OBJECTIVES:
Evidence of cerebrovascular complications in COVID-19 requiring venovenous extracorporeal membrane oxygenation (ECMO) is limited. Our study aims to characterize the prevalence and risk factors of stroke secondary to COVID-19 in patients on venovenous ECMO.
DESIGN:
We analyzed prospectively collected observational data, using univariable and multivariable survival modeling to identify risk factors for stroke. Cox proportional hazards and Fine-Gray models were used, with death and discharge treated as competing risks.
SETTING:
Three hundred eighty institutions in 53 countries in the COVID-19 Critical Care Consortium (COVID Critical) registry.
PATIENTS:
Adult COVID-19 patients who were supported by venovenous ECMO.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Five hundred ninety-five patients (median age [interquartile range], 51 yr [42–59 yr]; male: 70.8%) had venovenous ECMO support. Forty-three patients (7.2%) suffered strokes, 83.7% of which were hemorrhagic. In multivariable survival analysis, obesity (adjusted hazard ratio [aHR], 2.19; 95% CI, 1.05–4.59) and use of vasopressors before ECMO (aHR, 2.37; 95% CI, 1.08–5.22) were associated with an increased risk of stroke. Forty-eight-hour post-ECMO Paco2–pre-ECMO Paco2/pre-ECMO Paco2 (relative ΔPaco2) of negative 26% and 48-hour post-ECMO Pao2–pre-ECMO Pao2/pre-ECMO Pao2 (relative ΔPao2) of positive 24% at 48 hours of ECMO initiation were observed in stroke patients in comparison to relative ΔPaco2 of negative 17% and relative ΔPao2 of positive 7% in the nonstroke group. Patients with acute stroke had a 79% in-hospital mortality compared with 45% mortality for stroke-free patients.
CONCLUSIONS:
Our study highlights the association of obesity and pre-ECMO vasopressor use with the development of stroke in COVID-19 patients on venovenous ECMO. Also, the importance of relative decrease in Paco2 and moderate hyperoxia within 48 hours after ECMO initiation were additional risk factors.
Keywords: COVID-19, extracorporeal membrane oxygenation, intracranial hemorrhage, severe acute respiratory syndrome-related coronavirus, stroke, venovenous extracorporeal membrane oxygenation
KEY POINTS
Question: What are the prevalence and risk factors for developing stroke in COVID-19 patients supported by venovenous extracorporeal membrane oxygenation (ECMO)?
Findings: In an international registry analysis, we found that obesity and pre-ECMO use of vasopressors were associated with stroke development. The relative reduction of Paco2 and increased Pao2 within 48 hours were observed in patients with stroke on venovenous ECMO.
Meaning: These findings highlight the importance of close hemodynamic monitoring and the abrupt change in Co2 and oxygen around ECMO initiation.
Extracorporeal membrane oxygenation (ECMO) is recommended as a rescue intervention for COVID-19–related acute respiratory distress syndrome (ARDS) refractory to conventional measures (1, 2). In a recent analysis of 4,812 COVID-19 patients enrolled in the Extracorporeal Life Support Organization (ELSO) registry, the in-hospital mortality at 90 days after ECMO initiation ranged from 36.9% to 51.9% (3). This study reported that 1% of COVID-19 ECMO patients developed ischemic strokes, and nearly 6% experienced a hemorrhagic stroke. Patients with ischemic or hemorrhagic strokes have higher mortality and long-term disabilities than those without (4, 5). The stroke mechanism could be partially attributed to COVID-19 infection or its complications. In a meta-analysis of 108,571 COVID-19 patients, 1.4% had evidence of acute stroke, of whom 38% of those presented with stroke at admission (6). In a cluster study of 2,908 COVID-19 patients, the authors proposed the severity of COVID-19 infection and the associated coagulopathy as possible risk factors for ischemic strokes (7).
Besides particular considerations concerning COVID-19, multiple explanations for neurologic complications in venovenous ECMO patients have been proposed. The interaction between the blood and the artificial surfaces of the ECMO circuit can cause consumptive thrombocytopenia and von Willebrand factor deficiency (8, 9). The use of the neck veins to place large ECMO cannulas might predispose patients to venous congestion or cerebral venous sinus thrombosis, and paradoxical thromboembolism can occur with intracardiac shunts (10). In addition, in hypercapnic respiratory failure, the rate and the magnitude of the drop in Paco2 may predispose patients to strokes (11). Finally, due to COVID-19–associated prothrombotic state, higher anticoagulation targets are usually may be required.
The COVID-19 Critical Care Consortium (COVID Critical) is an international registry enrolling COVID-19 patients admitted to ICUs from more than 370 hospitals in 53 countries (12, 13). The study’s primary purpose is to report the prevalence and risk factors of cerebrovascular complications in COVID-19 patients supported by venovenous ECMO.
METHODS
Study Design, Settings, and Patients
The COVID-19 Critical Care Consortium (COVID Critical) international registry was launched in January 2020 in response to COVID-19 pandemic (Clinical trial: ACTRN12620000421932, Study protocol approved by the Alfred Hospital Ethics Committee, Melbourne, Australia [Project: 62066, Local reference: 108/20]). According to Australian legislation, informed consent was waived because of the observational nature of data collection, utilization of de-identified patient information, and the minimal risk for patients. All methods were carried out in accordance with relevant guidelines, regulations and with the Helsinki Declaration of 1975. No experimental protocols or interventions were implemented as part of this study. The full study protocol is accessible online (14). Data collection and entry were carried out by the local site investigators and the study coordinators as per the study protocol. The International Severe Acute Respiratory and Emerging Infection Consortium (15) and the Short PeRiod IncideNce sTudy of Severe Acute Respiratory Infection network (16) data collection forms were completed on hospital admission. Additional information related to respiratory support, ECMO, management, and complications were collected and entered the Research Electronic Data Capture hosted by the University of Oxford, Oxford, United Kingdom.
