Abstract
Background
Intra‐uterine insemination (IUI), in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) are frequently used fertility treatments for couples with male subfertility. The use of these treatments has been subject of discussion. Knowledge on the effectiveness of fertility treatments for male subfertility with different grades of severity is limited. Possibly, couples are exposed to unnecessary or ineffective treatments on a large scale.
Objectives
To evaluate the effectiveness and safety of different fertility treatments (expectant management, timed intercourse (TI), IUI, IVF and ICSI) for couples whose subfertility appears to be due to abnormal sperm parameters.
Search methods
We searched for all publications that described randomised controlled trials (RCTs) of the treatment for male subfertility. We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, PsycINFO and the National Research Register from inception to 14 April 2015, and web‐based trial registers from January 1985 to April 2015. We applied no language restrictions. We checked all references in the identified trials and background papers and contacted authors to identify relevant published and unpublished data.
Selection criteria
We included RCTs comparing different treatment options for male subfertility. These were expectant management, TI (with or without ovarian hyperstimulation (OH)), IUI (with or without OH), IVF and ICSI. We included only couples with abnormal sperm parameters.
Data collection and analysis
Two review authors independently selected the studies, extracted data and assessed risk of bias. They resolved disagreements by discussion with the rest of the review authors. We performed statistical analyses in accordance with the guidelines for statistical analysis developed by The Cochrane Collaboration. The quality of the evidence was rated using the GRADE methods. Primary outcomes were live birth and ovarian hyperstimulation syndrome (OHSS) per couple randomised.
Main results
The review included 10 RCTs (757 couples). The quality of the evidence was low or very low for all comparisons. The main limitations in the evidence were failure to describe study methods, serious imprecision and inconsistency.
IUI versus TI (five RCTs)
Two RCTs compared IUI with TI in natural cycles. There were no data on live birth or OHSS. We found no evidence of a difference in pregnancy rates (2 RCTs, 62 couples: odds ratio (OR) 4.57, 95% confidence interval (CI) 0.21 to 102, very low quality evidence; there were no events in one of the studies).
Three RCTs compared IUI with TI both in cycles with OH. We found no evidence of a difference in live birth rates (1 RCT, 81 couples: OR 0.89, 95% CI 0.30 to 2.59; low quality evidence) or pregnancy rates (3 RCTs, 202 couples: OR 1.51, 95% CI 0.74 to 3.07; I2 = 11%, very low quality evidence). One RCT reported data on OHSS. None of the 62 women had OHSS.
One RCT compared IUI in cycles with OH with TI in natural cycles. We found no evidence of a difference in live birth rates (1 RCT, 44 couples: OR 3.14, 95% CI 0.12 to 81.35; very low quality evidence). Data on OHSS were not available.
IUI in cycles with OH versus IUI in natural cycles (five RCTs)
We found no evidence of a difference in live birth rates (3 RCTs, 346 couples: OR 1.34, 95% CI 0.77 to 2.33; I2 = 0%, very low quality evidence) and pregnancy rates (4 RCTs, 399 couples: OR 1.68, 95% CI 1.00 to 2.82; I2 = 0%, very low quality evidence). There were no data on OHSS.
IVF versus IUI in natural cycles or cycles with OH (two RCTs)
We found no evidence of a difference in live birth rates between IVF versus IUI in natural cycles (1 RCT, 53 couples: OR 0.77, 95% CI 0.25 to 2.35; low quality evidence) or IVF versus IUI in cycles with OH (2 RCTs, 86 couples: OR 1.03, 95% CI 0.43 to 2.45; I2 = 0%, very low quality evidence). One RCT reported data on OHSS. None of the women had OHSS.
Overall, we found no evidence of a difference between any of the groups in rates of live birth, pregnancy or adverse events (multiple pregnancy, miscarriage). However, most of the evidence was very low quality.
There were no studies on IUI in natural cycles versus TI in stimulated cycles, IVF versus TI, ICSI versus TI, ICSI versus IUI (with OH) or ICSI versus IVF.
Authors' conclusions
We found insufficient evidence to determine whether there was any difference in safety and effectiveness between different treatments for male subfertility. More research is needed.
Keywords: Female; Humans; Male; Pregnancy; Birth Rate; Coitus; Fertilization; Infertility, Male; Ovulation Induction; Insemination, Artificial; Insemination, Artificial/methods; Randomized Controlled Trials as Topic
Plain language summary
Treatments for male subfertility
Review question
Cochrane authors reviewed the evidence about the effectiveness of different treatments for couples with male subfertility.
Background
Intra‐uterine insemination (IUI), in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) are frequently‐used fertility treatments for couples with low male fertility (subfertility). In IUI, the man's sperm is prepared and placed in the womb (uterus). Thus, the sperm is close to the place where the embryo is made (conception site). IUI can be performed with or without ovarian hyperstimulation (OH). In an OH cycle, women receive drugs to stimulate the ovaries (the organs that produce the eggs (called oocytes)) to increase the number of available oocytes. The main side effects of these drugs are multiple pregnancy (production of two or more embryos (early stage in the development of a baby)) and ovarian hyperstimulation syndrome (OHSS; the ovaries produce too many eggs). In IVF and ICSI, the fertilisation (where the egg and sperm are together and produce an embryo) is outside the body. The oocytes are retrieved from the woman using an ultrasound‐guided needle, piercing the vaginal wall to reach the ovaries. Through this needle, follicular fluid, which contains the oocyte, can be aspirated. It is common to remove between 10 and 15 oocytes. In IVF, the eggs are mixed with the sperm in a culture dish. In ICSI, sperm are injected directly into the oocytes to cause fertilisation. The fertilised oocytes are treated for two to six days in a medium that contains nutrients and are then placed in the uterus.
Study characteristics
We searched medical databases for randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) investigating male subfertility. We found 10 randomised controlled trials, all comparing different treatments for couples with male subfertility, with a total of 757 couples. The studies evaluated the following treatment options: timed intercourse (TI; where sex occurred at a recommended time in the menstrual cycle) (with or without OH), IUI (with or without OH), IVF and ICSI. The evidence was current to April 2015. We were mainly interested in how many women had live births and OHSS.
Key results
We found no evidence of a difference in live birth or pregnancy rates between treatments. We also found no evidence of a difference between any of the groups in rates of adverse effects (multiple pregnancy, miscarriage). Available data on OHSS was too limited for us to draw any conclusions.
Quality of the evidence
Most of the evidence was of low or very low quality. The main limitations were failure to describe study methods, small sample sizes and inconsistency in how trials were conducted. Evidence was available for only six of the 14 comparisons that we evaluated. More research is needed.
Summary of findings
Summary of findings for the main comparison. IUI in natural cycles compared to TI in natural cycles for male subfertility.
| IUI in natural cycles compared to TI in natural cycles for male subfertility | ||||||
| Patient or population: couples with male subfertility Settings: single centre (Australia, Italy) Intervention: IUI in natural cycles Comparison: TI in natural cycles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of couples (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TI in natural cycles | IUI in natural cycles | |||||
| Live birth rate | Not reported in any included studies | ‐ | ‐ | |||
| OHSS | Not reported in any included studies | ‐ | ‐ | |||
| Pregnancy rate per couple (all cycles) Follow‐up: 9‐12 months | 0 per 1000 | 0 per 1000 (0 to 0) | OR 4.57 (0.21 to 101.61) | 62 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUI: intra‐uterine insemination; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; TI: timed intercourse. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was very serious: 1. Francavilla 2009, allocation concealment: high risk (on chronological basis), 2. Francavilla 2009, other bias: high risk (no stratification by diagnosis category of subfertility). 2 There was very serious imprecision, with small sample sizes and very few events.
Summary of findings 2. IUI in stimulated cycles compared to TI in stimulated cycles for male subfertility.
| IUI in stimulated cycles compared to TI in stimulated cycles for male subfertility | ||||||
| Patient or population: couples with male subfertility Settings: single centre (Greece, Italy, The Netherlands) Intervention: IUI in stimulated cycles Comparison: TI in stimulated cycles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of couples (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TI in stimulated cycles | IUI in stimulated cycles | |||||
| Live birth rate per couple (all cycles) Follow‐up: 3 months | 220 per 1000 | 200 per 1000 (78 to 421) | OR 0.89 (0.30 to 2.59) | 81 (1 study) | ⊕⊕⊝⊝ low1 | ‐ |
|
OHSS per couple Follow‐up: 6 months |
See comment | See comment | Not estimable | 59 (1 study) |
⊕⊕⊝⊝ low1 | ‐ |
| Pregnancy rate per couple (all cycles) Follow‐up: 3‐6 months | 175 per 1000 | 243 per 1000 (136 to 395) | OR 1.51 (0.74 to 3.07) | 202 (3 studies) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| Multiple pregnancy rate per couple Follow‐up: 3 months | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.15 (0.12 to 79.69) | 81 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| Miscarriage rate per couple Follow‐up: 3 months | 73 per 1000 | 75 per 1000 (15 to 300) | OR 1.03 (0.19 to 5.42) | 81 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUI: intra‐uterine insemination; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; TI: timed intercourse. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was very serious imprecision, with small sample size. 2 Inconsistency was serious between Melis 1995 (favoured TI + OH) and Gregoriou 1996 and Nan 1994 (favoured IUI + OH). 3 There was very serious imprecision, with small sample sizes and findings were compatible with substantial benefit in either group.
Summary of findings 3. IUI in stimulated cycles compared to TI in natural cycles for male subfertility.
| IUI in stimulated cycles compared to TI in natural cycles for male subfertility | ||||||
| Patient or population: couples with male subfertility Settings: single centre (Italy) Intervention: IUI in stimulated cycles Comparison: TI in natural cycles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of couples (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| TI in natural cycles | IUI in stimulated cycles | |||||
| Live birth rate per couple (all cycles) Follow‐up: 9 months | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.14 (0.12 to 81.35) | 44 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| OHSS | Not reported in any included studies | ‐ | ||||
| Pregnancy rate per couple (all cycles) Follow‐up: 9 months | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.14 (0.12 to 81.35) | 44 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUI: intra‐uterine insemination; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; TI: timed intercourse. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was very serious: 1. Allocation concealment: high risk (on chronological basis), 2. Other bias: high risk (no stratification by diagnosis category of subfertility). 2 There was very serious imprecision, with small sample sizes and very few events.
Summary of findings 4. IUI in stimulated cycles compared to IUI in natural cycles for male subfertility.
| IUI in stimulated cycles compared to IUI in natural cycles for male subfertility | ||||||
| Patient or population: couples with male subfertility Settings: single and multicentre (Italy, the Netherlands, USA) Intervention: IUI in stimulated cycles Comparison: IUI in natural cycles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of couples (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IUI in natural cycles | IUI in stimulated cycles | |||||
| Live birth rate per couple (all cycles) Follow‐up: 6‐9 months | 172 per 1000 | 218 per 1000 (138 to 326) | OR 1.34 (0.77 to 2.33) | 346 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
| OHSS | Not reported in any included studies | ‐ | ‐ | |||
| Pregnancy rate per couple (all cycles) Follow‐up: 4‐9 months | 148 per 1000 | 226 per 1000 (148 to 329) | OR 1.68 (1.00 to 2.82) | 399 (4 studies) | ⊕⊝⊝⊝ very low1,3,4 | ‐ |
| Miscarriage rate per couple Follow‐up: 6‐9 months | 53 per 1000 | 56 per 1000 (11 to 238) | OR 1.06 (0.20 to 5.63) | 115 (2 studies) | ⊕⊝⊝⊝ very low5,6 | ‐ |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUI: intra‐uterine insemination; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was very serious: 1. Francavilla 2009, allocation concealment: high risk (on chronological basis), 2. Arici 1994, Francavilla 2009, and Guzick 1999, other bias: high risk (no stratification by diagnosis category of subfertility. 2 Inconsistency was serious between Cohlen 1998a and Goverde 2000 (favoured IUI in natural cycles) and Arici 1994, Francavilla 2009, and Guzick 1999 (favoured IUI in stimulated cycles). 3 There was serious imprecision, with small sample sizes. 4 Inconsistency was serious between Cohlen 1998a (favoured IUI in natural cycles) and Arici 1994, Francavilla 2009, and Guzick 1999 (favoured IUI in stimulated cycles). 5 Risk of bias was very serious: Francavilla 2009, allocation concealment: high risk (on chronological basis) and other bias: high risk (no stratification by diagnosis category of subfertility). 6 There was serious imprecision, findings were compatible with no benefit in either group.
Summary of findings 5. IVF compared to IUI in natural cycles for male subfertility.
| IVF compared to IUI in natural cycles for male subfertility | ||||||
| Patient or population: couples with male subfertility Settings: single centre (the Netherlands) Intervention: IVF Comparison: IUI in natural cycles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of couples (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IUI in natural cycles | IVF | |||||
| Live birth rate per couple (all cycles) Follow‐up: 6 months | 407 per 1000 | 346 per 1000 (147 to 618) | OR 0.77 (0.25 to 2.35) | 53 (1 study) | ⊕⊕⊝⊝ low1 | ‐ |
| OHSS | Not reported in any included studies | ‐ | ‐ | |||
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUI: intra‐uterine insemination; IVF: in vitro fertilisation; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was very serious imprecision, with small sample sizes.
Summary of findings 6. IVF compared to IUI in stimulated cycles for male subfertility.
| IVF compared to IUI in stimulated cycles for male subfertility | ||||||
| Patient or population: couples with male subfertility Settings: single and multicentre (the Netherlands) Intervention: IVF Comparison: IUI in stimulated cycles | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of couples (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| IUI in stimulated cycles | IVF | |||||
| Live birth rate per couple (all cycles) Follow‐up: 6‐12 months | 452 per 1000 | 460 per 1000 (262 to 669) | OR 1.03 (0.43 to 2.45) | 86 (2 studies) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| OHSS per couple Follow‐up: 6 months | See comment | See comment | Not estimable | 36 (1 study) | ⊕⊝⊝⊝ very low1,2 | No OHSS occurred |
| Pregnancy rate per couple (all cycles) Follow‐up: 6 months | 611 per 1000 | 666 per 1000 (341 to 886) | OR 1.27 (0.33 to 4.97) | 36 (1 study) | ⊕⊝⊝⊝ very low1,2 | ‐ |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IUI: intra‐uterine insemination; IVF: in vitro fertilisation; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was serious: Bensdorp 2015, other bias: high risk (no stratification by diagnosis category of subfertility). 2 There was very serious imprecision, with small sample sizes.
Background
Description of the condition
Male subfertility is a common condition among subfertile couples. It has been estimated to be directly responsible for approximately 30% of problems with conception and to be a contributory factor in 50% (Crosignani 1994; Hull 1985). Over time, different definitions of male subfertility have been used. A normal quality semen sample was described as having a sperm concentration of 20 million/mL or greater, total motility 50% or greater, normal morphology in 50% or greater and no sperm antibodies (WHO 1987). In 1992, The World Health Organization (WHO) changed its criteria for normal sperm morphology from 50% to 30% (WHO 1992). When strict criteria for morphology were used, greater than 14% was considered normal (Kruger 1993). Since 2010, the reference values for a normal quality semen sample have been revised and the most important changes to the reference limits were semen volume of 1.5 mL or greater, a sperm concentration of 15 million/mL or greater, total motility 40% or greater and normal morphology in 4% or greater (Cooper 2010; WHO 2010). Despite the worldwide use of the WHO criteria, these are unable to distinguish men who are likely to father a child from men who are not. However, the correlation that has been established is a continuous one between total motile sperm count (TMSC) and the probability of natural conception (van der Steeg 2011). In couples undergoing intra‐uterine insemination (IUI), the TMSC also appears to have a consistent, direct relationship with the pregnancy rate, but there is no definite predictive threshold for success (Tijani 2010). The post‐wash TMSC probably has the most predictive value because it reflects both sperm concentration and motility as well as the effects of sperm processing (van Weert 2004). Because of the different definitions for male subfertility worldwide it is difficult to estimate what proportion of fertility treatments are associated with this indication, or how it affects the overall success rate.
Description of the intervention
IUI is a frequently used fertility treatment for couples with male subfertility (Cohlen 2005; Goverde 2000). In IUI, a small volume of prepared semen is injected trans‐cervically into the uterine cavity around the expected time of ovulation. The rationale behind this procedure is to bypass the cervix and to bring the semen closer to the released oocyte. In addition, washing of semen and the selection of motile sperm (by semen preparation) might further increase the chances of fertilisation (Duran 2002a). It has been argued that the method of sperm preparation might influence the probability of conception (Duran 2002b), but there is insufficient evidence to recommend any specific preparation technique (Boomsma 2007). IUI can be used with or without ovarian hyperstimulation (OH), which increases the number of available oocytes at the site of conception. It has also been suggested that it would overcome subtle ovulation disorders that cannot be detected by routine investigations (Zikopoulos 2005). OH is achieved by administering drugs such as anti‐oestrogens (e.g. clomiphene citrate) or gonadotrophins, sometimes combined with gonadotrophin‐releasing hormone (GnRH) agonists or, more recently, antagonists (Cantineau 2007).
In natural cycles, the pre‐ovulatory luteinising hormone (LH) surge is the best indicator of the initiation of ovulation (WHO 1980). Ovulation occurs 35 to 38 hours after the onset of the LH rise in blood (Hoff 1983; Testart 1982). In stimulated cycles, the chances of adequate timing are increased by the administration of an ovulatory triggering injection of human chorionic gonadotrophin (hCG). In order to time the hCG injection, the diameter of the largest follicle (mostly 16 to 18 mm) is determined with sonographic measurements. It has been determined that the largest follicle is the most probable to rupture and will do so approximately 38 hours after the hCG injection (Andersen 1995; Martinez 1991). Therefore, it is most favourable to inseminate around 35 to 45 hours after hCG administration.
Other, more invasive and expensive, fertility treatments for couples with male subfertility are in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). Both methods use controlled ovarian hyperstimulation (COH), which pursues three main objectives: hypophyseal activity suppression, multiple follicle growth stimulation and ovulation induction. Hypophyseal activity suppression, by a GnRH agonist or antagonist, prevents premature ovulation and allows for the timed collection of mature oocytes. Follicle‐stimulating hormone (FSH), sometimes combined with LH, is used to stimulate the growth of multiple follicles. Ovulation is induced by hCG or a GnRH agonist and is indicated when multiple follicles of 16 mm or greater are present with sonographic measurements. The optimal timing for ovulation induction remains uncertain, and more studies are necessary to explore the optimal timing (Mochtar 2011; Tarlatzis 2006). Oocyte harvesting is performed approximately 36 hours after hCG or GnRH agonist administration. In IVF, the retrieved oocytes and spermatozoa are put together in a culture dish to achieve fertilisation; for ICSI, a single selected sperm is injected directly into the cytoplasm of the oocyte. The purpose of ICSI is to overcome a potential failure of the sperm to activate the oocyte to initiate fertilisation. After fertilisation, the fertilised oocytes are cultured in a growth medium for two to six days and monitored for embryonic development. Based on morphological criteria for quality, one or two embryos are transferred into the uterine cavity and supernumerary good‐quality embryos are cryopreserved. Luteal phase supplementation with progesterone or hCG is necessary to sustain endometrial stimulation.
How the intervention might work
IUI, IVF and ICSI are used to improve the live birth rates in couples experiencing male subfertility.
