Abstract
Background
The use of omics technologies in human transfusion medicine has improved our understanding of the red blood cell (RBC) storage lesion(s). Despite significant progress towards understanding the storage lesion(s) of human RBCs, a comparison of basal and post-storage RBC metabolism across multiple species using omics technologies has not yet been reported, and is the focus of this study.
Materials and methods
Blood was collected in a standard bag system (CPD-SAG-Mannitol) from dogs (n=8), horses, bovines, and donkeys (n=6). All bags were stored at 4°C for up to 42 days (i.e., the end of the shelf life in Italian veterinary clinics) and sampled weekly for metabolomics analyses. In addition, data comparisons to our ongoing Zoomics project are included to compare this study’s results with those of non-human primates and humans.
Results
Significant interspecies differences in RBC metabolism were observed at baseline, at the time of donation, with bovine showing significantly higher levels of metabolites in the tryptophan/kynurenine pathway; dogs showing elevated levels of high-energy compounds (especially adenosine triphosphate and S-adenosyl-methionine) and equine (donkey and horse) RBCs showing almost overlapping phenotypes, with the highest levels of free branched chain amino acids, glycolytic metabolites (including 2,3-diphosphoglycerate), higher total glutathione pools, and elevated metabolites of the folate pathway compared to the other species. Strikingly, previously described metabolic markers of the storage lesion(s) in humans followed similar trends across all species, though the rate of accumulation/depletion of metabolites in energy and redox metabolism varied by species, with equine blood showing the lowest degree of storage lesion(s).
Discussion
These results interrogate RBC metabolism across a range of mammalian species and improve our understanding of both human and veterinary blood storage and transfusion.
Keywords: comparative biology, erythrocyte, hemolysis, metabolomics
KEY POINTS.
Fresh red blood cells from canines, bovines and equines (horses and donkeys) have different energy and redox metabolism;
Storage under blood bank conditions exacerbates stress to cow RBCs, activates antioxidant pathways in dogs, while equine RBCs maintain energy and redox homeostasis up to 42 days.
INTRODUCTION
Blood transfusion is a life-saving intervention, not only for millions of humans around the world every year, but also for animals. Canines, equines, and bovines are species of veterinary interest requiring transfusion following scheduled surgeries (e.g., as part of procedures targeting oncological, hematological, or cardiovascular disease) or upon trauma and hemorrhage (e.g., vehicle-animal accidents, coagulopathies, penetrating wounds in police or military service dogs or equines)1. In all these cases, care is limited to vein-to-vein transfusion, or transfusion of blood products that are regulated by guidelines that were designed for human blood-derived therapeutics2.
Research animal species are investigated as surrogate models for human RBC storage3,4, given the opportunity to perform interventional studies under controlled conditions for donor and recipient animals. Indeed, most human studies designed to test the quality of novel blood products are not only costly and confounded by inter-donor variability5, but are also limited to autologous studies in healthy donor volunteers6, with limitations detailed elsewhere7. Models of trauma, shock, and resuscitation using packed RBCs in mammals, such as non-human primates (e.g., baboons or macaques), or smaller rodents (e.g., rats8, mice9, or guinea pigs10) have sparked interest in performing comparative transfusion biology studies over the past few years. Using omics technologies, we embarked on the Zoomics project11,12, an initiative fostered by both scientific interest and translational relevance for transfusion medicine applications in veterinary species as potential models of human RBC storage and transfusion. Recently, omics studies in human transfusion medicine have improved our understanding of RBC storage lesion(s)13. A growing body of studies has shown that the metabolism of packed red blood cells (pRBCs) is affected by storage duration, processing strategies (e.g., leukofiltration), donor exposures14. The latter include diet (e.g., fatty acid composition); exposure to alcohol, caffeine, and taurine-rich caffeinated beverages; smoking; over the counter-drugs that do not result in donor deferral; genetics (e.g., sex15, ethnicity, G6PD status16); and other biological factors (e.g., age, body mass index)5,15,17,18. Despite significant advances in understanding human pRBC quality over regulatory-defined storage durations, limited omics data are available to help inform comparative and veterinary medicine-based blood storage and transfusion. Studies in murine models of storage and post-transfusion recovery (i.e., the percentage of transfused RBCs still circulating 24 h post-transfusion) contributed to identifying dysregulated iron homeostasis, and iron-dependent oxidation of membrane lipids, as a critical regulator of RBC storage quality and post-transfusion performance3. Of note, genetic heterogeneity across multiple mouse strains was sufficient to drive differences in blood storage quality and post-transfusion RBC recovery3. As such, we anticipate that investigating RBC storage quality across multiple mammalian species with even greater genetic diversity would further contribute to our understanding of species-specific RBC metabolic characteristics. This information may also help to define the translational underpinnings of the storage lesion(s) across various species and potentially guide the development of novel storage strategies tailored towards the metabolic needs of RBCs from different species.
RBC aging in vivo or in vitro (i.e., under refrigerated storage) may provide a critical cellular and organismal aging model in humans and other mammals, since RBC lifespan varies across species and studies correlated RBC aging with the longevity of a given species19. For example, canine RBCs have an average lifespan of 110–120 days, comparable to humans, but longer than other species we had recently investigated as part of the Zoomics project: macaques (98±21 days)20, baboons (~100 days)21, guinea pigs (77–91 days)22, and mice (55–60 days3). Dog RBCs are relatively large (7 μm diameter (comparable to human RBCs); mean cell volume (MCV) of 64–76 fL) with have a hemoglobin level of 14.1–20.1 g/dL. The normal RBC count in dogs is 4.8–9.3 million/microliter depending on breed, with a hematocrit of 41–58% and <1.5% reticulocytes. In healthy equines, RBCs have a longer average lifespan (140–160 days)23. Equines have a lower hematocrit24 of 36–46%, with a RBC count of 6.6–9.7 million/microliter (depending on breed and altitude), MCV of 43–55 fL, and hemoglobin of 11.8–15.9 g/dL. Bovines have lower hemoglobin levels (8.7–12.4 g/dL) and hematocrits (25–33%), but comparable cell counts (5.0–7.2 million/microliters). Bovine RBCs are also smaller (MCV of 38–51 fL), but circulate longer than humans and canines (130–160 days)25.
