Abstract
A 63-year-old man presented to the emergency department with low back pain, perineal and genital numbness, together with bilateral lower limb paraesthesia and urinary retention. He was admitted under the orthopaedic service for investigation of suspected cauda equina syndrome. Magnetic resonance imaging of his spine did not reveal any evidence of cauda equina compression. Magnetic resonance imaging of his brain demonstrated nonspecific multiple hyperintensities in the right frontotemporal and left temporo-occipital regions. Computed tomography of his chest, abdomen, and pelvis did not identify any evidence of malignancy. Cerebrospinal fluid from a lumbar puncture showed a high leucocyte count (predominantly lymphocytes). Viral cerebrospinal fluid polymerase chain reaction was positive for varicella zoster virus. A diagnosis of varicella zoster virus myeloradiculitis (Elsberg syndrome) was established and the patient was treated with intravenous aciclovir. Unfortunately, the patient succumbed to a devastating intracerebral haemorrhage during his inpatient stay, probably due to vasculopathy from the underlying varicella zoster virus infection. This case describes a rare infectious mimic of cauda equina syndrome. Elsberg syndrome is an infectious syndrome characterised by bilateral lumbosacral myeloradiculitis, with varicella zoster virus being a well-recognised aetiological agent. We discuss the relevant literature in detail and identify the key, cautionary lessons learned from this case.
Keywords: Central Nervous System Infections, Cauda Equina Syndrome, Varicella Zoster Virus Infection, Radiculopathy
Background
Cauda equina syndrome is a spinal emergency characterised by a combination of neuromuscular and urogenital symptoms resulting from damage to lumbosacral nerve roots.1 It is typically caused by compressive aetiology such as lumbar disc herniation; non-compressive mechanisms may be of ischaemic, inflammatory or infectious origin.1 Although compressive aetiologies commonly account for patients presenting with cauda equina syndrome, consideration of infectious causes as differential diagnoses is typically restricted to immunocompromised patients.1 In this case, however, the patient was immunocompetent.
Case history
A right-handed 63-year-old man attended the emergency department with a two-week history of low back pain, perineal numbness, and bilateral sciatica. He was able to pass urine but unable to control flow; his bowel function was normal. Sexual dysfunction was undocumented. His medical history consisted of alopecia totalis, inguinal hernia repair and knee arthroscopy. There were no risk factors for immunodeficiency.
On examination, the patient was oriented, apyrexial and did not display any rashes or features of meningitis. Cranial nerve and upper limb examinations were unremarkable. A lower limb examination noted altered gait with reduced sensation in L3–5 dermatomes bilaterally. Power was MRC 5/5 in all myotomes with normal reflexes and downgoing plantars. Digital rectal examination confirmed reduced perianal sensation and anal tone. The patient developed urinary retention and was catheterised; catheter tug sensation was intact. He was then admitted under the orthopaedic service for investigation of cauda equina syndrome.
Blood tests revealed a leucocytosis with a neutrophilia of 70% and a C-reactive protein of 5. Magnetic resonance imaging (MRI) of his lumbosacral spine (Figure 1A), however, demonstrated a normal appearance of the spinal cord and cauda equina, with small disc protrusions at L3–4 and L4–5. MRI of the cervicothoracic spine (Figure 1B) only showed degenerative changes with no evidence of cord compression, while the MRI brain (Figure 1C,D) demonstrated multiple, nonspecific hyperintensities in the right frontotemporal and left temporooccipital regions. These were thought to either be inflammatory, haemorrhagic or neoplastic in origin. Computed tomography (CT) of his chest, abdomen and pelvis did not identify any evidence of malignancy. A neurology consult was requested and a lumbar puncture was subsequently performed. Nerve conduction studies are not available at our institution and hence were not done.