Why it is important to do this review
The use of fertility treatments in male subfertility has been under debate. Some authors consider that IUI should be offered as first‐line therapy before IVF and ICSI are offered (Bhattachary 2000; Cohlen 2005; Goverde 2000; Gregoriou 1996; Nan 1994). Other authors have questioned its effectiveness in male subfertility (Guzick 1998). It has also been suggested that IUI in male subfertility would be advantageous only when a certain threshold value of motile sperm count can be achieved (van Voorhis 2001; van Weert 2004). When OH is used to enhance the effectiveness of IUI, the prevalence of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy rates increases. The most recent National Institute for Health and Care Excellence (NICE) guideline states that for mild male subfertility, routine use of IUI, either with or without OH is not appropriate. Instead, expectant management for two years is recommended, before considering IVF (NICE 2013). Other authors recommend expectant management (for at least six months) over IUI (or another fertility treatment) in couples with unexplained subfertility or moderate male factor (TMSC greater than three million) and a good or intermediate prognosis for natural conception (Hunault 2004; Steures 2006). IVF, introduced in the late 1970s as a treatment for tubal infertility, was also proposed as a therapeutic option for male subfertility (Cohen 1984). Direct and randomised comparisons between IVF and IUI are scarce and in favour of the latter in terms of cost‐effectiveness (Bensdorp 2015; Goverde 2000; Tjon‐Kon‐Fat 2015). However, there is no clear cutoff value for semen quality to support the choice for IVF or IUI. ICSI provided the possibility of genetic offspring even to people with severely compromised semen parameters (Palermo 1992). More recently, the use of ICSI has increased, also for men with borderline or even normal semen characteristics, without clear evidence of its benefits (Bhattachary 2001; Kim 2007) or even its possible harm (Boulet 2015). The cutoff values for semen parameters used to decide between conventional IVF and ICSI are generally experience‐based (Tournaye 2012), and vary per country/centre/laboratory. Performing a split IVF‐ICSI cycle in which sibling oocytes are either inseminated conventionally or micro‐injected, may prevent complete fertilisation failure in one out of four IVF cycles for moderate male factor subfertility (Kihaile 2003; van der Westenlaken 2005).
This review investigated the benefits and disadvantages of expectant management or timed intercourse (TI), IUI with or without OH, IVF and ICSI in couples with male subfertility.
Objectives
To evaluate the effectiveness and safety of different fertility treatments (expectant management, timed intercourse (TI), IUI, IVF and ICSI) for couples whose subfertility appears to be due to abnormal sperm parameters.
Methods
Criteria for considering studies for this review
Types of studies
We included both published and unpublished randomised controlled trials (RCTs). We assessed the method of randomisation to determine whether the studies were truly randomised. In the case of cross‐over trials, we only included them if pre‐cross‐over data were available. We incorporated trials that included a subset of participants with male subfertility if data were available for that subset.
Types of participants
Couples with male subfertility who had been trying to conceive for at least one year were eligible for inclusion. We included all couples with male factor subfertility, including oligo‐, terato‐, asthenospermia, or a combination of these, preferably measured by two separate semen samples. Routine fertility evaluation should have consisted of confirmed ovulatory status (basal body temperature (BBT) chart, mid‐luteal progesterone or sonographic evidence of ovulation) and low risk for tubal pathology according to the medical history (Coppus 2007).
Types of interventions
We included RCTs with at least one of the following comparisons:
IUI versus TI or expectant management both in natural cycles;
IUI versus TI both in cycles with OH;
IUI in natural cycles versus TI in cycles with OH;
IUI in cycles with OH versus TI or expectant management in natural cycles;
IUI in natural cycles versus IUI in cycles with OH;
IVF versus TI or expectant management in natural cycles;
IVF versus TI in cycles with OH;
IVF versus IUI in natural cycles;
IVF versus IUI in cycles with OH;
ICSI versus TI or expectant management in natural cycles;
ICSI versus TI in cycles with OH;
ICSI versus IUI in natural cycles;
ICSI versus IUI in cycles with OH;
ICSI versus IVF.
We excluded trials comparing methods using insemination other than IUI, such as intracervical insemination (ICI), gamete intrafallopian transfer (GIFT) and fallopian tube sperm perfusion. In addition, we excluded trials comparing different types of ovarian stimulation protocol, as this is the subject of a different review (Cantineau 2007).
Types of outcome measures
Primary outcomes
Live birth rate, defined as delivery of a live foetus after 20 completed weeks of gestational age, per couple.
Incidence of OHSS per couple.
Secondary outcomes
(Clinical) pregnancy rates, defined as evidence of a gestational sac, confirmed by ultrasound, per couple.
Multiple pregnancy rates.
Miscarriage rates.
Total fertilisation failure rates per couple during IVF.
Search methods for identification of studies
Electronic searches
We searched for all published and unpublished RCTs that described (or might have described) treatments for male subfertility with no language restrictions. Marian Showell, Trials Search Co‐ordinator of the Cochrane Gynaecology and Fertility Group, performed a search of the following:
the Cochrane Menstrual Disorders and Subfertility Group Specialised Register of controlled trials (inception to April 2015) (Appendix 1);
the Cochrane Central Register of Controlled Trials (CENTRAL; inception to April 2015) (Appendix 2);
MEDLINE (inception to April 2015) (Appendix 3);
EMBASE (inception to April 2015) (Appendix 4);
PsycINFO (inception to April 2015) (Appendix 5);
CINAHL (inception to April 2015) (Appendix 6).
We placed no language restrictions.
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying RCTs, which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Section 6.4.11) (Higgins 2011). We combined the EMBASE, PsycINFO and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
Other electronic sources of trials included the following:
-
trial registers for ongoing and registered trials:
ClinicalTrials.gov, a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home), and the WHO International Clinical Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx);
conference abstracts in the Web of Knowledge (wokinfo.com/);
OpenSIGLE database for grey literature from Europe (opensigle.inist.fr/);
LILACS database, as a source of trials from the Portuguese and Spanish speaking world (regional.bvsalud.org/php/index.php?lang=en) (choose 'LILACS' in 'all sources' drop‐down box);
PubMed (www.ncbi.nlm.nih.gov/pubmed/).
We searched the databases using the medical subject headings (MeSH terms) and keywords in Appendix 7.
Searching other resources
We checked the reference lists of all identified studies for relevant articles. We performed a handsearch of abstracts of the American Society for Reproductive Medicine (1999 to April 2015) and the European Society for Human Reproduction and Embryology (1997 to April 2015) meetings.
When important information was lacking from the original publications, we tried to contact the authors. We incorporated additional information in the review.
Data collection and analysis
Selection of studies
After screening the titles and abstracts retrieved by the search, we obtained full texts of all potentially eligible studies. Two review authors (MC and MvW) independently selected the trials to be included according to the above‐mentioned criteria. We resolved disagreements by consensus or through arbitration by a third review author (AB). We documented the selection process with a PRISMA flow chart (Figure 1).
1.
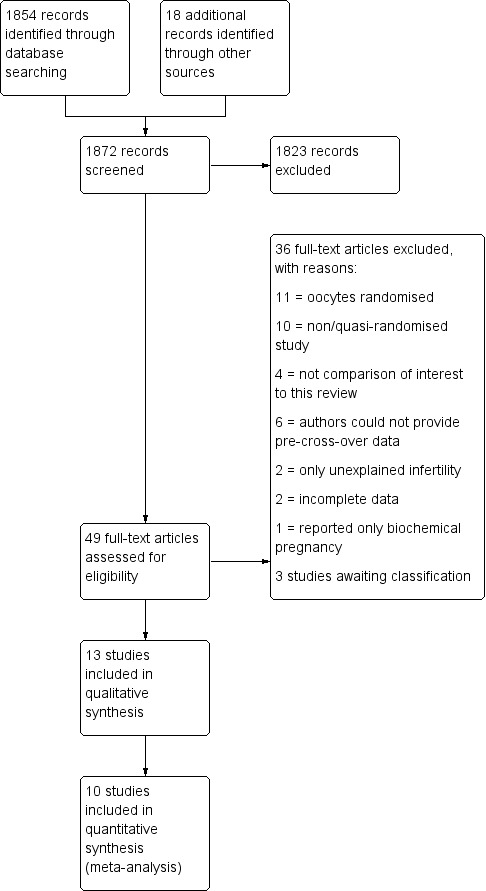
Study flow diagram.
Data extraction and management
The same two review authors independently used a data extraction form to extract data from published reports. We resolved disagreements by consensus or through arbitration by a third review author (AB). This data extraction form included information on the type of study, quality of the selected studies, types of participants, types of interventions and the types of outcome measures (Appendix 8). An analysis of agreement between the two review authors on assessment of the method of randomisation and study design resulted in 100% agreement.
Assessment of risk of bias in included studies
As part of the data collection process, two review authors (MC and MvW) independently extracted data for trial characteristics that have been recognised as potential sources of bias, such as the method used in generating the allocation sequence, how allocation was concealed and differences in drop‐out rates between study arms. We used the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011). Where there was uncertainty, we contacted the authors to clarify aspects of study design. We resolved disagreements by consensus or through arbitration by a third review author (AB).
Two review authors independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 using the following domains (Higgins 2011):
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessors);
attrition bias (incomplete outcome data);
reporting bias (selective reporting);
other bias.
These domains were assessed to have:
high risk of bias;
unclear risk of bias;
low risk of bias.
We resolved disagreements by discussion or by consulting a third review author. We described all judgements fully and presented the conclusions in the 'Risk of bias' table, which was incorporated into the interpretation of review findings by means of sensitivity analyses.
We judged that blinding of the researcher, the personnel or the participants could not influence any of the outcomes. Therefore, we assessed all included trials at low risk of bias for blinding.
Measures of treatment effect
We performed statistical analyses in accordance with the guidelines for statistical analysis developed by Cochrane, outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011). All outcomes were binary. We expressed results for each included study as Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CI).
Unit of analysis issues
The primary analysis was per couple randomised. If an included study only reported per cycle data, we contacted the author for additional information. We planned to include studies that could not provide us with per couple data in the review but not in the meta‐analysis, and describe them separately. We included both parallel group and cross‐over trials in the analysis. For cross‐over trials, we used only pre‐cross‐over data. Furthermore, we counted multiple live births (e.g. twins or triplets) as one live birth event.
Dealing with missing data
For missing data, we attempted to contact the authors. When we could not obtain the missing data from the authors, we explained the assumptions we made in the extraction and analysis of the data. We analysed only the available data.
Assessment of heterogeneity
We noted statistical heterogeneity between the results of different studies by visually inspecting the scatter in the data points on the graphs and the overlap in their CIs and using the I2 statistics. Following the Cochrane Handbook for Systematic Reviews of Interventions, we judged an I2 value greater than 50% to indicate substantial heterogeneity. We used a random‐effects model for sensitivity analysis and explored the original trials for clinical and methodological heterogeneity.
Assessment of reporting biases
Publication bias might influence the interpretation of the pooled results. To detect publication bias, we used a funnel plot, plotting sample size versus effect size, if there were sufficient studies. This plot is only relevant when five or more studies per comparison are included. The graph is symmetrical when bias is absent.
Data synthesis
If appropriate, we combined the data in a meta‐analysis using Review Manager 5 (RevMan 2014), using a fixed‐effect model and presenting OR with 95% CI. For reporting purposes, we translated primary outcomes to absolute risks. We considered live birth rate and pregnancy outcomes as a positive consequence of treatment. For adverse outcomes such as OHSS, multiple pregnancy rate, miscarriage rate and total fertilisation failure, which are negative consequences, higher numbers were considered to be detrimental (increased odds signify relative harm). This needs to be take into consideration when interpreting the analyses.
Subgroup analysis and investigation of heterogeneity
A priori, we planned to perform separate subgroup analyses if there were more than two studies in each subgroup, for trials with different ovarian stimulation protocols (oral ovulation induction agents (anti‐oestrogens) versus gonadotrophins (FSH, human menopausal gonadotropin (hMG)).
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes, to examine stability regarding the pooled outcomes.
Restriction to studies without high risk of bias.
Use of a random‐effects model.
Use of risk ratio (RR) rather than OR.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro software. This table evaluated the overall quality of the body of evidence for the review outcomes using GRADE criteria (study limitations, i.e. risk of bias, consistency of effect, imprecision, indirectness and publication bias). We justified, documented and incorporated judgements about evidence quality (high, moderate or low) into reporting of results for each outcome.
Results
Description of studies
Results of the search
The search strategy identified 2778 studies; after removing duplicates, 1854 studies remained. Handsearching identified another 18 studies. One review author (MC) screened the titles and abstracts and selected 49 studies for further evaluation. We excluded 36 studies with reasons and three studies were awaiting classification. Finally, the review included 10 studies (see Figure 1).
Included studies
Study design
Four of the 10 studies used a parallel design (Bensdorp 2015; Goverde 2000; Guzick 1999; Melis 1995). Bensdorp 2015 and Guzick 1999 published no separate data for male subfertility, but after correspondence with the first author and the author of another review (Veltman‐Verhulst 2012), we could extract relevant data. Six studies used a cross‐over design (Arici 1994; Cohlen 1998a; Francavilla 2009; Gregoriou 1996; Kerin 1984; Nan 1994). We only pooled pre‐cross‐over data in the meta‐analysis. Three studies were three‐arm trials (Bensdorp 2015; Francavilla 2009; Goverde 2000).
Elementary details concerning the studies are displayed in the Characteristics of included studies table.
Participants
The number of participants (couples) reported in the 10 included studies was 757. The sample size ranged from 21 to 254 couples.
Interventions
1. IUI versus TI or expectant management both in natural cycles
We extracted suitable data from one trial comparing IUI versus TI (Kerin 1984). The authors of Francavilla 2009 supplied unpublished pre‐cross‐over data. In Kerin 1984, one of the treatment arms instructed the participants to have "a single act of vaginal intercourse on the day the couple thought they were most fertile as detected by symptom thermal methods of ovulation detection", which can be considered to be expected management. No specifications regarding the number of outcomes were reported for this treatment arm.
2. IUI versus TI both in cycles with OH
One parallel trial addressed IUI versus TI both in cycles with OH (Melis 1995), and another two trials provided data after the first treatment period after one (Gregoriou 1996) and three (Nan 1994) cycles.
3. IUI in natural cycles versus TI in cycles with OH
We found no trials comparing IUI in natural cycles versus TI in cycles with OH.
4. IUI in cycles with OH versus TI or expectant management in natural cycles
The authors of Francavilla 2009 supplied unpublished pre‐cross‐over data.
5. IUI in cycles with OH versus IUI in natural cycles
Two parallel trials (Goverde 2000; Guzick 1999), and three cross‐over trials reported and provided data comparing IUI in natural cycles versus IUI in cycles with OH (Arici 1994; Cohlen 1998a; Francavilla 2009). Arici 1994 and Francavilla 2009 submitted unpublished data to another review from which we could extract separate data. Cohlen 1998a provided pre‐cross‐over per couple data.
6. IVF versus TI or expectant management in natural cycles
We found no trials comparing IVF versus TI or expectant management in natural cycles.
7. IVF versus TI in cycles with OH
We found no trials comparing IVF versus TI in cycles with OH.
8. IVF versus IUI in natural cycles
One parallel trial compared IVF versus IUI in natural cycles (Goverde 2000).
9. IVF versus IUI in cycles with OH
Two parallel trials compared IVF versus IUI in cycles with OH (Bensdorp 2015; Goverde 2000).
10. ICSI versus TI or expectant management in natural cycles
We found no trials comparing ICSI versus TI or expectant management in natural cycles.
11. ICSI versus TI in cycles with OH
We found no trials comparing ICSI versus TI in cycles with OH.
12. ICSI versus IUI in natural cycles
We found no trials comparing ICSI versus IUI in natural cycles.
13. ICSI versus IUI in cycles with OH
We found no trials comparing ICSI versus IUI in cycles with OH.
14. ICSI versus IVF
We found no trials comparing ICSI versus IVF.
Outcomes
Five studies provided our main outcome of interest; live birth rate per couple (Bensdorp 2015; Francavilla 2009; Goverde 2000; Guzick 1999; Melis 1995).The other five studies could provide data on pregnancy per couple (Arici 1994; Cohlen 1998a; Gregoriou 1996; Kerin 1984; Nan 1994). As most trials did not mention the results after each cycle separately, it was not possible to calculate cumulative pregnancy rates.
Two studies supplied information about OHSS in the mild male subfertility population (Bensdorp 2015; Nan 1994). For four studies, the OHSS data was not provided separately for the population with mild male subfertility (Francavilla 2009; Goverde 2000; Guzick 1999; Melis 1995). One study only provided the post‐cross‐over OHSS data (Cohlen 1998a).
Seven studies reported adverse outcomes (Bensdorp 2015; Cohlen 1998a; Goverde 2000; Guzick 1999; Kerin 1984; Melis 1995; Nan 1994). Six studies reported miscarriage or abortion (Bensdorp 2015; Cohlen 1998a; Francavilla 2009; Goverde 2000; Guzick 1999; Melis 1995). Eight studies reported multiple pregnancies (Bensdorp 2015; Cohlen 1998a; Francavilla 2009; Goverde 2000; Guzick 1999; Melis 1995; Kerin 1984; Nan 1994), and four studies reported ectopic pregnancies (Goverde 2000; Guzick 1999; Kerin 1984; Melis 1995). Two studies did not state any adverse outcomes (Arici 1994; Gregoriou 1996). Often the details on adverse effects were not provided for male subfertility separately, or at the end of the trial of post‐cross‐over. Therefore, we could not use these data in the review.
Excluded studies
We excluded 36 studies for the following reasons: 10 studies were not RCTs (retrospective or commentary design, not randomised or quasi randomised) (Elizur 2004; Galle 1990; Goverde 2001; Hewitt 1985; Moolenaar 2015; Nulsen 1993; Plachot 2002; Prentice 1995; Xie 2015; Zayed 1997). Eleven studies randomised oocytes instead of couples (Aboulghar 1995; Aboulghar 1996; Fan 2012; Fishel 2000; Kastrop 1999; Kihaile 2003; Li 2004; Pisarska 1999; Tournaye 2002; van der Westerlaken 2006; Verheyen 1999). In four studies, none of the comparisons of interest was included (Cruz 1986; Friedman 1989; Karlström 2000; Melis 1987). Two studies did not include male subfertility participants (Agarwal 2004; Elzeiny 2014). Two studies published incomplete data (Buvat 1990; Soliman 1993) and one study reported on biochemical pregnancies only (Evans 1991). To this date, we have received no response from the authors for additional information. Six studies with a cross‐over design could not supply their pre‐cross‐over data (Crosignani 1994; Ho 1989; Ho 1992; Kirby 1991; Martinez 1991; te Velde 1989). It is advocated that a particular concern with the cross‐over design is the risk of a carry‐over effect (Elbourne 2002; Khan 1996; McDonnell 2004). It may have been a source of bias and, therefore, we excluded these studies from the review. See the Characteristics of excluded studies table.
Studies awaiting classification
Three studies used a cross‐over design where no pre‐cross‐over data were published (Aribarg 1995; Jaroudi 1998; Kerin 1987). We attempted to contact authors to get these pre‐cross‐over data, but to this date, we have received no response.
Risk of bias in included studies
Figure 2 presents our judgements about each methodological quality item, presented as percentages across all included studies, and Figure 3 summarises our judgements about each methodological quality item for each included study.
2.
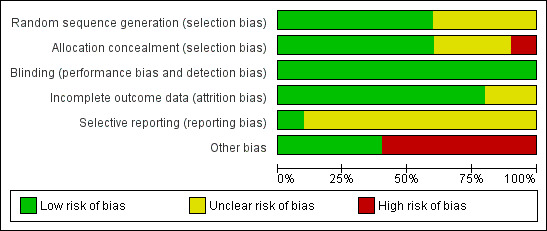
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
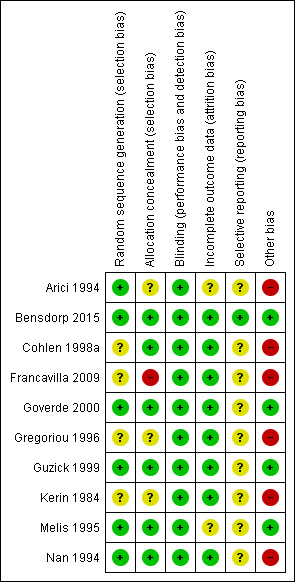
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Study design
Of the 10 studies, four had a parallel design (Bensdorp 2015; Goverde 2000; Guzick 1999; Melis 1995). Six studies were of cross‐over alternating design, thus couples were initially randomised to one of the interventions and then alternated between treatment arms on each cycle. In Gregoriou 1996, the cross‐over took place after three cycles.