RBC metabolism regulates their lifespan in circulation and during refrigerator storage. Uncoupling adenosine triphosphate (ATP)-generating steps in glycolysis or NADPH-generating steps in the pentose phosphate pathway due to genetic abnormalities (e.g., pyruvate kinase deficiency26 or glucose 6-phosphate dehydrogenase deficiency27, respectively) promote intra- and extravascular hemolysis, the latter by splenic sequestration and erythrophagocytosis. These phenomena are recapitulated, or even exacerbated, by refrigerated storage13, resulting in rapid RBC clearance following transfusion28. Multiple studies demonstrated that oxidant stress affecting hemoglobin, glycolytic enzymes (e.g., glyceraldehyde 3-phospahte dehydrogenase29), and antioxidant enzymes (e.g. peroxiredoxin 2) can drive the RBC “storage lesion(s)”13. However, the onset and severity of these lesions are affected by processing strategies, including by specific storage additives13,30. These trends are well established in human31, non-human primate11,32, and rodent RBCs (e.g., mice3, rats8); however, little is known about the effects of refrigerated storage on canine, equine, and bovine RBCs. Veterinary medicine guidelines in individual countries (like Italy) state that RBCs from all the animals studied here be stored in Citrate Phosphate Dextrose anticoagulants, supplemented with storage additive Saline Adenine Glucose Mannitol (CPD-SAGM); the latter was developed for human RBCs in 198133 and only eventually implemented in veterinary transfusion medicine practices. The concept of comparative erythrocyte metabolism is not novel34. For example, a solid body of literature shows different glucose uptake and glycolytic activity -as determined via activity assays for hexokinase and phosphofructokinase activity assays- in RBCs from dogs, horses and cattle35. NMR-based studies showed that, in mammalian erythrocytes, the amount of glucose metabolized through the pentose phosphate pathway (PPP) ranges from 2.1 to 7.0% of the total glucose utilized36. Similar targeted investigations had been performed to investigate interspecies heterogeneity in pyrmidine 5’-nucleotidase activity in mammalian erythrocytes. However, no metabolomics study has been performed to date on fresh RBCs from multiple species of veterinary interest. In addition, no omics study has shown whether and to what extent these differences are impacting the metabolome of stored RBCs, differences that would likely manifest themselves when RBCs are stored in the same additive designed for storage of human RBCs. Here we hypothesize that RBC metabolic heterogeneity at baseline (time of donation) across multiple species drives species-specific rates and severity of the metabolic storage lesion(s). First, the present study defines RBC metabolic similarities and differences in canines, bovines, and equines. These data were then compared to human and non-human primate RBC metabolic results from our ongoing studies11,12,32 to improve understanding of species-specific basal RBC metabolic differences and of time-dependent storage-based differences. The Zoomics project is intended to advance our understanding of species-specific RBC characteristics, identify translational models of human transfusion, optimize human storage RBC quality, and work towards developing novel storage formulations to meet the RBC energetic needs required for veterinary blood transfusion11,32.
MATERIALS AND METHODS
Ethical statement
All experimental protocols were approved by the University of Teramo IACUC under Protocol 24321 on 05/10/2021 (2021-UNTECLE-0024321), as part of the following project: “Analisi lipidomica, proteomica e metabolomica delle unità di eritrociti concentrate stoccati di cane, gatto, cavallo, asino e bovino nell’ambito della valutazione delle lesioni da stoccaggio” (which translates to “Lipidomics, proteomics, and metabolomics analysis of stored packed RBCs from dogs, cats, horses, donkeys, and bovines for the evaluation of the storage lesion[s]”).
Blood collection, processing and storage
Dog (n=8), cow, donkey, and horse (n=6, for each species) blood was processed, stored, and sampled similarly, as per current veterinary practice. Thus, ~450 mL of whole blood was collected in Citrate Phosphate Dextrose and centrifuged at 2,000 × g for 10 minutes; plasma was removed and 0.45 mL of Saline Adenine Glucose Mannitol (SAGM) was added for every 1 mL of packed RBCs. RBCs in CPD-SAGM preservative solution (total volume ~250–300 mL) were transferred to a sterile customized single port bag through a sterile self-sealing sampling site coupler port (Fenwal, Lake Zurich, IL, USA). Half of the collected blood was individually processed by passage through a leukoreduction filter to achieve log4 WBC and log2.5 PLT removal from the unit (Haemonetics, Braintree, MA, USA). The volume-modified storage bags (Hemanext, Lexington, MA, USA) were designed to hold 200 mL volumes and approximate the plasticizer composition of standard units that incorporate polyvinylchloride (PVC) and phthalate (DEHP and MEHP) plasticizers. Processing procedures were performed in a biosafety cabinet under aseptic conditions the morning of the blood collection and RBCs were refrigerator stored (4–6°C). Following sterile weekly sampling of each unit on days 0, 7, 14, 21, 28, 35, and 42, RBCs were separated from supernatants by centrifugation (2,500 rpm).
Ultra-high-pressure liquid chromatography-mass spectrometry (MS) metabolomics
Frozen RBC and supernatant aliquots (50 μL) were extracted 1:10 and 1:25, respectively, in ice cold extraction solution (methanol:acetonitrile:water 5:3:2 v/v/v). Samples were vortexed and insoluble material pelleted, as described11,32. Analyses were performed using a Vanquish UHPLC coupled online to a Q Exactive mass spectrometer (Thermo Fisher, Bremen, Germany). Samples were analyzed using a 1 min and 5 minute gradient-based method, as described11,32. Data analysis was performed through the auxilium of the software MAVEN. Graphs and statistical analyses (either two-way ANOVA or repeated measures ANOVA) were prepared with GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA), GENE E (Broad Institute, Cambridge, MA, USA), and MetaboAnalyst 5.0.
