Abstract
Introduction
Management of malignant small bowel obstruction (mSBO) is challenging. The decision to perform an operation evaluates the perceived chance of success against a patient’s fitness for operation. The aim of this study was to characterise the mSBO patient population in a tertiary UK centre and assess the patient’s treatment pathway including use and effects of palliative surgery, total parenteral nutrition (TPN), Gastrografin and dexamethasone as well as preoperative stratification.
Methods
Patients were included if they had mSBO confirmed on computed tomography imaging due to a primary or metastatic neoplasm. Data were collected on pathway and management, and Cox proportional hazard methods were utilised to observe effects on survival.
Results
Ninety-four patients were included, with 104 inpatient episodes. Mean age was 67.4 (SD 13.7), with 57 (60.6%) females. Most (89.4%) had only one admission for mSBO. Eighty-four (89.4%) patients died over the ten-year period, 18 (17.3%) within 30 days of admission. Fifty patients (53.1%) underwent operative management: 70% bypass, 24% stoma formation and 6% open-close laparotomies. Log rank testing of survival probability analysis was significant (p = 0.00018), with 50% survival probability at 107.32 days for operative management and 47.87 days for non-operative.
Discussion and Conclusion
Operative management forms part of the treatment pathway for a significant proportion of patients with mSBO, offering a survival benefit, though quality of survival is not known. Case selection is good, with few open-close laparotomies. Trials of non-operative interventions such as Gastrografin and dexamethasone are not utilised fully.
Keywords: Intestinal obstruction, Neoplasms, General surgery, Colorectal surgery
Introduction
Malignant small bowel obstruction (mSBO) can occur anywhere from the ligament of Trietz through to the ileocaecal valve. Most cases of mSBO are caused by peritoneal disease or carcinomatosis. Commonly this is a result of metastatic disease to the peritoneum from abdominal or pelvic malignancy. Spread of disease across the peritoneum throughout the abdomen can cause obstruction at multiple levels of the bowel.1 However, primary malignant intra-abdominal disease can also cause mSBO through local advancement either via intraluminal obstruction, extramural compression or invasion of innervating plexi disturbing normal peristalsis.2
Standards of therapy for treating mSBO disease do not currently exist.3 Surgery in these patients can be difficult and result in detrimental outcomes even in the setting of palliation. As a result, alternative options for treatment are sought. For very proximal bowel obstruction, stenting procedures have become a useful means of controlling symptoms to avoid invasive surgery. The same methods can be applied in distal large bowel obstruction. Aggressive medical regimens, such as concurrent total parenteral nutrition (TPN) and high-dose chemotherapy, have been explored but to no avail.4 Oral water-soluble contrast agents (Gastrografin) are used in adhesional small bowel obstruction to predict resolution of obstruction and need for surgery. This practice could be transferred to the mSBO population, although the benefit in this group is unclear.5 The use of corticosteroids, specifically in the mSBO population, has been shown to relieve obstruction without any benefit on survival.6
Operative intervention is employed to relieve mSBO. However, the patient demographic is complex with a variation in health status, primary disease, method of bowel obstruction and level or extent of disease. The decision to operate is challenging and individualistic. It involves careful assessment of patient factors (patient’s wishes, frailty, prognosis, quality of life, comorbidities, expectation, ie risk of failure of operation, need for stoma) and technical aspects (previous surgery, cause of obstruction, multilevel obstruction, length of small bowel proximal to obstruction). A judgement is therefore made based on a patient’s overall fitness for operative intervention and the perceived technical chance of success. Preoperative factors that could help to inform this decision would be important.
The aims of this study were to characterise the patient population presenting with mSBO to a tertiary UK centre, to assess the patients’ treatment pathway and to include the frequency of operative management and other interventions (Gastrografin, steroids, TPN). Furthermore, we sought to characterise the patient group that benefits maximally from palliative surgery.