This study used data from February 19, 2020, to December 4, 2021. The enrollment eligibility criteria were adult patients (18 yr old or older), confirmed acute COVID-19 by polymerase chain reaction, and the need for invasive mechanical ventilation and venovenous ECMO support. We excluded patients with confirmed stroke before ECMO initiation and patients on venoarterial ECMO. The primary outcomes were stroke development and mortality after 90 days from ECMO initiation.
Data Collection
The collected data included demographics, comorbid conditions, geographic location and the date of admission and discharge from the ICU, the date of ECMO initiation and discontinuation, the Acute Physiology Score II, and the Sequential Organ Failure Assessment (SOFA) score. Also, we collected data relevant to pre-ECMO support: mechanical ventilation parameters, use of prone positioning, neuromuscular blockade, systemic anticoagulation, and vasoactive medications. Regarding ECMO support, we focused on the cannulation strategy and complications during the ECMO run, such as cardiac arrest, neurologic complications, other organ failures, hemorrhagic events, and secondary infections. Finally, center-specific data included the country, economic status, and the number of patients on ECMO support.
Outcomes
The primary outcomes were clinically diagnosed stroke and its mortality at 90 days from ECMO initiation. The secondary outcomes were the duration of mechanical ventilation and ECMO support, ICU length of stay, hospital length of stay, and the discharge disposition (alive, dead, discharged home, ongoing hospitalization, palliative discharge, transfer, or unknown).
Statistical Analysis
Baseline characteristics of patients, characteristics of ECMO support, and complications were summarized using medians and interquartile range (IQR) for continuous variables and counts and percentages for categorical variables. The association between risk factors of interest and any type of stroke was analyzed through survival analysis, as well as joint modeling of survival outcomes and longitudinal biomarkers. Survival models for the time from ECMO initiation to any stroke included cause-specific Cox proportional hazards models censored at death, discharge, or 90 days. Since death can preclude stroke, Fine-Gray models, with death treated as a competing risk, were also used. Fine-Gray models estimate subdistribution hazard ratios (subHRs) of each covariate and survival outcome, which are interpreted as the effect of the covariates on the cumulative frequency of the events over time (17).
Risk factors for stroke in the models included covariates determined at the initiation of ECMO: age, sex, number of days ventilated pre-ECMO, ethnicity, smoking status, comorbidities at baseline including obesity, diabetes, hypertension, and chronic cardiac disease, pre-ECMO use of vasoactive medicines, cannula type, the ratio of Pao2/Fio2 (log2-transformed), SOFA score, platelet count, the relative change in Pao2 and Paco2 at ECMO initiation, defined as (arterial blood gas levels at 24–48 hr post-initiation–pre-initiation)/pre-initiation, World Health Organization economic region (high vs upper middle vs lower middle), and pandemic era. Time-varying covariates were ECMO support (during vs after) and anticoagulant use after ECMO initiation.
Initially, separate univariable models were fitted to risk factors. Then, a multivariable model was fitted, including all risk factors that had less than 20% of missing data. Finally, joint models using a Bayesian approach were investigated, comprising a competing risk survival model and longitudinal models for ECMO support biomarkers. The survival model component of the joint model created separate strata for the competing risk outcomes of stroke and death. Covariates in the survival model were reduced, excluding those which were of lesser clinical interest (e.g., smoking), had small numbers, and caused variance inflation or lack of convergence in the models. Robust ses were used to account for clustering by the site in all survival models using an independence working correlation within a generalized estimating model. Longitudinal models included Pao2, Paco2, and platelet count as biomarkers, which were log2-transformed due to skewness. Models were used to estimate hazard ratios (HRs), or subHRs for Fine-Gray models, and 95% CIs, or 95% credible intervals for joint models.
Sensitivity analyses included Fine-Gray models treating both death and discharge as competing risks and penalized joint models. Penalization reduced the effect of covariates of lesser importance which allowed the biomarker which was most strongly associated with stroke or death to be identified out of the three included in the joint model. Ridge and horseshoe priors with different types of shrinkage methods were used in the penalization. R Version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses. The R package “survival” (version 3.2-13) was used for survival analysis and “JMBayes2” (version 0.1-8) for joint modeling.
RESULTS
Five hundred ninety-five patients with COVID-19–associated severe ARDS required venovenous ECMO support in different countries (Supplementary Table 1, http://links.lww.com/CCM/H324), and 43 patients (7.2%) developed cerebrovascular complications. There were 100 sites with a median of three patients (IQR, 1–6) at each site. Most of the patients were White (38.7%) and male (70.8%) with a median (IQR) age of 51 years (42–59 yr) (Supplementary Table 2, http://links.lww.com/CCM/H324). The time of onset of symptoms and the duration of mechanical ventilation before ECMO were comparable between those who developed stroke and those who did not. Patients with stroke were more likely obese (65.1%) and active smokers (23.3%) and had a history of chronic cardiac disease (9.3%) versus those without stroke (43.3%, 13.8%, and 4.5%, respectively). Vasoactive drugs were used before ECMO in 69% of patients who developed stroke versus 50.5% of those who did not. The overall in-hospital mortality of the entire cohort was 47.2%, with 79.1% mortality in the stroke group compared with 44.7% in the nonstroke group. The median duration of ICU length of stay was 17 days (8–28 d), and hospital length of stay was 14 days (4–29 d) in the stroke group, which was shorter than in the nonstroke group (34 d [20–52 d] and 33 d [20–52], respectively) (Supplementary Table 2, http://links.lww.com/CCM/H324). The duration of ECMO and mechanical ventilation were less in the stroke group (15 d [5–22 d] and 20 d [10–32 d]) in comparison to the nonstroke group (19 and 27 d). Most venovenous ECMO survivors without stroke were discharged (30.9%). However, of the stroke survivors, most of them were transferred to other facilities (9.3%).