RESULTS
Interspecies differences in RBC metabolism across dogs, cows, donkey,s and horses
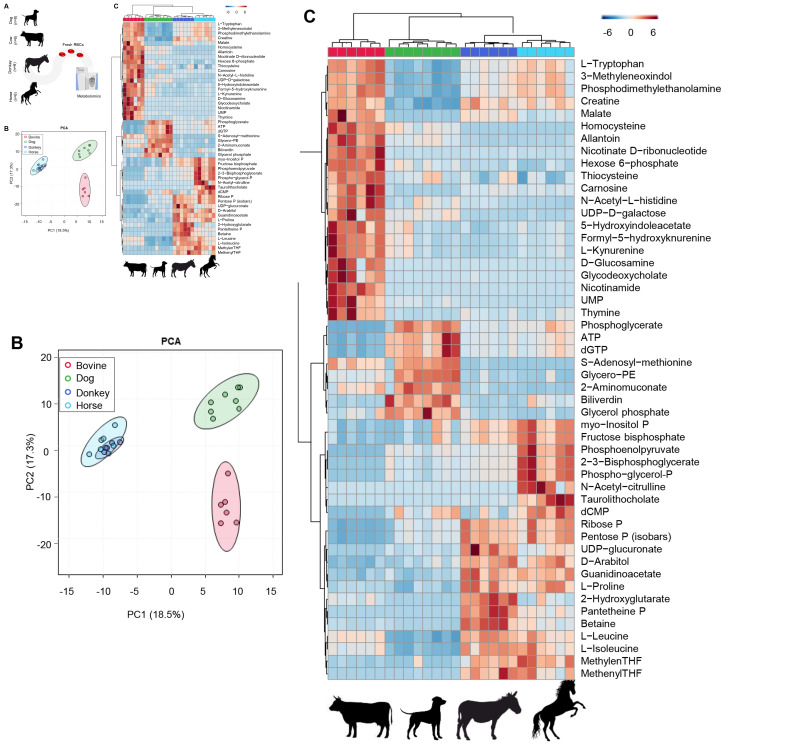
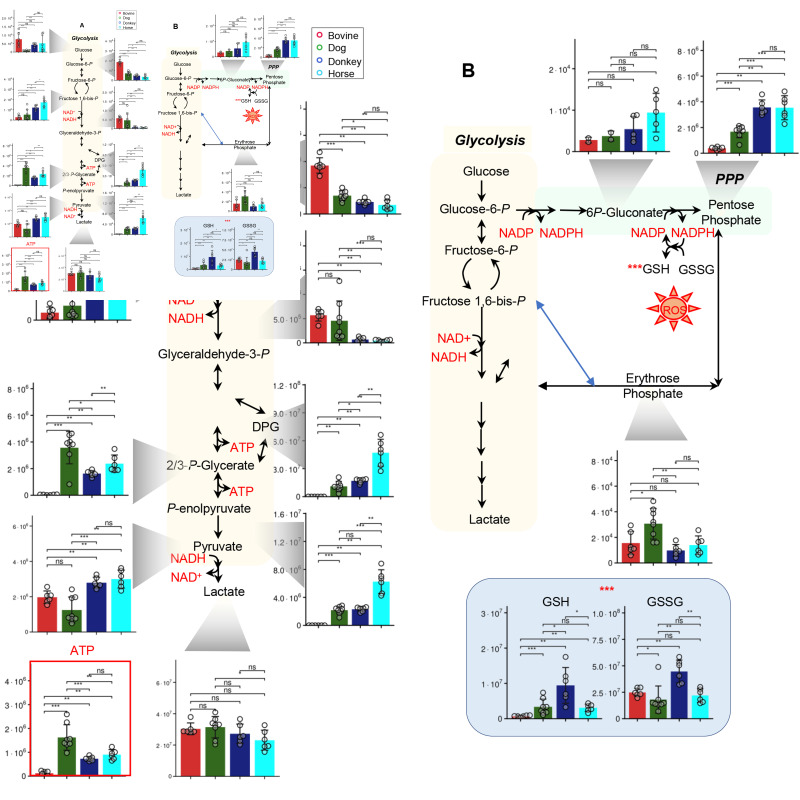
Metabolomics analyses were performed on leukofiltered, freshly drawn RBCs from dogs (n=8), cows, donkeys, and horses (n=6 for these three species; Figure 1.A, Online Supplementary Table SI). Significant interspecies differences were observed in the basal RBC metabolomes, as determined by principal component analysis (Figure 1.B), Specifically, dogs, cows, and equines (donkeys and horses) clustered separately, with principal component (PC)1 explaining 18.5% of the total variance and discriminating between equines and the other species, and PC2 discriminating between cows and the other species (17.3% of the total variance). These results were further confirmed by hierarchical clustering analysis (a highlight of the top 50 metabolites by ANOVA is in Figure 1.C). Cow RBCs had the highest levels of metabolites in the tryptophan/kynurenine pathway across all species (including tryptophan, kynurenine, formyl-hydroxykynurenine, methyleneoxindol, hydroxyindoleacetate, Figure 1.C). Dogs showed elevated levels of high-energy compounds, especially adenosine triphosphate (ATP) and S-adenosylmethionine (Figure 1.C). Equine RBCs showed almost overlapping phenotypes. Fresh RBCs from donkeys and horses were characterized by the highest levels of free branched chain amino acids, glycolytic metabolites (including Rapoport-Luebering-derived 2,3-diphosphoglycerate - DPG and phosphoenolpyruvate - Figure 2.A), higher total glutathione pools (reduced and oxidized, especially in donkeys - Figure 2.B), PPP products (ribose phosphate and pentose phosphate isobars), and elevated metabolites of the folate pathway as compared to the other species (Figure 1). Cow RBCs had the highest levels of deaminated purines, including xanthine and allantoin (Online Supplementary Figure S1.A-B). Free amino acids were significantly elevated in fresh donkey and horse RBCs, as compared to the other groups (Online Supplementary Figure S1.C).
Figure 1.
Interspecies differences in red blood cell metabolism across dogs, cows, donkeys and horses
Metabolomics analyses were performed on freshly drawn red blood cells from dogs (n=8), cows, donkeys and horses (n=6 for each one of the three species. (A) Significant baseline interspecies differences were observed in the metabolomes of fresh red blood cells, as determined by principal component analysis (B) and hierarchical clustering analysis of the top 50 metabolites by ANOVA (C).
Figure 2.
Significant interspecies differences in red blood cell glycolysis, pentose phosphate pathway and glutathione metabolism
Fresh red blood cells from cows (red), dogs (green), donkeys (dark blue) and horses (light blue) significantly differ in baseline levels of metabolites involved in central energy and redox metabolism. Asterisks indicate significance based on ANOVA with post-hoc multiple column comparisons (ns: not significant; * p <0.05; ** p < 0.01; *** p < 0.001).