Methods
This retrospective cohort study examined data from 1 October 2009 to 1 October 2019 at Queen Elizabeth Hospital Birmingham, UK. Initial inclusion required a diagnosis of intra-abdominal cancer and small bowel obstruction using ICD-9/10 codes. In addition, the words ‘small bowel obstruction’ or ‘SBO’ and ‘cancer’ or ‘malign-’ were searched in discharge letters, imaging requests and reports. This approach maximised inclusion of the intended patients, given the known problems of miscoding of admission diagnoses.7 From those initially included, any patients without computed tomography (CT) imaging supporting small bowel obstruction due to malignant disease were excluded. Basic patient demographics were collected alongside data on procedures, complications, therapies and readmissions. Where possible, the place of death was obtained from the medical records.
Statistical analysis was performed using the statistical programming language R 3.6.38 with the following packages: tableone, survival, survminer, ggplot2. Data were visually inspected using Q-Q plots and assessed with Shapiro–Wilk testing for normality. Parametric data are displayed as mean with standard deviation (SD) and non-parametric data are displayed as median with interquartile range (IQR). Summary statistics were compared using Student’s t-test for parametric or Wilcoxon signed-rank test for non-parametric data. Cox proportional hazards testing was conducted along with Kaplan–Meier curves with log rank testing to visualise survival probability. Preoperative factors associated with operative, as opposed to non-operative, management were assessed and tested for significance by univariate and multivariate analysis.
Results
Patient demographics
Ninety-four patients with malignant small bowel obstruction were included (Table 1). Mean age was 67 years (SD ± 14), and 57 patients were female (61%). Eighty-four patients had one admission for malignant small bowel obstruction, but some patients had two or three admissions (n = 9 and 1, respectively). Most admissions were as an emergency (100 out of 104, 96%). The remaining four were semi-elective admissions with subacute obstructions, requiring urgent surgery. Eighty-four (89%) patients died during the ten-year study period, 18 (17%) of these dying within 30 days of presentation. Deaths were equally split between hospice (21, 25%) and home (21, 25%), though most in the ‘unknown’ category are patients who likely died at home. Twenty patients (24%) died in hospital, either from this admission or during an unrelated admission.
Table 1 .
Demographics of included cohort
| Overall | |
|---|---|
| Number of patients (n) | 94 |
| Age (mean (SD)) | 67.39 (13.72) |
| Male gender (%) | 37 (39.4) |
| BMI (kg/m2) (mean (SD)) | 24.34 (4.58) |
| Weight change from the highest weight recorded in the 1 year preceding the admission (kg) (mean (SD)) | −6.12 (6.05) |
| Charlson index (mean (SD)) | 8.12 (2.05) |
| Number of admissions (n) | 104 |
| Total number of admissions per patient with SBO due to malignancy (%) | |
| 1 | 84 (89.4) |
| 2 | 9 (9.6) |
| 3 | 1 (1.1) |
| Admission was emergency (%) | 100 (96.2) |
| Admission under directorate (%) | |
| Hepatobiliary (HPB) surgery | 4 (3.8) |
| Medicine | 4 (3.8) |
| Oncology | 25 (24.0) |
| General surgery | 69 (66.3) |
| Urology | 2 (1.9) |
| Death (%) | 84 (89.4) |
| Death within 30 days of admission (%) | 18 (17.3) |
| Patient died during an admission (%) | 13 (14.6) |
| Days to death from first admission (median [IQR]) | 66.95 [35.75, 129.18] |
| Location of death (%) | |
| Convent | 1 (1.2) |
| Home | 21 (25.0) |
| Hospice | 21 (25.0) |
| Hospital | 20 (23.8) |
| Nursing home | 1 (1.2) |
| Unknown | 20 (23.8) |
| Length of stay (median [IQR]) | 19.12 [10.68, 30.09] |
| Discharge destination (%) | |
| Community hospital | 4 (3.8) |
| Home | 72 (69.2) |
| Hospice | 13 (12.5) |
| Nursing home | 2 (1.9) |
| Not known | 13 (12.5) |
| Chemotherapy post-discharge (%) | 26 (27.6) |
In patients who had died, there were 67 [IQR 36–129] days from the patient’s first admission with malignant SBO to death. The median length of stay was 19 days (IQR 11–30). Most patients were discharged home (72, 69%): 13 discharged to hospice, 4 to community hospital for rehabilitation or low-level care, and 2 to nursing homes. Thirteen patients had an unknown discharge location.
