like UV exposure fostered the expression of the immune checkpoint programmed death-ligand 1 (PD-L1) resulting in a diminished CD8+ T cell–dependent cytotoxicity in melanoma (205). These findings are consistent with the observation that HMGB-1 is more highly expressed in cancer patients insensitive to immune checkpoint inhibitors compared with patients that respond to therapy (206). In addition, the RAGE ligands S100A8 and S100A9 released in advanced pancreatic ductal adenocarcinoma lesions induce an expansion of a subset of monocytic MDSCs, a heterogeneous population of myeloid cells with immune suppressive activity (207); however, the involvement of RAGE was hypothesized but not assessed in this study (207). Supporting these findings, Wuren et al demonstrated that RAGE contributes to lung tumor growth and metastatic burden by recruiting and promoting the biological functions of MDSCs, thus establishing an immune indolent microenvironment permissive to disease progression; these effects involve the RAGE ligands S100A8/S100A9 and NF-κB signaling activated in the hematopoietic compartment (208). On the other hand, a recent interesting study showed that S100A9 elicits immunosuppressive actions in a RAGE-independent manner in BCRA1-mutated breast cancer (209), thus suggesting that further mechanisms may contribute to immune evasion in certain tumor contexts. However, data collected from a transgenic animal model of spontaneous murine pancreatic ductal adenocarcinoma indicate that RAGE promotes the accumulation of MDSCs (210). Notably, these effects may be mediated not only by RAGE but also by the TLR4, which serves as a binding molecule for several ligands of RAGE. These findings further suggest that a cooperative interaction between RAGE and TLRs may be implicated in the MDSC regulatory role, as observed in colorectal cancer (211).
RAGE and IIGFs Cross-talk in Meta-inflammation
Accumulating evidence establishes cooperation between RAGE and IIGFs in driving metabolic-dependent inflammatory responses. Nearly 15 years ago, a breakthrough study by Unoki and collaborators identified for the first time the role of RAGE in prompting insulin resistance mediated by ROS in adipocytes (212). Later on, additional research groups confirmed these findings and identified certain ligands of RAGE that, together with ROS, may induce insulin resistance (180, 213). In this condition, an aberrant activation of IIGFs occurs. For instance, when insulin action is impaired in peripheral target tissues, the establishment of a compensatory hyperinsulinemia contributes not only to the metabolic actions, but also to the mitogenic effects elicited by insulin through IR and IGF-1R. Furthermore, during hyperinsulinemia an increased activation of IGF-1/IGF-1R is detected because of augmented hepatic production and systemic bioavailability of IGF-1 (214). The resulting hyperactivation of IIGFs contributes to foster proliferative, invasive, and survival responses in both premalignant and malignant contexts (215).
Interestingly, RAGE activation induces the transcriptional regulation of IGF-1, thereby contributing to amplifying IIGFs signaling (216). In parallel, an increased activation of IGF-1R is observed upon stimulation with AGEs (217). Together, these findings support bi-directional interaction between RAGE and IIGFs (Fig. 5), a concept reinforced by the evidence that most of RAGE-dependent translational effectors are also activated by IIGFs.
Figure 5.
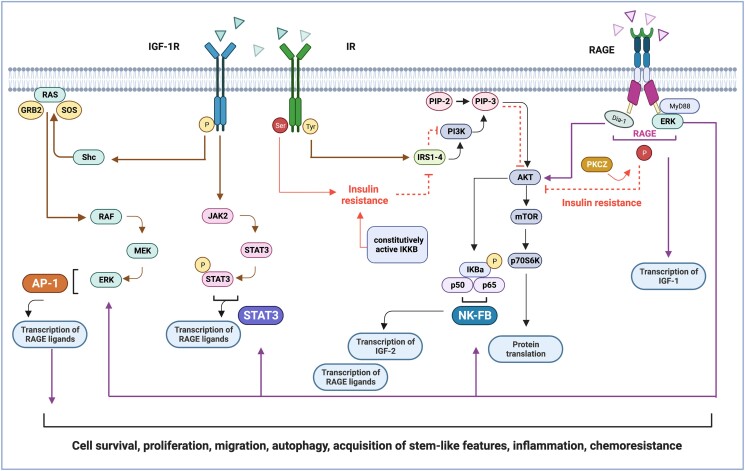
Signaling cross-talk between IIGFs and RAGE. Schematic representation of the translational mediators shared by IIGFs and RAGE which culminate in NF-κB, activator protein -1 and STAT3-mediated gene transcription. Ligands belonging to IIGFs induce receptor phosphorylation at tyrosine level (in yellow). The subsequent engagement of IRSs (1-4) promotes the activation of the PI3K/AKT/mTOR and the RAS/RAF/MEK/ERK cascades, toward gene transcription and protein translation. IIGFs-mediated signals also involve the recruitment of NF-κB and JAK2/STAT3 pathway, which in turn promote the transcription of RAGE ligands (like S100A7), as well as the transcription of IIGFs ligands (like IGF-2). During insulin resistance, insulin receptor phosphorylation at serine (in red), rather than tyrosine residues, inhibits the activation of IRS-1/PI3K/AKT pathway, thus repressing insulin-mediated signals. RAGE activation contributes to insulin resistance through the engagement of PKC-ζ (PKCZ), which directly inhibits IRS-1/PI3K/AKT signaling. RAGE activation also induces the transcription of IGF-1, thus increasing its availability. Parallel, an increased phosphorylation of IGF-1R is evidenced upon activation of the AGE/RAGE axis, thereby amplifying IIGFs signaling. Abbreviations: AKT, protein kinase B; AP-1, activator protein 1; Dia1, diaphanous-related formin 1; ERK, extracellular signal-regulated kinase; GRB2, growth factor receptor–bound protein 2; IGF-1R, insulin-like growth factor-1 receptor; IKBa, I kappa b kinase type a; IKKB, inhibitor of nuclear factor kappa-B kinase subunit beta; IR, insulin receptor; JAK, Janus kinase; IR-A, insulin receptor isoform A, IR-B, insulin receptor isoform B; IRS, insulin-receptor substrate; MEK, mitogen-activated protein kinase kinase; MyD88, myeloid differentiation primary response 88; NF-κB, NF-κB, nuclear factor kappa-light-chain-enhancer; PI3K, phosphatidylinositol 3-kinase; PKC-ζ, protein kinase c zeta type; PIP-2, phosphatidyl inositol 4,5-bisphosphate; PIP-3, phosphatidylinositol (3-5)-trisphosphate; RAS, rat sarcoma family of proteins; RAF, rapidly accelerated fibrosarcoma protein; RAGE, receptor for advanced glycation end products; STAT, signal transducer and activator of transcription; shc, Src homology/collagen protein; SOS, son of sevenless protein.
NF-kB Pathway Is Engaged by the Network of RAGE and IIGFs Signaling
The NF-κB family consists of a group of evolutionary conserved transcription factors activated in response to a heterogeneous plethora of cellular stressors to regulate immune and inflammatory responses and protect from damage. Nevertheless, constitutive activation of the NF-κB pathway is associated with diverse disease states, including cancer. The regulation of the cell transcriptional machinery by NF-κB implies the cooperation of all the 5 structurally related members constituting the NF-κB family: NF-κB1 (also known as p50), NF-κB2 (also known as p52), RelA (also known as p65), RelB, and c-Rel (218). The NF-κB proteins are usually sequestered in the cytoplasm by a family of inhibitory proteins of the IκB family members, which prevent NF-κB nuclear translocation and gene transcription. However, upon several stimuli like cytokines and PRRs, IκBα is phosphorylated by a multisubunit IκB kinase (IKK) complex and thereafter degraded by means of proteasomal ubiquitination; and subsequently, a rapid and transient nuclear translocation of the canonical members p50/RelA and p50/c-Rel occurs, thus switching on gene transcription. Numerous studies have highlighted the role of the NF-κB pathway in transducing signals initiated by RAGE activation in diverse pathological conditions including cancer. For example, NF-κB activation represents a signaling event engaged in response to various RAGE ligands, as it has been demonstrated for AGEs, HMGB-1, several S100 proteins, and HSP70. For instance, the binding of RAGE by its ligands AGEs, S100P and S100A8/A9 induced transcriptional effects and biological responses dependent on NF-κB activation in pancreatic cancer cells (219). In addition, extracellular HSP70 binds RAGE to trigger NF-κB proinflammatory gene expression in human lung cancer cells (220). Notably, in pancreatic cancer unliganded RAGE maintains oncogenic KRas signaling through a feed-forward stimulatory mechanism that involves NF-κB–mediated inflammation (221). This transduction pathway was also involved in the establishment of therapy resistance in acute leukemia cells subjected to chemotherapy. Mechanistically, the release of HMGB-1 from autophagic cancer cells prompted NF-κB–mediated action toward reduced drug sensitiveness (222). In addition, AGE/RAGE signaling activates a particular NF-κB–dependent transcriptional signature implicated in collagen deposition (223), indicating that gene pattern instructed upon RAGE activation may induce deep ECM remodeling that depends on the specific engagement of NF-κB. Not surprisingly, NF-κB action in cancer may include the recruitment of noninflammatory but yet tumor-promoting pathways. In this context, NF-κB pathway is activated in conditions of metabolic deregulations associated with alterations of the IIGF pathways. In fact, in breast cancer cells, IGF-1 was shown to induce proliferative effects through the PI3K/AKT signaling and IRS-2-mediated NF-κB transcriptional activity (224). Microarray data analysis coupled with enrichment pathway analysis indicated that the signaling network IGF-1/PI3K/NFκB/ERK is associated with aggressive features of high-grade serous epithelial ovarian cancer, including reduced sensitivity to platinum-based treatments (225). In addition, the CD74-NRG1 gene fusion product, a hybrid gene resulting from structural DNA rearrangements and leading to aberrant ErbB signaling, has been shown to hyperactivate NF-κB signaling pathway; in turn, the dysfunctional NF-κB response engages IIGFs, as evidenced by the enhanced secretion of IGF2 and the increased phosphorylation of IGF-1R (226). Corroborating these observations, the transcription of IGF2 appears to rely on the occupation of candidate NF-κB binding site motifs present in the human IGF2 promoter (227).
Taken together, these observations place NF-κB signaling among the transduction pathways shared by RAGE and IIGFs toward cancer progression through the modulation of metabolic and inflammatory responses. Conceivable with its action of transcription factor implicated in cell response to stress, NF-κB regulates metabolic pathways mainly during stressful conditions like overnutrition (228). A paradigmatic example is provided by the evidence that (1) NF-κB contributes to the inflammatory response instigated by macrophages in conditions of obesity and (2) NF-κB is involved in the establishment of insulin resistance. In this regard, it has been shown that components of the NF-κB pathway like the noncanonical IKK kinase IKK3, which regulates the late phase of the NF-κB transcriptional activity, is required for the establishment of obesity induced by high-fat diet (229). Interestingly, a milestone study on the role of NF-κB in the regulation of insulin sensitivity demonstrated that mice heterozygous for IKKb (Ikk2+/−) are protected against insulin resistance in response to high-fat diet and genetically induced obesity (230). Furthermore, diacylglycerol derived from fatty acids engages PKC signaling toward the B-cell lymphoma 10–mediated activation of NF-κB and insulin resistance (231), which is also observed when a constitutively active form of IKKb aberrantly engages NF-κB activity (232). In these conditions, insulin resistance, hyperinsulinemia, high fatty acids, and glucose intolerance are detected (232).
JAK/STAT Pathway Contributes to the Network of RAGE and IIGFs Signaling
Several membrane receptors, including cytokine and growth factor receptors, convey stimulatory signals through the Janus kinase (JAK)/STAT signaling pathway. First identified when characterizing the molecular mechanisms implicated in interferon signaling, the JAK/STAT family includes 4 members of JAK (TYK2, Jak-1, Jak-2, Jak-3) and 7 members of STAT (STAT1, STAT2, STAT3, STAT4, STAT5 (a/b), STAT6), which regulate gene transcription, thereby inducing several biological responses implicated in tumorigenesis and neoplastic progression (233). JAK/STAT–mediated gene transcription is initiated when the JAK kinase domain, characterized by tyrosine catalytic activity, recruits the SH2-like domain of other JAK/STAT proteins to form homodimers or heterodimers that move in the cell nucleus to be recruited to candidate sequences within the promoter of target genes (233). Several RAGE ligands including AGEs, S100A7, HMGB-1, S100A4 have been shown to recruit the JAK/STAT signaling cascade and promote stimulatory effects in cancer. For instance, the AGE methylglyoxal promotes proliferative and migratory effects in breast cancer and acute myeloid leukemia cells through a RAGE-dependent engagement of STAT3 (150, 234). In addition, the RAGE ligand HMGB-1 may induce the release of IL-6 through the engagement of JAK2/STAT3 signaling pathway toward anticancer therapeutic resistance (235). Further extending these findings, the HMGB-1/RAGE pathway promoted the activation of an inflammatory gene program in the tumor microenvironment consisting of IL-23, IL-17, and IL-6, which engaged STAT3 signaling thereby fostering melanoma tumor growth (236). STAT3 is also implicated in the upregulation of the RAGE ligands S100A8 and S100A9, leading to the accumulation of MDSCs and antitumor immunity (237). Likewise, an S100B/RAGE driven activation of STAT3 obliterates the immune fractions of microglia in malignant gliomas, thus corroborating the involvement of STAT3 in the promotion of immunosuppressive functions prompted by RAGE (238). Therefore, it is conceivable to postulate the role of RAGE-mediated JAK/STAT signaling in tumor immune modulation. In an effort to disclose the role of the RAGE/STAT3 pathway at the crossroad between altered inflammation and metabolic dysfunction, Kang and coworkers found that RAGE-mediated autophagic programs in pancreatic cancer leads to IL-6-induced STAT3 activation and localization at the mitochondria, where pSTAT3 mobilizes the pool of available ATP thus supporting cell proliferation (161). As the effects mediated by RAGE on the engagement of inflammatory factors in the context of altered energy pathways occur during the early stages of pancreatic cancer lesions, it could be postulated that RAGE/STAT3 signaling represents a pathogenic molecular hub bridging together inflammation and metabolism toward the early development of pancreatic malignancy (161). The bidirectional interaction elicited between metabolic and inflammatory pathways through the RAGE/STAT3 signaling is evidenced in diverse investigations aimed at clarifying the tumor-promoting role elicited by the RAGE ligand S100A7 in cancer. First, the calgranulin S100A7 has been shown to promote mammary tumorigenesis by coordinating the transcriptional profile of breast cancer toward metastatic and inflammatory programs in vitro; for example, enhanced lung metastasis and TAM recruitment were observed in mice models of breast cancer after activation of the S100A7/RAGE/STAT3 pathway (239). In S100A7-mediated inflammatory actions, it has been shown that STAT3 directly regulates the transcriptional activation of the human S100A7 gene, as demonstrated in breast cancer cells stimulated with the proinflammatory cytokines oncostatin-M and IL-6 (240). These data suggest that STAT3 may either respond to S100A7 in triggering tumor inflammation and recruitment of immune cells, either reinforce the inflammatory microenvironment by fueling S100A7 transcription in an autoregulatory loop. Interestingly, the interrogation of a coculture system based on carcinoma adipose stromal cells and breast cancer cells showed that paracrine interactions between breast cancer cells and adipose stromal cells are facilitated by inflammatory mediators and involve the activation of the S100A7/RAGE/STAT3 pathway to prompt aggressive features (10). These observations point at STAT3 as an effector of multiple converging nodes of aberrant metabolic and inflammatory cascades that facilitate microenvironmental responses involved in cancer progression. In line with these observations, the IGF-1/IGF-1R signaling, which is frequently deregulated in conditions of obesity, diabetes and cancer, induces in breast cancer cells the STAT3-dependent upregulation of S100A7, which is extracellularly released to prime adjacent endothelial cells toward a RAGE-mediated angiogenic switch (99). Therefore, STAT3 may represent an alternate transduction factor engaged by IGF-1R, along with the classical PI3K/AKT and ERK1/2 signaling cascades, to mediate stimulatory actions in cancer cells through the activation of RAGE signaling (99). Likewise, insulin itself is known to activate STAT3 to convey intracellular messages of proliferation, as observed in keratinocytes (241). On the other hand, STAT3 has been implicated in the establishment of insulin resistance in mice fed a high-fat diet (242), which are known to depend on RAGE for the accumulation of body weight, the development of insulin resistance and of altered glucose tolerance (25).
