Abstract
Background
There are limited data about the range of diseases, natural history, age-appropriate end-points and optimal care for children with pulmonary hypertension (PH), including the need for developing high-quality patient registries of children with diverse forms of PH to enhance care and research. Our objective was to characterise the distribution and clinical features of diseases associated with paediatric PH, including natural history, evaluation, therapeutic interventions and outcomes, as defined by the World Symposium on Pulmonary Hypertension (WSPH) classification.
Methods
1475 patients were enrolled into a multisite registry across the Pediatric Pulmonary Hypertension Network (PPHNet), comprised of eight interdisciplinary PH programmes.
Results
WSPH Groups 1 (pulmonary arterial hypertension (PAH)) and 3 (lung or hypoxia-related PH) were the most common primary classifications (45% and 49% of subjects, respectively). The most common Group 3 conditions were bronchopulmonary dysplasia and congenital diaphragmatic hernia. Group 1 disease was predominantly associated with congenital heart disease (60% of Group 1 cases) and idiopathic PAH (23% of Group 1 cases). In comparison with Group 1, Group 3 subjects had better disease resolution (hazard ratio 3.1; p<0.001), tended to be younger at diagnosis (median (interquartile range) age 0.3 (0.0–0.6) versus 1.6 (0.1–6.9) years; p<0.001) and were more often male (57% versus 45%; p<0.001). Down syndrome, the most common genetic syndrome in the PPHNet Registry, constituted 11% of the entire PH cohort.
Conclusions
We find a striking proportion of paediatric PH patients with Group 3 disorders, reflecting the growing recognition of PH in diverse developmental lung diseases. Greater precision of clinical phenotyping based on disease-specific characterisation may further enhance care and research of paediatric PH.
Shareable abstract (@ERSpublications)
To characterise the clinical features of diseases associated with paediatric pulmonary hypertension, including natural history, evaluation and therapeutic interventions, longitudinal data from 1500 children from major PH programmes were analysed https://bit.ly/3ff3zjV
Introduction
Pulmonary hypertension (PH) and related pulmonary vascular diseases (PVDs) are associated with severe morbidities and high mortality in children, but little is known about the natural history, outcomes, age-appropriate end-points and optimal care for children with diverse forms of PH [1–4]. Although there have been major advances in the field, PH continues to cause significant morbidity and mortality in diverse pulmonary, cardiac, haematological and other systemic disorders of childhood [2–4]. The long-term outcomes of children with severe PH remain poor despite the availability of new drug therapies, as most clinical trials have been performed in adult patients and these drugs are used off-label for paediatric PH.
Despite some similarities, many aspects of PH in children are distinct from adult PH [3–5]. Paediatric PH is often intrinsically linked to issues of lung growth and development, as well as many genetic, prenatal and early postnatal influences [2], and there are marked differences in the epidemiology of paediatric and adult PH [6–12]. The Pediatric Task Force at the recent 6th World Symposium on Pulmonary Hypertension (WSPH; Nice, France, 2018) reported that striking differences exist in function, structure, genetics and responsiveness to therapies between adults and children with PH [4]. Randomised clinical trials and case studies that address the safety and efficacy of PH therapies in children are rare, as most pharmaceutical studies have focused on the adult population and primarily in patients with a fairly limited range of associated conditions.
Paediatric PH is associated with diverse disorders that have distinct pathogenetic mechanisms, clinical courses and outcomes from adult disease that remain poorly characterised [2–5]. There remains a major need to understand the diversity of paediatric PH, the prevalence of each disease within PH groups, disease-specific features (such as age of onset, diagnostic and therapeutic approaches, interventions, and outcomes), and the utility of the current classification systems to better characterise comorbidities and to identify phenotypes or subgroups within each of the disease categories. Given this disease heterogeneity, high-quality patient registries with large numbers of patients and related databases of children with diverse forms of PH are necessary to better define the epidemiology of paediatric PH, disease subgroups, and the current status of PH evaluation and therapies.
Over the past decades, the original World Health Organization (WHO) classification system of PH has progressively evolved to characterise different forms of PH, as most recently updated at the WSPH in 2018 [13]. Although primarily designed for use in adult PH, the current version of the WSPH classification has been amended in response to the Pediatric Task Force of the WSPH to design a comprehensive system that is readily useful for children [4]. The importance of developing a useful classification for children includes the need for clarifying disease phenotypes, encouraging novel understanding of disease causation and pathobiology, enhancing diagnostic evaluations, improving correlations of phenotype and therapeutic responsiveness, and optimising clinical trial design. Most importantly, such systems may further promote successful outcomes by facilitating strategies more directly supportive of “precision medicine” [14–19].
Based on the WSPH classification, past registry reports of children with PH have provided important insights into the spectrum of paediatric PH [6–11]. These registries are partly limited, however, by the relatively narrow scope of patient selection by PH group, the inherently biased nature of referrals, relatively small patient numbers, cardiac catheterisation as a required inclusion criteria and the nature of clinical practice of PH centres participating in the registries that may not reflect broader, multidisciplinary programmes. Due to these limitations, past registries may not directly capture the “real-world” nature of PH in children, in which multiple factors may contribute to underlying disease severity and outcomes.
We sought to define the epidemiology of patients based on the WSPH classification using a multicentre prospective registry of paediatric PH patients. Specifically, in order to measure the scope and current landscape of paediatric PH, we developed a multisite registry with the following goals: 1) determine the proportion of newborns, infants and children with PH of diverse aetiologies as defined by the WSPH classification system; 2) fully characterise and define clinical features, current diagnostic and therapeutic approaches, and outcomes of the diverse conditions associated with paediatric PH; and 3) characterise the outcomes, age of disease onset and disease progression or resolution, within each of the WSPH diagnostic categories.
Methods
Study design
We developed an extensive registry enrolling subjects with diverse forms of paediatric PH from eight well-established academic PH centres throughout North America that constitute the Pediatric Pulmonary Hypertension Network (PPHNet) [2]. The multicentre registry captured electronic reports of comprehensive clinical data from October 2014 to April 2020, which was partly supported by a National Heart, Lung, and Blood Institute-funded U01 program. The study protocol was approved by the institutional review boards of all participating centres, and all study participants, parents and/or guardians signed informed consent. Assent was obtained in patients according to institutional guidelines. Inclusion criteria included newborns, infants, older children and adolescents who were diagnosed prior to 18 years of age as having PH, which was defined according to established guidelines for paediatric PH from the American Heart Association (AHA)/American Thoracic Society (ATS) [5]. We followed the diagnostic criteria based on the AHA/ATS Consensus Guidelines Statement, which was developed from member sites of PPHNet along with others (table 1 in [5]). These criteria are similar to the WSPH criteria, which state that “the definition of PH in children has been the same as in adults, i.e. mean pulmonary arterial pressure (mPAP) ⩾25 mmHg. In the normal fetal circulation, PAP is similar to systemic pressure and rapidly falls after birth, achieving levels that are similar to the adult by 2–3 months of age. Due to variability in pulmonary haemodynamics during post-natal transition, paediatric PH has been defined as mPAP ⩾25 mmHg after 3 months of age” [4]. We further consider estimated PAP over half-systemic in infants younger than 3 months of age as meeting the criteria [4, 5]. We did not use the newer WSPH standard of mPAP >20 mmHg, as this change in recommendations came after the initiation of the current study. Echocardiographic or haemodynamic evidence of PH was required for all enrolled subjects. Although cardiac catheterisation was often performed as part of the diagnostic evaluation, it was not required for enrolment and differences between sites may reflect different practice patterns or scope of practice [5].
TABLE 1.