Allocation
The methods of randomisation or allocation concealment were generally poor in the published information, which might increase the risk for selection bias. However, we received additional information about allocation methods for some studies.
Random sequence generation
Five studies mentioned the use of a computer‐generated program for randomisation (Arici 1994; Bensdorp 2015; Goverde 2000; Guzick 1999; Melis 1995). One study used a random number table, not further specified (Nan 1994). The random sequence generation remained unclear for the other studies (Cohlen 1998a; Francavilla 2009; Gregoriou 1996; Kerin 1984). Sixty percent of the studies were at low risk of bias and 40% of the studies were at unclear risk of bias.
Allocation concealment
Four studies explicitly stated concealment of allocation (Bensdorp 2015; Cohlen 1998a; Goverde 2000; Melis 1995). After we had received additional information about allocation, we deemed two other trials at low risk of bias in this domain (Guzick 1999; Nan 1994). Concealment of allocation was done by the use of sealed opaque envelopes, locked computer files or white and black discs from a blinded bag. We deemed one study at high risk allocation concealment (Francavilla 2009). Concealment of allocation was done on chronological basis. The concealment of allocation was unclear for the other studies (Arici 1994; Gregoriou 1996; Kerin 1984). Sixty percent of the studies were at low risk of bias, 10% of the studies were at high risk of bias and 30% of the studies were at unclear risk of bias.
Blinding
None of the studies reported blinding. In trials comparing TI versus IUI or IUI versus IVF it is of course impossible to blind the participants. In trials of IUI with and without OH, blinding could technically be performed. However, often stimulation is administered intramuscularly, so blinding might be considered unethical. All studies were at low risk of bias with respect to blinding as we determined that it was unlikely to influence our review outcomes.
Incomplete outcome data
Nine studies reported information on drop‐outs, cancelled cycles, or both (Arici 1994; Bensdorp 2015; Cohlen 1998a; Francavilla 2009; Goverde 2000; Guzick 1999; Kerin 1984 ; Melis 1995; Nan 1994). The number of drop‐outs varied from 0% to 25%, the number of cancelled cycles varied from 4% to 19%. One study reported the drop‐out of 17 couples before the start of the first treatment cycle (failed to return, refused randomisation, other subfertility factors) and included 75% (56/75) of the couples in their analysis (Arici 1994). One study reported the drop‐out of 11 couples and included 88% (81/92) of the couples in their analysis (Melis 1995). Six studies reported on their drop‐outs and analysed 100% of the couples included in their study (Bensdorp 2015; Cohlen 1998a; Goverde 2000; Gregoriou 1996; Kerin 1984; Nan 1994). The proportion of analysed couples remained unclear in two studies (Francavilla 2009; Guzick 1999).
Four studies stated that the most important reasons for cancelling a cycle were a premature or missed LH surge and OHSS (Cohlen 1998a; Goverde 2000; Gregoriou 1996; Nan 1994). Furthermore, Goverde 2000 reported that in 37 cycles there was no fertilisation after insemination of the aspirated oocytes during IVF. Melis 1995 stated that the most important reasons for cancelling a cycle were a poor response to ovulation induction and exaggerated response to ovulation induction.
Eighty percent of the studies were at low risk of bias and 20% of the studies were at unclear risk of bias.
Selective reporting
A total of 50% of the included studies reported live birth rates. The remaining studies defined (clinical) pregnancy rates (see Characteristics of included studies table). Ten percent of the studies were at low risk of bias and 90% of the studies were at unclear risk of bias.
Other potential sources of bias
Six studies used a cross‐over design and there might be selectivity in availability of the data (Arici 1994; Cohlen 1998a; Francavilla 2009; Gregoriou 1996; Kerin 1984; Nan 1994). Forty percent of the studies were at low risk of bias and 60% of the studies were at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6.
Overall the meta‐analyses included 10 studies with 757 couples. Three studies were three‐arm trials, in which each full group has been used twice in a pair‐wise comparison between arms.
1. IUI versus TI or expectant management both in natural cycles
Two studies compared IUI with TI both in natural cycles (Kerin 1984; Francavilla 2009).
Live birth rate per couple
Neither of the studies reported on live births.
OHSS
Neither of the studies reported on OHSS.
Pregnancy rate per couple
Both studies reported clinical pregnancy rate. There was no evidence of a difference in pregnancy rate per couple for IUI versus TI in natural cycles (2 trials, 62 couples: OR 4.57, 95% CI 0.21 to 101.61; very low quality evidence). There were no events in one of the studies (Figure 4; Analysis 1.1).
4.
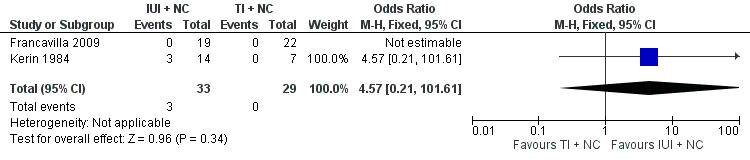
Forest plot of comparison: 1 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in natural cycles (NC), outcome: 1.1 Pregnancy rate per couple (all cycles).
1.1. Analysis.
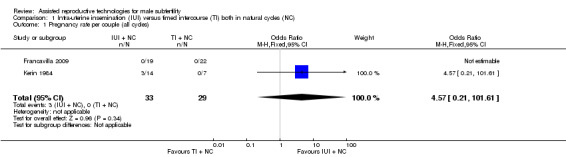
Comparison 1 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in natural cycles (NC), Outcome 1 Pregnancy rate per couple (all cycles).
Multiple pregnancy
Neither of the studies reported on multiple pregnancy.
Miscarriage
Neither of the studies reported on miscarriage.
2. IUI versus TI both in cycles with OH
Three studies compared IUI with TI both in cycles with OH (Gregoriou 1996; Melis 1995; Nan 1994).
Live birth rate per couple
One study reported on live birth rate. There was no evidence of a difference in live birth rate per couple for IUI versus TI in stimulated cycles (1 trial, 81 couples: OR 0.89, 95% CI 0.30 to 2.59; low quality evidence) (Analysis 2.1). In absolute terms, this result implied that a 22% success rate using TI with OH would become between 2% and 38% using IUI with OH.
2.1. Analysis.
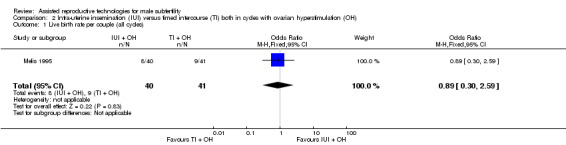
Comparison 2 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in cycles with ovarian hyperstimulation (OH), Outcome 1 Live birth rate per couple (all cycles).
OHSS
OHSS occurred in none of the cycles (Nan 1994) (Analysis 2.2).
2.2. Analysis.
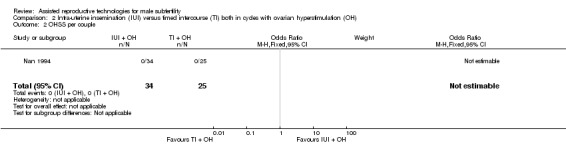
Comparison 2 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in cycles with ovarian hyperstimulation (OH), Outcome 2 OHSS per couple.
Pregnancy rate per couple
There was no evidence of a difference in pregnancy rate per couple for IUI versus TI in stimulated cycles (3 trials, 202 couples: OR 1.51, 95% CI 0.74 to 3.07; I2 = 11%, very low quality evidence) (Figure 5; Analysis 2.3).
5.
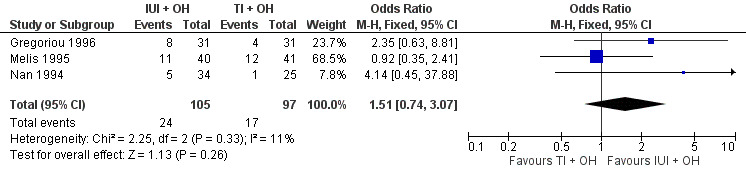
Forest plot of comparison: 2 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in cycles with ovarian hyperstimulation (OH), outcome: 2.3 Pregnancy rate per couple (all cycles).
2.3. Analysis.
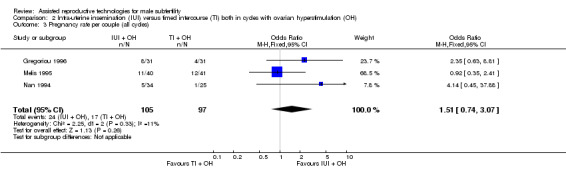
Comparison 2 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in cycles with ovarian hyperstimulation (OH), Outcome 3 Pregnancy rate per couple (all cycles).
Multiple pregnancy
There was no evidence of a difference in multiple pregnancy rate between IUI and TI in stimulated cycles (1 trial, 81 couples: OR 3.15, 95% CI 0.12 to 79.69; low quality evidence) (Analysis 2.4).
2.4. Analysis.
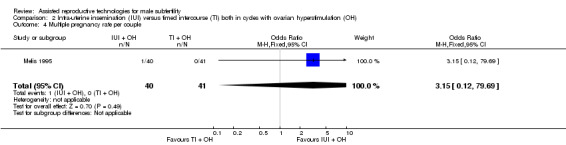
Comparison 2 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in cycles with ovarian hyperstimulation (OH), Outcome 4 Multiple pregnancy rate per couple.
Miscarriage
There was no evidence of a difference in miscarriage rate per couple for IUI versus TI in stimulated cycles (1 trial, 81 couples: OR 1.03, 95% CI 0.19 to 5.42; low quality evidence) (Analysis 2.5).
2.5. Analysis.
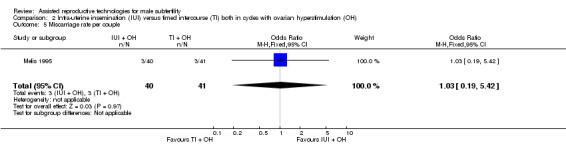
Comparison 2 Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in cycles with ovarian hyperstimulation (OH), Outcome 5 Miscarriage rate per couple.
3. IUI in natural cycles versus TI in cycles with OH
We found no trials comparing IUI in natural cycles versus TI in cycles with OH.
4. IUI in cycles with OH versus TI or expectant management in natural cycles
One study compared IUI with OH versus TI with natural cycles (Francavilla 2009).
Live birth rate per couple
One study reported on live birth rate. There was no evidence of a difference in live birth rate per couple for IUI with OH versus TI in natural cycles (1 trial, 44 couples: OR 3.14, 95% CI 0.12 to 81.35; very low quality evidence) (Analysis 4.1). In the TI group, there were no live births (0 of 29 couples), in the IUI group, 9% of couples had a live birth (3/33 couples).
4.1. Analysis.
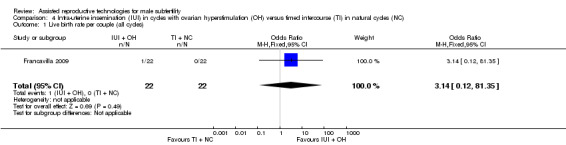
Comparison 4 Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus timed intercourse (TI) in natural cycles (NC), Outcome 1 Live birth rate per couple (all cycles).
OHSS
There were no (pre‐cross‐over) data available.
Pregnancy rate per couple
There was no evidence of a difference in pregnancy rate per couple for IUI with OH versus TI in natural cycles (1 trial, 44 couples: OR 3.14, 95% CI 0.12 to 81.35; very low quality evidence) (Figure 6; Analysis 4.2).
6.

Forest plot of comparison: 4 Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus timed intercourse (TI) in natural cycles (NC), outcome: 4.2 Pregnancy rate per couple (all cycles).
4.2. Analysis.
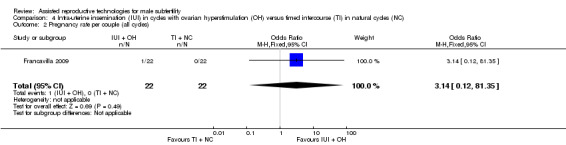
Comparison 4 Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus timed intercourse (TI) in natural cycles (NC), Outcome 2 Pregnancy rate per couple (all cycles).
Multiple pregnancy
There were no (pre‐cross‐over) data available.
Miscarriage
There were no miscarriages reported.
5 IUI in cycles with OH versus IUI in natural cycles
Five studies compared IUI in cycles with OH with IUI in natural cycles (Arici 1994; Cohlen 1998a; Francavilla 2009; Goverde 2000; Guzick 1999).
Live birth rate per couple
One study reported on live births per treatment arm (Goverde 2000). Francavilla 2009 and Guzick 1999 provided data on live birth rate for the male subfertility group after we contacted them. There was no evidence of a difference in live birth rate per couple for IUI with OH versus IUI in natural cycles (3 trials, 346 couples: OR 1.34, 95% CI 0.77 to 2.33; I2 = 0%, very low quality evidence) (Analysis 5.1). In absolute terms, this result implied that a 17% success rate using IUI in natural cycles would become between 13% and 30% using IUI with OH.
5.1. Analysis.
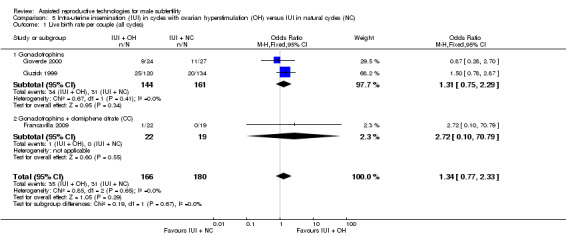
Comparison 5 Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus IUI in natural cycles (NC), Outcome 1 Live birth rate per couple (all cycles).
OHSS
None of the studies reported on OHSS.
Pregnancy rate per couple
Four studies reported on pregnancy rate per couple, after one cycle (Arici 1994; Cohlen 1998a; Francavilla 2009) or several cycles (Guzick 1999). There was no evidence of a difference in pregnancy rate per couple for IUI with OH versus IUI in natural cycles (4 trials, 399 couples: OR 1.68, 95% CI 1.00 to 2.82; I2 = 0%, very low quality evidence) (Figure 7; Analysis 5.2).
7.
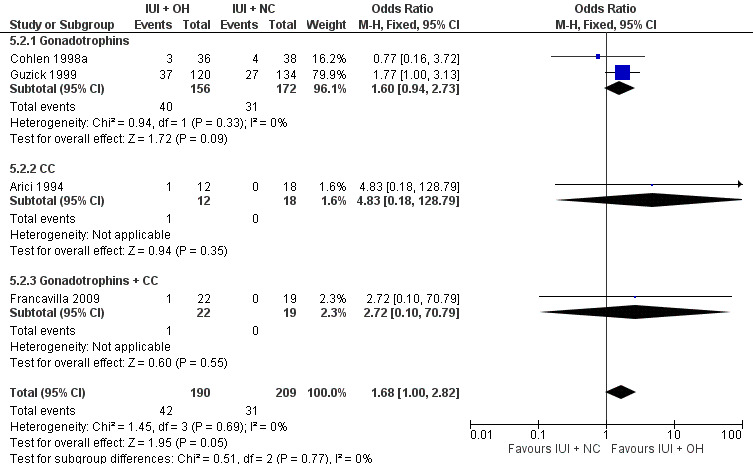
Forest plot of comparison: 5 Intra‐uterine insemination (IUI) in natural cycles (NC) versus IUI in cycles with ovarian hyperstimulation (OH) versus IUI in natural cycles (NC), outcome: 5.2 Pregnancy rate per couple (all cycles).
5.2. Analysis.
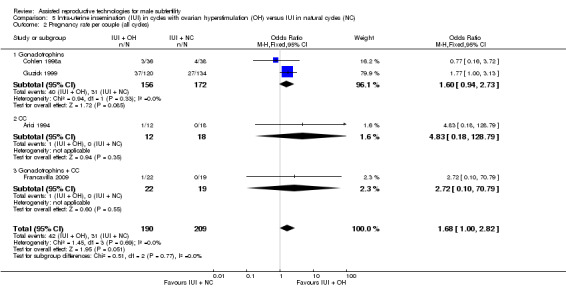
Comparison 5 Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus IUI in natural cycles (NC), Outcome 2 Pregnancy rate per couple (all cycles).
Multiple pregnancy
None of the studies reported on multiple pregnancy.
Miscarriage
Two studies reported on miscarriage rate (Cohlen 1998a; Guzick 1999). There was no evidence of a difference in miscarriage rate per couple for IUI with OH versus IUI in natural cycles (2 trials, 115 couples: OR 1.06, 95% CI 0.20 to 5.63; very low quality evidence). There were no events in one of the studies (Analysis 5.3).
5.3. Analysis.
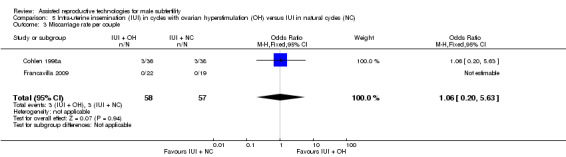
Comparison 5 Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus IUI in natural cycles (NC), Outcome 3 Miscarriage rate per couple.
6. IVF versus TI or expectant management in natural cycles
We found no trials comparing IVF versus TI or expectant management in natural cycles.
7. IVF versus TI in cycles with OH
We found no trials comparing IVF versus TI in cycles with OH.
8. IVF versus IUI in natural cycles
One study compared IVF with IUI in natural cycles (Goverde 2000).
Live birth rate per couple
There was no evidence of a difference in live birth rate per couple for IVF versus IUI in natural cycles (1 trial, 53 couples: OR 0.77, 95% CI 0.25 to 2.35; low quality evidence) (Figure 8; Analysis 8.1). In absolute terms, this result implied that a 41% success rate using IUI in natural cycles would become between 9% and 61% using IVF.
8.

Forest plot of comparison: 8 In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in natural cycles (NC), outcome: 8.1 Live birth rate per couple (all cycles).
8.1. Analysis.
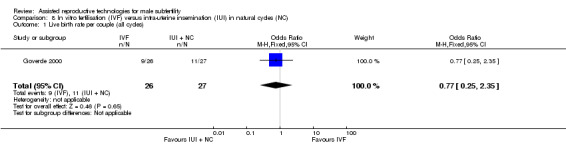
Comparison 8 In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in natural cycles (NC), Outcome 1 Live birth rate per couple (all cycles).
OHSS
Severe OHSS occurred in three women of the IVF group for the whole study arm of whom the majority had unexplained subfertility. It was unclear whether any of the couples with mild male subfertility developed OHSS.
Pregnancy rate per couple
None of the studies reported on pregnancy rate.
Multiple pregnancy
None of the studies reported on multiple pregnancy.
Miscarriage
None of the studies reported on miscarriage.
Total fertilisation failure
Total fertilisation failure occurred in 37 IVF cycles (male and unexplained subfertility).
9. IVF versus IUI in cycles with OH
Two studies compared IVF with IUI with OH cycles (Bensdorp 2015; Goverde 2000).
Live birth rate per couple
There was no evidence of a difference in live birth rate per couple for IVF versus IUI cycles with OH (2 trials, 86 couples: OR 1.03, 95% CI 0.43 to 2.45; I2 = 0%, very low quality evidence) (Figure 9; Analysis 9.1). In absolute terms, this result implied that a 45% success rate using IUI cycles with OH would become between 25% and 66% using IVF.
9.
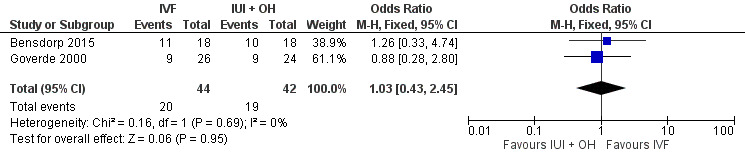
Forest plot of comparison: 9 In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH), outcome: 9.1 Live birth rate per couple (all cycles).