In the context of obesity, a chronic activation of the JAK/STAT3 pathway is known to promote SOCS3 binding to IRS-1 and -2, which leads to their ubiquitin-mediated degradation, facilitating insulin resistance (243). Hence, the prolonged activation of RAGE occurring during obesity and/or diabetes may contribute to the STAT3-dependent impairment of insulin function, and the subsequent establishment of hyperinsulinemia. High levels of circulating insulin may contribute to the detrimental actions elicited by this hormone in cancer contexts, which have been extensively demonstrated (93). Notably, STAT3 is implicated in the stimulatory responses elicited by hyperglycemia (244), a condition well-known to be characterized by aberrant RAGE signaling toward inflammation and disease progression (23).
PI3K/AKT and MEK/ERK Pathways Function Within the Network of RAGE and IIGFs Signaling
The interaction of RAGE with its ligands triggers signaling cascades that include the PI3K/AKT/mTOR and the ERK1/2 transduction pathways, implicated in the transmission of signals from extracellular stimuli to the intracellular transcriptional machinery in normal and cancer cells. Upon RAGE ligand binding, stimulation of both the PI3K/AKT and the MAPK signaling cascades converge on NF-κB, either triggering AP1-dependent gene transcription and/or STAT3 activation.
In prostate cancer cells, AGE/RAGE interaction has been shown to activate PI3K/AKT signaling leading to the degradation of retinoblastoma protein and proliferative effects (245). The engagement of the PI3K/AKT/mTOR pathway occurs also for other RAGE ligands implicated in tumor progression, like S100A4, S100A7, and HMGB-1 (99, 246, 247). However, certain ligands like S100A11 bypass PI3K/AKT activation to engage mTOR signaling through the adaptor protein MyD88, which interacts with RAGE cytoplasmic tail upon ligand binding, to recruit downstream signaling cascades (132, 248). In parallel with PI3K/AKT/mTOR pathway, RAGE recruits ERK1/2 signaling cascade by either directly binding to the cytoplasmic D-domain–like docking site (249), or by activating the Rho family small G-proteins and cdc42/Rac (21). ERK activation appears to be required for the acquisition of migratory, invasive, and mesenchymal-like phenotypes observed after RAGE overexpression in lung cancer cells, as well as for the accumulation of TAMs and formation of lung metastasis (187). In addition, the HMGB-1/RAGE pathway induces ERK activation toward autophagy activation and cell survival in lung adenocarcinoma cells exposed to nutrient depletion (250), indicating that ERK1/2 represents 1 of the signaling nodes employed by RAGE axis to cope with metabolic stressors. Similarly, the RAGE ligands S100A7, HMGB-1, S100P, and S100A4 trigger ERK1/2 signaling to promote stimulatory actions in breast, colorectal, pancreatic, gastric, renal and thyroid cancer (99, 159, 251-254). A study by Kang et al, showed that HMGB-1 released by necrotic fibroblasts induces a metabolic reprogramming in adjacent pancreatic cancer cells consisting of enhanced OXPHOS-dependent energetic flux and ATP production (161). Such actions support cell proliferation and migration, and appear to depend on RAGE localization at the mitochondrial complex I, its phosphorylation at Ser377, and its interaction with pERK1/2 (161). This interesting study provides evidence for the role of the inflammatory microenvironment in instigating metabolic rearrangements permissive for cancer progression through the involvement of RAGE-dependent ERK1/2 signaling.
The PI3K/AKT and the MAPK signaling pathways are also engaged by the IIGFs to prompt aggressive features in diverse types of tumors. While the PI3K/AKT signaling is mainly implicated in insulin-mediated responses, the ERK1/2 pathway mostly responds to IGFs. These signaling pathways are implicated in the regulation of the energetic metabolism of cancer cells induced by IIGFs. In fact, in breast cancer cells engineered for the overexpression of IGF2, an increased activation of ERK1/2 and AKT is detected, together with higher metabolic capability and enhanced proliferative and invasive properties (43). Both ERK1/2 and AKT signaling cascades are implicated also in the IGF1-induced activation of the S100A7/RAGE pathway which leads to increased breast tumor angiogenesis (99). These data establish the involvement of IIGFs in activating RAGE axis through ERK1/2 and AKT signaling in cancer.
The relationship between RAGE and IIGFs is further supported by evidence that RAGE knockdown attenuates insulin resistance in response to stressful conditions like hypoxia, as demonstrated by the inhibition of IRS-1 serine phosphorylation, the increased activation of AKT, and the improvement of glucose uptake (255). Also, the RAGE ligand HMGB-1 halts insulin-induced AKT phosphorylation, used as a readout for insulin sensitivity/resistance, through RAGE and TLR4 (256).
One might postulate that RAGE axis controls insulin signaling and promotes a metabolic shift toward insulin resistance, associated with decreased AKT activation and the establishment of compensatory hyperinsulinemia, which triggers stimulatory actions in cancer cells.
The molecular mechanisms potentially implicated in this action suggest that PKC-ζ may serve as a main actor in the regulation of insulin sensitivity by RAGE. First, it has been shown that PKC-ζ phosphorylates RAGE at Ser391 after ligand binding; also, PKC-ζ appears to be implicated in the phosphorylation of IRS-1 at Ser318 and Ser570, which support the downstream inhibitory effect observed during prolonged hyperinsulinemia, paving the way to insulin resistance (257). Also, in conditions that mimic a hyperglycemic and hyperinsulinemic environment, the stimulation of IRS-1 Ser318 and AKT Thr34 by PKC-ζ dramatically impacts on insulin capability to uptake glucose by adipocytes (258). Taken together, these observations implicate PKC-ζ as a molecular effector of both RAGE-signaling and insulin resistance through the direct inhibition of IRS-1/AKT activation.
Further investigations are warranted to clarify the role of RAGE in insulin-rich milieu, as the data suggest that microenvironmental conditions implicated in the establishment of insulin resistance and aberrant insulin signaling are initiated by inflammation and may involve the RAGE pathway to generate a permissive niche for tumor progression. Therefore, in some contexts RAGE may play a dual tumor promoting role, by instigating an obesity-driven inflammatory microenvironment on 1 side, and by perpetuating the main trigger of insulin resistance and hyperinsulinemia on the other side.
On this basis, RAGE cross-talk with signaling pathways activated in response to aberrant IIGFs may provide a valuable target for molecular intervention, particularly in those cancer types associated with meta-inflammation.
“Un-RAGE-ing” Meta-inflammation: Therapeutic Opportunities
The evidence that RAGE KO mice are viable and healthy, and with no signs of altered embryonic development (259) suggests that targeting aberrant RAGE signaling may represent a reasonably feasible approach to halt a number of pathological conditions associated with altered inflammation and metabolism, including cancer.
Specific RAGE inhibition strategies with known anticancer activity in preclinical and clinical studies are summarized in Table 1 and include (1) small molecules like FPS-ZM1 (N-benzyl-N-cyclohexyl-4-chlorobenzamide); (2) RAGE neutralizing antibodies; (3) RAGE DNA oligonucleotide aptamers; (4) RAGE binding peptide; (5) soluble RAGE (sRAGE).
Table 1.
Schematic example of inhibitors of RAGE axis with anticancer properties
| RAGE inhibitor/inhibition strategy | Target of inhibition | Cancer type | Effect | Ref. |
|---|---|---|---|---|
| FPS-ZM1 | RAGE | Breast cancer | Reduced cell migration and invasion; decreased tumor growth, metastasis formation and angiogenesis | (260) |
| RAGE | Pancreatic cancer | Reduced tumor growth | (221) | |
| RAGE/HMGB1 | Melanoma | Restoration of anticancer immunity | (205) | |
| RAGE/S100A9 | Colorectal cancer | Restoration of anticancer immunity | (211) | |
| RAGE/S1007 | Breast cancer | Reduced tumor angiogenesis | (99) | |
| RAGE/S1008-A9 | TNBC | Reduced cell proliferation, migration and colony formation | (261) | |
| RAGE/HMGB-1 | Cervical cancer | Reduced LPS-dependent malignant transformation | (262) | |
| RAGE/HMGB-1 | Pancreatic cancer | Reduced tumor growth in combination with gemcitabine | (263) | |
| RAGE/S100P | Nasopharyngeal carcinoma | Reduced cell proliferation and migration | (264) | |
| RAGE/AGEs | TNBC | Reduced cell migration and invasion | (265) | |
| TTP-488 | RAGE/AGEs | Prostate cancer | Reduced tumor formation and cell migration | (174) |
| TTP-488 analogue | RAGE | TNBC | Reduced cell proliferation and survival | (266) |
| RAP | RAGE | Pancreatic cancer and glioma | Reduced tumor growth and metastasis | (267) |
| S100P/RAGE | Pancreatic cancer | Reduced cell proliferation, survival, migration, and invasion | (267) | |
| RAGE | Pancreatic cancer | Delayed tumor development | (152) | |
| RAGE/S100A14 | Esophageal squamous cell carcinoma | Reduced cell proliferation and survival | (268) | |
| RBP | RAGE | Glioblastoma | Reduced tumor growth and angiogenesis | (269) |
| RAGE aptamer | RAGE/S100AB | Colorectal cancer | Reduced tumorigenesis | (270) |
| AGE/RAGE | Melanoma | Reduced tumor growth and liver metastasis | (271) | |
| RAGE | Melanoma | Reduced tumor growth and angiogenesis | (272) | |
| siRNA | RAGE | Breast cancer | Reduced cell proliferation | (273) |
| RAGE/HMGB-1 | Pancreatic cancer | Reduced tumor growth | (161) | |
| RAGE/AGE | Prostate | Reduced cell proliferation | (245) | |
| shRNA | RAGE/HMGB-1 | Breast cancer | Reduced expression of immune checkpoint inhibitors, reduced cell invasion | (274) |
| RAGE/HMGB-1 | Hepatocarcinoma | Reduced cell invasion | (275) | |
| Genetic ablation | RAGE | Hepatocarcinoma | Reduced tumorigenesis | (276) |
| ODNs | RAGE/AGE | Colon cancer | Reduced cell growth, invasion and migration | (277) |
| Primary acute myeloid leukemia | Inhibition of AGE-induced cell growth | (234) | ||
| Neutralizing antibody | RAGE/HMGB-1 | Gastric cancer | Decreased cell survival | (278) |
| RAGE/HMGB-1 | Colorectal | Reduced EMT | (279) | |
| RAGE/AGE | Breast cancer | Reduced cell proliferation | (150, 151) | |
| RAGE/AGE | Colorectal cancer and hepatic | Decreased cell proliferation, migration and invasion | (157, 280) | |
| RAGE/S100A7 | Breast cancer | Reduced cell proliferation, reduced tumor growth and metastasis | (188) | |
| RAGE/sulfated glycosaminoglycans | Lung cancer | Reduced metastasis | (281) | |
| RAGE/HMGB-1 | Thyroid cancer | Reduced expression of the oncogenic cluster mir221/222, implicated in the regulation of cell proliferation | (282) | |
| ADCs | RAGE | Endometrial cancer | Selective cytotoxicity in vitro and in vivo | (283) |
| Pioglitazone | RAGE | Hepatic cancer | Reduced cell proliferation | (284) |
| Chloroquine | RAGE | Pancreatic ductal adenocarcinoma | Reduced tumor growth in vitro and in vivo | (285) |
| BMS-687681 | RAGE | Pancreatic ductal adenocarcinoma | Enhanced efficacy of immunotherapy and radiotherapy | (286) |
| Cromolyn | RAGE/S100P | Pancreatic cancer | Inhibition of tumor growth increased effectiveness of gemcitabine | (287) |
| Papaverine | RAGE/HMGB-1 | Glioblastoma | Reduced cell proliferation and migration | (288) |
| RAGE/HMGB-1 | Fibrosarcoma | Reduced cell proliferation, migration and invasion | (289) | |
| RAGE/HMGB-1 | Glioblastoma | Reduced tumor growth, enhanced radiosensitivity, enhanced efficacy of temozolomide | (290) | |
| Heparin | RAGE/HMGB-1 | Fibrosarcoma | Reduced tumorigenesis and metastasis formation | (291) |
| Quercetin | RAGE/HMGB-1 | Breast cancer | Protection from necrotic insult, induction of apoptosis | (292) |
| Pancreatic cancer | Enhanced gemcitabine effectiveness | (293) | ||
| Metformin | AGE/RAGE interaction | Breast cancer | Reduced expression of angiogenic mediators, reduced cell proliferation | (294) |
| AACOCF3 | RAGE/S100A7 | Breast cancer | Reduced tumor burden and enhanced, immunosuppressive environment | (295) |
| sRAGE | S100A4 | Melanoma | Reduced cell transmigration | (296) |
| HMGB-1 | Glioma | Reduced tumor growth and metastasis formation | (297) | |
| Neutralizing antibody | HMGB-1 | Pancreatic cancer | Reduced tumor growth | |
| Oral cancer | Reduced bone destruction | (161, 298) | ||
| Head and neck cancer | Reduced bone pain associated with bone invasion | (299) | ||
| Colorectal cancer | Reduced tumor growth and metastasis formation | (300) | ||
| Prostate cancer | Sensitization to paclitaxel | (301) | ||
| Ethyl pyruvate | HMGB-1 | Gallbladder cancer | Reduced cell proliferation | (302) |
| Thoracic cancers | Protection from radiation-induced damage | (303) | ||
| Lung cancer | Reduced growth, invasion and migration of nonsmall-cell lung cancer cells | (304) | ||
| Glycyrrhizin | HMGB-1 | Esophageal carcinoma | Reduced tumor growth and angiogenesis | (305) |
| Colorectal cancer | Reduced tumorigenesis and inflammation | (306) | ||
| Invasive breast, lung and cervical cancer | Increased efficacy of anti-PD-1 based immunotherapy | (307) | ||
| Prostate cancer | Sensitization to paclitaxel | (301) | ||
| Gastric cancer | Reduced cell proliferation | (278) | ||
| shRNA | HMGB-1 | Hepatocellular carcinoma | Tumor growth | (308) |
| HMGB-1 | TNBC | Reduced cell viability Reduced EMT transition |
(309) | |
| Neutralizing antibody | S100A4 | Pancreatic cancer | Reduced tumor growth and angiogenesis | (310) |
| Breast cancer | Reduced tumor progression, premetastatic niche formation and T cell infiltration | (311) | ||
| Sulindac | S100A4 | Colon cancer | Reduced metastasis formation | (312) |
| Calcimycin | S100A4 | Colon cancer | Reduced metastasis formation | (313) |
| Peptibody | S100A8/S100A9 | Lymphoma | Reduced tumor growth associated with depletion of MDSCs | (314) |
| Genetic ablation | S100A8/S100A9 | Hepatic cancer | Reduced cell proliferation and tumor growth | (315) |
| Tasquinimod | S100A9 | Prostate cancer | Prolonged survival of patients with metastatic castration–resistant prostate cancer | Clinical trial (316) |
| ONO-2506 | S100B | Glioma | Reduced TAM infiltration increased survival in animal models of glioma | (317) |
| siRNA | S100B | Ovarian cancer | Reduced self-renewal in vitro and tumorigenicity in vivo | (318) |
| Neutralizing antibody | S100B | Colon cancer | Reduced cell proliferation, migration and invasion | (319) |
| Duloxetine | S100B | Glioma | Reduced trafficking of tumor-associated myeloid-derived cells reduced tumor growth | (320) |
| Pentamidine | S100B | Melanoma | Reduced ATP production in ATP chemosensitivity assays | (321) |
| Neutralizing antibody | S100A7 | Colorectal cancer | Reduced cell migration and metastasis formation | (322) |
| Luteolin and quercetin | S100A7 | Squamous carcinoma | Reduced cell migration and invasion | (323) |
| DNA aptamer | S100P | Colon cancer | Reduced cell proliferation, migration and EMT reduced tumor growth | (324) |
| AGEs | Melanoma | Reduced tumor growth | (325) | |
| siRNA | S100P | Nasopharyngeal carcinoma | Reduced cell proliferation, migration and colony formation | (326) |
| PENVE | S100B | Colon cancer | Increased apoptotic rate | (327) |
| Aminoguanidine | Methylglyoxal | Thyroid cancer | Reduced cell migration, invasion and EMT | (328) |
Abbreviations: ADC, antibody drug conjugate; AGE, advanced glycation end product; ATP, adenosine triphosphate; EMT, epithelial mesenchymal transition; HMGB-1, high mobility group box 1; LPS, lipopolysaccharide; MDSC, myeloid derived suppressor cell; ODN, S-oligodeoxynucleotide; PENVE, penthamidine vehiculation; PD-1, programmed cell death protein 1; RAGE, receptor for advanced glycation end products; RAP, RAGE antagonist peptide; RBP, RAGE binding peptide; siRNA, small interfering RNA; shRNA, short hairpin RNA; sRAGE, soluble RAGE; TAM, tumor-associated macrophages; TNBC, triple negative breast cancer.