Demographic findings of the PPHNet Registry cohort by primary World Symposium on Pulmonary Hypertension (WSPH) group
| Overall (n=1475) | WSPH group | p-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Group 1 (n=663) | Group 2 (n=54) | Group 3 (n=720) | Group 4 (n=8) | Group 5 (n=30) | |||
|
| |||||||
| Site | <0.001 | ||||||
| Colorado | 222 (15) | 92 (14) | 2 (4) | 123 (17) | 0 (0) | 5 (17) | |
| Alberta | 94 (6) | 58 (9) | 6 (11) | 28 (4) | 1 (13) | 1 (3) | |
| Stanford | 101 (7) | 61 (9) | 6 (11) | 34 (5) | 0 (0) | 0 (0) | |
| Boston | 286 (19) | 90 (14) | 10 (19) | 178 (25) | 2 (25) | 6 (20) | |
| UCSF | 111 (8) | 58 (9) | 2 (4) | 47 (7) | 2 (25) | 2 (7) | |
| CHOP | 260 (18) | 109 (16) | 7 (13) | 139 (19) | 1 (13) | 4 (13) | |
| Vanderbilt | 139 (9) | 50 (8) | 5 (9) | 75 (10) | 2 (2) | 7 (23) | |
| Columbia | 262 (18) | 145 (22) | 16 (30) | 96 (13) | 0 (0) | 5 (17) | |
| Male | 758 (51) | 296 (45) | 31 (57) | 413 (57) | 3 (38) | 15 (50) | <0.001 |
| Age at diagnosis, years | |||||||
| Mean±SD | 2.9±4.7 | 4.3±5.4 | 4.3±5.6 | 1.1±2.8 | 14.2±5.8 | 6.3±6.3 | <0.001 |
| Median (IQR) | 0.5 (0.1–3.6) | 1.5 (0.3–6.9) | 1.4 (0.5–5.8) | 0.3 (0.0–0.6) | 16.0 (14.8–17.1) | 4.4 (0.4–12.1) | <0.001 |
| Hispanic | 0.24 | ||||||
| Yes | 235 (16) | 115 (17) | 8 (15) | 108 (15) | 1 (13) | 3 (10) | |
| No/Unknown | 1240 (84) | 548 (73) | 46 (85) | 612 (85) | 7 (88) | 27 (90) | |
| Race group | <0.001 | ||||||
| White | 899 (61) | 400 (60) | 36 (67) | 444 (62) | 4 (50) | 15 (50) | |
| Black | 191 (13) | 66 (10) | 6 (11) | 105 (15) | 3 (38) | 11 (37) | |
| Asian | 135 (9) | 84 (13) | 3 (6) | 45 (6) | 0 (0) | 3 (10) | |
| Other/Unknown | 250 (17) | 113 (17) | 9 (17) | 126 (18) | 1 (13) | 1 (3) | |
Data are presented as n (%), unless otherwise stated; column percentages for each characteristic total 100%. UCSF: University of California San Francisco; CHOP: Children’s Hospital of Philadelphia. The p-value tests equality of the distributions of each characteristic between Group 1 and Group 3 (p<0.05 is significant).
Although patient evaluation and care was directed by each site, clinical management generally followed the consensus recommendations and guidelines for paediatric PH as previously published [4, 5, 20]. This further included the approach of PPHNet sites to the management of PH in former preterm infants with chronic lung disease, which has been the subject of intense discussions and became the standard of care for our centres prior to this work [20].
Case report forms for data collection were developed in working groups among the PPHNet investigators, and included extensive clinical, demographic, haemodynamic data, laboratory information, drug therapies and outcomes, along with other parameters (as described in the following data collection and analysis section). Accuracy and completeness of data entry into the electronic database was monitored throughout the data collection period through biweekly data quality committee meetings, development and review of data quality queries, and coordination with site principal investigators.
Clinical data were entered into the secure web-based platform (Medidata; www.medidata.com) and included group assignment as based on designation according to the classification from the 5th WSPH held in Nice in 2013, which was established at the time of the initiation of the PPHNet Registry [2]. Each patient was assessed and classified by site investigators by primary aetiology into one of the five of the established groups: Group 1, pulmonary arterial hypertension (PAH); Group 2, PH associated with left heart disease; Group 3, PH associated with lung and hypoxia-related causes; Group 4, chronic thromboembolic PH (CTEPH); and Group 5, systemic and multifactorial disorders (figure 1).
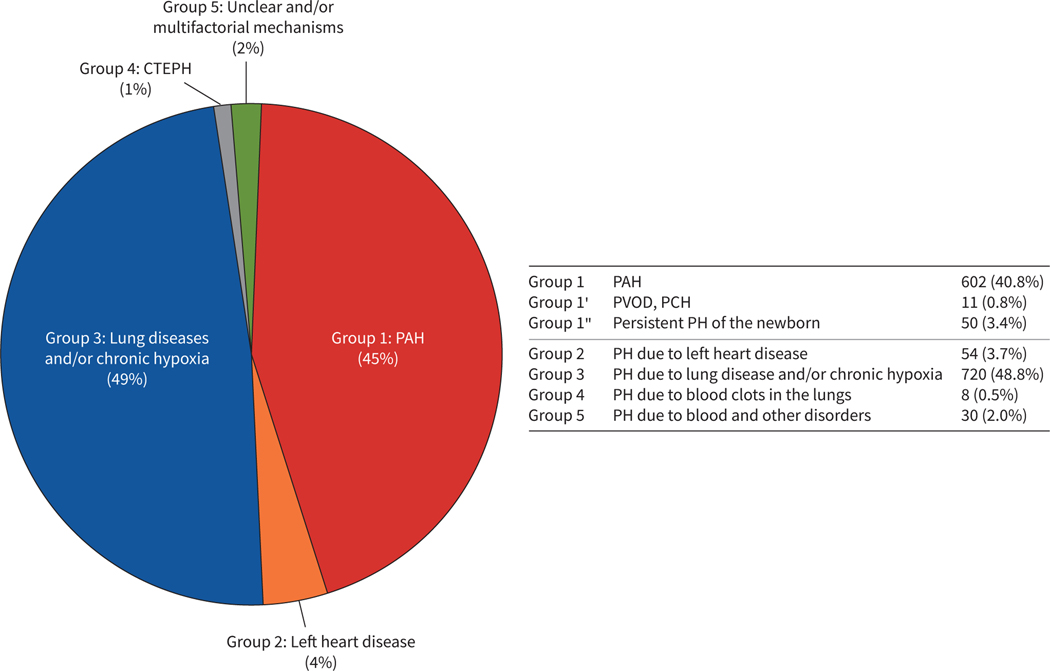
FIGURE 1.
Distribution of children with pulmonary hypertension (PH) according to the World Symposium on Pulmonary Hypertension (WSPH) classification system. The proportions of subjects with paediatric PH are depicted according to the 2013 WSPH classification (n=1475 subjects). Group 3 disorders (lung or hypoxia-related PH) constitute the largest proportion of subjects in the PPHNet Registry followed closely by Group 1 (pulmonary arterial hypertension (PAH)). CTEPH: chronic thromboembolic PH; PVOD: pulmonary veno-occlusive disease; PCH: pulmonary capillary haemangiomatosis.