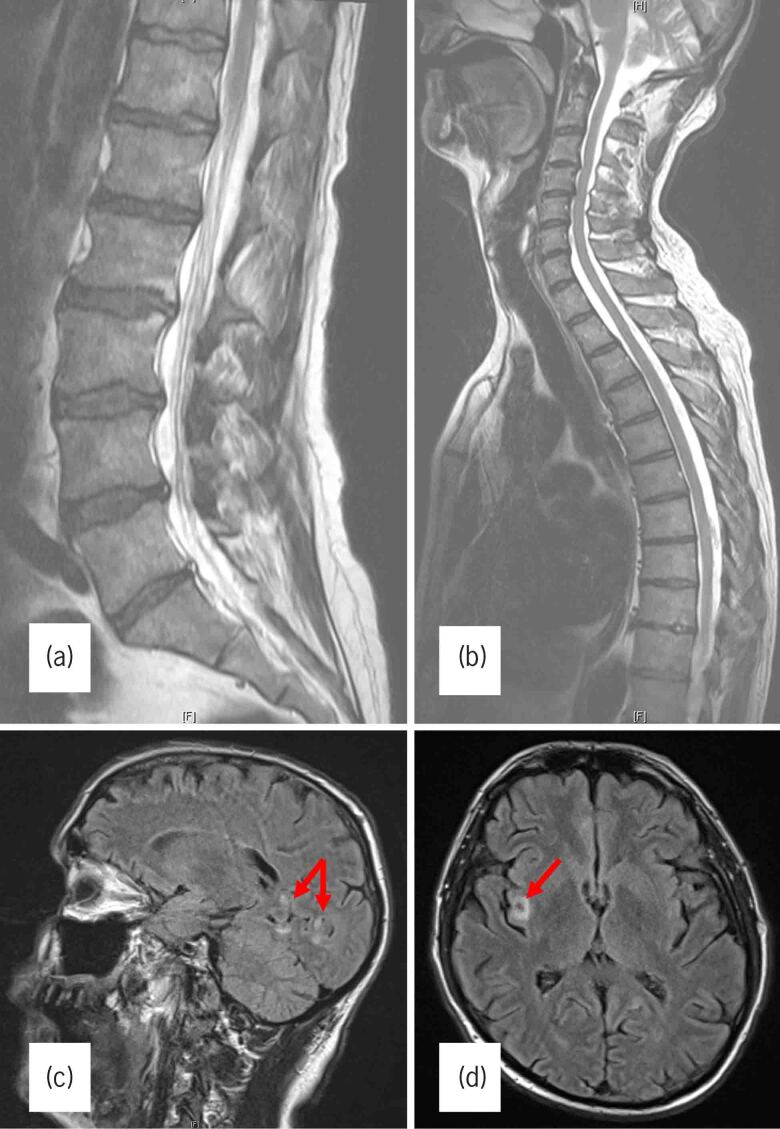
Figure 1 .
(A) T2-weighted sagittal magnetic resonance image (MRI) of the lumbar spine showing small disc protrusions at L3–4 and L4–5 but no cauda equina compression. (B) T2-weighted sagittal MRI of the cervicothoracic spine showing no compressive lesion affecting the proximal cord. (C) Sagittal fluid attenuation inversion recovery (FLAIR) MRI sequence demonstrating hyperintense lesions (red arrows) in the temporooccipital region. (D) Axial FLAIR MRI sequence showing a similar hyperintense lesion (red arrow) in the right insula.
The cerebrospinal fluid was turbid in appearance, with a normal opening pressure of 11, elevated glucose (4.76mmol/l) and protein (2.00g/l). A raised white cell count of 276, with 95% lymphocytes, and red cells of 910 were also noted, raising suspicion of a traumatic tap. The sample remained sterile after 48 hours. The patient was therefore empirically commenced on intravenous aciclovir, amoxicillin and cefotaxime to treat for central nervous system infection.
Viral cerebrospinal fluid polymerase chain reaction results were available on day 12 of his admission, which was positive for varicella zoster virus (VZV). A diagnosis of VZV myeloradiculitis (Elsberg syndrome) was established and aciclovir was continued.
The patient was nursed in isolation and he remained neurologically stable during the first two weeks of his inpatient stay. Unfortunately, on day 20 of his admission, his Glasgow Coma Score (GCS) suddenly dropped to 6/15 and a CT of his head (Figure 2) demonstrated an extensive intracerebral haemorrhage in the right frontotemporal regions with associated hydrocephalus. His consciousness rapidly deteriorated to GCS 3/15 with a fixed and dilated right pupil. Neurosurgical opinion noted the extensive haematoma and poor neurological function and, because of the grim prognosis, no further intervention was offered. The patient died a few hours later.
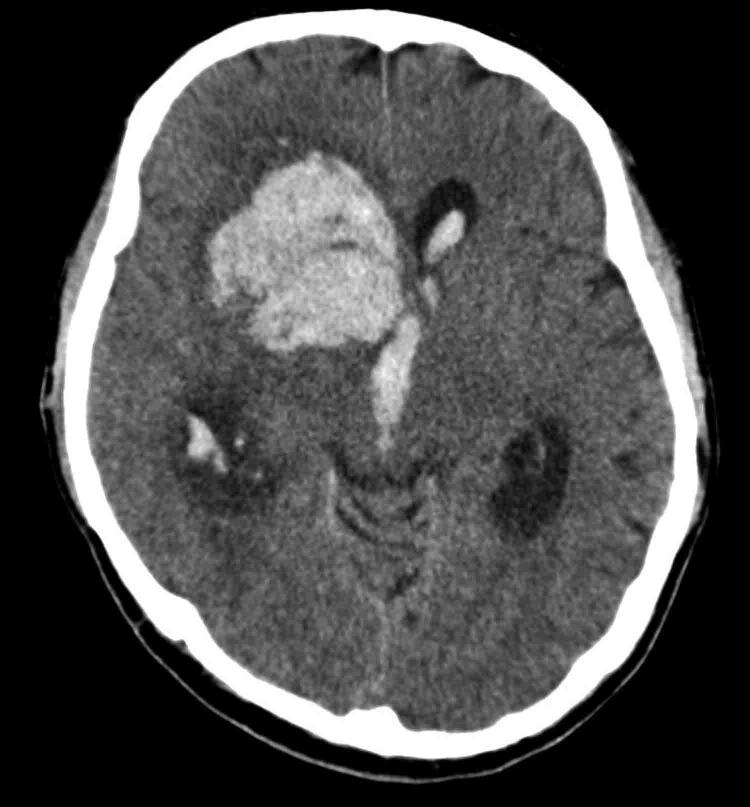
Figure 2 .