In addition, repurposing strategies have proposed that RAGE-mediated action in cancer is halted also by several FDA-approved drugs such as metformin, pioglitazone, sulindac, chloroquine, heparin (Table 1).
The global anticancer mechanisms elicited by RAGE inhibitors have been described extensively; however, an in depth focus on available strategies known to target RAGE-mediated aberrant inflammation and deranged metabolism might be useful to better exploit current anticancer therapeutic opportunities and further develop novel approaches, particularly in the context of neoplastic diseases characterized by a strong inflammatory component and/or undue metabolic imbalances.
Inhibiting RAGE
The small peptide FPS-ZM1 (N-benzyl-N-cyclohexyl-4-chlorobenzamide), which inhibits the interaction between RAGE V domain and ligands, interferes with RAGE-mediated transduction inflammatory responses in various tumor contexts. For instance, in murine models of breast cancer, the administration of FPS-ZM1 was shown to prevent inflammatory cell recruitment, as demonstrated by a reduction in both tumor-associated leukocytes and macrophages (260). This effect, was accompanied by a reduced metastatic burden at lung and liver, thus suggesting that FPS-ZM1 may be further investigated for its ability to facilitate antitumor immunity and thwart metastatic dissemination.
Likewise, among the anticancer actions elicited by FPS-ZM1, the reduced levels of the immune checkpoint inhibitor PD-L1 appear to be accountable for the restoration of anticancer immunity observed in melanocytes exposed to UVs-triggered inflammatory stimuli (205). In agreement with these findings, the treatment with FPS-ZM1 was able to prevent S100A9-induced MDSCs chemotaxis and migration in colorectal cancer, indicating that RAGE inhibition normalizes the immunosuppressive milieu generated in response to inflammatory-prone tumor niches (211).
With the aim to further assess the ability of RAGE-blocking strategies to normalize aberrant inflammation, Nasser et al employed a transgenic mouse model of breast cancer engineered to mimic the hyperactivation of RAGE signaling through the overexpression of S100A7. In this model system, the administration of a RAGE neutralizing antibody halted the recruitment of TAMs at the tumor site and hampered metastasis formation, an effect observed also after administration of sRAGE, which serves as a scavenger for RAGE ligands thereby preventing the detrimental consequences of membrane RAGE activation (188). In addition, the administration of both sRAGE and a RAGE neutralizing antibody induced a substantial decrease of M2 macrophages accumulation in the lungs. Therefore, the inhibition of metastasis formation occurs, at least in part, through the decrease of inflammatory cells recruitment (188). Extending these findings, an anti-RAGE peptide was shown to restore immune recognition in cervical cancer by preventing dysfunctional plasmacytoid dendritic cells activity in the tumor microenvironment (204).
Along with their acknowledged action in preventing inflammatory damage and immune escape, several RAGE inhibitors have been shown to normalize the response of cancer cells to abnormal energy metabolism. For instance, in high glucose environments a RAGE blocking antibody was shown to prevent glyco-metabolic responses associated with lung cancer progression (153). This beneficial action mainly relied on the inhibition of NOXs activity and with the suppression of inflammatory responses (153). In addition, a RAGE antagonist peptide was shown to prevent the proliferative effects induced in pancreatic cancer cells by CML, whose levels may increase in neoplastic diseases and in diabetes (152).
Similarly, a RAGE-blocking antibody halted the activation of transcriptional programs conducive to glycolytic and anabolic activity induced by AGEs in colorectal and liver cancer cells, thus contributing to block aberrant proliferation rates (157, 280). Further extending these findings, depleting the RAGE ligand S100A4 reduced mitochondrial respiration, with a drastic shift of cellular metabolism to glycolysis. This rendered lung cancer cells more sensitive to glycolysis inhibitors and decreased tumor growth and metastatic rates in animal models of lung cancer (168).
Evidence that RAGE regulates the metabolic features of the tumor microenvironment comes from a study showing RAGE inhibition exhausts the stimulatory cross-talk between cancer cells and CAFs (174). More specifically, the orally bioavailable RAGE inhibitor TTP-488 decreases nutrition-associated and glycoxidation-mediated tumorigenesis in vivo, also by repressing fibroblasts activation (174).
Inhibition of RAGE activation may also normalize the detrimental tumor microenvironment. Indeed, a neutralizing antibody directed at the RAGE ligand HMGB-1 abrogated the activation of CAFs and their glycolytic energetic asset, thus abolishing CAF-dependent production of lactate and subsequent migration of adjacent breast cancer cells (175).
While the potential of inhibiting RAGE/ligand axis to control aberrant metabolic reprogramming and halt disease progression has been largely supported, additional efforts need to address whether the inhibition of this crucial signaling may reverse the inflammation-dependent metabolic imbalances of cancer cells. Some support for this potential action comes from a study showing that a RAGE neutralizing antibody and a HMGB-1 inhibitor are able to block anabolic reactions instructed upon microenvironmental inflammation, by restraining mitochondrial ATP production toward decreased pancreatic tumor growth in vitro and in vivo (161). The proposed model suggests that inflammatory mediators like HMGB-1, released by necrotic tumor areas, foster energy metabolism by shifting the energetic cell machinery toward a RAGE-mediated anabolic phenotype, which represents a suitable pharmacological target.
Hence, RAGE inhibition could breach the solid link between inflammatory signals coming from the tumor microenvironment (HMGB-1) and aberrant metabolic responses (high ATP production) enacted by cancer cells. These findings establish a robust rational for further exploring the potential of anti-RAGE strategies in neoplastic diseases associated with imbalanced metabolism and inflammation.
Inhibiting RAGE-IIGFs Cross-talk
While accumulating evidence points at RAGE signaling as an emerging drug target to normalize aberrant meta-inflammation in cancer, research should focus more on whether targeting aberrant RAGE pathway may be of even greater benefit in conditions of deregulated IIGF signaling.
Although cancer presents with more severe features in patients with concomitant diabetes and/or obesity, the current oncological approach in this subpopulation of individuals does not generally differ from that of nonobese, nondiabetic patients. The lack of a comprehensive understanding of the molecular interactions and biological responses determining cell fate in cancer patients with coexistent metabolic imbalances is mostly accountable for such clinical gap.
The opportunity to halt RAGE signaling in cancer types associated with dysregulation of IIGFs is supported by evidence collected in diverse models characterized by aberrant IIGF activation. For instance, the genetic deficiency of RAGE prevents the negative effects elicited by high-fat diet on energy expenditure, weight gain, and adipose tissue inflammation (25). In addition, in animal models of diet-induced obesity, RAGE deletion abrogates the establishment of insulin resistance, a condition associated with relative enhancement of circulating insulin levels and depression of IR signaling achieved through multiple mechanisms (ie, downregulation of IR and phosphorylation of IR in serine rather than tyrosine residues) (25). Likewise, interrupting the molecular interaction between RAGE cytoplasmic domain and its downstream transduction effector diaphanous Related Formin 1 (DIAPH1) has been proven effective in halting diabetic complications (329).
On the other hand, insulin increases the expression of both full-length RAGE and esRAGE, together with enhancing the shedding of sRAGE from membrane-bound receptor in monocytes (330). These observations suggest that different patterns of reciprocal regulation between RAGE and IR occur in distinct cell types and in peculiar metabolic environments, highlighting that cell- and tissue-specific pharmacological manipulation may be required to prevent aberrant RAGE/IIGFs cross-talk.
Notably, RAGE phosphorylation upon ligand binding occurs through the involvement of an atypical PKC isoform named PKC-ζ (132), a serine/threonine kinase, which is also a downstream effector of insulin signaling pathway. In fact, in adipocytes exposed to hyperglycemia and hyperinsulinemia PKC-ζ contributes to the repression of IR signaling and insulin resistance (257, 331), effects reversed by a cell-permeable and specific PKC-ζ- inhibitory peptide (258). In addition, in hyperglycemic environments PKC-ζ facilitates IGF-1 action (332), thus corroborating the role of this transduction mediator in conveying stimulatory signals propagated during dysregulation of IIGFs.
Considering its role in cancer-related inflammation, chemoresistance and metastasis, PKC-ζ may represent 1 of the emerging RAGE-associated molecular effectors linking insulin resistance/hyperinsulinemia with aggressive malignant features (333). Diverse investigations have indicated that PKC-ζ inhibition contributes to reducing tumor growth and metastatic progression in vitro and in vivo (334, 335), but further studies are needed to clarify whether antagonizing PKC-ζ signaling may be useful in the context of IIGF deregulation.
Additional transduction pathways, activated downstream or independent of PKC-ζ, may amplify the detrimental actions instigated by RAGE/IIGFs cross-talk, thus representing crucial druggable targets to consider in combination therapies. Likewise, the evidence that certain intracellular transduction pathways (including ERK1/2, PI3K, STAT3, and NF-κB) are shared by RAGE and IIGFs supports the concept that the cooperation between these complex signaling axes may contribute to the activation of multiple biological responses encompassing altered metabolism and inflammation.
On the other hand, in breast cancer the RAGE inhibitor FPS-ZM1 was sufficient to normalize aberrant angiogenesis induced by IGF-1/IGF-1R (99), thus indicating that targeting RAGE may represent a promising strategy to terminate aberrant IIGFs signaling in anticancer settings. In this context, while blocking IIGFs in cancer may lead to undesired hyperglycemia, ultimately triggering prediabetes, targeting RAGE could represent a more feasible and well-tolerated option. As tempting as this speculation may sound, additional research investigations will have to address whether a pharmacological effort aimed at targeting shared nodes of interactions between IIGFs and RAGE systems might be of benefit in selected populations of cancer patients. In this regard, clinical studies are assessing whether nutritional approaches meant to rectify aberrant IIGFs and RAGE signaling might be helpful in reducing cancer risk or cancer prognosis.
For instance, in the last decade studies have shown that calorie restriction may elicit beneficial effects in cancer patients through multiple mechanisms, including the normalization of altered metabolome and inflammation (336). In this regard, calorie restriction adjusts aberrant meta-inflammation through the decrease of IGF1-induced activation of the NF-κB/S100A9 signaling in pancreatic cancer (337). Likewise, calorie restriction mimetics reduce the bioavailability of IGF-1 and reinstate anticancer immunosurveillance thus inhibiting tumor growth in vivo and improving chemotherapy efficacy (338). A similar scenario has been proposed when employing fasting regimens (336, 339). Extending these findings, in dietary interventions enacted during obesity, sRAGE might be used as a biomarker to predict outcome in terms of weight loss and improvement of insulin resistance (340).