Data collection and analysis
Descriptive statistics include counts (percentages) for categorical variables and mean with standard deviation or median (interquartile range (IQR)) for continuous variables. Patient and disease characteristics and management factors for WSPH Groups 1 versus 3 were compared using a Chi-squared test for categorical measures and the t-test or Wilcoxon rank sum test for continuous measures. Kaplan–Meier methodology was used to estimate the distribution of time from the diagnosis of PH to the composite outcome of death or lung transplant, with group comparisons by the log-rank test. The distribution of time to death or transplant using the entire cohort was estimated using risk set delayed-entry methodology. The Fine–Gray subdistribution proportional hazards model was used to estimate time from the diagnosis of PH to the earliest occurrence of a qualifying event in a competing risks framework (death versus lung transplant versus PH resolution). The competing risks analysis was restricted to the incident case cohort, defined as cases with a PH diagnosis on or after the inception of the PPHNet Registry (October 2014). For PH patients in WSPH Groups 1 and 3 (with the exception of Eisenmenger syndrome patients), PH resolution was defined as the cessation of PH-targeted medications (calcium channel blockers, endothelin receptor agonists, phosphodiesterase type 5 inhibitors, prostacyclin, inhaled nitric oxide and selexipag). For patients with PH-targeted medication stops and restarts, the latest date on record for cessation of PH-targeted medications was used as the event time, not the initial date of cessation. In addition, all tables in this article that depict Group 1 include Groups 1′ and 1″, unless otherwise noted. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.5.1 (www.r-project.org).
Results
Overall cohort
The analytic cohort was comprised of 1475 patients (table 1). The majority of patients were diagnosed between 2005 and 2017 (supplementary figure S1). There were 782 patients diagnosed prior to October 2014 (designated as the prevalent cohort) and 693 diagnosed in October 2014 or later (incident cohort). The cohort was 51% male, with a median (IQR) age at diagnosis of PH of 0.5 (0.1–3.6) years. The racial distribution was 61% White, 13% Black, 9% Asian and 17% Other/Unknown; 16% of patients were of Hispanic ethnicity. The range of enrolment of patients from the eight registry sites was 94–286 patients, and the distribution of subjects from each centre that comprise total enrolment within the primary categories of Group 1 (PAH) and Group 3 (lung or hypoxia-related PH) varied from 8% to 22% and 4% to 25%, respectively, which likely represent differences in scope of practice among the PH programmes. We recruited both incident and prevalent cases into the PPHNet Registry. Approximately half (53% (782 patients)) were diagnosed prior to the start of the Registry in October 2014 and the remaining 47% (693 patients) were diagnosed after the start of the Registry, with the vast majority enrolled within months of PH diagnosis. We examined the distribution of WSPH classification for the two groups and found no major systematic differences. With respect to persistent PH of the newborn (PPHN) (a total of 50 patients), 22% of those enrolled were prevalent (11 patients) and 78% were incident (39 patients). There were 58 Group 2 patients and 44% versus 56% of these cases were prevalent versus incident compared with the overall ratio of 53%/47%.
Figure 1 and tables 1–3 present the distribution of subjects enrolled in the PPHNet Registry according to the WSPH classification system. Patients categorised in Group 3 disease (lung or hypoxia-related PH) comprised 49% of subjects. Group 1 (PAH) was the second largest group, which included 45% of enrolled patients. Group 1 diseases were predominantly associated with congenital heart disease (CHD) (60%) and idiopathic PAH (23%). The other groups, including Group 2 (left heart disease; n=54) and Group 5 (systemic diseases; n=30), were far more infrequent and only eight subjects with Group 4 (CTEPH) were identified during the study period. As a result, further characterisation and analysis primarily focused on Group 1 and 3 disorders.
TABLE 3.
Diseases associated with paediatric pulmonary hypertension (PH) with World Symposium on Pulmonary Hypertension (WSPH) Group 3 as the primary classification
| Group 3: PH due to lung diseases and/or chronic hypoxia | 718 (100) |
| 3.1 Chronic obstructive pulmonary disease | 4 (0.6) |
| 3.2 Interstitial lung disease | 29 (4.0) |
| 3.2.1 Alveolar capillary dysplasia | 3 (0.4) |
| 3.2.2 Pulmonary interstitial glycogenosis | 6 (0.8) |
| 3.2.3 Surfactant protein deficiency | 4 (0.6) |
| 3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern | 13 (1.8) |
| 3.4 Sleep disordered breathing | 18 (2.5) |
| 3.5 Alveolar hypoventilation disorders | 3 (0.4) |
| 3.6 Chronic exposure to high altitude | 3 (0.4) |
| 3.7 Developmental abnormalities | 651 (90.4) |
| 3.7.1 Bronchopulmonary dysplasia | 320 (44.4) |
| 3.7.2 Congenital cystic adenomatoid malformation | 7 (1.0) |
| 3.7.3 Congenital diaphragmatic hernia | 256 (35.6) |
Data are presented as n (%); all percentages are based on WSPH Group 3 (n=718) as the denominator. Subclasses may include overlapping or concurrent WSPH classifications.
Characterisation by WSPH group
As shown in table 1, Group 3 patients were more often male compared with Group 1 patients (57% versus 45%; p<0.001). Median (IQR) age at diagnosis was younger (p<0.001) for Group 3 (0.3 (0–0.6) years) versus Group 1 patients (1.6 (0.3–6.9) years). White subjects constituted 61% of the overall cohort, which was higher than the proportion of Black (13%), Asian (9%) or Other/Unknown (17%). There was no association between WSPH group and Hispanic ethnicity; however, race was associated with WSPH group (p<0.001), even after adjusting for study site (p=0.008). Black race was more common in Group 3 than Group 1 (15% versus 10%; site-adjusted p=0.009) and Asian race was more common in Group 1 than Group 3 (13% versus 6%; site-adjusted p=0.001). There was no difference in the distribution of White subjects between Groups 1 and 3. Descriptive statistics for subgroups within Groups 1 and 3 (the largest primary classifications) are provided in tables 2 and 3, respectively. CHD constituted nearly 60% of Group 1 disease, with idiopathic and heritable PAH diagnosed in 23% and 6% of Group 1 patients, respectively (table 2). The majority of Group 1 patients were characterised by: Eisenmenger syndrome in 4.1%, systemic-to-pulmonary shunts in 7.7% and post-operative PAH in 19.1% (combined partial and total repair). Subjects with PPHN (Group 1″) represented only 7.6% of the study population.
TABLE 2.
Diseases associated with paediatric pulmonary hypertension (PH) patients with World Symposium on Pulmonary Hypertension (WSPH) Groups 1, 1′ and 1″ as primary classification
| Group 1: Pulmonary arterial hypertension (PAH) | 602 (90.8) |
| 1.1 Idiopathic | 148 (22.3) |
| 1.2 Heritable | 40 (6.1) |
| 1.3 Drug and toxins induced | 2 (0.3) |
| 1.4 Associated with PAH | 410 (61.8) |
| 1.4.1 Connective tissue disease | 9 (1.4) |
| 1.4.2 HIV infection | 0 (0) |
| 1.4.3 Portal hypertension | 6 (0.9) |
| 1.4.4 Congenital heart disease# | 390 (58.8) |
| 1.4.4.1 Type | 312 (47.1) |
| 1.4.4.1.1 Simple pre-tricuspid shunts | 93 (14.0) |
| 1.4.4.1.1.1 Atrial septal defect | 78 (11.7) |
| 1.4.4.1.1.2 Total or partial unobstructed anomalous pulmonary venous return | 15 (2.3) |
| 1.4.4.1.2 Simple post-tricuspid shunts | 112 (16.9) |
| 1.4.4.1.2.1 Ventricular septal defect | 77 (11.6) |
| 1.4.4.1.2.2 Patent ductus arteriosus | 54 (8.1) |
| 1.4.4.1.3 Combined shunts | 35 (5.3) |
| 1.4.4.1.4 Complex congenital heart disease | 121 (18.2) |
| 1.4.4.1.4.1 Complete atrioventricular septal defect | 52 (7.8) |
| 1.4.4.1.4.2 Truncus arteriosus | 3 (0.5) |
| 1.4.4.1.4.3 Single ventricle physiology with unobstructed pulmonary blood flow | 20 (3.0) |
| 1.4.4.1.4.4 Transposition of the great arteries | 16 (2.4) |
| 1.4.4.2 Direction of shunt | 99 (14.9) |
| 1.4.4.2.1 Predominantly systemic-to-pulmonary | 51 (7.7) |
| 1.4.4.2.2 Predominantly pulmonary-to-systemic (Eisenmenger’s) | 27 (4.1) |
| 1.4.4.2.3 Bidirectional | 19 (2.9) |
| 1.4.4.3 Repair status | 170 (25.6) |
| 1.4.4.3.1 Unoperated | 43 (6.5) |
| 1.4.4.3.2 Partial repair | 18 (2.7) |
| 1.4.4.3.3 Complete repair | 109 (16.4) |
| 1.4.5 Schistosomiasis | 0 (0) |
| Group 1′: Pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis | 11 (1.7) |
| Group 1″: Persistent PH of the newborn | 50 (7.6) |
Data are presented as n (%); all percentages are based on WSPH Groups 1, 1′ and 1″ (n=663) as the denominator. Category counts may be larger than the sum of counts for associated subclasses, due to the classification of disease for some patients only at a high-level subgroup.