9.1. Analysis.
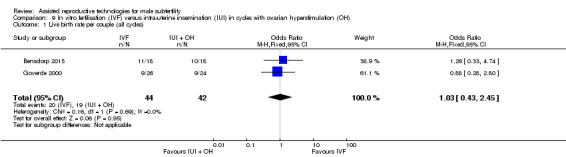
Comparison 9 In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH), Outcome 1 Live birth rate per couple (all cycles).
OHSS
OHSS occurred in none of the IVF or IUI with OH cycles (Bensdorp 2015) (Analysis 9.2).
9.2. Analysis.
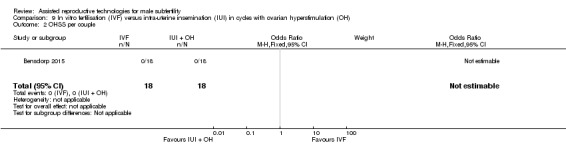
Comparison 9 In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH), Outcome 2 OHSS per couple.
Pregnancy rate per couple
There was no evidence of a difference in pregnancy rate per couple for IVF versus IUI cycles with OH (1 trial, 36 couples: OR 1.27, 95% CI 0.33 to 4.97; low quality evidence) (Analysis 9.3).
9.3. Analysis.
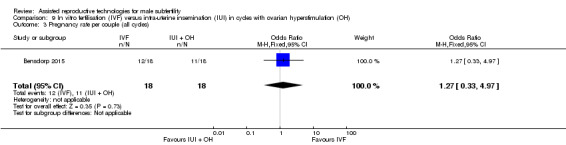
Comparison 9 In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH), Outcome 3 Pregnancy rate per couple (all cycles).
Multiple pregnancy
Bensdorp 2015 reported two twins, one in the IUI with OH group, one in the IVF with single embryo transfer group.
Miscarriage
Bensdorp 2015 reported two miscarriages, one in the IUI with OH group, one in the IVF with single embryo transfer group.
Total fertilisation failure
Goverde 2000 reported total fertilisation failure in 37 IVF cycles (male and unexplained subfertility).
10. ICSI versus TI or expectant management in natural cycles
We found no trials comparing ICSI versus TI or expectant management in natural cycles.
11. ICSI versus TI in cycles with OH
We found no trials comparing ICSI versus TI in cycles with OH.
12. ICSI versus IUI in natural cycles
We found no trials comparing ICSI versus IUI in natural cycles.
13. ICSI versus IUI in cycles with OH
We found no trials comparing ICSI versus IUI in cycles with OH.
14. ICSI versus IVF
We found no trials comparing ICSI versus IVF.
Sensitivity analyses
The use of RRs and use of a random‐effects model did not substantially alter the findings for any of the comparisons and outcomes.
Discussion
Summary of main results
The aim of this review was to investigate the effectiveness and safety of treatments for couples with male subfertility with regard to live birth rates. Because RCTs are considered to provide the best assessment of the effectiveness of treatments (Johnson 2003), we included only RCTs in this review. The meta‐analyses could include 10 studies, see Table 1; Table 2; Table 3; Table 4; Table 5; and Table 6. These studies reported data on six of the proposed comparisons and included 757 couples with male subfertility who underwent 4400 cycles. The trials in this review revealed that there is no evidence that one of the treatment options is superior to another. However, the available evidence is limited due to small sample size and lack of high quality trials.
Overall completeness and applicability of evidence
The primary outcome for this review was live birth rate per couple. Not all of the trials reported this outcome. Furthermore, evidence was available for only six of the 14 comparisons that we evaluated. We found RCTs to compare expectant management or TI with IUI, IUI with and without OH and IUI with IVF. Unfortunately, we found no RCTs comparing IVF and ICSI. Although these treatments are used on a large scale for male subfertility globally, we only found studies comparing IVF and ICSI in couples with male subfertility with random allocation of oocytes to fertilisation by insemination or injection only (Aboulghar 1995; Aboulghar 1996; Fan 2012; Fishel 2000; Kastrop 1999; Kihaile 2003; Li 2004; Pisarska 1999; Tournaye 2002; van der Westerlaken 2006; Verheyen 1999). Embryos, irrespective of mode of fertilisation, were transferred according to quality. Therefore, we could draw no conclusions on the effect of IVF or ICSI on pregnancy rate in these studies.
Only a few studies reported on adverse effects. Globally, OHSS and multiple pregnancy rates are considered to be an adverse outcome in subfertility practice (Dias 2006; Healy 2004). The risk of perinatal mortality and maternal morbidity associated with multiple pregnancy has become increasingly unacceptable. Therefore, the aim in fertility treatment is shifting from focusing on pregnancy rates alone to the birth of healthy term singletons (Fauser 2005). The use of OH, as part of the IUI treatment, increases the number of available oocytes at the site of conception and thereby might increase the prevalence of OHSS and multiple pregnancy rates. We could not establish on what scale IUI with OH influence OHSS and multiple pregnancy rates in this review. In the literature, only a few studies reported on the differences in multiple pregnancy rates between fertility treatments (Bensdorp 2015; Mansour 2014; Practice Committee of the ASRM 2012; Sullivan 2013). Bensdorp 2015 found no difference in OHSS or multiple pregnancy rate between IUI with OH and IVF with single embryo transfer in couples experiencing male and unexplained subfertility. Unfortunately, other studies did not report on OHSS rates for couples with male subfertility separately and, therefore, we could draw no firm conclusion from this study for couples experiencing male subfertility, due to the lack of power.
WHO criteria are often applied when defining normal semen quality, but they have little prognostic value. Pregnancy has been achieved with IUI with semen that was below these thresholds (Dickey 1999), and also men whose sperm met these standards have been found infertile (Hamilton 2015). In addition, the distinction between male subfertility (semen parameters below the levels of normality defined by WHO) and unexplained subfertility (semen parameters above the levels of normality defined by WHO) is indefinite. Many trials have been performed to analyse the relationship between semen quality and parameters or the TMSC and natural fertility. For clinical practice, it would be useful to have a test that could distinguish subfertile men with good chances of conception from those with poor odds, rather than discriminating between fertile and subfertile men (Verhoeve 2006). There is evidence for a continuous correlation between TMSC and the probability of natural conception (van der Steeg 2011). The predictive capacity of threshold values for (post‐wash) TMSC, progressive motility as well as the role of sperm morphology are yet to be established (Matorras 1995; Ombelet 1997). It appears that semen quality contributes to the effectiveness of IUI (Duran 2002a; Ombelet 2003; Steures 2004; Tijani 2010; Wainer 2004), and that there is a threshold below which IUI is no longer effective (Dickey 1999; van Weert 2004). Furthermore, semen quality seems to play a role in predicting total fertilisation failure in IVF cycles (Repping 2002; Rhemrev 2001).
Other screening tests have been proposed in male subfertility, such as sperm deoxyribonucleic acid (DNA) integrity tests. There are several techniques to measure gross sperm DNA fragmentation (e.g. the sperm chromatin structure assay (SCSA), the sperm chromatin dispersion test (SCD), the TUNEL (terminal deoxyribonucleotide transferase‐mediated dUTP‐X Nick end‐labelling) assay and the Comet assay). An association between the presence of DNA abnormalities in sperm and pregnancy outcome has been established (Avendano 2010; Bakos 2008; Duran 2002a; Simon 2014). In view of the debatable accuracy of these tests to predict pregnancy rates, they do not seem to be of use in practice (Practice Committee of the ASRM 2013).
Quality of the evidence
The quality of the evidence for most comparisons was low or very low. The method of randomisation and allocation concealment were unclear in some trials. Blinding could not be performed due to the nature of the interventions, but this was unlikely to affect the outcomes in this review. The trials included in the meta‐analysis had several limitations, which were most prominent in the oldest studies. These studies had small sample sizes, used a cross‐over design, had a limited duration of follow‐up that was unequal between the studies and the definition of male subfertility and the clinical protocols used varied among the studies. Methodological quality within studies with a cross‐over design in fertility trials have been under debate. A cross‐over design could result in an overestimation of the treatment effect (Khan 1996; Norman 2000). Whether this overestimation could be statistically corrected for or whether it is clinically relevant remains unclear (Cohlen 1998b; McDonnell 2004; Vail 2003), therefore, we only used the pre‐cross‐over data. Furthermore, most studies have determined pregnancy rates as the endpoint, while live birth rate was our primary outcome. The latest updated Cochrane guidelines for analysing and presenting results emphasise the use of pregnancy and live birth per woman or couple in the meta‐analysis. However, in practice such data are not always available.
Potential biases in the review process
Our searches aimed to identify all potentially eligible studies. Besides the potential biases discussed above, there might be some bias due to differential definitions of male subfertility. The trials used different definitions with respect to the numbers of semen samples required, how many and which parameters were assessed, and the thresholds that were subsequently applied for inclusion. Most of the trials used the WHO criteria, in accordance with the year of the study performed.
Agreements and disagreements with other studies or reviews
Conclusion of the previous version of this review and another review were in line with our findings (Bensdorp 2007a; Tournaye 2012). The Cochrane review on the use of IUI in couples experiencing unexplained subfertility found evidence of a higher pregnancy rate in IUI versus TI, both in stimulated cycles (Veltman‐Verhulst 2012). This review also found a higher live birth rate in IUI with OH cycles versus IUI in natural cycles. There was no evidence of a difference in multiple pregnancy rates. In the Cochrane review on the use of IVF versus expectant management or IUI with OH in couples experiencing unexplained subfertility the evidence of a difference was inconclusive (Pandian 2012).
Authors' conclusions
Implications for practice.
The data outlined in this review demonstrated that for the treatment of couples with male subfertility, the evidence from randomised controlled trials is insufficient. No firm conclusions can be drawn about the relative effectiveness of expectant management, intra‐uterine insemination (IUI), with or without ovarian hyperstimulation (OH), in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). More research is needed.
Implications for research.
There is need for large prospective multicentre trials with adequate concealment of allocation and comparing the effectiveness of different treatments for couples with male subfertility. In our opinion, priority should be to assess relative merits of ICSI versus IVF.
Data should be reported as the live birth rate per couple or at least as the ongoing pregnancy rate per couple. Adverse events should also be reported. Cut‐off values of sperm characteristics such as total motile sperm count before and after preparation could be explored in future trials.
What's new
| Date | Event | Description |
|---|---|---|
| 21 January 2016 | New citation required and conclusions have changed | The scope of the review was extended to more invasive treatments for male subfertility (IVF and ICSI) |
| 21 January 2016 | New search has been performed | More extended review plus updated up to 13 April 2015. Added studies: Bensdorp 2015; Francavilla 2009. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 11 November 2008 | Amended | Converted to new review format. |
| 23 August 2007 | New citation required and conclusions have changed | Substantive amendement |
Acknowledgements
Many special thanks to Marian Showell and the staff of the Cochrane Gynaecology and Fertility Group office for their support, performing the electronic searches and their valuable comments during the writing of this review. Thanks to Professor E. te Velde and Professor J.D.F. Habbema, who contributed to the first publication of the protocol (Cohlen 1998).
Appendices
Appendix 1. MDSG search strategy
From inception to 14 April 2015
Keywords CONTAINS "subfertility‐male" or "idiopathic asthenospermia" or "idiopathic oligozoospermia" or "oligo‐asthenozoospermia" or "Oligoasthenospermia" or "oligoasthenoteratozoospermia"or"oligospermia"or"oligozoospermia" or "asthenospermia" or "asthenozoospermia"or"azoospermia"or"varicocele"or"varicocele‐embolization"or"varicocele ligation" or "varicocele‐outcome" or "varicocelectomized " or "varicocelectomy" or "Male" or "male factor" or "male fertility" or "male infertility" or "male subfertility" or "unexplained infertility" or "unexplained subfertility" or "teratozoospermic" or "sperm damage" or "sperm disorders" or "sperm DNA damage" or "sperm DNA integrity" or "sperm extraction techniques" or "sperm motility" AND Keywords CONTAINS "IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic morphologically selected sperm injection" or "superovulation" or "superovulation induction" or "IUI" or "insemination, intrauterine " or "Intrauterine Insemination" or "ART" or "artificial insemination" or "assisted reproduction techniques" or "controlled ovarian hyperstimulation" or "controlled ovarian stimulation" or "COH" or "COH IUI" or "ovulation induction" or "ovulation stimulation" or "timed intercourse" or "expectant management" or "Natural cycle" or "natural cycles" or "coitus" or "wait and see" or Title CONTAINS"IVF" or "ICSI" or "in‐vitro fertilisation " or "in‐vitro fertilisation procedure" or "in vitro fertilization" or "intracytoplasmic sperm injection" or "intracytoplasmic morphologically selected sperm injection" or "superovulation" or "controlled ovarian hyperstimulation" (22 hits)
Appendix 2. CENTRAL search strategy
From inception to 14 April 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (1753) 2 embryo transfer$.tw. (1135) 3 vitro fertili?ation.tw. (1571) 4 ivf.tw. (2386) 5 icsi.tw. (910) 6 intracytoplasmic sperm injection$.tw. (518) 7 (blastocyst adj2 transfer$).tw. (121) 8 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (2457) 9 assisted reproduct$.tw. (510) 10 artificial insemination.tw. (97) 11 iui.tw. (380) 12 intrauterine insemination$.tw. (489) 13 ovulation induc$.tw. (566) 14 (ovari$ adj2 stimulat$).tw. (966) 15 superovulat$.tw. (154) 16 ovarian hyperstimulation.tw. (668) 17 COH.tw. (162) 18 (ovari$ adj2 induction).tw. (32) 19 timed intercourse.tw. (38) 20 expectant management.tw. (318) 21 natural cycle$.tw. (106) 22 exp Coitus/ (270) 23 coitus.tw. (92) 24 intra‐uterine insemination$.tw. (41) 25 watchful waiting.tw. (226) 26 or/1‐25 (6305) 27 exp male infertility/ (506) 28 (asthenozoospermia or oligospermia or azoospermia).tw. (219) 29 Asthenospermia.tw. (33) 30 Teratospermia.tw. (2) 31 (male$ adj2 subfertil$).tw. (66) 32 (male$ adj2 infertil$).tw. (360) 33 (subfertil$ adj2 men).tw. (22) 34 (infertil$ adj2 men).tw. (142) 35 (male$ adj2 fertility).tw. (59) 36 oligoasthenoteratozoospermi$.tw. (17) 37 (idiopathic adj3 infertil$).tw. (80) 38 (idiopathic adj3 subfertil$).tw. (11) 39 Oligozoospermi$.tw. (99) 40 Aspermi$.tw. (2) 41 Teratospermia.tw. (2) 42 unexplained subfertility.tw. (15) 43 unexplained infertility.tw. (274) 44 or/27‐43 (1183) 45 26 and 44 (553)
Appendix 3. MEDLINE search strategy
From inception to 14 April 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (33481) 2 embryo transfer$.tw. (8656) 3 vitro fertili?ation.tw. (17653) 4 ivf.tw. (17313) 5 icsi.tw. (5854) 6 intracytoplasmic sperm injection$.tw. (5221) 7 (blastocyst adj2 transfer$).tw. (598) 8 exp reproductive techniques, assisted/ or exp insemination, artificial/ or exp ovulation induction/ (54694) 9 assisted reproduct$.tw. (9783) 10 artificial insemination.tw. (5138) 11 iui.tw. (1280) 12 intrauterine insemination$.tw. (1897) 13 ovulation induc$.tw. (3509) 14 (ovari$ adj2 stimulat$).tw. (5151) 15 superovulat$.tw. (2972) 16 ovarian hyperstimulation.tw. (4003) 17 COH.tw. (1191) 18 (ovari$ adj2 induction).tw. (232) 19 timed intercourse.tw. (108) 20 expectant management.tw. (1780) 21 natural cycle$.tw. (929) 22 exp Coitus/ (6553) 23 coitus.tw. (2519) 24 intra‐uterine insemination$.tw. (185) 25 watchful waiting.tw. (1698) 26 or/1‐25 (83004) 27 exp male infertility/ (23103) 28 (asthenozoospermia or oligospermia or azoospermia).tw. (5858) 29 Asthenospermia.tw. (281) 30 Teratospermia.tw. (143) 31 (male$ adj2 subfertil$).tw. (625) 32 (male$ adj2 infertil$).tw. (8353) 33 (subfertil$ adj2 men).tw. (439) 34 (infertil$ adj2 men).tw. (3434) 35 (male$ adj2 fertility).tw. (4268) 36 oligoasthenoteratozoospermi$.tw. (301) 37 (idiopathic adj3 infertil$).tw. (940) 38 (idiopathic adj3 subfertil$).tw. (62) 39 Oligozoospermi$.tw. (1809) 40 Aspermi$.tw. (220) 41 Teratospermia.tw. (143) 42 unexplained subfertility.tw. (78) 43 unexplained infertility.tw. (1577) 44 or/27‐43 (32348) 45 26 and 44 (7523) 46 randomized controlled trial.pt. (391583) 47 controlled clinical trial.pt. (89189) 48 randomized.ab. (316291) 49 randomised.ab. (62637) 50 placebo.tw. (165382) 51 clinical trials as topic.sh. (172188) 52 randomly.ab. (228234) 53 trial.ti. (136301) 54 (crossover or cross‐over or cross over).tw. (63611) 55 or/46‐54 (994662) 56 exp animals/ not humans.sh. (4023388) 57 55 not 56 (916956) 58 45 and 57 (667)
Appendix 4. EMBASE search strategy
From inception to 14 April 2015
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (55222) 2 embryo$ transfer$.tw. (13466) 3 in vitro fertili?ation.tw. (21550) 4 icsi.tw. (10216) 5 intracytoplasmic sperm injection$.tw. (6647) 6 (blastocyst adj2 transfer$).tw. (1178) 7 ivf.tw. (26204) 8 exp infertility therapy/ or exp artificial insemination/ or exp intrauterine insemination/ or exp ovulation induction/ (80903) 9 assisted reproduct$.tw. (14085) 10 artificial insemination.tw. (4861) 11 iui.tw. (2090) 12 intrauterine insemination$.tw. (2619) 13 ovulation induc$.tw. (4466) 14 (ovari$ adj2 stimulat$).tw. (7397) 15 superovulat$.tw. (3129) 16 ovarian hyperstimulation.tw. (5490) 17 COH.tw. (1597) 18 (ovar$ adj2 induction).tw. (305) 19 timed intercourse.tw. (166) 20 expectant management.tw. (2456) 21 natural cycle$.tw. (1323) 22 exp coitus/ (5440) 23 coitus.tw. (2448) 24 intra‐uterine insemination$.tw. (303) 25 watchful waiting.tw. (2368) 26 or/1‐25 (108525) 27 exp male infertility/ (32048) 28 (asthenozoospermia or oligospermia or azoospermia).tw. (6986) 29 Asthenospermia.tw. (340) 30 Teratospermia.tw. (177) 31 (male$ adj2 subfertil$).tw. (772) 32 (male$ adj2 infertil$).tw. (10861) 33 (subfertil$ adj2 men).tw. (496) 34 (infertil$ adj2 men).tw. (4297) 35 (male$ adj2 fertility).tw. (4919) 36 oligoasthenoteratozoospermi$.tw. (386) 37 (idiopathic adj3 infertil$).tw. (1242) 38 (idiopathic adj3 subfertil$).tw. (72) 39 Oligozoospermi$.tw. (2043) 40 Aspermi$.tw. (195) 41 Teratospermia.tw. (177) 42 unexplained subfertility.tw. (100) 43 unexplained infertility.tw. (2132) 44 or/27‐43 (41854) 45 26 and 44 (11456) 46 Clinical Trial/ (842028) 47 Randomized Controlled Trial/ (366567) 48 exp randomization/ (65714) 49 Single Blind Procedure/ (19913) 50 Double Blind Procedure/ (119287) 51 Crossover Procedure/ (42210) 52 Placebo/ (253674) 53 Randomi?ed controlled trial$.tw. (113414) 54 Rct.tw. (16495) 55 random allocation.tw. (1392) 56 randomly allocated.tw. (21964) 57 allocated randomly.tw. (2006) 58 (allocated adj2 random).tw. (720) 59 Single blind$.tw. (15500) 60 Double blind$.tw. (148801) 61 ((treble or triple) adj blind$).tw. (434) 62 placebo$.tw. (211189) 63 prospective study/ (283895) 64 or/46‐63 (1442620) 65 case study/ (31021) 66 case report.tw. (278028) 67 abstract report/ or letter/ (918503) 68 or/65‐67 (1221392) 69 64 not 68 (1403772) 70 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5242944) 71 69 not 70 (1348753) 72 45 and 71 (1405)