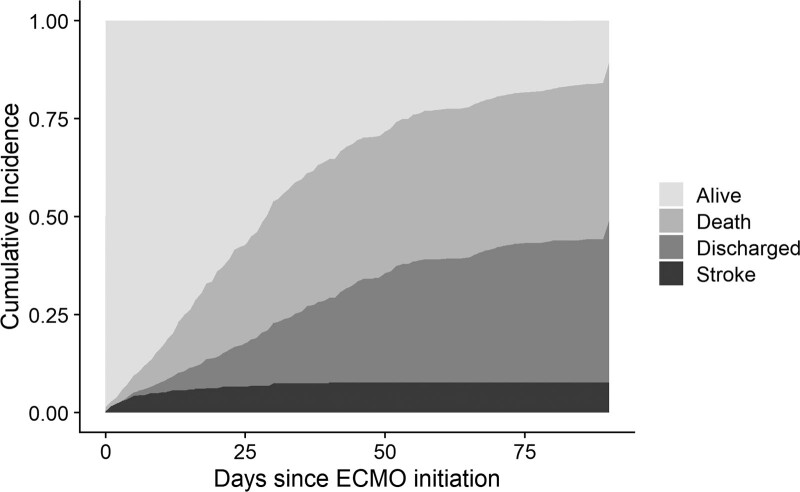
The majority of strokes occurred within 30 days from ECMO initiation (Fig. 1), and the median (IQR) time to stroke was 5 days (2–12 d). Strokes were predominantly hemorrhagic (36 patients [83.7%]). Three patients (7%) suffered an ischemic stroke, and four patients (9.3%) had a stroke of undetermined type (Supplementary Table 3, http://links.lww.com/CCM/H324). In addition to obesity and active smoking, other risk factors, either pre-ECMO or during the ECMO support, were examined. Most of the ECMO patients (83.4%) were supported via two cannulas configuration. There were no significant differences in terms of cannula size and cannulation site, and the use of anticoagulation between the cohorts (Supplementary Tables 4 and 5, http://links.lww.com/CCM/H324). The pre-ECMO Paco2 was higher in the stroke group with a geometric mean of 68 mm Hg (1.3 mm Hg) versus 58 mm Hg (1.4 mm Hg) in the nonstroke group (Table 1). Within 48 hours of ECMO initiation, the decrease in Paco2 in the stroke group was higher than that of the decrease in Paco2 in the nonstroke group: a median change in Paco2 of –17 mm Hg (–32 to –7 mm Hg) in comparison to a change of –9 mm Hg (–25 to –1 mm Hg). Relative ΔPaco2 is defined as 48-hr post-ECMO Paco2–pre-ECMO Paco2/pre-ECMO Paco2. The stroke group had a median reduction in relative ΔPaco2 of 26% (–11% to –40%) versus 17% (–2% to –36%) for the nonstroke patients. Furthermore, there was a larger increase of Pao2 within 48 hours in the stroke group when compared with the nonstroke group (12 mm Hg [–2 to 31 mm Hg] vs 5 mm Hg [–10 to 21 mm Hg]), translating to a 48-hour post-ECMO Pao2–pre-ECMO Pao2/pre-ECMO Pao2 (relative ΔPao2) increase of 24% (–2% to 50%) versus 7% (–15% to 34%), respectively.
Figure 1.
Cumulative frequency plot for stroke and death. Day 0 is the first day of extracorporeal membrane oxygenation (ECMO) support. Most strokes occurred within 30 d of initiating ECMO.
TABLE 1.
Arterial Blood Gas Before and 48 Hours After the Initiation of Extracorporeal Membrane Oxygenation for Patients Who Experienced Stroke Versus Those Without Stroke
| Variables | No Stroke (n = 552) | Stroke (n = 43) | Total (n = 595) |
|---|---|---|---|
| Pre-ECMO arterial blood gas | |||
| Pao2, mm Hg | 69.2 (1.4) | 63.5 (1.4) | 68.7 (1.4) |
| Paco2, mm Hg | 58.4 (1.4) | 67.7 (1.3) | 59.1 (1.4) |
| Pao2/Fio2 ratio | 79.4 (1.5) | 69.5 (1.5) | 78.7 (1.5) |
| Post-ECMO arterial blood gas (48 hr) | |||
| Pao2, mm Hg | 73.8 (1.3) | 74.8 (1.3) | 73.9 (1.3) |
| Paco2, mm Hg | 46.6 (1–2) | 47 (41–56.3) | 47 (41–54) |
| Pao2/Fio2 ratio | 130 (86.2–176.4) | 139.3 (87.9–165) | 130 (86.5–175.9) |
| Pre- and post-ECMO difference in oxygen tension, mm Hg | 5 (–10 to 21) | 12.0 (–2 to 31) | 5.0 (–9 to 22) |
| Pre- and post-ECMO difference in carbon dioxide tension, mm Hg | –9.0 (–25 to –1) | –17 (–32 to –7) | –9 (–26 to –1) |
| 48-hr post-ECMO Pao2–pre-ECMO Pao2/pre-ECMO Pao2, % | 7 (–15 to 34) | 24 (–2 to 50) | 7 (–14 to 37) |
| 48-hr post-ECMO Paco2–pre-ECMO Paco2/pre-ECMO Paco2, % | –17 (–36 to –2) | –26 (–40 to –11) | –17 (–37 to –3) |
ECMO = extracorporeal membrane oxygenation.