Significant interspecies differences in stored red blood cell metabolism
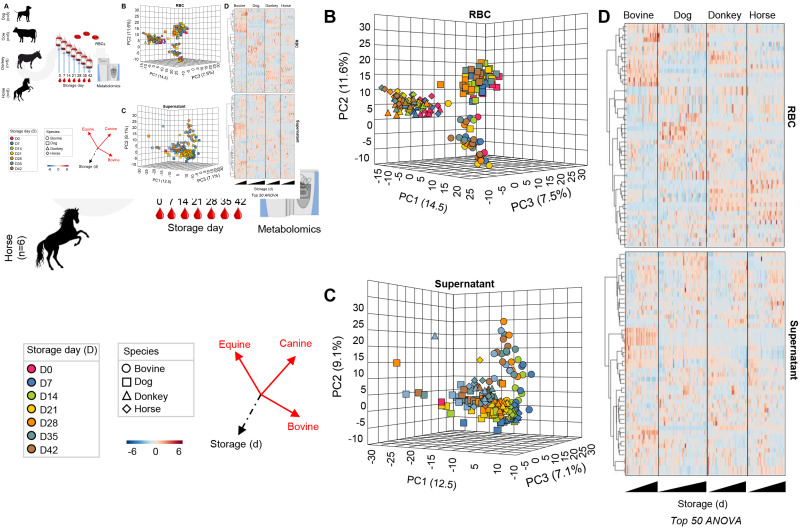
Leukofiltered RBCs from cows, dogs, donkeys, and horses were stored in CPD-SAGM for 42 days (Figure 3.A). Weekly samples were collected using sterile technique for metabolomics analyses. There was a significant species-dependent effect (PC1, explaining 14.5% of the total variance, separated dogs from cows from the other species, while PC2, 11.6% of the variance, separated equines from the other species) of the metabolome of the RBCs (Figure 3.B) and, to a lesser extent and more clearly for bovines, supernatants (Figure 3.C). These results were confirmed by hierarchical clustering analysis of significant metabolites by repeated measure ANOVA for each species (Figure 3.D).
Figure 3.
Significant interspecies differences in stored red blood cell metabolism
Leukofiltered red blood cells from cows (red), dogs (green), donkeys (dark blue) and horses (light blue) were stored in CPD-SAGM for 42 days (A). Sterile weekly sampling generated samples for metabolomics analyses via UHPLC-MS. Results indicate a significant impact of storage duration and species on red blood cell (B) and supernatant (C) metabolites, as determined by principal component analysis and hierarchical clustering analysis of significant metabolites by repeated measure ANOVA (D).
Of note, the metabolic markers defining the human RBC storage lesion(s)37 accumulated or decreased at different rates in the stored RBCs from the other species. Specifically, ATP was consumed in stored RBCs of all species, although horses showed high levels of ATP until storage day 5 and only minimal decreases by storage day 42 (Online Supplementary Figure S2). Hypoxanthine, a marker of ATP breakdown and deamination38, accumulated the most in dogs, which had the highest basal ATP levels before storage (Online Supplementary Figure S2). Line plots indicate median (solid line) ± interquartile range (lighter, dotted lines) for top significant metabolites by Two-way ANOVA across all four species. Line plots are color coded according to the scheme in the bottom right corner of the figure. Lactate levels were comparably greater in dogs, horses, and donkeys, with dog RBCs also showing preserved levels of S-adenosylmethionine (SAM) throughout storage and the highest levels of free linoleic acid (fatty acid – FA 18:2) by the end of storage (Online Supplementary Figure S2). Cow RBCs exhibited the sharpest decline in SAM, the lowest levels of ATP and oxononaoic acid, and the highest levels of prostaglandins (especially D2), suggestive of the slowest energy metabolism and the highest oxidant stress in this species throughout storage (Online Supplementary Figure S2).
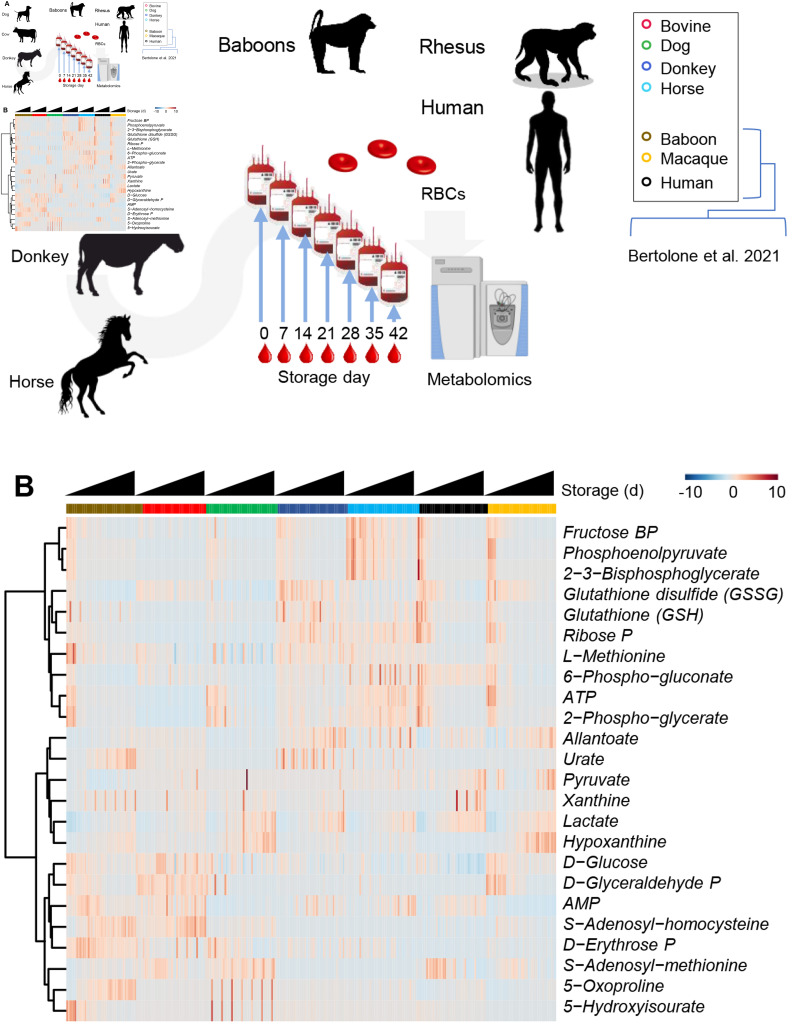
Comparison of storage metabolomics data from cows, dogs, donkeys, and horses to published data in humans and non-human primates
Metabolomics data from the current RBC storage studies were normalized across species to measurements in fresh RBCs. We then compared the data from the present study to published RBC storage data from previous studies in humans and non-human primates (i.e., baboons and macaques)11,32 (Figure 4.A). For visualization purposes, the results were graphed as a heat map (Figure 4.B) or overlapping line plots, color-coded by species and divided by key metabolic pathways relevant to RBC storage biology and post-transfusion performances (hemolysis and post-transfusion recovery) based on prior publications15,27,39 (Figures 5–6).
Figure 4.
Comparative analysis of the storage lesion in cows, dogs, horses and donkeys vs previously published data from human and non-human primates
Metabolomics analyses from the present study were compared against published literature in humans and non-human primates, baboons and rhesus macaques12,17,23 (A). In B, hierarchical clustering of red cell metabolomics data from the present and previous studies12,17,23, as a function of storage and species.