Appendix 5. PsycINFO search strategy
From inception to 14 April 2015
1 exp reproductive technology/ (1385) 2 in vitro fertili?ation.tw. (568) 3 ivf‐et.tw. (17) 4 (ivf or et).tw. (101465) 5 icsi.tw. (50) 6 intracytoplasmic sperm injection$.tw. (42) 7 (blastocyst adj2 transfer$).tw. (4) 8 assisted reproduct$.tw. (590) 9 artificial insemination.tw. (227) 10 iui.tw. (24) 11 intrauterine insemination$.tw. (19) 12 ovulation induc$.tw. (22) 13 (ovari$ adj2 stimulat$).tw. (49) 14 ovarian hyperstimulation.tw. (10) 15 COH.tw. (75) 16 superovulat$.tw. (5) 17 (ovari$ adj2 induction).tw. (5) 18 timed intercourse.tw. (5) 19 expectant management.tw. (20) 20 natural cycle$.tw. (41) 21 exp "Sexual Intercourse (Human)"/ (12585) 22 coitus.tw. (767) 23 intra‐uterine insemination$.tw. (0) 24 watchful waiting.tw. (117) 25 or/1‐24 (116058) 26 exp Infertility/ (1743) 27 (asthenozoospermia or oligospermia or azoospermia).tw. (38) 28 (male$ adj2 subfertil$).tw. (6) 29 (male$ adj2 infertil$).tw. (165) 30 (infertil$ adj2 men).tw. (73) 31 (male$ adj2 fertility).tw. (125) 32 oligoasthenoteratozoospermi$.tw. (1) 33 Asthenospermia.tw. (2) 34 (idiopathic adj3 infertil$).tw. (12) 35 Oligozoospermi$.tw. (4) 36 Aspermi$.tw. (5) 37 unexplained subfertility.tw. (1) 38 unexplained infertility.tw. (27) 39 or/26‐38 (1930) 40 random.tw. (43215) 41 control.tw. (335612) 42 double‐blind.tw. (18726) 43 clinical trials/ (8513) 44 placebo/ (4032) 45 exp Treatment/ (610630) 46 or/40‐45 (936137) 47 25 and 39 and 46 (180)
Appendix 6. CINAHL search strategy
From inception to 14 April 2015
| # | Query | Results |
| S60 | S58 AND S59 | 31 |
| S59 | EM 2014* or EM 2015* | 439,602 |
| S58 | S45 AND S57 | 264 |
| S57 | S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 | 954,451 |
| S56 | TX allocat* random* | 4,243 |
| S55 | (MH "Quantitative Studies") | 13,282 |
| S54 | (MH "Placebos") | 9,173 |
| S53 | TX placebo* | 33,620 |
| S52 | TX random* allocat* | 4,243 |
| S51 | (MH "Random Assignment") | 38,985 |
| S50 | TX randomi* control* trial* | 85,907 |
| S49 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 763,614 |
| S48 | TX clinic* n1 trial* | 170,899 |
| S47 | PT Clinical trial | 77,668 |
| S46 | (MH "Clinical Trials+") | 186,062 |
| S45 | S26 AND S44 | 930 |
| S44 | S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 | 3,570 |
| S43 | TX unexplained infertility | 102 |
| S42 | TX unexplained subfertility | 20 |
| S41 | TX Oligozoospermi* | 28 |
| S40 | TX (idiopathic adj3 subfertil*) | 3 |
| S39 | TX (idiopathic adj3 subfertil*) | 0 |
| S38 | TX(idiopathic N3 infertil*) | 30 |
| S37 | TX oligoasthenoteratozoospermi* | 9 |
| S36 | TX (male fertil*) | 259 |
| S35 | TX (infertil* N2 men) | 169 |
| S34 | TX (subfertil* N2 men) | 18 |
| S33 | TX (male* N2 infertil*) | 485 |
| S32 | TX (male* N2 subfertil*) | 34 |
| S31 | TX Asthenospermia | 3 |
| S30 | TX (asthenozoospermia or oligospermia or azoospermia) | 141 |
| S29 | TX sperm* | 3,014 |
| S28 | (MH "Sperm Motility") OR (MH "Spermatozoa") OR (MH "Sperm Count") OR "sperm" | 2,161 |
| S27 | "male infertility" | 327 |
| S26 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 8,262 |
| S25 | TX intra‐uterine insemination | 9 |
| S24 | TX coitus | 1,743 |
| S23 | (MM "Coitus") | 762 |
| S22 | TX natural cycle* | 118 |
| S21 | TX expectant management | 398 |
| S20 | TX timed intercourse | 19 |
| S19 | TX (ovari* N2 induction) | 12 |
| S18 | TX COH | 62 |
| S17 | TX ovarian hyperstimulation | 333 |
| S16 | TX superovulat* | 23 |
| S15 | TX ovulation induc* | 574 |
| S14 | TX intrauterine insemination | 149 |
| S13 | TX IUI | 79 |
| S12 | TX artificial insemination | 453 |
| S11 | TX assisted reproduct* | 1,296 |
| S10 | (MM "Insemination, Artificial") | 242 |
| S9 | (MM "Reproduction Techniques+") | 3,949 |
| S8 | TX intracytoplasmic sperm injection* | 234 |
| S7 | TX embryo* N3 transfer* | 769 |
| S6 | TX ovar* N3 hyperstimulat* | 336 |
| S5 | TX ovari* N3 stimulat* | 246 |
| S4 | TX IVF or TX ICSI | 1,248 |
| S3 | (MM "Fertilization in Vitro") | 1,445 |
| S2 | TX vitro fertilization | 2,849 |
| S1 | TX vitro fertilisation | 266 |
Appendix 7. Other electronic sources search strategy (PubMed)
timed intercourse; expectant management; natural cycle; intrauterine; intra uterine; intra‐uterine; insemination; inseminate; IUI; artificial insemination; AI; artificial insemination husband; AIH; ovarian hyperstimulation; in vitro fertilization; IVF; intracytoplasmic sperm injection; ICSI; male infertility; male subfertility; oligoasthenoteratozoospermia; oligospermia; asthenospermia; teratospermia; (randomised controlled trial [Publication Type], controlled clinical trials [Publication Type], randomised controlled trials, random allocation, double‐blind method, single‐blind method, clinical trial [Publication Type], clinical trials, (clinical AND trial*)).
Appendix 8. Data extraction table
Type of studies Randomised controlled trials (RCTs) only. Trial quality 1. Randomisation:
truly randomised, e.g. blocked randomisation list, on‐site computer system, centralised randomisation scheme, random number tables or drawing lots;
stated without further description, or not stated.
2. Concealment of allocation:
adequate (low risk of bias), e.g. sealed opaque envelopes or third party randomisation;
inadequate (high risk of bias), e.g. open list of random numbers, open envelops, tables;
stated without further description or not stated (unclear risk of bias)
3. Study design:
parallel design, cross‐over design or not clear (we included only parallel group studies or pre‐cross‐over data in the meta‐analysis);
single centre or multicentre.
4. Blinding:
if appropriate, were the couple, the care provider and the outcome assessor blinded?
5. Analysis:
by intention to treat (ITT);
power calculation (prospective power calculation, no power calculation or not stated).
6. Drop‐outs:
number or percentage of drop‐outs;
reasons for and details on drop‐outs (selective drop‐out?).
7. Cancelled cycles:
number or percentage of cancelled cycles;
reasons for cancelled cycles.
Study participants
8. Prognostic factors:
type of subfertility;
woman's age;
duration of subfertility;
primary or secondary subfertility.
9. Male subfertility:
definition;
number of semen samples
10. Basic fertility work‐up:
regular menstrual cycles with basal body temperature (BBT) charts, normal mid‐luteal progesterone or sonographic evidence of ovulation;
patent tubes on hysterosalpingography or laparoscopy, or low risk for tubal pathology according to the medical history (Coppus 2007);
postcoital test.
11. Previous fertility treatment
12. Exclusion criteria
Type of interventions
13. Comparison of treatment:
timed intercourse or expectant management (with or without ovarian hyperstimulation (OH));
intra‐uterine insemination (IUI) (with or without OH);
in vitro fertilisation (IVF);
intracytoplasmic sperm injection (ICSI).
14. Stimulation protocols:
type and dosage of drugs for mild OH;
days of ovarian stimulation;
use and timing of ovulation induction;
cancellation criteria, risk of multiple pregnancies or ovarian hyperstimulation syndrome (OHSS);
use of luteal support.
15. Semen sample preparation techniques:
amount of semen injected, number of motile spermatozoa;
method of sperm preparation (washing and centrifugation technique, swim up technique, other).
16. Insemination characteristics
use of single or double insemination;
number of treatment cycles;
actual timing of IUI (time form luteinising hormone (LH) surge, time from human chorionic gonadotrophin (hCG) administration to IUI).
Type of outcome measures
17. Primary outcomes:
live birth rate per couple;
incidence OHSS.
18 Secondary outcomes:
pregnancy rate per couple;
incidence of multiple pregnancies;
incidence of miscarriage;
incidence of total fertilisation failure during IVF.
Data and analyses
Comparison 1. Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in natural cycles (NC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pregnancy rate per couple (all cycles) | 2 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.57 [0.21, 101.61] |
Comparison 2. Intra‐uterine insemination (IUI) versus timed intercourse (TI) both in cycles with ovarian hyperstimulation (OH).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.30, 2.59] |
| 2 OHSS per couple | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pregnancy rate per couple (all cycles) | 3 | 202 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.74, 3.07] |
| 4 Multiple pregnancy rate per couple | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.12, 79.69] |
| 5 Miscarriage rate per couple | 1 | 81 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.19, 5.42] |
Comparison 4. Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus timed intercourse (TI) in natural cycles (NC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.12, 81.35] |
| 2 Pregnancy rate per couple (all cycles) | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.12, 81.35] |
Comparison 5. Intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH) versus IUI in natural cycles (NC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 3 | 346 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.77, 2.33] |
| 1.1 Gonadotrophins | 2 | 305 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.75, 2.29] |
| 1.2 Gonadotrophins + clomiphene citrate (CC) | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.72 [0.10, 70.79] |
| 2 Pregnancy rate per couple (all cycles) | 4 | 399 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.00, 2.82] |
| 2.1 Gonadotrophins | 2 | 328 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.94, 2.73] |
| 2.2 CC | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.83 [0.18, 128.79] |
| 2.3 Gonadotrophins + CC | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.72 [0.10, 70.79] |
| 3 Miscarriage rate per couple | 2 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.20, 5.63] |
Comparison 8. In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in natural cycles (NC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 1 | 53 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.25, 2.35] |
Comparison 9. In vitro fertilisation (IVF) versus intra‐uterine insemination (IUI) in cycles with ovarian hyperstimulation (OH).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple (all cycles) | 2 | 86 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.43, 2.45] |
| 2 OHSS per couple | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pregnancy rate per couple (all cycles) | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.33, 4.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arici 1994.
| Methods | Design: cross‐over alternating Pre‐cross‐over data: available Power calculation: not stated ITT: no ITT Number of couples randomised: 75 Number of couples analysed: 56 Number of couples included in this review: 30 Number of started cycles: not stated Number of completed cycles: 95 Number of drop‐outs: 17 before starting first treatment cycles (failed to return (n = 9), refused randomisation (n = 5), other subfertility factors (n = 3)) and 27 during the study (moved out of the geographical area (n = 3), failed to return (n = 6), cross‐over (n = 18)). 2 couples became pregnant before the initiation of the first treatment cycle Number of cancelled cycles: not stated Centre: single‐centre, private infertility practice of the University of Texas, Southwestern Medical Center at Dallas, TX, USA |
|
| Participants | Couples: male (n = 26) and unexplained (n = 30) subfertility Definition male subfertility: sperm concentration < 20 million/mL, total motility < 50%, normal morphology < 50%, or a combination of these (WHO 1987) Number of semen samples: 2 Age of women (whole group): mean 33 years (range 24‐41) Duration of subfertility: mean 3.5 years (range 2.4‐5.5) Primary/secondary subfertility: not stated Ovulatory status: BBT, luteal progesterone > 10 ng/mL or in‐phase late luteal endometrial biopsy Tubal patency: DLS, HSG, or both PCT: not stated Previous treatment: endocrinologically/surgically correctable factors were treated, no previous ART Exclusion criteria: sperm antibodies |
|
| Interventions | Comparison: IUI with OH cycles vs. IUI in natural cycles Treatment duration: maximum of 4 cycles Method OH: CC 50 mg days 5‐9 Timing ovulation for IUI in natural cycles: LH surge urine Timing ovulation for IUI + OH cycles: measurement follicles > 18 mm Ovulation induction (IUI + OH cycles): hCG 10,000 IM when ≥ 1 follicles 18 mm Number of IUI per cycle: 1 or 2 Timing IUI in natural cycles: first on day of LH peak, a second next day when possible Timing IUI + OH cycles: single IUI 32 hours after injection Sperm preparation: wash (human tubal fluid) and centrifugation Number of inseminated spermatozoa: not stated Cancellation criteria: women exhibiting an anovulatory cycle at any time during the study |
|
| Outcomes | PR per couple for the first cycle, PR per completed cycle OHSS: not stated Miscarriage rate: not stated Multiple PR: not stated Ectopic PR: not stated Definition/diagnosis pregnancy: gestational sac confirmed by USS |
|
| Notes | Large number of drop‐outs. Authors supplied unpublished pre‐cross‐over data. No stratification by diagnosis category of subfertility, unequal division of couples between treatment options | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Less than 95% of the couples included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, adverse effects not stated |
| Other bias | High risk | Cross‐over design |
Bensdorp 2015.
| Methods | Design: parallel study Power calculation (for whole group): stated ITT: done Number of couples randomised: 602 (male subfertility, n = 57) Number of couples analysed: 602 (male subfertility, n = 57) Number of couples included in this review: 36 Number of started cycles: 104 Number of completed cycles: 97 Number of drop‐outs: 4 (personal reasons (n = 2), medical reasons (n = 2)) Number of cancelled cycles: 7 (no embryo transfer (n = 4), no IUI (n = 3)) Centre: multicentre, 17 centres, the Netherlands |
|
| Participants | Couples: unexplained (n = 545) and mild male (n = 57) subfertility Definition male subfertility: pre‐wash TMSC 3‐10 million Number of semen samples: not stated Mean age of women (whole group): IUI + OH 34 years (SD ± 3.67), IVF‐SET 33 years (SD ± 3.39), IVF‐MNC 33 years (SD ± 3.50) Duration of subfertility (mean (IQR) for whole group): IUI + OH 2.30 years (1.82‐3.13), IVF‐SET 2.13 years (1.73‐3.01), IVF‐MNC 2.14 years (1.77‐2.81) Primary/secondary subfertility: mixed Ovulatory status: done Tubal patency: chlamydia antibody test, HSG or DLS PCT: not stated Previous treatment: none Exclusion criteria: anovulation, double‐sided tubal disease, severe endometriosis, premature ovarian failure and endocrine disorders |
|
| Interventions | Comparison: IVF‐SET (n = 18) vs. IVF‐MNC (n = 21) vs. IUI in cycles with OH (n = 18) Treatment duration: maximum 12 months (3 cycles of IVF‐SET plus subsequent cryo cycles, 6 cycles of IVF‐MNC or 6 cycles of IUI with COH) Method OH for IVF‐SET: long or short agonist or antagonist protocol (adhere to local stimulation protocols), COH using FSH 150 IU Method OH for IVF‐MNC: daily injections of GnRH antagonist 0.25 mg and FSH 150 IU when the leading follicle had a diameter of ≥ 14 mm Method OH for IUI: CC 100 mg (cycle day 3‐7) or FSH 75 IU (daily) Timing ovulation for IVF‐SET: measurement of ≥ 2 follicles of ≥ 18 mm Timing ovulation for IVF‐MNC: measurement of a follicle of 17‐18 mm Timing ovulation for IUI + OH cycles: measurement of at least 1 follicle of 17‐18 mm Ovulation induction for IVF: hCG 10,000 IU Ovulation induction for IUI + OH cycles: hCG 5000 IU Number of IUI per cycle: 1 Timing IUI + OH cycles: 36 hours after hCG Semen preparation: not stated Number of inseminated spermatozoa: not stated Embryo transfer: 2‐4 days after oocyte retrieval 1 good‐quality embryo or 2 embryos if no good embryos were available After results of pilot study only SET Luteal phase support (IVF): hCG 1500 IU on day 5, 8 and 11 after oocyte retrieval Cancellation criteria IUI: OHSS (> 3 follicles ≥ 16 mm or > 5 follicles > 12 mm) Cancellation criteria IVF: not stated |
|
| Outcomes | Live birth and PR per couple OHSS: stated Miscarriage rate: stated Multiple PR: stated Ectopic PR: not stated Definition/diagnosis pregnancy: confirmed by USS |
|
| Notes | Power calculation: 200 couples were needed per treatment group to obtain an 80% power to detect a difference of 12.5% between IUI with COH and IVF‐SET Inclusion criteria: women aged 18‐38 years, an unfavourable prognosis for natural conception (Hunault < 30%) and diagnosis of unexplained or mild male subfertility. Author supplied separate data for male subfertility. No stratification by diagnosis category of subfertility |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A web‐based generated program |
| Allocation concealment (selection bias) | Low risk | Unique numbers with allocation code |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol available (Bensdorp 2009) |
| Other bias | Low risk | No other bias |
Cohlen 1998a.
| Methods | Design: cross‐over alternating Pre‐cross‐over data: available Power Calculation: stated ITT: done Number of couples randomised: 74 Number of couples analysed: 74 Number of couples included in this review: 74 Number of started cycles: 320 Number of completed cycles: 308 Number of drop‐outs: 6 (personal reasons) Number of cancelled cycles: 12 (premature or missed LH surge (n = 7), OHSS (n = 5)) Centre: single centre, Utrecht, the Netherlands |
|
| Participants | Couples: male subfertility Definition male subfertility: concentration < 20 million/mL, motility < 40%, normal morphology < 40%, or a combination of these Number of semen samples: ≥ 2 Age of women: 30.7 years (range 24‐39) Duration of subfertility: 3.1 years (range 2‐9) Primary/secondary subfertility: mixed Ovulatory status: BBT and luteal progesterone > 9.7 ng/mL Tubal patency: HSG, DLS, or both PCT: done Previous treatment: not stated Exclusion criteria: sperm antibodies, cervical factor |
|
| Interventions | Comparison: IUI with OH cycles vs. IUI in natural cycles Treatment duration: maximum 6 cycles Method OH: HMG 75 IU/day up to HMG 150 IU/day starting on cycle day 3 Timing ovulation for IUI in natural cycles: LH surge blood Timing ovulation for IUI + OH cycles: measurement follicles ≥ 18 mm or LH surge blood Ovulation induction (IUI + OH cycles): hCG 5000 IU Number of IUI per cycle: 1 Timing IUI in natural cycles: 26 hours after LH surge Timing IUI + OH cycles: 38‐40 hours after hCG Sperm preparation: wash (Ham's F10) and Percoll Number of inseminated spermatozoa: no conception observed below threshold of < 1 million motile spermatozoa Cancellation criteria: ≥ 4 follicles ≥ 18 mm or oestradiol > 1635 pg/mL, premature LH surge, no LH surge detected |
|
| Outcomes | PR and live birth rate per started and completed cycle OHSS rate: stated Miscarriage rate: stated Multiple PR: stated Ectopic PR: not stated Definition/diagnosis pregnancy: hCG in urine + USS at 6‐7 weeks |
|
| Notes | Power calculation: 150 cycles per treatment would be needed to detect an 8% difference (numbers based on previous studies) between natural cycles vs. stimulated cycles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data, adequate description of drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Cross‐over design |
Francavilla 2009.