Non-contrast computed tomography of the head demonstrating a large right frontotemporal intracerebral haemorrhage with intraventricular extension and associated hydrocephalus.
Discussion
VZV, also known as human herpesvirus (HHV3), is a neurotropic organism with a double-stranded DNA genome. It is a leading cause of viral encephalitis and meningitis. Other rarer central nervous system manifestations include myelitis, cerebellitis, stroke-related syndromes and cranial nerve palsies.2 VZV central nervous system disease can occur in both immunocompetent and immunosuppressed individuals, yet will tend to cause more considerable neurological sequelae and mortality in the latter group.2
Elsberg syndrome is a rare infectious syndrome that mimics cauda equina syndrome.3 It is an acute, bilateral lumbosacral myeloradiculitis, which characteristically occurs secondary to herpes virus infection. Most commonly, HSV-2 is the causative pathogen, but VZV is also a well-recognised aetiological agent.3 Elsberg syndrome is diagnosed by the clinical, radiological or electrophysiological presence of radiculitis, plus or minus myelitis, with evidence of infection by a causative virus, where alternative aetiology has been excluded.3 This patient presented with bilateral altered lower limb neurology and bladder dysfunction in the presence of VZV infection, and thus exhibited core characteristics of Elsberg syndrome. There was also evidence of VZV-related vasculopathy in this patient,2 with respect to his intracerebral haemorrhage. VZV infection has been identified as an important risk factor for stroke.2 Therefore, in the absence of conventional risk factors, it is likely that the acute VZV infection led to this patient’s devastating intracerebral haemorrhage.
There are several learning points from this case. First, as cauda equina syndrome was the primary differential diagnosis, lumbosacral MRI is a priority and should be performed urgently. In the UK, national guidelines recommend that MRI scanning should be available out of hours at all hospitals to definitively diagnose cauda equina syndrome; however, the lack of available radiographers has meant that this standard is routinely missed.4 As such, MRI was performed 72 hours after this patient’s admission, which is considerably longer than the median time of 13 hours taken to scan nationally.4 If there is no compression in the lumbar spine, it is essential to identify whether a proximal lesion may be causative by imaging the rest of the central nervous system, as in this case.
Having excluded compressive lesions, nondiscogenic differentials such as infections, although very rare,5 should be considered. A lumbar puncture and relevant blood tests should be performed and specialist input from neurology colleagues sought. Although the diagnosis of Elsberg syndrome was established in this case, the diagnostic uncertainty meant that treatment was only initiated from day 8 of his admission. This case report should, therefore, serve as a reminder to clinicians to consider extra-spinal differentials promptly, as well as involving specialist colleagues in cases where a definitive diagnosis has not been established.
References
- 1.Panos G, Watson DC, Karydis Iet al. Differential diagnosis and treatment of acute cauda equina syndrome in the human immunodeficiency virus positive patient: a case report and review of the literature. J Med Case Reports 2016; 10: 165. 10.1186/s13256-016-0902-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grahn A, Studahl M. Varicella-zoster virus infections of the central nervous system: prognosis, diagnostics and treatment. J Infect 2015; 71: 281–293. 10.1016/j.jinf.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Savoldi F, Kaufmann TJ, Flanagan EP. et al. Elsberg syndrome: a rarely recognized cause of cauda equina syndrome and lower thoracic myelitis. Neurol Neuroimmunol Neuroinflamm 2017; 4: e355. 10.1212/NXI.0000000000000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutton M. Spinal Services: GIRFT Programme National Specialty Report. London: NHS Getting It Right First Time; 2019. https://gettingitrightfirsttime.co.uk/girft-reports (cited October 2020). [Google Scholar]
- 5.Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve 2007; 35: 529–531. 10.1002/mus.20696 [DOI] [PubMed] [Google Scholar]