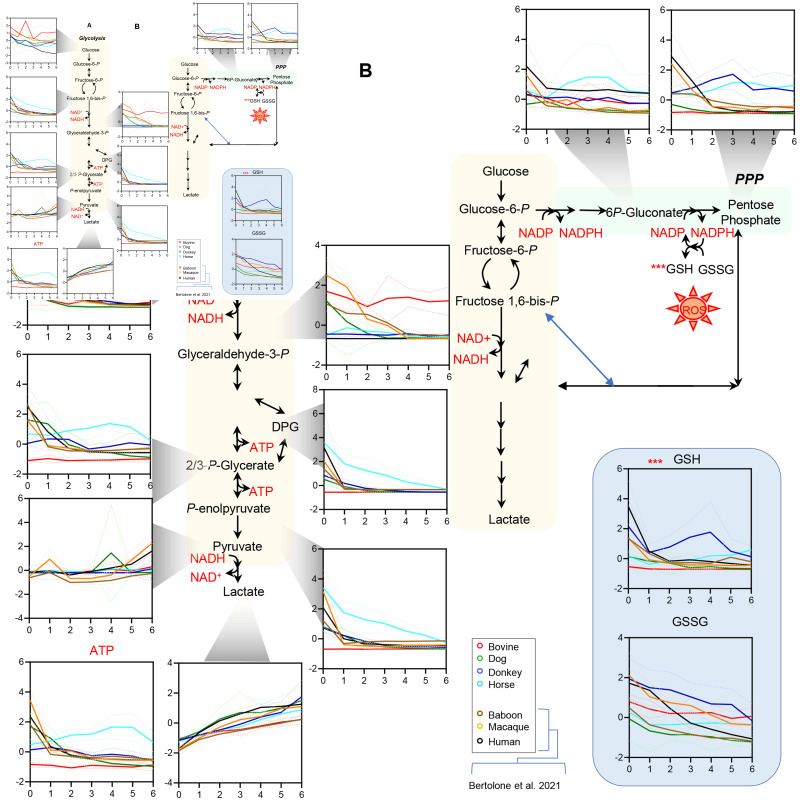
Figure 5.
Interspecies differences in the red blood cell storage lesion to glycolysis (A), the pentose phosphate pathway and glutathione pools (B), based on data from the present study (cows, dogs, donkeys and horses) and previous studies (humans, baboons, rhesus macaques) of the Zoomics project12,17,23
Line plots indicate median (solid line) + interquartile range (lighter, dotted lines) for top significant metabolites by Two-way ANOVA across all four species. Line plots are color coded according to the scheme in the bottom right corner of the figure.
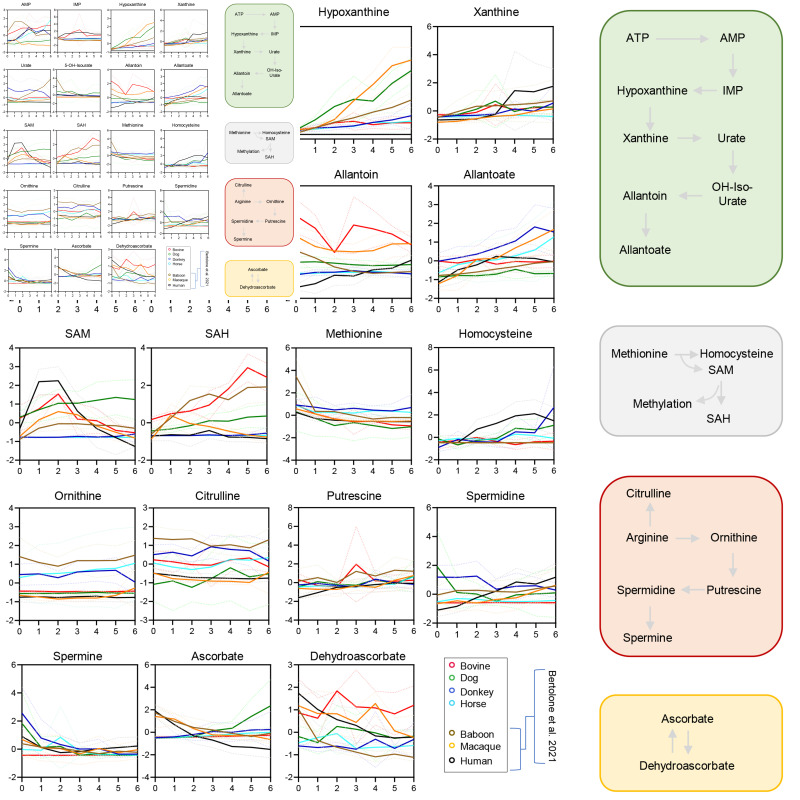
Figure 6.
Interspecies differences in the red blood cell storage lesion to purine deamination and oxidation, methionine and arginine metabolism, based on data from the present study (cows, dogs, donkeys and horses) and previous studies (humans, baboons, rhesus macaques) of the Zoomics project12,17,23
Line plots indicate median (solid line) + interquartile range (lighter, dotted lines) for top significant metabolites by Two-way ANOVA across all four species. Line plots are color coded according to the scheme in the bottom right corner of the figure.
Glycolytic metabolites, especially fructose bisphosphate, DPG, phosphoglycerate, phosphoenolpyruvate, and ATP were the highest in horses throughout storage, despite similar declining trends across all species (Figure 5.A). Horses and donkeys showed the highest levels of PPP metabolites of the oxidative phase (6-phosphoglucomnate) and ribose phosphate (including pentose phosphate isobars, Figure 5.B). This observation corresponded to significantly higher levels of reduced and total glutathione in donkeys, as compared to the other species throughout storage, despite storage-dependent depletion of glutathione pools in all species (Figure 5.C). Cows showed the lowest levels of glycolytic metabolites and glutathione pools, despite being characterized by the highest levels of glucose and glyceraldehyde 3-phosphate across all species, suggesting a potential early metabolic bottleneck at the step catalyzed by the redox sensitive29 enzyme glyceraldehyde 3-phosphate dehydrogenase. Indeed, cow RBCs were also characterized by the highest levels of purine oxidation products, inosine monophosphate (IMP), hypoxanthine, allantoin, and allantoate (Figure 6), but not urate; the latter is an antioxidant that showed the highest levels in donkeys and baboons throughout storage (Figure 6). Of note, humans had the highest levels of SAM and the lowest levels of S-Adenosylhomocysteine (SAH) throughout storage (Figure 6), suggestive of the lowest rate of oxidant stress-induced methylation-dependent repair of isoaspartyl-damage to proteins40. Human RBCs also had the highest levels of polyamines (spermidine and spermine) and the lowest levels of ornithine (Figure 6), suggesting species-specific differences in arginine metabolism.