Inpatient pathway and management
In total, 85 (82%) admissions were due to bowel obstruction from metastatic disease, corresponding to 76 patients; 65 had peritoneal metastatic disease. Metastases to ovarian (n = 3), retroperitoneal (n = 5) and bowel (n = 3) locations contributed to obstruction in the rest. The remaining 18 patients had a primary cancer causing small bowel obstruction, 13 of whom had mechanical obstructions from locally advanced disease causing luminal compression, and 5 had obstruction due to locally advanced disease within the small bowel itself. Forty-nine patients (47%) had obstruction at a single-level rather than multi-level disease, as reported on CT imaging (Table 2).
Table 2 .
Inpatient admission details, operation and operative complications
| Overall | |
|---|---|
| Number of admissions (n) | 104 |
| Admission for obstruction due to metastasis or local metastasis (%) (out of all 104 admissions) | 85 (81.7) |
| Obstruction of bowel at single level (%) (of 94 patients) | 49 (52.12) |
| Patient received operation for obstruction (%) (of 94 patients) | 50 (53.19) |
| Days to operation from admission (median [IQR]) | 5.81 [2.29, 11.88] |
| Operation type (%) | |
| Bypass | 35 (70.0) |
| Open and close | 3 (6.0) |
| Ileostomy formation | 12 (24.0) |
| Complications postop (%) | |
| Ileus (%) | 4 (3.8) |
| High output stoma (%) | 4 (3.8) |
| Wound infection/dehiscence (%) | 7 (6.7) |
| VTE (PE or DVT) (%) | 2 (1.9) |
| Return to theatre (%) | 3 (2.9) |
| Stroke (%) | 1 (1.0) |
| Fistula or anastomotic leak (%) | 5 (4.8) |
| Arrythmia (%) | 4 (3.8) |
| Infection (chest or urinary) (%) | 5 (4.8) |
| Number of operations with one or more complication (% of operations) | 24 (48%) |
| Went to ITU postsurgery (%) | 17 (34.7) |
| If admitted to ITU, length of stay ITU postsurgery (median [IQR]) | 3.0 [1.5, 5.0] |
| Oral water-soluble contrast (Gastrografin) used during inpatient episode (%) | 18 (17.3) |
| Dexamethasone used during inpatient episode (%) | 34 (32.7) |
| TPN used during admission (%) | 22 (21.2) |
| If used, number of days of TPN (median [IQR]) | 13.50 [10.00, 23.25] |
VTE: venous thromboembolism; PE: pulmonary embolism; DVT: deep vein thrombosis; ITU: intensive therapy unit; TPN: total parenteral nutrition
Fifty patients (53%) underwent surgical management (Table 2). Of these cases, 70% were bypass operations, 24% were ileostomy formation and three patients had an open-close laparotomy where continuing the operation was deemed likely to cause injury or be futile. In patients who received an operation, the average time between admission and operation was six days (IQR 2–12). In total, 24 (48%) operations had at least one complication. Seventeen patients (35% of patients who had an operation) were admitted to the intensive therapy unit (ITU) postoperatively, with a median length of stay of 3 days (IQR 1.5–5).
Regarding all admissions, Gastrografin was used in 18 (17%), and IV dexamethasone was given in 34 (33%). Those given dexamethasone were less likely to receive surgical management (p < 0.001) (24% on dexamethasone proceed to surgery versus 60% not given dexamethasone who proceed to surgery). There was no such difference with Gastrografin (p > 0.05). Twenty-two (23.4%) patients received TPN for a median of 13.5 days (IQR 10–23). After discharge, 26 patients (27.65%) received chemotherapy.
When examining the impact of TPN on survival as a univariate factor, it is significant (p = 0.036), but this is not the case when correcting for operative intervention in a Cox proportional hazards model (p = 0.16). In our study, only four patients of the 44 (9%) non-surgically managed patients received TPN. This subset is too small to examine the effect of TPN in patients who did not have surgery. In contrast, of those patients that had operative management (n = 50), 17 (34%) received TPN.
Characteristics of cancers
For 22 patients, this was the first presentation of cancer (Table 3). Of those with a known previous diagnosis of cancer, 78% had received previous surgery with curative intent. This was 620 days (IQR 352–1,731) prior to a first presentation of malignant small bowel obstruction.
