Abstract
Case
We present a case of a 70-year-old woman with simultaneous periprosthetic joint infection (PJI) of both hips and left knee due to a bilateral psoas abscess. The patient underwent debridement and implants removal with the consequent reimplantation in a sequential six-stage revision surgery. At four years of follow-up and in spite of the patient’s comorbidities and current PJI presentation, she maintains full activities of daily living without restrictions.
Conclusion
Accurate and early diagnosis of a psoas abscess is crucial. This case report provides experience of a complex scenario, the decision-making involved and the outcomes of an underdiagnosed complication.
Keywords: Periprosthetic joint infection, Total hip arthroplasty, Total knee arthroplasty, Psoas abscess, Simultaneous periprosthetic joint infection
Background
Periprosthetic joint infection (PJI) continues to be challenging and requires great experience for its treatment.1,2 The PJI rate is approximately 1% to 2% and is associated with high morbidity and mortality.3,4 The currently most accepted treatment for chronic PJI is two-stage revision surgery, with 83% to 95% success rate.5,6
The psoas abscess is a rare entity and can lead to an undesired evolution with sepsis or even the patient's death in some untreated cases.7 The aetiology is multifactorial, and abdominal infections, Crohn's disease and spinal surgical procedures have been described as possible infectious origins. They can be classified as primary if they are a consequence of haematogenous dissemination or secondary if they are associated with a contiguous infection.8
A few case reports of two or more simultaneous PJIs in the same patient have been described in the literature.9 To our knowledge, we present the first patient with a chronic and simultaneous PJI of both hips and left knee due to a bilateral psoas abscess who was treated with six-stage revision surgery.
The patient was informed that data concerning the case would be submitted for publication, and she provided consent.
Case history
A 70-year-old woman with a body mass index of 35kg/m2 was referred to our emergency department complaining about bilateral hip and left knee chronic pain.
Her previous medical history revealed non-simultaneous primary arthroplasties of both hips and left knee two and seven years ago, respectively. Six months after her right total hip arthroplasty (THA), she was diagnosed with spondylodiscitis, which was medically treated with antibiotics. After one year of follow-up, she evolved with a bilateral psoas abscess that was surgically treated with percutaneous drainage at another institution. Cultures from the fluid drainage were positive for Staphylococcus aureus. According to the antibiogram, the patient was given intravenous antibiotics (ATBs) for six weeks with apparent clinical improvement. She received vancomycin (1g/12 hours) for six weeks, followed by trimethoprim-sulfamethoxazole (TMS) (160–800mg/12 hours) orally for six months.
The patient was afebrile but had pain on active and passive range of motion (ROM) of the three joints with inability to ambulate. The physical exam revealed erythema and sinus tracts with spontaneous purulent drainage in both her hips and left knee (Figure 1a–c). Having suspended ATB treatment three weeks ago, laboratory examination showed an elevated erythrocyte sedimentation rate (ESR, 120mm/h) and C-reactive protein (CRP, 75mg/dL); the white blood cell count was 14,000cells/mm3 with 85% polymorphonuclear neutrophils (PMNs). Anteroposterior (AP) and lateral (L) view radiographs of both hips and left knee were performed. The x-rays showed dislocation of her right THA and the acetabular cup loosening with a Paprosky 3A10 bone defect. The left THA had similar characteristics, with acetabular component loosening and a Paprosky 2C bone deficiency, but without prosthetic dislocation (Figure 2). Finally, the left total knee arthroplasty (TKA) had no evident signs of loosening (Figure 3a and b).
Figure 1 .

Clinical images showing the patient in the left lateral decubitus position evidencing a sinus tract, erythema and purulent fluid in the right hip (a). Image of the left hip with a sinus tract at the distal end of the previous incision (b). Image of the left knee showing the sinus tract (c).
Figure 2 .