: subclasses for congenital heart disease are not mutually exclusive, as more than one heart defect may be associated with a single patient.
Within the Group 3 category, infants with developmental lung disease, especially bronchopulmonary dysplasia (BPD) and congenital diaphragmatic hernia (CDH), were the largest subgroups, representing 22% and 17% of all PPHNet Registry subjects, respectively (44% and 36% within the Group 3 cohort, respectively) (table 3). Although subjects with interstitial lung disease accounted for 4% of patients in Group 3, no patients with cystic fibrosis were enrolled, which may reflect the later onset of PH outside of the paediatric age range in this important subgroup. Secondary WSPH classifications are displayed in supplementary table S3.
Genetic causes of identified cases of heritable PAH (table 4) and genetic syndromes associated with PH (table 5 and supplementary table S1) are provided. Down syndrome was the most common diagnosis among the genetic syndromes (11% of all PPHNet Registry subjects) and BMPR2 gene mutation was the most frequent cause of the known heritable cases (17 out of 40 patients; or 43% of identified cases with heritable disease in the PPHNet Registry).
TABLE 4.
Genetic findings reported as the primary World Symposium on Pulmonary Hypertension (WSPH) classification
| Group 1: Pulmonary arterial hypertension (PAH) | |
| 1.2 Heritable | 40 (100) |
| 1.2.1 BMPR2 | 17 (43) |
| 1.2.2 ALK1/ENG (with or without hereditary haemorrhagic telangiectasia) | 5 (13) |
| 1.2.3 SMAD9 | 0 (0) |
| 1.2.4 CAV1 | 1 (3) |
| 1.2.5 KCNK3 | 1 (3) |
| 1.2.6 Unknown | 6 (15) |
| 1.2.7 Other | 10 (25) |
| TBX4 | 6 (15) |
| GDF2 mutation | 2 (5) |
| Other/family history | 2 (5) |
Data are presented as n (%); all percentages are based WSPH Group 1.2 heritable disease (n=40) as the denominator.
TABLE 5.
Genetic syndromes associated with paediatric pulmonary hypertension
| Overall (n=1475) | WSPH group | p-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Group 1 (n=663) | Group 2 (n=54) | Group 3 (n=720) | Group 4 (n=8) | Group 5 (n=30) | |||
|
| |||||||
| Down | 158 (10.7) | 116 (17.5) | 2 (3.7) | 33 (4.6) | 0 (0) | 7 (23.3) | <0.001 |
| DiGeorge | 21 (1.4) | 15 (2.3) | 1 (1.9) | 5 (0.7) | 0 (0) | 0 (0) | 0.015 |
| Noonan | 7 (0.5) | 3 (0.5) | 1 (1.9) | 2 (0.3) | 0 (0) | 1 (3.3) | 0.59 |
| Russell-Silver | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 0.34 |
| Trisomy 18 | 8 (0.6) | 7 (1.1) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 0.027 |
| CHARGE | 7 (0.5) | 2 (0.3) | 0 (0) | 4 (0.6) | 0 (0) | 1 (3.3) | 0.46 |
| VACTERL | 4 (0.3) | 0 (0) | 1 (1.9) | 2 (0.3) | 0 (0) | 1 (3.3) | 0.17 |
| Scimitar | 3 (0.2) | 2 (0.3) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 0.53 |
| Prader-Willi | 4 (0.3) | 2 (0.3) | 0 (0) | 2 (0.3) | 0 (0) | 0 (0) | 0.95 |
| Other # | 36 (2.4) | ||||||
Data are presented as n (%), unless otherwise stated; percentages represent the percentage of patients with the listed syndrome. WSPH: World Symposium on Pulmonary Hypertension.
other disorders are listed in supplementary table S1. The p-value tests equality of the percentage of patients with each syndrome in the Group 1 versus Group 3 cohorts (p<0.05 is significant).
Associations with WSPH group
Differences in diagnostic evaluations were noted between the WSPH groups (supplementary table S2). Group 1 subjects had higher rates of ECG studies (76% versus 51%; p<0.001) and cardiac catheterisations at PH diagnosis than Group 3 patients (70% versus 30%; p<0.001), whereas Group 1 subjects more often had pulmonary function testing (7% versus 2%; p<0.001) and chest computed tomography scans (26% versus 13%; p<0.001). At cardiac catheterisation, median (IQR) pulmonary vascular resistance index was higher in Group 1 (9.0 (5.0–16.1) WU) than in Group 3 subjects (5.8 (4.3–8.3) WU; p<0.01) (supplementary table S2). Assessment of 6-min walk distance (6MWD) was less common in Group 3 (1%) than Group 1 (12%) patients, which likely reflects age differences between groups.