Table 3 .
Characteristics of cancers in patient cohort
| Overall | |
|---|---|
| First presentation of cancer (%) | 22 (23.4) |
| In those with known cancer, who had previous curative surgery performed for the primary (%)? | |
| N | 16 (22.2%) |
| Y | 56 (77.7%) |
| Days from curative surgery to malignant SBO (median [IQR]) | 620.20 [351.50, 1,731.25] |
| Primary cancer location (%) | |
| Appendix | 3 (3.2) |
| Breast | 4 (4.3) |
| Colorectal | 46 (48.9) |
| Gynaecological | 17 (18.1) |
| Not known | 2 (2.1) |
| Peritoneal | 1 (1.1) |
| Skin | 2 (2.1) |
| Small bowel | 5 (5.3) |
| Small bowel NET | 1 (1.1) |
| UGI | 6 (6.4) |
| Urological | 7 (7.4) |
SBO: small bowel obstruction; NET: neuroendocrine tumour
Most primary cancers were colorectal, followed by gynaecological then urological (n = 46, 17 and 7, respectively). Most patients had a form of peritoneal metastasis (n = 74).
Operative and non-operative management
There was a significant association between crude survival and surgical management. This remained when correcting for patient comorbidities using the Charlson score.9 Having a palliative operation for malignant small bowel obstruction was associated with a hazard ratio (HR) of 0.384 (95% CI 0.24–0.61, p < 0.001). Figure 1 shows the survival curve of the 94 patients included in this study. There was no difference in survival whether the obstruction was caused by a metastatic deposit or local advancement of a primary. There was also no difference in survival between the different primary cancers. Therefore, these were not added to the multivariate Cox proportional hazards model. There was a small but significant difference between median survival for those who had an operation (117 days; 95% CI 74.1–235.2) versus those who did not receive an operation (49 days; 95% CI 41.0–91.9) (log rank test, p = 0.00018).
Figure 1 .
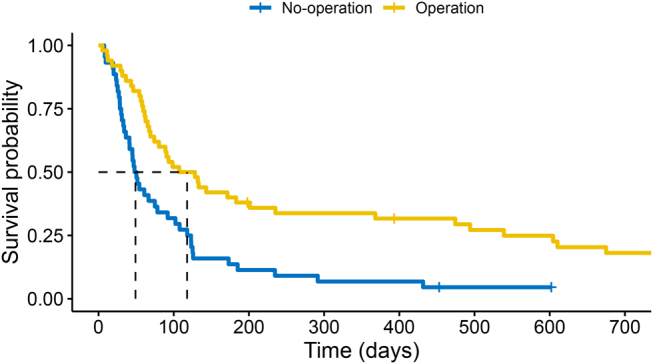
Survival curve of patients who had operative management during admission for malignant bowel obstruction and those without operative management; 0.5 (50%) survival probability (shown by dashed lines) at 107.32 days for operative management and 47.87 days for non-operative management (overall log rank test, p = 0.00018)
Factors associated with operative management
Owing to the difference in survival with operative management, we identified any preoperative factors that led to the decision in favour of operative intervention (Table 4). The specialty that the patient was admitted under made a significant difference to management (p = 0.004), mainly with General Surgery associated with operative management and Oncology associated with non-operative management. If this was the first presentation of cancer, surgical management was more likely (p = 0.005). Patients with a primary colorectal cancer were more likely to receive operative management (31, 62%) than not (14, 32%).
Table 4 .