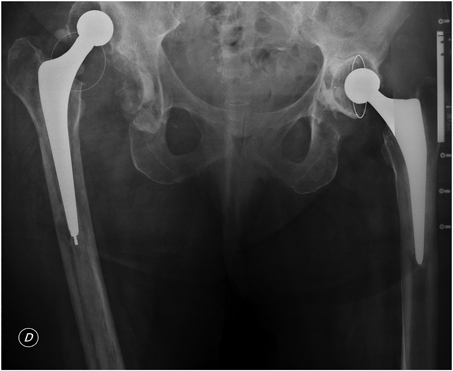
Anteroposterior pelvic radiograph showing right total hip arthroplasty (THA) dislocation, and cup loosening and bone deficiency of both THAs
Figure 3 .

Anteroposterior (a) and lateral (b) view radiographs showing the left total knee arthroplasty (TKA) at the onset of symptoms without evident signs of loosening
With a PJI diagnosis of the three arthroplasties, surgical treatment was indicated in six sequential stages. The second day after admission, the first-stage revision surgery of her right THA was performed through a posterolateral approach with the additional requirement of an extended trochanteric osteotomy. A tobramycin-impregnated cement spacer was implanted (Figure 4), and culture samples were sent for bacteriological and mycological evaluation, as well as for frozen section analysis. Seven and fourteen days after the aforementioned procedure, the patient underwent the first-stage revision surgery of her left THA and left TKA, respectively. A similar tobramycin-impregnated cement spacer was implanted in both procedures (Figure 4). The immediate rehabilitation protocol included early mobilisation 24 hours after surgery and ambulation with a wheelchair without weight-bearing.
Figure 4 .
Anteroposterior (a–c) and lateral (d) postoperative views after first-stage revision surgery showing the tobramycin-impregnated cement spacer implanted
As expected, cultures from the debrided periprosthetic tissue and fluid from the three joints confirmed a methicillin-sensitive Staphylococcus aureus (MSSA) infection. According to the antibiogram, the patient was given intravenous antibiotics (ATBs) for six weeks with clinical improvement. She received ceftriaxone (1g/day) and completed the 6-week protocol with TMS (160–800mg/12 hours) and rifampicin (300mg/12 hours) orally. The postoperative period was uneventful, and the patient was discharged three weeks after the first procedure and continued rehabilitation and treatment with ATBs under strict supervision.
At eight months of follow-up and without receiving ATB treatment, laboratory results showed a decreased ESR (15mm/h) and CRP (5mg/dL). The physical examination indicated proper wound healing without signs of active PJI. Owing to the favourable evolution, the second-stage revision surgery of each joint was indicated 60 days after the first stage.
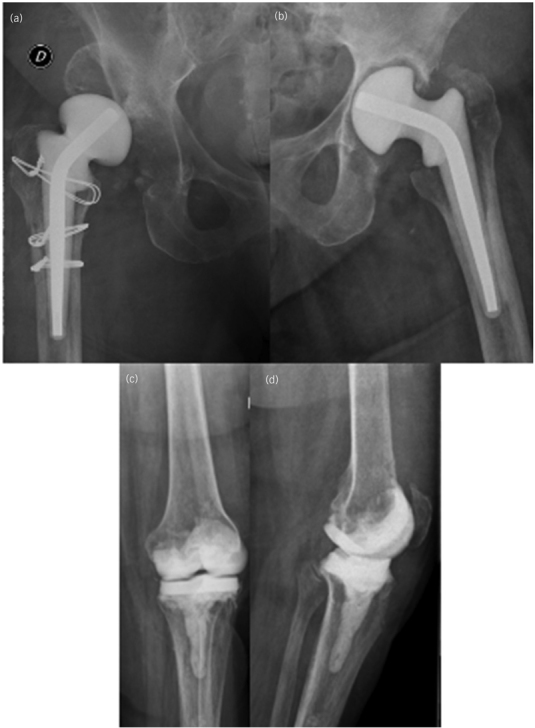
The second stage of the right THA was performed using a Trabecular Metal Acetabular Revision Shell (TMARS, Zimmer Biomet, Warsaw, IN, USA) and a cemented polished femoral stem (CPT, Zimmer Biomet) with the impaction bone grafting (IBG) technique (Figure 5a). On the left hip, a TMARS (Zimmer Biomet) and a cementless distally fixed modular fluted stem (ZMR, Zimmer Biomet) were used (Figure 5b). Finally, the sixth-stage for the left TKA was performed with tibial tubercle osteotomy (TTO), providing reliable and safe exposure during the revision surgery. Long cemented stems were used in both femoral and tibial components. Distal and posterior augments were required in the femoral side, as well as medial and lateral augments in the tibial baseplate due to secondary bone loss. A constrained total stabilised prosthesis was implanted (Optetrak, Exactech, Gainesville, FL, USA) as a consequence of soft tissue instability (Figure 6a and b).