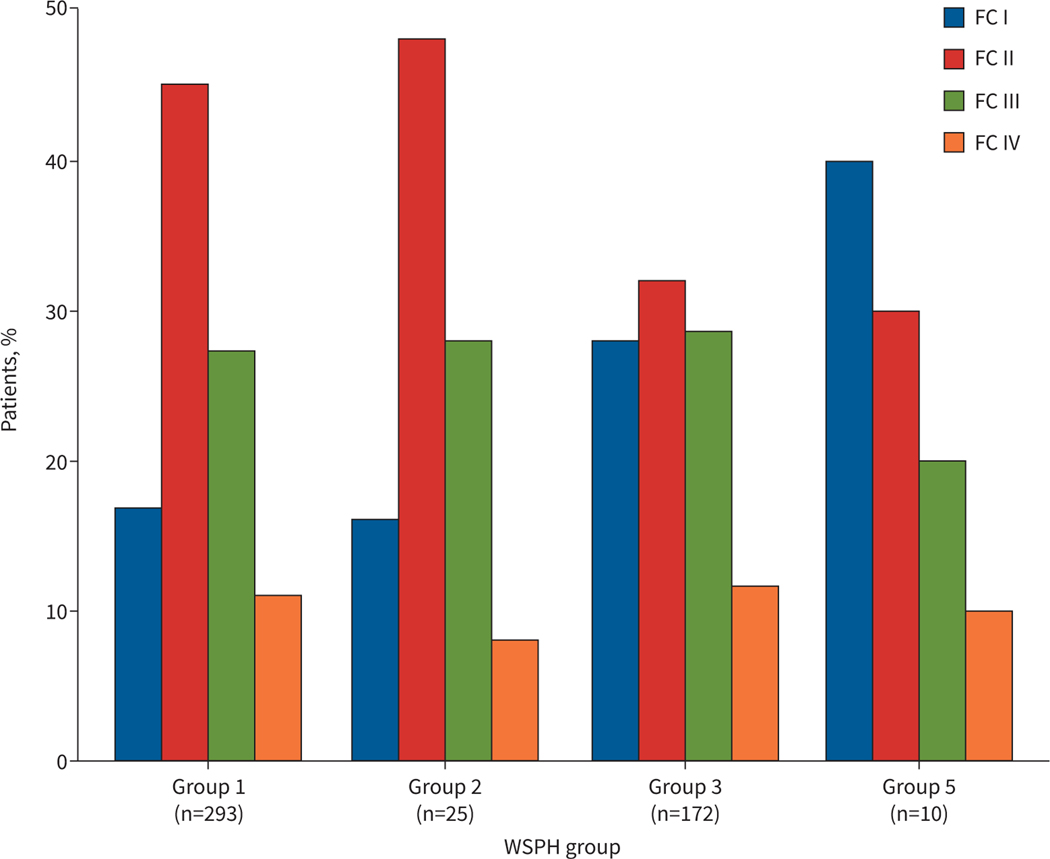
Functional class (FC) at diagnosis as assessed by the standard WHO definitions and by age-related recommendations (the Pediatric FC [21]) are shown in figure 2 and supplementary table S4. At diagnosis, the distributions of FC for the 503 patients evaluated by WHO criteria differed for Groups 1 and 3 (p=0.011), primarily due to the lower percentage of patients in FC I (17% versus 28%, respectively). For both groups, over one-third were in FC groups III and IV (38% and 40%, respectively). According to assessment with the Panama Pediatric FC criteria [22], which was used for the evaluation of 791 subjects, 58% of the overall cohort presented as meeting FC IIIa, IIIb and IV criteria, which was more common (p<0.001) in Group 3 (68%) than Group 1 (48%) (supplementary table S4).
FIGURE 2.
World Health Organization (WHO) Functional Class (FC) at the time of pulmonary hypertension diagnosis (n=503). The distributions of WHO FC for World Symposium on Pulmonary Hypertension (WSPH) Group 1 and Group 3 differ (p=0.011). Group 4 is omitted due to few patients (n=3).
Medication use in the overall cohort and according to WSPH group is shown in table 6. Medications are reported from the time of enrolment into the PPHNet Registry, at a median of 1.3 years following diagnosis of PH. Group 3 patients were less likely to be treated with PH medications than Group 1 (50% versus 78%; p<0.001). The proportions of patients on monotherapy were similar (37% for Group 3 and 34% for Group 1), but more subjects in Group 1 were treated with combination therapy (table 3). Group 1 patients who received PH medications were more likely to be treated with endothelin receptor antagonists (34% versus 10%; p<0.001) and prostacyclin or prostacyclin derivatives (27% versus 6%; p<0.001). At enrolment, 62% of Group 1 and 47% of Group 3 patients were prescribed PH-associated medications (p<0.001). Among those taking other medications, aspirin use was more common in Group 1 (16% versus 3%; p<0.001), as well as coumadin (10% versus 1%; p<0.001).
TABLE 6.
Respiratory therapy and medications at time of enrolment for the entire cohort and by primary World Symposium on Pulmonary Hypertension (WSPH) group
| Overall (n=1475) | WSPH group# | p-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Group 1 (n=663) | Group 2 (n=54) | Group 3 (n=720) | Group 4 (n=8) | Group 5 (n=30) | |||
|
| |||||||
| Median (IQR) time since PH diagnosis, years | 1.3 (0.2–4.8) | 1.6 (0.1–5.6) | 0.8 (0–4.1) | 1.2 (0.3–4.0) | 0.2 (0–3.8) | 1.1 (0.2–4.3) | 0.45 |
| Any respiratory treatment ¶ | 945 (64) | 363 (55) | 22 (41) | 537 (75) | 5 (63) | 18 (60) | <0.001 |
| On any PH medication | 932 (63) | 515 (78) | 34 (63) | 361 (50) | 4 (50) | 18 (60) | <0.001 |
| CCB | 71 (5) | 46 (7) | 1 (2) | 19 (3) | 0 (0) | 5 (17) | <0.001 |
| ERA | 313 (21) | 227 (34) | 5 (9) | 74 (10) | 2 (25) | 5 (17) | <0.001 |
| PDE5 inhibitor | 819 (56) | 447 (68) | 32 (59) | 323 (45) | 2 (25) | 15 (50) | <0.001 |
| Prostacyclin | 232 (16) | 180 (27) | 5 (9) | 46 (6) | 0 (0) | 1 (3) | <0.001 |
| Inhaled nitric oxide | 60 (4) | 30 (5) | 3 (6) | 25 (4) | 2 (25) | 0 (0) | 0.31 |
| Selexipag | 1 (0.1) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.30 |
| PH medications | <0.001 | ||||||
| Monotherapy | 526 (36) | 226 (34) | 23 (43) | 263 (37) | 3 (38) | 11 (37) | |
| Combination | 406 (28) | 289 (44) | 11 (20) | 98 (14) | 1 (14) | 7 (23) | |
| None | 542 (37) | 147 (22) | 20 (37) | 359 (50) | 4 (50) | 12 (40) | |
| Monotherapy | 526 | 226 | 23 | 263 | 3 | 11 | 0.005 |
| CCB | 29 (6) | 16 (7) | 1 (4) | 10 (4) | 0 (0) | 2 (18) | |
| ERA | 32 (6) | 17 (8) | 0 (0) | 13 (5) | 1 (33) | 1 (9) | |
| PDE5 inhibitor | 425 (81) | 167 (74) | 21 (91) | 228 (87) | 1 (33) | 8 (73) | |
| Prostacyclin | 18 (3) | 13 (6) | 1 (4) | 4 (2) | 0 (0) | 0 (0) | |
| Inhaled nitric oxide | 22 (4) | 13 (6) | 0 (0) | 8 (3) | 1 (33) | 0 (0) | |
| Selexipag | 0 | 0 | |||||
| On any PH-associated medication | 805 (55) | 409 (62) | 40 (74) | 337 (47) | 4 (50) | 15 (50) | <0.001 |
| PGE1 | 13 (1) | 2 (0.3) | 0 (0) | 11 (2) | 0 (0) | 0 (0) | 0.018 |
| Dopamine | 34 (2) | 15 (2) | 1 (2) | 18 (3) | 0 (0) | 0 (0) | 0.78 |
| Milrinone | 32 (2) | 13 (2) | 1 (2) | 18 (3) | 0 (0) | 0 (0) | 0.50 |
| Aspirin | 144 (10) | 103 (16) | 15 (28) | 24 (3) | 0 (0) | 2 (7) | <0.001 |
| Coumadin | 78 (5) | 63 (10) | 5 (9) | 5 (0.7) | 3 (38) | 2 (7) | <0.001 |
| Digoxin | 91 (6) | 59 (9) | 9 (17) | 21 (3) | 0 (0) | 2 (7) | <0.001 |
| Diuretics | 565 (38) | 261 (39) | 34 (63) | 259 (36) | 1 (13) | 10 (33) | 0.19 |
| Iron supplement | 155 (11) | 55 (8) | 4 (7) | 94 (13) | 0 (0) | 2 (7) | 0.003 |
| Plavix | 4 (0.3) | 4 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.037 |
| Thyroid replacement | 65 (4) | 35 (5) | 3 (6) | 24 (3) | 0 (0) | 3 (10) | 0.07 |
Data are presented as n (%), unless otherwise stated; percentages represent the percentage of patients prescribed the listed treatment within each WSPH group. IQR: interquartile range; PH: pulmonary hypertension; CCB: calcium channel blocker; ERA: endothelin receptor agonist; PDE5: phosphodiesterase type 5; PGE1: PGE1: prostaglandin E1.
medications data were missing for n=1 patient in Group 1, and respiratory therapy data were missing for n=1 patient in Group 1 and n=1 patient in Group 3;
respiratory treatments are defined as supplemental oxygen, airway medications and noninvasive or invasive respiratory support. The p-value reflects the comparison of the percentage of patients on each medication or tests equality of the distributions of the medication types for the Group 1 versus Group 3 cohorts (p<0.05 is significant).