Differences in preoperative characteristics of patients in the operative management vs non-operative management groups
| Operative management | Non-operative management | p-value | |
|---|---|---|---|
| n | 50 | 44 | |
| Gender (%) | 0.001 | ||
| Male | 28 (56.0) | 9 (20.5) | |
| Female | 22 (44.0) | 35 (79.5) | |
| Age (mean (SD)) | 68.11 (14.34) | 66.56 (13.10) | 0.586 |
| Charlson index (mean (SD)) | 8.10 (2.31) | 8.14 (1.73) | 0.932 |
| Primary cancer location (%) | 0.001 | ||
| Appendix | 0 (0.0) | 3 (6.8) | ns |
| Breast | 0 (0.0) | 4 (9.1) | ns |
| Colorectal | 31 (62.0) | 14 (31.8) | 0.0041 |
| Gynaecological | 3 (6.0) | 15 (34.1) | 0.0006 |
| Not known | 0 (0.0) | 1 (2.3) | ns |
| Peritoneal | 0 (0.0) | 1 (2.3) | ns |
| Skin | 2 (4.0) | 0 (0.0) | ns |
| Small bowel | 4 (8.0) | 1 (2.3) | ns |
| Small bowel NET | 2 (4.0) | 0 (0.0) | ns |
| UGI | 4 (8.0) | 2 (4.5) | ns |
| Urological | 4 (8.0) | 3 (6.8) | ns |
| Admission was emergency (%) | 47 (94.0) | 43 (97.7) | 0.703 |
| First presentation of cancer (%) | 18 (36.0) | 4 ( 9.1) | 0.005 |
| Obstruction of bowel at single level (%) | 28 (56.0) | 16 (36.4) | 0.09 |
| Previous curative surgery performed for the primary (%) | 28 (56.0) | 29 (65.9) | 0.441 |
| Days from curative surgery to mSBO presentation (median [IQR]) | 620.20 [362.19, 1,911.14] | 699.13 [354.01, 1,676.08] | 0.911 |
| Albumin level on admission (g/l) (mean (SD)) | 40.43 (6.46) | 36.75 (6.35) | 0.007 |
| Albumin level preoperatively (g/l) (mean (SD)) | 32.56 (7.82) | NA | |
| Weight on admission (kg) (mean (SD)) | 69.0 (14.3) | 62.85 (12.9) | 0.035 |
| BMI on admission (kg/m2) (mean (SD)) | 24.7 (4.65) | 23.9 (4.5) | 0.376 |
| Weight change from the highest weight recorded in the 1 year preceding the admission (mean (SD)) | −6.41 (6.60) | −5.86 (5.57) | 0.721 |
| Ascites on admission (%) | 7 (14.0) | 14 (31.8) | 0.069 |
| Radiotherapy to pelvis or abdomen previously (%) | 4 (8.0) | 3 (6.8) | 1 |
Primary cancer category is tested with individual Fisher exact tests; p-values below 0.0045 were deemed significant (as per Bonferroni correction)
NET: neuroendocrine tumour; UGI: upper gastrointestinal; mSBO: malignant small bowel obstruction
Patients with a gynaecological primary cancer were more likely to be managed non-operatively (15, 34%), than operatively (3, 6%). There was also a difference in gender between the operative groups, which could be explained by the observed differences in pathophysiology of gynaecological primary disease. When included in a multivariate binomial logistic regression model along with primary cancer, and level of obstruction (single or multi), the effect of gender was not significant (p = 0.12). The effect on operative approach of having a gynaecological primary cancer is significant in the model and associated with non-operative management (p = 0.03).
Albumin level at admission was significantly different between the operative and non-operative management groups, 40.3g/l (SD ± 6.46) and 36.8g/l (SD ± 6.35), respectively (p = 0.007). Albumin levels at admission had a significant association with survival in a univariate Cox proportional hazards model, but the effect size was modest with an HR = 0.96 (p = 0.0125), suggesting that for every 1g/l increase in albumin the risk of death decreases by 4%. However, when included within a multivariate model with surgical management, the significance was removed (p = 0.0587). In the group of patients that did not have surgical management, admission albumin had no association with survival (p = 0.38). In those who had surgical management, there was no association between preoperative albumin and survival (p = 0.0597).
Weight on admission was also significantly different between surgical and non-surgical management groups (69kg (SD ± 14) vs 63kg (SD ± 13), respectively; p = 0.035). However, this difference was not observed with BMI (p > 0.05). In addition, weight on admission played no factor in survival (p = 0.4). Presence of ascites (p = 0.069) and previous radiotherapy to the pelvis were not different in the two groups (Table 4).