Figure 5 .

Anteroposterior (a) view of the right hip revision surgery, showing reconstruction with a trabecular metal acetabular revision shell and a cemented polished femoral stem with IBG technique at one year of follow-up. Anteroposterior (b) view of the left hip revision surgery, showing reconstruction with a trabecular metal acetabular revision shell and a cementless distally fixed modular fluted stem at one year of follow-up.
Figure 6 .

Anteroposterior (a) and lateral (b) views of the left knee revision surgery, showing reconstruction after tibial tubercle osteotomy (TTO) with long cemented stems in both femoral and tibial components and a constrained total stabilised prosthesis as a consequence of soft tissue instability at one year of follow-up.
The rehabilitation protocol included early mobilisation 24 hours after surgery and ambulation with a walker and partial weight-bearing for 45 days due to the IBG technique on the right hip and the TTO on the left knee. After that, we encouraged the patient to progressively weight-bear as tolerated, depending on the evolution of graft incorporation and healing of the osteotomy seen on follow-up radiographs.
At one-year of follow-up, AP and L views showed well-fixed components without signs of loosening in either revision arthroplasty (Figures 5 and 6). The physical examination showed good ROM in both hips and a stable, well-aligned and fully extended knee with 95° of flexion.
At the present time, and after four years of follow-up, the patient presented to our outpatient clinic without referring pain and was able to perform activities of daily living without restrictions. Physical examination revealed a new sinus tract on her right hip, and a new joint aspiration was indicated; cultures showed MSSA. After careful evaluation and because of the patient’s comorbidities and current PJI presentation, suppressive ATB therapy with oral TMS (160–800mg/12 hours) was indicated under strict multidisciplinary supervision.
Discussion
The iliopsoas abscess is a rare entity characterised by the presence of purulent material in the muscle compartment. According to current literature, mortality in primary abscesses is 2.4%, and in secondary abscesses rises to 19%.11
Primary abscesses arise from a distant site of infection, which disseminate via the haematogenous or lymphatic drainage system, whereas secondary abscesses develop as a consequence of dissemination of infection from contiguous layers.12 The aetiology is multiple, with kidney failure, diabetes, intravenous drug abuse and spinal procedures being some of the predisposing factors described.13
In primary abscesses, Staphylococcus aureus is the most common organism involved.14,15 Secondary abscesses can be monomicrobial or polymicrobial, and the organisms isolated most often in polymicrobial infections are Enterobacteriaceae species. In our patient, the haematogenous dissemination can explain the left knee infection, and PJI in both hips can be a consequence of contiguous dissemination from the iliopsoas bursa. The iliopsoas bursa and the synovial hip capsule are in direct contact, and a distal psoas abscess can disseminate to the hip joint from such contiguous tissue.16,17
There is a close relationship between the presentation of PJI and the time elapsed since the psoas abscess was diagnosed. Dauchy et al analysed the time passed between the arthroplasty and the psoas abscess diagnosis. The authors found a significant difference in the septic complication rate the later the diagnosis occurs.18
According to the Musculoskeletal Infection Society, the definition of PJI is based on major and minor criteria. The current PJI diagnosis is confirmed by the presence of one major criterion or three minor criteria.19
Two-stage revision surgery is the most accepted treatment for PJI.20 The first stage consists of removing the infected implants and implanting a cemented spacer with a high local concentration of antibiotics. It is essential to know the specific characteristics of the ATB,21 including the availability in powder form, the adequate dilution in cement, thermostability and sensitivity to the microorganism. After six weeks of ATB therapy and according to the patient's evolution, the second stage can be performed implanting a new prosthesis.