Interestingly, an ongoing clinical study aims to clarify whether endocrine therapy in combination with an AGE inhibitor can improve insulin resistance and lower circulating levels of sRAGE, AGE, and other inflammatory mediators in patients with metastatic breast cancer (ClinicalTrials.gov identifier: NCT03092635). More specifically, results collected from this interventional clinical study will contribute to unveil the efficacy of anti-AGE regimen in obliterating inflammation-induced insulin resistance and the progression of breast cancer (ClinicalTrials.gov identifier: NCT03092635).
With a similar purpose, a randomized, phase II, clinical study has been designed to establish whether metformin in combination with an AGE inhibitor (oligomeric procyanidin complex) may contribute to control prostate cancer progression through the reduction of AGEs levels in patients receiving androgen deprivation therapy (ClinicalTrials.gov identifier: NCT02946996). In addition, ongoing clinical trials are attempting to determine whether (1) AGEs intake may correlate with inflammation-induced DNA damage in cancer patients (ClinicalTrials.gov identifier: NCT04716764) and (2) AGE-lowering strategies may prevent tumor recurrence (ClinicalTrials.gov identifier: NCT05265715).
Notably, results coming from a clinical trial evaluating the potential effect of time-restricted feeding on the inhibition of AGE/RAGE axis may help in deciphering whether the manipulation of this signaling pathway will be useful in women at high risk of developing breast cancer (ClinicalTrials.gov identifier: NCT05038137).
Not surprisingly, these dietary regimens also elicit a remarkable impact on the IIGF pathway, as a low-AGE diet improves insulin sensitivity and restrains inflammation in overweight and diabetic patients, compared with a high-AGE, isocaloric diet (341, 342). Similar beneficial effects are also achieved when restricting AGEs intake in obese individual with metabolic syndrome (343, 344). Of note, in a rodent model of insulin resistance, the antidiabetic drug pioglitazone was shown to improve insulin sensitivity by suppressing the AGE/RAGE/NF-κB pathway (345).
Other drugs interfering with IIGF have been shown to exert an inhibitory effect on RAGE pathway, including metformin (294), dipeptidyl peptidase-4 inhibitors (346-348), glucagon-like peptide 1 agonists (349, 350), and SGLT2 inhibitors (351-353). These beneficial effects appear to depend on the drug's RAGE-dependent anti-inflammatory action rather than on achieving better glycemic control. Despite holding great potential, only a few of these RAGE and IIGF-normalizing strategies have been tested in cancer patients, with some encouraging preliminary results obtained in preclinical models (294, 348).
On the other hand, the beneficial effect of certain RAGE- and IIGFs- targeting drugs was poor, as recently demonstrated for metformin in a randomized clinical trial performed in breast cancer patients (354). Collectively, these observations suggest that further preclinical and clinical evidence are needed to validate the therapeutic potential of anticancer strategies aimed at inhibiting RAGE signaling and its cross-talk with IIGFs, particularly in cancer patients affected by metabolic conditions like obesity and diabetes.
Conclusion
Meta-inflammation is a relevant feature in obesity, diabetes, and cancer. As a notable effector of meta-inflammation, RAGE signaling is emerging a promising actionable pharmacological target, particularly in malignancies associated with deregulated IIGFs.
Epidemiological evidence, preclinical studies and clinical data suggest that RAGE does not merely represent a signaling molecule activated in cancer cells because of imbalanced lipid and glucose homeostasis during obesity and diabetes; it also serves as a pivotal initiator of cellular derangements driving inflammation-dependent cancer progression. In this scenario, cancer could be included among the well-acknowledged complications of diabetes, similar to nephropathy, neuropathy, retinopathy, and cardiovascular disease. The risk for developing these long-term complications in treated patients persists even after glucose normalization, suggesting that a form of metabolic memory may take over, despite the pharmacological glucose-lowering intervention. RAGE represents an early instigator of such metabolic memory, which is known to rely strictly on the generation of oxidative stress, nonenzymatic glycation of proteins and the perpetuation of inflammation. In such inflammatory environment, RAGE also contributes to build-up a form of trained innate immunity, in which cells keep memory of past signals and propagate autoinflammatory processes upon training, toward worse outcomes of diabetes, obesity, and cancer. Based on these observations, targeting RAGE holds the promise to eradicate metabolic and immune memory, thus paving the way for a better control of neoplastic disease, in conditions characterized by aberrant metabolism and inflammation.
In this complex but captivating scenario, further investigations are warranted, as shedding light on RAGE action in IIGF-rich environment provides a fertile and exciting ground for future research efforts in basic and clinical oncology.
Abbreviations
- AGE
advanced glycation end-product
- ATP
adenosine triphosphate
- CAF
cancer associated fibroblast
- CML
Nε-carboxymethyllysine
- COX-2
cyclooxygenase 2
- CSC
cancer stem cell
- DAMP
danger-associated molecular pattern molecule
- ECM
extracellular matrix
- EMT
epithelial mesenchymal transition
- HMGB-1
high-mobility group box 1
- HIF
hypoxia inducible factor
- IGF
insulin-like growth factor
- IIGFs
insulin/insulin-like growth factor system
- IKK
IκB kinase
- IL
interleukin
- INS
insulin
- IR
insulin receptor
- IRS
insulin receptor substrate
- JAK
Janus kinase
- MAPK
mitogen-activated protein kinase
- MDSC
myeloid-derived suppressor cell
- MMP
metalloproteinase
- NET
neutrophil extracellular trap
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PPR
pattern recognition receptor
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
- T2DM
type 2 diabetes mellitus
- TAM
tumor-associated macrophage
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
Contributor Information
Veronica Vella, Endocrinology Unit, Department of Clinical and Experimental Medicine, University of Catania, Garibaldi-Nesima Hospital, 95122 Catania, Italy.
Rosamaria Lappano, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy.
Eduardo Bonavita, IRCCS Humanitas Research Hospital, Laboratory of Cellular and Molecular Oncoimmunology, 20089 Rozzano, Italy.
Marcello Maggiolini, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy.
Robert Bryan Clarke, Manchester Breast Centre, Division of Cancer Sciences, University of Manchester, Manchester M204GJ, UK.
Antonino Belfiore, Endocrinology Unit, Department of Clinical and Experimental Medicine, University of Catania, Garibaldi-Nesima Hospital, 95122 Catania, Italy.
Ernestina Marianna De Francesco, Endocrinology Unit, Department of Clinical and Experimental Medicine, University of Catania, Garibaldi-Nesima Hospital, 95122 Catania, Italy.
Funding
The research leading to these results has received funding from AIRC under Start-Up 2018—ID. 21651—E.M.D.F.; and Start-Up 2021—ID. 25828—E.B.; A.B. was supported by Fondazione AIRC (IG 23369) and by MUR under PNRR M4C2I1.3 Heal Italia project PE00000019 CUP B73C22001250006 (of University of Catania). A.B. and R.L. were supported by Ministero della Salute, Italy, grant RF-2019-12368937. A.B., M.M., and R.L. were supported by Fondazione AIRC (IG 23369 to A.B., IG 21322 to M.M., and IG27386 to R.L.); M.M. and R.L. acknowledge (1) the special award—namely, Department of Excellence 2018-2022 (Italian Law 232/2016)—to the Department of Pharmacy, Health, and Nutritional Sciences of the University of Calabria (Italy); (2) PON Ricerca e Competitività 2007-2013 and the “Sistema Integrato di Laboratori per L’Ambiente—(SILA) PONa3_00341).
Disclosures
Nothing to disclose.
References
- 1. Furman D, Campisi J, Verdin E, et al. . Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41(1):33‐52. [DOI] [PubMed] [Google Scholar]
- 3. Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843‐850. [DOI] [PubMed] [Google Scholar]
- 4. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177‐185. [DOI] [PubMed] [Google Scholar]
- 5. Kaaks R, Kühn T. Obesity and cancer—the evidence is fattening up. Nat Rev Endocrinol. 2014;10(11):644‐645. [DOI] [PubMed] [Google Scholar]
- 6. López-Suárez A. Burden of cancer attributable to obesity, type 2 diabetes and associated risk factors. Metabolism. 2019;92:136‐146. [DOI] [PubMed] [Google Scholar]
- 7. Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta-analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61(10):2140‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song YC, Lee SE, Jin Y, Park HW, Chun K-H, Lee H-W. Classifying the linkage between adipose tissue inflammation and Tumor growth through cancer-associated adipocytes. Mol Cells. 2020;43:763‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lashinger LM, Ford NA, Hursting SD. Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J Nutr. 2014;144(2):109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lapeire L, Hendrix A, Lambein K, et al. . Cancer-Associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and jak/STAT3 signaling. Cancer Res. 2014;74(23):6806‐6819. [DOI] [PubMed] [Google Scholar]
- 11. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zewdu A, Casadei L, Pollock RE, Braggio D. Adipose Tumor microenvironment. In: Birbrair A, ed. Tumor Microenvironments in Organs. Advances in Experimental Medicine and Biology. Springer International Publishing; 2020:73‐86 [DOI] [PubMed] [Google Scholar]
- 13. Filippello A, Urbano F, Di Mauro S, et al. . Chronic exposure to palmitate impairs insulin signaling in an intestinal L-cell line: a possible shift from GLP-1 to glucagon production. Int J Mol Sci. 2018;19(12):E3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vinciguerra F, Baratta R, Farina MG, et al. . Very severely obese patients have a high prevalence of type 2 diabetes mellitus and cardiovascular disease. Acta Diabetol. 2013;50(3):443‐449. [DOI] [PubMed] [Google Scholar]
- 16. Zeng C, Li Y, Ma J, Niu L, Tay FR. Clinical/translational aspects of advanced glycation End-products. Trends Endocrinol Metabol. 2019;30(12):959‐973. [DOI] [PubMed] [Google Scholar]
- 17. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586‐623. [DOI] [PubMed] [Google Scholar]
- 18. Ekyalongo RC, Yee D. Revisiting the IGF-1R as a breast cancer target. NPJ Precis Oncol. 2017;1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmidt AM, Vianna M, Gerlach M, et al. . Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267(21):14987‐14997. [PubMed] [Google Scholar]
- 20. Neeper M, Schmidt AM, Brett J, et al. . Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(21):14998‐15004. [PubMed] [Google Scholar]
- 21. Hudson BI, Lippman ME. Targeting RAGE signaling in inflammatory disease. Annu Rev Med. 2018;69(1):349‐364. [DOI] [PubMed] [Google Scholar]
- 22. Gebhardt C, Riehl A, Durchdewald M, et al. . RAGE Signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205(2):275‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palanissami G, Paul SFD. RAGE and its ligands: molecular interplay between glycation, inflammation, and hallmarks of cancer—a review. Horm Cancer. 2018;9(5):295‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng Z, Zhu L, Wu J. RAGE signalling in obesity and diabetes: focus on the adipose tissue macrophage. Adipocyte. 2020;9(1):563‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song F, Hurtado del Pozo C, Rosario R, et al. . RAGE Regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes (2014) 63(6):1948‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019;51(1):27‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10(4):369‐373. [DOI] [PubMed] [Google Scholar]
- 28. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436‐444. [DOI] [PubMed] [Google Scholar]
- 29. Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic interplay in the Tumor microenvironment. Cancer Cell. 2021;39(1):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batty MJ, Chabrier G, Sheridan A, Gage MC. Metabolic hormones modulate macrophage inflammatory responses. Cancers (Basel). 2021;13(18):4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li P, Wu M, Wang J, Sui Y, Liu S, Shi D. NAC Selectively inhibit cancer telomerase activity: A higher redox homeostasis threshold exists in cancer cells. Redox Biol. 2016;8:91‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hönigova K, Navratil J, Peltanova B, Polanska HH, Raudenska M, Masarik M. Metabolic tricks of cancer cells. Biochim Biophys Acta Rev Cancer. 2022;1877(3):188705. [DOI] [PubMed] [Google Scholar]
- 33. Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11(4):173‐186. [DOI] [PubMed] [Google Scholar]
- 34. Korbecki J, Simińska D, Gąssowska-Dobrowolska M, et al. . Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. IJMS. 2021;22(19):10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes. 2009;33(1):54‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66(13):6699‐6707. [DOI] [PubMed] [Google Scholar]
- 37. Wen H, Gris D, Lei Y, et al. . Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cinti S, Mitchell G, Barbatelli G, et al. . Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347‐2355. [DOI] [PubMed] [Google Scholar]
- 39. Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64(1):45‐57. [DOI] [PubMed] [Google Scholar]
- 40. Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7(15):2346‐2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Das UN. Essential fatty acids and their metabolites as modulators of stem cell biology with reference to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2011;30(3-4):311‐324. [DOI] [PubMed] [Google Scholar]
- 42. Xu H, Barnes GT, Yang Q, et al. . Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vella V, Nicolosi ML, Giuliano M, Morrione A, Malaguarnera R. Insulin receptor isoform A modulates metabolic reprogramming of breast cancer cells in response to IGF2 and insulin stimulation. Cells. 2019;8(9):1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127(1):43‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oslowski CM, Hara T, O'Sullivan-Murphy B, et al. Thioredoxin-Interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16(2):265‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95(3):727‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384(9945):755‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arnold M, Pandeya N, Byrnes G, et al. . Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103‐1123. [DOI] [PubMed] [Google Scholar]
- 50. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol Mech Dis. 2016;11(1):421‐449. [DOI] [PubMed] [Google Scholar]
- 51. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111‐2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nguyen MTA, Favelyukis S, Nguyen A-K, et al. . A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279‐35292. [DOI] [PubMed] [Google Scholar]
- 54. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72(1):219‐246. [DOI] [PubMed] [Google Scholar]