Clinical outcomes
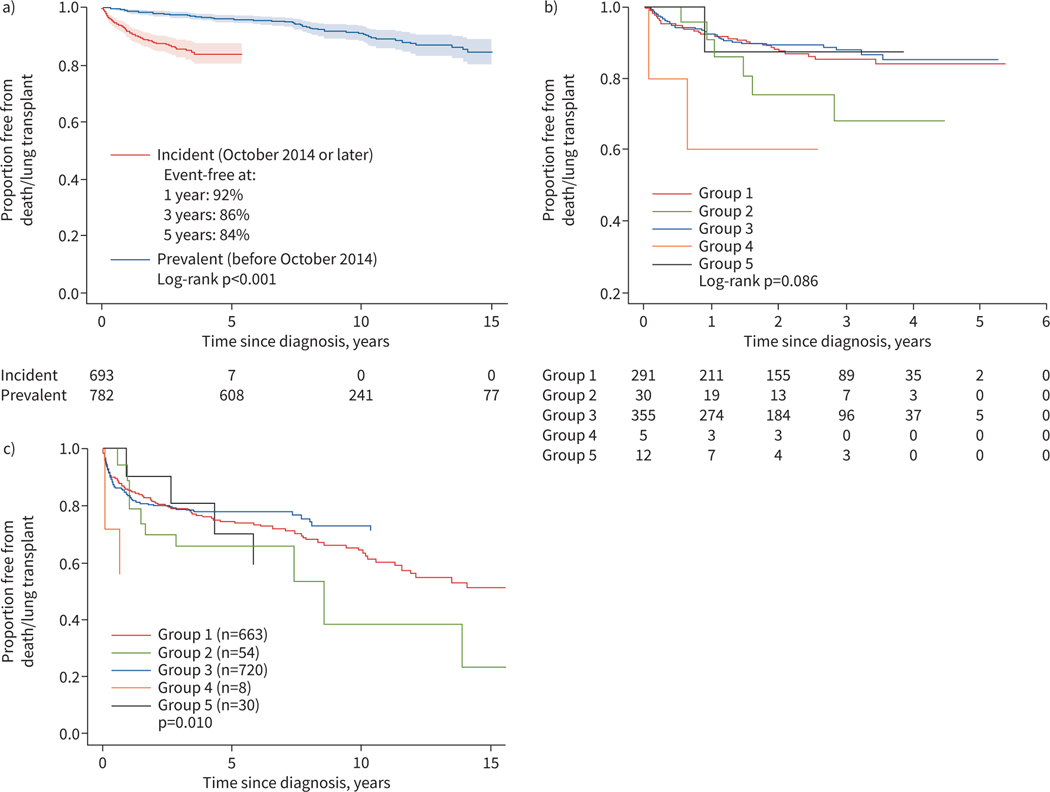
The median (IQR) follow-up duration of the transplant-free survivors was 4.1 (2.0–7.4) years. We first examined lung transplant-free survival for differences according to the inception date of the PPHNet Registry (October 2014). The composite incidence of death or lung transplant was lower (p<0.001) for the prevalent cohort (i.e. those diagnosed prior to October 2014), suggesting the expected survivor bias in enrolment, compared with the incident cohort (diagnosed later) (figure 3a). However, the distribution of WSPH groups was similar for the two cohorts. In the incident cohort, which had a median follow-up of 2 years, overall transplant-free survival was 92% (95% CI 90–94%) at 1 year and 84% (95% CI 80–87%) at 5 years. In the incident cohort, the difference in the distribution of death/transplant by WSPH group did not achieve statistical significance (p=0.09) (figure 3b). At 3 years post-diagnosis, transplant-free survival was 85% for Group 1 and 88% for Group 3. Group 2 (PH due to left heart disease, n=54) had 68% (95% CI 41–85%) transplant-free survival at 3 years. Based on the entire cohort with extended follow-up, we found a significant difference (p=0.010) in the distributions of death/transplant by WSPH group (figure 3c).
FIGURE 3.
Freedom from death/lung transplant for a) prevalent versus incident cases (defined as pulmonary hypertension diagnosis before versus after the inception date of the PPHNet Registry), b) by World Symposium on Pulmonary Hypertension (WSPH) group based on the incident cohort and c) by WSPH group for the full cohort. Follow-up is truncated at 15 years. Four deaths in the diagnosis prior to 2014 cohort occurred after 15 years and are not shown (n=3 Group 1 patients and n=1 Group 2 patient). Shaded regions denote pointwise 95% confidence bands.
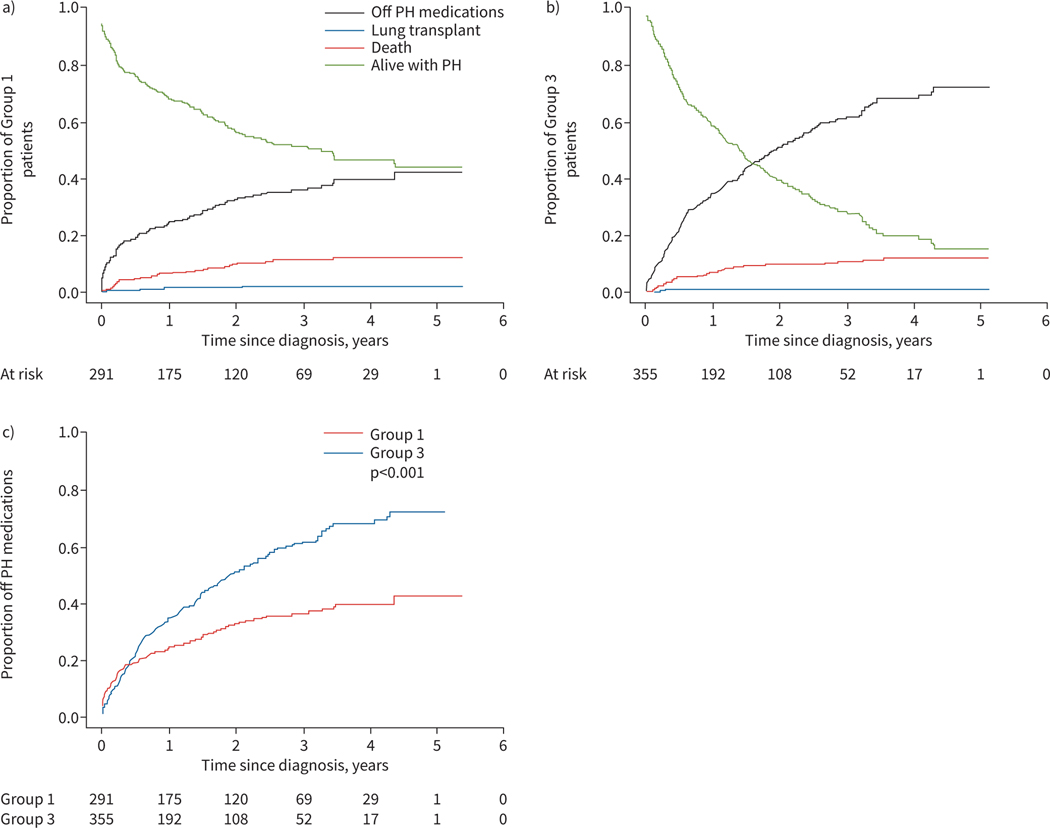
A competing risks analysis based on the Groups 1 and 3 incident cohort was performed. Outcomes, which included death, lung transplant and resolved PH (defined as cessation of PH medications; see Methods), are depicted in figure 4. For Group 1, 5-year event rates were 12% mortality, 2% lung transplant, 42% PH resolution and 44% alive transplant-free with PH. As shown, the patterns for Groups 1 and 3 disease differ with respect to disease resolution, with Group 3 subjects having a higher rate of PH disease resolution, as defined by being alive without requiring PH-targeted medications (resolution hazard ratio 3.1, 95% CI 2.6−3.7; p<0.001) than Group 1 patients. By 5 years post-diagnosis, event rates for Group 3 were 12% mortality, 1% lung transplant, 72% PH resolution and 15% alive transplant-free with PH. Figure 3a displays transplant-free survival for the entire PPHNet Registry cohort. While an underestimate of total events, there is a pattern of greater late mortality in Group 1 patients compared with Group 3 patients.