In patients with acute symptoms (more than three to four weeks) and with an isolated low-virulence microorganism, debridement, antibiotic therapy and implant retention (DAIR) is a valid option. Some authors reported a success rate of 71%,22 being lower in cases where methicillin-resistant Staphylococcus aureus (MRSA) is identified.23 Patients who do not meet these characteristics should be treated with the two-stage procedure. Arthroplasty surgeons must be aware of the high false-negative rate, which has been reported in almost 18% of PJI cases.24
As we already know, PJI is a significant cause of mortality with a reported rate that ranges from 2.7% to 18%.25 In addition, it is associated with poorer patient-reported outcomes and higher healthcare costs.26
Finally, in some specific cases, surgical treatment cannot be achieved because of the patient's comorbidities and the increased risk of complications related to the surgical procedure. In these patients, prolonged suppressive ATB therapy is often used to control the infection.27 The treatment aims to maintain an asymptomatic patient with a functional prosthesis, but not necessarily free of infection. Rao et al28 reported an 86% success rate at medium-term follow-up.
Our case describes a severe and unusual simultaneous PJI of three total joint arthroplasties, secondary to a psoas abscess in the same patient. It is crucial to notice that the chances of successful treatment increase with an accurate and early diagnosis involving multidisciplinary management that includes clinicians, infectologists and hip surgeons. Despite the high complication and mortality rates, we believe that two-stage revision surgery is the correct treatment in patients with multiple simultaneous PJI.
Acknowledgements
The study was performed at the Italian Hospital of Buenos Aires, Argentina. All authors certify that their institution has approved the reporting of this case. All investigations were conducted in conformity with ethical principles of research.
References
- 1.Parisi TJ, Konopka JF, Bedair HS. What is the long-term economic societal effect of periprosthetic infections after THA? A Markov analysis. Clin Orthop Relat Res 2017; 475: 1891–1900. 10.1007/s11999-017-5333-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004; 351: 1645–1654. 10.1056/NEJMra040181 [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Lau E, Schmier Jet al. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 2008; 23: 984–991. 10.1016/j.arth.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 4.Perfetti DC, Boylan MR, Naziri Qet al. Have periprosthetic hip infection rates plateaued? J Arthroplasty 2017; 32: 2244–2247. 10.1016/j.arth.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 5.Berend KR, Lombardi AV Jr, Morris MJet al. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res 2013; 471: 510–518. 10.1007/s11999-012-2595-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb JE, Schleck CD, Larson DRet al. Mortality of elderly patients after two-stage reimplantation for total joint infection: a case-control study. J Arthroplasty 2014; 29: 2206–2210. 10.1016/j.arth.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 7.Sadat-Ali M, al-Habdan I, Ahlberg A. Retrofascial nontuberculous psoas abscess. Int Orthop 1995; 19: 323–326. 10.1007/BF00181120 [DOI] [PubMed] [Google Scholar]
- 8.Ricci MA, Rose FB, Meyer KK. Pyogenic psoas abscess: worldwide variations in etiology. World J Surg 1986; 10: 834–843. 10.1007/BF01655254 [DOI] [PubMed] [Google Scholar]