DISCUSSION
These studies show that the basal metabolomes of RBCs from cows, dogs, donkeys, and horses are significantly different, with horses and donkeys clustering together and cows showing the most unique phenotype of all the species compared. Though limited to four species, these data suggest that genetic heterogeneity across mammalian species is recapitulated at the metabolic level. Some differences are indicative of species-specific metabolic peculiarities, rather than (red blood) cell-intrinsic metabolic reprogramming, since such changes can be reflected in the RBC metabolome as a result of their transit through the vasculature and the uptake/release of small molecule metabolites through their complex system of metabolite and ion transporters (77 of which were reported in human RBCs41). The basal differences in the RBC metabolome across these species are interestingly associated with the animal phenotypes, with equine and canine RBCs showing stronger antioxidant capacity (the PPP and glutathione system in equines) or energy metabolism (ATP and other high-energy phosphate compounds in dogs). In contrast, cow RBCs had higher basal levels of carboxylic acids (e.g., malate) and tryptophan/kynurenine metabolites; the latter is a metabolic signature that, in humans, is associated with interferon-driven inflammation and responses to viral infection42,43. Although speculative at this stage, these data pinpoint at a dysregulation of the kynurenine pathway in cows, similar to that reported in humans with Down syndrome44. Based on this kynurenine pathway dysregulation in cow RBCs, and given that this pathway is upstream to NAD synthesis, a rate-limiting cofactor in energy metabolism, which, in RBCs, is involved in the redox reactions of methemoglobin metabolism, it is interesting to speculate that cow RBCs are energetically challenged at steady state, in the absence of other perturbations.
Refrigerator storage exacerbates baseline metabolic phenotypes, with cow RBCs showing the highest and fastest accumulation of RBC metabolic changes consistent with oxidative storage lesions (especially eicosanoids [pro-inflammatory prostaglandins]). In contrast, equine RBCs had the highest levels of glycolytic metabolites and high-energy phosphate compounds, including ATP and DPG, suggesting that equine RBCs, even upon storage, have evolved (spontaneously or through human breeding-driven selection over the course of millennia) mechanisms to maximize the synthesis of metabolites involved in oxygen uptake and release kinetics30 to, thus, maximize performance in the face of rapid bouts of exercises/workloads. Since refrigerated storage seems to have the lowest impact on energy metabolism in donkeys and horses among all species tested in this study, and human and non-human primates studied previously11,32, it is interesting to speculate that our findings may be functionally relevant to equine RBC storage biology, with implications for equine exercise performance and even blood doping strategies for racehorses45. At the opposite end of the spectrum, cow RBCs had the highest amounts of free glucose and the lowest glycolytic rates across all species tested in this study, with an apparent metabolic blockade at the level of fructose 1,6-bisphosphate, suggesting a potential bottleneck at the metabolic step catalyzed by glyceraldehyde 3-phospahte dehydrogenase (GAPDH). Of note, this particular step depends on free NAD (see comments above on the kynurenine pathway). In addition, storage of human RBCs promotes the (progressively irreversibly) oxidation of GAPDH29, a phenomenon that may be exacerbated in cow RBCs under refrigerated storage conditions. Consistent with the exacerbated oxidant stress in stored cow RBCs compared to the other species, cow RBCs were also characterized by the highest levels of purine oxidation products, inosine monophosphate (IMP), hypoxanthine -a marker of poor post transfusion recovery38, allantoin, and allantoate- but not the antioxidant urate46.
Dog RBCs showed the highest SAM levels and SAM/SAH ratios throughout storage (lowest in cows). This is relevant in light of the role this pathway plays in oxidant stress-induced protein isoaspartyl-damage repair, a mechanism that compensates for the inability of RBCs to synthesize new proteins, by repairing damaged components through consumption of methyl-group donors, like SAM and its precursor, methionine40.
In addition to energy metabolism, the most notable feature across all species tested in this and prior Zoomics studies was the significantly higher basal (and storage-dependent) activation of the PPP in equines, especially horses. In humans, genetic polymorphisms that result in lower G6PD enzyme activity, the rate-limiting enzyme of the PPP, are associated with increased susceptibility to spontaneous or oxidant stress-induced hemolysis and lower post-transfusion hemoglobin increments15,16,47. Taken together, these results suggest an increased resistance to oxidant stress in equine RBCs, an observation with potentially important implications in comparative biology studies of redox metabolism.
The present study has several limitations. For example, these metabolomics analyses were not accompanied by functional or morphological investigations, such as determination of hemolysis, oxygen kinetics, RBC morphology by scanning electron microscopy, vesiculation rates, deformability, and post-transfusion recovery. However, we hope that studies like this one will spark sufficient interest to support the development of more comprehensive programs to investigate these unexplored aspects of RBC storage biology in species of veterinary interest, including exotic and wild animals. In addition, the extensive understanding of the genetics of certain animals based on selective breeding programs (e.g., with dogs, cows, and horses) would allow further dissection of genetic contributions to specific metabolic pathways. Another limitation of the current study is that these metabolomics analyses were based on high-throughput screening of several hundred samples48, an approach that makes such studies cost-affordable; nonetheless, this limits the scope to steady state observations in the absence of incubation of RBCs with stable isotope labeled substrates. In addition, here we investigated RBC storage biology under conditions that mimic human RBC storage, including using leukofiltration. Leukofiltration may be performed in blood units from species of veterinary interest, albeit not routinely, and few studies have investigated the impact of leukofiltration on animal blood storage49–51. As another limitation, the small group sizes studied did not allow us to determine the impact of other biological variables, such as sex or age, on stored RBC metabolism. Finally, although the animals were of the same breed and were chosen by the same breeding (horses, cows, and donkeys), genetic heterogeneity across breeds, such as for dogs, was not considered here, although it is relevant to murine RBC storage biology3,4; thus, this approach may pave the way for follow up studies addressing this issue52. Despite these limitations, these data are not only relevant per se, owing to their impact on veterinary science, but also translationally relevant to understand how genetic divergency across mammals influences the severity of the storage lesion(s), with the final goal to improve RBC processing and storage strategies for humans and veterinary species. Indeed, our data show that storing RBCs from different species in the same additive (SAGM in Italy) exacerbates inter-species differences in erythrocyte metabolism at baseline and upon refrigerated storage.