FIGURE 4.
Display of competing risks estimates including death, lung transplant, alive with pulmonary hypertension (PH) and alive off PH medications for the PPHNet Registry World Symposium on Pulmonary Hypertension (WSPH) a) Group 1 and b) Group 3 incident cohort. c) In comparison with Group 1 disease, longitudinal follow-up of patients within Group 3 demonstrates a pattern of greater rates of cessation of PH medications with lower mortality with time.
Examination of the entire cohort revealed that Group 1 patients who had PH disease resolution compared with those without resolution were diagnosed at an earlier age with PH (median 0.3 versus 3.6 years), more likely to have PPHN (Group 1″; 23% versus 2%) and were more often in Pediatric FC I or II (59% versus 48%). They did not differ with respect to the presence of CHD or with respect to gestational age. The Group 3 patients who had PH disease resolution compared with those without resolution were diagnosed with PH at a somewhat earlier age (median 0.2 versus 0.4 years), were more likely to have CDH (41% versus 25%) and were more likely to be in WHO FC I or II (69% versus 48%).
Discussion
Despite advances in our understanding of its pathobiology and the growing availability of PH-specific drug therapies, PH and related PVDs in children continue to cause significant morbidity and mortality. Paediatric PH is highly heterogeneous and has been understudied. Relatively little is known about the basic disease mechanisms, natural history, long-term outcomes, age-appropriate clinical end-points and optimal therapeutic strategies for children with PH and clinically important subsets [2–5]. To address these critical problems and to assess disease phenotypes, we developed an extensive patient registry of children with PH at large interdisciplinary programmes and characterised PH-related diseases as categorised by the 5th WSPH classification system from 2013. Although slightly modified during the 6th WSPH in 2018 [4], the system outlined in the previous symposium was used for this study due to the timing of the initiation of the PPHNet Registry. In contrast with past registry studies [6–11], we report that WSPH Group 3 disease (lung or hypoxia-associated PH) constitutes nearly half of the cases of paediatric PH, reflecting a greater recognition of preterm infants at risk for BPD, the impact of CDH and Down syndrome, and genetic causes of developmental lung diseases. This large dataset confirms substantial differences in the types and outcomes of PH between paediatric and adult patients, justifying discussions for the need to develop a new paediatric-specific classification system for PH patients.
We found important differences in demographic factors and clinical outcomes based on PH subtype, including better transplant-free survival and disease resolution, as defined by subjects who are alive off PH medications, in the Group 3 cohort in comparison with Group 1 disease. Subjects with Group 3 disease tended to be younger at diagnosis, were more often male and more frequently Black race than Group 1 children. Rates of cardiac catheterisation were lower in Group 3 than Group 1 children (30% versus 70%) and Group 3 subjects were more often treated with monotherapy versus combined PH-targeted therapies in comparison with Group 1 patients. Overall, these findings highlight the striking contribution of lung-related disease to the spectrum of paediatric PH and differences of treatment approach, and given the high prevalence of this PH type, underscore the important need for focused research and multicentre randomised trials of this understudied group.
High-quality patient registries of children with diverse forms of PH are important to better define the epidemiology and clinical features of paediatric PH. A helpful classification system would serve to enhance approaches to patient care and research that will improve the early identification or anticipation of PH in at-risk subjects; provide the correct primary diagnosis; guide the effective evaluation and therapies; enable rapid recognition of critical features, common comorbidities and disease modifiers; and develop disease-specific strategies. Improving the recognition of disease phenotypes provides a basis for establishing common approaches for understanding and treating subgroups of patients with PH to meet critical challenges of providing personalised care, and to provide a useful, pragmatic, consistent and easy to understand system that can be readily applied by clinicians from multiple sites.
Based on concerns that the WSPH classification system, largely an adult-focused system, may not sufficiently identify the phenotypic heterogeneity of childhood PH or meet the needs of paediatric providers, the Pediatric Task Force of the Pulmonary Vascular Research Institute (PVRI) proposed a novel classification that may prove useful as a paediatric-specific system [22]. The goals of the PVRI Panama Classification System are to highlight the phenotypic heterogeneity of PH and related PVDs throughout childhood and the impact on diagnosis, treatment and research. However, whether this system has greater clinical utility than the recently updated version of the WSPH classification system as recently modified by the Pediatric Task Force of the WSPH is uncertain [4].
In the Spanish paediatric PH registry, 61% of patients had PAH (Group 1), 14% had PH with left heart disease (Group 2), 18% had PH associated with respiratory disease (Group 3), 1% had CTEPH (Group 4) and 4.5% had multifactorial factors (Group 5), including metabolic diseases [7]. Of significance is that 31% of the patients had PH associated with multiple aetiologies, suggesting the need for more accurate phenotyping beyond traditional classification schemes alone. This study showed that independent risk factors for mortality included aetiology group, age at diagnosis younger than 2 years, advanced FC and high right atrial pressure as assessed by cardiac catheterisation. Among several unique observations from the Spanish registry is the important association of CHD in patients with lung disease or left heart disease with worse outcomes; notably, Group 2 PAH was not included in the Tracking Outcomes and Practice in Pediatric Pulmonary Hypertension (TOPP) registry [6]. As observed in other registries, patients with Eisenmenger syndrome or those with left-to-right shunts had better survival than patients with PAH and coincidental CHD or post-operative PAH. The PPHNet Registry has also confirmed previous findings that patients with Shone’s complex or isolated pulmonary vein stenosis have a very poor prognosis [6–9].
The development of high-quality patient registries represents only the beginning of the extensive work that must be done to more precisely phenotype and characterise the natural history, disease course, response to therapies and late outcomes of paediatric PH. Rigorous registries provide an essential foundation for supporting deeper explorations of patient phenotypes and better identification of patient populations for clinical trials, as well as establishing useful study end-points and meaningful surrogates to enhance understanding and development of novel therapeutic strategies. These issues reflect the need to include collaborations with multidisciplinary teams (e.g. pulmonologists, neonatologists, intensivists, haematologists and others) to cast a far larger net that will more accurately reflect the epidemiology and nature of paediatric PH.