- 9.Gunaratne GD, Khan RJ, Tan C, Golledge C. Bilateral prosthetic hip joint infections associated with a Psoas abscess. A case report. J Orthop Case Rep 2016; 6: 3–6. 10.13107/jocr.2250-0685.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown NM, Foran JRH, Valle CJDet al. The inter-observer and intra-observer reliability of the Paprosky femoral bone loss classification system. J Arthroplasty 2014; 29: 1482–1484. 10.1016/j.arth.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 11.Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80: 459–462. 10.1136/pgmj.2003.017665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santaella RO, Fishman EK, Lipsett PA. Primary vs. secondary iliopsoas abscess. Presentation, microbiology, and treatment. Arch Surg 1995; 130: 1309–1313. 10.1001/archsurg.1995.01430120063009 [DOI] [PubMed] [Google Scholar]
- 13.Mückley T, Schütz T, Kirschner Met al. Psoas abscess: the spine as a primary source of infection. Spine (Phila Pa 1976) 2003; 28: 106–113. 10.1097/01.BRS.0000050402.11769.09 [DOI] [PubMed] [Google Scholar]
- 14.Casa DJ, Guskiewicz KM, Anderson SAet al. National athletic trainers’ association position statement: preventing sudden death in sports. J Athl Train 2012; 47: 96–118. 10.4085/1062-6050-47.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flavin NE, Gomez M. Fever, pain, and a limp: a case of a psoas and spinal epidural abscess caused by methicillin-resistant Staphylococcus aureus in a diabetic patient. J Natl Med Assoc 2009; 101: 84–86. 10.1016/S0027-9684(15)30793-8 [DOI] [PubMed] [Google Scholar]
- 16.Steinbach LS, Schneider R, Goldman ABet al. Bursae and abscess cavities communicating with the hip: diagnosis using arthrography and CT. Radiology 1985; 156: 302–303. 10.1148/radiology.156.2.4011891 [DOI] [PubMed] [Google Scholar]
- 17.Buttaro M, Della Valle A G, Piccaluga F. Psoas abscess associated with infected total hip arthroplasty. J Arthroplasty 2002; 17: 230–234. 10.1054/arth.2002.28734 [DOI] [PubMed] [Google Scholar]
- 18.Dauchy FA, Dupon M, Dutronc Het al. Association between psoas abscess and prosthetic hip infection: a case-control study. Acta Orthop 2009; 80: 198–200. 10.3109/17453670902947424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parvizi J, Gehrke T. International consensus group on periprosthetic joint infection. definition of periprosthetic joint infection. J Arthroplasty 2014; 29: 1331. 10.1016/j.arth.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 20.Charette RS, Melnic CM. Two-stage revision arthroplasty for the treatment of prosthetic joint infection. Curr Rev Musculoskelet Med 2018; 11: 332–340. 10.1007/s12178-018-9495-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rava A, Bruzzone M, Cottino Uet al. Hip spacers in two-stage revision for periprosthetic joint infection: A review of literature. Joints 2019; 7: 56–63. 10.1055/s-0039-1697608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odum S, Fehring T, Lombardi Aet al. Periprosthetic infection consortium. irrigation and debridement for periprosthetic infections: does the organism matter? J Arthroplasty 2011; 26: 114–118. 10.1016/j.arth.2011.03.031 [DOI] [PubMed] [Google Scholar]
- 23.Bradbury T, Fehring T, Taunton Met al. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty 2009; 24: 103–107. 10.1016/j.arth.2009.04.028 [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Adeli B, Zmistowski Bet al. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am 2012; 94: e104. 10.2106/JBJS.K.01417 [DOI] [PubMed] [Google Scholar]
- 25.Berbari EF, Hanssen AD, Duffy MCet al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998; 27: 1247–1254. 10.1086/514991 [DOI] [PubMed] [Google Scholar]
- 26.Parvizi J, Pawasarat IM, Azzam KAet al. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty 2010; 25: 103–107. 10.1016/j.arth.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 27.Kapadia BH, Berg RA, Daley JAet al. Periprosthetic joint infection. Lancet 2016; 387: 386–394. 10.1016/S0140-6736(14)61798-0 [DOI] [PubMed] [Google Scholar]
- 28.Rao N, Crossett LS, Sinha RK, Le Frock JL. Long-term suppression of infection in total joint arthroplasty. Clin Orthop Relat Res 2003; 414: 55–60. 10.1097/01.blo.0000087321.60612.cf [DOI] [PubMed] [Google Scholar]