- 55. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239‐3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549‐555. [DOI] [PubMed] [Google Scholar]
- 58. Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21(11):799‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166‐6173. [DOI] [PubMed] [Google Scholar]
- 60. Casanova-Acebes M, Dalla E, Leader AM, et al. . Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. 2021;595(7868):578‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheng S-C, Quintin J, Cramer RA, et al. . mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodríguez-Prados J-C, Través PG, Cuenca J, et al. . Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605‐614. [DOI] [PubMed] [Google Scholar]
- 63. Tannahill GM, Curtis AM, Adamik J, et al. . Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heiden MG V, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tan Z, Xie N, Cui H, et al. . Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol. 2015;194(12):6082‐6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vats D, Mukundan L, Odegaard JI, et al. . Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huang SC-C, Everts B, Ivanova Y, et al. . Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(9):846‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu Z-G. ROS Play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23(7):898‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16(7):407‐420. [DOI] [PubMed] [Google Scholar]
- 70. Vandanmagsar B, Youm Y-H, Ravussin A, et al. . The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Shen X, Liu J, et al. . High glucose mediates NLRP3 inflammasome activation via upregulation of ELF3 expression. Cell Death Dis. 2020;11(5):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gianfrancesco MA, Dehairs J, L’homme L, et al. . Saturated fatty acids induce NLRP3 activation in human macrophages through K+ efflux resulting from phospholipid saturation and Na, K-ATPase disruption. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(7):1017‐1030. [DOI] [PubMed] [Google Scholar]
- 73. Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48(6):1038‐1050. [DOI] [PubMed] [Google Scholar]
- 74. Dror E, Dalmas E, Meier DT, et al. . Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol. 2017;18(3):283‐292. [DOI] [PubMed] [Google Scholar]
- 75. Eizirik DL, Sandler S, Welsh N, Juntti-Berggren L, Berggren P-O. Interleukin-1β-induced stimulation of insulin release in mouse pancreatic islets is related to diacylglycerol production and protein kinase C activation. Mol Cell Endocrinol. 1995;111(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 76. Meyerovich K, Fukaya M, Terra LF, Ortis F, Eizirik DL, Cardozo AK. The non-canonical NF-κB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia. 2016;59(3):512‐521. [DOI] [PubMed] [Google Scholar]
- 77. Lewitt M, Dent M, Hall K. The insulin-like growth factor system in obesity, insulin resistance and type 2 diabetes mellitus. J Clin Med. 2014;3(4):1561‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chidambaram S, Velloso FJ, Rothbard DE, et al. . Subventricular zone adult mouse neural stem cells require insulin receptor for self-renewal. Stem Cell Reports. 2022;17(6):1411‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ziegler AN, Feng Q, Chidambaram S, et al. . Insulin-like growth factor II: an essential adult stem cell niche constituent in brain and intestine. Stem Cell Rep. 2019;12(4):816‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Belfiore A, Malaguarnera R, Vella V, et al. . Insulin receptor isoforms in physiology and disease: an updated view. Endocr Rev. 2017;38(5):379‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mancarella C, Pasello M, Ventura S, et al. . Insulin-Like growth factor 2 mRNA-binding protein 3 is a novel post-transcriptional regulator of Ewing sarcoma malignancy. Clin Cancer Res. 2018;24(15):3704‐3716. [DOI] [PubMed] [Google Scholar]
- 82. Stanicka J, Rieger L, O'Shea S , et al. FES-related tyrosine kinase activates the insulin-like growth factor-1 receptor at sites of cell adhesion. Oncogene. 2018;37(23):3131‐3150. [DOI] [PubMed] [Google Scholar]
- 83. Wu Y, Brodt P, Sun H, et al. . Insulin-Like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70(1):57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Holly JMP, Biernacka K, Perks CM. The neglected insulin: IGF-II, a metabolic regulator with implications for diabetes, obesity, and cancer. Cells (2019) 8(10):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alfaro-Arnedo E, López IP, Piñeiro-Hermida S, et al. . IGF1R Acts as a cancer-promoting factor in the tumor microenvironment facilitating lung metastasis implantation and progression. Oncogene. 2022;41(28):3625‐3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Perks CM, Zielinska HA, Wang J, et al. . Insulin receptor isoform variations in prostate cancer cells. Front Endocrinol. 2016;7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Peng Y, Li F, Zhang P, et al. . IGF-1 promotes multiple myeloma progression through PI3K/Akt-mediated epithelial-mesenchymal transition. Life Sci. 2020;249:117503. [DOI] [PubMed] [Google Scholar]
- 88. Song D, Cismas S, Crudden C, et al. . IGF-1R is a molecular determinant for response to p53 reactivation therapy in conjunctival melanoma. Oncogene. 2022;41(4):600‐611. [DOI] [PubMed] [Google Scholar]
- 89. Morrione A, Valentinis B, Xu S, et al. . Insulin-like growth factor II stimulates cell proliferation through the insulin receptor. Proc Natl Acad Sci USA. 1997;94(8):3777‐3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sciacca L, Mineo R, Pandini G, Murabito A, Vigneri R, Belfiore A. In IGF-I receptor-deficient leiomyosarcoma cells autocrine IGF-II induces cell invasion and protection from apoptosis via the insulin receptor isoform A. Oncogene. 2002;21(54):8240‐8250. [DOI] [PubMed] [Google Scholar]
- 91. Sprynski AC, Hose D, Kassambara A, et al. . Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24(11):1940‐1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang G, Li X, Zhang L, et al. . The expression and role of hybrid insulin/insulin-like growth factor receptor type 1 in endometrial carcinoma cells. Cancer Genet Cytogenet. 2010;200(2):140‐148. [DOI] [PubMed] [Google Scholar]
- 93. Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18(4):R125‐R147. [DOI] [PubMed] [Google Scholar]
- 94. Vella V, De Francesco EM, Lappano R, et al. . Microenvironmental determinants of breast cancer metastasis: focus on the crucial interplay between estrogen and insulin/insulin-like growth factor signaling. Front Cell Dev Biol. 2020;8:608412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cirillo F, Pellegrino M, Talia M, et al. . Estrogen receptor variant ERα46 and insulin receptor drive in primary breast cancer cells growth effects and interleukin 11 induction prompting the motility of cancer-associated fibroblasts. Clin Transl Med. 2021;11(11):e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Avino S, Marco PD, Cirillo F, et al. . Stimulatory actions of IGF-I are mediated by IGF-IR cross-talk with GPER and DDR1 in mesothelioma and lung cancer cells. Oncotarget. 2016;7(33):52710‐52728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. De Francesco EM, Sims AH, Maggiolini M, Sotgia F, Lisanti MP, Clarke RB. GPER Mediates the angiocrine actions induced by IGF1 through the HIF-1α/VEGF pathway in the breast tumor microenvironment. Breast Cancer Res. 2017;19(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vella V, Nicolosi ML, Cantafio P, et al. . DDR1 Regulates thyroid cancer cell differentiation via IGF-2/IR-A autocrine signaling loop. Endocr Relat Cancer. 2019;26(1):197‐214. [DOI] [PubMed] [Google Scholar]
- 99. Muoio MG, Talia M, Lappano R, et al. . Activation of the S100A7/RAGE pathway by IGF-1 contributes to angiogenesis in breast cancer. Cancers (Basel). 2021;13(4):621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vella V, Giuliano M, La Ferlita A, et al. . Novel mechanisms of Tumor promotion by the insulin receptor isoform A in triple-negative breast cancer cells. Cells. 2021;10(11):3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Erdmann H, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. J Physiol Endocrinol Metab. 2008;294(3):E568‐E575. [DOI] [PubMed] [Google Scholar]
- 102. Brismar K, Fernqvist-Forbes E, Wahren J, Hall K. Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J Clin Endocrinol Metabol. 1994;79(3):872‐878. [DOI] [PubMed] [Google Scholar]
- 103. Attia N, Tamborlane WV, Heptulla R, et al. . The metabolic syndrome and insulin-like growth factor I regulation in adolescent obesity. J Clin Endocrinol Metabol. 1998;83(5):1467‐1471. [DOI] [PubMed] [Google Scholar]
- 104. Nam S, Lee E, Kim K, et al. . Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes. 1997;21(5):355‐359. [DOI] [PubMed] [Google Scholar]
- 105. Ferry RJ Jr., Cerri RW, Cohen P. Insulin-Like growth factor binding proteins: new proteins, new functions. Horm Res Paediatr (1999) 51(2):53‐67. [DOI] [PubMed] [Google Scholar]
- 106. Vella V, Giuliano M, Nicolosi ML, et al. . DDR1 Affects metabolic reprogramming in breast cancer cells by cross-talking to the insulin/IGF system. Biomolecules. 2021;11(7):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang C, An Y, Wang Y, et al. . Insulin-like growth factor-I activates NFκB and NLRP3 inflammatory signalling via ROS in cancer cells. Mol Cell Probes. 2020;52:101583. [DOI] [PubMed] [Google Scholar]
- 108. Eyre R, Alférez DG, Santiago-Gómez A, et al. . Microenvironmental IL1β promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat Commun. 2019;10(1):5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ieronymaki E, Theodorakis EM, Lyroni K, et al. . Insulin resistance in macrophages Alters their metabolism and promotes an M2-like phenotype. J Immunol. 2019;202(6):1786‐1797. [DOI] [PubMed] [Google Scholar]
- 110. Knuever J, Willenborg S, Ding X, et al. . Myeloid cell-restricted insulin/IGF-1 receptor deficiency protects against skin inflammation. J Immunol. 2015;195(11):5296‐5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ireland L, Santos A, Campbell F, et al. . Blockade of insulin-like growth factors increases efficacy of paclitaxel in metastatic breast cancer. Oncogene. 2018;37(15):2022‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gombos A, Metzger-Filho O, Dal Lago L, Awada-Hussein A. Clinical development of insulin-like growth factor receptor–1 (IGF-1R) inhibitors: at the crossroad? Invest New Drugs. 2012;30(6):2433‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31(1):805. [DOI] [PubMed] [Google Scholar]
- 114. Abou-Alfa GK, Capanu M, O’Reilly EM, et al. . A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60(2):319‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Langer CJ, Novello S, Park K, et al. . Phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non–small-cell lung cancer. J Clin Oncol. 2014;32(19):2059‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fuchs CS, Azevedo S, Okusaka T, et al. . A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26(5):921‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gradishar WJ, Yardley DA, Layman R, et al. . Clinical and translational results of a phase II, randomized trial of an anti–IGF-1R (cixutumumab) in women with breast cancer that progressed on endocrine therapy. Clin Cancer Res. 2016;22(2):301‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Haluska P, Shaw HM, Batzel GN, et al. . Phase I dose escalation study of the anti–insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13(19):5834‐5840. [DOI] [PubMed] [Google Scholar]
- 119. Tolcher AW, Sarantopoulos J, Patnaik A, et al. . Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27(34):5800‐5807. [DOI] [PubMed] [Google Scholar]
- 120. van Garderen E, Schalken JA. Morphogenic and tumorigenic potentials of the mammary growth hormone/growth hormone receptor system. Mol Cell Endocrinol. 2002;197(1-2):153‐165. [DOI] [PubMed] [Google Scholar]
- 121. Møller N, Jørgensen JOL. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152‐177. [DOI] [PubMed] [Google Scholar]
- 122. Buck E, Gokhale PC, Koujak S, et al. . Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9(10):2652‐2664. [DOI] [PubMed] [Google Scholar]
- 123. Buck E, Eyzaguirre A, Rosenfeld-Franklin M, et al. . Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68(20):8322‐8332. [DOI] [PubMed] [Google Scholar]
- 124. Li H, Singh Batth L, Qu X, et al. . IGF-IR signaling in epithelial to mesenchymal transition and targeting IGF-IR therapy: overview and new insights. Mol Cancer. 2017;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hudson BI, Carter AM, Harja E, et al. . Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22(5):1572‐1580. [DOI] [PubMed] [Google Scholar]
- 126. Zhang L, Bukulin M, Kojro E, et al. . Receptor for advanced glycation End products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283(51):35507‐35516. [DOI] [PubMed] [Google Scholar]
- 127. Kan S, Wu J, Sun C, Hao J, Wu Z. Correlation between RAGE gene promoter methylation and diabetic retinal inflammation. Exp Ther Med. 2017;15(1):242‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tesařová P, Kalousová M, Jáchymová M, Mestek O, Petruželka L, Zima T. Receptor for advanced glycation end products (RAGE)—soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Invest. 2007;25(8):720‐725. [DOI] [PubMed] [Google Scholar]
- 129. Jing R, Cui M, Wang J, Wang H. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): a new biomarker for lung cancer. Neoplasma. 2010;57(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 130. Krechler T, Jáchymová M, Mestek O, Žák A, Zima T, Kalousová M. Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clin Biochem. 2010;43(10-11):882‐886. [DOI] [PubMed] [Google Scholar]
- 131. Allegra A, Musolino C, Pace E, et al. . Evaluation of the AGE/sRAGE axis in patients with multiple myeloma. Antioxidants. 2019;8(3):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sakaguchi M, Murata H, Yamamoto K, et al. . TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. 2011;6(8):e23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Teissier T, Boulanger É. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology. 2019;20(3):279‐301. [DOI] [PubMed] [Google Scholar]
- 134. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805‐820. [DOI] [PubMed] [Google Scholar]
- 135. Shin JJ, Lee EK, Park TJ, Kim W. Damage-associated molecular patterns and their pathological relevance in diabetes mellitus. Ageing Res Rev. 2015;24(Pt A):66‐76. [DOI] [PubMed] [Google Scholar]
- 136. Heijmans J, Büller NVJA, Muncan V, van den Brink GR. Rage mediated DAMP signaling in intestinal tumorigenesis. Oncoimmunology. 2012;1(7):1165‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Riehl A, Németh J, Angel P, Hess J. The receptor RAGE: bridging inflammation and cancer. Cell Commun Signal. 2009;7(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Turovskaya O, Foell D, Sinha P, et al. . RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29(10):2035‐2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation End products. J Biol Chem. 1997;272(26):16498‐16506. [DOI] [PubMed] [Google Scholar]
- 140. Chawla D, Bansal S, Banerjee BD, Madhu SV, Kalra OP, Tripathi AK. Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvasc Res. 2014;95:1‐6. [DOI] [PubMed] [Google Scholar]