Previous publications from the few existing registries have begun to identify differences in the aetiology of PAH between adults and children [6–11]. Past reports from paediatric registries have primarily emphasised that WSPH Group 1 disorders, including idiopathic PAH, heritable PAH and PAH associated with CHD, constitute the majority of cases. For example, of the 362 patients included in the pharmaceutical-sponsored TOPP registry, 88% had PAH, including 57% with idiopathic PAH or familial PAH and 36% with PH associated with CHD [6]. Only 42 of the enrolled patients (12%) had PH associated with respiratory diseases, with BPD being the most frequent disorder. This is likely due to the requirement for cardiac catheterisation, as well as bias in registry enrolment due to scope of practice.
A potential limitation of these data is the use of the 5th WSPH classification system from 2013, rather than the most recent revision that followed the 6th WSPH in 2018, which was held after the initiation of this study. Although there may be some changes in disease classification [4], these specific changes are unlikely to significantly alter the findings of this report and allow for more direct comparisons with previously published registry data, as Group 1 and Group 3 diagnoses remained consistent between the two versions [4]. As this study required identification and consent for enrolment, not all patients enrolled at study sites represent sequential cases, which may have contributed to enrolment bias. Finally, these data further reflect the bias of case referrals to PH programmes for care at each site, e.g. many cases of milder forms of PPHN are managed without consultation.
Overall, we provide a characterisation of the scope of paediatric PH through an established patient registry as provided from a network of multidisciplinary academic centres that collectively provide the largest clinical experience with paediatric PH to date. This large dataset confirms substantial differences in the types and outcomes of PH between paediatric and adult patients, justifying discussions for the need to develop a new paediatric-specific classification system for PH patients.
As with previous registries, limitations include biased or selective enrolment that reflects differences in clinical practice, which raises questions regarding our ability to cast a sufficiently “wide net” as enrolled subjects depended upon multiple factors, including practice pattern, role of consultant services with PH and others. In addition, both prevalent and incident cases were enrolled, which will be useful for future association analyses, but we have restricted our event rate estimates in this article to the incident cohort. As discussed, cardiac catheterisation was performed in most but not all subjects, which better reflects current practice in many centres, especially in the setting of premature infants with lung disease (BPD) and developmental lung disorders, such as CDH, alveolar capillary dysplasia and others. Although academic programmes included within PPHNet provide interdisciplinary groups of investigators at each site, practice variations exist. There is a persistent need to more precisely define disease subgroups through computable phenotyping and/or use of biological markers (“-omics”) to provide endotypes within groups or to provide unique and distinct clusters between/among groups. We speculate that combining data from established patient registries with novel strategies that include data extraction from electronic health records and patient-reported outcomes will likely further enhance our ability to perform deeper phenotyping and personalised therapies in the near future.
Supplementary Material
Acknowledgements:
We are grateful for contributions of other members of the Pediatric Pulmonary Hypertension Network (PPHNet), including: Allan Everett and Lew Romer (Johns Hopkins University Medical School), Delphine Yung (University of Washington School of Medicine), Stephanie Handler (University of Wisconsin Milwaukee), Nidhy Varghese (Texas Children’s Hospital), Russel Hirsch (Cincinnati Children’s Hospital and Medical Center), Robin Steinhorn (University of California San Diego), Rolf Berger (University Medical College, Groningen, The Netherlands), Wendy Chung (Columbia University School of Medicine), David Cornfield (Stanford University School of Medicine), Csaba Galambos (University of Colorado) and Minmin Lu (Boston Children’s Hospital).
Conflict of interest:
S.H. Abman has nothing to disclose. M.P. Mullen reports grants from the National Heart, Lung, and Blood Institute, during the conduct of the study; personal fees from Actelion, outside the submitted work. L.A. Sleeper reports grants from the National Heart, Lung, and Blood Institute (subcontract from University of Colorado), during the conduct of the study. E.D. Austin has nothing to disclose. E.B. Rosenzweig reports grants from the National Institutes of Health, during the conduct of the study. J.P. Kinsella has nothing to disclose. The University of Colorado contracts with Actelion, Bayer, Gilead and United Therapeutics for D. Ivy to be a consultant and preform clinical research trials. R.K. Hopper has nothing to disclose. J.U. Raj has nothing to disclose. J. Fineman has nothing to disclose. R.L. Keller reports grants from the National Heart, Lung, and Blood Institute, during the conduct of the study. A. Bates has nothing to disclose. U.S. Krishnan has nothing to disclose. C.M. Avitabile has nothing to disclose. A. Davidson has nothing to disclose. M.D. Natter reports grants from the National Heart, Lung, and Blood Institute, during the conduct of the study. K.D. Mandl reports no specific conflicts, however in the interest of full disclosure, reports personal fees from Merck, and philanthropy from Eli Lily to his lab, during the conduct of the study.
Support statement:
This work was supported by grant U01 HL12118 (Data Fusion: A Sustainable, Open Source Registry Advancing Pediatric Pulmonary Vascular Disease Research; K.D. Mandl and S.H. Abman) from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or NIH. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Rosenzweig EB, Widlitz AC, Barst RJ. Pulmonary arterial hypertension in children. Pediatr Pulmonol 2004; 38: 2–22. [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Raj U. Towards improving the care of children with pulmonary hypertension: rationale for developing a Pediatric Pulmonary Hypertension Network. Prog Pediatr Cardiol 2009; 27: 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins IM, Moore TM, Blaisdell CJ, et al. National Heart, Lung, and Blood Institute Workshop: improving outcomes for pulmonary vascular disease. Circulation 2012; 125: 2165–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019; 53: 1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132: 2037–2099. [DOI] [PubMed] [Google Scholar]
- 6.Berger RM, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Cerro Marin MJ, Rotes AS, Ogando AR, et al. Assessing pulmonary hypertensive vascular disease in childhood: data from the Spanish registry. Am J Respir Crit Care Med 2014; 190: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 8.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001–2006. Heart 2009; 95: 312–317. [DOI] [PubMed] [Google Scholar]
- 9.Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012; 125: 113–122. [DOI] [PubMed] [Google Scholar]
- 10.van Loon RL, Roofthooft MT, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterisation during the period 1991 to 2005. Circulation 2011; 124: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 11.Moledina S, Hislop AA, Foster H, et al. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart 2010; 96: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 12.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43: 5S–12S. [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019; 53: 1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erzurum S, Rounds SI, Stevens T, et al. Strategic plan for lung vascular research: an NHLBI-ORDR Workshop Report. Am J Respir Crit Care Med 2010; 182: 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman J, Rich S, Abman S, et al. Enhancing insights into pulmonary vascular disease (PVD) through a precision medicine approach. A joint NHLBI–Cardiovascular Medical Research and Education Fund Workshop Report. Am J Respir Crit Care Med 2017; 195: 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin ED, West J, Loyd JE, et al. Molecular medicine of pulmonary arterial hypertension from population genetics to precision medicine and gene editing. Am J Respir Crit Care Med 2017; 195: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015; 372: 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haendel MA, Chute CG, Robinson PN. Classification, ontology, and precision medicine. N Engl J Med 2018; 379: 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dweik RA, Rounds S, Erzurum SC, et al. An official American Thoracic Society statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med 2014; 189: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan U, Feinstein J, Adatia I, et al. Evaluation and management of pulmonary hypertension in children with bronchopulmonary dysplasia. J Pediatrics 2017; 188: 24–34. [DOI] [PubMed] [Google Scholar]
- 21.Lammers AE, Adatia I, Del Cerro MJ, et al. Functional classification of pulmonary hypertension in children: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ 2011; 1: 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Cerro MJ, Abman S, Diaz G, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ 2011; 1: 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.