Discussion and conclusion
In this study of 94 patients presenting with mSBO to a tertiary centre, we characterised the patient population and elements of their treatment pathway. Most patients had only one admission with mSBO (89%), which was typically an emergency. Over the ten-year study period, 96% of patients had died. Only a small proportion died during an admission (14.6%). Location of death was split between home (25%) and hospice (25%), though the ‘unknown’ category (23.8%) likely contains those who passed away at home without documentation. The median length of stay was just less than 3 weeks (19 days; IQR 11–30). This study also involved some rare primary cancer sources of malignant small bowel obstruction, such as skin melanoma (two patients, 2%) and breast cancer (four patients, 4.3%).
Our data illustrate that palliative surgery forms a significant part of the treatment of mSBO, with approximately half of patients undergoing operations (50 patients, 53.2%). On average, this operation happens within the first week of admission. Both the improved survival benefit in operated patients and the lack of open-close laparotomies (three patients, 6% of operated cases) suggests that cases are well selected for surgery. However, palliative operations were associated with at least one complication (24 patients, 48% of operated cases) and need for ITU level postoperative care. The role of laparoscopy to avoid open-close laparotomies is an issue of contention. A laparoscopic approach to adhesional SBO can be attempted and may have some advantages.10 However, in the context of malignant SBO it would likely be difficult to enter the abdomen safely, and relieving obstruction would be more complicated than division of a simple band adhesion.
The outcomes in operated patients led us to focus on preoperative characteristics (Table 4). Patients with a gynaecological primary were more likely to be treated with non-operative management. This is likely the result of differences in disease process. Gynaecological cancers are more likely to present with omental or peritoneal disease, particularly epithelial ovarian cancer, which arises from the serosal lining of the ovary and as a result communicates with the peritoneum.11,12 Previous studies have also suggested that patients with colorectal primary disease have more favourable outcomes than gynaecological disease.13,14 However, in our study, there were no differences between colorectal, gynaecological and other primary cancers in survival outcomes. This held true even if the data were coerced into colorectal and non-colorectal groups (p = 0.56). Interestingly, age and Charlson index values did not differ significantly between operative and non-operative groups. In addition, time from previous curative surgery to presentation was not different, which is a contradiction to some of the available literature.15 Operative management was more common during the initial presentation of cancer. This could be confounded by disease severity, with surgery being less of an option in a patient who had already received multiple lines of therapy. First presentation had no effect on crude mortality (p = 0.55), which contradicts the results of Blair et al.14
Other studies have suggested that lower albumin levels are associated with reduced survival in patients undergoing surgery for mSBO.14,16 However, these studies focused specifically on a subgroup of patients with mSBO who underwent surgery. In our cohort, we found no association between admission albumin level and survival, when adjusted for surgical management.
Ascites has been associated with worse outcome;14 however, our analysis showed no difference between outcomes in patients with or without ascites (p = 0.069). Very few of our patients (n = 7, 7.45%) received radiotherapy to the pelvis or abdomen as part of their original cancer treatment. This has been shown in gynaecological cancers to have a negative effect on mortality,14,17 but there was no effect on survival in our study (p > 0.05).
Water-soluble contrast, such as Gastrografin, was used in only 17.3% of admissions. This number is low considering its efficacy in adhesional small bowel obstruction. A recent systematic review reports a paucity of evidence for its use specifically in mSBO.5 Dexamethasone is also used in patients with mSBO for symptom relief. In our study it showed no survival benefit (p = 0.1). Other studies have found it difficult to conclude a benefit for dexamethasone on symptom relief,18 but Cochrane report on evidence in one of their reviews that corticosteroids may help relieve mSBO.6
A number of patients (n = 22) received TPN, often for a period of around 2 weeks (median 13 days; IQR 10–23). There is limited evidence for the use of TPN, but some small studies have reported a modest benefit,19–22 which appears to be removed when undergoing concurrent chemotherapy.4,20 TPN also comes under scrutiny in a 2018 Cochrane review investigating home TPN trials for inoperable malignant bowel obstruction.23 In our study, if examined alone, TPN shows a benefit, but when included in a multivariate model with operative management, the significance is removed (p = 0.16). In this study, only four patients received TPN in the non-surgical management group, too small for analysis.