CONCLUSIONS
Our results prompt the question as to whether alternative storage protocols should be implemented in routine veterinary transfusion practices, including the development of ad hoc additive solutions tailored towards the metabolic needs of RBCs from different species.
Supplementary Information
ACKNOWLEDGMENTS
Research reported in this publication was supported by funds from R01HL146442, R01HL149714, and R01HL148151 by the National Heart, Lung and Blood Institutes (AD, SLS). Zoomics is part of the MIRAGES project: Metabolic Investigation of Red blood cells as a function of Aging, Genetics and Environment, sponsored by the National Heart, Lung and Blood Institutes (R21HL150032 to AD).
Footnotes
AUTHORS’ CONTRIBUTIONS
AM, MDT, FR, MTA collected and stored the samples. SLS, PWB, AD provided essential materials and methods to perform the study. MM, TN, AD performed metabolomics analyses (untargeted and targeted quantitative). AD performed data analysis and prepared figures and tables. AD wrote the first draft of the manuscript, which was revised by all the other authors. All the Authors contributed to finalizing the manuscript.
DISCLOSURE OF CONFLICT OF INTEREST
Though unrelated to the contents of this manuscripts, the authors declare that AD and TN are founders of Omix Technologies Inc. AD is also a consultant for Altis Biosciences LLC., Rubius Inc. and Forma Inc. AD and SLS are both consultants for Hemanext Inc. SLS is also a consultant for Tioma, Inc., TCIP, Inc., and the Executive Director of the Worldwide Initiative for Rh Disease Eradication (WIRhE). All the other authors disclose no conflicts of interest relevant to this study.
REFERENCES
- 1.Davidow B. Transfusion medicine in small animals. Vet Clin North Am Small Anim Pract. 2013;43:735–756. doi: 10.1016/j.cvsm.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Stefani A, Capello K, Carminato A, Wurzburger W, Furlanello T, Bertazzo V, et al. Effects of leukoreduction on storage lesions in whole blood and blood components of dogs. J Vet Intern Med. 2021;35:936–945. doi: 10.1111/jvim.16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howie HL, Hay AM, de Wolski K, Waterman H, Lebedev J, Fu X, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019;3:2272–2285. doi: 10.1182/bloodadvances.2019000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–148. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Alessandro A, Culp-Hill R, Reisz JA, Anderson M, Fu X, Nemkov T, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2019;59:89–100. doi: 10.1111/trf.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 7.Francis RO, Mahajan S, Rapido F, La Carpia F, Soffing M, Divgi C, et al. Reexamination of the chromium-51-labeled posttransfusion red blood cell recovery method. Transfusion. 2019;59:2264–2275. doi: 10.1111/trf.15310. [DOI] [PubMed] [Google Scholar]
- 8.Williams AT, Jani VP, Nemkov T, Lucas A, Yoshida T, Dunham A, et al. Transfusion of anaerobically or conventionally stored blood after hemorrhagic shock. Shock. 2020;53:352–362. doi: 10.1097/SHK.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollenberg SM. Mouse models of resuscitated shock. Shock. 2005;24(Suppl 1):58–63. doi: 10.1097/01.shk.0000191415.02085.48.24. [DOI] [PubMed] [Google Scholar]
- 10.Miller HI, Parker JL. The guinea pig as a model in shock research. Prog Clin Biol Res. 1989;299:277–286. [PubMed] [Google Scholar]
- 11.Bertolone L, Shin HK, Stefanoni D, Baek JH, Gao Y, Morrison EJ, et al. ZOOMICS: comparative metabolomics of red blood cells from old world monkeys and humans. Front Physiol. 2020;11:593841. doi: 10.3389/fphys.2020.593841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertolone L, Shin HKH, Baek JH, Gao Y, Spitalnik SL, Buehler PW, et al. ZOOMICS: comparative metabolomics of red blood cells from guinea pigs, humans, and non-human primates during refrigerated storage for up to 42 days. Front Physiol. 2022;13:845347. doi: 10.3389/fphys.2022.845347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida T, Prudent M, D’Alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17:27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemkov T, Stefanoni D, Bordbar A, Issaian A, Palsson BO, Dumont LJ, et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight. 2021;6:e146175. doi: 10.1172/jci.insight.146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Alessandro A, Fu X, Kanias T, Reisz JA, Culp-Hill R, Guo Y, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2021;106:1290–1302. doi: 10.3324/haematol.2020.246603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzounakas VL, Kriebardis AG, Georgatzakou HT, Foudoulaki-Paparizos LE, Dzieciatkowska M, Wither MJ, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152–165. doi: 10.1016/j.freeradbiomed.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Mykhailova O, Olafson C, Turner TR, D’Alessandro A, Acker JP. Donor-dependent aging of young and old red blood cell subpopulations: Metabolic and functional heterogeneity. Transfusion. 2020;60:2633–2646. doi: 10.1111/trf.16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazegh K, Fang F, Bravo MD, Tran JQ, Muench MO, Jackman RP, et al. Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion. 2021;61:435–448. doi: 10.1111/trf.16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaestner L, Minetti G. The potential of erythrocytes as cellular aging models. Cell death and differentiation. Cell Death Differ. 2017;24:1475–1477. doi: 10.1038/cdd.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonseca LL, Alezi HS, Moreno A, Barnwell JW, Galinski MR, Voit EO. Quantifying the removal of red blood cells in Macaca mulatta during a Plasmodium coatneyi infection. Malar J. 2016;15:410. doi: 10.1186/s12936-016-1465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valeri CR, Pivacek LE, Cassidy GP, Ragno G. Volume of RBCs, 24- and 48- hour posttransfusion survivals, and the lifespan of (51)Cr and biotin-X-N-hydroxysuccinimide (NHS)-labeled autologous baboon RBCs: effect of the anticoagulant and blood pH on (51)Cr and biotin-X-NHS elution in vivo. Transfusion. 2002;42:343–348. doi: 10.1046/j.1537-2995.2002.00071.x. [DOI] [PubMed] [Google Scholar]
- 22.Edmondson PW, Wyburn JR. The erythrocyte life-span, red cell mass and plasma volume of normal guinea-pigs as determined by the use of (51) Chromium, (32)Phosphorus Labelled Di-isopropyl Fluorophosphonate and (131)Iodine Labelled human serum albumin. Br J Exp Pathol. 1963;44:72–80. [Google Scholar]