- 141. Ko S-Y, Ko H-A, Shieh T-M, et al. . Cell migration is regulated by AGE-RAGE interaction in human oral cancer cells in vitro. PLoS One. 2014;9(10):e110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498(2-3):99‐111. [DOI] [PubMed] [Google Scholar]
- 143. Bierhaus A, Schiekofer S, Schwaninger M, et al. . Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes. 2001;50(12):2792‐2808. [DOI] [PubMed] [Google Scholar]
- 144. Brett J, Schmidt AM, Yan SD, et al. . Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143(6):1699‐1712. [PMC free article] [PubMed] [Google Scholar]
- 145. Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323(3):475‐488. [DOI] [PubMed] [Google Scholar]
- 146. Bartling B. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis. 2004;26(2):293‐301. [DOI] [PubMed] [Google Scholar]
- 147. Liu W, Ouyang S, Zhou Z, et al. . Identification of genes associated with cancer progression and prognosis in lung adenocarcinoma: analyses based on microarray from oncomine and the cancer genome atlas databases. Mol Genet Genomic Med. 2019;7(2):e00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Downs CA. Analysis of RAGE proteome and interactome in lung adenocarcinoma using PANTHER and STRING databases. Biol Res Nurs. 2021;23(4):698‐707. [DOI] [PubMed] [Google Scholar]
- 149. Ren F, Zhao Q, Liu B, et al. . Transcriptome analysis reveals GPNMB as a potential therapeutic target for gastric cancer. J Cell Physiol. 2020;235(3):2738‐2752. [DOI] [PubMed] [Google Scholar]
- 150. Sharaf H, Matou-Nasri S, Wang Q, et al. . Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochim Biophys Acta. 2015;1852(3):429‐441. [DOI] [PubMed] [Google Scholar]
- 151. Matou-Nasri S, Sharaf H, Wang Q, et al. . Biological impact of advanced glycation endproducts on estrogen receptor-positive MCF-7 breast cancer cells. Biochim Biophy Acta. 2017;1863(11):2808‐2820. [DOI] [PubMed] [Google Scholar]
- 152. Menini S, Iacobini C, de Latouliere L, et al. . The advanced glycation end-product Nɛ -carboxymethyllysine promotes progression of pancreatic cancer: implications for diabetes-associated risk and its prevention: AGEs as a diabetes-related risk factor for pancreatic cancer. J Pathol. 2018;245(2):197‐208. [DOI] [PubMed] [Google Scholar]
- 153. Liao Y-F, Yin S, Chen Z-Q, Li F, Zhao B. High glucose promotes tumor cell proliferation and migration in lung adenocarcinoma via the RAGE-NOXs pathway. Mol Med Rep. 2018;17(6):8536‐8541. [DOI] [PubMed] [Google Scholar]
- 154. Yang X, Liu Y, Mani H, et al. . Biologic evaluation of diabetes and local recurrence in Non-small cell lung cancer. Pathol Oncol Res. 2017;23(1):73‐77. [DOI] [PubMed] [Google Scholar]
- 155. Luo J, Hendryx M, Qi L, Ho GY, Margolis KL. Pre-existing diabetes and lung cancer prognosis. Br J Cancer. 2016;115(1):76‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhou Y, Liu S-X, Zhou Y-N, Wang J, Ji R. Research on the relationship between RAGE and its ligand HMGB1, and prognosis and pathogenesis of gastric cancer with diabetes mellitus. Eur Rev Med Pharmacol Sci. 2021;25(3):1339‐1350. [DOI] [PubMed] [Google Scholar]
- 157. Chen H, Wu L, Li Y, et al. . Advanced glycation end products increase carbohydrate responsive element binding protein expression and promote cancer cell proliferation. Mol Cell Endocrinol. 2014;395(1-2):69‐78. [DOI] [PubMed] [Google Scholar]
- 158. Lashinger LM, Rossi EL, Hursting SD. Obesity and resistance to cancer chemotherapy: interacting roles of inflammation and metabolic dysregulation. Clin Pharmacol Ther. 2014;96(4):458‐463. [DOI] [PubMed] [Google Scholar]
- 159. Huang C-Y, Chiang S-F, Chen WT-L, et al. . HMGB1 Promotes ERK-mediated mitochondrial drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis. 2018;9(10):1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Guo X, He D, Zhang E, et al. . HMGB1 Knockdown increases MM cell vulnerability by regulating autophagy and DNA damage repair. J Exp Clin Cancer Res. 2018;37(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kang R, Tang D, Schapiro NE, et al. . The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33(5):567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kang R, Tang D, Lotze MT, Zeh HJ III. AGER/RAGE-mediated autophagy promotes pancreatic tumorigenesis and bioenergetics through the IL6-pSTAT3 pathway. Autophagy. 2012;8(6):989‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lo J-F, Yu C-C, Chiou S-H, et al. . The epithelial-mesenchymal transition mediator S100A4 maintains cancer-initiating cells in head and neck cancers. Cancer Res. 2011;71(5):1912‐1923. [DOI] [PubMed] [Google Scholar]
- 164. Li Y, Wang J, Song K, et al. . S100a4 promotes hepatocellular carcinogenesis by intensifying fibrosis-associated cancer cell stemness. OncoImmunology. 2020;9(1):1725355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Qian F, Xiao J, Gai L, Zhu J. HMGB1-RAGE Signaling facilitates ras-dependent yap1 expression to drive colorectal cancer stemness and development. Mol Carcinog. 2019;58(4):500‐510. [DOI] [PubMed] [Google Scholar]
- 166. De Francesco EM, Maggiolini M, Tanowitz HB, Sotgia F, Lisanti MP. Targeting hypoxic cancer stem cells (CSCs) with doxycycline: implications for optimizing anti-angiogenic therapy. Oncotarget. 2017;8(34):56126‐56142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Horiuchi A, Hayashi T, Kikuchi N, et al. . Hypoxia upregulates ovarian cancer invasiveness via the binding of HIF-1α to a hypoxia-induced, methylation-free hypoxia response element of S100A4 gene. Int J Cancer. 2012;131(8):1755‐1767. [DOI] [PubMed] [Google Scholar]
- 168. Liu L, Qi L, Knifley T, et al. . S100a4 alters metabolism and promotes invasion of lung cancer cells by up-regulating mitochondrial complex I protein NDUFS2. J Biol Chem. 2019;294(18):7516‐7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Moon B-S, Jeong W-J, Park J, Kim TI, Min DS, Choi K-Y. Role of oncogenic K-Ras in cancer stem cell activation by aberrant Wnt/β-catenin signaling. J Natl Cancer Inst. 2014;106(2):djt373. [DOI] [PubMed] [Google Scholar]
- 170. Kang R, Hou W, Zhang Q, et al. . RAGE Is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death Dis. 2014;5(10):e1480‐e1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Goh JY, Feng M, Wang W, et al. . Chromosome 1q21.3 amplification is a trackable biomarker and actionable target for breast cancer recurrence. Nat Med. 2017;23(11):1319‐1330. [DOI] [PubMed] [Google Scholar]
- 172. Monteiro C, Miarka L, Perea-García M, et al. . Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism. Nat Med. 2022;28(4):752‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jang M, Oh SW, Lee Y, Kim JY, Ji ES, Kim P. Targeting extracellular matrix glycation to attenuate fibroblast activation. Acta Biomater. 2022;141:255‐263. [DOI] [PubMed] [Google Scholar]
- 174. Krisanits BA, Woods P, Nogueira LM, et al. . Non-enzymatic glycoxidation linked with nutrition enhances the tumorigenic capacity of prostate cancer epithelia through AGE mediated activation of RAGE in cancer associated fibroblasts. Transl Oncol. 2022;17:101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chen Y, Cai L, Guo X, et al. . HMGB1-activated Fibroblasts promote breast cancer cells metastasis via RAGE/aerobic glycolysis. NEO. 2021;68(1):71‐78. [DOI] [PubMed] [Google Scholar]
- 176. Ren Y, Cao L, Wang L, et al. . Autophagic secretion of HMGB1 from cancer-associated fibroblasts promotes metastatic potential of non-small cell lung cancer cells via NFκB signaling. Cell Death Dis. 2021;12(10):858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhao X-L, Lin Y, Jiang J, et al. . High-mobility group box 1 released by autophagic cancer-associated fibroblasts maintains the stemness of luminal breast cancer cells: cross-talk between autophagic CAFs and BCICs. J Pathol. 2017;243(3):376‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hurtado del Pozo C, Ruiz HH, Arivazhagan L, et al. . A receptor of the immunoglobulin superfamily regulates adaptive thermogenesis. Cell Rep. 2019;28(3):773‐791.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gaens KHJ, Goossens GH, Niessen PM, et al. . Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34(6):1199‐1208. [DOI] [PubMed] [Google Scholar]
- 180. Monden M, Koyama H, Otsuka Y, et al. . Receptor for advanced glycation End products regulates adipocyte hypertrophy and insulin sensitivity in mice. Diabetes. 2013;62(2):478‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Nativel B, Marimoutou M, Thon-Hon VG, et al. . Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: contribution to chronic inflammation in fat tissue. PLoS One. 2013;8(9):e76039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sakurai M, Miki Y, Takagi K, et al. . Interaction with adipocyte stromal cells induces breast cancer malignancy via S100A7 upregulation in breast cancer microenvironment. Breast Cancer Res. 2017;19(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Gonzalez Suarez N, Fernandez-Marrero Y, Torabidastgerdooei S, Annabi B. EGCG prevents the onset of an inflammatory and cancer-associated adipocyte-like phenotype in adipose-derived mesenchymal stem/stromal cells in response to the triple-negative breast cancer secretome. Nutrients. 2022;14(5):1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wagner M, Bjerkvig R, Wiig H, Dudley AC. Loss of adipocyte specification and necrosis augment tumor-associated inflammation. Adipocyte. 2013;2(3):176‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Chen X, Zhang L, Zhang IY, et al. . RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014;74(24):7285‐7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ray R, Jangde N, Singh SK, Sinha S, Rai V. Lysophosphatidic acid-RAGE axis promotes lung and mammary oncogenesis via protein kinase B and regulating tumor microenvironment. Cell Commun Signal. 2020;18(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chen M-C, Chen K-C, Chang G-C, et al. . RAGE Acts as an oncogenic role and promotes the metastasis of human lung cancer. Cell Death Dis. 2020;11(4):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Nasser MW, Wani NA, Ahirwar DK, et al. . RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Res. 2015;75(6):974‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Rojas A, Araya P, Romero J, et al. . Skewed signaling through the receptor for advanced glycation End-products Alters the proinflammatory profile of Tumor-associated macrophages. Cancer Microenviron. 2018;11(2-3):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Rojas A, Delgado-López F, Perez-Castro R, et al. . HMGB1 Enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumor Biol. 2016;37(3):3321‐3329. [DOI] [PubMed] [Google Scholar]
- 191. Huber R, Meier B, Otsuka A, et al. . Tumour hypoxia promotes melanoma growth and metastasis via high mobility group box-1 and M2-like macrophages. Sci Rep. 2016;6(1):29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Araya P, Romero J, Delgado-López F, et al. . HMGB1 Decreases CCR-2 expression and migration of M2 macrophages under hypoxia. Inflamm Res. 2019;68(8):639‐642. [DOI] [PubMed] [Google Scholar]
- 193. Simard J-C, Cesaro A, Chapeton-Montes J, et al. . S100a8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-κB1. PLoS One. 2013;8(8):e72138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yin X-F, Zhang Q, Chen Z-Y, et al. . NLRP3 In human glioma is correlated with increased WHO grade, and regulates cellular proliferation, apoptosis and metastasis via epithelial-mesenchymal transition and the PTEN/AKT signaling pathway. Int J Oncol. 2018;53(3):973‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Westbom C, Thompson JK, Leggett A, et al. . Inflammasome modulation by chemotherapeutics in malignant mesothelioma. PLoS One. 2015;10(12):e0145404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Li Z, Fu W-J, Chen X-Q, et al. . Autophagy-based unconventional secretion of HMGB1 in glioblastoma promotes chemosensitivity to temozolomide through macrophage M1-like polarization. J Exp Clin Cancer Res. 2022;41(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22(3):173‐187. [DOI] [PubMed] [Google Scholar]
- 198. Miller-Ocuin JL, Liang X, Boone BA, et al. . DNA Released from neutrophil extracellular traps (NETs) activates pancreatic stellate cells and enhances pancreatic tumor growth. OncoImmunology. 2019;8(9):e1605822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zha C, Meng X, Li L, et al. . Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med. 2020;17(1):154‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Boone BA, Orlichenko L, Schapiro NE, et al. . The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015;22(6):326‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Sionov RV, Fainsod-Levi T, Zelter T, Polyansky L, Pham CT, Granot Z. Neutrophil cathepsin G and tumor cell RAGE facilitate neutrophil anti-tumor cytotoxicity. OncoImmunology. 2019;8(9):e1624129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Narumi K, Miyakawa R, Ueda R, et al. . Proinflammatory proteins S100A8/S100A9 activate NK cells via interaction with RAGE. J Immunol. 2015;194(11):5539‐5548. [DOI] [PubMed] [Google Scholar]
- 203. Kusume A, Sasahira T, Luo Y, et al. . Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology. 2009;76(4):155‐162. [DOI] [PubMed] [Google Scholar]
- 204. Demoulin S, Herfs M, Somja J, Roncarati P, Delvenne P, Hubert P. HMGB1 Secretion during cervical carcinogenesis promotes the acquisition of a tolerogenic functionality by plasmacytoid dendritic cells: cervical HMGB1 induces tolerogenic functionality in tumor-associated pDCs. Int J Cancer. 2015;137(2):345‐358. [DOI] [PubMed] [Google Scholar]
- 205. Wang W, Chapman NM, Zhang B, et al. . Upregulation of PD-L1 via HMGB1-activated IRF3 and NF-κB contributes to UV radiation-induced immune suppression. Cancer Res. 2019;79(11):2909‐2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Gebhardt C, Sevko A, Jiang H, et al. . Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. 2015;21(24):5453‐5459. [DOI] [PubMed] [Google Scholar]
- 207. Basso D, Fogar P, Plebani M. The S100A8/A9 complex reduces CTLA4 expression by immature myeloid cells: implications for pancreatic cancer-driven immunosuppression. OncoImmunology. 2013;2(6):e24441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wuren T, Huecksteadt T, Beck E, et al. . The receptor for advanced glycation endproducts (RAGE) decreases survival of tumor-bearing mice by enhancing the generation of lung metastasis-associated myeloid-derived suppressor cells. Cell Immunol. 2021;365:104379. [DOI] [PubMed] [Google Scholar]
- 209. Li J, Shu X, Xu J, et al. . S100A9-CXCL12 activation in BRCA1-mutant breast cancer promotes an immunosuppressive microenvironment associated with resistance to immunotherapy. Nat Commun. 2022;13(1):1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Vernon PJ, Loux TJ, Schapiro NE, et al. . The receptor for advanced glycation End products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells. J Immunol. 2013;190:1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Huang M, Wu R, Chen L, et al. . S100a9 regulates MDSCs-mediated immune suppression via the RAGE and TLR4 signaling pathways in colorectal carcinoma. Front Immunol. 2019;10:2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007;76(2):236‐244. [DOI] [PubMed] [Google Scholar]