The national audit of small bowel obstruction (NASBO) captured data on malignant small bowel obstruction also.24 They showed similar rates of surgical intervention in these patients (47.8% surgical management, versus 53.2% in our study) and similar in-hospital mortality. The NASBO audit also showed that Gastrografin was poorly utilised in their cohort. However, they defined small bowel obstruction occasionally on just plain film radiographs (19%), whereas we utilised a stricter case definition approach of requiring a CT scan. As a prospective audit, 30-day outcomes were captured, but this study is able to capture a longer period post-hospitalisation to examine crude mortality and detect a difference. In addition, this study is able to examine discharge destination and dexamethasone use. A recent systematic review of this topic, in a majority gynaecological primary cohort, has also shown similar rates of operative management and an improvement in survival with operative management.25
Lastly, all of these points are an illustration that the care of these patients is a complex process and should involve a multidisciplinary team approach. Effective palliation and symptom control are the domain of oncologists and palliative care physicians. The involvement of the palliative care team with these patients is generally at admission for those with metastatic disease. The inpatient palliative care team help manage patients’ symptoms, attend to psychological and spiritual needs, and may be involved in the discharge planning process. Community support and discharge from hospital involve palliative care teams, occupational therapy, physiotherapy and social services. Evidence of their involvement in our study is clear, in that approximately one quarter of patients died in a hospice. Decisions concerning parenteral nutrition are handled by dedicated nutritional teams and gastroenterologists. The decision regarding surgical treatment, and sometimes the coordination of care of these complex patients, remains the responsibility of the general surgeon. However, as a complex group, these patients should be discussed in a multidisciplinary meeting.
As a retrospective study, the data were not recorded with the purpose of this study in mind. This results in some limitations. For example, there may be confounding from variables that were not measured relevant to selection for operative management. For example, a prospective study would evaluate preoperative risk scores and performance status. Retrospective studies are also prone to misclassification bias, as information may be incorrectly recorded. In addition, there is no way of ascertaining quality of life, or whether the survival benefit observed is problem free. These are important considerations. Consequently, patients would benefit from further study looking at quality of life, and need for further medical or surgical interventions. Given the complex nature of the joint patient–doctor decision to proceed to surgery in mSBO, a randomised controlled trial (RCT) evaluating operative intervention would be inappropriate. However, there is scope for larger RCTs evaluating the effect of TPN, dexamethasone and Gastrografin in mSBO patients, thereby aiding clear evidence-based decision-making.
In conclusion, palliative surgery forms part of the pathway for mSBO in approximately half of patients in our study. Although surgery is associated with improved survival, this represents selection bias of patients who are fit for intervention and have a perceived chance of a successful operation. Factors associated with selection for operative management were colorectal cancer as the primary cancer, mSBO as the first presentation of cancer and high albumin. Although these factors may be taken into account, the decision to proceed to surgery in mSBO is complex and should involve multidisciplinary decision-making. Non-operative measures, such as use of Gastrografin and corticosteroids, are probably underutilised.
Acknowledgement
With thanks to Mohammed Rob for help with data extraction.
References
- 1.Laval G, Marcelin-Benazech B, Guirimand Fet al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage 2014; 48: 75–91. 10.1016/j.jpainsymman.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 2.Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res 2012; 4: 159–169. 10.2147/CMAR.S29297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koisser K. [Palliative surgery of malignant small bowel obstruction after colorectal cancer]. Wien Med Wochenschr 2019; 169: 381–386. [DOI] [PubMed] [Google Scholar]