Data are geometric mean (sd) or median (interquartile range) for changes pre- to post-ECMO.
Survival Analysis
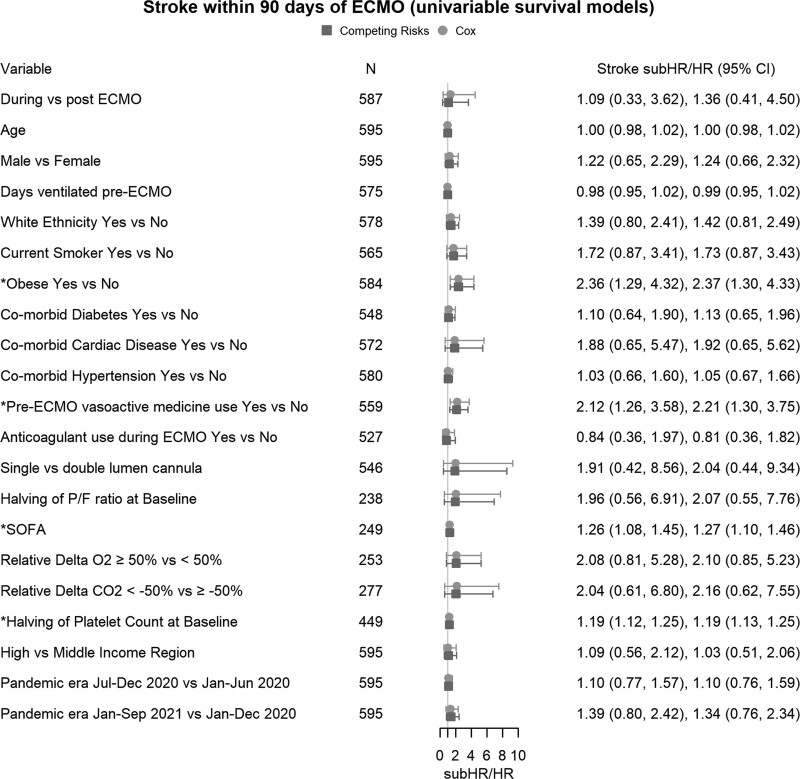
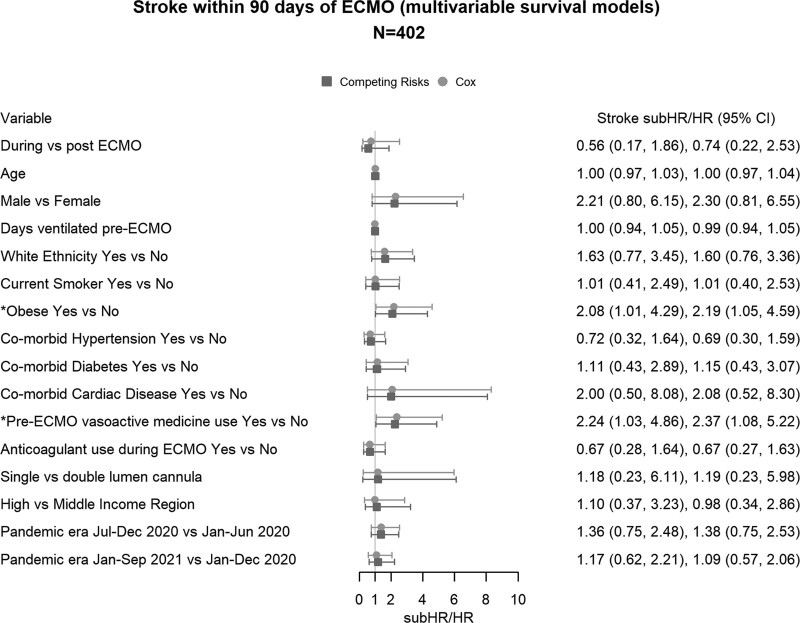
Univariable cause-specific survival analysis identified the following risk factors for stroke at ECMO start: the presence of obesity (n = 584; HR, 2.37; 95% CI, 1.30–4.33), use of vasoactive medications before ECMO (n = 559; HR, 2.21; 95% CI, 1.30–3.75), higher SOFA (n = 249; HR, 1.27; 95% CI, 1.10–1.46), and lower platelet count (n = 449; HR, 1.19; 95% CI, 1.13–1.25) (Fig. 2). There was a nonsignificant increase in the risk of stroke with more than 50% reduction in relative ΔPaco2 (n = 277; HR, 2.16; 95% CI, 0.62–7.55) and 50% increase in relative ΔPao2 (n = 253; HR, 2.10; 95% CI, 0.85–5.23). Multivariable survival analysis included the variables with less than 20% missing data (n = 402, 33 strokes). Obesity (HR, 2.19; 95% CI, 1.05–4.59) and the use of vasoactive medications before ECMO (HR, 2.37; 95% CI, 1.08–5.22) were independently associated with an increased risk of stroke (Fig. 3). Results from the Fine-Gray models with death (Figs. 2 and 3) and death and discharge as competing risks (Supplementary Fig. 1, http://links.lww.com/CCM/H324) were similar to the cause-specific Cox models.