- 23.Miglio A, Gavazza A, Siepi D, Bagaglia F, Misia A, Antognoni MT. Hematological and biochemical reference intervals for 5 adult hunting dog breeds using a blood donor database. Animals (Basel) 2020;10:1212. doi: 10.3390/ani10071212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miglio A, Falcinelli E, Mezzasoma AM, Cappelli K, Mecocci S, Gresele P, et al. Effect of first long-term training on whole blood count and blood clotting parameters in thoroughbreds. Animals. 2021;11:447. doi: 10.3390/ani11020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roland L, Drillich M, Iwersen M. Hematology as a diagnostic tool in bovine medicine. J Vet Diagn Invest. 2014;26:592–598. doi: 10.1177/1040638714546490. [DOI] [PubMed] [Google Scholar]
- 26.Roy MK, Cendali F, Ooyama G, Gamboni F, Morton H, D’Alessandro A. Red blood cell metabolism in pyruvate kinase deficient patients. Front Physiol. 2021;12:735543. doi: 10.3389/fphys.2021.735543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis RO, D’Alessandro A, Eisenberger A, Soffing M, Yeh R, Coronel E, et al. Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J Clin Invest. 2020;130:2270–2285. doi: 10.1172/JCI133530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roussel C, Morel A, Dussiot M, Marin M, Colard M, Fricot-Monsinjon A, et al. Rapid clearance of storage-induced microerythrocytes alters transfusion recovery. Blood. 2021;137:2285–2298. doi: 10.1182/blood.2020008563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisz JA, Wither MJ, Dzieciatkowska M, Nemkov T, Issaian A, Yoshida T, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128:e32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 30.Donovan K, Meli A, Cendali F, Park KC, Cardigan R, Stanworth S, et al. Stored blood has compromised oxygen unloading kinetics that can be normalized with rejuvenation and predicted from corpuscular side-scatter. Haematologica. 2022;107:298–302. doi: 10.3324/haematol.2021.279296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Alessandro A, Fu X, Kanias T, Reisz JA, Culp-Hill R, Guo Y, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2021;106:1290–1302. doi: 10.3324/haematol.2020.246603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanoni D, Shin HKH, Baek JH, Champagne DP, Nemkov T, Thomas T, et al. Red blood cell metabolism in Rhesus macaques and humans: comparative biology of blood storage. Haematologica. 2020;105:2174–2186. doi: 10.3324/haematol.2019.229930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91:13–9. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko JJ. Comparative erythrocyte metabolism. Adv Vet Sci Comp Med. 1974;18:117–153. [PubMed] [Google Scholar]
- 35.Arai T, Washizu T, Sagara M, Sako T, Nigi H, Matsumoto H, et al. D-glucose transport and glycolytic enzyme activities in erythrocytes of dogs, pigs, cats, horses, cattle and sheep. Res Vet Sci. 1995;58:195–196. doi: 10.1016/0034-5288(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 36.Magnani M, Piatti E, Dachà M, Fornaini G. Comparative studies of glucose metabolism on mammals’ red blood cells. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1980;67:139–142. doi: 10.1016/0305-0491(80)90282-5. [DOI] [Google Scholar]
- 37.Paglia G, D’Alessandro A, Rolfsson Ó, Sigurjónsson ÓE, Bordbar A, Palsson S, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128:e43–50. doi: 10.1182/blood-2016-06-721688. [DOI] [PubMed] [Google Scholar]
- 38.Nemkov T, Sun K, Reisz JA, Song A, Yoshida T, Dunham A, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103:361–372. doi: 10.3324/haematol.2017.178608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Alessandro A, Yoshida T, Nestheide S, Nemkov T, Stocker S, Stefanoni D, et al. Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion. 2020;60:786–798. doi: 10.1111/trf.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reisz JA, Nemkov T, Dzieciatkowska M, Culp-Hill R, Stefanoni D, Hill RC, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion. 2018;58:2978–2991. doi: 10.1111/trf.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordbar A, Jamshidi N, Palsson BO. iAB-RBC-283: A proteomically derived knowledge-base of erythrocyte metabolism that can be used to simulate its physiological and patho-physiological states. BMC Syst Biol. 2011;5:110. doi: 10.1186/1752-0509-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorgdrager FJH, Naudé PJW, Kema IP, Nollen EA, Deyn PPD. Tryptophan metabolism in inflammaging: from biomarker to therapeutic target. Front Immunol. 2019;10:2565. doi: 10.3389/fimmu.2019.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5:e140327. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers RK, Culp-Hill R, Ludwig MP, Smith KP, Waugh KA, Minter R, et al. Trisomy 21 activates the kynurenine pathway via increased dosage of interferon receptors. Nat Commun. 2019;10:4766. doi: 10.1038/s41467-019-12739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkin T, Baoutina A, Hamilton N. Equine performance genes and the future of doping in horseracing. Drug Test Anal. 2017;9:1456–1471. doi: 10.1002/dta.2198. [DOI] [PubMed] [Google Scholar]
- 46.Tzounakas VL, Karadimas DG, Anastasiadi AT, Georgatzakou HT, Kazepidou E, Moschovas D, et al. Donor-specific individuality of red blood cell performance during storage is partly a function of serum uric acid levels. Transfusion. 2018;58:34–40. doi: 10.1111/trf.14379. [DOI] [PubMed] [Google Scholar]
- 47.Page GP, Kanias T, Guo YJ, Lanteri MC, Zhang X, Mast AE, et al. Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage. J Clin Invest. 2021;131:e146077. doi: 10.1172/jci146077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nemkov T, Yoshida T, Nikulina M, D’Alessandro A. High-throughput metabolomics platform for the rapid data-driven development of novel additive solutions for blood storage. Front Physiol. 2022;13:833242. doi: 10.3389/fphys.2022.833242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidow EB, Montgomery H, Mensing M. The influence of leukoreduction on the acute transfusion-related complication rate in 455 dogs receiving 730 packed RBCs: 2014–2017. J Vet Emerg Crit Care (San Antonio) 2022;32:479–490. doi: 10.1111/vec.13175. [DOI] [PubMed] [Google Scholar]
- 50.Antognoni MT, Marenzoni ML, Misia AL, Avellini L, Chiaradia E, Gavazza A, et al. Effect of leukoreduction on hematobiochemical parameters and storage hemolysis in canine whole blood units. Animals (Basel) 2021;11:925. doi: 10.3390/ani11040925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefani A, Capello K, Carminato A, Wurzburger W, Furlanello T, Bertazzo V, et al. Effects of leukoreduction on storage lesions in whole blood and blood components of dogs. J Vet Intern Med. 2021;35:936–945. doi: 10.1111/jvim.16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Creevy KE, Akey JM, Kaeberlein M, Promislow DEL. An open science study of ageing in companion dogs. Nature. 2022;602:51–57. doi: 10.1038/s41586-021-04282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.