- 213. Guzmán-Ruiz R, Ortega F, Rodríguez A, et al. . Alarmin high-mobility group B1 (HMGB1) is regulated in human adipocytes in insulin resistance and influences insulin secretion in β-cells. Int J Obes. 2014;38(12):1545‐1554. [DOI] [PubMed] [Google Scholar]
- 214. Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin–IGF axis. Trends Endocrinol Metabol. 2006;17(8):328‐336. [DOI] [PubMed] [Google Scholar]
- 215. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915‐928. [DOI] [PubMed] [Google Scholar]
- 216. Kirstein M, Aston C, Hintz R, Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992;90(2):439‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yang S-J, Chen C-Y, Chang G-D, et al. . Activation of Akt by advanced glycation end products (AGEs): involvement of IGF-1 receptor and caveolin-1. PLoS One. 2013;8(3):e58100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sun S-C, Chang J-H, Jin J. Regulation of nuclear factor-κB in autoimmunity. Trends Immunol. 2013;34(6):282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kadasah S, Thiyagarajan S, Vetter S, Leclerc E. Activation of the receptor for glycation end products (RAGE) by its ligands in pancreatic cancer cells. FASEB J. 2019;33(S1):476.11. [Google Scholar]
- 220. Somensi N, Brum PO, de Miranda Ramos V, et al. . Extracellular HSP70 activates ERK1/2, NF-kB and pro-inflammatory gene transcription through binding with RAGE in A549 human lung cancer cells. Cell Physiol Biochem. 2017;42(6):2507‐2522. [DOI] [PubMed] [Google Scholar]
- 221. Azizan N, Suter MA, Liu Y, Logsdon CD. RAGE maintains high levels of NFκB and oncogenic Kras activity in pancreatic cancer. Biochem Biophys Res Commun. 2017;493(1):592‐597. [DOI] [PubMed] [Google Scholar]
- 222. Lai W, Li X, Kong Q, et al. . Extracellular HMGB1 interacts with RAGE and promotes chemoresistance in acute leukemia cells. Cancer Cell Int. 2021;21(1):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Peng Y, Kim J-M, Park H-S, et al. . AGE-RAGE signal generates a specific NF-κB RelA “barcode” that directs collagen I expression. Sci Rep. 2016;6(1):18822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Wu S. Interaction between nuclear insulin receptor substrate-2 and NF-κB in IGF-1 induces response in breast cancer cells. Oncol Rep. 2010;24(6):1541‐1550. [DOI] [PubMed] [Google Scholar]
- 225. Koti M, Gooding RJ, Nuin P, et al. . Identification of the IGF1/PI3K/NF κB/ERK gene signalling networks associated with chemotherapy resistance and treatment response in high-grade serous epithelial ovarian cancer. BMC Cancer. 2013;13(1):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Murayama T, Nakaoku T, Enari M, et al. . Oncogenic fusion gene CD74-NRG1 confers cancer stem cell–like properties in lung cancer through a IGF2 autocrine/paracrine circuit. Cancer Res. 2016;76(4):974‐983. [DOI] [PubMed] [Google Scholar]
- 227. Tominaga K, Shimamura T, Kimura N, et al. . Addiction to the IGF2-ID1-IGF2 circuit for maintenance of the breast cancer stem-like cells. Oncogene. 2017;36(9):1276‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kracht M, Müller-Ladner U, Schmitz ML. Mutual regulation of metabolic processes and proinflammatory NF-κB signaling. J Allergy Clin Immunol. 2020;146(4):694‐705. [DOI] [PubMed] [Google Scholar]
- 229. Chiang S-H, Bazuine M, Lumeng CN, et al. . The protein kinase IKKɛ regulates energy balance in obese mice. Cell. 2009;138(5):961‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yuan M, Konstantopoulos N, Lee J, et al. . Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293(5535):1673‐1677. [DOI] [PubMed] [Google Scholar]
- 231. Van Beek M, Oravecz-Wilson KI, Delekta PC, et al. . Bcl10 links saturated fat overnutrition with hepatocellular NF-κB activation and insulin resistance. Cell Rep. 2012;1(5):444‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Cai D, Yuan M, Frantz DF, et al. . Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11(2):183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Zeinalzadeh E, Valerievich Yumashev A, Rahman HS, et al. . The role of Janus kinase/STAT3 pathway in hematologic malignancies with an emphasis on epigenetics. Front Genet. 2021;12:703883. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 234. Kim JY, Park HK, Yoon JS, et al. . Advanced glycation end product (AGE)-induced proliferation of HEL cells via receptor for AGE-related signal pathways. Int J Oncol. 2008;33(3):493‐501. [PubMed] [Google Scholar]
- 235. Zhu X, Liu L, Wang Y, et al. . lncRNA MIAT/HMGB1 axis is involved in cisplatin resistance via regulating IL6-mediated activation of the JAK2/STAT3 pathway in nasopharyngeal carcinoma. Front Oncol. 2021;11:651693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Tang Q, Li J, Zhu H, Li P, Zou Z, Xiao Y. Hmgb1-IL-23-IL-17-IL-6-Stat3 axis promotes tumor growth in murine models of melanoma. Mediators Inflamm. 2013;2013:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Cheng P, Corzo CA, Luetteke N, et al. . Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205(10):2235‐2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Zhang L, Liu W, Alizadeh D, et al. . S100b attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59(3):486‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Nasser MW, Qamri Z, Deol YS, et al. . S100a7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res. 2012;72(3):604‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. West NR, Watson PH. S100a7 (psoriasin) is induced by the proinflammatory cytokines oncostatin-M and interleukin-6 in human breast cancer. Oncogene. 2010;29(14):2083‐2092. [DOI] [PubMed] [Google Scholar]
- 241. Gartsbein M, Alt A, Hashimoto K, Nakajima K, Kuroki T, Tennenbaum T. The role of protein kinase C δ activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation. J Cell Sci. 2006;119(3):470‐481. [DOI] [PubMed] [Google Scholar]
- 242. Zhang L, Chen Z, Wang Y, Tweardy DJ, Mitch WE. Stat3 activation induces insulin resistance via a muscle-specific E3 ubiquitin ligase Fbxo40. Am J Phys -Endocrinol Metabol. 2020;318(5):E625‐E635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Wunderlich CM, Hövelmeyer N, Wunderlich FT. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAKSTAT. 2013;2(2):e23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wallbillich JJ, Josyula S, Saini U, et al. . High glucose-mediated STAT3 activation in endometrial cancer is inhibited by metformin: therapeutic implications for endometrial cancer. PLoS One. 2017;12(1):e0170318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Bao J-M, He M-Y, Liu Y-W, et al. . AGE/RAGE/akt pathway contributes to prostate cancer cell proliferation by promoting Rb phosphorylation and degradation. Am J Cancer Res. 2015;5(5):1741‐1750. [PMC free article] [PubMed] [Google Scholar]
- 246. Wang H, Duan L, Zou Z, et al. . Activation of the PI3K/akt/mTOR/p70S6K pathway is involved in S100A4-induced viability and migration in colorectal cancer cells. Int J Med Sci. 2014;11(8):841‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Tang C-H, Keng Y-T, Liu J-F. HMGB-1 induces cell motility and α5β1 integrin expression in human chondrosarcoma cells. Cancer Lett. 2012;322(1):98‐106. [DOI] [PubMed] [Google Scholar]
- 248. Takamatsu H, Yamamoto K, Tomonobu N, et al. . Extracellular S100A11 plays a Critical role in spread of the fibroblast population in pancreatic cancers. Oncol Res. 2019;27(6):713‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003;550(1-3):107‐113. [DOI] [PubMed] [Google Scholar]
- 250. Su Z, Wang T, Zhu H, et al. . HMGB1 Modulates Lewis cell autophagy and promotes cell survival via RAGE-HMGB1-erk1/2 positive feedback during nutrient depletion. Immunobiology. 2015;220(5):539‐544. [DOI] [PubMed] [Google Scholar]
- 251. Fuentes MK, Nigavekar SS, Arumugam T, et al. . RAGE Activation by S100P in colon cancer stimulates growth, migration, and cell signaling pathways. Dis Colon Rectum. 2007;50(8):1230‐1240. [DOI] [PubMed] [Google Scholar]
- 252. Tang T, Wang S, Cai T, et al. . High mobility group box 1 regulates gastric cancer cell proliferation and migration via RAGE-mTOR/ERK feedback loop. J Cancer. 2021;12(2):518‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lin L, Zhong K, Sun Z, Wu G, Ding G. Receptor for advanced glycation end products (RAGE) partially mediates HMGB1-ERKs activation in clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2012;138(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 254. Medapati MR, Dahlmann M, Ghavami S, et al. . RAGE Mediates the pro-migratory response of extracellular S100A4 in human thyroid cancer cells. Thyroid. 2015;25(5):514‐527. [DOI] [PubMed] [Google Scholar]
- 255. Tang Y, Wang J, Cai W, Xu J. RAGE/NF-κB pathway mediates hypoxia-induced insulin resistance in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2020;521(1):77‐83. [DOI] [PubMed] [Google Scholar]
- 256. Jiang Y, Steinle JJ. HMGB1 Inhibits insulin signalling through TLR4 and RAGE in human retinal endothelial cells. Growth Factors. 2018;36(3-4):164‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Moeschel K, Beck A, Weigert C, et al. . Protein kinase C-ζ-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004;279(24):25157‐25163. [DOI] [PubMed] [Google Scholar]
- 258. Lu H, Bogdanovic E, Yu Z, et al. . Combined hyperglycemia- and hyperinsulinemia-induced insulin resistance in adipocytes is associated with dual signaling defects mediated by PKC-ζ. Endocrinology. 2018;159(4):1658‐1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Al-Robaiy S, Weber B, Simm A, et al. . The receptor for advanced glycation end-products supports lung tissue biomechanics. Am J Physiol Lung Cell Mol Physiol. 2013;305(7):L491‐L500. [DOI] [PubMed] [Google Scholar]
- 260. Kwak T, Drews-Elger K, Ergonul A, et al. . Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene. 2017;36(11):1559‐1572. [DOI] [PubMed] [Google Scholar]
- 261. Rigiracciolo DC, Nohata N, Lappano R, et al. . Focal Adhesion Kinase (FAK)-hippo/YAP transduction signaling mediates the stimulatory effects exerted by S100A8/A9-RAGE system in triple-negative breast cancer (TNBC). J Exp Clin Cancer Res. 2022;41(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. You L, Cui H, Zhao F, et al. . Inhibition of HMGB1/RAGE axis suppressed the lipopolysaccharide (LPS)-induced vicious transformation of cervical epithelial cells. Bioengineered. 2021;12(1):4995‐5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Swami P, O’Connell KA, Thiyagarajan S, et al. . Inhibition of the receptor for advanced glycation End products enhances the cytotoxic effect of gemcitabine in murine pancreatic tumors. Biomolecules. 2021;11(4):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Wang C, Wang X, Han A, Wang Y, Jiang H. Proof-of-concept study investigating the role of S100P-RAGE in nasopharyngeal carcinoma. Exp Ther Med. 2021;21(5):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Santolla MF, Talia M, Cirillo F, et al. . The AGEs/RAGE transduction signaling prompts IL-8/CXCR1/2-mediated interaction between Cancer-Associated Fibroblasts (CAFs) and breast cancer cells. Cells. 2022;11(15):2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Xie J, Xu H, Wu X, Xie Y, Lu X, Wang L. Design, synthesis and anti-TNBC activity of azeliragon triazole analogues. Bioorg Med Chem Lett. 2021;54:128444. [DOI] [PubMed] [Google Scholar]
- 267. Arumugam T, Ramachandran V, Gomez SB, Schmidt AM, Logsdon CD. S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin Cancer Res. 2012;18(16):4356‐4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Jin Q, Chen H, Luo A, Ding F, Liu Z. S100a14 stimulates cell proliferation and induces cell apoptosis at different concentrations via receptor for advanced glycation end products (RAGE). PLoS One. 2011;6(4):e19375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Choi E, Oh J, Lee D, et al. . A ternary-complex of a suicide gene, a RAGE-binding peptide, and polyethylenimine as a gene delivery system with anti-tumor and anti-angiogenic dual effects in glioblastoma. J Control Release. 2018;279:40‐52. [DOI] [PubMed] [Google Scholar]
- 270. Zheng J, Zhu W, He F, Li Z, Cai N, Wang H-H. An aptamer-based antagonist against the receptor for advanced glycation End-products (RAGE) blocks development of colorectal cancer. Mediators Inflamm. 2021;2021:9958051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Nakamura N, Matsui T, Ishibashi Y, et al. . RAGE-aptamer Attenuates the growth and liver metastasis of malignant melanoma in nude mice. Mol Med. 2017;23(1):295‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Nakamura N, Matsui T, Nishino Y, Sotokawauchi A, Higashimoto Y, Yamagishi S-I. Long-Term local injection of RAGE-aptamer suppresses the growth of malignant melanoma in nude mice. J Oncol. 2019;2019:7387601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Radia A-M, Yaser A-M, Ma X, et al. . Specific siRNA targeting receptor for advanced glycation end products (RAGE) decreases proliferation in human breast cancer cell lines. Int J Mol Sci. 2013;14(4):7959‐7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Amornsupak K, Thongchot S, Thinyakul C, et al. . HMGB1 mediates invasion and PD-L1 expression through RAGE-PI3K/AKT signaling pathway in MDA-MB-231 breast cancer cells. BMC Cancer. 2022;22(1):578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Gong W, Wang Z-Y, Chen G-X, Liu Y-Q, Gu X-Y, Liu W-W. Invasion potential of H22 hepatocarcinoma cells is increased by HMGB1-induced tumor NF-κB signaling via initiation of HSP70. Oncol Rep. 2013;30(3):1249‐1256. [DOI] [PubMed] [Google Scholar]
- 276. Pusterla T, Nèmeth J, Stein I, et al. . Receptor for advanced glycation endproducts (RAGE) is a key regulator of oval cell activation and inflammation-associated liver carcinogenesis in mice. Hepatology. 2013;58(1):363‐373. [DOI] [PubMed] [Google Scholar]
- 277. Kuniyasu H, Chihara Y, Kondo H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int J Cancer. 2003;104(6):722‐727. [DOI] [PubMed] [Google Scholar]
- 278. Zhang Q-Y, Wu L-Q, Zhang T, Han Y-F, Lin X. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol Rep. 2015;33(4):1630‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Zhu L, Li X, Chen Y, Fang J, Ge Z. High-mobility group box 1: a novel inducer of the epithelial-mesenchymal transition in colorectal carcinoma. Cancer Lett. 2015;357(2):527‐534. [DOI] [PubMed] [Google Scholar]
- 280. Deng R, Wu H, Ran H, et al. . Glucose-derived AGEs promote migration and invasion of colorectal cancer by up-regulating Sp1 expression. Biochim Biophys ActaGen Subj. 2017;1861(5):1065‐1074. [DOI] [PubMed] [Google Scholar]
- 281. Mizumoto S, Takahashi J, Sugahara K. Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J Biol Chem. 2012;287(23):18985‐18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Mardente S, Mari E, Massimi I, et al. . HMGB1-induced cross talk between PTEN and miRs 221/222 in thyroid cancer. Biomed Res Int. 2015;2015:512027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Healey GD, Pan-Castillo B, Garcia-Parra J, et al. . Antibody drug conjugates against the receptor for advanced glycation end products (RAGE), a novel therapeutic target in endometrial cancer. J Immunother Cancer. 2019;7(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Yang Y, Zhao L-H, Huang B, et al. . Pioglitazone, a PPARγ agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol Carcinog. 2015;54(12):1584‐1595. [DOI] [PubMed] [Google Scholar]