| Methods | Design: cross‐over alternating Pre‐cross‐over data: not stated Power calculation: stated ITT: not stated Number of couples randomised: not stated Number of couples analysed: 73 Number of couples included in this review: 63 Number of started cycles: 384 Number of completed cycles: 384 Number of drop‐outs: not stated Number of cancelled cycles: none Centre: single centre, L'Aquila, Italy |
|
| Participants | Couples: male subfertility (OAT) (n = 63), immunological subfertility (n = 10) Definition male subfertility: motile sperm count < 10 million/mL (due to oligozoospermia (< 20 million/mL), asthenozoospermia (< 50% progressive motility)), teratozoospermia (normal sperm morphology < 15%), immunological subfertility, or a combination of these Number of semen samples: ≥ 2 Age of women: ≤ 40 years Duration of subfertility: ≥ 2 years Primary/secondary subfertility: primary Ovulatory status: mid‐luteal phase progesterone ≥ 10 ng/mL, day 3 FSH < 10 IU/mL Tubal patency: HSG, DLS, or both PCT: done Previous treatment: not stated Exclusion criteria: < 1 million motile spermatozoa after semen preparation |
|
| Interventions | Comparison: TI in natural cycles vs. IUI with OH cycles vs. IUI in natural cycles Treatment duration: maximum 9 cycles (6 IUI cycles) Method OH: CC 50 mg/day (cycle day 3‐7) and hMG 75 IU/day (cycle day 8 and 9) Timing of ovulation: LH surge urine or measurement of at least 1 follicle ≥ 20 mm when no LH surge was detected Ovulation induction (when no LH surge was detected): hCG 10,000 IU Number of IUI per cycle: 1 or 2 Timing IUI: the day after LH surge and in 2 consecutive days if the LH surge was detected in the evening or 39‐41 hours after hCG Timing intercourse: the day after LH surge Sperm preparation: swim up procedure Number of inseminated spermatozoa: not stated Cancellation criteria: not stated |
|
| Outcomes | PR per completed cycle, authors supplied live birth rates per completed cycle OHSS: stated Miscarriage rate: stated Multiple PR: stated Ectopic PR: not stated Definition/diagnosis pregnancy: intrauterine gestational sac detected by USS |
|
| Notes | Authors supplied unpublished pre‐cross‐over data. No stratification by diagnosis category of subfertility (male subfertility and immunological subfertility) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | On chronological basis |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors supplied unpublished pre‐cross‐over data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Cross‐over design |
Goverde 2000.
| Methods | Design: parallel study Power calculation (for whole group): stated ITT: done Number of couples randomised: 258 Number of couples analysed: 258 Number of couples included in this review: 77 Number of started cycles: 963 (male subfertility = 293) Number of completed cycles: 184 Number of drop‐outs (whole group): > 10% Number of cancelled cycles (whole group): > 10% Centre: single centre, Vrije Universitieit Medical Centre, Amsterdam, the Netherlands |
|
| Participants | Couples: male (n = 77) and unexplained (n = 179) subfertility Definition male subfertility: TMSC of < 20 million progressively motile sperm Number of semen samples: 3 out of 5 Mean age of women (only male subfertility): IUI + OH 31.7 years (SD ± 3.92), IUI in natural cycle 31.6 years (SD ± 3.73), IVF 32.1 years (SD ± 4.20) Duration of subfertility (only male subfertility): IUI + OH 4.2 years (SD ± 1.9), IUI in natural cycle 3.9 years (SD ± 1.7), IVF 4.5 years (SD ± 2.8) Primary/secondary subfertility: mixed Ovulatory status: BBT, endometrial biopsy Tubal patency: DLS + HSG PCT: done Previous treatment: not stated Exclusion criteria: cycle disorders, untreated endometriosis, bilateral occluded tubes or semen sample yielded < 1 million progressively motile spermatozoa after processing, > 20% carried antibodies or > 50% had no acrosome |
|
| Interventions | Comparison: IUI with OH cycles vs. IUI in natural cycles vs. IVF Treatment duration: maximum 6 cycles Method OH for IUI: FSH 75 IU (starting dose) Method OH for IVF: women < 38 years: 'long' protocol: GnRH agonist and FSH or hMG 150‐225 IU; women > 38 years: 'short' protocol Timing ovulation for IUI in natural cycles: LH surge urine Timing ovulation for IUI + OH cycles: measurement 1‐3 follicles > 18 mm or LH surge urine Timing ovulation for IVF: measurement at least 1 follicle > 18 mm and 3 follicles > 16 mm Ovulation induction (IUI + OH cycles and IVF): hCG 10,000 IU Number of IUI per cycle: 1 Timing IUI in natural cycles: 20‐30 hours after LH surge Timing IUI + OH cycles: 20‐30 hours after LH surge, 40‐42 hours after hCG when no LH surge was detected Semen preparation: Percoll gradient technique Number of inseminated spermatozoa: not stated Embryo transfer: 48‐72 hours after oocyte retrieval: women ≤ 35 years: maximum 2 embryos; women > 35 years: maximum 3 embryos Luteal phase support (IVF): 3 doses of progesterone 200 mg/day intravaginally, in case of breakthrough bleeding hCG 1500 IU every 48 hour Cancellation criteria IUI: > 3 follicles of ≥ 18 mm or > 6 follicles of ≥ 14 mm Cancellation criteria IVF: serum oestradiol > 20,000 nmol/L |
|
| Outcomes | Live birth rate per couple (PR include only pregnancies that resulted in at least 1 live birth) OHSS: stated Miscarriage rate: not stated Multiple PR: stated Ectopic PR: not stated Definition/diagnosis pregnancy: LH urine and USS confirmation |
|
| Notes | Power calculation: 80 couples were needed per treatment group to obtain a 90% power to detect a difference of 9% between IUI and IVF. Stratification for woman's age, duration of subfertility, diagnosis, category of subfertility, presence of either 1 or 2 ovaries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Numbered masked and sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data, adequate description of drop‐outs/cancellations |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Gregoriou 1996.
| Methods | Design: cross‐over after 3 cycles Pre‐cross‐over data: available Power calculation: not stated ITT: not stated Number of couples randomised: 62 Number of couples analysed: 62 Number of couples included in this review: 62 Number of started cycles: 314, before cross‐over 172 Number of completed cycles: 258, before cross‐over 143 Number of drop‐outs: not stated Number of cancelled cycles: 56 Centre: single centre, Athens, Greece |
|
| Participants | Couples: male subfertility Definition male subfertility: sperm concentration < 20 million/mL, progressive motility < 30%, normal morphology < 40%, or a combination of these Number of semen samples: 3 Age of women: mean 30.5 years (SD ± 2.6) Duration of subfertility: mean 5.8 years (SD ± 3.9) Primary/secondary subfertility: mixed Ovulatory status: BBT, luteal progesterone ≥ 32 nmol/L and in‐phase endometrial biopsy Tubal patency: HSG and DLS PCT: not stated Previous treatment: not stated Exclusion criteria: abnormal serum levels of testosterone, dehydroepiandrosterone‐sulphate, prolactin or thyroid‐stimulating hormone in women |
|
| Interventions | Comparison: IUI with OH cycles vs. TI with OH Treatment duration: maximum 6 cycles Method OH: day 3‐9 hMG 75 IU/day, if no increase in serum oestradiol was observed, dose increased to hMG 150 IU/day for next 5 days Timing ovulation: measurement of follicle > 16 mm and oestradiol ≤ 5500 pmol/L, 24 hours after last hMG Ovulation induction: hCG 10,000 IU Number of IUI per cycle: 1 Timing IUI: 36‐40 hours after hCG administration Timing intercourse: 36‐40 hours after hCG administration Sperm preparation: wash (Ham's 10) 2‐layer Percoll gradient (40% and 90%) Number of inseminated spermatozoa: not stated Cancellation criteria: OH |
|
| Outcomes | PR per completed and per started cycle OHSS: not stated Miscarriage: not stated Multiple PR: not stated Ectopic PR: not stated Definition/diagnosis pregnancy: hCG serum and gestational sac on USS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data (pre‐ and after cross‐over) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, adverse effects not stated |
| Other bias | High risk | Cross‐over design |
Guzick 1999.
| Methods | Design: parallel Power calculation: not stated ITT: unclear Number of couples randomised: 932 Number of couples analysed: not stated Number of couples included in this review: 254 Number of started cycles: 4676 Number of completed cycles: 2678 Number of drop‐outs: 167 Number of cancelled cycles: 292 Centre: multicentre, 10 clinical sites, USA |
|
| Participants | Couples: male and unexplained subfertility Definition male subfertility: sperm concentration < 20 million/mL, motility < 50% Number of semen samples: not stated Age of women (whole group): 32 years (SD ± 4) Duration of subfertility: IUI in natural cycle 34 months (SD ± 4), IUI with OH cycle 35 (SD ± 5) Primary/secondary subfertility: mixed Ovulatory status: in phase endometrial biopsy Tubal patency: DLS + HSG PCT: not stated Previous treatment: none Exclusion criteria: antisperm antibodies |
|
| Interventions | Comparison: IUI with OH cycles vs. IUI in natural cycles (ICI in natural cycles vs. ICI with OH cycles) Treatment duration: maximum 6 cycles Method OH: FSH 150 IU days 3‐7, from day 8 onwards dose adjusted Timing ovulation for IUI in natural cycles: LH surge urine Timing ovulation for IUI + OH cycles: measurement of 2 follicles ≥ 18 mm, serum oestradiol concentration 500‐3000 pg/mL Ovulation induction (IUI + OH cycles): hCG 10,000 IU Number of IUI per cycle: 1 Timing IUI in natural cycles: day after LH surge Timing IUI + OH cycles: 36‐40 hours after hCG Sperm preparation: Ham's F‐10 Number of inseminated spermatozoa: not stated Cancellation criteria: if no surge in urinary excretion LH for IUI in natural cycle or for IUI + OH if serum oestradiol after 3 days > 3000 pg/mL |
|
| Outcomes | Live birth per couple or cycle, PR per couple or cycle OHSS rate: stated Miscarriage rate: stated Multiple PR: stated Ectopic PR: stated Definition/diagnosis pregnancy: hCG measured on day 15 and 17 |
|
| Notes | Author provided separate data for male subfertility, but states that randomisation might not hold | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted block procedure |
| Allocation concealment (selection bias) | Low risk | Locked computer files |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Author supplied separate data for male subfertility |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Kerin 1984.
| Methods | Design: cross‐over alternating Pre‐cross‐over data: partly extractable Power calculation: not stated ITT: not stated Number of couples randomised: 35 Number of couples analysed: 35 Number of couples included in this review: 21 Number of started cycles: not stated Number of completed cycles: 39 Number of drop‐outs: not stated Number of cancelled cycles: not stated Centre: single centre, Adelaide, Australia |
|
| Participants | Couples: male subfertility Definition male subfertility: ≥ 2 of the following criteria: sperm density < 40 million/mL, motility < 45%, normal morphology < 40%, < 60 million motile spermatozoa Number of semen samples: ≥ 3 Age of women: not stated Duration of subfertility: > 3 years, not further specified Primary/secondary subfertility: not stated Ovulatory status: luteal progesterone > 20 nmol/L Tubal patency: laparoscopic tubal dye insufflation test PCT: done Previous treatment: not stated Exclusion criteria: positive PCT |
|
| Interventions | Comparison: IUI in natural cycles vs. TI in natural cycles vs. natural cycles Treatment duration: maximum 12 cycles Timing ovulation natural cycles: symptothermal methods Timing ovulation TI in natural cycles and IUI in natural cycles: LH surge Ovulation induction: none Timing IUI: day of LH surge Timing intercourse: day after LH surge Number of inseminations: 1 Sperm preparation: Wittingham's T6 medium wash with swim‐up Number of inseminated spermatozoa: stated Cancellation criteria: not stated |
|
| Outcomes | PR per completed cycle OHSS: not stated Miscarriage rate: not stated Multiple PR: not stated Ectopic PR: not stated Definition/diagnosis pregnancy: not further defined |
|
| Notes | Pre‐cross‐over data only partly available. No reply from author, received letter back as wrongly addressed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pre‐cross‐over data partly available |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, adverse effects not stated |
| Other bias | High risk | Cross‐over design |
Melis 1995.
| Methods | Design: parallel Power calculation: not stated ITT: no Number of couples randomised: 200 Number of couples analysed: 184 Number of couples included in this review: 81 Number of started cycles: not stated Number of completed cycles: 462, 213 for male subfertility Number of drop‐outs/cancelled cycles: 16; 11 for male subfertility (family problems (n = 5), poor response to ovulation induction (n = 3), exaggerated response to ovulation induction (n = 8) Centre: single centre, Cagliari, Italy |
|
| Participants | Couples: male (n = 92) and unexplained (n = 108) subfertility Definition male subfertility: sperm concentration 10‐20 million/mL, progressive motility 15‐25%, total motility 30‐50%, normal morphology 30‐50% Number of semen samples: ≥ 2 Age of women: 34.2 years (SD ± 4.8, range 27‐36) Duration of subfertility: 51.2 months (SD ± 14.3) Primary/secondary subfertility: not stated Ovulatory status: in‐phase endometrial biopsy, USS evidence ovulation, female endocrine profile Tubal patency: DLS, HSG PCT: done Previous treatments: all couples had received 3 cycles CC‐induced TI and 3 cycles CC‐induced IUI Exclusion criteria: severe male subfertility, female factor subfertility |
|
| Interventions | Comparison: IUI with OH cycles vs. TI with OH cycles Treatment duration: maximum 3 cycles Method OH: 3 ampoules FSH starting from cycle day 3, personally adjusted to endocrine monitoring and USS Timing of ovulation induction: measurement of at least 2 follicle ≥ 16 mm and oestradiol 800‐1500 pg/mL Ovulation induction: 10.000 hCG 36 hours after last injection FSH Number of IUI per cycle: 1 Timing of IUI: 30‐36 hours after hCG Timing intercourse: 12 hours after hCG Sperm preparation: wash (Menezo B2) and swim unconventional layering technique Number of inseminated spermatozoa: not stated Cancellation criteria: oestradiol > 1500 pg/mL or poor response to OH |
|
| Outcomes | PR per couple PR per completed cycle OHSS: stated Miscarriage rate: stated Multiple PR: stated Ectopic PR: stated Definition/diagnosis pregnancy: hCG (> 25 IU/L) in serum always confirmed by USS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Less than 95% of the couples included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Nan 1994.
| Methods | Design: cross‐over alternating Pre‐cross‐over data: available Power calculation: not stated ITT: not stated Number of couples randomised: 76 Number of couples analysed: 76 Number of couples included in this review: 59 Number of started cycles: 249 Number of completed cycles: 202 Number of drop‐outs: not stated Number of cancelled cycles: 47 Centre: single centre, University Hospital Utrecht, the Netherlands |
|
| Participants | Couples: male subfertility Definition male subfertility: sperm concentration < 20 million/mL, total motility < 40%, normal morphology < 40%, or a combination of these Number of semen samples: 4 Age of women: 32 years (range 24‐39) Duration of subfertility: 4.5 years (range 2‐10) Primary/secondary subfertility: mixed Ovulatory status: BBT, luteal progesterone ≥ 31 nmol/L Tubal patency: DLS, HSG PCT: done Previous fertility treatment: not stated Exclusion criteria: sperm antibodies |
|
| Interventions | Comparison: IUI with OH cycles vs. TI with OH cycles Treatment duration: maximum 6 cycles Method OH: 150 IU HMG/day starting from cycle day 3 Timing ovulation: measurement of leading follicle ≥ 18 mm and LH surge Ovulation induction: hCG 10,000 IU Number of IUI per cycle: 1 Timing IUI: 38‐40 hours after hCG injections or following morning in case LH surged Timing intercourse: evening next day, or same evening in case LH surged Method of semen preparation; Wash (Ham's F10) and Percoll gradient technique Number of inseminated spermatozoa: not stated Cancellation criteria: ≥ 4 follicles ≥ 18 mm or oestradiol > 6000 pmol/L |
|
| Outcomes | Live birth per cycle, PR per completed cycle, PR per started cycle OHSS: stated Miscarriage rate: stated Multiple PR: stated Ectopic PR: not stated Abruptio placenta: stated Definition/diagnosis pregnancy: HCG urine and USS confirmation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding stated, but outcome was not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Cross‐over design |
ART: assisted reproductive technique; BBT: basal body temperature; CC: clomiphene citrate; COH: controlled ovarian hyperstimulation; DLS: diagnostic laparoscopic surgery; FSH: follicle‐stimulating hormone; GnRH: gonadotropin‐releasing hormone; hCG: human chorionic gonadotrophin; hMG: human menopausal gonadotrophin; HSG: hysterosalpingography; ICI: intra‐cervical insemination; IQR: interquartile range; IM: intramuscular; ITT: intention to treat; IU: international unit; IUI: intra‐uterine insemination; IVF: in vitro fertilisation; LH: luteinising hormone; MNC: modified natural cycle; n: number of couples; OAT: oligoasthenoteratozoospermia; OH: ovarian hyperstimulation; OHSS: ovarian hyperstimulation syndrome; PCT: post coital test; PR: pregnancy rate; SD: standard deviation; SET: single embryo transfer; TI: timed intercourse; TMSC: total motile sperm count; USS: ultrasound scan.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboulghar 1995 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Aboulghar 1996 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Agarwal 2004 | Unexplained subfertility couples |
| Buvat 1990 | Number of couples receiving IUI and TI was not stated |
| Crosignani 1994 | Authors could not provide pre‐cross‐over data |
| Cruz 1986 | Different comparison: IUI vs. ICI |
| Elizur 2004 | Not an RCT, but a retrospective study |
| Elzeiny 2014 | Unexplained subfertility couples |
| Evans 1991 | Biochemical pregnancies only, no response from the author |
| Fan 2012 | Oocytes were randomised between IVF and ICSI, no outcome data available per couple |
| Fishel 2000 | Oocytes were randomised between IVF and ICSI, no outcome data available per couple |
| Friedman 1989 | Preliminary report, different comparison: IUI vs. ICI |
| Galle 1990 | Not an RCT, but an observational study |
| Goverde 2001 | Not an RCT, correspondence |
| Hewitt 1985 | Not an RCT, an observational study |
| Ho 1989 | Authors could not provide pre‐cross‐over data |
| Ho 1992 | Authors could not provide pre‐cross‐over data |
| Karlström 2000 | Compares IUI both with different forms of OH |
| Kastrop 1999 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Kihaile 2003 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Kirby 1991 | Authors could not provide pre‐cross‐over data |
| Li 2004 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Martinez 1991 | Authors could not provide pre‐cross‐over data |
| Melis 1987 | Different comparison: ICI in natural cycle vs. ICI with OH |
| Moolenaar 2015 | Not an RCT, but a retrospective study |
| Nulsen 1993 | Not an RCT: quasi randomised, biochemical pregnancies only |
| Pisarska 1999 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Plachot 2002 | Not an RCT: oocytes were quasi randomised between IVF and ICSI |
| Prentice 1995 | Not an RCT: quasi randomised, based on hospital case record number |
| Soliman 1993 | Incomplete data on treatment of control group |
| te Velde 1989 | Authors could not provide pre‐cross‐over data |
| Tournaye 2002 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| van der Westerlaken 2006 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Verheyen 1999 | Oocytes were randomly divided between IVF and ICSI, no outcome data available per couple |
| Xie 2015 | Not an RCT |
| Zayed 1997 | Not an RCT: quasi randomised, patient preference |
ICI: intra‐cervical insemination; ICSI: intracytoplasmic sperm injection; IUI: intra‐uterine insemination; IVF: in vitro fertilisation; OH: ovarian hyperstimulation; RCT: randomised controlled trial; TI: timed intercourse.