Figure 2.
Univariable survival models of the risk factors for stroke. The combined estimates for cause-specific Cox model hazard ratios (HRs), Fine-Gray competing risk subdistribution HRs (subHRs) and number (n) for each model are shown in the forestplot. ECMO = extracorporeal membrane oxygenation, P/F ratio = the ratio of Pao2 to Fio2, relative delta Paco2 = 48-hr post-ECMO Paco2–pre-ECMO Paco2/pre-ECMO Paco2, relative delta Pao2 = 48-hr post-ECMO Pao2–pre-ECMO Pao2/pre-ECMO Pao2, SOFA = Sequential Organ Failure Assessment.
Figure 3.
Multivariable survival models of the risk factors with less than 50% missing data were included in a multivariate survival model. ECMO = extracorporeal membrane oxygenation, HR = hazard ratio, subHR = subdistribution hazard ratio.
Competing Risk Joint Models for Stroke
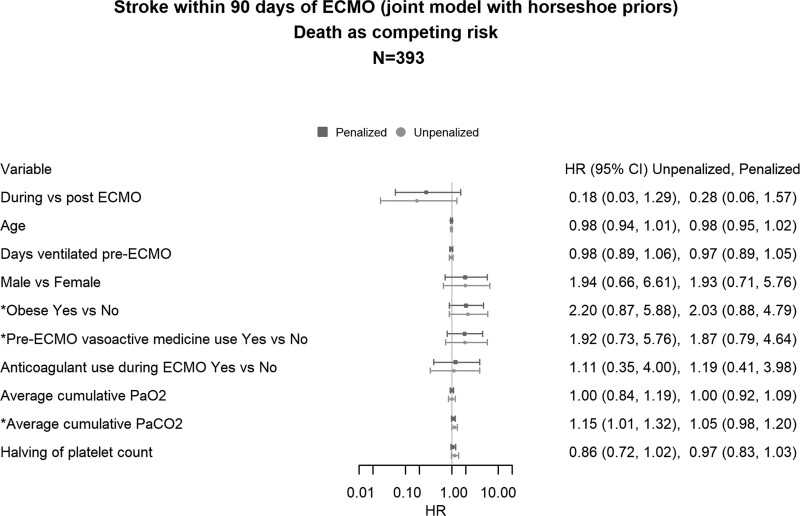
In the survival component of the joint model, using all available data (n = 472, 39 strokes), obesity (HR, 2.19; 95% CI, 1.05–4.60) and the use of vasoactive medications (HR, 2.11; 95% CI, 1.02–4.33) before ECMO initiation continued to be associated with an increased risk of stroke (Supplementary Fig. 2, http://links.lww.com/CCM/H324). The best-fitting joint model (n = 393, 35 strokes) used horseshoe priors as a penalty. In the unpenalized model, higher average cumulative Paco2 levels 48 hours after ECMO initiation were associated with an increased risk of stroke (HR, 1.15; 95% CI, 1.01–1.32) but were no longer associated after penalization (HR, 1.05; 95% CI, 0.98–1.20). However, of the three biomarkers included in the joint model, penalization identified that cumulative Paco2 was most associated with the risk of stroke. In joint models, obesity (HR, 2.03; 95% CI, 0.88–4.79) and the use of vasoactive medications (HR, 1.87; 95% CI, 0.79–4.64) were no longer associated with stroke, but the wider CIs compared with the survival models were attributed to the smaller sample size (n = 393) due to the exclusion of patients with missing data (Fig. 4). Arterial blood gas trajectories for Pao2 and Paco2 are plotted in Supplementary Figures 3 and 4 (http://links.lww.com/CCM/H324).
Figure 4.
The unpenalized and penalized models, the best-fitting model used horseshoe priors, followed by the unpenalized model, while the ridge priors had the worst fit. Hazard ratios (HRs) for the best fitting model with horseshoe penalized model are provided for both stroke and death outcomes in the forestplot. The forestplot for penalized HRs with horseshoe priors, showed that the regression coefficients shrunk closer to the null and that all HRs for stroke now included 1.0, including Paco2 for stroke. Average cumulative Paco2 = the average Paco2 during the extracorporeal membrane oxygenation support, average cumulative Pao2 = the average Pao2 during the extracorporeal membrane oxygenation support, ECMO = extracorporeal membrane oxygenation.
DISCUSSION
Among 595 patients with severe ARDS from COVID-19 supported by venovenous ECMO, the frequency of stroke within 90 days was 7.2%. Development of stroke was associated with substantially higher in-hospital mortality (79% at 90 d), which is higher than the reported mortality in patients without stroke (45%). We identified several risk factors associated with stroke development, including obesity, pre-ECMO use of vasoactive medication, high SOFA score, low platelet count, higher Paco2 levels before ECMO support, and the higher ΔPaco2. These findings emphasize several clinically relevant points and spark the need for future studies. Since obesity and the use of vasoactive medications were associated with an increased risk of stroke, mainly hemorrhagic, this finding emphasizes the need for more robust hemodynamic monitoring of those patients to assess the degree of hypovolemia and not relying solely on parameters that could be influenced by patient body habitus such as central venous pressure. Also, it emphasizes the risk of ischemia-reperfusion injury that represents the impairment of cerebral autoregulation associated with these risk factors. Whether gradual correction of pre-ECMO hypercarbia and hypoxemia when starting venovenous ECMO will reduce the risk of stroke is an important research question for both animal studies and prospective clinical research (18).