- 285. Chen R-J, Lyu Y-J, Chen Y-Y, et al. . Chloroquine potentiates the anticancer effect of pterostilbene on pancreatic cancer by inhibiting autophagy and downregulating the RAGE/STAT3 pathway. Molecules. 2021;26(21):6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Wang J, Saung MT, Li K, et al. . CCR2/CCR5 inhibitor permits the radiation-induced effector T cell infiltration in pancreatic adenocarcinoma. J Exp Med. 2022;219(5):e20211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98(24):1806‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Inada M, Shindo M, Kobayashi K, et al. . Anticancer effects of a non-narcotic opium alkaloid medicine, papaverine, in human glioblastoma cells. PLoS One. 2019;14(5):e0216358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. El-Far AHAM, Munesue S, Harashima A, et al. . In vitro anticancer effects of a RAGE inhibitor discovered using a structure-based drug design system. Oncol Lett. 2018;15(4):4627‐4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Inada M, Sato A, Shindo M, et al. . Anticancer Non-narcotic opium alkaloid papaverine suppresses human glioblastoma cell growth. Anticancer Res. 2019;39(12):6743‐6750. [DOI] [PubMed] [Google Scholar]
- 291. Takeuchi A, Yamamoto Y, Munesue S, et al. . Low molecular weight heparin suppresses receptor for advanced glycation end products-mediated expression of malignant phenotype in human fibrosarcoma cells. Cancer Sci. 2013;104(6):740‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Dhumale SS, Waghela BN, Pathak C. Quercetin protects necrotic insult and promotes apoptosis by attenuating the expression of RAGE and its ligand HMGB1 in human breast adenocarcinoma cells. IUBMB Life. 2015;67(5):361‐373. [DOI] [PubMed] [Google Scholar]
- 293. Lan C-Y, Chen S-Y, Kuo C-W, Lu C-C, Yen G-C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food Drug Anal. 2019;27(4):887‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Metformin inhibits advanced glycation end products (AGEs)-induced growth and VEGF expression in MCF-7 breast cancer cells by suppressing AGEs receptor expression via AMP-activated protein kinase. Horm Metab Res. 2013;45(5):387‐390. [DOI] [PubMed] [Google Scholar]
- 295. Mishra S, Charan M, Shukla RK, et al. . cPLA2 blockade attenuates S100A7-mediated breast tumorigenicity by inhibiting the immunosuppressive tumor microenvironment. J Exp Clin Cancer Res. 2022;41(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Herwig N, Belter B, Pietzsch J. Extracellular S100A4 affects endothelial cell integrity and stimulates transmigration of A375 melanoma cells. Biochem Biophys Res Commun. 2016;477(4):963‐969. [DOI] [PubMed] [Google Scholar]
- 297. Taguchi A, Blood DC, del Toro G, et al. . Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354‐360. [DOI] [PubMed] [Google Scholar]
- 298. Sakamoto Y, Okui T, Yoneda T, et al. . High-mobility group box 1 induces bone destruction associated with advanced oral squamous cancer via RAGE and TLR4. Biochem Biophys Res Commun. 2020;531(3):422‐430. [DOI] [PubMed] [Google Scholar]
- 299. Nakamura T, Okui T, Hasegawa K, et al. . High mobility group box 1 induces bone pain associated with bone invasion in a mouse model of advanced head and neck cancer. Oncol Rep. 2020;44(6):2547‐2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Huang M, Geng Y, Deng Q, et al. . Translationally controlled tumor protein affects colorectal cancer metastasis through the high mobility group box 1-dependent pathway. Int J Oncol. 2018;53(4):1481‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Lei X, Hu X, Zhang T, et al. . HMGB1 Release promotes paclitaxel resistance in castration-resistant prostate cancer cells via activating c-Myc expression. Cell Signal. 2020;72:109631. [DOI] [PubMed] [Google Scholar]
- 302. Li M-L, Wang X-F, Tan Z-J, et al. . Ethyl pyruvate administration suppresses growth and invasion of gallbladder cancer cells via downregulation of HMGB1-RAGE axis. Int J Immunopathol Pharmacol. 2012;25(4):955‐965. [DOI] [PubMed] [Google Scholar]
- 303. Chen B, Na F, Yang H, et al. . Ethyl pyruvate alleviates radiation-induced lung injury in mice. Biomed Pharmacother. 2017;92:468‐478. [DOI] [PubMed] [Google Scholar]
- 304. Liu Q, Huo Y, Zheng H, Zhao J, Jia L, Wang P. Ethyl pyruvate suppresses the growth, invasion and migration and induces the apoptosis of non-small cell lung cancer cells via the HMGB1/RAGE axis and the NF-κB/STAT3 pathway. Oncol Rep. 2019;42(2):817‐825. [DOI] [PubMed] [Google Scholar]
- 305. Kam NG, Wu KC, Dai W, et al. . Peritumoral B cells drive proangiogenic responses in HMGB1-enriched esophageal squamous cell carcinoma. Angiogenesis. 2022;25(2):181‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Wang G, Hiramoto K, Ma N, et al. . Glycyrrhizin attenuates carcinogenesis by inhibiting the inflammatory response in a murine model of colorectal cancer. Int J Mol Sci. 2021;22(5):2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Hubert P, Roncarati P, Demoulin S, et al. . Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer. 2021;9(3):e001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Chen Y, Lin C, Liu Y, Jiang Y. HMGB1 Promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2. Tumour Biol. 2016;37(4):4399‐4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Wang L, Kang F-B, Wang J, Yang C, He D-W. Downregulation of miR-205 contributes to epithelial-mesenchymal transition and invasion in triple-negative breast cancer by targeting HMGB1-RAGE signaling pathway. Anticancer Drugs. 2019;30(3):225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Hernández JL, Padilla L, Dakhel S, et al. . Therapeutic targeting of tumor growth and angiogenesis with a novel anti-S100A4 monoclonal antibody. PLoS One. 2013;8(9):e72480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Grum-Schwensen B, Klingelhöfer J, Beck M, et al. . S100A4-neutralizing antibody suppresses spontaneous tumor progression, pre-metastatic niche formation and alters T-cell polarization balance. BMC Cancer. 2015;15(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Stein U, Arlt F, Smith J, et al. . Intervening in β-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia. 2011;13(2):131‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Sack U, Walther W, Scudiero D, et al. . S100A4-induced cell motility and metastasis is restricted by the Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells. Mol Biol Cell. 2011;22(18):3344‐3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Qin H, Lerman B, Sakamaki I, et al. . Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med. 2014;20(6):676‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. De Ponti A, Wiechert L, Schneller D, et al. . A pro-tumorigenic function of S100A8/A9 in carcinogen-induced hepatocellular carcinoma. Cancer Lett. 2015;369(2):396‐404. [DOI] [PubMed] [Google Scholar]
- 316. Pili R, Häggman M, Stadler WM, et al. . Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol. 2011;29(30):4022‐4028. [DOI] [PubMed] [Google Scholar]
- 317. Wang H, Zhang L, Zhang IY, et al. . S100b promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clin Cancer Res. 2013;19(14):3764‐3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Yang T, Cheng J, Yang Y, et al. . S100b mediates stemness of Ovarian cancer stem-like cells through inhibiting p53. Stem Cells. 2017;35(2):325‐336. [DOI] [PubMed] [Google Scholar]
- 319. Seguella L, Capuano R, Pesce M, et al. . S100b protein stimulates proliferation and angiogenic mediators release through RAGE/pAkt/mTOR pathway in human colon adenocarcinoma caco-2 cells. Int J Mol Sci. 2019;20(13):E3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Capoccia E, Cirillo C, Marchetto A, et al. . S100B-p53 disengagement by pentamidine promotes apoptosis and inhibits cellular migration via aquaporin-4 and metalloproteinase-2 inhibition in C6 glioma cells. Oncol Lett. 2015;9(6):2864‐2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Smith J, Stewart B-J, Glaysher S, et al. . The effect of pentamidine on melanoma ex vivo. Anticancer Drugs. 2010;21(2):181‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Padilla L, Dakhel S, Adan J, et al. . S100a7: from mechanism to cancer therapy. Oncogene. 2017;36(49):6749‐6761. [DOI] [PubMed] [Google Scholar]
- 323. Fan J-J, Hsu W-H, Lee K-H, et al. . Dietary flavonoids luteolin and Quercetin inhibit migration and invasion of squamous carcinoma through reduction of Src/Stat3/S100A7 signaling. Antioxidants (Basel). 2019;8(11):E557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Sun W, Luo L, Fang D, et al. . A novel DNA aptamer targeting S100P induces antitumor effects in colorectal cancer cells. Nucleic Acid Ther. 2020;30(6):402‐413. [DOI] [PubMed] [Google Scholar]
- 325. Ojima A, Matsui T, Maeda S, et al. . DNA Aptamer raised against advanced glycation end products inhibits melanoma growth in nude mice. Lab Invest. 2014;94(4):422‐429. [DOI] [PubMed] [Google Scholar]
- 326. Liu Y, Wang C, Shan X, et al. . S100p is associated with proliferation and migration in nasopharyngeal carcinoma. Oncol Lett. 2017;14(1):525‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Seguella L, Rinaldi F, Marianecci C, et al. . Pentamidine niosomes thwart S100B effects in human colon carcinoma biopsies favouring wtp53 rescue. J Cell Mol Med. 2020;24(5):3053‐3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Antognelli C, Moretti S, Frosini R, Puxeddu E, Sidoni A, Talesa VN. Methylglyoxal acts as a Tumor-promoting factor in anaplastic thyroid cancer. Cells. 2019;8(6):E547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Manigrasso MB, Rabbani P, Egaña-Gorroño L, et al. . Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci Transl Med. 2021;13(621):eabf7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Lam JKY, Wang Y, Shiu SWM, Wong Y, Betteridge DJ, Tan KCB. Effect of insulin on the soluble receptor for advanced glycation end products (RAGE). Diabet Med. 2013;30(6):702‐709. [DOI] [PubMed] [Google Scholar]
- 331. Ravichandran LV, Esposito DL, Chen J, Quon MJ. Protein kinase C-ζ phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J Biol Chem. 2001;276(5):3543‐3549. [DOI] [PubMed] [Google Scholar]
- 332. Xi G, Shen X, Maile LA, Wai C, Gollahon K, Clemmons DR. Hyperglycemia enhances IGF-I-stimulated Src activation via increasing Nox4-derived reactive oxygen species in a PKCζ-dependent manner in vascular smooth muscle cells. Diabetes. 2012;61(1):104‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Rimessi A, Patergnani S, Ioannidi E, Pinton P. Chemoresistance and cancer-related inflammation: two hallmarks of cancer connected by an atypical link, PKCζ. Front Oncol. 2013;3:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Zhou P, Qin J, Li Y, et al. . Combination therapy of PKCζ and COX-2 inhibitors synergistically suppress melanoma metastasis. J Exp Clin Cancer Res. 2017;36(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Dey A, Islam SMA, Patel R, Acevedo-Duncan M. The interruption of atypical PKC signaling and Temozolomide combination therapy against glioblastoma. Cell Signal. 2021;77:109819. [DOI] [PubMed] [Google Scholar]
- 336. Valdemarin F, Caffa I, Persia A, et al. . Safety and feasibility of fasting-mimicking diet and effects on nutritional Status and circulating metabolic and inflammatory factors in cancer patients undergoing active treatment. Cancers (Basel). 2021;13(16):4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Harvey AE, Lashinger LM, Hays D, et al. . Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression in an insulin-like growth factor-1-dependent manner. PLoS One. 2014;9(5):e94151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Pietrocola F, Pol J, Vacchelli E, et al. . Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30(1):147‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Cheng C-W, Adams GB, Perin L, et al. . Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Hagen I, Schulte DM, Müller N, et al. . Soluble receptor for advanced glycation end products as a potential biomarker to predict weight loss and improvement of insulin sensitivity by a very low calorie diet of obese human subjects. Cytokine. 2015;73(2):265‐269. [DOI] [PubMed] [Google Scholar]
- 341. Mark AB, Poulsen MW, Andersen S, et al. . Consumption of a diet low in advanced glycation End products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care. 2014;37(1):88‐95. [DOI] [PubMed] [Google Scholar]
- 342. Uribarri J, Cai W, Ramdas M, et al. . Restriction of advanced glycation End products improves insulin resistance in human type 2 diabetes. Diabetes Care. 2011;34(7):1610‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Goudarzi R, Sedaghat M, Hedayati M, Hekmatdoost A, Sohrab G. Low advanced glycation end product diet improves the central obesity, insulin resistance and inflammatory profiles in Iranian patients with metabolic syndrome: a randomized clinical trial. J Diabetes Metab Disord. 2020;19(2):1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Vlassara H, Cai W, Tripp E, et al. . Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2016;59(10):2181‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Liu X, Luo D, Zheng M, Hao Y, Hou L, Zhang S. Effect of pioglitazone on insulin resistance in fructose-drinking rats correlates with AGEs/RAGE inhibition and block of NAPDH oxidase and NF kappa B activation. Eur J Pharmacol. 2010;629(1-3):153‐158. [DOI] [PubMed] [Google Scholar]
- 346. Sarker MK, Lee JH, Lee DH, Chun K-H, Jun H-S. Attenuation of diabetic kidney injury in DPP4-deficient rats; role of GLP-1 on the suppression of AGE formation by inducing glyoxalase 1. Aging (Albany NY) 2020;12:593‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Wang X, Ke J, Zhu Y-J, et al. . Dipeptidyl peptidase-4 (DPP4) inhibitor sitagliptin alleviates liver inflammation of diabetic mice by acting as a ROS scavenger and inhibiting the NFκB pathway. Cell Death Discov. 2021;7(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Yamagishi S, Fukami K, Matsui T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc Diabetol. 2015;14(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Zhao W, Zhang X, Zhou Z, et al. . Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression. Mol Med Rep. 2018;17:5202‐5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Puddu A, Mach F, Nencioni A, Viviani GL, Montecucco F. An emerging role of glucagon-like peptide-1 in preventing advanced-glycation-end-product-mediated damages in diabetes. Mediators Inflamm. 2013;2013:591056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Steven S, Oelze M, Hanf A, et al. . The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47(9):686‐692. [DOI] [PubMed] [Google Scholar]
- 353. Yang L, Liang B, Li J, et al. . Dapagliflozin alleviates advanced glycation end product induced podocyte injury through AMPK/mTOR mediated autophagy pathway. Cell Signal. 2022;90:110206. [DOI] [PubMed] [Google Scholar]
- 354. Goodwin PJ, Chen BE, Gelmon KA, et al. . Effect of metformin vs placebo on invasive disease–free survival in patients with breast cancer: the MA.32 randomized clinical trial. JAMA. 2022;327(20):1963. [DOI] [PMC free article] [PubMed] [Google Scholar]