Characteristics of studies awaiting assessment [ordered by study ID]
Aribarg 1995.
| Methods | Design: cross‐over alternating Pre‐cross‐over data: not stated Power calculation: not stated ITT: not done, could not be extracted Number of couples randomised: not stated Number of couples analysed: 50 Number of started cycles: not stated Number of completed cycles: 495 Number of drop‐outs: not stated Number of cancelled cycles: not stated Centre: single centre, Chulalongkorn University, Bangkok, Thailand |
| Participants | Couples: male subfertility Definition male subfertility: sperm concentration 1‐20 million/mL, motility < 50%, normal morphology < 30%, or a combination of these (WHO 1992) Number of semen samples: 2 Age of women: mean 25.5 years (range 23‐37) Duration of subfertility: mean 3.7 years (range 2‐15) Primary/secondary subfertility: not stated Ovulatory status: BBT Tubal patency: DLS and HSG PCT: not stated Previous treatments: not stated Exclusion criteria: severe oligospermia < 1 million/mL, semen with evidence of bacterial infection, women with endometriosis, hormonal, tubal or ovulatory disturbance diagnosed |
| Interventions | Comparison: IUI with OH cycles vs. TI in natural cycles Treatment duration: maximum 4‐6 cycles Method of OH: CC 100 mg/day (cycle day 3‐7) Ovulation induction: none Number of IUI per cycle: 1, sometimes 2 Timing of IUI: timed by USS, BBT and LH surge urine Timing of intercourse: evening of the day of the LH surge and on the following day Sperm preparation: wash and swim up, Ham's F‐10 medium Number of inseminated spermatozoa: no conception observed below threshold of < 5 million motile spermatozoa Cancellation criteria: not stated |
| Outcomes | PR per completed cycle OHSS: not stated Miscarriage rate: stated Multiple PR: stated Ectopic PR: not stated Definition/diagnosis pregnancy: hCG in blood and confirmation by USS, clinical examination |
| Notes | Quality of spermatozoa in terms of their concentration and motility before and after sperm washing was compared No pre‐cross‐over data available, no reply from author |
Jaroudi 1998.
| Methods | Design: cross‐over alternating Pre‐cross‐data: not stated Power calculation: not stated ITT: not stated Number of couples randomised: 36 Number of couples analysed: 36 Number of started cycles: not stated Number of completed cycles: 110 Number of drop‐outs: not stated Number of cancelled cycles: not stated Centre: single centre, Riyadh, Saudi Arabia |
| Participants | Couples: male subfertility Definition male subfertility: sperm count 1‐20 million/mL or motility 10‐30% with > 1 million total spermatozoa Number of semen samples: 3 Age of women: 27 years (SD ± 3.7) Duration of subfertility: 6.5 years (SD ± 3.0) Primary/secondary subfertility: not stated Ovulatory status: not stated Tubal patency: HSG PCT: not stated Previous treatment: not stated Exclusion criteria: not stated |
| Interventions | Comparison: IUI with OH cycles vs. TI with OH cycles Treatment duration: maximum 6 cycles Method OH: hMG 150 IU/day started from cycle day 3 (adjusting to woman's response), buserelin acetate spray 500 μg/day started from cycle day 2 Timing of ovulation: measurement of 3‐5 follicles > 15 mm and appropriate plasma oestradiol concentration (800 pmol/L per follicle) Ovulation induction: hCG 10,000 IU Number of IUI per cycle: 1 Timing of IUI: 34 hours after hCG Timing of intercourse: 36 hours after hCG Luteal support: progesterone 200 mg/day Sperm preparation: Percoll gradient technique Number of inseminated spermatozoa: not stated Cancellation criteria: not stated |
| Outcomes | PR per completed cycle OHSS: not stated Miscarriage rate: not stated Multiple PR: not stated Ectopic PR: not stated Definition/diagnosis pregnancy: USS 6‐7 weeks' gestational sac |
| Notes | No pre‐cross‐over data available, no reply from author |
Kerin 1987.
| Methods | Design: cross‐over alternating Pre‐cross‐over data: not stated Power calculation: not stated ITT: not stated Number of couples randomised: not stated Number of couples analysed: not stated Number of started cycles: not stated Number of completed cycles: 509 Number of drop‐outs: not stated Number of cancelled cycles: not stated Centre: single centre, Los Angeles, USA |
| Participants | Couples: male subfertility Definition male subfertility: assessment of at least 2 abnormalities:
Number of semen samples: 3 Age of women: < 41 years, not specified Duration of subfertility: ≥ 3 years, not specified Primary/secondary subfertility: primary Ovulatory status: normal endocrine profile (LH, FSH, prolactin) Tubal patency: tested, not further specified PCT: not stated Previous treatment: not stated Exclusion criteria: sperm antibodies, endometriosis, sperm count < 100,000 after preparation |
| Interventions | Comparison: IUI in natural cycles vs. TI in natural cycles Treatment duration: maximum 12 cycles Timing of ovulation: LH surge urine Ovulation induction: none Number of IUI per cycle: 1 Timing of IUI: first of second day after LH surge Timing of intercourse: day of LH surge Sperm preparation: swim up procedure Number of inseminated spermatozoa: stated Cancellation criteria: not stated |
| Outcomes | PR per completed cycle OHSS: not stated Miscarriage rate: stated Multiple PR: not stated Ectopic PR: stated Definition/diagnosis pregnancy: not stated |
| Notes | Stratified for moderate semen defect and severe semen defect Compared IUI within 24 hours and within 48 hours after LH surge No pre‐cross‐over data available, no reply from author |
BBT: basal body temperature; CC: clomiphene citrate; DLS: diagnostic laparoscopic surgery; FSH: follicle‐stimulating hormone; hCG: human chorionic gonadotrophin, hMG: human menopausal gonadotrophin; HSG: hysterosalpingography; ITT: intention to treat; IUI: intra‐uterine insemination; LH: luteinising hormone; OH: ovarian hyperstimulation; OHSS: ovarian hyperstimulation syndrome; PCT= post coital test, PR: pregnancy rate; SD: standard deviation; TI: timed intercourse; USS: ultrasound scan.
Differences between protocol and review
None.
Contributions of authors
M Cissen: took the lead in rewriting the protocol. Performed the literature search, selected trials and performed data extraction and analysis. Wrote the review.
AJ Bensdorp: primary author of the previous publication of the review (Bensdorp 2007b). Assisted in rewriting the protocol.
BJ Cohlen: primary author of the first publication of the review (Cohlen 1998; Cohlen 2000). Substantial contribution writing update.
JP de Bruin: formulation of research question; substantial contribution writing update.
S Repping: formulation of research question; substantial contribution writing update.
M van Wely: helped in rewriting the protocol and writing the review. As the second review author, she selected trials and performed data extraction and analysis.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
None known for any of the review authors.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Arici 1994 {published data only}
- Arici A, Byrd W, Bradshaw K, Kutteh WH, Marshburn P, Carr BR. Evaluation of clomiphene citrate and human chorionic gonadotropin treatment: a prospective, randomized, crossover study during intrauterine insemination cycles. Fertility & Sterility 1994;61:314‐8. [DOI] [PubMed] [Google Scholar]
Bensdorp 2015 {published data only}
- Bensdorp AJ, Tjon‐Kon‐Fat RI, Bossuyt PM, Koks CA, Oosterhuis GJ, Hoek A, et al. Prevention of multiple pregnancies in couples with unexplained or mild male subfertility: randomised controlled trial of in vitro fertilisation with single embryo transfer or in vitro fertilisation in modified natural cycle compared with intrauterine insemination with controlled ovarian hyperstimulation. BMJ 2015;350:7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohlen 1998a {published data only}
- Cohlen BJ, Velde ER, Kooij RJ, Looman CWN, Habbema JDF. Controlled ovarian hyperstimulation and intrauterine insemination for treating male subfertility: a controlled study. Human Reproduction 1998;13:1553‐8. [DOI] [PubMed] [Google Scholar]
Francavilla 2009 {published data only}
- Francavilla F, Sciarretta F, Sorgentone S, Necozione S, Santucci R, Barbonetti A, et al. Intrauterine insemination with or without mild ovarian stimulation in couples with male subfertility due to oligo/astheno‐ and/or teratozoospermia or antisperm antibodies: a prospective cross‐over trial. Fertility & Sterility 2009;92:1009‐11. [DOI] [PubMed] [Google Scholar]
Goverde 2000 {published data only}
- Goverde AJ, Lambalk CB, McDonnel J, Schats R, Homburg R, Vermeiden JPW. Further considerations on natural or mild hyperstimulation cycles for intrauterine insemination treatment: effects on pregnancy and multiple pregnancy rates. Human Reproduction 2005;20(11):3141‐6. [DOI] [PubMed] [Google Scholar]
- Goverde AJ, McDonnell J, Vermeiden JPW, Schats R, Rutten FFH, Schoemaker J. Intrauterine insemination or in‐vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost‐effectiveness analysis. Lancet 2000;355:13‐8. [DOI] [PubMed] [Google Scholar]
Gregoriou 1996 {published data only}
- Gregoriou O, Vitoratos N, Papadias C, Konidaris S, Gargaropoulos A, Rizos D. Pregnancy rates in gonadotrophin stimulated cycles with timed intercourse or intrauterine insemination for the treatment of male subfertility. European Journal of Obstetrics & Gynaecology and Reproductive Biology 1996;64:213‐6. [DOI] [PubMed] [Google Scholar]
Guzick 1999 {published data only}
- Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor‐Litvak P, Steinkampf MP, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. New England Journal of Medicine 1999;340(3):177‐83. [DOI] [PubMed] [Google Scholar]
Kerin 1984 {published data only}
- Kerin JFP, Peek J, Warnes GM, Kirby C, Jeffrey R, Matthews CD. Improved conception rate after intrauterine insemination of washed spermatozoa from men with poor quality semen. Lancet 1984;1:533‐5. [DOI] [PubMed] [Google Scholar]
Melis 1995 {published data only}
- Melis GB, Paoletti AM, Ajossa S, Guerriero S, Depau GF, Mais V. Ovulation induction with gonadotropins as sole treatment in infertile couples with open tubes: a randomized prospective comparison between intrauterine insemination and timed vaginal intercourse. Fertility & Sterility 1995;64:1088‐93. [DOI] [PubMed] [Google Scholar]
Nan 1994 {published data only}
- Nan PM, Cohlen BJ, Velde ER, Kooij RJ, Eimers JM, Zonneveld P, et al. Intra‐uterine insemination or timed intercourse after ovarian stimulation for male subfertility? A controlled study. Human Reproduction 1994;9:2022‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aboulghar 1995 {published data only}
- Aboulghar MA, Mansour RT, Serour GI, Amin YM. The role of intracytoplasmic sperm injection (ICSI) in the treatment of patients with borderline semen. Human Reproduction 1995;10(11):2829‐30. [DOI] [PubMed] [Google Scholar]
Aboulghar 1996 {published data only}
- Aboulghar MA, Mansour RT, Serour GI, Sattar MA, Amin YM. Intracytoplasmic sperm injection and conventional in vitro fertilization for sibling oocytes in cases of unexplained infertility and borderline semen. Journal of Assisted Reproduction and Genetics 1996;13(1):38‐42. [DOI] [PubMed] [Google Scholar]
Agarwal 2004 {published data only}
- Agarwal S, Mittal S. A randomised prospective trial of intrauterine insemination versus timed intercourse in superovulated cycles with clomiphene. Indian Journal of Medical Research 2004;6:519‐22. [PubMed] [Google Scholar]
Buvat 1990 {published data only}
- Buvat J, Buvat‐Herbaut M, Marcolin G, Guittard C, Herbaut JC, Louvet AL, et al. Male subfertility: randomized comparison of intra‐uterine insemination versus timed intercourse after superovulation in the female. [Hypofertilite masculine: comparaison randomisee insemination intra‐uterine versus rapport sexuel programme apres superovulation de la femme]. Contraception Fertilite Sexualite 1990;18:435‐8. [Google Scholar]
Crosignani 1994 {published data only}
- Crosignani PG, Walters DE. Clinical pregnancy and male subfertility; the ESHRE multicentre trial on the treatment of male subfertility. Human Reproduction 1994;9:1112‐8. [DOI] [PubMed] [Google Scholar]
Cruz 1986 {published data only}
- Cruz RI, Kemmann E, Brandeis VT, Becker KA, Beck M, Beardsley L, et al. A prospective study of intrauterine insemination of processed sperm from men with oligo‐astheno spermia in superovulated women. Fertility & Sterility 1986;46:673‐7. [DOI] [PubMed] [Google Scholar]
Elizur 2004 {published data only}
- Elizur SE, Levron J, Seidman DS, Kees S, Levran D, Dor J. Conventional in vitro fertilization versus intracytoplasmic sperm injection for sibling oocytes in couples with mild oligoteratoasthenozoospermia and couples with normal sperm. Fertility & Sterility 2004;82(1):241‐3. [DOI] [PubMed] [Google Scholar]
Elzeiny 2014 {published data only}
- Elzeiny H, Garrett C, Toledo M, Stern K, McBain J, Baker HWG. A randomised controlled trial of intra‐uterine insemination versus in vitro fertilisation in patients with idiopathic or mild male infertility. Australian and New Zealand Journal of Obstetrics and Gynaecology 2014;54:156‐61. [DOI] [PubMed] [Google Scholar]
Evans 1991 {published data only}
- Evans J, Wells C, Gregory L, Walker S. A comparison of intrauterine insemination, intraperitoneal insemination, and natural intercourse in superovulated women. Fertility & Sterility 1991;56:1183‐7. [PubMed] [Google Scholar]
Fan 2012 {published data only}
- Fan W, Li SW, Li L, Huang Z, Ma Q, Wang Y, et al. Outcome of conventional IVF and ICSI on sibling oocytes in the case of isolated teratozoospermia. Journal of Assisted Reproduction and Genetics 2012;29:905‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fishel 2000 {published data only}
- Fishel S, Aslam I, Lisi F, Rinaldi L, Timson J, Jacobson M, et al. Should ICSI be the treatment of choice for all cases of in‐vitro conception?. Human Reproduction 2000;15(6):1278‐83. [DOI] [PubMed] [Google Scholar]
Friedman 1989 {published data only}
- Friedman A, Haas S, Kredentser J, Stewart E, Schiff I. A controlled trial of intrauterine insemination for cervical factor and male factor: a preliminary report. International Journal of Fertility 1989;34:199‐203. [PubMed] [Google Scholar]
Galle 1990 {published data only}
- Galle PC, McRae MA, Colliver JA, Alexander JS. Sperm washing and intrauterine insemination for cervical factor, oligospermia, immunological infertility and unexplained infertility. Journal of Reproductive Medicine 1990;35(2):116‐22. [PubMed] [Google Scholar]
Goverde 2001 {published data only}
- Goverde AJ. IUI: the treatment of choice in idiopathic and male subfertility. Biomedical Pharmacotherapy 2001;55:70‐1. [Google Scholar]
Hewitt 1985 {published data only}
- Hewitt J, Cohen J, Krishnaswamy V, Fehilly CB, Steptoe PC, Walters DE. Treatment of idiopathic infertility, cervical mucus hostility, and male infertility: artificial insemination with husband's semen or in vitro fertilization?. Fertility & Sterility 1985;44(3):350‐5. [DOI] [PubMed] [Google Scholar]
Ho 1989 {published data only}
- Ho PC, Poon IML, Chan SYW, Wang C. Intrauterine insemination is not useful in oligoasthenospermia. Fertility & Sterility 1989;51(4):682‐4. [DOI] [PubMed] [Google Scholar]
Ho 1992 {published data only}
- Ho PC, So WK, Chan YF, Yeung WSB. Intrauterine insemination after ovarian stimulation as a treatment for subfertility because of subnormal semen: a prospective randomized controlled trial. Fertility & Sterility 1992;58:995‐9. [DOI] [PubMed] [Google Scholar]
Karlström 2000 {published data only}
- Karlström PO, Bergh T, Lundkvist Ö. Addition of gonadotrophin‐releasing hormone agonist and/or two inseminations with husband's sperm do not improve the pregnancy rate in superovulated cycles. Acta Obstetricia et Gynaecologica Scandinavica 2000;79:37‐42. [PubMed] [Google Scholar]
Kastrop 1999 {published data only}
- Kastrop PMM, Weima SM, Kooij RJ, Velde ER. Comparison between intracytoplasmic sperm injection and in‐vitro fertilization (IVF) with high insemination concentration after total fertilization failure in a previous IVF attempt. Human Reproduction 1999;14(1):65‐9. [DOI] [PubMed] [Google Scholar]
Kihaile 2003 {published data only}
- Kihaile PE, Misumi J, Hirotsuru K, Kumasako Y, Kisanga RE, Utsunomiya T. Comparison of sibling oocyte outcomes after intracytoplasmic sperm injection and in vitro fertilization in severe teratozoospermic patients in the first cycle. International Journal of Andrology 2003;26:57‐62. [DOI] [PubMed] [Google Scholar]
Kirby 1991 {published data only}
- Kirby CA, Flaherty SP, Godfrey BM, Warnes GM, Matthews CD. A prospective trial of intrauterine insemination of motile spermatozoa versus timed intercourse. Fertility & Sterility 1991;56:102‐7. [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li Z, Lin H, Xiao W, Wang Y. Fertilization of IVF/ICSI using sibling oocytes from couples with subfertile male or unexplained infertility. Journal of Huazhong University of Science and Technology 2004;24(4):365‐8. [DOI] [PubMed] [Google Scholar]
Martinez 1991 {published data only}
- Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JP, Schoemaker J. Intrauterine insemination does and clomiphene citrate does not improve fecundity in couples with infertility due to male or idiopathic factors: a prospective, randomized, controlled study. Fertility & Sterility 1990;53(5):847‐53. [DOI] [PubMed] [Google Scholar]
- Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JPW, Schoemaker J. Pregnancy rates after timed intercourse or intrauterine insemination after human menopausal gonadotropin stimulation or normal ovulatory cycles: a controlled study. Fertility & Sterility 1991;55:258‐65. [DOI] [PubMed] [Google Scholar]
Melis 1987 {published data only}
- Melis GB, Paoletti AM, Strigini F, Fabris FM, Canale D, Fioretti P. Pharmacologic induction of multiple follicular development improves the success rate of artificial insemination with husband's semen in couples with male‐related or unexplained infertility. Fertility & Sterility 1987;47(3):441‐5. [DOI] [PubMed] [Google Scholar]
Moolenaar 2015 {published data only}
- Moolenaar LM, Cissen M, Bruin JP, Hompes PG, Repping S, Veen F, et al. Cost‐effectiveness of assisted conception for male subfertility. Reproductive Biomedicine Online 2015;30(6):659‐66. [DOI] [PubMed] [Google Scholar]
Nulsen 1993 {published data only}
- Nulsen JC, Walsh S, Dumez S, Metzger DA. A randomized and longitudinal study of human menopausal gonadotropin with intrauterine insemination in the treatment of infertility. Obstetrics and Gynecology 1993;82:780‐6. [PubMed] [Google Scholar]
Pisarska 1999 {published data only}
- Pisarska MD, Casson PR, Cisneros PL, Lamb DJ, Lipshultz LI, Buster JE, et al. Fertilization after standard in vitro fertilization versus intracytoplasmic sperm injection in subfertile males using sibling oocytes. Fertility & Sterility 1999;71(4):627‐32. [DOI] [PubMed] [Google Scholar]