The frequency of stroke in this study is consistent with the previously published studies either in COVID-19 or severe ARDS due to other etiologies (11, 19, 20). The predominant stroke type was hemorrhagic (83.7%), which contrasts sharply with the type of strokes reported in COVID-19 patients who did not receive venovenous ECMO support (4). In a meta-analysis of 16 studies of COVID-19 patients, the frequency of stroke was 1.1%, and 96.6% were ischemic (21). The higher overall frequency of stroke and the hemorrhagic nature of most of the strokes in COVID-19 patients on venovenous ECMO may reflect the severity of illness, the intensity of the inflammatory response, and the need for systemic anticoagulation (4). Once a stroke develops, the outcome is worse than expected for COVID-19 patients on ECMO. In a systematic review of 1,322 patients from twelve case reports and cohort studies of COVID-19 patients supported by venovenous ECMO, the mortality of patients who suffered neurologic complications was 92% (22). This is consistent with other studies of patients who have strokes during ECMO. In a meta-analysis of 25 studies for different modes of ECMO, the development of hemorrhagic stroke was associated with a relative mortality risk of 1.27–4.43 (23). Similarly, in H1N1 patients supported with venovenous ECMO, hemorrhagic stroke was the most common cause of death (24).
Multiple factors could predispose COVID-19 patients on ECMO to stroke. Our analysis showed that obesity and pre-ECMO use of vasopressors are associated stroke. Obesity may predispose patients to well-established mechanisms underlying stroke, such as atherosclerotic and hypercoagulable pathophysiology (25). In COVID-19 patients, obesity is associated with poor outcomes (26). This may be due to the harmful effects of obesity on pulmonary mechanics as well as a higher basal metabolic rate (27). Also, adipose tissue has proinflammatory characteristics that intensify the systemic inflammatory response, and obese patients could have impaired adaptive immune responses (28). Hence, the higher tendency for stroke in COVID-19 in ECMO patients could reflect the intense inflammatory response that predisposes patients to coagulation and metabolic derangements (29). Also, lung involvement in severe ARDS causes hypoxemia and hypercapnia, which impair the autoregulatory mechanisms of the nervous system.
Our study highlighted that using vasopressors before initiating venovenous ECMO corresponds to stroke development. The need for vasopressors could reflect the severity of illness in stroke patients. However, in our study, there was no significant difference in the severity of illness in stroke and the nonstroke populations as conveyed by the Acute Physiology and Chronic Health Evaluation II or SOFA scores. More importantly, using vasopressors reduces cerebral blood flow by increasing cerebral vascular resistance and cerebral oxygen requirements (30). In addition, COVID-19 infection induces systemic inflammation, disrupts the cerebral microcirculation by inducing endothelial dysfunction, and increases the production of nitric oxide and inflammatory cytokines, further exacerbating the preexisting neuroinflammation and inducing thrombotic events (31).
An abrupt reduction in Paco2 around the time of ECMO initiation may be mechanistically linked to cerebrovascular complications (32). Cerebral vascular tone and autoregulation are sensitive to hyper- and hypocapnia. Hypercapnia causes cerebral vasodilation and edema, while hypocapnia causes vasoconstriction and a decrease in cerebral blood flow (33). Also, hypocapnia increases cerebral excitability and metabolic rate, which predisposes patients to seizure activity and increases cerebral oxygen demand (34). In a retrospective study of 11,972 patients enrolled in ELSO registry, a significant relative reduction (> 50%) in Paco2 in the first 24 hours after ECMO initiation was associated with neurologic complications (11).
Similarly, the level of Pao2 has significant clinical importance. Hypoxemia causes an increase in cerebral blood flow to maintain adequate oxygen delivery (35). Subsequent hyperoxia after placement on ECMO increases the risk of neurologic complications. In a study of 765 patients on venovenous ECMO, the presence of moderate hyperoxia (Pao2 101–300 mm Hg) was associated with increased mortality (36). The proposed mechanisms were the increase in reactive oxygen species and the accompanying vasoconstriction that will diminish the end-organ perfusion (37). Regarding stroke, a multicenter retrospective analysis of 2,894 ischemic and hemorrhagic stroke patients on mechanical ventilation revealed that hyperoxia was associated with increased mortality (38).
Our study showed that ECMO configurations (double-lumen vs dual-cannulas) had minimal effect on stroke development. Despite the theoretical risk of impaired cerebral venous drainage by the ECMO cannulas, results from an analysis of 6,834 patients enrolled in the ELSO registry point toward the minimal effect of cannulation strategy on neurologic complications (20). Given the missing data, the current analysis was not powered to elucidate the effect of anticoagulation and cannulation strategies on the frequency of stroke development. Our study had limited information regarding the vasopressors’ dose and duration. In addition, multiple limitations are intrinsic to the study’s retrospective nature. It is an observational study with the potential presence of many unmeasured confounders. Also, the multicenter design of the study did not account for the difference in the protocols and management strategies among the centers, and this heterogeneity is not fully controlled in our retrospective study. To address this, we used robust ses clustered by site to account for potentially correlated outcomes within each site. Furthermore, some of our estimates for the hazard of stroke development had wide 95% CIs indicating uncertainty in the effect size. We tried to overcome these challenges, particularly in missing data, through a staged approach using univariable analysis followed by multivariable and joint modeling, including data with less than 20% missingness, conducting sensitivity analyses and checking for consistency across models. As an example, the 95% CIs for obesity and pre-ECMO vasopressor drug use were above 1.0 in all models except the joint model (with the smallest sample size), but the point estimates for the HRs were close to 2.0 across models. In contrast, 95% CIs for sex contained 1.0 for all models. HR estimates and 95% CIs for the longitudinal biomarkers can be obtained from the unpenalized joint model, while the multivariable survival model may be used for the remaining covariates since the estimates are more precise and more covariates were included. To provide additional evidence for the risk factors where the lower 95% is very close to 1, replication in an independent cohort, potentially with fewer centers to reduce heterogeneity in the data, would be useful. We could not conduct stroke subtype analysis because of the small number of stroke patients.