- 4.Chouhan J, Gupta R, Ensor Jet al. Retrospective analysis of systemic chemotherapy and total parenteral nutrition for the treatment of malignant small bowel obstruction. Cancer Med 2016; 5: 239–247. 10.1002/CAM4.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syrmis W, Richard R, Jenkins-Marsh Set al. Oral water soluble contrast for malignant bowel obstruction. Cochrane Database Syst Rev 2018; 3: CD012014. 10.1002/14651858.CD012014.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feuer DJ, Broadley KE. Corticosteroids for the resolution of malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2000; 2017. 10.1002/14651858.CD001219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang KL, Lucyk K, Quan H. Coder perspectives on physician-related barriers to producing high-quality administrative data: a qualitative study. C Open 2017; 5: E617. 10.9778/cmajo.20170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.R Foundation for Statistical Computing. R Core Team (2016). R: A Language and Environment for Statistical Computing, 2013. 10.1038/sj.hdy.6800737 [DOI] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 10.Sajid MS, Khawaja AH, Sains Pet al. A systematic review comparing laparoscopic vs open adhesiolysis in patients with adhesional small bowel obstruction. Am J Surg 2016; 212: 138–150. 10.1016/j.amjsurg.2016.01.030 [DOI] [PubMed] [Google Scholar]
- 11.Fagotti A, Gallotta V, Romano Fet al. Peritoneal carcinosis of ovarian origin. World J Gastrointest Oncol 2010; 2: 102–108. 10.4251/wjgo.v2.i2.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halkia E, Spiliotis J, Sugarbaker P. Diagnosis and management of peritoneal metastases from ovarian cancer. Gastroenterol Res Pract 2012; 2012. 10.1155/2012/541842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas SM, Merrie AEH. Resection of peritoneal metastases causing malignant small bowel obstruction. World J Surg Oncol 2007; 5: 122. 10.1186/1477-7819-5-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair SL, Chu DZJ, Schwarz RE. Outcome of palliative operations for malignant bowel obstruction in patients with peritoneal carcinomatosis from nongynecological cancer. Ann Surg Oncol 2001; 8: 632–637. 10.1007/s10434-001-0632-1 [DOI] [PubMed] [Google Scholar]
- 15.Goto T, Takano M, Aoyama Tet al. Outcomes of palliative bowel surgery for malignant bowel obstruction in patients with gynecological malignancy. Oncol Lett 2012; 4: 883–888. 10.3892/ol.2012.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryan DN, Radbod R, Berek JS. An analysis of surgical versus chemotherapeutic intervention for the management of intestinal obstruction in advanced ovarian cancer. Int J Gynecol Cancer 2006; 16: 125–134. 10.1111/j.1525-1438.2006.00283.x [DOI] [PubMed] [Google Scholar]
- 17.Feuer DJ, Broadley KE, Shepherd JH, Barton DPJ. Systematic review of surgery in malignant bowel obstruction in advanced gynecological and gastrointestinal cancer. Gynecol Oncol 1999; 75: 313–322. 10.1006/gyno.1999.5594 [DOI] [PubMed] [Google Scholar]
- 18.Hardy J, Ling J, Mansi Jet al. Pitfalls in placebo-controlled trials in palliative care: dexamethasone for the palliation of malignant bowel obstruction. Palliat Med 1998; 12: 437–442. 10.1191/026921698666334766 [DOI] [PubMed] [Google Scholar]
- 19.Duerksen DR, Ting E, Thomson Pet al. Is there a role for TPN in terminally ill patients with bowel obstruction? Nutrition 2004; 20: 760–763. 10.1016/j.nut.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 20.Brard L, Weitzen S, Strubel-Lagan SLet al. The effect of total parenteral nutrition on the survival of terminally ill ovarian cancer patients. Gynecol Oncol 2006; 103: 176–180. 10.1016/j.ygyno.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 21.Diver E, O’Connor O, Garrett Let al. Modest benefit of total parenteral nutrition and chemotherapy after venting gastrostomy tube placement. Gynecol Oncol 2013; 129: 332–335. 10.1016/j.ygyno.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 22.Bozzetti F. The role of parenteral nutrition in patients with malignant bowel obstruction. Support Care Cancer 2019; 27: 4393–4399. 10.1007/s00520-019-04948-1 [DOI] [PubMed] [Google Scholar]
- 23.Sowerbutts AM, Lal S, Sremanakova Jet al. Home parenteral nutrition for people with inoperable malignant bowel obstruction. Cochrane Database Syst Rev 2018; 2018. 10.1002/14651858.CD012812.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake TM, Lee MJ, Sayers AEet al. Outcomes following small bowel obstruction due to malignancy in the national audit of small bowel obstruction. Eur J Surg Oncol 2019; 45: 2319–2324. 10.1016/j.ejso.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Banting SP, Waters PS, Peacock Oet al. Management of primary and metastatic malignant small bowel obstruction, operate or palliate. A systematic review. ANZ J Surg. n.d.;n/a. 10.1111/ans.16188 [DOI] [PubMed] [Google Scholar]