Plachot 2002 {published data only}
- Plachot M, Belaisch‐Allart J, Mayenga JM, Chouraqui A, Tesquier L, Serkine AM. Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Human Reproduction 2002;17(3):362‐9. [DOI] [PubMed] [Google Scholar]
Prentice 1995 {published data only}
- Prentice A, Sacks GP, Morton NC, Deary AJ, Smith SK. Controlled ovarian stimulation (superovulation) and intrauterine insemination for the treatment of unexplained and minor male factor infertility. Human Reproduction 1995;10(Abstract book 2):112. [Google Scholar]
Soliman 1993 {published data only}
- Soliman S, Daya S, Collins J, Jarrell J. A randomized trial of in vitro fertilization versus conventional treatment for infertility. Fertility & Sterility 1993;59(6):1239‐44. [DOI] [PubMed] [Google Scholar]
te Velde 1989 {published data only}
- Velde ER, Kooy RJ, Waterreus JJH. Intrauterine insemination of washed husband's spermatozoa: a controlled study. Fertility & Sterility 1989;51:182‐5. [DOI] [PubMed] [Google Scholar]
Tournaye 2002 {published data only}
- Tournaye H, Verheyen G, Albano C, Camus M, Landuyt L, Devroey P, et al. Intracytoplasmic sperm injection versus in vitro fertilization: a randomized controlled trial and a meta‐analysis of the literature. Fertility & Sterility 2002;78(5):1030‐7. [DOI] [PubMed] [Google Scholar]
van der Westerlaken 2006 {published data only}
- Westerlaken L, Naaktgeboren N, Verburg H, Dieben S, Helmerhorst FM. Conventional in vitro fertilization versus intracytoplasmic sperm injection in patients with borderline semen: a randomized study using sibling oocytes. Fertility & Sterility 2006;85(2):395‐400. [DOI] [PubMed] [Google Scholar]
Verheyen 1999 {published data only}
- Verheyen G, Tournaye H, Staessen C, Vos A, Vandervorst M, Steirteghem A. Controlled comparison of conventional in‐vitro fertilization and intracytoplasmic sperm injection in patients with asthenozoospermia. Human Reproduction 1999;14(9):2313‐9. [DOI] [PubMed] [Google Scholar]
Xie 2015 {published data only}
- Xie BG, Huang YH, Zhu WJ, Jin S. Comparison of the outcome of conventional in vitro fertilization and intracytoplasmic sperm injection in moderate male infertility from ejaculate. Urologia Internationalis 2015;94(1):111‐6. [DOI] [PubMed] [Google Scholar]
Zayed 1997 {published data only}
- Zayed F, Lenton EA, Cooke ID. Comparison between stimulated in‐vitro fertilization and stimulated intrauterine insemination for the treatment of unexplained and mild male factor infertility. Human Reproduction 1997;12(11):2408‐13. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aribarg 1995 {published data only}
- Aribarg A, Sukcharoen N. Intrauterine insemination of washed spermatozoa for treatment of oligozoospermia. International Journal of Andrology 1995;18(Suppl 1):62‐6. [DOI] [PubMed] [Google Scholar]
Jaroudi 1998 {published data only}
- Jaroudi KA, Hollanders H, Sieck U, Zahrani A, Al‐Nour A, Atared A. Superovulation and intrauterine insemination for male factor infertility: a controlled randomized study. Middle East Fertility Society Journal 1998;3(3):254‐9. [Google Scholar]
Kerin 1987 {published data only}
- Kerin J, Quinn P. Washed intrauterine insemination in the treatment of oligospermic infertility. Seminars in Reproductive Endocrinology 1987;5:23‐33. [Google Scholar]
Additional references
Andersen 1995
- Andersen AG, Als‐Nielsen BM, Hornenes PJ, Franch Andersen L. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Human Reproduction 1995;10:3202‐5. [DOI] [PubMed] [Google Scholar]
Avendano 2010
- Avendano C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertility & Sterility 2010;94(2):549‐57. [DOI] [PubMed] [Google Scholar]
Bakos 2008
- Bakos HW, Thompson JG, Feil D, Lane M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. International Journal of Andrology 2008;31(5):518‐26. [DOI] [PubMed] [Google Scholar]
Bensdorp 2009
- Bensdorp AJ, Slappendel E, Koks C, Oosterhuis J, Hoek A, Hompes P, et al. The INeS study: prevention of multiple pregnancies: a randomised controlled trial comparing IUI COH versus IVF e SET versus MNC IVF in couples with unexplained or mild male subfertility. BMC Women's Health 2009;9(35):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattachary 2000
- Bhattachary S. Cost‐effective treatment of couples with subfertility. Lancet 2000;355(1):2. [DOI] [PubMed] [Google Scholar]
Bhattachary 2001
- Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, et al. Conventional in‐vitro fertilisation vs intracytoplasmic sperm injection for the treatment of non‐male‐factor infertility: a randomised controlled trial. Lancet 2001;357(9274):2075‐9. [DOI] [PubMed] [Google Scholar]
Boomsma 2007
- Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004507.pub3] [DOI] [PubMed] [Google Scholar]
Boulet 2015
- Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA 2015;313(3):255‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantineau 2007
- Cantineau AEP, Cohlen BJ. Ovarian stimulation protocols (anti‐oestrogens, gonadotrophins with and without GnRH agonists/ antagonists) for intrauterine insemination (IUI) in women with subfertility. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005356.pub2] [DOI] [PubMed] [Google Scholar]
Cohen 1984
- Cohen J, Fehilly CB, Fishel SB, Edwards RG, Hewitt J, Rowland GF, et al. Male infertility successfully treated by in‐vitro fertilisation. Lancet 1984;8388:1239‐40. [DOI] [PubMed] [Google Scholar]
Cohlen 1998b
- Cohlen BJ, Velde ER, Looman CW, Eijckemans R, Habbema JD. Crossover or parallel design in infertility trials? The discussion continues. Fertility & Sterility 1998;70(1):40‐5. [DOI] [PubMed] [Google Scholar]
Cohlen 2005
- Cohlen BJ. Should we continue performing intrauterine inseminations in the year 2004?. Gynecologic and Obstetric Investigation 2005;59(1):3‐13. [DOI] [PubMed] [Google Scholar]
Cooper 2010
- Cooper TG, Noonan E, Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Human Reproduction Update 2010;16(3):231‐45. [DOI] [PubMed] [Google Scholar]
Coppus 2007
- Coppus SF, Verhoeve HR, Opmeer BC, Steeg JW, Steures P, Eijkemans MJ, et al. Identifying subfertile ovulatory women for timely tubal patency testing: a clinical decision rule based on medical history. Human Reproduction 2007;22(10):2685‐92. [DOI] [PubMed] [Google Scholar]
Dias 2006
- Dias S, McNamee R, Vail A. Evidence of improving quality of reporting of randomized controlled trials in subfertility. Human Reproduction 2006;10:2617‐27. [DOI] [PubMed] [Google Scholar]
Dickey 1999
- Dickey RP, Pyrzak R, Lu PY, Taylor SN, Rye PH. Comparison of the sperm quality necessary for successful intrauterine insemination with World Health Organization threshold values for normal sperm. Fertility & Sterility 1999;17:684‐9. [DOI] [PubMed] [Google Scholar]
Duran 2002a
- Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Human Reproduction 2002;17:3122‐8. [DOI] [PubMed] [Google Scholar]
Duran 2002b
- Duran HE, Morshedi M, Kruger T, Oehninger S. Intrauterine insemination: a systematic review on determinants of success. Human Reproduction Update 2002;8(4):373‐84. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Fauser 2005
- Fauser BCJM, Devroey P, Macklon NS. Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet 2005;365:1807‐16. [DOI] [PubMed] [Google Scholar]
Guzick 1998
- Guzick DS, Sullivan MW, Adamson GD, Cedars MI, Falk RJ, Peterson EP, et al. Efficacy of treatment for unexplained infertility. Fertility & Sterility 1998;70(2):207‐13. [DOI] [PubMed] [Google Scholar]
Hamilton 2015
- Hamilton JA, Cissen M, Brandes M, Smeenk JMJ, Bruin JP, Kremer JA, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Human Reproduction 2015;30(5):1110‐21. [DOI] [PubMed] [Google Scholar]
Healy 2004
- Healy D. Damaged babies from assisted reproductive technologies: focus on BESST (birth emphasizing a successful singleton at term) outcome. Fertility & Sterility 2004;3:512‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Greens S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoff 1983
- Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. Journal of Clinical Endocrinology and Metabolism 1983;4:792‐6. [DOI] [PubMed] [Google Scholar]
Hull 1985
- Hull MGR, Glazener CMA, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment and outcome of infertility. British Medical Journal 1985;291:1693‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunault 2004
- Hunault C, Habbema JD, Eijkemans MJ, Collins JA, Evers JL, Velde ER. Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Human Reproduction 2004;19:2019‐26. [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson NP, Proctor M, Farquhar CM. Gaps in the evidence for fertility treatment ‐ an analysis of the Cochrane Menstrual Disorders and Subfertility Group database. Human Reproduction 2003;18(5):947‐54. [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Collins JA, Walter SD. Empirical evidence of bias in infertility research: overestimation of treatment effect in crossover trials using pregnancy as the outcome measure. Fertility & Sterility 1996;65:939‐45. [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim HH, Bundorf MK, Behr B, McCallum SW. Use and outcomes of intracytoplasmic sperm injection for non‐male factor infertility. Fertility & Sterility 2007;88(3):622‐8. [DOI] [PubMed] [Google Scholar]
Kruger 1993
- Kruger TF, Du Toit TC, Franken DR, Acosta AA, Oehninger SC, Menkveld R, et al. A new computerized method of reading sperm morphology (strict criteria) is as efficient as technician reading. Fertility & Sterility 1993;59(1):202‐9. [DOI] [PubMed] [Google Scholar]
Mansour 2014
- Mansour R, Ishihara O, Adamson GD, Dyer S, Mouzon J, Nygren KG, et al. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2006. Human Reproduction 2014;29:1536‐51. [DOI] [PubMed] [Google Scholar]
Matorras 1995
- Matorras R, Corcostequi B, Perez C, Mandiola M, Mendoza R, Rodriguez‐Escudero FJ. Sperm morphology analysis (strict criteria) in male infertility is not a prognostic factor in intrauterine insemination with husband's sperm. Fertility & Sterility 1995;63(3):608‐11. [DOI] [PubMed] [Google Scholar]
McDonnell 2004
- McDonnell J, Goverde AJ, Vermeiden JPW. The place of the crossover design in infertility trials: a maximum likelihood approach. Human Reproduction 2004;19(11):2537‐44. [DOI] [PubMed] [Google Scholar]
Mochtar 2011
- Mochtar MH, Custers IM, Koks CA, Bernardus RE, Verhoeve HR, Mol BW, et al. Timing oocyte collection in GnRH agonists down‐regulated IVF and ICSI cycles: a randomized clinical trial. Human Reproduction 2011;26(5):1091‐6. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Fertility problems: assessment and treatment for people with fertility problems. Clinical guideline published by the RCOG press 2013; Vol. 156, issue https://www.nice.org.uk/guidance/cg156.
Norman 2000
- Norman GR, Daya S. The alternating‐sequence design (or multiple‐period crossover) trial for evaluating treatment efficacy in infertility. Fertility & Sterility 2000;74(2):319‐24. [DOI] [PubMed] [Google Scholar]
Ombelet 1997
- Ombelet W, Vandeput H, Janssen M, Cox A, Vossen C, Pollet H, et al. Treatment of male infertility due to sperm surface antibodies: IUI or IVF. Human Reproduction 1997;12(6):1165‐70. [DOI] [PubMed] [Google Scholar]
Ombelet 2003
- Ombelet W. Semen quality and intrauterine insemination. Reproductive BioMedicine Online 2003;7(4):465‐92. [DOI] [PubMed] [Google Scholar]
Palermo 1992
- Palermo G, Joris H, Devroey P, Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17‐8. [DOI] [PubMed] [Google Scholar]
Pandian 2012
- Pandian Z, Gibreel A, Bhattacharya S. In vitro fertilisation for unexplained subfertility. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD003357.pub3] [DOI] [PubMed] [Google Scholar]
Practice Committee of the ASRM 2012
- Practice Committee of the American Society for Reproductive Medicine. Multiple gestation associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertility & Sterility 2012;97:825‐34. [DOI] [PubMed] [Google Scholar]
Practice Committee of the ASRM 2013
- Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertility & Sterility 2013;99:673‐7. [DOI] [PubMed] [Google Scholar]
Repping 2002
- Repping S, Weert JM, Mol BWJ, Vries JWA, Veen F. Use of the total motile sperm count to predict total fertilization failure in in vitro fertilization. Fertility & Sterility 2002;78(1):22‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhemrev 2001
- Rhemrev JPT, Lens JW, McDonnell J, Schoemaker J, Vermeiden JPW. The postwash total progressively motile sperm cell count is a reliable predictor of total fertilization failure during in vitro fertilization treatment. Fertility & Sterility 2001;76(5):884‐91. [DOI] [PubMed] [Google Scholar]
Simon 2014
- Simon L, Liu L, Murphy K, Ge S, Hotaling J, Aston KI, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Human Reproduction 2014;29(5):904‐17. [DOI] [PubMed] [Google Scholar]
Steures 2004
- Steures P, Steeg JW, Mol BW, Eijkemans MJ, Veen F, Habbema JD, et al. Prediction of an ongoing pregnancy after intrauterine insemination. Fertility & Sterility 2004;82(1):45‐51. [DOI] [PubMed] [Google Scholar]
Steures 2006
- Steures P, Steeg JW, Hompes PGA, Habbema DF, Eijkemans MJC, Broekmans FJ, et al. Intrauterine insemination with controlled ovarian hyperstimulation versus expectant management for couples with unexplained subfertility and an intermediate prognosis: a randomised clinical trial. Lancet 2006;368:216‐21. [DOI] [PubMed] [Google Scholar]
Sullivan 2013
- Sullivan EA, Zegers‐Hochschild F, Mansour R, Ishihara O, Mouzon J, Nygren KG, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: assisted reproductive technology 2004. Human Reproduction 2013;28:1375‐90. [DOI] [PubMed] [Google Scholar]
Tarlatzis 2006
- Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Human Reproduction Update 2006;12(4):333‐40. [DOI] [PubMed] [Google Scholar]
Testart 1982
- Testart J, Frydman R. Minimum time lapse between luteinizing hormone surge or human chorionic gonadotrophin administration and follicular rupture. Fertility & Sterility 1982;37:50. [DOI] [PubMed] [Google Scholar]
Tijani 2010
- Tijani HA, Bhattacharya S. The role of intrauterine insemination in male infertility. Human Fertility 2010;13:226‐32. [DOI] [PubMed] [Google Scholar]
Tjon‐Kon‐Fat 2015
- Tjon‐Kon‐Fat RI, Bensdorp AJ, Bossuyt PM, Koks C, Oosterhuis GJ, Hoek A, et al. Is IVF‐served two different ways‐more cost‐effective than IUI with controlled ovarian hyperstimulation?. Human Reproduction 2015;30(10):2331‐9. [DOI] [PubMed] [Google Scholar]
Tournaye 2012
- Tournaye H. Male factor infertility and ART. Asian Journal of Andrology 2012;14:103‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18(5):1000‐4. [DOI] [PubMed] [Google Scholar]
van der Steeg 2011
- Steeg JW, Steures P, Eijkemans MJC, Habbema JD, Hompes PG, Kremer JAM, et al. Role of semen analysis in subfertile couples. Fertility & Sterility 2011;95(3):1013‐9. [DOI] [PubMed] [Google Scholar]
van der Westenlaken 2005
- Westenlaken L, Helmerhorst F, Dieben S, Naaktgeboren N. Intracytoplasmic sperm injection as a treatment for unexplained total fertilization failure or low fertilization after conventional in vitro fertilization. Fertility & Sterility 2005;83:612‐7. [DOI] [PubMed] [Google Scholar]
van Voorhis 2001
- Voorhis BJ, Barnett M, Sparks AET, Syrop CH, Rosenthal G, Dawson J. Effect of the total motile sperm count on the efficacy and cost‐effectiveness of intrauterine insemination and in vitro fertilization. Fertility & Sterility 2001;75(4):661‐8. [DOI] [PubMed] [Google Scholar]
van Weert 2004
- Weert J‐M, Repping S, Voorhis BJ, Veen F, Bossuyt PMM, Mol BWJ. Performance of the postwash total motile sperm count as a predictor of pregnancy at the time of intrauterine insemination: a meta‐analysis. Fertility & Sterility 2004;82(3):612‐20. [DOI] [PubMed] [Google Scholar]
Veltman‐Verhulst 2012
- Veltman‐Verhulst SM, Cohlen BJ, Hughes E, Heineman MJ, Velde E. Intra‐uterine insemination for unexplained subfertility. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD001838.pub4] [DOI] [PubMed] [Google Scholar]
Verhoeve 2006
- Verhoeve HR, Veen F, Mol BW. Diagnostic tests in reproductive medicine. Reviews in Gynaecological and Perinatal Practice 2006;6:20‐5. [Google Scholar]
Wainer 2004
- Wainer R, Albert M, Dorion A, Bailly M, Bergere M, Lombroso R, et al. Influence of the number of motile spermatozoa inseminated and of their morphology on the success of intrauterine insemination. Human Reproduction 2004;9:2060‐5. [DOI] [PubMed] [Google Scholar]
WHO 1980
- World Health Organization Task Force Investigators. Temporal relationships between ovulation and defined changes in the concentration of plasma estradiol‐17 beta, luteinizing hormone, follicle stimulating hormone and progesterone. American Journal of Obstetrics and Gynecology 1980;138(4):382‐90. [PubMed] [Google Scholar]
WHO 1987
- World Health Organization. Laboratory Manual for Semen Analysis and Sperm Cervical Mucus Interaction, revised edition. London/New York: Cambridge University Press, 1987. [Google Scholar]
WHO 1992
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm Cervical Mucus Interaction. Cambridge: Cambridge University Press, 1992. [Google Scholar]
WHO 2010
- World Health Organization. WHO Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization, 2010. [Google Scholar]
Zikopoulos 2005
- Zikopoulos K, Kaponis A, Adonakis G, Sotiriadis A, Kalantaridou S, Georgiou I, et al. A prospective randomized study comparing gonadotropin‐releasing hormone agonists or gonadotropin‐releasing hormone antagonists in couples with unexplained infertility and/or mild oligozoospermia. Fertility & Sterility 2005;83(5):1354‐62. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bensdorp 2007a
- Bensdorp A, Cohlen BJ, Heineman MJ, Vanderkerckhove P. Intra‐uterine insemination for male subfertility. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD000360.pub3] [DOI] [PubMed] [Google Scholar]
Bensdorp 2007b
- Bensdorp A, Cohlen BJ, Heineman MJ, Vanderkerckhove P. Intra‐uterine insemination for male subfertility. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000360.pub4] [DOI] [PubMed] [Google Scholar]
Cohlen 1998
- Cohlen BJ, Vanderkerckhove P, Velde ER, Habbema JDF. Timed intercourse versus intra‐uterine insemination with or without ovarian hyperstimulation for subfertility in men. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000360] [DOI] [PubMed] [Google Scholar]
Cohlen 2000
- Cohlen BJ, Vanderkerckhove P, Velde ER, Habbema JDF. Timed intercourse versus intra‐uterine insemination with or without ovarian hyperstimulation for subfertility in men. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000360.pub2] [DOI] [PubMed] [Google Scholar]