CONCLUSIONS
Our multicenter, observational study demonstrated that the rate of stroke in COVID-19 patients supported by venovenous ECMO is low yet confers a poor outcome. Obesity and pre-ECMO use of vasopressors were highly associated with stroke. Also, our report emphasizes the importance of rapid decreases in Paco2 within 48 hours of ECMO initiation as well as the impact of moderate hyperoxia in the development of stroke. The findings of our analysis could be encouraging to the scientific community to conduct further transitional and clinical research to further highlight our findings and the potential underlying mechanisms.
ACKNOWLEDGMENTS
We recognize the crucial importance of the International Severe Acute Respiratory and Emerging Infection Consortium and Short PeRiod IncideNce sTudy of Severe Acute Respiratory Infection networks for the development and expansion of the COVID-19 Critical Care Consortium. We thank the generous support we received from Extracorporeal Life Support Organization and Extracorporeal Membrane Oxygenation Network. We owe Li Wenliang, MD, from the Wuhan Central Hospital, an eternal debt of gratitude for reminding the world that doctors should never be censored during a pandemic. Finally, we acknowledge all members of the COVID-19 Critical Care Consortium, the COVID Critical Care statistical team for sharing code and advice, and various collaborators.
Supplementary Material
Footnotes
*See also p. 1098.
New affiliation for Dr. Suen: School of Pharmacy and Medical Sciences, Griffith University, Brisbane, QLD, Australia.
Drs. Zaaqoq, Griffee, Kelly, Heinsar, Lorusso, Peek, and Cho conceived the study. Drs. Kelly, Heinsar, Suen, Li Bassi, Jacobs, and White collected the data. Drs. Zaaqoq, Griffee, Kelly, Peek, and Cho conducted data analysis. Drs. Zaaqoq, Griffee, Kelly, Fanning, Jacobs, Peek, and Cho drafted the article. All authors helped to revise the draft of the article. All authors read and approved the final article.
The Bill & Melinda Gates Foundation (Grant number INV-034765), The University of Queensland, The Wesley Medical Research, The Prince Charles Hospital Foundation, and The Health Research Board of Ireland.
Drs. Kelly’s, Heinsar’s, and Suen’s institutions received funding from the Bill and Melinda Gates Foundation. Drs. Kelly, Heinsar, Suen, and Fraser received support for article research from the Bill and Melinda Gates Foundation. Dr. Heinsar received funding from The Prince Charles Hospital Foundation. Dr. Suen is funded by the Advance Queensland fellowship program. Dr. Li Bassi is a recipient of the Biomedicine International training research programme for excellent clinician-scientists (BITRECS) fellowship; the “BITRECS” project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754550 and from the “La Caixa” Foundation (identification number 100010434), under the agreement LCF/PR/GN18/50310006. Dr. Jacobs received funding from SpecialtyCare and the American Academy of Dermatology. Dr. Fraser’s institution received funding from Fisher & Paykel, Mallinckrodt Pharmaceuticals, and Lendlease; he received funding from De Motu Cordis, Philips Electronics, International Society for Heart and Lung Transplantation, and General Practice Training Queensland. Dr. Lorusso’s institution received funding from Medtronic, LivaNova, Getinge, Eurosets, Corcym, Hemocue, and Xenios. Dr. Cho received support for article research from the National Institutes of Health; he is funded by the National Heart, Lung, and Blood Institute 1K23HL157610. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
COVID-19 Critical Care Consortium (COVID Critical) are listed in the Supplementary Material (http://links.lww.com/CCM/H324).
The datasets used during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Alhazzani W, Møller MH, Arabi YM, et al. : Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020; 48:e440–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badulak J, Antonini MV, Stead CM, et al. : Extracorporeal membrane oxygenation for COVID-19: Updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 2021; 67:485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization: Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international extracorporeal life support organization registry. Lancet (London, England). 2021; 398:1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho SM, Canner J, Caturegli G, et al. : Risk factors of ischemic and hemorrhagic strokes during venovenous extracorporeal membrane oxygenation: Analysis of data from the extracorporeal life support organization registry. Crit Care Med. 2021; 49:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho SM, Canner J, Chiarini G, et al. : Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: Results from the extracorporeal life support organization registry. Crit Care Med. 2020; 48:e897–e905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nannoni S, de Groot R, Bell S, et al. : Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke. 2021; 16:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esenwa C, Cheng NT, Luna J, et al. : Biomarkers of coagulation and inflammation in COVID-19-associated ischemic stroke. Stroke. 2021; 52:e706–e709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heilmann C, Geisen U, Beyersdorf F, et al. : Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS). Intensive Care Med. 2012; 38:62–68 [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner MV, Philipp A, Lubnow M, et al. : Hemostatic changes during extracorporeal membrane oxygenation: A prospective randomized clinical trial comparing three different extracorporeal membrane oxygenation systems. Crit Care Med. 2016; 44:747–754 [DOI] [PubMed] [Google Scholar]
- 10.Mazzeffi M, Kon Z, Menaker J, et al. : Large dual-lumen extracorporeal membrane oxygenation cannulas are associated with more intracranial hemorrhage. ASAIO J. 2019; 65:674–677 [DOI] [PubMed] [Google Scholar]
- 11.Cavayas YA, Munshi L, Del Sorbo L, et al. : The early change in Pa(CO(2)) after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am J Respir Crit Care Med. 2020; 201:1525–1535 [DOI] [PubMed] [Google Scholar]
- 12.Rubin R: Global effort to collect data on ventilated patients with COVID-19. JAMA. 2020; 323:2233–2234 [DOI] [PubMed] [Google Scholar]
- 13.COVID-19 Critical Care Consortium: COVID-19 Critical Care Consortium Observational Study Incorporating the Extracorporeal Membrane Oxygenation for 2019 Novel Coronavirus Acute Respiratory Disease (ECMOCARD). 2020. Available at: https://www.covid-critical.com/. Accessed November 26, 2020
- 14.Li Bassi G, Suen J, Barnett AG, et al. : The COVID-19 Critical Care Consortium observational study: Design and rationale of a prospective, international, multicenter, observational study. medRxiv Preprint posted online June 2, 2020. doi: 10.1101/2020.05.29.20115253 [Google Scholar]
- 15.Dunning JW, Merson L, Rohde GGU, et al. ; ISARIC Working Group 3, ISARIC Council: Open source clinical science for emerging infections. Lancet Infect Dis. 2014; 14:8–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy S: Using research to prepare for outbreaks of severe acute respiratory infection. BMJ Global Health. 2019; 4:e001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC, Fine JP: Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017; 36:4391–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aubron C, DePuydt J, Belon F, et al. : Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Inten Care. 2016; 6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho SM, Premraj L, Fanning J, et al. : Ischemic and hemorrhagic stroke among critically ill patients with coronavirus disease 2019: An International Multicenter Coronavirus Disease 2019 Critical Care Consortium Study. Crit Care Med. 2021; 49:e1223–e1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorusso R, Belliato M, Mazzeffi M, et al. : Neurological complications during veno-venous extracorporeal membrane oxygenation: Does the configuration matter? A retrospective analysis of the ELSO database. Crit Care. 2021; 25:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamakawa M, Kuno T, Mikami T, et al. : Clinical characteristics of stroke with COVID-19: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020; 29:105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannapadi NV, Jami M, Premraj L, et al. : Neurological complications in COVID-19 patients with ECMO support: A systematic review and meta-analysis. Heart Lung Circ. 2022; 31:292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher-Sandersjöö A, Thelin EP, Bartek J, et al. : Incidence, outcome, and predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: A systematic and narrative review. Front Neurol. 2018; 9:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies A, Jones D, Bailey M, et al. ; Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators: Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009; 302:1888–1895 [DOI] [PubMed] [Google Scholar]
- 25.Kernan WN, Inzucchi SE, Sawan C, et al. : Obesity: A stubbornly obvious target for stroke prevention. Stroke. 2013; 44:278–286 [DOI] [PubMed] [Google Scholar]
- 26.Hajifathalian K, Kumar S, Newberry C, et al. : Obesity is associated with worse outcomes in COVID-19: Analysis of early data from New York City. Obesity (Silver Spring, Md). 2020; 28:1606–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonnet A, Chetboun M, Poissy J, et al. ; LICORN and the Lille COVID-19 and Obesity study group: High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring, Md). 2020; 28:1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sattar N, McInnes IB, McMurray JJV: Obesity is a risk factor for severe COVID-19 infection: Multiple potential mechanisms. Circulation. 2020; 142:4–6 [DOI] [PubMed] [Google Scholar]
- 29.Kowalewski M, Fina D, Słomka A, et al. : COVID-19 and ECMO: The interplay between coagulation and inflammation—a narrative review. Critical Care. 2020; 24:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brassard P, Seifert T, Secher NH: Is cerebral oxygenation negatively affected by infusion of norepinephrine in healthy subjects? Br J Anaesth. 2009; 102:800–805 [DOI] [PubMed] [Google Scholar]
- 31.McAlpine LS, Zubair AS, Maran I, et al. : Ischemic stroke, inflammation, and endotheliopathy in COVID-19 patients. Stroke. 2021; 52:e233–e238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts BW, Karagiannis P, Coletta M, et al. : Effects of PaCO2 derangements on clinical outcomes after cerebral injury: A systematic review. Resuscitation. 2015; 91:32–41 [DOI] [PubMed] [Google Scholar]
- 33.Battisti-Charbonney A, Fisher J, Duffin J: The cerebrovascular response to carbon dioxide in humans. J Physiol. 2011; 589:3039–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huttunen J, Tolvanen H, Heinonen E, et al. : Effects of voluntary hyperventilation on cortical sensory responses. Electroencephalographic and magnetoencephalographic studies. Exp Brain Res. 1999; 125:248–254 [DOI] [PubMed] [Google Scholar]
- 35.Harris AD, Murphy K, Diaz CM, et al. : Cerebral blood flow response to acute hypoxic hypoxia. NMR Biomed. 2013; 26:1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munshi L, Kiss A, Cypel M, et al. : Oxygen thresholds and mortality during extracorporeal life support in adult patients*. Crit Care Med. 2017; 45:1997–2005 [DOI] [PubMed] [Google Scholar]
- 37.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. : Association between arterial hyperoxia and outcome in subsets of critical illness: A systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015; 43:1508–1519 [DOI] [PubMed] [Google Scholar]
- 38.Rincon F, Kang J, Maltenfort M, et al. : Association between hyperoxia and mortality after stroke: A multicenter cohort study. Crit Care Med. 2014; 42:387–396 [DOI] [PubMed] [Google Scholar]